Summary
Background
Pregnant women with SARS-CoV-2 infection experience higher rates of stillbirth and preterm birth. A unique pattern of chronic histiocytic intervillositis (CHI) and/or massive perivillous fibrin deposition (MPFD) has emerged, coined as SARS-CoV-2 placentitis.
Methods
The aim of this study was to describe a cohort of placentas diagnosed with SARS-CoV-2 placentitis during October 2020-March 2021. Cases with a histological diagnosis of SARS-CoV-2 placentitis and confirmatory immunohistochemistry were reported. Maternal demographic data, pregnancy outcomes and placental findings were collected.
Findings
59 mothers delivered 61 infants with SARS-CoV-2 placentitis. The gestational age ranged from 19 to 41 weeks with most cases (78.6%) being third trimester. 30 infants (49.1%) were stillborn or late miscarriages. Obese mothers had higher rates of pregnancy loss when compared with those with a BMI <30 [67% (10/15) versus 41% (14/34)]. 47/59 (79.7%) mothers had a positive SARS-CoV-2 PCR test either at the time of labour or in the months before, of which 12 (25.5%) were reported to be asymptomatic. Ten reported only CHI, two cases showed MPFD only and in 48 placentas both CHI and MPFD was described.
Interpretation
SARS-CoV2 placentitis is a distinct entity associated with increased risk of pregnancy loss, particularly in the third trimester. Women can be completely asymptomatic and still experience severe placentitis. Unlike ‘classical’ MPFD, placentas with SARS-CoV-2 are generally normal in size with adequate fetoplacental weight ratios. Further work should establish the significance of the timing of maternal SARS-CoV-2 infection and placentitis, the significance of SARS-CoV2 variants, and rates of vertical transmission associated with this pattern of placental inflammation.
Funding
There was not funding associated with this study.
Keywords: COVID-19, Chronic histiocytic intervillositis, Massive perivillous fibrin deposition, Placentitis, SARS-CoV2, Stillbirth
Research in context.
Evidence before this study
Published studies from UK Obstetric Surveillance System (UKOSS) showed that stillbirth incidence was three times higher among women affected by SARS-CoV-2. Placental compromise was considered likely to be one of the determining factors in the outcome of pregnancies affected by the virus through a pattern of injury called ‘SARS-CoV-2 placentitis’. A PubMed literature search was conducted using the terms: SARS-CoV2 placentitis, stillbirth and COVID-19, COVID-19 and pregnancy, SARS-CoV2 and pregnancy. Relevant articles published in English between March 2020 and 1st August 2021 were retrieved.
Added value of this study
Our study confirmed that SARS-CoV2 placentitis is a distinct pathological entity, usually characterised by the presence of massive perivillous fibrin deposition and chronic histiocytic intervillositis, and associates with increased risk of pregnancy loss, particularly in the third trimester. Pregnancy loss was higher among mothers with a high BMI.
Implications of all the available evidence
Our result may have relevance in the implementation of diagnostic and therapeutic options in these women. The findings add to the evidence base, but are preliminary and will need further confirmation.
Alt-text: Unlabelled box
Introduction
In July 2020, a study published by UK Obstetric Surveillance System (UKOSS) indicated that the incidence of stillbirth (11.5 per 1000 total births) among women affected by SARS-CoV-2 virus was almost three times the national rate (4.1 per 1000 total births).1, 2 Since then, the incidence of pregnancy complications such as spontaneous abortion, stillbirths and premature delivery have increased. This may be related to the higher transmission rate of new variants of SARS-CoV-2.3,4
In the second UKOSS study conducted from March to August 2020, the estimated incidence of hospitalisation with symptomatic SARS-CoV-2 was calculated to be 2.0 per 1000 pregnancies.4 In this study women hospitalised with symptomatic SARS-CoV-2 were more likely to be overweight or obese and/or to be of Black, Asian or another minority ethnic group and/or and to have a relevant medical comorbidity, compared to pregnant women not infected with SARS-CoV-2. Although hospitalised pregnant women with symptomatic infection were more likely to be admitted to intensive care and require caesarean section delivery, the risk of poor outcomes was small.4 There was no significant increase in the number of stillbirths or neonatal deaths between March and August 2020 with 3% (n = 31) of hospitalised pregnant women with SARS-CoV-2 reporting pregnancy loss, compared to < 1% (n = 2) of those without SARS-CoV-2.4
Provisional data from UKOSS are concerning for an increased risk of severe illness in hospitalised pregnant women, though this is yet, to our knowledge, to be presented in a peer reviewed publication.
Placental compromise as a consequence of SARS-CoV2 maternal infection is likely to be one of the determining factors in the outcome of pregnancies affected by the virus. Various histopathological patterns have been described in the setting of SARS-CoV-2 infection including maternal vascular malperfusion, fetal vascular malperfusion, massive perivillous fibrin deposition, and inflammatory patterns including acute chorioamnionitis, chronic deciduitis, chronic villitis and chronic histiocytic intervillositis.5 In the United Kingdom (UK), specialist paediatric and perinatal pathologists have noticed an increasing incidence of a rare placental pathology similar to chronic histiocytic intervillositis (CHI) associated with massive perivillous fibrin deposition (MPFD). This was first described in Ireland by Linehan et al., who demonstrated the presence of the virus within the syncytiotrophoblast compartment of the placenta and termed the pattern of injury ‘SARS-CoV-2 placentitis’.6 SARS-CoV-2 placentitis is suggested by other groups to be associated with increased risks of vertical transmission of the virus.7,8
Although both CHI and MPFD were known to affect the placentas of pregnant women before the SARS-CoV-2 pandemic, the incidence of both conditions is very low, reported as <1% of all pregnancies.9 Non-SARS-CoV2 associated CHI and MPFD are both presumed to have an immunological basis, carry a very high recurrence risk, and are associated with intrauterine growth restriction in the fetus.9 Whilst MPFD can be seen with some histiocytic infiltrates, in pre-pandemic placentas, the association of diffuse CHI and MPFD has seldom been reported.10
The study aim was to report on the collective experience of paediatric and perinatal pathologists across the UK with those placental histological features present in the context of SARS-CoV2 infection. Our objective was to characterise a cohort of placentas diagnosed with SARS-CoV-2 placentitis during the Alpha wave an describe the pregnancy outcome.
Methods
Study design and participants
Paediatric and Perinatal Pathologists from ten tertiary centres identified cases in which there was a histological diagnosis SARS-CoV-2 placentitis. This included cases of CHI or/and MPFD with SARS-CoV-2 infection proven with PCR (in 47/59 mothers and in 15/61 placentas). SARS-CoV-2 infection was confirmed in all placentas by positive immunohistochemical staining of the syncytiotrophoblast. With the exception of one case from March 2020, all other cases were from deliveries occurring between October 2020 and March 2021, during the peak of the Alpha wave. Maternal demographic data including ethnicity and Body Mass Index (BMI), were collected, along with maternal co-morbidities, and pregnancy outcomes including gestational age, birth weight and SARS-CoV-2 status of both the mother and baby. Placental findings including weight and histological diagnosis were also collected. The histological and demographic features were discussed during virtual meetings, and all authors had access to the database.
Transmission electron microscopy
In four cases, ultrathin sections of the placenta were examined by transmission electron microscopy.
In these four cases the tissue had been primarily fixed in 10% phosphate buffered formalin and processed to wax as per standard histological protocol. A portion was removed from the wax, chopped into mm cubes, dewaxed in xylene, rehydrated through graded alcohols to distilled water, followed by post-fixation in 2% aqueous osmium tetroxide; dehydrated through graded alcohols, acetone and into TAAB EMIX medium grade epoxy resin.
Following heat polymerisation (16 h at 65°Centigrades), resin blocks were sectioned using a Reichert ultracut E ultramicrotome at 0.6 µm, stained using 1% toluidine blue in 1% sodium tetraborate and examined light microscopically. The most appropriate block selected and thin-sectioned using a Diatome diamond knife at 85 nm. Thin-sections were stained in saturated uranyl acetate in 99% ethanol, and Reynold's lead citrate.
Sections were examined on a Philips 400 transmission electron microscope and photographed using images taken using AMT 16 megapixel mid mount digital camera XR16. Images taken in TIF format and converted to JPG with minimum compression.
In one case Covid-19 spike protein and RNA scope signal was detected and findings were published as a case report.11
Ethics
The Department of Clinical Governance considered that the study did not fall under the category of original research as no investigations were undertaken for research purposes and there was no control group established. The cases (demographics and histological findings) were anonymously discussed at online meetings. Clinical Governance concluded that this multicentric study did not require Ethical approval. Patients’ consent was not required as no histological review was undertaken and the cases remained anonymised. The study was approved as a national audit project by Sheffield Children's NHS FT Clinical Governance, Registration SE1642.
Role of the Founding source:
No funding was received. Sophie Stenton and Marta C Cohen had full access to the data. All authors agreed to submit the study for publication.
Results
59 mothers delivered 61 infants in which each placenta had a pathological diagnosis of MPFD+/- CHI with positive immunohistochemical staining for SARS-CoV-2 spike protein in the syncytiotrophoblast. With the exception of one case from March 2020, all other cases were from deliveries between October 2020 and March 2021. The gestational age was available in 60 cases (except one un-booked pregnancy) and ranged from 19 to 41 completed weeks with a mean of 30 completed weeks. 48 cases were third trimester placentas (defined as 28 weeks and beyond), of which five had reached term (defined as 37 completed weeks and beyond), with the remaining known gestational ages second trimester placentas. Table 1 presents the pregnancy outcome and birthweight (this latter was not always available).
Table 1.
Pregnancy outcome.
| Case Number | Month of delivery | Gestation (weeks +days) | Pregnancy Outcome | Birthweight | Personalised Growth Chart Calculation | WHO Growth Chart Centiles |
|---|---|---|---|---|---|---|
| 1 | Dec-20 | 32 | stillbirth | 1590 | 8th | – |
| 2 | Dec-20 | 41 | stillbirth | 2180 | <1st | – |
| 3 | Dec-20 | 25 | stillbirth | 615 | 1st | – |
| 4 | Dec-20 | 29 | livebirth | 1225 | – | 50–75 |
| 5 (TWIN) | Jan-21 | 31 | stillbirth | 1630 | 11th | – |
| 6 (TWIN) | Jan-21 | 31 | stillbirth | 1440 | 2nd | – |
| 7 | Jan-21 | 36 | livebirth | 2570 | 16th | – |
| 8 | Jan-21 | 34 | livebirth | 2130 | 22nd | – |
| 9 | Jan-21 | 33 | stillbirth | 1700 | 3rd | – |
| 10 | Jan-21 | 31 | livebirth | 1950 | – | 75–91 |
| 11 | Jan-21 | 34 | livebirth | not stated | – | Not known |
| 12 | Jan-21 | 34 | livebirth | not stated | – | Not known |
| 13 | Feb-21 | 39 | livebirth | 3625 | – | 75–90th |
| 14 | Feb-21 | 36 | livebirth | 2300 | – | 9–25th |
| 15 | Mar-21 | 34 | livebirth | 1550 | – | 2–9th |
| 16 | Mar-21 | 35 | livebirth | 2530 | – | 50–75 |
| 17 | Mar-21 | 31 | livebirth | 1460 | – | 25–50 |
| 18 | Jan-21 | 20 | Miscarriage | 320 | Not calculable | Not calculable- |
| 19 | April 2020 | 25 | stillbirth | 762 | 16 | N/A |
| 20 | Jan-21 | 29 | stillbirth | 1174 | 2 | N/A |
| 21 | Feb-21 | 19 | Miscarriage | 162 | Not calculable | Not calculable |
| 22 | Dec 2020 | 26 | stillbirth | 760 | 4 | – |
| 23 | Dec 2020 | 35 | stillbirth | 2000 | 3 | – |
| 24 | Jan-21 | 27 | Livebirth | 718 | 1 | – |
| 25 | Jan-21 | 30 | stillbirth | 1170 | 1 | – |
| 26 | Jan-21 | 33 | stillbirth | Not known | Not calculable | Not calculable |
| 27 | Feb-21 | Unknown | stillbirth | 1100 | Not calculable | Not calculable |
| 28 | Nov-20 | 35+6 | Livebirth | 2195 g | 4.3 | – |
| 29 | Dec-20 | 30+5 | Livebirth | 1500 g | 60 | – |
| 30 | Jan-21 | 25+4 | Stillbirth | 775 g | 32 | – |
| 31 | Mar-20 | 32+4 | Livebirth | 1680 | – | 25–50 |
| 32 | Nov-20 | 29+3 | Stillbirth | 1220 | – | 25–50 |
| 33 | Mar-21 | 36+4 | Livebirth | 2280 | – | 9–25 |
| 34 | Feb-21 | 34+4 | Livebirth | 2060 | 50 | – |
| 35 | Dec-20 | 32+4 | Stillbirth | 2010 | – | 75–90 |
| 36 | Jan-21 | 36+4 | Stillbirth | 2780 | – | 50–75 |
| 37 | Feb-21 | 32 | Livebirth | 1364 | – | 9–25 |
| 38 | Nov-20 | 26+4 | Livebirth | 700 | – | 9–25 |
| 39 | Mar-21 | 32+6 | Livebirth | 1890 | 58.07 | 75–91 |
| 40 | Mar-21 | 30+6 | Livebirth | 1320 | – | 25–50 |
| 41 | Mar-21 | 34+4 | Livebirth | 2180 | – | 50–75 |
| 42 | Mar-21 | 37+6 | Livebirth | 2550 | – | 9–25 |
| 43 | Oct-20 | 34+2 | Stillbirth | 2020 | 11.7 | – |
| 44 | Dec-20 | 31 | Stillbirth | 1395 | – | 25–50 |
| 45 | Oct-20 | 37+3 | Stillbirth | 3150 | – | 50–75 |
| 46 | Jun-20 | 37+3 | Livebirth | 2775 | – | 25–50 |
| 47 (TWIN) | Jan-21 | 28+5 | Livebirth | 1390 | – | 75–91 |
| 48 (TWIN) | Jan-21 | 28+5 | Livebirth | 1090 | – | 25–50 |
| 49 | Jan-21 | 31+6 | Livebirth | 1930 | – | 75–91 |
| 50 | Jan-21 | 33+2 | Livebirth | 1500 | – | 9 |
| 51 | Jan-21 | 34+6 | Livebirth | 3050 | – | 91–98 |
| 52 | Jan-21 | 33+2 | Livebirth | 1734 | – | 09–25 |
| 53 | Mar-21 | 41+4 | Livebirth | 2220 | – | <0.4 |
| 54 | Feb-21 | 28 | Stillbirth | 980 | – | 09 to 25 |
| 55 | Jan-21 | 31 | Stillbirth | 1450 | – | 09 to 25 |
| 56 | Jan-21 | 21+6 | Miscarriage | 428 | Not calculable | Not calculable |
| 57 | Jan-21 | 22+1 | Miscarriage | 373 | Not calculable | Not calculable |
| 58 | Jan-21 | 27 | Stillbirth | 855 g | – | 9–25 |
| 59 | Jan-21 | 23+1 | Miscarriage | XX | Not calculable | Not calculable |
| 60 | Jan-21 | 32+6 | Stillbirth | 1738 | – | 50th |
| 61 | Feb-21 | 28+1 | Stillbirth | 910 | 9–25th | – |
Maternal demographics (see Table 2)
Table 2.
Maternal demographics.
| Case Number | Pregnancy Outcome | Maternal ethnicity | Maternal BMI | Maternal co-morbidities | Maternal COVID Status | Maternal Symptoms? Asymptomatic? | Date of Positive Test in relation to delivery |
|---|---|---|---|---|---|---|---|
| 1 | Stillbirth | White/Caucasian | 18 | – | positive | symptomatic | – |
| 2 | Stillbirth | Asian | 19.6 | Hypertension | positive | symptomatic | – |
| 3 | Stillbirth | Black African or Afro Caribbean | 22.7 | – | – | – | – |
| 4 | Livebirth | Asian | 37 | – | positive | – | – |
| 5 + 6 (TWINs) | Stillbirth | Black African or Afro Caribbean | 27.7 | SLE, hyperthyroid | positive | asymptomatic | – |
| 7 | Livebirth | White/Caucasian | 25.5 | – | positive | symptomatic | – |
| 8 | Livebirth | Black African or Afro Caribbean | 28.7 | – | positive | symptomatic | – |
| 9 | Stillbirth | Asian | 24.4 | – | positive | asymptomatic | – |
| 10 | Livebirth | – | – | – | positive | Critical illness with intensive care | – |
| 11 | Livebirth | Black African or Afro Caribbean | 26.9 | Sickle cell trait | positive | symptomatic | 10 days |
| 12 | Livebirth | – | – | – | – | – | – |
| 13 | Livebirth | – | – | – | positive | – | – |
| 14 | Livebirth | Asian | 26.9 | – | positive | asymptomatic | – |
| 15 | Livebirth | White/Caucasian | 24 | – | negative | – | – |
| 16 | Livebirth | White/Caucasian | 21.5 | – | positive | symptomatic | – |
| 17 | Livebirth | Black African or Afro Caribbean | 24.5 | – | negative | – | – |
| 18 | Miscarriage | White/Caucasian | 36 | Hypertension and Hepatitis C | positive | – | 2 days |
| 19 | Stillbirth | White/Caucasian | 27.9 | – | positive | symptomatic | – |
| 20 | Stillbirth | White/Caucasian | 22.9 | Anticardiolipin antibodies, recurrent miscarriages | positive | Asymptomatic | – |
| 21 | Miscarriage | Black African or Afro Caribbean | 27.4 | Rheumatoid arthritis, intra-cranial hypertension, anxiety, depression, congenital heart disease | positive | Symptomatic | – |
| 22 | stillbirth | Asian | 55.2 | hypertension, asthma, hypothyreosis, multiple miscarriages | positive | Critical illness requiring intensive care | – |
| 23 | Stillbirth | Asian | 27 | Anaemia | positive | Symptomatic | 9 days |
| 24 | Livebirth | White/Caucasian | 26.6 | – | Positive | Symptomatic | >1 month and on delivery day |
| 25 | Stillbirth | White/Caucasian | 30.1 | Ex-smoker | Positive | Symptomatic | |
| 26 | Stillbirth | Asian | 27 | positive | Asymptomatic | 0 day | |
| 27 | Stillbirth | White/Caucasian | 39 | Smoker | positive | Asymptomatic | |
| 28 | Livebirth | White/Caucasian | 21.79 | – | Positive | Symptomatic | 10 days |
| 29 | Livebirth | White/Caucasian | 26.49 | – | – | Critical illness requiring intensive care | 0 days |
| 30 | Stillbirth | White/Caucasian | 22 | – | Positive. | – | – |
| 31 | Livebirth | Asian | – | Congenital heart disease, Stroke | Positive | Symptomatic | 0 days |
| 32 | Stillbirth | White/Caucasian | 43.2 | – | – | – | – |
| 33 | Livebirth | – | – | – | – | – | – |
| 34 | Livebirth | Asian | 50 | HELLP | Positive | – | >1 month |
| 35 | Stillbirth | White/Caucasian | 35 | – | Positive | Asymptomatic | – |
| 36 | Stillbirth | White/Caucasian | 39.1 | Gestational Diabetes | Positive | Symptomatic | – |
| 37 | Livebirth | White/Caucasian | 42 | Smoker | Positive | Asymptomatic | 2 weeks |
| 38 | Livebirth | Asian | 22.9 | ‘Cardiac murmur’ | Positive | Symptomatic | >2 months |
| 39 | Livebirth | White/Caucasian | 18.4 | – | – | – | – |
| 40 | Livebirth | White/Caucasian | 25 | – | Positive | – | – |
| 41 | Livebirth | – | – | Abruption | Positive | – | November 2020 |
| 42 | Livebirth | White/Caucasian | 27 | – | – | – | – |
| 43 | Stillbirth | White/Caucasian | 27.3 | – | Positive | Asymptomatic | 10 days- |
| 44 | Stillbirth | White/Caucasian | 30 | – | Positive | Asymptomatic | – |
| 45 | Stillbirth | White/Caucasian | 39.4 | – | Positive | Symptomatic | – |
| 46 | Livebirth | White/Caucasian | 29.4 | – | Positive | – | – |
| 47+48 (TWIN) | Livebirth | White/Caucasian | 27.22 | – | Positive | – | |
| 49 | Livebirth | White/Caucasian | 34.6 | – | Positive | Symptomatic | 0 days |
| 50 | Livebirth | White/Caucasian | 22.7 | Sjogren's syndrome | – | – | |
| 51 | Livebirth | White/Caucasian | 41.91 | – | Positive | – | 0 days |
| 52 | Livebirth | White/Caucasian | 26 | – | Positive | Asymptomatic | 3 weeks |
| 53 | Livebirth | White/Caucasian | 29 | Uterine fibroids | – | – | |
| 54 | Stillbirth | White/Caucasian | 21.9 | Depression and post-traumatic stress disorder | – | Symptomatic | |
| 55 | Stillbirth | White/Caucasian | 42 | – | Positive | Symptomatic | 1 day |
| 56 | Miscarriage | – | – | – | Positive | – | 2 weeks |
| 57 | Miscarriage | – | – | Childhood leukaemia and thyroid carcinoma | Positive | – | >2 weeks |
| 58 | Stillbirth | White/Caucasian | 27 | – | Positive | Symptomatic | |
| 59 | Miscarriage | White/Caucasian | – | – | Positive | Asymptomatic | >one week |
| 60 | Stillbirth | White/Caucasian | – | – | Positive | Symptomatic | One week |
| 61 | Stillbirth | White/Caucasian | 27.2 | Deep Vein Thrombosis | Positive | – | One week |
Ethnicity
Maternal ethnicity was available in 52/59 cases, with 36 identifying as Caucasian (‘white British’ or ‘white, European’) and 16 of Black and minority ethnic groups (BAME; 10 Asian, 6 Black Caribbean or Black African).
BMI
BMI was available in 49 mothers, ranging from 18 to 55.2 kg/m2. Of these, 14 (28.5%) had a BMI of 18.5–25 kg/m2, 20 (40.8%) had a BMI of between 25 and 30 kg/m2. and 15 were obese; two (4.1%) had a BMI of 30–35 kg/m2 and 13 (26.4%) were >35 kg/cm2.
Co-morbidities
Thirty-eight had no reported previous medical history. 14 had some form of cardiovascular risk factor including hypertension (n = 3), and one each of deep vein thromboses, anti-cardiolipin antibodies, sickle cell trait, smoking in pregnancy, ex-smoker, gestational diabetes, Systemic Lupus Erythematous (SLE), and Haemolysis and Elevated Liver enzymes and Low Platelets (HELLP Syndrome). One mother had a ‘heart murmur’ and two others had congenital heart disease.
Three had known histories of depression, childhood malignancy, and Sjogren's syndrome.
Antenatal complications
Six women had experienced reduced fetal movements in the current pregnancy (as clinical evidence of fetal compromise). Two reported antepartum haemorrhage and one presented with oligohydramnios.
Maternal SARS-CoV-2 status
47/59 (79.7%) mothers had a positive SARS-CoV-2 Polymerase Chain Reaction (PCR) test either at the time of labour or in the months before. Two mothers (3.4%) had tested negative for SARS-CoV-2 at the time of delivery and were not known to have had SARS-CoV2 at any stage of their pregnancy. In 10 instances (16.9%), maternal SARS-CoV2 status was either not known to clinicians or unavailable.
Of the 47 mothers with a positive SARS-CoV-2 PCR test, 12 (25.5%) were reported to be asymptomatic. 22 (46.8%) reported symptoms at some stage including pyrexia (n = 10), breathlessness and cough (n = 3) and headaches (n = 2). Three had severe illness requiring intensive care admission. In the remaining 13 cases, symptoms were unknown (27.6%).
In 22 cases the timing of a SARS-CoV-2 positive test could be calculated in relation to the delivery date: five mothers tested positive on the day of delivery and four tested positive less than 7 days prior to delivery. Six mothers tested positive between 7 and 14 days prior to delivery, seven tested positive more than 2 weeks prior to delivery, including one who tested positive four months before delivery.
Placental features (see Table 3)
Table 3.
Placental pathology.
| Case Number | Gestation (weeks + days) | Placenta percentile | Fetoplacental weight centile | Fibrin Deposition | Intervillositis |
|---|---|---|---|---|---|
| 1 | 32 | 10–25 | 50–75 | Diffuse | Present |
| 2 | 41 | <3 | 10–25 | Diffuse | Absent |
| 3 | 25 | 25–50 | 25 | Diffuse | Present |
| 4 | 29 | 10–25 | 50–75 | Diffuse | Present |
| 5 (TWIN) | 31 | 25–50 a | Not calculable | Diffuse | Absent |
| 6 (TWIN) | 31 | 25–50 a | Not calculable | Diffuse | Absent |
| 7 | 36 | 25 | 50 | Diffuse | Absent |
| 8 | 34 | 50–75 | 10–25 | Diffuse | Present |
| 9 | 33 | 25–50 | 25–50 | Diffuse | Present |
| 10 | 31 | 75–90 | 10–25 | Diffuse | Present |
| 11 | 34 | 75 | Not calculable | Diffuse | Present |
| 12 | 34 | 25–50 | Not calculable | Diffuse | Present |
| 13 | 39 | >95 | <3rd | Diffuse | Present |
| 14 | 36 | 10–25 | 50–75 | >50% | Present |
| 15 | 34 | <10 | 10–25 | Diffuse | Present |
| 16 | 35 | 50–75 | 25–50 | Diffuse | Present |
| 17 | 31 | 50–75 | 5 | 25% | absent |
| 18 | 20 | 75–90 | Not calculable | Diffuse | Present |
| 19 | 25 | 25 | 75 | Diffuse (90%) | Present |
| 20 | 29 | 50 | 25–50 | Diffuse (100%) | Present |
| 21 | 19 | Not calculable | Not calculable | Diffuse (90%) | Present |
| 22 | 26 | 90 | 25–50 | Diffuse (95%) | Present |
| 23 | 35 | 25 | 25–50 | Diffuse (100%) | Present |
| 24 | 27 | 25–50 | 50 | Multifocal (50%) | Present |
| 25 | 30 | 10 | 50 | Diffuse (100%) | Present |
| 26 | 33 | 50 | Not calculable | Multifocal (50%) | Present |
| 27 | Unbooked | Not calculable | Not calculable | Diffuse (80%) | Present |
| 28 | 35+6 | 5–10 | 75–90 | >30% | Present |
| 29 | 30+5 | 10–25 | 75–90 | <10% | Present |
| 30 | 25+4 | 50 | 50–75 | ∼99% | Present |
| 31 | 32+4 | 10–25 | 50–75 | 60–70% | Present |
| 32 | 29+3 | 50–75 | 25 | 50% | Absent |
| 33 | 36+4 | 90–95 | 10–25 | 30–40% | Present |
| 34 | 34+4 | 25–50 | 25–50 | 50% | Present |
| 35 | 32+4 | 50–75 | 25–50 | 90% | Present |
| 36 | 36+4 | 25–50 | 50–75 | 90% | Present |
| 37 | 32 | 25–50th | 10–25 | 30% | Absent |
| 38 | 26+4 | 10–25 | 50–75 | 50% | Absent |
| 39 | 32+6 | 50–75 | 25–50 | 50% | Present |
| 40 | 30+6 | 25–50 | 25–50 | 20% | Present |
| 41 | 34+4 | 10–25 | 75–90 | 15% | Present |
| 42 | 37+6 | 5–10 | 75–90 | 95% | Present |
| 43 | 34+2 | 5–10 | 90–95 | 10% | Present |
| 44 | 31 | 25–50 | 25–50 | Absent | Present |
| 45 | 37+3 | 25–50 | 75–90 | 75–80% | Present |
| 46 | 37+3 | <3 | >97 | 10% | Present |
| 47 (TWIN) | 28+5 | 25–50 | 75–90 | 40% | Present |
| 48 (TWIN) | 28+5 | 25–50 | 75–90 | 40% | Present |
| 49 | 31+6 | 50–75 | 50–75 | Not documented | Present |
| 50 | 33+2 | 10–25 | 25–50 | 45–50% | Present |
| 51 | 34+6 | 90–95 | 50 | Not documented | Present |
| 52 | 33+2 | 50–75 | 3–5 | 40% | Present |
| 53 | 41+4 | 3–5 | 10–25 | >60% | Absent |
| 54 | 28 | 75–90 | 5–10 | Diffuse | Absent |
| 55 | 31 | 25–50 | 50–75 | 70% | Present |
| 56 | 21+6 | Not calculable | Not calculable | Diffuse (90%) | Present |
| 57 | 22+1 | <10 | 50–75 | Diffuse (90%) | Present |
| 58 | 27 | 25–50 | 50–75 | Multifocal | Present |
| 59 | 23+1 | 10–25 | Not calculable | Diffuse (90%) | Present |
| 60 | 32+6 | 50–75 | 10–25 | Absent | Present |
| 61 | 28+1 | 25–50 | 50–75 | Diffuse (100%) | Absent |
Overall placental weight percentile calculated for MCDA twin.
There were 57 singleton placentas and 2 twin placentas; one dichorionic diamniotic (DCDA) and one monochorionic diamniotic (MCDA).
Placental weights
Placental trimmed weights (without membranes and cords) are routinely measured as part of placental pathology reporting, and the American Registry of Pathology (ARP) standards for placental weight percentiles and feto-placental weight ratios are used.12 Three placental weight percentiles could not be calculated due to gestational age being omitted (n = 1) or gestational age below 22 weeks (n = 2), for which AFIP tables are not standardised. In this series, 47 out of the remaining 58 (81%) placentas had a normal placental weight for gestation (defined as 10–90th percentile). Eight of 58 (13.8%) were less than the 10th percentile, and three (5.2%) were reported greater than the 90th percentile.
Feto-placental weight ratio could be calculated in 51 placentas. Of these, 45 (88%) had normal fetoplacental weight ratios (defined as 10–90th percentiles), four (8%) were low (<10th percentile), and two (4%) were greater than the 90th percentile.
Histology
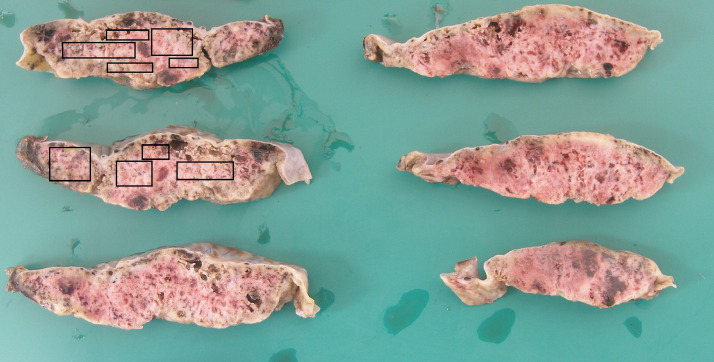
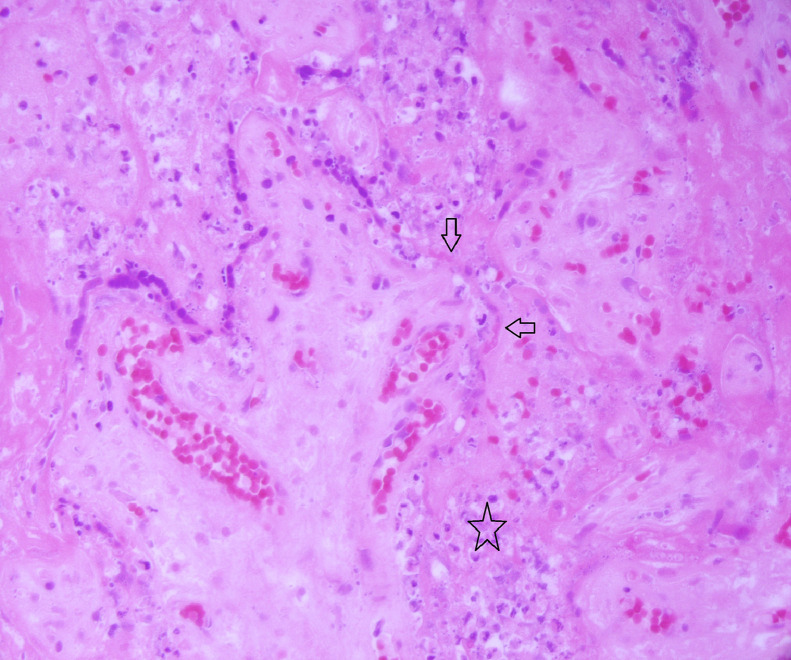
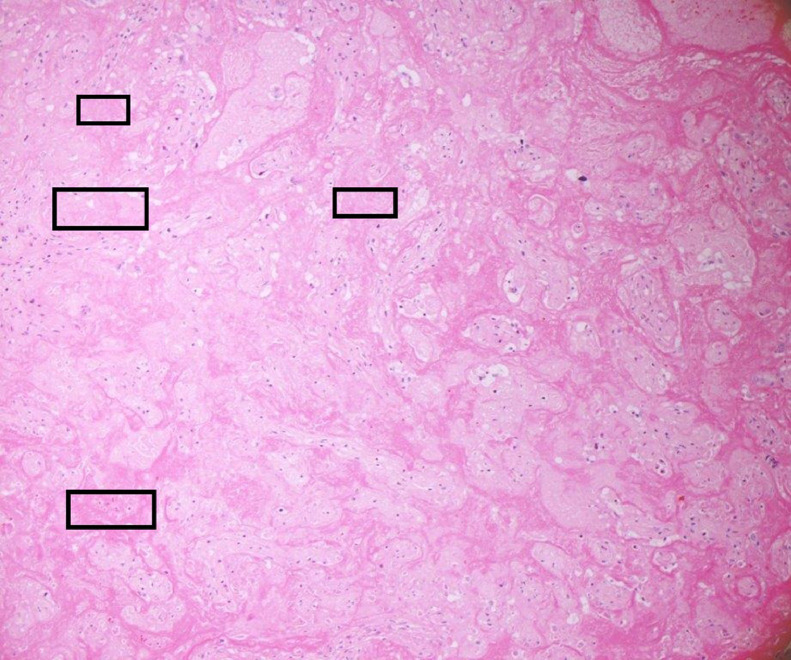
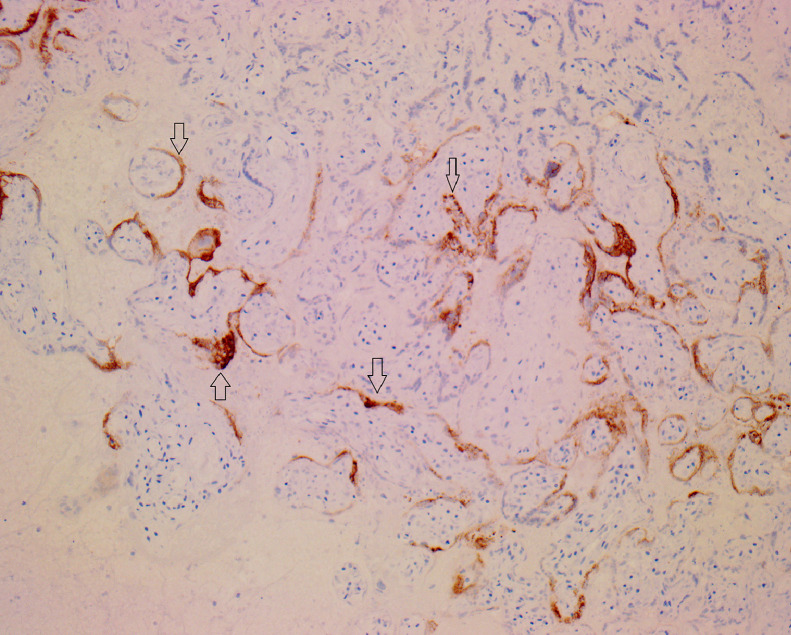
In two cases, the degree of macroscopic fibrin deposition was not described (3.3%) and in a further seven (11.5%), fibrin deposition was described as affecting less than 25% of the parenchyma. One (1.6%) reported ‘multifocal’ fibrin, but a percentage was not documented. Figure 1 depicts macroscopic appearances of a placenta with characteristic features of extensive fibrin deposition. MPFD was reported in 50 placentas (82%) and in 35 of these, fibrin deposition was reported as ‘diffuse’ or specified as involving more than 50% of the parenchyma. Histological examination revealed concurrent CHI in 48 of these cases (79% of all placentas in the series). Ten reported only CHI (16%). Figure 2, Figure 3, Figure 4 show the distinctive features displayed by the combined presence of MPVFD and CHI (Figure 2), syncytiotrophoblast necrosis (Figure 3) and the presence of dense perivillous fibrin without chronic histiocytic infiltrates as seen in one of the two cases which presented exclusively with MPVFD (Figure 4).
Figure 1.
Macroscopic image of term placenta, serially sectioned, showing extensive fibrin deposition throughout the parcenchyma.
Figure 2.
Haematoxylin and eosin stained section at x 200 magnification showing a placenta affected by an inflammatory cell infiltrates within the intervillous space with necrosis of the trophoblast and perivillous fibrin deposition.
Figure 3.
Haematoxylin and eosin stained section at x 400 magnification showing trophoblast necrosis characterised by ‘smudging’ of the nuclei and karyorrhectic debris within the intervillous space.
Figure 4.
Immunohistochemical staining of placental parenchyma with SARS-CoV2 immunohistochemical stain (x200 magnification) with expression in the syncytiotrophoblast.
Other reported placental pathology included chronic deciduitis (n = 4; 6.6%), chronic chorioamnionitis (n = 8; 13%) fetal vascular malperfusion (2; 3.2%), eosinophilic/T-cell vasculitis (n = 1; 1.6%) intervillous haemorrhage (n = 4; 6.6%), acute chorioamnionitis (n = 1; 1.6%), chronic villitis (n = 2; 3.2%), maternal vascular malperfusion (n = 1; 1.6%), decidual vasculopathy (n = 2; 3.2%) and chorangiosis (n = 1; 1.6%).
All placentas demonstrated positive SARS-CoV-2 immunohistochemical expression in the syncytiotrophoblast, 14 (23%) also had SARS-CoV-2 positivity by PCR on placental tissue. This is shown in Figure 5.
Figure 5.
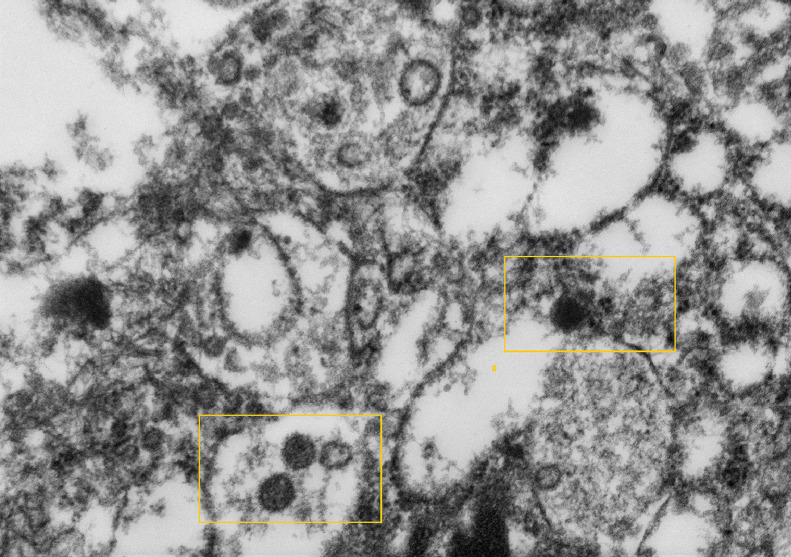
High magnification electron microscopy image of formalin fixed paraffin processed trophoblast cytoplasm showing possible Covid19 virion budding into endoplasmic reticulum (at 3 o'clock) and a couple of vacuoles containing 3 and 2 possible complete virus particles (at 7 o'clock and 11 o'clock). The spherical virus particles contain black dots, which are cross-sections through the viral nucleocapsid (as shown within rectangles).
One case that was also published separately as a case report also had in situ hybridisation, proving both the presence and the replication of virus in the syncitiotrophoblasts.11
Electron microscopy
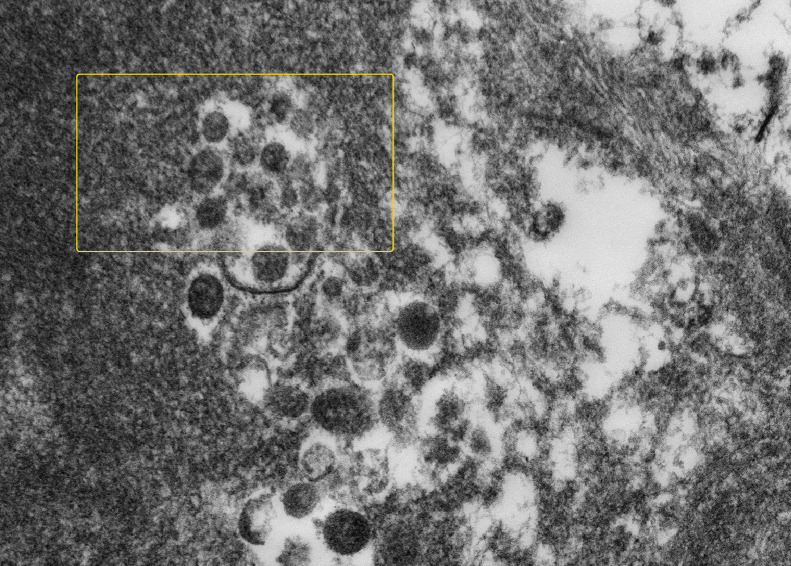
Transmission electron microscopy was performed on 4 formalin-fixed paraffin processed placentas. A large number of particles measuring between 87 and 178 nm in diameter with spikes measuring 17 nm reminiscent of SARS-CoV213 were found within the extravillous polymerised fibrin (Figure 6) and within trophoblast cells (Figure 7) Both original magnification x20,000.
Figure 6.
High magnification electron microscopy image of formalin fixed paraffin processed possible multiple Covid19 virions in apex of cytotrophoblast cell within vacuoles (as shown within rectangle).
Figure 7.
High magnification electron microscopy image of formalin fixed paraffin processed possible multiple Covid19 virions in perivillous fibrin lying free and within a vacuole (at 10 o'clock) (as shown within rectangle).
Pregnancy outcome
Of 61 infants delivered to 59 pregnancies, 30 were stillborn or miscarried (49.1%).
Six infants (four livebirths and two stillbirths) tested positive for SARS-CoV-2 by PCR following delivery (timing not specified). Five infants (two livebirths and three stillbirths) tested negative following delivery (timing not specified). For the remaining 50 infants the SARS-CoV-2 status was not known.
Of the 12 mothers who were known to be SARS-CoV-2 positive but who were reportedly asymptomatic (12 mothers, but 13 infants due to twin pregnancies) 75% ended in pregnancy loss (n = 9), compared to pregnancy loss rate of 50% in women who were symptomatic (n = 11).
Obese mothers (defined as BMI>30 kg/m2) had higher rates of pregnancy loss when compared with those with a BMI <30 [67% (10/15) versus 41% (14/34) respectively]
For BAME groups 8/16 (50%) pregnancies ended in pregnancy loss (one miscarriage, seven stillbirths). This was similar to the Caucasian mothers reporting 19/36 (52.7%) of pregnancy loss (one miscarriage and 18 stillbirths).
For 20 infants in which a personalised growth centile was provided; 11 were < 9th centile for the stated gestational age, and nine were reported to be within normal limits for the stated gestation (defined as 10–90th centile).
There were 31 cases in which a personalised growth centile was not available and growth was assessed using the UK WHO growth charts for close fetal and neonatal monitoring.14 In cases in which the sex of the baby was not specified, both centiles for male and female were calculated and the lower percentile was selected. Using this method, three infants were less than the 9th percentile for the stated gestation, 25 were within normal limits (10–90th centile), and one baby was reported to be >90th centile for the stated age.
A birthweight was either not recorded or a centile could not be calculated in nine cases, including one early gestation, one monochorionic twin placenta and one of unknown gestation.
Discussion
To our knowledge, this is the largest audited case series of SARS-CoV-2 placentitis. 79% of placentas showed both MPFD with CHI, with 16% reporting just CHI and 3.3% only showing MPFD.
Assessing how the timing of SARS-CoV-2 infection correlates with the histological features is challenging, given the data was not available in just over one third of cases and is further complicated by lack of symptoms, nadirs in testing and delay in investigations in relation to acquisition of infection. However, it should be noted that 15/22 (68%) had a positive test within two weeks of delivery.
In this series many of the placentas complicated by SARS-COV2 placentitis were from mothers in whom no symptoms of SARS-CoV-2 infection were reported, however we did not know the symptomatology in 13 of 47 known SARS-CoV-2 positive mothers so conclusions cannot be reliably drawn. However, it is possible that only a fraction of placentas from mothers with SARS-CoV-2 infection are referred for pathological examination – and only in the case of poor placental outcomes. Therefore, the true number of asymptomatic mothers with SARS-CoV-2 placentitis may be even higher than our data has shown.
In a recent UK cohort study, mothers with SARS-CoV-2 infection were reported to be nearly three times as likely to experience a preterm birth or stillbirth compared with mothers without SARS-CoV-2 infection.1,2 The same paper highlighted the risks to women of BAME, high BMI and those with higher levels of social deprivation. Despite the known risks to women from BAME backgrounds with SARS-CoV-2 infection,4 there were similar rates of pregnancy loss with SARS-CoV-2 placentitis in BAME women as Caucasians in our cohort (50% and 52.7% respectively).
The proportion of BAME women in our data was significantly higher than the national average at 32%.15 This may be due to referral bias as some of the tertiary centres involved in this study provide services to areas in which there are much higher rates of BAME groups than the national average. Furthermore, the data in this study is from 10 participating referral centres out of >20 centres in the UK. It is therefore not possible to determine whether women from BAME groups are over-represented in our results.
There were higher rates of pregnancy loss in obese mothers compared with women with a BMI <30. It is well documented that obesity is a risk factor for severe SARS-CoV-2 infection and in isolation increases the risk of stillbirth,16 and our results therefore would not be unexpected. It should however, be noted that as we do not have a control population of which to compare and given the variability in the demographics, like our comments relating to ethnicity, it is not possible to determine whether obese mothers are over represented our case series.
The placental weights were largely within normal limits for the gestational age (79%) and had normal feto-placental weight ratios (88%). This is in contrast to the pattern typically described in classical pre-pandemic MPFD in which placentas are described as small and firm.9 Furthermore pre-pandemic MPFD is also often associated with intra-uterine growth restriction.9 The histological picture in MPFD in SARS-CoV-2 placentitis differs from the pre-pandemic’ MPFD mainly due to the presence of prominent CHI in the former, and the absence of extravillous trophoblast. Significant trophoblast necrosis was only described in a proportion of cases (28%). However, it is possible that this proportion is not truly reflective; many pathologists include this in the general histological description of SARS-CoV-2 placentitis (not reporting this feature separately) and in some instances the clinical significance may not have been appreciated on initial reporting as it can be a focal finding. The intervillous fibrin also appears less dense, without migration of extravillous trophoblast and can at times be associated with a mixed inflammatory cell infiltrate.
The pathophysiological mechanism behind the development of MPFD in the placentas of some mothers infected with SARS-CoV-2 remains to be elucidated. However, it may be possible that a synergistic effect of the immunological dysregulation induced by the virus and an underlying or acquired thrombophilia in the mother, or the fetus, triggers the CHI – trophoblast necrosis- MPFD pathway.
When calculable in 51 cases, the majority of babies in our cohort (34/51; 67%) were reported to have normal birth weight (defined as 10–90th centile on personalised or WHO generalised growth charts as available). There were however a greater proportion of small infants when using the personalised growth chart; 9/20 as opposed to 3/31 when using the WHO growth charts. This variation in infant weight likely reflects a degree of referral bias. Growth restriction (<10th percentile) is one of the referral criteria for placental examination according to the Royal College of Pathologists.17 Placentas from infants who are small for gestation are more likely to be received, regardless of SARS-CoV2 status of the mother. Further, these cases are more likely to have a personalised growth centile calculated as part of referral practices. It is still not uniformly common practice for placentas to be referred for pathological examination purely on the basis of SARS-CoV2 infection, particularly if the mother is negative at the time of delivery, is asymptomatic or if the baby appears normally grown and healthy.
There was limited information on the number of babies testing positive for SARS-CoV-2. Details of 11 babies were available to the pathologists with six testing positive and five negatives, though the timing of the testing was not specified. At least one case of vertical transmission was confirmed in our series (paper under submission). No known cases of vertical transmission have been reported in the coronaviruses Severe Acute Respiratory Syndrome and Middle Eastern Respiratory Syndrome, though relatively few pregnancy cases were reported.18
A systematic review and meta-analysis of over 1000 neonates born to SARS-CoV-2 positive mothers, showed relatively low rates of vertical transmission, with 0.7–7% of neonates testing positive.19 These results should be interpreted with caution given the risk of false positives from maternal contamination of the samples if nasopharyngeal swabs rather than umbilical cord blood are used. McDevitt et al. reported on universal screening at their hospital including neonates: eight tested positive for SARS-CoV-2, all born to asymptomatic mothers, seven of whom had tested negative on admission.20 The neonatal tests were subsequently repeated, and each was reported to be negative. This was a curious finding, which raises the possibility of sample contamination and technical failure of the Real Time-PCR tests, though also could reflect previous maternal SARS-CoV-2 infection which has resolved, though placental infection persists. Placental histology unfortunately was not available for correlation in any of these instances.
The proposed mechanism of placental infection relies on the presence of ACE2 receptors on syncytiotrophoblast,21 with viral endocytosis supported by a host of transmembrane proteins including the transmembrane serine proteases (TMPRSS). Infection then triggers both a direct and indirect immunological response with the release of inflammatory cytokines.22 Why the placentas of some women are affected in this way and others are not, is yet unestablished.
An investigation team from Yale compared the placental transcriptome in SARS-CoV-2 infected cases with matched controls, demonstrating that infected cases showed increased expression of genes associated with immune responses, suggesting a robust local response to respiratory infection even in the absence of localised placental infection.23 The study reported changes in gene expression including upregulation of HSPA1A, which encodes the heat shock protein Hsp70, a proposed alarmin that has been implicated previously in placental vascular diseases and preeclampsia. The Yale team found a significant increase in the interactions between immune cells at the maternal-fetal interface in SARS-CoV-2 infected cases compared with non-infected controls. This is in keeping with the identification in our cases of SARS-CoV2 in the trophoblast -at the feto:maternal interface – both by histochemistry and electron microscopy.18 Placental immune responses during maternal respiratory SARS-CoV-2 infection may contribute to the poor pregnancy outcomes and that active infection at the maternal-fetal interface is not required for immune activation at this distant site.23
We propose that CHI may be the initial response to SARS-CoV-2 infection, with fibrin deposition a secondary feature and/or associated with trophoblast necrosis. A study from Chen and Roberts in pre-pandemic placentas suggests that the pathologic increase in perivillous fibrin is a reaction to trophoblast damage/necrosis.9 Prospective longitudinal studies are required to establish the pathophysiology further. This may be challenging given the number of women that can be completely asymptomatic for infection. However, all of the women whose placentas are included in this study are expected to be followed up closely during subsequent pregnancies and it will be interesting to see how many cases of MPFD or CHI recur outside of the pandemic.
Mothers with gestations affected by CHI often have elevated serum alpha-fetal protein and/or alkaline phosphatase due to placental damage.9 These markers could potentially be used in the routine care and follow-up of SARS-CoV-2 infected pregnant women.
The limitations of our work include the inability to place our findings in the full context of SARS-CoV2 in pregnancy; namely comparison with outcomes of maternal SARS-CoV2 infection without the finding of SARS-CoV2 placentitis, and the lingering question of what proportion of SARS-CoV2 positive mothers develop placentitis? These are difficult areas of interest to address as there is referral bias in placental examination, variation in each hospital policy including testing of asymptomatic patients, nadirs in positive SARS-Cov2 PCR tests and of course a host of epidemiological data and risks to consider. A well-designed prospective study, with a control population and a cohort of mothers with SARS-CoV2 infection without placentitis could provide some progress in this area, though all mothers symptomatic or not, would require SARS-CoV2 PCR and each placenta, regardless of pregnancy outcome would need to be assessed to generate accurate meaningful data. Another limitation of this national audit project was the lack of independent review and quantitative analysis of cases. Further, the histopathological pattern of SARS-CoV-2 placentitis in relation to SARS-CoV-2 variants may be another area of interest which is yet to be explored, given the rise in Delta variant at the time of writing. Utilisation of genomic sequencing and clinicopathological correlation is needed for further clarification.
To conclude, we have presented the largest case series of SARS-CoV-2 placentitis to date. In half of cases, the pregnancy ended in stillbirth or late miscarriage. Importantly, mothers who were reported as asymptomatic for SARS-CoV-2 showed the same placenta histological pattern and experienced a similar incidence of pregnancy loss to those reported as symptomatic. Obese mothers had higher rates of stillbirth compared to non-obese mothers with the same placental histology. Whilst most infants were of normal weight, the results of personalised growth centiles and generalised growth centiles varied greatly and may be affected by a degree of referral bias. Further work is required to establish the significance of the timing of maternal SARS-CoV-2 infection and its relation to SARS-CoV-2 placentitis, the significance of SARS-CoV2 variants in pregnancy, and rates of vertical transmission associated with this particular pattern of placental inflammation.
UKOSS findings have also shown that vaccine up-take in pregnant women is low compared to the general population, and that most hospitalised pregnant women are not fully vaccinated. The experiences of UK perinatal and paediatric pathologists have recently been acknowledged by Public Health England, and pregnant women are strongly advised to be vaccinated against SARS-CoV-2 infection.24
Data sharing statement
The authors will share Individual participant data that underlie the results reported in this article, after de-identification (text, tables and figures) as well as the audit project. Applicants willing to receive the data should apply between1 and 12 months after the manuscript has been published and should demonstrate that the proposed use of the data has been approved by an independent review committee (“learned intermediary”) identified for this purpose. The data should be requested to the corresponding author Marta.Cohen@nhs.net
Contributors
SS and MCC had the idea for and designed the study with the help of all co-authors, applied to Clinical Governance for a National Audit authorization, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. BW performed and interpreted the electron microscopy analysis. All authors submitted cases, participated at the online meetings where all cases were discussed, critically revised the manuscript for intellectual content and gave final approval for the version to be published. SS chaired all meetings and performed the analysis. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of interests
We declare no competing interests.
Funding
There was not funding associated with this study.
References
- 1.Magee L.A., Khalil A., von Dadleszen P. Covid-19: UK Obstetric Surveillance System (UKOSS) study in context. BMJ. 2020;370:m2915. doi: 10.1136/bmj.m2915. [DOI] [PubMed] [Google Scholar]
- 2.Office for National Statistics. Births in England and Wales: 2018-2019. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthsummarytablesenglandandwales. Accessed 16 August 2021
- 3.Kazemi S.N., Hajikhani B., Didar H., et al. COVID-19 and cause of pregnancy loss during the pandemic: a systematic review. PLoS ONE. 2020;16 doi: 10.1371/journal.pone.0255994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vousden N., Bunch K., Morris E., et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS) PLoS ONE. 2020;16 doi: 10.1371/journal.pone.0251123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong Y.P., Khong T.Y., Tan G.C. The effects of COVID-19 on placenta and pregnancy: what do we know so far? Diagnostics (Basel) 2021;11:94–107. doi: 10.3390/diagnostics11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linehan L., O'Donoghue K., Dineen S., White J., Higgins J.R., Fitzgerald B. SARS-CoV-2 placentitis: an uncommon complication of maternal COVID-19. Placenta. 2021;15:261–266. doi: 10.1016/j.placenta.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz D.A., Morotti D. Placental pathology of COVID-19 with and without fetal and neonatal infection: trophoblast necrosis and chronic histiocytic intervillositis as risk factors for transplacental transmission of SARS-CoV-2. Viruses. 2020;12:1308. doi: 10.3390/v12111308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz D.A., Baldewijns M., Benachi A., et al. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (COVID-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in live-born and stillborn infants. Arch Pathol Lab Med. 2021;145:517–528. doi: 10.5858/arpa.2020-0771-SA. https//:doi.org/10.5858/arpa.2020-0771-SA. [DOI] [PubMed] [Google Scholar]
- 9.Chen A., Roberts D.J. Placental pathologic lesions with a significant recurrence risk - what not to miss! APMIS. 2018;126:589–601. doi: 10.1111/apm.12796. https//:doi.org/10.1111/apm.12796. [DOI] [PubMed] [Google Scholar]
- 10.Weber M.A., Nikkels P.G., Hamoen K., Duvekot J.J., de Krijger R.R. Co-occurrence of massive perivillous fibrin deposition and chronic intervillositis: case report. Pediatr Dev Pathol. 2006;9:234–238. doi: 10.2350/06-01-0019.1. https//:doi.org/10.2350/06-01-0019.1. [DOI] [PubMed] [Google Scholar]
- 11.Marton T., Hargitai B., Hunter K., Pugh M., Murray P. Massive perivillous fibrin deposition and chronic histiocytic intervillositis a complication of SARS-CoV-2 infection. Pediatr Dev Pathol. June 2021 doi: 10.1177/10935266211020723. https//:doi.org/10.1177/10935266211020723. [DOI] [PubMed] [Google Scholar]
- 12.Kraus F.T., Redline R.W., Gersell D.J., Nelson D.M., Dicke J.M. American Registry of Pathology; Washington DC: 2004. Placental Pathology. [Google Scholar]
- 13.Dittmayer C, Meinhardt J, Radbruch H, Radke J, Heppner B, Heppner F. Why misinterpretation of electron micrographs in SARS-CoV-2-infected tissue goes viral. Lancet. 2020;396:e64–e65. doi: 10.1016/S0140-6736(20)32079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.https://www.rcpch.ac.uk/resources/uk-who-growth-charts-neonatal-infant-close-monitoring-nicm. Accessed 05 Feburary 2022
- 15.HM Government UK. Ethnicity facts and figures. https://www.ethnicity-facts-figures.service.gov.uk. Accessed 6 September 2021.
- 16.Stubert J., Reister F., Hartmann S., Janni W. The risks associated with obesity in pregnancy. Dtsch Arztebl Int. 2018;115:276–283. doi: 10.3238/arztebl.2018.0276. https://doi.org/10.3238/arztebl.2018.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Royal College of Pathologists . Royal College of Pathologists; London: 2019. Tissue Pathway for Histopathological Examination of the Placenta.https://www.rcpath.org/uploads/assets/ec614dfa-007c-4a93-8173cb202a071a72/G108-Tissue-pathway-for-histopathological-examination-of-the-placenta.pdf Accessed 1 August 2021. [Google Scholar]
- 18.Zhu Z., Lian X., Su X., Wu W., Marraro G.A., Zeng Y. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res. 2020;21:224. doi: 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotlyar A.M., Grechukhina O., Chen A., et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224 doi: 10.1016/j.ajog.2020.07.049. 35-53.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDevitt K.E.M., Ganjoo N., Mlangeni D., Pathak S. Outcome of universal screening of neonates for COVID-19 from asymptomatic mothers. J Infect. 2020;81:452–482. doi: 10.1016/j.jinf.2020.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni W., Yang X., Yang D., et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhury M.A., Hossain N., Kashem M.A., Shahid M.A., Alam A. Immune response in COVID-19: a review. J Infect Public Health. 2020;13:1619–1629. doi: 10.1016/j.jiph.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu-Culligan A., Chavan A.R., Vijayakumar P., Irshaid L., Courchaine E.M., Milano K.M., et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (New York, N.Y.) 2020 doi: 10.1016/j.medj.2021.04.016. 591–610.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Public Health England. Safety of COVID-19 vaccines when given in pregnancy. 2020. https://www.gov.uk/government/publications/safety-of-covid-19-vaccines-when-given-in-pregnancy. Accessed 1 August 2021.