Abstract
Some studies have addressed the prevalence of human papillomavirus (HPV) in head and neck cancer in South America; however, no studies have systematically gathered prevalence and conducted a meta-analysis. This study aims to estimate the prevalence of HPV in oral and oropharyngeal squamous cell carcinomas in South America. We performed a systematic review and metaanalysis using the following databases: PubMed, Embase, Lilacs, Medline, Scopus, and Web of Science. Data were extracted and analyzed using random-effects models to estimate the pooled prevalence of HPV. We identified 209 nonduplicated studies, of which 38 were selected. The overall prevalence of HPV was 24.31% (95% CI 16.87–32.64; I2 = 96%, pheterogeneity <0.001). HPV prevalence in oropharyngeal cancer was 17.9% (95% CI 7.6–31.4; I2 = 96%, pheterogeneity <0.001) and that in oral cavity cancer was 23.19% (95% CI 14.94–32.63; I2 = 94%, pheterogeneity <0.001). We found an overall prevalence of HPV in 24.31% of oral and oropharyngeal squamous cell carcinomas in South American patients. The prevalence of HPV was 17.9% for oropharyngeal cancer and 23.19% for oral cavity cancer.
Key words: Squamous cell carcinoma of head and neck, oral cavity squamous cell carcinoma, oropharyngeal squamous cell carcinoma, prevalence, meta-analysis
Introduction
In 2020, the International Agency for Research on Cancer (IARC) registered 476,125 new cases of oropharyngeal and oral cavity cancer worldwide, of which 98,412 belonged to oropharynx and 377,713 belonged to the oral cavity.1 In South America, with approximately 7292 new oropharyngeal cancer cases and 14,191 new oral cavity cancer cases, the combined incidence was estimated as 1.97% of all new cases recorded in 2020.1
According to the National Cancer Institute (INCA), cancers of the oral cavity and oropharynx had an incidence in Brazil of 10.69 per 100,000 inhabitants in 2020 and represented the fifth highest incidence among men.2
Squamous cell carcinoma (SCC) is the most common histological type of oral cancer,3,4 and even with advances achieved in treatment regimes, mortality remains high after 5 years.5 Despite recognizing a multifactorial process, the risk of developing head and neck cancer (HNC) is 30 times greater for individuals who smoke and drink.6 Several studies have reported human papillomavirus (HPV) as a relevant risk factor for HNC development, particularly for oropharynx.7–9
More than 170 types of HPV are known, and they are divided into two large groups: high-risk (hrHPV) and low-risk (lrHPV). The most common genotypes in head and neck cancers are HPV16 and HPV18.10,11 A meta-analysis published in 2014, with data from 44 countries, recorded a pooled prevalence of 29.5% for HPV DNA in HNCSCC; in the oral cavity and oropharynx, the pooled prevalences were 24.2% and 45.8%, respectively.11 Moreover, HPV16 DNA could contribute to 82.2% of all cases of HPV-positive DNA.11 A recent meta-analysis of Brazilian studies estimated an overall prevalence of 11.89% for different types of HPV in the oral cavity and 4.69% for hrHPVs.12
Methods for detecting and diagnosing HPV can target viral DNA or RNA, oncoproteins, cellular proteins, and serum antibodies specific to HPV.13 The detection of messenger RNA E6/E7 (mRNA) is considered as gold standard for identifying HPV.13 Among DNA detection techniques, the most cited are direct hybridization, such as Southern blotting, in situ hybridization (ISH), and polymerase chain reaction (PCR);13–15 whereas, among the biomarker detecting methods, the most common is immunohistochemical (IHC) detection of overexpression of p16INK4a.13–15
Furthermore, the literature results vary for the association between HPV and oral cavity and oropharyngeal cancers. Few studies have verified the prevalence of HPV in HNCC in South American countries. Considering the need for studies that collate the data recorded in this region, as well as the variation of data from Brazil and other South American countries concerning the rest of the world, the present study aimed to conduct a systematic review and meta-analysis to estimate the prevalence of HPV in oral and oropharyngeal squamous cell carcinoma in South America.
Materials and methods
We performed a systematic review and meta-analysis, where three authors carried out the initial searches on the following databases: Embase, Lilacs, Medline, Scopus, and Web of Science. We searched for articles in the following languages: English, Portuguese, and Spanish.
The following terms were used to search in the titles, abstracts, and keywords: (“HPV” OR “papillomavirus” OR “papillomaviridae”) AND ((“head” AND “neck”) OR “oropharynx” OR “oropharyngeal” OR “tongue” OR “mouth” OR “oral” OR “oral cavity”) AND (“cancer” OR “tumor” OR “neoplasms” OR (“carcinoma” AND “squamous” AND “cell”)) AND (“Brazil” OR “Brazilian” OR “Argentina” OR “Bolivia” OR “Chile” OR “Colombia” OR “Ecuador” OR “Guyana” OR “Paraguay” OR “Peru” OR “Suriname” OR “Uruguay” OR “Venezuela”).
Initially, the search in each database resulted in 494 records. After the first screening and exclusion of duplications, 209 studies were selected to read the titles and abstracts. Of these, 52 studies were selected from a review of titles and abstracts. After analyzing these 52 articles’ references, another 2 were selected for detailed assessment. To control the data collection process, each of the 54 studies selected for detailed assessment received a sequential code ranging from A01 to A54. After reading the full texts, 38 articles were selected for qualitative and quantitative reviews.
The search and selection stages of the studies are presented in Appendix A and are illustrated in Figure 1.
Exclusion criteria
The following exclusion criteria were established: articles not written in English, Portuguese, or Spanish; articles in which the correlation between HPV and oral cavity squamous cell carcinoma (OSCC) or oropharynx squamous cell carcinoma (OPSCC) were not found; review articles with or without meta-analysis or case reports; animal studies; search results with incomplete information or those without access to full text; studies with incomplete or inaccurate information that would make it impossible to extract the necessary information reliably; duplicate studies; studies that revealed strong evidence of sample overlap; and studies whose samples had less than five cases of OSCC or OPSCC per country.
Data extraction
In articles where the lesion subsites were not classified into the oropharynx and oral cavity, the following grouping was applied, according to the ICD-O classification:16 oropharynx (C01.9, 02.4, 09.0–10.9), oral cavity (C00.0–00.9, 02.0–02.3, 02.8–03.1, 03.9– 04.1, 04.8–05.0, 05.8–06.2, 06.8–06.9).
Data collection was performed only for viral DNA detection techniques in studies where HPV diagnostic technique involved more than one detection method and the criterion for diagnosis did not include combination of results.
Data analysis
The collected data were tabulated using STATA IC version 16.17 The combined prevalence was estimated using the R metaprop package, version 3.6.1,18 via the Rstudio interface, version 1.2.1335.19 The following parameters were used for statistical analysis: inverse variance method, DerSimonian-Laird estimator for the variance between studies, Jackson’s method for calculating confidence intervals, arcsine transformation for gross proportions, and Clopper–Pearson CI to estimate the confidence intervals (95% CI) for each of the studies. Heterogeneity tests (I2) between studies were performed, and Cochran’s Q test was used to establish the significance of heterogeneity. These parameters refer to the random- effects analysis, considering the heterogeneity of our sample (p<0.01).
Figure 1.
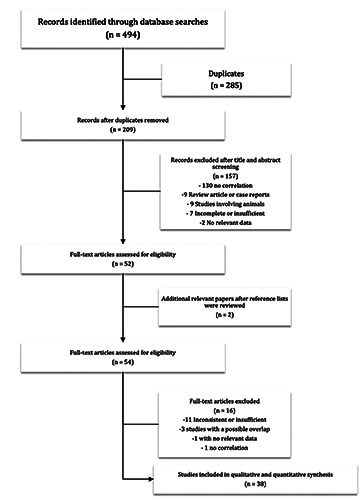
Flow chart of study selection.
Heterogeneity was analyzed using meta-regression and stratification. The explanatory variables selected for the meta-regression analysis included the country of origin of the studies, types of HPV investigated, type of material collected, and method for diagnosing HPV. Stratification included all subgroups of the metaregression and the patient’s sex and profile (smokers and alcohol consumers). For the type of material collected, the analysis included in this document involves two groups: material fixed by freezing or inclusion in paraffin, and exfoliation off mucosa or swab. As for the diagnostic method, four subgroups were evaluated: HPV DNA detection techniques, HPV and IHC DNA alone, HPV and IHC DNA combined, and IHC. Meta-regression analysis was performed using the metareg package 18 via moderator test. Subgroup comparisons were calculated using the Q test for differences in subgroups of the metaprop package.18
The R codes, dataset, and the complete list of nonduplicate records with the respective reasons for exclusion can be found in Mendeley Data doi:10.17632/9ss9d42f74.1.
Results
We identified 209 unduplicated records. After reading the titles and abstracts, 52 studies were included. The references of these 52 articles were revised, and another two studies were added, adding up to 54 full texts selected for reading. After reading, 38 articles were selected for the qualitative and quantitative reviews. The selected studies were published between 1998 and August 2020 and included patients assessed between 1970 and 2019. Brazil has the largest number of studies (n=28),20-47 followed by Colombia (n=4),48–51 Argentina (n=2),52,53 Chile (n=2),54,55 and Venezuela (n=2)56,57 presented only two studies each.
The patients’ sample resulting from the 38 studies included 2788 individuals–1643 cases of OSCC and 1145 of OPSCC. Brazil had highest number of cases (2329 cases), followed by Colombia (190 cases), Chile (102 cases), and Argentina (101 cases), and Venezuela had the lowest number (66 cases) (Table 1).
The pooled prevalence of HPV for OSCCs (33 studies)20-23,25-27,30-40,42-57 and OPSCC (14 studies)20-22,24,25,28-30,34,37,38,40,41,51 was estimated at 24.31% (95% CI 16.87–32.64) (Figure 2). In Brazil, this prevalence was estimated to be 21.15% (95% CI, 12.83– 30.90). The highest prevalence was observed in Venezuela (57.6% [95% CI 45.57–69.18]), Argentina (42.57% [95% CI 33.13– 52.30]), and Colombia (30.27% [95% CI 16.14–46.63]). Chile had the lowest prevalence (10.77% [95% CI 5.53–17.48]) (Table 2). Nevertheless, notably, with the exception of Brazil, all other countries presented few studies for analysis and a reduced number of cases (Table 2).
The pooled prevalence for HPV16 in OSCC and OPSCC was 12.8% (95% CI 8.7–17.5) (Figure 3) (23 studies).20-25,27,30,34– 40,46,48–50,52,53,55,56 In Brazil (16 studies),20-25,27,30,34–40,46 this prevalence was estimated at 9.6% (95% CI 6.1-13.9). Chile55 (6.2% [95% CI 2.0–12.6]) and Venezuela55 (44% [95% CI 30.5–57.8]) presented only one study each for this criterion. Argentina considered two studies52,53 (9.9% [95% CI 4.8–16.4]) and Colombia presented three studies48-50 (38.6% [95% CI 13.8–67.2]). In the metaregression analysis, studies from Colombia and Venezuela, using the data obtained from Argentinian studies as a reference, revealed statistical significance for heterogeneity moderation in the pooled prevalence of HPV16 in OPSCCs and OSCCs, thereby contributing positively to the calculated percentage (p<0.05). Except for countries’ stratification in the set of studies that investigated HPV16 infection, none of the other stratifications revealed significant results for moderating heterogeneity (p>0.05). This information is presented in Table 2.
The pooled prevalence of HPV in OSCCs was estimated at 23.19% (95% CI 14.94–32.63) (Figure 5) (33 studies).20,22,23,25-27,30–40,42–57 In Brazil (23 studies),20,22,23,25-27,30–40,42–47 this prevalence was 18.71% (95% CI 9.51–30.15) (Table 2). Argentina52,53 (42.57% [95% CI 33.13–52.30]), Chile54,55 (10.77% [95% CI 5.53–17.48]), and Venezuela56,57 (57.60% [45.57–69.18]) presented only two studies each. Colombia48–51 (31.80% [95% CI 17.61– 48.97]) maintained the four initial studies (Table 2).
The subgroup analysis by type of material collected and HPV investigated did not reveal any statistical significance between the pooled prevalence (p>0.05). About 22.64% of the tissue samples fixed by frozen or paraffin embedding techniques (35 studies)20– 41,43–46,48–52,54–57 presented a positive diagnosis for some type of HPV (95% CI 15.13–31.17). In the samples of mucosa scraping or swab (3 studies),42,47,53 this percentage was 44.22% (95% CI 24.15–65.32). In studies that investigated only hrHPV (9 studies), 20,24,25,27–30,35,50 the calculated prevalence was 16.45% (95% CI 05.03–32.75), those that investigated infection for both hrHPV and lrHPV (29 studies),21-23,26,31-34,36–49,51–57 the pooled prevalence was 27.14% (18.31–36.99). In studies where data collection considered only the results of molecular tests to detect HPV DNA (34 studies),21-23,25-27,30-57 the combined prevalence of HPV DNA was 24.67% (95% CI 17.13–33.10). Moreover, only two studies applied the combined methodology between PCR and IHC to diagnose HPV, considering only positive cases in which the p16 biomarker and viral DNA were present.20,24 The pooled prevalence of HPV was 4.15% (95% CI 1.37–8.34). In study A16,29 detection of HPV involved viral DNA molecular detection techniques and IHC to detect the p16 biomarker; however, it was not specified which technique tested cases, and the pooled HPV prevalence in that study, in the random-effects model, was estimated at 59.07% (95% CI 52.18–65.71). Furthermore, Study A2228 was the only one included in this review in which HPV was diagnosed only via IHC; the pooled prevalence of the cases in this study, in the randomeffects model, was 35.29% (95% CI 22.89–48.80). This information is detailed in Table 2.
Eventually, Table 2 details the stratification by sex and profile of the cases analyzed (smokers and alcoholics). In studies where cases were stratified by sex, the pooled prevalence of HPV in OPSCC and OSCC for men (19 studies)20,21,23,24,26,27,29-31,39-41,44,46,49,50,54,55,57 was 19.11% (95% CI 9.37–31.29), and for women (19 studies),20,21,23,24,27,29-31,39,40,41,44,46,49,50,54–57 it was 22.35% (95% CI 11.85–35.04). Among smokers considering 947 cases (13 studies),20,21,23,24,27-29, 38,39,41,44,49,56 the pooled prevalence was 20.75% (95% CI 9.21–35.44) and for alcoholics, with 720 cases (10 studies),20,21,23,24,27,29,41,44,49,56 the pooled prevalence of HPV was 22.23% (95% CI 8.23–40.62).
Discussion
This review included 38 studies that examined the prevalence of HPV DNA and p16INK4a positivity, alone or in combination, in OPSCC and OSCC in patients from South American countries.
Based on 2788 cases, 1145 OPSCC and 1643 OSCC, we found that approximately 25% of the cases were associated with HPV infection. The highest pooled prevalence was observed in Venezuela (57.6%), and Chile had the lowest percentage (10.77%). Nevertheless, notably, the number of cases registered for countries, except for Brazil, was reasonably low, making the sample more subject to random variations.
Table 1.
General panel of studies selected for review.
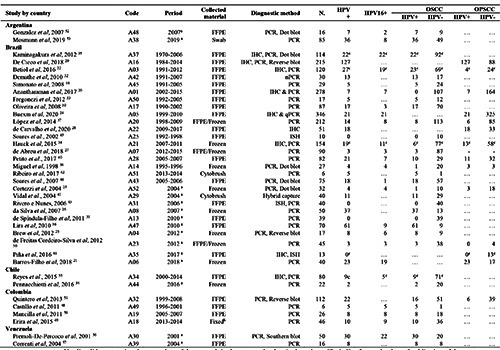
aStudies did not mention the exact date of the research in the text, so the date in the column "Period" refers to the date of publication of the study. bThe study does not specify which fixation technique was adopted, informing only that the genetic material was an AllPrep DNA/RNA/ ProteinQuiagen® kit. cResults for HPV DNA only. N = Total cases was 2788. HPV+ = HPV positive, the total number of cases was 630. HPV16+ = HPV genotype 16 positive, the total number of cases was 206. OSCC/HPV+ = Squamous cell carcinomas of the oral cavity with HPV+, the total number of cases was 388. OSCC/HPV- = Squamous cell carcinomas of the oral cavity with HPV negative, the total number of cases was 1643. OPSCC/HPV+ = Squamous cell carcinomas of the oropharynx with HPV +, the total number of cases was 242 OPSCC/HPV+ = Squamous cell carcinomas of the oral cavity with HPV-, the total number of cases for OPSCC / HPV- was 1145.
Table 2.
Pooled prevalence of human papillomavirus in squamous cell carcinomas of the oral cavity (OSCC) and oropharynx (OPSCC) in South American countries by stratification variables.
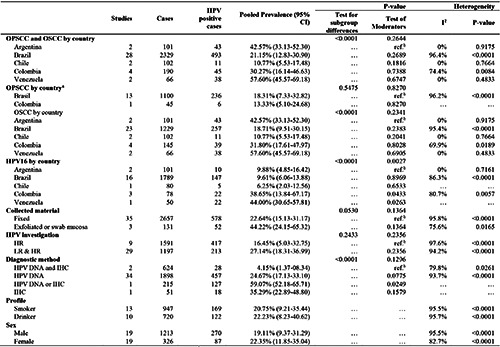
aOnly Brazil and Colombia presented eligible studies. bReference value for the moderator test. HR = high-risk; LR = low-risk. HPV = human papillomavirus. HPV DNA = HPV DNA detection techniques. IHC = immunohistochemical detection of overexpression of p16. HPV DNA or IHC = HPV DNA or IHC, non-combined techniques. DNA and IHC = HPV DNA and IHC, combined techniques.
Figure 2.
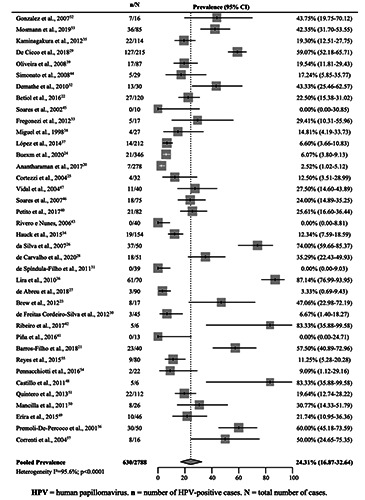
Pooled prevalence of human papillomavirus in squamous cell carcinomas of the oral cavity and oropharynx in South American countries.
In Europe, in 2014, the pooled prevalence of HPV in HNCs was estimated at 40%, and the highest and lowest percentages were observed in one of the oropharynx and oral cavity subsites, that is tonsil (66.4%) and tongue (25.7%), respectively.58 In another meta-analysis that analyzed records from 43 countries, the prevalence of HPV in non-oropharyngeal cancers was 21.8%.59 Although they did not specifically mention the term oral cavity, we can infer that the prevalence observed in the present study (24.2%) was relatively closer to the averages recorded in our study.
In contrast to other studies, the pooled prevalence of OPSCCs in our meta-analysis was low. A recent study investigating the global prevalence of HPV in OPSCC patients reported a pooled percentage of 44.8%. Among the countries analyzed, Brazil was the only representative of South America, with the lowest prevalence (11.1%);60 however, notably, the aforementioned study analyzed only 15 cases worldwide, and data from Brazil were collected from a single article with 63 cases.34 In 2015, a meta-analysis was published that demonstrated the increased prevalence of HPV in OPSCCs over four periods in 23 countries: i. pre 1995: 32.3%; 1995–1999: 37%; 2000–2004: 51.8%; and 2005–2014: 52.9%.9 Another meta-analysis with the same theme, published in 2013, calculated a global prevalence of 47.7% for HPV infection in OPSCCs.59 Data from the South American countries were not included.
Figure 3.
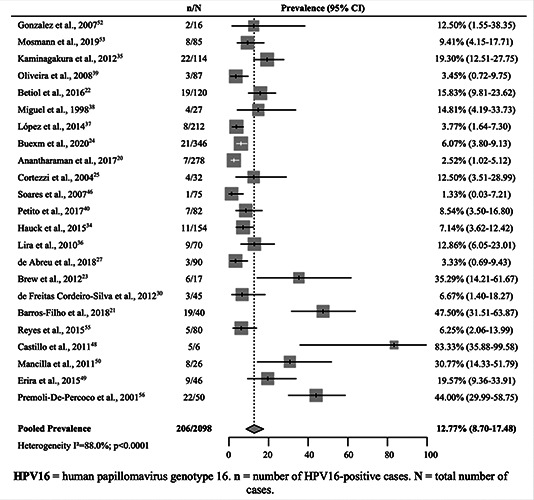
Pooled prevalence of human papillomavirus genotype 16 in squamous cell carcinomas of the oral.
A recently published meta-analysis revealed that the IHC tests for p16INK4a and PCR for detecting HPV DNA are highly sensitive (94% and 98%, respectively), but with moderate specificity (83% and 84%, respectively), and that the combination of these two revealed high sensitivity (98%) and high specificity (96%) for HPV diagnosis in OPSCC.15 In the present study, the cases tested by IHC or HPV DNA had the highest prevalence of HPV (59.07%) in the random-effects model, followed by cases tested by only IHC (35.29%); however, it is relevant to note that these two situations only presented one study (A1629 and A2228). The lowest prevalence was observed in studies in which HPV was diagnosed using a combination of DNA and IHC techniques (4.15%), but only two studies presented this diagnostic methodology (A0120 and A0524).
In our meta-analysis, the pooled prevalence of HPV among smokers was 20.75% and 22.23% for alcoholics. The consumption of beverages and smoking increases the risk of developing cancers of the oropharynx and oral cavity.6 In addition, evidence suggests an association between smoking and HPV carcinogenesis and a worse prognosis in smokers’ overall survival with HNC HPV positive cases.61,62
The main limitation of our study is the heterogeneity of the sample. Nevertheless, even if more factors were stratified, heterogeneity would undoubtedly be maintained. Despite a broad research tactic, the initial number of 209 records without duplication, of which only 52 complete studies were selected for reading, is reasonably low if we consider that no filter was included to limit the period of publication. Another factor to be highlighted as limiting a more effective analysis was the deficiency of information presented in a considerable part of the analyzed studies, of 38 studies, 15 did not mention the period corresponding to the collection of the samples, making any analysis impossible about the evolution of prevalence over time. Data that could support heterogeneity analysis could not be extracted from many of the studies analyzed because the data presented did not provide sufficient detail to stratify the information. Cofactors such as sex, age, smoking, alcoholism, and risky sexual behavior have not been reported in many reviewed articles. Even when approached, they did not present sufficient details for the quantitative analysis. Indeed, the simple provision of raw data with metadata would be sufficient to improve the information collected. However, only three of the studies analyzed provided supplementary material for consultation.
In conclusion, we found an overall prevalence of HPV in 24.31% of oral and oropharyngeal squamous cell carcinomas in South American patients. The prevalence of HPV was 17.9% for oropharyngeal cancer and 23.19% for oral cavity cancer in South American patients.
Figure 4.
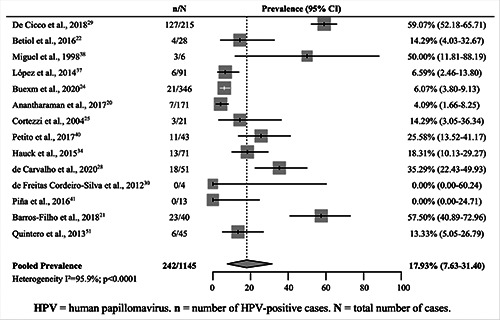
Pooled prevalence of human papillomavirus in squamous cell carcinomas of the oropharynx in South American countries.
Figure 5.
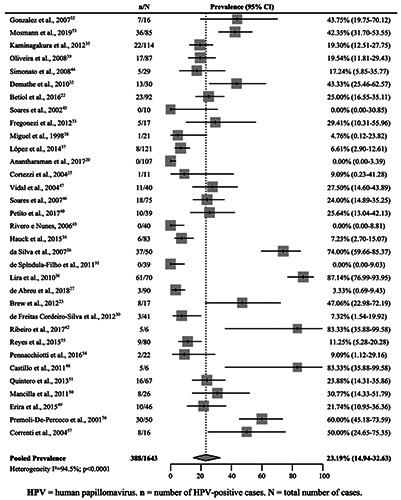
Pooled prevalence of human papillomavirus in squamous cell carcinomas of the oral cavity in South American countries.
Acknowledgements
The authors would like to thank the Fundação Universidade Federal de Rondônia (UNIR) and all those who directly or indirectly collaborated to carry out this work.
References
- 1.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Int Agency Res Cancer 2020. Available at: https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=0&include_nmsc_other=0&projection=natural-earth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&show_ranking=0&rotate=%255B10%252C0%255D. [Google Scholar]
- 2.INCA. [Estimativa 2020: incidência de câncer no Brasil.][Article in Portuguese.] Available at: https://www.inca.gov.br/publicacoes/livros/estimativa-2020-incidencia-de-cancer-no-brasil [Google Scholar]
- 3.Motta RDR, Zettler CG, Cambruzzi E, et al. Ki-67 and p53 correlation prognostic value in squamous cell carcinomas of the oral cavity and tongue. Braz J Otorhinolaryngol 2009;75:544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira de Lima MA, Lima da Silva CG, Barem Rabenhorst SH. Association between human papillomavirus (HPV) and the oral squamous cell carcinoma: a systematic review. J Bras Patol e Med Lab 2014;50:75–84. [Google Scholar]
- 5.Chiesa F, Ostuni A, Grigolato R, et al. Head and Neck Cancer Prevention. In: Head and Neck Cancer, Springer, New York, USA, 2016, p. 41-55. [Google Scholar]
- 6.American Cancer Society. Cancer Facts & Figures 2019. Available at: https://www.cancer.org/content/dam/cancerorg/research/cancer-facts-and-statistics/annual-cancer-factsand-figures/2019/cancer-facts-and-figures-2019.pdf [Google Scholar]
- 7.Garland EM, Parker LC. Human Papillomavirus–Associated Oropharyngeal Squamous Cell Carcinoma: A Review. Physician Assist Clin 2016;1:465–77. [Google Scholar]
- 8.Young D, Xiao CC, Murphy B, et al. Increase in head and neck cancer in younger patients due to human papillomavirus (HPV). Oral Oncol 2015;51:727–30. [DOI] [PubMed] [Google Scholar]
- 9.Stein AP, Saha S, Kraninger JL, et al. Prevalence of Human Papillomavirus in Oropharyngeal Cancer. Cancer J 2015;21:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonawane K, Suk R, Chiao EY, et al. Oral Human Papillomavirus Infection: Differences in Prevalence Between Sexes and Concordance With Genital Human Papillomavirus Infection, NHANES 2011 to 2014. Ann Intern Med 2017;167:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol 2014;15:1319–31. [DOI] [PubMed] [Google Scholar]
- 12.Colpani V, Soares Falcetta F, Bacelo Bidinotto A, et al. Prevalence of human papillomavirus (HPV) in Brazil: A systematic review and meta-analysis. PLoS One 2020;15:e0229154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westra WH. Detection of human papillomavirus (HPV) in clinical samples: Evolving methods and strategies for the accurate determination of HPV status of head and neck carcinomas. Oral Oncol 2014;50:771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis JS, Beadle B, Bishop JA, et al. Human papillomavirus testing in head and neck carcinomas guideline from the college of American pathologists. Arch Pathol Lab Med 2018;142:559– 97. [DOI] [PubMed] [Google Scholar]
- 15.Prigge E-S, Arbyn M, von Knebel Doeberitz M, et al. Diagnostic accuracy of p16 INK4a immunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and metaanalysis. Int J Cancer 2017;140:1186–98. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. 2013. International Classification of Diseases for Oncology (ICD-O). 3rd ed. Available at: https://apps.who.int/iris/handle/10665/96612 [Google Scholar]
- 17.StataCorp. 2019. Stata Statistical Software: Release 16. College Station, USA: StataCorp. LLC. [Google Scholar]
- 18.R Core Team. 2019. R: A Language and Environment for Statistical Computing. Vienna, Austria. [Google Scholar]
- 19.RStudio Team. 2018. RStudio: Integrated Development Environment for R. RStudio PBC, Boston, USA. [Google Scholar]
- 20.Anantharaman D, Abedi-Ardekani B, Beachler DC, et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int J Cancer 2017;140:1968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barros-Filho MC, Reis-Rosa LA, Hatakeyama M, et al. Oncogenic drivers in 11q13 associated with prognosis and response to therapy in advanced oropharyngeal carcinomas. Oral Oncol 2018;83:81–90. [DOI] [PubMed] [Google Scholar]
- 22.Betiol JC, Sichero L, Costa HO de O, et al. Prevalence of human papillomavirus types and variants and p16INK4a expression in head and neck squamous cells carcinomas in São Paulo, Brazil. Infect Agent Cancer 2016;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brew MC, Trapp R, Hilgert JB, et al. Human papillomavirus and oral squamous cell carcinoma in a south Brazilian population. Exp Mol Pathol 2012;93:61–5. [DOI] [PubMed] [Google Scholar]
- 24.Buexm LA, Soares-Lima SC, Brennan P, et al. HPV impact on oropharyngeal cancer patients treated at the largest cancer center from Brazil. Cancer Lett 2020;477:70–5. [DOI] [PubMed] [Google Scholar]
- 25.Cortezzi SS, Provazzi PJ, Sobrinho JS, et al. Analysis of human papillomavirus prevalence and TP53 polymorphism in head and neck squamous cell carcinomas. Cancer Genet Cytogenet 2004;150:44–9. [DOI] [PubMed] [Google Scholar]
- 26.Da Silva CEX dos SR, Da Silva IDCG, CERRI A, et al. Prevalence of human papillomavirus in squamous cell carcinoma of the tongue. Oral Surgery, Oral Med Oral Pathol Oral Radiol Endodontology 2007;104:497–500. [DOI] [PubMed] [Google Scholar]
- 27.de Abreu PM, Có ACG, Azevedo PL, et al. Frequency of HPV in oral cavity squamous cell carcinoma. BMC Cancer 2018;18:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Carvalho AC, Perdomo S, dos Santos W, et al. Impact of genetic variants in clinical outcome of a cohort of patients with oropharyngeal squamous cell carcinoma. Sci Rep 2020;10:9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Cicco R, Melo Menezes R, Nicolau UR, et al. Impact of human papillomavirus status on survival and recurrence in a geographic region with a low prevalence of HPV-related cancer: A retrospective cohort study. Head Neck 2020;42:93–102. [DOI] [PubMed] [Google Scholar]
- 30.de Freitas Cordeiro-Silva M, Stur E, Agostini LP, et al. Promoter hypermethylation in primary squamous cell carcinoma of the oral cavity and oropharynx: a study of a Brazilian cohort. Mol Biol Rep 2012;39:10111–9. [DOI] [PubMed] [Google Scholar]
- 31.de Spíndula-Filho JV, da Cruz AD, Oton-Leite AF, et al. Oral squamous cell carcinoma versus oral verrucous carcinoma: an approach to cellular proliferation and negative relation to human papillomavirus (HPV). Tumor Biol 2011;32:409–16. [DOI] [PubMed] [Google Scholar]
- 32.Demathe A Bernabé DG Garcia JF,et al. [Comparação entre dois métodos de detecção de DNA de papilomavírus humano em carcinoma epidermoide de lábio.][Article in Portuguese.] J Bras Patol e Med Lab 2010;46:85–90. [Google Scholar]
- 33.Fregonezi PAG, Silva TGA, Simões RT, et al. Expression of nonclassical molecule human leukocyte antigen–G in oral lesions. Am J Otolaryngol 2012;33:193–8. [DOI] [PubMed] [Google Scholar]
- 34.Hauck F, Oliveira-Silva M, Dreyer JH, et al. Prevalence of HPV infection in head and neck carcinomas shows geographical variability: a comparative study from Brazil and Germany. Virchows Arch 2015;466:685–93. [DOI] [PubMed] [Google Scholar]
- 35.Kaminagakura E, Villa LL, Andreoli MA, et al. High-risk human papillomavirus in oral squamous cell carcinoma of young patients. Int J Cancer 2012;130:1726–32. [DOI] [PubMed] [Google Scholar]
- 36.Lira RCP, Miranda FA, Guimarães MCM, et al. BUBR1 expression in benign oral lesions and squamous cell carcinomas: correlation with human papillomavirus. Oncol Rep 2010;23:1027–36. [DOI] [PubMed] [Google Scholar]
- 37.López RVM, Levi JE, Eluf-Neto J, et al. Human papillomavirus (HPV) 16 and the prognosis of head and neck cancer in a geographical region with a low prevalence of HPV infection. Cancer Causes Control 2014;25:461–71. [DOI] [PubMed] [Google Scholar]
- 38.Miguel REV, Villa LL, Cordeiro AC, et al. Low prevalence of human papillomavirus in a geographic region with a high incidence of head and neck cancer. Am J Surg 1998;176:428–9. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira LR, Ribeiro-Silva A, Ramalho LN, et al. HPV infection in Brazilian oral squamous cell carcinoma patients and its correlation with clinicopathological outcomes. Mol Med Rep 2008;1:123–9. [PubMed] [Google Scholar]
- 40.Petito G, Carneiro MA dos S, Santos SH de R, et al. Human papillomavirus in oral cavity and oropharynx carcinomas in the central region of Brazil. Braz J Otorhinolaryngol 2017;83:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piña AR, Jimenez LS, Mariano FV, et al. Human papillomavirus in tonsillar squamous cell carcinomas from Guatemala and Brazil. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;121:412–8. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro MGM, Marcolino LD, Ramos BR de A, et al. High prevalence of human papillomavirus (HPV) in oral mucosal lesions of patients at the Ambulatory of Oral Diagnosis of the Federal University of Sergipe, Northeastern Brazil. J Appl Oral Sci 2017;25:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivero ERC, Nunes FD. HPV in oral squamous cell carcinomas of a Brazilian population: amplification by PCR. Braz Oral Res 2006;20:21–4. [DOI] [PubMed] [Google Scholar]
- 44.Simonato LE, Garcia JF, Sundefeld MLMM, et al. Detection of HPV in mouth floor squamous cell carcinoma and its correlation with clinicopathologic variables, risk factors and survival. J Oral Pathol Med 2008;37:593–8. [DOI] [PubMed] [Google Scholar]
- 45.Soares CP, Malavazi I, Reis RI dos, et al. [Presença do papilomavirus humano em lesões malignas de mucosa oral.][Article in Portuguese.] Rev Soc Bras Med Trop 2002;35:439–44. [DOI] [PubMed] [Google Scholar]
- 46.Soares RC, Oliveira MC, Souza LB, et al. Human papillomavirus in oral squamous cells carcinoma in a population of 75 Brazilian patients. Am J Otolaryngol 2007;28:397–400. [DOI] [PubMed] [Google Scholar]
- 47.Vidal AK de L, Caldas Júnior A de F, Mello RJV de, et al. HPV detection in oral carcinomas. J Bras Patol e Med Lab 2004;40. [Google Scholar]
- 48.Castillo A, Koriyama C, Higashi M, et al. Human papillomavirus in upper digestive tract tumors from three countries. World J Gastroenterol 2011;17:5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erira A, Motta LA, Chala A, et al. [Genotipificación, niveles de expresión y estado físico del virus del papiloma humano en pacientes colombianos con cáncer de células escamosas en la cavidad oral.][Article in Spanish.] Biomédica 2015;36:14.27622789 [Google Scholar]
- 50.Mancilla LI, Carrascal E, Mario Tamayo O, et al. Role of Human Papillomavirus Type 16 in Squamous Cell Carcinoma of Upper Aerodigestive Tracts in Colombian Patients. Int J Cancer Res 2011;7:222–32. [Google Scholar]
- 51.Quintero K, Giraldo GA, Uribe ML, et al. Human papillomavirus types in cases of squamous cell carcinoma of head and neck in Colombia. Braz J Otorhinolaryngol 2013;79:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gónzalez J V., Gutiérrez RA, Keszler A, et al. [Virus papiloma humano en lesiones orales.][Article in Spanish.] Med (Buenos Aires) 2007;67:363–8. [Google Scholar]
- 53.Mosmann JP, Talavera AD, Criscuolo MI, et al. Sexually transmitted infections in oral cavity lesions: Human papillomavirus, Chlamydia trachomatis, and Herpes simplex virus. J Oral Microbiol 2019;11:1632129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pennacchiotti G, Sáez R, Martínez MJ, et al. [Prevalencia del virus papiloma humano en pacientes con diagnóstico de carcinoma escamoso de la cavidad oral.][Article in Spanish.] Rev Chil Cirugía 2016;68:137–42. [Google Scholar]
- 55.Reyes M, Rojas-Alcayaga G, Pennacchiotti G, et al. Human papillomavirus infection in oral squamous cell carcinomas from Chilean patients. Exp Mol Pathol 2015;99:95–9. [DOI] [PubMed] [Google Scholar]
- 56.Premoli-De-Percoco G, Ramirez JL. High risk human papillomavirus in oral squamous carcinoma: evidence of risk factors in a Venezuelan rural population. Preliminary report. J Oral Pathol Med 2001;30:355–61. [DOI] [PubMed] [Google Scholar]
- 57.Correnti M, Rivera H, Cavazza M. Detection of human papillomaviruses of high oncogenic potential in oral squamous cell carcinoma in a Venezuelan population. Oral Dis 2004;10:163–6. [DOI] [PubMed] [Google Scholar]
- 58.Abogunrin S, Di Tanna GL, Keeping S, et al. Prevalence of human papillomavirus in head and neck cancers in European populations: a meta-analysis. BMC Cancer 2014;14:968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and non-oropharyngeal head and neck cancer-systematic review and meta-analysis of trends by time and region. Head Neck 2013;35:747–55. [DOI] [PubMed] [Google Scholar]
- 60.Mariz BALA, Kowalski LP, William WN, et al. Global prevalence of human papillomavirus-driven oropharyngeal squamous cell carcinoma following the ASCO guidelines: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2020;156:103116. [DOI] [PubMed] [Google Scholar]
- 61.Chen SY, Massa S, Mazul AL, et al. The association of smoking and outcomes in HPV-positive oropharyngeal cancer: A systematic review. Am J Otolaryngol 2020;41:102592. [DOI] [PubMed] [Google Scholar]
- 62.Sinha P, Karadaghy OA, Doering MM, et al. Survival for HPVpositive oropharyngeal squamous cell carcinoma with surgical versus non-surgical treatment approach: A systematic review and meta-analysis. Oral Oncol 2018;86:121–31. [DOI] [PubMed] [Google Scholar]


