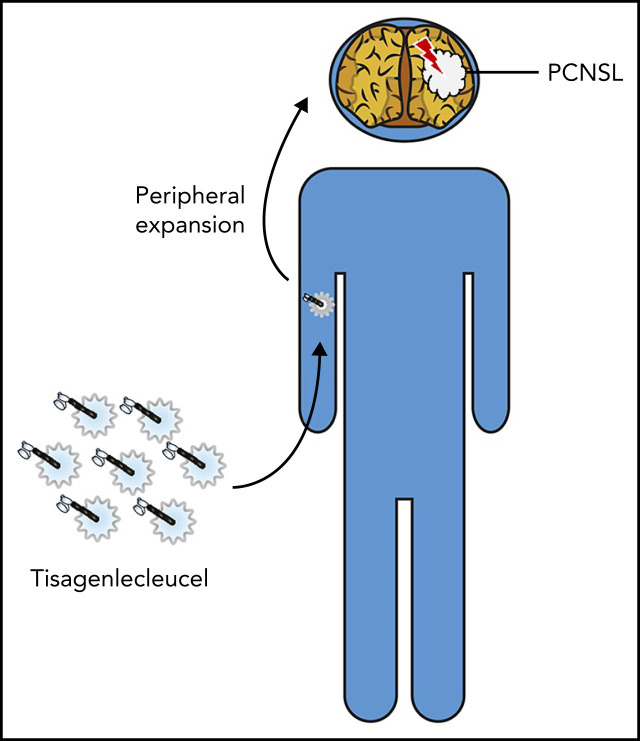
Relapsed/refractory primary central nervous system (CNS) lymphoma (PCNSL) has a terrible prognosis. Frigault et al reported encouraging results with CAR-T cell therapy with tisangenlecleucel in 12 patients with relapsed PCNSL. CAR-T cells expanded in the periphery and transited to the CNS. The 58% response rate, including 50% complete responses and 43% sustained responses, with manageable episodes of cytokine release syndrome and neurotoxicity suggest this is a safe and effective therapy for this otherwise poorly responsive disease.
Key Points
Tisagenlecleucel demonstrated safety and efficacy in primary CNS lymphoma.
CAR-T cells were noted to expand and traffic to the CNS in the absence of systemic disease.
Visual Abstract
Abstract
CD19-directed chimerical antigen receptor T-cell (CAR-T) products have gained US Food and Drug Administration approval for systemic large B-cell lymphoma. Because of concerns about potential immune cell-associated neurotoxicity syndrome (ICANS), patients with primary central nervous system (CNS) lymphoma (PCNSL) were excluded from all pivotal CAR-T studies. We conducted a phase 1/2 clinical trial of tisagenlecleucel in a highly refractory patients with PCNSL and significant unmet medical need. Here, we present results of 12 relapsed patients with PCNSL who were treated with tisagenlecleucel and followed for a median time of 12.2 months (range, 3.64-23.5). Grade 1 cytokine release syndrome was observed in 7/12 patients (58.3%), low-grade ICANS in 5/12 (41.6%) patients, and only 1 patient experienced grade 3 ICANS. Seven of 12 patients (58.3%) demonstrated response, including a complete response in 6/12 patients (50%). There were no treatment-related deaths. Three patients had ongoing complete remission at data cutoff. Tisagenlecleucel expanded in the peripheral blood and trafficked to the CNS. Exploratory analysis identified T-cell, CAR T, and macrophage gene signatures in cerebrospinal fluid following infusion when compared with baseline. Overall, tisagenlecleucel was well tolerated and resulted in a sustained remission in 3/7 (42.9%) of initial responders. These data suggest that tisagenlecleucel is safe and effective in this highly refractory patient population. This trial was registered at www.clinicaltrials.gov as #NCT02445248.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment of relapsed or refractory (r/r) systemic large B-cell lymphomas (LBCL). Three CD19-directed CAR-T products are currently US Food and Drug Administration (FDA) approved based on phase 2 clinical data: axicabtagene ciloleucel (Study Evaluating the Safety and Efficacy of KTE-C19 in Adult Participants With Refractory Aggressive Non-Hodgkin Lymphoma [ZUMA-1] trial), tisagenlecleucel (Study of Efficacy and Safety of CTL019 in Adult DLBCL Patients [JULIET] trial), and lisocabtagene maraleucel (Safety and Efficacy of the SurVeil Drug-Coated Balloon [TRANSCEND] trial), with overall response rates up to ∼80%.1-3 Because of concerns for increased and potentially life-threatening immune effector cell-associated neurotoxicity syndrome (ICANS) and potentially impaired T-cell trafficking to the central nervous system (CNS) in the absence of systemic disease, patients with primary CNS lymphoma (PCNSL) were excluded from these initial pivotal studies. Consequently, all 3 products carry a limitation of use in the PCNSL patient population per their FDA labels. Because of these exclusions, little is known about the treatment-related toxicities and therapeutic potential of the currently available commercial CD19-directed CAR-T products in this challenging patient population.
PCNSL is an extranodal non-Hodgkin lymphoma confined to the brain, leptomeninges, eyes, and/or spinal cord without evidence of systemic disease.4 Although pathologically similar to systemic LBCL, the prognosis of newly diagnosed PCNSL is inferior to that of other non-Hodgkin lymphoma subtypes with 5- and 10-year overall survival rates of 29.3% and 21.6%, respectively.5 Less favorable outcomes in PCNSL are even more pronounced in patients over the age of 60 years, who make up 50% of newly diagnosed cases.6,7 In general, the prognosis for patients with r/r PCNSL is poor, with a median survival of approximately 4 months.8,9 Therapies for r/r CNS disease have largely been based on cytotoxic chemotherapy, small-molecule inhibitors, or radiation therapy with limited curative potential.10 Additionally, because of the intensity of these salvage regimens, patients who do survive are at high risk of developing treatment-related toxicity and disabling cognitive dysfunction, raising the question of how to balance therapy intensification with mitigation of side effects.11
The anti-CD19 CAR T-cell therapy tisagenlecleucel was approved in 2018 based on the single-group, open-label, multicenter, international phase 2 JULIET study.2 Two recent retrospective series using investigational and commercial CAR-T constructs demonstrated safety, and potential efficacy, of such an approach in PCNSL and acknowledged the unmet need for prospective studies investigating this rare and difficult patient population.12,13 Based on real-world data from the Center for International Blood and Marrow Transplant Research, demonstrating low rates of severe ICANS with tisagenlecleucel in systemic lymphoma,14 as well as our previously reported retrospective series demonstrating safety and efficacy of tisagenlecleucel in secondary CNS lymphoma,15 we conducted a prospective phase 1/2 study of tisagenlecleucel in patients with PCNSL to evaluate safety and efficacy in a highly refractory patient population with significant unmet medical need. We also conducted exploratory analyses of CAR T-cell expansion, trafficking, and memory phenotypes in the blood and cerebral spinal fluid (CSF).
Methods
Study design
We conducted a phase 1/2 study of tisagenlecleucel in adults with relapsed or refractory PCNSL with financial and drug product support by Novartis. The initial phase 1 run-in was designed to assess 6 patients for treatment-related toxicities unique to the PCNSL patient population at the FDA-approved CAR-T dose of 0.6 to 6.0e8 CAR+ T cells. Pausing criteria included any death not related to disease progression within 30 days of tisagenlecleucel infusion, 2 or more instances of grade 4 cytokine release syndrome (CRS), a single instance of grade 4 ICANS, cerebral edema, or other grade 4 CNS event such as hemorrhage. Following data safety monitoring committee review, an additional 6 patients were treated as part of the phase 2 expansion. The protocol was approved by the Dana Farber/Harvard Cancer Center institutional review board and the trial was performed in accordance with the principles of the Declaration of Helsinki. To be eligible for enrollment, patients had to be 18 years of age or older and have had progression or relapse following systemic high-dose methotrexate-based therapy. Because of the inferior outcomes in older patients, patients >60 years of age were eligible if they were intolerant of first-line therapy for grade 3+ kidney or liver injury. All patients needed a confirmed diagnosis of PCNSL without evidence of systemic disease. Confirmation of CD19 positivity was not required and patients were required to have a left ventricular ejection fraction >40%, creatinine clearance >30 mL/min at time of screening, and an Eastern Cooperative Oncology Group performance status of 0 to 2 before leukapheresis.
After providing written informed consent, patients were enrolled and underwent leukapheresis. Bridging therapy was allowed, but a 2-week washout from lymphodepleting agents was required before cell collection. Corticosteroids were allowed up to a daily dexamethasone equivalent of 4 mg throughout leukapheresis and CAR-T cell infusion. Patients who were treated with ibrutinib as their most recent therapy, and who had demonstrated refractory disease despite Bruton tyrosine kinase inhibition (BTKi), were allowed to continue treatment up until 3 months after CAR-T cell infusion given benefits of inducible T-cell kinase inhibition on T-cell function.16,17 For lymphodepleting chemotherapy (LDC), patients received a regimen of fludarabine (25 mg/m2) and cyclophosphamide (250 mg/m2) daily on days -5, -4, and -3 of infusion. Patients received a dose of 0.6 to 6.0 × 108 tisagenlecleucel CAR+ T cells in line with the current FDA approval for systemic LBCL. All patients were administered seizure prophylaxis unless already treated with antiepileptic drugs. Growth factor support was allowed starting on day +7 in the absence of CRS/ICANS. All patients had repeat baseline assessments before LDC and after any subsequent bridging therapy.
End points
The primary end point of this study was tolerability and toxicity including the rate and grade of CRS and ICANS per the 2019 American Society of Transplantation and Cellular Therapy consensus criteria.18 Secondary end points included overall response rate and complete response (CR) rate to tisagenlecleucel per the International PCNSL Collaborative Group criteria, which included magnetic resonance imaging and CSF assessments monthly for 6 months, and quarterly up to 24 months. Radiographic responses were assessed independently by our tumor imaging metrics core in collaboration with neuro-oncology. All patients will be followed for 15 years per FDA-mandated long-term follow-up requirements. Exploratory end points included long-term efficacy, expansion, persistence, and phenotype of tisagenlecleucel, cytokine and RNA profiling of blood, and CSF and CNS trafficking of CAR-T cells.
Statistical analysis
Analyses were primarily descriptive in keeping with the safety end point of this study. Frequency and rate of CRS and ICANS as well as summary statistics of patient demographics and treatment history are reported. An interim safety review was performed following the first 6 patients treated, and the study was subsequently expanded to further investigate efficacy. Only patients who were infused were included in the efficacy analysis. Stopping rules included 2 or more instances of grade 4 CRS or grade 4 organ-specific toxicities, a single instance of grade 4 ICANS, or treatment-related death.
Correlative studies
Samples for correlative studies were collected throughout the treatment course including baseline peripheral blood mononuclear cells, serum, and CSF analysis. Study mandated CSF sampling was obtained at baseline, day +7, and month 1 to investigate both tisagenlecleucel trafficking to the CNS and CNS cytokine profiles. Tisagenlecleucel flow cytometry phenotyping was performed using cryopreserved peripheral blood mononuclear cells stained with CD3-APC-H7, CD4-V450, CD8-V500c CD45RA-BV605, CD95-PE-Cy7, CCR7-FITC, CD14-PerCP-Cy5.5 (BD Biosciences), exhaustion markers PD-1-BV786, TIM-3-BV711, LAG-3-AF647, and a soluble CD19-PE detection reagent (BioLegend). 7-AAD was used to exclude dead cells. CSF infiltrate immunophenotyping was performed using CD3-APC-H7, CD4-V450, CD8-V500c, CD56-APC, CD19-PE-Cy7, CD22-PE-Cy7, CD11b-AF700, CD14-PerCP-Cy5.5, CD16-FITC, CD45-BV786, and soluble CD19-PE. Expression profiling of the CSF infiltrates were performed on 5 × 103 to 4.5 × 104 cells lysed in 1/3 RLT buffer using the Nanostring CAR-T characterization panel, with an additional customized 29 gene drop in panel. Data were analyzed using nSolver Advanced analysis software. Luminex cytokine profiling was performed on patient serum and CSF using a customized 27plex assay (R&D Systems) (interleukin-1a [IL-1a], IL-1b, IL-2, IL-5 IL-6, IL-7, IL-8/CXCL8, IL-10, IL-12 p70, IL-13, IL-15, IL-17A, IL-18, IL-33, CXCL10/IP-10, CCL2/MCP-1, IL-6Ra, IL-1RA, CCL3/MIP-1a, CCL4/MIP-1b, interferon-α, interferon-γ, tumor necrosis factor-α, granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor, IL-2R α/CD25, S100B).
Results
Patients
Between December 2019 and the data cutoff date, November 2021, a total of 13 patients were enrolled and 12 were infused. One patient experienced disease progression following CAR-T manufacturing failure (Escherichia coli contamination) and elected to transition to hospice rather than attempt repeat leukapheresis. Of the 12 infused patients, the median time from leukapheresis to infusion was 33 days (range, 27-37) and the median time from infusion to data cutoff was 12.2 months (range, 3.64-23.5). Baseline patient characteristics demonstrated a median age of 63 years (range, 34-81) (Table 1). Most patients had parenchymal involvement, whereas 1 patient had isolated leptomeningeal disease confirmed by magnetic resonance imaging and CSF analysis. Five of 12 patients had an Eastern Cooperative Oncology Group performance status of 2 or greater at time of infusion because of progressive neurological disease. The majority of patients had the nongerminal center B-cell subtype of diffuse LBCL by Hans criteria. Patients were heavily pretreated before study enrollment and received a median of 4 prior lines of antineoplastic therapy (range, 2-9; supplemental Table 1 available on the Blood Web site). All patients had progressed or failed first-line high-dose methotrexate; 3 had a history of prior thiotepa-based autologous stem cell transplant (ASCT). All 12 patients had BTKi and/or immunomodulatory drug refractory disease as part of temozolomide, etoposide, Doxil, dexamethasone, ibrutinib, and rituximab (n = 6); venetoclax, ibrutinib, prednisone, obinutuzumab, and Revlimid (n = 4); and/or as monotherapy (n = 8) and 4 patients had received prior partial radiotherapy. All patients had measurable disease by magnetic resonance and/or cytology/flow cytometry at the time of lymphodepletion (preinfusion).
Table 1.
Demographics and clinical characteristics of treated patients
| Characteristics | Patients (n = 12) |
|---|---|
| Median age (range), y | 63 (34-81) |
| Male:female | 7:5 |
| Infused/enrolled | 12/13 |
| ECOG performance status, no. % | |
| 0-1 | 7/12 |
| 2+ | 5/12 |
| Disease location | |
| Parenchymal | 11/12 |
| Leptomeningeal enhancement/CSF+ | 2/12 |
| Cell of origin | |
| Germinal center B-cell type | 1/12 |
| Nongerminal center B-cell type | 11/12 |
| Median no. of previous lines of antineoplastic therapy (range) | 4 (2-9) |
| Prior methotrexate-based regimen | |
| Yes | 12/12 |
| No | 0/12 |
| Prior thiotepa-based ASCT | |
| Yes | 3/12 |
| No | 9/12 |
| BTKi refractory | |
| Yes | 12/12 |
| No | 0/12 |
| IMiD refractory * | |
| Yes | 4/12 |
| No | 8/12 |
| TEDDI-R refractory | |
| Yes | 6/12 |
| No | 6/12 |
| Prior radiotherapy | |
| Yes | 4/12 |
| No | 8/12 |
| Bridging therapy (including high-dose steroids) | |
| Yes | 12/12 |
| No | 0/12 |
| Median vein-to-vein time, d | 33 (27-37) |
ECOG, Eastern cooperate oncology group performance status; TEDDI-R, temozolomide, etoposide, Doxil, dexamethasone, ibrutinib, and rituximab.
4 patients received IMiD as part of venetoclax, ibrutinib, prednisone, obinutuzumab, and lenalidomide.
Safety
All patients required bridging therapy, including steroids, for symptomatic disease and disease-related cerebral edema following leukapheresis and before initiation of LDC. Attempts were made to completely wean patients off steroids before lymphodepletion. However, 4 patients required ongoing steroid use for symptomatic disease characterized by left-sided weakness (patient 002), lower extremity weakness (patient 009), worsening cranial nerve deficits (patient 011), and intractable headaches (patient 015). Three of the 4 patients who required ongoing steroid use were able to reduce steroids to physiologic dosing by month 1 and demonstrated a response at first assessment. The fourth patient experienced disease progression during initial hospitalization and was transitioned to hospice care. Of patients who experienced toxicity, all but 1 experienced low-grade CRS and/or ICANS (Table 2). In the 12 patients infused with tisagenlecleucel, grade 1 CRS was observed in 7 patients with a median onset of 4 days (range, 1-5) following tisagenlecleucel infusion and a median duration of 2 days (range, 2-8). No patient required tocilizumab or other management for CRS. ICANS developed in 6 of the 12 patients (50%), all of whom received at least 1 dose of dexamethasone; the median time of onset was 5 days (range, 3-11) and the median duration was 3 days (range, 1-11). Grade 1 ICANS was seen in 3 patients (25%) and characterized primarily by mild confusion. Grade 2 ICANS was seen in 2 patients (16.7%) and was characterized by word-finding difficulty and decreased level of consciousness. Patient 002 developed grade 3 ICANS (8%); this patient had presented with worsening left-sided weakness and progressive thalamic lymphoma with increased T2/fluid-attenuated inversion recovery (FLAIR) hyperintensity 1 week before lymphodepleting chemotherapy, necessitating high-dose steroids before initiation of lymphodepleting chemotherapy. The patient was eventually tapered to 4 mg of dexamethasone daily before tisagenlecleucel infusion but developed acute worsening of left-sided weakness postinfusion, increased focal FLAIR signal abnormality, and an immune effector call-associated encephalopathy score of 5/10 on day +5. With 10 mg dexamethasone every 6 hours, the patient’s immune effector call-associated encephalopathy score normalized, although progressive motor function decline was notable on day +11 and was associated with increased T2/FLAIR signal on magnetic resonance imaging (supplemental Figure 1A). Because of our inability to differentiate ICANS from disease progression by imaging, and concern for worsening mass effect, the patient received involved field radiotherapy to the tumor site and ongoing steroid treatment and had improvement in motor function by day +28. Subsequent CSF analysis demonstrated increased levels of IL-6 (94 pg/mL), CD25 (357 pg/mL), and granulocyte macrophage colony-stimulating factor (80 pg/mL) compared with peripheral blood during peak toxicity (supplemental Figure 1B). The patient was tapered off steroids and remains in a CR 22 months after infusion.
Table 2.
Rates of CRS and ICANS
| Characteristics | Patients (n = 12) |
|---|---|
| CRS * | |
| Any CRS | 7/12 |
| Grade 1 | 7/12 |
| Grade 2 | — |
| Grade 3 | — |
| Grade 4 | — |
| Required tocilizumab | — |
| Median onset of CRS (day postinfusion) | 4 |
| Median duration of CRS (day postinfusion) | 2 |
| ICANS * | |
| Any ICANS | 6/12 |
| Grade 1 | 3/12 |
| Grade 2 | 2/12 |
| Grade 3 | 1/12 |
| Grade 4 | — |
| Required corticosteroids | |
| At time of infusion for disease control† | 4/12 |
| Additional provided for ICANS following infusion | 6/12 |
| Median onset (day postinfusion) | 5 |
| Median duration (day postinfusion) | 3 |
Cytokine release syndrome and immune cell-associated neurotoxicity syndrome were graded with the use of the American Society of Transplantation and Cellular Therapy 2019 consensus criteria.
Maximum dexamethasone dose allowed at time of infusion was 4 mg daily.
Efficacy
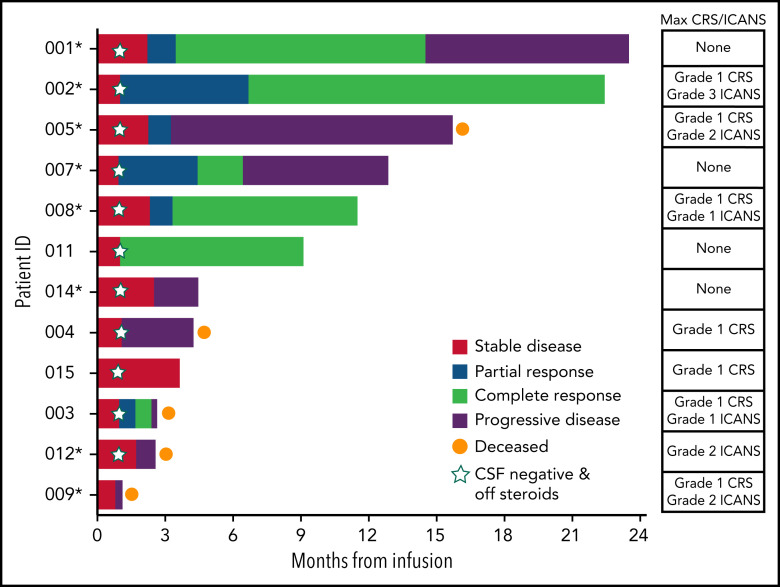
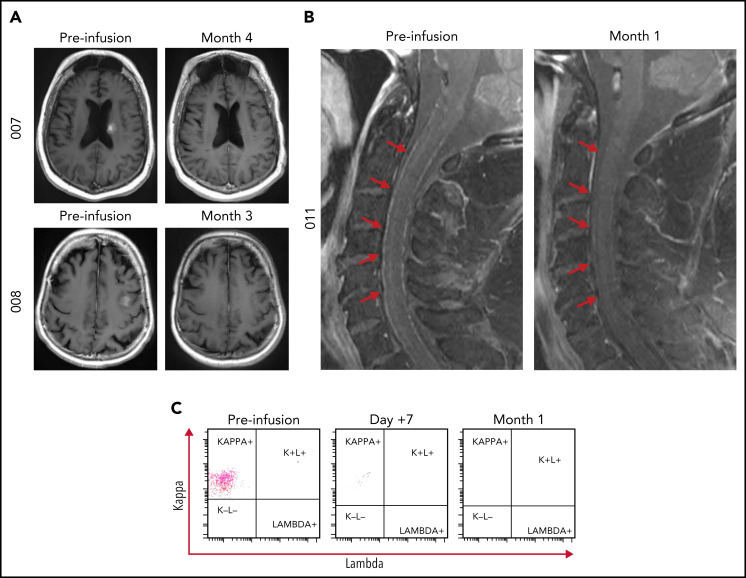
Responses were observed in 7/12 patients and consisted of 6 CRs and 1 partial response per the International PCNSL Collaborative Group (Figure 1). With a median time of 12.2 months from infusion to data cutoff, 7/12 patients were alive and 3/12 patients have not experienced disease progression. The median time to response was within 1 month, with patients obtaining their best response by a median of 3.4 months at time of data cutoff. All responders had negative CSF by cytology, flow cytometry, immunoglobulin H sequencing, and MYD88 polymerase chain reaction. No responder, which included patients who required steroids at time of infusion for symptomatic disease, required corticosteroids above adrenal replacement dosing by day +28. Areas of response included large intraparenchymal masses (Figure 2A; supplemental Figure 2) as well as leptomeningeal and CSF involvement (Figure 2B-C).
Figure 1.
Swimmer’s plot of evaluable patients following tisagenlecleucel infusion and associated CRS/ICANS. Response to most recent therapy included in parentheses. 001 (fourth line; PR), 002 (third line; PD), 005 (fourth line; PR), 007 (fourth line; PD), 008 (second line; SD), 011 (fifth line; PD), 014 (sixth line; PD), 004 (fourth line; PD), 015 (sixth line; SD), 003 (fourth line; PD), 012 (fifth line; PD), and 009 (fourth line; PD). PD, progressive disease; PR, partial response; SD, stable disease. *Continued ibrutinib use through month 3.
Figure 2.
Representative magnetic resonance imaging of responders at baseline and initial time point of best overall response. (A) Patient 007 baseline scan demonstrating a 1.6 cm × 1.3 cm × 1.1 cm enhancing mass within the left posterior caudate body bordering the left lateral ventricle with complete resolution of enhancing lesion at month 4. Patient 008 baseline scan demonstrating a 1.4 cm × 1.4 cm × 0.5 cm enhancing mass in the left middle frontal gyrus corresponding to biopsy proven CNS lymphoma with complete resolution at month 3. (B) Patient 011 demonstrating leptomeningeal enhancement within the spinal cord and (C) CD19+ CD20+ CD5− CD10−/dim leptomeningeal disease with monotypic κ immunoglobulin light chain expression at baseline, day +7 and month 1 demonstrating resolution following tisagenlecleucel infusion.
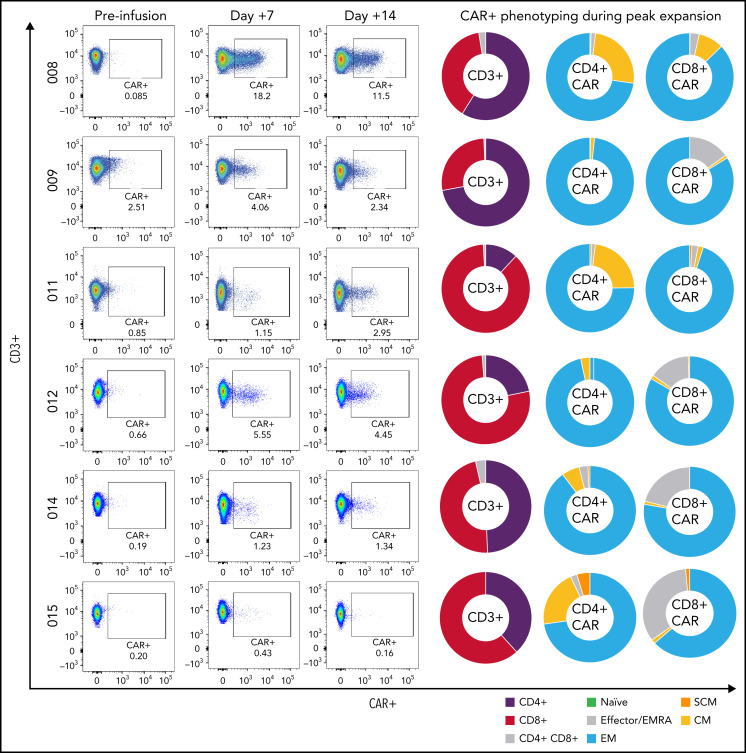
Cell expansion and phenotyping
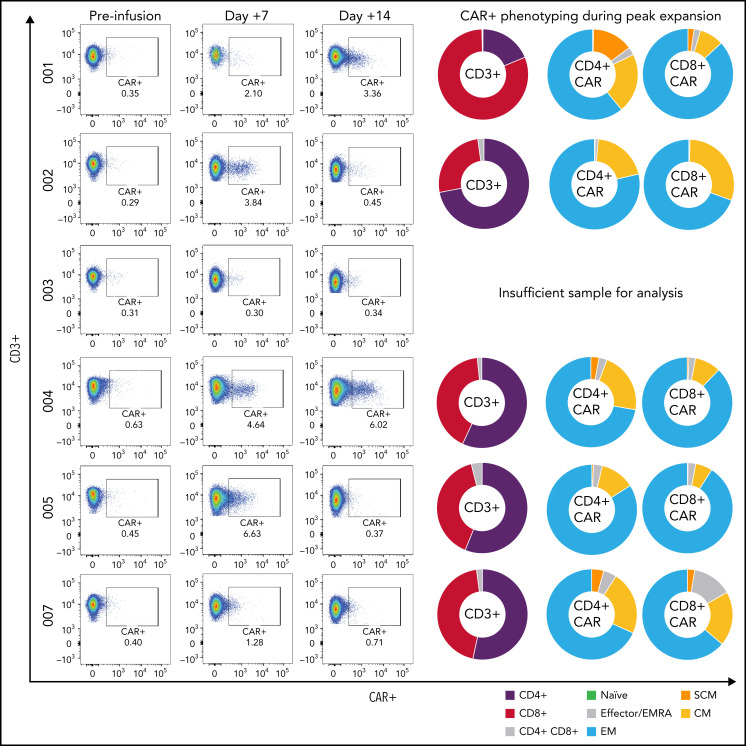
Tisagenlecleucel expansion in peripheral blood was observed and measurable by flow cytometry in all but 1 of the patients; in 1 case, CAR-T levels comprised 18.2% of CD45+ lymphocytes in the peripheral blood (1226 CAR+ cells/µL blood) (Figure 3, patient 008). Peripheral expansion of CAR T cells was noted in patients despite concurrent use of dexamethasone at time of infusion. Patients demonstrated a predominance of CD4+ CAR-T expansion with varying degrees of memory phenotypes (TSCM, TCM, TEMRA, TEM, and TN) (Figure 3). Markers of T-cell exhaustion, such as PD-1, TIM-3, and LAG-3 were identified during peak expansion (supplemental Figure 3). All patients who remain in an ongoing response continued to demonstrate B-cell aplasia as defined as <1% B cells by flow analysis of their peripheral blood.
Figure 3.
Peripheral CAR-T expansion and phenotyping. Expansion of tisagenlecleucel in peripheral blood and associated CAR-T phenotyping during peak expansion (day +7) demonstrating variable TNaïve, TEMRA, TEM, TCM, and TSCM phenotypes.
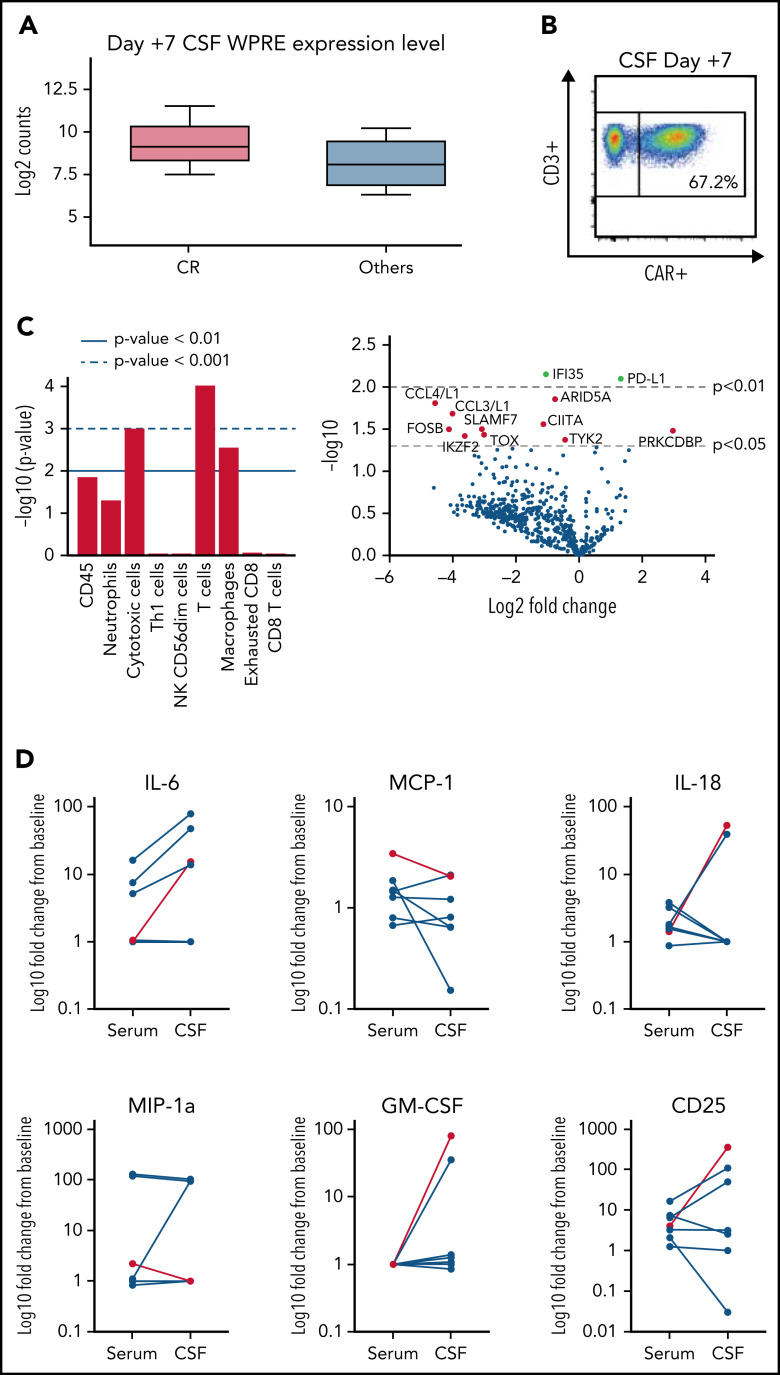
CSF trafficking and cytokine analysis
CAR-T cell trafficking into the CSF was noted in all patients who demonstrated expansion in peripheral blood. Patients who achieved a CR as their best response demonstrated higher levels of CAR transgene RNA in the CSF (Figure 4A). One patient demonstrated a relative higher level of tisagenlecleucel in the CSF than in peripheral blood based on CAR expression as a percent of CD45+ cells (67.2% vs 18.2%; Figure 4B). RNA profiling from CSF also identified increased expression of T-cell and macrophage gene pathways in patients who achieved a CR as best response. Downregulation of genes associated with inflammatory cytokines (CCL4/CCL3), T-cell exhaustion, effector functions, and Jak family members (Tyk2) was also observed in patients who ultimately achieved a CR (Figure 4C). Along with CNS trafficking of CAR-T cells, we also noted a trend toward increased inflammatory cytokines compared with preinfusion baseline within the serum and CNS 1 week after tisagenlecleucel infusion (Figure 4D).
Figure 4.
CSF and cytokine analysis of pre/post infusion samples. (A) Detection of tisagenlecleucel by RNA (Nanostring) demonstrating higher levels of the WPRE element in patients who achieved a CR. (B) Detection of tisagenlecleucel in the CSF of patient 008 (21 CAR+ cells/μL CSF) on day +7 by flow cytometry. (C) Pathway analysis and differential gene expression of patients who achieved a CR as best response vs others. Increase over non-CRs shown. (D) Representative serum and CSF cytokine analysis showing fold change from preinfusion baseline vs day +7. Grade 3 ICANS event shown in red.
Discussion
To our knowledge, this is the first and largest prospective study to demonstrate the safety and efficacy of commercially available CD19 CAR-T cells in PCNSL. It is also the first to address peripheral expansion, CNS trafficking, and mechanisms of toxicity in this rare patient population. Treatment was well tolerated and low-grade ICANS and CRS were manageable. No treatment-related deaths were observed and only 1 patient experienced severe grade 3 ICANS during treatment; no patient required tocilizumab for the management of CRS. Although additional follow-up is necessary, with a median follow-up time of 12.2 months from infusion, responses were seen in 7/12 patients (58.3%), including 4 responses beyond 6 months despite heavily pretreated disease. All infused subjects were able to discontinue corticosteroids within 28 days of tisagenlecleucel infusion. Responses were observed in patients who had progressed following high-dose methotrexate; ASCT; cytarabine and etoposide; ibrutinib monotherapy; temozolomide, etoposide, Doxil, dexamethasone, ibrutinib and rituximab; and venetoclax, ibrutinib, prednisone, obinutuzumab, and Revlimid. Tisagenlecleucel was tolerated safely in this older patient population (median age, 63 years) with 1 patient being 81 years old at time of infusion. Notably, this study demonstrated effective peripheral blood expansion and trafficking of tisagenlecleucel to the CNS compartment in the absence of systemic disease.
As we previously demonstrated in our series of secondary CNS lymphoma patients treated with tisagenlecleucel,15 the presence of CNS disease does not appear to predispose patients to severe ICANS. Although a single case of grade 3 ICANS was observed in the present study, this patient’s symptom onset was associated with peak CAR-T cell expansion and elevations in inflammatory CSF cytokines similar to that observed in systemic LBCL.19 Because of the acute nature of symptom presentation, and absence of real-time cytokine profiling, this patient received involved field radiotherapy in addition to high-dose steroids to address concerns of possible focal disease progression. This patient is showing an ongoing response >22 months from infusion, which is unlikely attributable to simply radiation effects given the high rate of out-of-radiation field recurrences seen in PCNSL,20 although a synergistic effect of CAR-T cells and radiation therapy cannot be ruled out in this instance. Our experience with both secondary (and now primary) CNS lymphoma emphasizes that the previously defined systemic risk factors for CRS, such as tumor burden and baseline inflammatory state, are more likely to predispose a patient to life-threatening ICANS than the mere presence of CNS disease. It also emphasizes the unique management challenges for this population and suggests that earlier use of CAR-T cells in a less refractory cohort may optimize long-term outcomes.
The high response rate and acceptable safety profile demonstrated in this study suggests that tisagenlecleucel may offer a valuable option to patients with relapsed or refractory PCNSL. Despite the small sample size, the number of patients described in this trial exceeds that which led to the inclusion of primary mediastinal B-cell lymphoma and follicular lymphoma grade 3B in the respective product labels for axicabtagene ciloleucel and lisocabtagene maraleucel (ZUMA-1, primary mediastinal B-cell lymphoma n = 8; TRANSCEND, follicular lymphoma grade 3B, n = 3).1,3 Interestingly, all responding patients who subsequently progressed demonstrated new sites of CNS disease rather than recurrence of prior lesions. Additional studies may need to explore earlier use of CAR-T cells as a potential consolidative approach for relapsed or refractory PCNSL patients who are not eligible for high-dose therapy and ASCT21 especially using noncurative bridging strategies such as BTKi/inducible T-cell kinase inhibition and PD-1 checkpoint inhibitors.22,23 Given the dual benefits of disease control and immunomodulatory augmentation of CAR-T function with such therapies,16,24-26 PCNSL may be a suitable disease to combine these synergistic approaches in a less refractory patient population. Regardless, these present data demonstrate the ongoing promise of CD19 directed CAR-T in hematologic malignancies and suggest that broadening access of tisagenlecleucel to patients with PCNSL may improve clinical outcomes in this patient population.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the patients who participated in the trial and the many nurses, staff, and providers of the Cellular Immunotherapy Program and the Massachusetts General Hospital Cancer Center. The authors would also like to thank the Karrie Kapoor Fund for Cellular Immunotherapy
This investigator-initiated trial was funded by Novartis. M.J.F. is supported by the National Institutes of Health National Cancer Institute grant K12CA087723. Novartis did not have any role in the collection, analysis or interpretation of data, the writing of this report, or decision to submit this manuscript for publication.
Footnotes
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.J.F., J.D., M.R., J.T.J, D.F., S.R.P., M.L., B.C., M.S., S.M., P.V.O., T.R.S., B.D., Z.D., A.E., T.T.B., and Y.B.V. provided patient care; M.J.F., K.G., D.C., K.S.C., K.A.L., F.P., G.D.D., K.K., E.L.E., and N.H. were involved in translational studies, data collection/acquisition and/or analysis; M.J.F., J.D., K.G., J.T.D., D.F., S.R.P., and B.C. were involved in clinical data interpretation; and M.J.F., J.D., K.G., M.R., J.T.J., D.F., S.R.P., D.C., K.S.C., K.A.L., G.D.D., K.K., E.V.E., M.B.L., B.C., N.H., F.P., M.S., S.A., P.V.O., T.R.S., B.D., Z.D., A.E., T.T.B., M.V.M., and Y.B.C. wrote the manuscript.
Conflict-of-interest disclosure: M.J.F. consulting for Kite/Gilead, BMS, Novartis, Iovance, and Arcellx. J.D. consults for Blue Earth Diagnostics, Magnolia, and Unum Therapeutics; has received research support from Beacon Biosignals, Boehringer Ingelheim, Bristol-Myers Squibb, Medimmune, Acerta Pharma, and Orbus Therapeutics; and has received royalties from Wolters Kluwer for serving as an author for UpToDate. J.T.J. consults and serves as a scientific advisory member for CereXis; consults for Navio Theragnostics and Health2047; and reports royalties from Elsevier. S.R.P. is a cofounder of NFlection Therapeutics and NF2 Therapeutics; consults for AstraZeneca and Akuous; and serves on the scientific advisory board of SonALAsense. F.P. is on the scientific advisory board of Cytek Biosciences. Y.-B.C. consults for Incyte, Gamida Cell, Jasper, and Novartis and serves on data and safety monitoring committees for Daiichi, Equillium, Celularity, Actinium, and Abbvie. M.V.M. is an inventor on patents related to adoptive cell therapies, held by Massachusetts General Hospital and University of Pennsylvania (some licensed to Novartis); holds equity in TCR2, Century Therapeutics, and 2SeventyBio; and has served as a consultant for multiple companies involved in cell therapies. The remaining authors declare no competing financial interests.
Correspondence: Matthew J. Frigault, Hematopoietic Cell Transplant and Cellular Therapy Program, Massachusetts General Hospital and Harvard Medical School, Zero Emerson Place, Suite 118, Office 127, Boston, MA 02114; e-mail: mfrigault@partners.org.
REFERENCES
- 1.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 3.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. [DOI] [PubMed] [Google Scholar]
- 4.Batchelor TT, DeAngelis LM. Lymphoma and Leukemia of the Nervous System, 2nd ed. New York, NY: Springer; 2013. [Google Scholar]
- 5.Green K, Hogg JP. Central Nervous System Lymphoma. Treasure Island, FL: StatPearls; 2021. [PubMed] [Google Scholar]
- 6.Kasenda B, Ferreri AJ, Marturano E, et al. First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL) – a systematic review and individual patient data meta-analysis. Ann Oncol. 2015;26(7):1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panageas KS, Elkin EB, Ben-Porat L, Deangelis LM, Abrey LE. Patterns of treatment in older adults with primary central nervous system lymphoma. Cancer. 2007;110(6):1338-1344. [DOI] [PubMed] [Google Scholar]
- 8.Jahnke K, Thiel E, Martus P, et al. ; German Primary Central Nervous System Lymphoma Study Group . Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol. 2006;80(2):159-165. [DOI] [PubMed] [Google Scholar]
- 9.Shibamoto Y, Ogino H, Suzuki G, et al. Primary central nervous system lymphoma in Japan: changes in clinical features, treatment, and prognosis during 1985-2004. Neuro-oncol. 2008;10(4):560-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han CH, Batchelor TT. Diagnosis and management of primary central nervous system lymphoma. Cancer. 2017;123(22):4314-4324. [DOI] [PubMed] [Google Scholar]
- 11.Ferreri AJM. Therapy of primary CNS lymphoma: role of intensity, radiation, and novel agents. Hematology Am Soc Hematol Educ Program. 2017;2017:565-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alcantara M, Houillier C, Blonski M, et al. CAR-T cell therapy in primary central nervous system lymphoma (PCNSL): the clinical experience of the French LOC network. Blood. 2022;139(5):792-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqi T, Wang X, Blanchard MS, et al. CD19-directed CAR T-cell therapy for treatment of primary CNS lymphoma. Blood Adv. 2021;5(20):4059-4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasquini MC, Hu ZH, Curran K, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma [published correction appears in Blood Adv. 2021;5(4):1136]. Blood Adv. 2020;4(21):5414-5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frigault MJ, Dietrich J, Martinez-Lage M, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood. 2019;134(11):860-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraietta JA, Beckwith KA, Patel PR, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127(9):1117-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127(8):3052-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locke FL, Neelapu SS, Bartlett NL, et al. Preliminary results of prophylactic tocilizumab after axicabtageneciloleucel (axi-cel; KTE-C19) treatment for patients with refractory, aggressive non-Hodgkin lymphoma (NHL). Blood. 2017;130(suppl 1):1547. [Google Scholar]
- 20.Shibamoto Y, Hayabuchi N, Hiratsuka J, et al. Is whole-brain irradiation necessary for primary central nervous system lymphoma? Patterns of recurrence after partial-brain irradiation. Cancer. 2003;97(1):128-133. [DOI] [PubMed] [Google Scholar]
- 21.DeFilipp Z, Li S, El-Jawahri A, et al. High-dose chemotherapy with thiotepa, busulfan, and cyclophosphamide and autologous stem cell transplantation for patients with primary central nervous system lymphoma in first complete remission. Cancer. 2017;123(16):3073-3079. [DOI] [PubMed] [Google Scholar]
- 22.Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117: 121-130. [DOI] [PubMed] [Google Scholar]
- 24.Hill BT, Roberts ZJ, Xue A, Rossi JM, Smith MR. Rapid tumor regression from PD-1 inhibition after anti-CD19 chimeric antigen receptor T-cell therapy in refractory diffuse large B-cell lymphoma. Bone Marrow Transplant. 2020;55(6):1184-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauthier J, Hirayama AV, Purushe J, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135(19):1650-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong EA, Melenhorst JJ, Lacey SF, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129(8):1039-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.