Abstract
The human genes for Interleukin 13 (IL-13) and its receptor alpha 1 (IL-13Rα1) are in chromosomal regions associated with Parkinson’s disease (PD). The interaction of IL-13 with its receptor increases the susceptibility of mouse dopaminergic neurons to oxidative stress. We identified two rare single SNPs in IL13 and IL13RA1 and measured their cytotoxic effects. rs148077750 is a missense leucine to proline substitution in IL13. It was found in individuals with early onset PD and no other known monogenic forms of the disease and is significantly linked with PD (Fisher’s exact test: p-value=0.01, odds ratio=14.2). rs145868092 is a leucine to phenylalanine substitution in IL13RA1 affecting a residue critical for IL-13 binding. Both mutations increased the cytotoxic activity of IL-13 on human SH-SY5Y neurons exposed to sublethal doses of hydrogen peroxide, t-butyl hydroperoxide or RLS3, an inducer of ferroptosis. Our data show that both rs148077750 and rs145868092 conferred a gain-of-function that may increase the risk of developing PD.
Keywords: Parkinson’s, Neuroinflammation, Oxidative stress, IL-13, IL13RA1, Neurons, Neurodegeneration, Ferroptosis, Oxytosis, Polymorphism
1. INTRODUCTION
Parkinson’s disease (PD) is neurodegenerative disorder characterized primarily by the progressive reduction of dopamine signaling in the basal ganglia resulting from the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc). Neuronal degeneration in PD is associated with several pathophysiological changes including mitochondrial dysfunction, oxidative stress, and protein misfolding (Dauer et al., 2003). Recently, inflammatory processes were also proposed to contribute to PD (Gelders et al., 2018).
We reasoned that if inflammation contributed to PD, then genes regulating inflammation could represent genetic factors for disease susceptibility. By combining data mining and literature search we found that genes encoding for human interleukin 13 receptor alpha 1 (IL-13Rα1), interleukin 13 (IL-13) and interleukin 4 (IL-4) are in chromosomal regions linked to PD (Foroud et al., 2006; Hicks et al., 2002; Li et al., 2019; Li et al., 2002; Martinez et al., 2004; Pankratz et al., 2002; Scott et al., 2001). IL-13Rα1 heterodimerizes with the interleukin receptor 4 alpha (IL-4Rα) forming a complex that can be activated by either IL-13 or IL-4 which regulates peripheral allergic and anthelminthic immune responses. We also demonstrated in the mouse brain that IL-13Rα1 is constitutively and uniquely expressed in midbrain dopaminergic neurons and that IL-13 is induced in the SN primarily by activated microglia during inflammation but also by neurons (Mori et al., 2016; Mori et al., 2017; Morrison et al., 2012). Finally, we showed that IL-13 mediated activation of IL-13Rα1 increased cellular susceptibility to oxidative stress in human and murine cells and that mice null for IL-13Rα1 are partially protected in inflammatory models of PD (Maher et al., 2018; Mori et al., 2016; Mori et al., 2017; Morrison et al., 2012). Based on this, and on the evidence that IL-13Rα1 is expressed also in human SN (Debinski et al., 2000), we hypothesized the existence of mutations in IL13RA1, IL4RA, IL13, or IL4 that may contribute to PD development or progression. Here, we describe the identification of two such candidates in individuals affected with PD. Both mutations, one in IL13 and one in IL13RA1, conferred a gain-of-function increasing neuronal vulnerability to oxidative stress.
2. RESULTS
2.1. Identification of rs148077750 and rs145868092
We searched for the existence of single nucleotide polymorphisms (SNP) in the exomes of two cohorts of idiopathic PD and non-PD individuals. The first comprised of 18 PD patients and 29 controls from the Italian island of Sicily, the second was the Parkinson’s Progression Markers Initiative (PPMI) cohort composed of 399 individuals diagnosed with PD, 59 individuals with scans without evidence for dopaminergic deficit (SWEDD), and 184 healthy controls. Screening was carried out for IL13, IL4, IL13RA1, IL4RA, for the most common forms of early onset PD in SNCA (PARK1/4), LRRK2 (PARK8) and TMEM230, and for other genes associated with PD: PARK 2, 3, 5, 6, 7, 8, 9, 11, 13, 14, 15, 17, 18, 20, 22, 23, GBA, MAPT, ADH1C, TBP, ATXN2, GLUD2, ATP7B, GRN, GCH1. By restricting our search to individuals carrying missense mutations in members of the IL-13 family that did not have a known monogenic variant of PD, and that had a minor allele frequency (MAF) in the general population < 0.05, the two variants rs148077750 and rs145868092 were selected for phenotypic characterization.
rs148077750 is an IL13 missense mutation (CTG→CCG) substituting Leucine at position 72 with Proline (Leu72Pro) identified in two male PD individuals, one from the Sicilian and one from the PPMI cohort, diagnosed with early onset PD at age 39 and 40. No healthy individual had this mutation in the two cohorts. rs148077750 is rare with a MAF of 0.0001 in the general population (27/279248 in the public database GnomAD (https://gnomad.broadinstitute.org/) (Karczewski et al., 2019). As both the Sicilian and the PPMI had moderate sample sizes, we further obtained the allele counts of the variant from the exomes in the gnomAD (v2.1.1) database (Lek et al., 2016). Since no individual disease phenotypes were available from gnomAD, we restricted the samples to the non-neuro group used as a control. To match the population, only non-Finnish European individuals were included (n=44239). In combination, we had 2 PD patients and 15 controls that were rs148077750 heterozygotes, and 417 PD patients and 44428 controls that were wild type. There were no homozygotes. rs148077750 was significantly associated with a PD diagnosis with an odds ratio (OR) of 14.2 (p-value = 0.01, Fisher’s exact test).
rs145868092 is an IL13RA1 missense mutation (TTA → TTC) that substitutes Leucine at position 319 with Phenylalanine (Leu319Phe). It was identified in a male from the Sicilian cohort diagnosed with PD at age 60. rs145868092 is a rare variant with a MAF of 0.0002 (39/176552 in GnomAD). Unlike rs148077750, further allele counts of the variant from the exomes in the gnomAD database failed to confer statistical significance (OR = 3.935, with p-value 0.2311, Fisher’s exact test). However, we decided to investigate the phenotype of this polymorphism because amino acid 319 is essential for the binding of IL-13 (Arima et al., 2005).
2.2. Cytotoxic activity of hIL-13Leu72 and hIL-13Pro72 (rs148077750)
We generated WT human recombinant IL-13 (hIL-13Leu72) and the rs148077750 variant (hIL-13Pro72) (Suppl Fig. 1) and compared their ability to potentiate the cytotoxic effects of oxidative stress. We used SH-SY5Y cells, a human neuroblastoma cell line expressing dopamine widely used in PD research (Banerjee et al., 2014; Gegg et al., 2009; Jiang et al., 2019; van der Merwe et al., 2017). SH-SY5Y cells also express IL-13Rα1 and IL-4Rα and were previously used by us to investigate the ability of IL-13 to potentiate oxidative damage (Maher et al., 2018). Cell viability was measured by the MTT assay in the presence of a sublethal concentration of hydrogen peroxide (H2O2) and t-butyl peroxide (tBOOH) calculated in titration experiments to be the maximal dose of oxidative agent that caused minimal damage (80 μM for H2O2 and 5 μM for tBOOH). Both hIL-13Leu72 and hIL-13Pro72 cytokines potentiated the cytotoxicity of H2O2 and tBOOH (Fig. 1). hIL-13Pro72 potentiated the cytotoxicity of H2O2 significantly more than hIL-13Leu72 did at 2.5 and 5 ng/mL (Fig. 1A). Similar results were seen with tBOOH with significant differences between hIL-13Pro72 and hIL-13Leu72 found at 2.5 and 5 ng/ml (Fig. 1B).
Figure 1. Recombinant human IL-13 potentiates oxidative stress-induced cell death in SH-SY5Y cells.
SH-SY5Y cells were treated with 0, 1, 2.5, or 5 ng/mL hIL-13Pro72 or hIL-13Leu72 in the presence of (A) 80 μM H2O2 or (B) 5 μM tBOOH or (C) 100 nM RSL3. Cell viability was assessed by the MTT assay 24 h later. Values (means ± SD). n = 6 (experiments were carried out 6 times with each treatment done in quadruplicate). (A) # = p < 0.05 vs. control (no IL-13). **** = p < 0.0001 vs. hIL-13Leu72. (B) # = p < 0.05 vs. control (no IL-13). ** = p = 0.0083 and **** = p < 0.0001 vs. hIL-13Leu72. (C) # = p < 0.05 vs. control (no IL-13). * = p = 0.0494, ** = p = 0.0070, and **** =p < 0.0001 vs. hIL-13Leu72.
tBOOH has been reported to induce ferroptosis (Wenz et al., 2018), a form of non-apoptotic regulated cell death associated with a prominent increase in lipid peroxidation (Lewerenz et al., 2018). Ferroptosis is linked to PD (Do Van et al., 2016; Guiney et al., 2017; Ito et al., 2017; Liddell et al., 2018) and can be induced by IL-13 in human airway epithelial cells (Wenzel et al., 2017). Thus, we measured the effects of hIL-13Leu72 and hIL-13Pro72 on cytotoxicity induced by RSL3, a compound that inhibits glutathione peroxidase 4 (GPX4) thereby increasing lipid peroxidation and cell death by ferroptosis (Ursini et al., 1982; Yang et al., 2016; Yang et al., 2014; Yang et al., 2008). Similar to H2O2 and tBOOH, we calculated and used the highest amount of RLS3 that caused minimal damage (100 nM). Both hIL-13Leu72 and hIL-13Pro72 potentiated RSL3 induced cell death (Fig. 1C) but hIL-13Pro72 was significantly more effective than hIL-13Leu72 at all concentrations tested.
These data indicate that rs148077750 conferred a gain of function increasing the ability of IL-13 to potentiate the cytotoxic action of mild oxidative stress as well as by exacerbating ferroptosis.
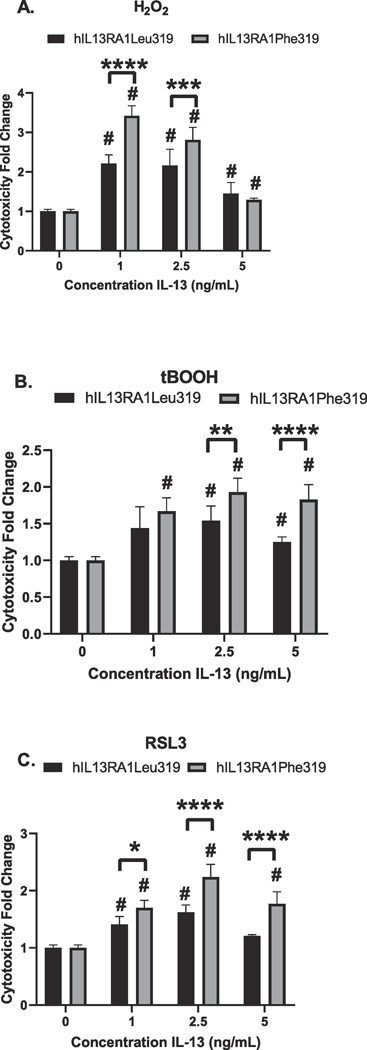
2.3. Cytotoxic activity of IL-13 on cells carrying hIL13RA1Leu319 or hIL13RA1Phe319 (rs145868092)
rs145868092 affects Leucine at position 319 (hIL13RA1Leu319), an amino acid essential for the binding of IL-13 (Arima et al., 2005) substituting it with Phenylalanine (hIL13RA1Phe319). WT SH-SY5Y (hIL13RA1Leu319) cells and cells carrying the rs145868092 (hIL13RA1Phe319) generated via CRISPR/Cas9 (Suppl Fig. 2) were used to measure the ability of IL-13 to potentiate H2O2, tBOOH or RLS3 cytotoxicity. IL-13 (hIL13Leu72) potentiated the cytotoxicity of hIL13RA1Leu319 and of hIL13RA1Phe319 cells (Fig. 2). However, the cytotoxicity was higher in hIL13RA1Phe319 cells with all insults tested. In the presence of H2O2, the cytotoxicity of hIL13RA1Phe319 was significantly higher than that of hIL13RA1Leu319 at 1 ng/mL and 2.5 ng/mL of IL-13 but not at 5 ng/mL (Fig. 2A). In the presence of tBOOH, cytotoxicity of hIL13RA1Phe319 was higher than that of hIL13RA1Leu319 at 2.5 and 5 ng/mL (Fig. 2B). In the presence of RLS3, cytotoxicity of hIL13RA1Phe319 was higher than that of hIL13RA1Leu319 at all concentrations tested (Fig. 2C). These data suggest that rs145868092 confers a gain of function increasing IL-13Rα1 mediated cytotoxicity and further support the hypothesis that IL-13Rα1 mediated cellular damage is caused by ferroptosis.
Figure 2. rs145868092 exacerbates IL-13 potentiation of oxidative stress-induced cell death in SH-SY5Y cells.
SH-SY5Y cells were untreated or treated with 0, 1, 2.5, or 5 ng/mL human IL-13 in the presence of (A) 80 μM H2O2 or (B) 5 μM tBOOH or (C) 100 nM RSL3. Cell viability was assessed by the MTT assay 24 h later. Values (means ± SD). n = 6 (experiments were carried out 6 times with each treatment done in quadruplicate). (A) # = p < 0.05 vs. control (no IL-13). *** = p = 0.0008 and **** = p < 0.0001 vs. hIL13RALeu319. (B) # = p < 0.05 vs. control (no IL-13). ** = p = 0.0083 and **** p < 0.0001 vs. hIL13RALeu319. (C) # = p < 0.05 vs. control (no IL-13). * = p = 0.0155 and **** = p < 0.0001 vs. hIL13RALeu319.
3. METHODS
3.1. Screening for SNPs of the IL-13 system
DNA from healthy subjects or individuals diagnosed with PD at the OASI Institute Maria SS, Troina (EN), Italy were screened using the Illumina Nextseq500 platform using the SeqCap Target Enrichment Kit (Personal Genomics). Data for the second cohort were from the Parkinson’s Progressive Marker’s Initiative (PPMI) exome sequence database of PD individuals and healthy controls (SWEDD individuals were excluded). Allele counts from the exome samples in the gnomAD (v2.1.1) database (https://gnomad.broadinstitute.org/) were also included in the analysis. GnomAD is a public database that aggregates and harmonizes both exome and genome sequencing data from a wide variety of large-scale sequencing projects. No individual disease phenotypes were made available from gnomAD to allow us to examine the PD cases in the dataset. We thus only included samples from individuals who were not ascertained for having a neurological condition in a neurological case/control study (“non-neuro” group) as controls. To match the population of the PPMI database, only non-Finnish European individuals were included.
3.2. Cell viability assays
Cell viability assays were carried out by the MTT assay using doses of IL-13 previously shown to potentiate oxidative damage (Maher et al., 2018; Morrison et al., 2012) (see supplemental methods for details).
3.3. Statistical analysis
Odds ratio test and Fischer’s exact test were used to determine the association of SNPs with PD diagnosis. Two-way ANOVA followed by Tukey’s post hoc test was used to determine significance (p < 0.05) in the cell viability assays using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, California USA).
4. DISCUSSION
Two rare SNPs in the genes encoding for human IL-13 and its receptor IL-13Rα1 were identified in individuals diagnosed with Parkinson’s disease. Both mutations caused a single amino acid substitution and the work presented here indicates that both confer a gain-of-function which increases cellular susceptibility to oxidative damage.
We and others showed that IL-13 can damage cells by increasing their susceptibility to oxidative stress (Morrison et al., 2012; Shin et al., 2004). We also showed that these actions are mediated by the Jak-Stat and the PI3 kinase-mTOR pathways activated by IL-13Rα1(Maher et al., 2018). Thus, the findings that hIL13Pro72 and hIL13RA1Phe319 enhance susceptibility to oxidative stress suggest that these variants may increase the binding affinity between IL-13 and IL-13Rα1, although this remains to be confirmed.
IL-13 is a Th2 cytokine classified as “anti-inflammatory” for its ability to lower the production of pro-inflammatory cytokines including some shown to contribute to the loss of dopaminergic cells. Thus, our findings may seem paradoxical at first since clinical and experimental evidence indicate that cellular damage is mediated by pro-inflammatory signals. IL-13 is also produced during inflammation and may indeed serve as an endogenous down-regulator of inflammation. However, the anti-inflammatory mechanisms of IL-13 include its ability to damage cells that promote inflammation including microglia that, upon activation, express IL-13Rα1 (Jeong et al., 2019; Park et al., 2009; Shin et al., 2004; Won et al., 2013; Yang et al., 2006). Dopaminergic neurons constitutively expressing IL-13Rα1 would be equally vulnerable. This suggests that the mechanisms that contribute to damage of dopaminergic neurons during neuroinflammation may not only depend on inflammation itself but also on the modality of its down-regulation.
IL-13 can damage cells expressing IL-13Rα1 only in the presence of oxidative stress (Morrison et al., 2012). Since the concomitance of both signals is less frequent than that of each signal alone, the conditions that can lead to IL-13-dependent damage may be relatively rare and/or may last for a limited period leading to minimal or modest neuronal loss. However, the eventual repetition of the event over time due to lifestyle, environmental and/or genetic factors could lead to progressive damage. In this scenario, the genetic variants identified and characterized here increase the probability that damage occurs by enhancing the activity of IL-13 or of IL-13Rα1 but their presence is neither necessary nor sufficient alone for IL-13-mediated damage because a sufficient amount of oxidative stress can damage neurons even in the absence of IL-13 and, by contrast, IL-13 alone is not cytotoxic. Thus, the penetrance of rs148077750 and rs145868092 may be partial and their identification difficult in GWAS studies alone (a recent GWAS and TWAS study identified the chromosomal region containing IL4 and IL13 as linked to PD (Li et al., 2019)). Similar limitations may apply to other regulators of inflammation and represent an obstacle for the identification of variants that can contribute to neurodegenerative diseases.
Previously, we showed that IL-13 could potentiate the toxicity of the general oxidants H2O2 and tBOOH in dopaminergic neurons in culture (Maher et al., 2018; Morrison et al., 2012). However, the relevance of this to PD remains an open question. Interestingly, tBOOH was found to activate a recently described cell death pathway called ferroptosis (Wenz et al., 2018) that shares many characteristics with a cell death pathway that we (Maher) described over 20 years ago and named oxytosis (Tan et al., 2001). Importantly, several studies have now linked ferroptosis to PD (Do Van et al., 2016; Guiney et al., 2017; Ito et al., 2017; Liddell et al., 2018). Ferroptosis is an iron-dependent cell death pathway that involves GSH depletion and lipid peroxidation (Lewerenz et al., 2018). These characteristics of ferroptosis are also observed in the DA neurons of the SN in PD and treatments that enhance GSH levels or chelate iron have shown some benefits in clinical trials in PD patients (Guiney et al., 2017). Thus, in this study we also tested RSL3, a well characterized inducer of ferroptosis through its ability to inhibit glutathione peroxidase 4, a critical enzyme for the removal of lipid peroxides (Yang et al., 2014). We show not only that IL13 can potentiate RSL3 toxicity in human SH-SY5Y cells but also that mutations in both IL-13 and its receptor that are associated with PD, show enhanced potentiation of RSL3-induced cell death.
Experiments were carried out on undifferentiated human SH-SY5Y cells because several groups reported that they are a better model than differentiated cells for studies on the effects of PD-related oxidative stress (Banerjee et al., 2014; Cheung et al., 2009; Jiang et al., 2019). While further studies using additional models are desirable to fully demonstrate the role of the mutations described in PD, including the use of primary or iPSC-derived dopaminergic neurons, the data collected here provide further support for the pathophysiological relevance of the IL-13 mediated potentiation of oxidative stress-induced cell death.
Supplementary Material
The genes for human IL13 and IL13RA1 are associated with Parkinson’s disease (PD)
rs148077750 is a missense mutation in IL13 identified in early onset PD
rs148077750 increasing neuronal vulnerability to oxidative stress and ferroptosis
rs145868092 is a missense mutation in IL13RA1 identified in one individual with PD
rs145868092 increasing neuronal vulnerability to oxidative stress and ferroptosis
ACKNOWLEDGEMENTS
Supported by the National Center for Advancing Translational Sciences, and the National Institutes of Health, through grant UL1 TR002550, TL1 TR002551, NS085155 and AA025095. We thank the Teyton and the Lazzerini-Denchi Labs at TSRI for help and advice.
Footnotes
DECLARATION OF COMPETING INTERESTS
The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arima K, et al. , 2005. Characterization of the interaction between interleukin-13 and interleukin-13 receptors. J Biol Chem 280, 24915–24922. [DOI] [PubMed] [Google Scholar]
- Banerjee K, et al. , 2014. Dopamine Cytotoxicity Involves Both Oxidative and Nonoxidative Pathways in SH-SY5Y Cells: Potential Role of Alpha-Synuclein Overexpression and Proteasomal Inhibition in the Etiopathogenesis of Parkinson’s Disease. Parkinsons Dis 2014, 878935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung YT, et al. , 2009. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 30, 127–135. [DOI] [PubMed] [Google Scholar]
- Dauer W, et al. , 2003. Parkinson’s disease: mechanisms and models. Neuron 39, 889–909. [DOI] [PubMed] [Google Scholar]
- Debinski W, et al. , 2000. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med 6, 440–449. [PMC free article] [PubMed] [Google Scholar]
- Do Van B, et al. , 2016. Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol Dis 94, 169–178. [DOI] [PubMed] [Google Scholar]
- Foroud T, et al. , 2006. Chromosome 5 and Parkinson disease. Eur J Hum Genet 14, 1106–1110. [DOI] [PubMed] [Google Scholar]
- Gegg ME, et al. , 2009. Silencing of PINK1 expression affects mitochondrial DNA and oxidative phosphorylation in dopaminergic cells. PLoS One 4, e4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelders G, et al. , 2018. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J Immunol Res 2018, 4784268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney SJ, et al. , 2017. Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochem Int 104, 34–48. [DOI] [PubMed] [Google Scholar]
- Hicks AA, et al. , 2002. A susceptibility gene for late-onset idiopathic Parkinson’s disease. Ann Neurol 52, 549–555. [DOI] [PubMed] [Google Scholar]
- Ito K, et al. , 2017. MPP+ induces necrostatin-1- and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells. Cell Death Discov 3, 17013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JY, et al. , 2019. Interleukin-4 and Interleukin-13 Exacerbate Neurotoxicity of Prothrombin Kringle-2 in Cortex In Vivo via Oxidative Stress. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, et al. , 2019. Proteomic Study of a Parkinson’s Disease Model of Undifferentiated SH-SY5Y Cells Induced by a Proteasome Inhibitor. Int J Med Sci 16, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski KJ, et al. , 2019. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv, 531210. [Google Scholar]
- Lek M, et al. , 2016. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, et al. , 2018. Oxytosis/Ferroptosis-(Re-) Emerging Roles for Oxidative Stress-Dependent Non-apoptotic Cell Death in Diseases of the Central Nervous System. Front Neurosci 12, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YI, et al. , 2019. Prioritizing Parkinson’s disease genes using population-scale transcriptomic data. Nat Commun 10, 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, et al. , 2002. Age at onset in two common neurodegenerative diseases is genetically controlled. Am J Hum Genet 70, 985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell JR, et al. , 2018. Nexus between mitochondrial function, iron, copper and glutathione in Parkinson’s disease. Neurochem Int 117, 126–138. [DOI] [PubMed] [Google Scholar]
- Maher P, et al. , 2018. Deciphering the pathways that protect from IL-13-mediated potentiation of oxidative stress-induced dopaminergic nerve cell death. Cytokine 103, 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, et al. , 2004. Genome-wide scan linkage analysis for Parkinson’s disease: the European genetic study of Parkinson’s disease. J Med Genet 41, 900–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, et al. , 2016. Neuroimmunology of the Interleukins 13 and 4. Brain Sci 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, et al. , 2017. Lack of interleukin-13 receptor alpha1 delays the loss of dopaminergic neurons during chronic stress. J Neuroinflammation 14, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BE, et al. , 2012. Cutting edge: IL-13Ralpha1 expression in dopaminergic neurons contributes to their oxidative stress-mediated loss following chronic peripheral treatment with lipopolysaccharide. J Immunol 189, 5498–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratz N, et al. , 2002. Genome screen to identify susceptibility genes for Parkinson disease in a sample without parkin mutations. Am J Hum Genet 71, 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, et al. , 2009. IL-13-induced oxidative stress via microglial NADPH oxidase contributes to death of hippocampal neurons in vivo. J Immunol 183, 4666–4674. [DOI] [PubMed] [Google Scholar]
- Scott WK, et al. , 2001. Complete genomic screen in Parkinson disease: evidence for multiple genes. Jama 286, 2239–2244. [DOI] [PubMed] [Google Scholar]
- Shin WH, et al. , 2004. Microglia expressing interleukin-13 undergo cell death and contribute to neuronal survival in vivo. Glia 46, 142–152. [DOI] [PubMed] [Google Scholar]
- Tan S, et al. , 2001. Oxytosis: A novel form of programmed cell death. Curr Top Med Chem 1, 497–506. [DOI] [PubMed] [Google Scholar]
- Ursini F, et al. , 1982. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta 710, 197–211. [DOI] [PubMed] [Google Scholar]
- van der Merwe C, et al. , 2017. Curcumin Rescues a PINK1 Knock Down SH-SY5Y Cellular Model of Parkinson’s Disease from Mitochondrial Dysfunction and Cell Death. Mol Neurobiol 54, 2752–2762. [DOI] [PubMed] [Google Scholar]
- Wenz C, et al. , 2018. t-BuOOH induces ferroptosis in human and murine cell lines. Arch Toxicol 92, 759–775. [DOI] [PubMed] [Google Scholar]
- Wenzel SE, et al. , 2017. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell 171, 628–641.e626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won SY, et al. , 2013. Interleukin-13/Interleukin-4-induced oxidative stress contributes to death of prothrombinkringle-2 (pKr-2)-activated microglia. J Neuroimmunol 265, 36–42. [DOI] [PubMed] [Google Scholar]
- Yang MS, et al. , 2006. Interleukin-13 enhances cyclooxygenase-2 expression in activated rat brain microglia: implications for death of activated microglia. J Immunol 177, 1323–1329. [DOI] [PubMed] [Google Scholar]
- Yang WS, et al. , 2016. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 113, E4966–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, et al. , 2014. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, et al. , 2008. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 15, 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.