Abstract
The retina and the optic nerve are considered extensions of the central nervous system (CNS) and thus can serve as the window for evaluation of CNS disorders. Spectral domain optical coherence tomography (OCT) allows for detailed evaluation of the retina and the optic nerve. OCT can non-invasively document changes in single retina layer thickness and structure due to neuronal and retinal glial cells (RGC) modifications in systemic and local inflammatory and neurodegenerative diseases. These can include evaluation of retinal nerve fibre layer and ganglion cell complex, hyper-reflective retinal spots (HRS, sign of activated microglial cells in the retina), subfoveal neuroretinal detachment, disorganization of the inner retinal layers (DRIL), thickness and integrity of the outer retinal layers and choroidal thickness. This review paper will report the most recent data on the use of OCT as a non invasive imaging biomarker for evaluation of the most common systemic neuroinflammatory and neurodegenerative/neurocognitive disorders in the adults and in paediatric population. In the adult population the main focus will be on diabetes mellitus, multiple sclerosis, optic neuromyelitis, neuromyelitis optica spectrum disorders, longitudinal extensive transverse myelitis, Alzheimer and Parkinson diseases, Amyotrophic lateral sclerosis, Huntington’s disease and schizophrenia. In the paediatric population, demyelinating diseases, lysosomal storage diseases, Nieman Pick type C disease, hypoxic ischaemic encephalopathy, human immunodeficiency virus, leukodystrophies spinocerebellar ataxia will be addressed.
Subject terms: Retina, Prognostic markers, Retinal diseases, Predictive markers
Abstract
视网膜和视神经是中枢神经系统 (CNS) 的延续, 因此可以作为评估CNS疾病的窗口。频域光学相干断层扫描 (SD-OCT) 可以对视网膜和视神经进行详细的评估。OCT可以无创性地记录系统性和局部炎症/神经退行性病变中, 由于神经元和视网膜胶质细胞 (RGC) 改变引起的视网膜单层厚度和结构的变化。OCT的观察的指征包括评估视网膜神经纤维层和神经节细胞复合体、视网膜高反射点 (HRS, 视网膜中小胶质细胞激活的征象) 、中心凹下神经视网膜脱离、视网膜内层结构紊乱 (DRIL) 、视网膜外层的厚度和完整性以及脉络膜厚度。本文将总结OCT作为无创成像生物标志物评估成人和儿童中最常见的系统性神经炎症和神经退行性病变/神经认知障碍的最新数据。在成人中, 我们最关注的疾病为糖尿病、多发性硬化症、视神经脊髓炎、视神经脊髓炎谱系障碍、纵向广泛横贯性脊髓炎、阿尔茨海默病和帕金森病、肌萎缩侧索硬化症、亨廷顿病和精神分裂症。在儿童中, 我们着重讨论的疾病有脱髓鞘疾病、溶酶体贮积病、尼曼-匹克病、缺氧缺血性脑病、人类免疫缺陷病毒、脑白质营养不良脊髓小脑性共济失调。
Introduction
Neurodegeneration involves progressive loss of neuronal structure associated with dysfunction in the central nervous system (CNS) [1]. The pathophysiologic mechanisms entailed in neurodegeneration are still being understood, but inflammation is believed to be involved [1]. Neuroinflammation is a reactive response of the CNS against conditions that affect homoeostasis and include infectious, traumatic, metabolic, ischaemic, immune-mediated, neoplastic and toxic stimuli, among others. Neuroinflammation/neurodegeneration can emerge in the course of intrinsic CNS diseases or systemic disorders [1, 2].
Depending on the nature, timing and duration of these stimuli as well as the response mechanisms of the CNS against them, inflammation can be beneficial or damaging [1, 2]. A prolonged neuroinflammatory response can be harmful and lead to neuronal damage, in part, due to reactive microglia and astrogliosis involving oligodendrocytes, neurons and endothelial cells [1, 2]. Neuronal death from progressive tissue damage in neuroinflammatory/neurodegenerative disorders can result from increased oxidative stress and impaired protein expression or axonal transport [1, 2] and involve glutamate excitotoxicity, CD8 T-cell-mediated cytotoxicity, antibodies against neuronal and axonal proteins, and apoptosis mediated by mitochondrial dysfunction [2]. Therefore, in the course of neurodegeneration, neuroinflammation can occur. In addition, neuronal damage can lead to the release of factors that trigger neuroinflammation [1–5], leading to a vicious cycle.
In adults and children, imaging, molecular and immuno-genetic tools have increased clinical knowledge and understanding of the pathophysiology of diseases and provide means to evaluate treatment responses. These tools also provide features that act as “biomarkers”. A biomarker is a feature that is an indicator of biological and pathogenic processes or responses to an exposure or intervention [6]. Imaging biomarkers provide information without being as invasive or expensive as tissue or serum biomarkers and are particularly useful in clinical practice both in adults and even more in paediatric practice. Determining non-invasive imaging biomarkers in neuroinflammatory/neurodegenerative disorders is, therefore, an important objective. The retina and the optic nerve have been considered extensions of the CNS and can provide a window to evaluate CNS diseases. Being amenable to imaging techniques, features on imaging can also yield biomarkers of diseases [7].
Optical coherence tomography (OCT) has emerged as a fast, non-invasive and affordable imaging modality to assess the retina and optic nerve and, therefore, lends itself to evaluate ocular changes in neuroinflammatory/neurodegenerative disorders [7–14]. Research on systemic diseases, such as diabetes mellitus, multiple sclerosis and CNS diseases, especially Alzheimer and Parkinson diseases, has identified inflammatory and neurodegenerative changes in the retina that can be evaluated through specific biomarkers, that recently has gained more importance.
This review paper will address the OCT biomarkers of neuroinflammation and neurodegeneration in systemic and CNS disease in both the adults and paediatric populations.
A review of the current evidence about OCT in adults and paediatric neuroinflammatory/neurodegenerative disorders was performed. A bibliographic search on the main database of National Centre for Biotechnology Information Database: (PubMed MEDLINE) was undertaken from May to September 2021, using “optical coherence tomography”, “neurodegenerative/neurodegeneration”, “neuroinflammatory/neuroinflammation”, “diabetes mellitus”, “sclerosis multiple”, “neurocognitive disorders”, “Alzheimer disease”, “Parkinson’s Disease”, “dementia”, “amyotrophic lateral sclerosis”, “Huntington’s disease”, “schizophrenia”,“pediatric”, “childhood”, “children” as key words. In addition, the name of each neuroinflammatory/neurodegenerative disease from Table 1, e and 3 was used as key word.
Table 1.
OCT biomarkers in adult neuroinflammatory/neurodegenerative disorders.
| Diseases | OCT biomarkers | Studies |
|---|---|---|
| DMO |
Increase: HRS, DRIL, INL thickness Decrease: NFL thickness, GCL thickness, capillary perfusion in DCP |
Vujosevic et al. [20], Vujosevic et al. [23], Van Dijk et al. [37], Van Dijk et al. [38], Barber et al. [39], Lynch et al. [40], Zafar et al. [43], Sun et al. [46], Van de Kreeke et al. [54], Scarinci et al. [57] |
| DR |
Increase: HRS, INL thickness Decrease: NFL thickness, GCL thickness |
Vujosevic et al. [20], Vujosevic et al. [23], Van Dijk et al. [37], Van Dijk et al. [38], Barber et al. [39], Lynch et al. [40], Zafar et al. [43], Sun et al. [46], Van de Kreeke et al. [54] |
| MS |
Increase: HRS, INL thickness, MMO Decrease: GCL-GCIPL thickness especially in the papillomacular bundle and the inferonasal area (early stages), temporal pNFL thickness (late stages), macular thickness and volume in nasal region, mNFL thickness |
Pilotto et al. [28]Knier et al. [41], Birkeldh et al. [61], Petzold et al. [62], Walter et al. [65], Ashtari et al. [66], Gelfand et al. [67], Saidha et al. [68] |
| MNO |
Increase: MMO Decrease: INL thickness, NFL thickness, GCL-GCIPL thickness, vascular density of the SCP and DCP |
Ashtari et al. [66], Syc et al. [75], Fernandes et al. [76], Gelfand et al. [79], Ratchford et al. [85] |
| AD | Decrease: INL thickness, NFL thickness, GCL-GCIPL thickness, macular volume, choroidal thickness | Chan et al. [96], Hinton et al. [100], Cheung et al. [101], Den Haan et al. [103], Trebbastoni et al. [105], Bulut et al. [106] |
| PD | Decrease: NFL thickness especially in the inferior disc quadrant with relative sparing of the nasal sector, GCL-GCIPL thickness, choroidal thickness, vascular density of the SCP | Harnois et al. [108], Chrysou et al. [111], Huang et al. [112], Kwapong et al. [116] |
| ALS | Decrease: NFL thickness | Cerveró et al. [118] |
| HD | Decrease: temporal NFL thickness, macular volume | Kersten et al. [119] |
| Schizophrenia | Decrease: peripapillary NFL thinning, macular thinning, macular volume | Chhablani et al. [109] |
DMO diabetic macular oedema, DR diabetic retinopathy, MS multiple sclerosis, MNO neuromyelitis optica, AD Alzheimer’s disease, PD Parkinson’s disease, ALS Amyotrophic lateral sclerosis, HD Huntington’s disease, HRS hyperreflective spots, DRIL Disorganization of retinal inner layers, INL inner nuclear layer, NFL nerve fibre layer, pNFL peripapillary NFL, mNFL macular NFL, SCP superficial capillary plexus, DCP deep capillary plexus, GCL ganglion cell layer, MMO microcystic macular oedema, GCL-GCIPL ganglion cell layer-ganglion cell inner plexiform layer.
OCT biomarkers of neuroinflammation and neurodegeneration
Spectral domain (SD)-OCT can be useful in evaluation of retinal glial cells (RGC) which undergo modifications during an inflammatory local and systemic process. The RGC include the microglial cells (the major resident immunocompetent cells in the CNS that share the phenotypic markers of monocytes and macrophages) [15–17] and the Müller cells (the major communicator between the neurons and the vessels) and astrocytes. For example, in diabetes mellitus (DM), which is considered a chronic, low grade inflammatory disease, the Müller cells and the microglial cells undergo mostly activation, reactive gliosis and release of inflammatory mediators [18–20]. Neuronal cells and astrocytes undergo apoptosis that leads to ganglion cell death with thinning of the inner retina [21–23].
Hyper-reflective retinal spots/dots/foci
The hyper-reflective retinal spots (HRS)/dots/foci in the retina visible on SD-OCT have been recently proposed as the clinical sign of aggregates of activated microglial cells, thus a sign of an inflammatory condition in the retina [24, 25]. An increase in the number of HRS has been reported in different retinal diseases characterized by the inflammation, including retinal vein occlusion (RVO), age related macular degeneration (AMD) etc [24, 26–29].
Also, in systemic conditions such as DM (Fig. 1) as well as in CNS disease such as multiple sclerosis (MS) an increase in the number of HRS has been documented [24, 29–31]. Histologic evidence from human donor eyes with AMD and acquired vitelliform lesions demonstrated that HRS visualized on SD-OCT may originate from both anteriorly migrated retinal pigment epithelium cells and lipid-filled cells (thought to correspond to activated microglia) [32–34]. In patients with diabetic macular oedema, a positive correlation between the number of HRS and aqueous humour concentration of soluble CD14 (a cytokine associated with immune response, expressed in microglia, monocytes and macrophages) was reported [35].
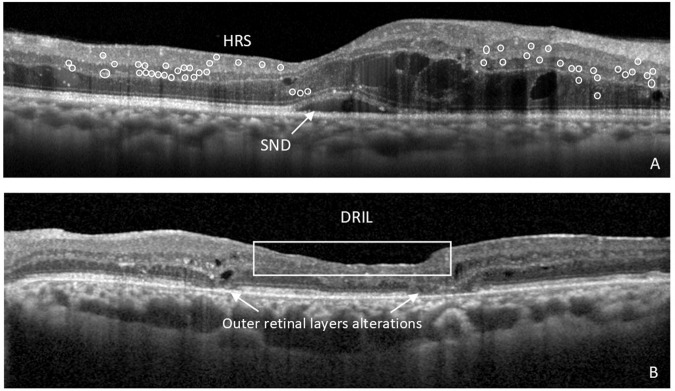
Fig. 1. OCT biomarkers of neuroinflammation and neurodegeneration in diabetic retinopathy (DR).
A Right eye of the patient with cystoid diabetic macular oedema (DMO) showing signs of neuroinflammation in the retina, such as subfoveal neuroretinal detachment (SND) and hyperreflective retinal spots (HRS), marked in circles. B Left eye of the patient with proliferative diabetic retinopathy (PDR) treated with numerous anti-VEGF intravitreal injections and vitrectomy, showing the presence of disorganization of the inner retinal layers (DRIL) and alterations in the integrity of outer retinal layers, in particular the external limiting membrane (ELM) and ellipsoid zone of the photoreceptors.
HRS related to microglia activation seem to have specific characteristics, such as small dimension (<30 μm), reflectivity similar to nerve fibre layer, absence of back-shadowing, and location in both inner and outer retina; additionally, they do not correspond to any specific lesion on fundus examination [24, 25] (Fig. 1). It is essential to differentiate the HRS related to microglia versus other lesions in the retina that can be visualized as HRS such as (precursors) of hard exudates; degenerated photoreceptors/macrophages correlated to outer retina disruption; anteriorly migrated RPE cells; retinal vessels [24–27, 29, 36].
Subfoveal neuroretinal detachment
Subfoveal neuroretinal detachment (SND) is detected on OCT as a hyporreflective area beneath the neuroretina, and has been described in different inflammatory local disorders of the retina (RVO, uveitis, AMD, etc…) and systemic diseases such as DM, and in particular in DMO (Fig. 1). In fact, in DMO, the presence of SND has been associated with higher levels of local inflammatory molecules, particularly IL-6 [37], as well a higher number of HRS in the retina. Long lasting SND may lead to photoreceptor damage and reduced visual function [36, 38].
Changes in thickness of the retina layers
The neuronal degeneration in the retina due to the loss of ganglion cells and amacrine cells has been reported on OCT as the thinning of the retinal nerve fibre layer (NFL) and ganglion cell layer (GCL). The reduced thickness of the inner retina layers has been reported both in the macula as well as around the optic disc [22, 39–42].
On the contrary, an increase in the inner nuclear layer (INL) has been reported as the sign of activation of the Müller cells, in patients with initial stages of DR and in MS [22, 43]. Also, modifications in the thickness of the outer retinal layers has been reported in different neuroinflammatory/neurodegenerative diseases [44–47].
Disorganization of the inner retinal layers (DRIL)
DRIL was proposed to present disorganization or destruction of cells within inner retinal layers, probably indicating a disruption of visual pathways from the photoreceptors to the ganglion cells [48]. DRIL is visible on OCT as the absence of detectable boundaries between the GC-inner plexiform layer IPL complex, INL and outer nuclear layer (ONL) and has been [48, 49]. Moreover, DRIL was proposed as a surrogate marker correlated with visual acuity in patients with existing or resolved centre-involving DMO, and a change in DRIL extension as an important prognostic and predictive biomarker of visual acuity response to treatment in DMO [48] (Fig. 1).
Diabetes Mellitus
In DM, chronic hyperglycaemia leads to an early damage of the neurovascular unit with consequent neurodegeneration and reactive gliosis, neuro-inflammation, microvascular damage and RPE and choroid pathology [50, 51]. The neurodegeneration in the retina in DM is caused by extracellular glutamate accumulation oxidative stress and reduction of neuroprotective factors synthesized by the retina [52].
Whether retinal neurodegeneration precedes or is independent of microvascular damage in the retina in DM is still not fully understood [53]; however, functional studies confirm an early neuronal dysfunction before the onset of clinically visible microvascular changes [54]. pERG amplitude, mfERG implicit time and oscillatory potentials are abnormal at early stages of DR or at a pre-clinical stage of DR [55]. A significant decrease in the thickness of NFL and GCL in both DM type 1 and type 2 even without clinical signs of DR or at initial stages of DR has been reported by several authors [22, 39–42, 45, 56]. These changes were reported in the macular and in the peripapillary area. Using OCT-angiography (OCT-A), the perifoveal capillary loss in superficial capillary plexus (SCP) had the highest correlation with inner retinal layer thickness in patients with DM type 1 and 2 without clinical signs of DR [57]. Moreover, a significant increase in the thickness of the inner nuclear layer, mostly composed by the nuclei of bipolar and Müller cells with consequent activation of inflammatory biomarkers had been previously documented [22].
HRS are increased in number in patients with DM (in both preclinical and early clinical DR) versus normal subjects and their number increases with the increasing severity of DR [25] (Fig. 1). In DMO with subfoveal neuroretinal detachment (SND), the increased number of HRS is superior versus DMO without SND confirming a more inflammatory pattern of oedema. The number of HRS decreases after anti-VEGF treatment [29] and even more after intravitreal treatment with dexamethasone implants [20]. In DMO with DRIL, intravitreal steroid treatment can decrease DRIL extension, potentially by promoting neuronal survival through suppressing microglial reactivity [31, 58]. Outer retina integrity including the external limiting membrane and ellipsoid zone integrity were reported as important biomarkers of visual function [45, 46]. Capillary nonperfusion on OCT-A in the deep capillary plexus (DCP) is associated with photoreceptor structural abnormalities and visual function loss in DR [59]. Choroidal thickness modification has remained a controversial issue as a sign of inflammatory changes in DR [60–62] (Table 1).
Multiple Sclerosis (MS)
In recent years, OCT has become a relevant device to support the diagnosis of MS and its monitoring over time. OCT has allowed to identify the involvement and changes in thickness of the retinal layers in the various stages of MS. Numerous studies have reported the onset of atrophy of GC and their axons, which correspond to ganglion cell layer - ganglion cell inner plexiform layer (GCL-GCIPL) and peri-papillary nerve fibre layer (pNFL) and macular NFL (mNFL) thinning, respectively. Thinning of pNFL has been shown to be the most reliable marker of axonal fibres degeneration in patients with MS whether associated with optic neuritis or not. Even patients with benign MS without symptoms have been reported to have NFL thinning [63]. The most involved area, at least in the late stages, is the temporal region [64], which is considered the main sign to differentiate affected patients from healthy people at the same age. In addition, they observed a reduction in the thickness and macular volume in its nasal lobe, an alteration that is in line with the decrease in NFL thickness. Such atrophy is of greater severity in patients with optic neuritis (ON) [65]. The possible occurrence of optic disc oedema limits the accuracy of using NFL as an index of inflammatory activity [65]. Recent studies also reported on poor reliability of pNFL analysis in the early stages of MS [66]. Another parameter that can be determined with the new OCT software is the thickness of the Bruch’s membrane opening minimum rim width (BMO-MRW), which is the minimum distance between BMO and ILM. This parameter allows to quantify the neuroretinal rim by evaluating Bruch’s membrane extension which is clinically invisible, and only visible with SD-OCT. It also takes into account the direction of the axon fibres and their effective thickness. Because BMO-MRW measurement is made perpendicular to the axis of the neural tissue, it takes into account the variable trajectory of axons over the point of measurement, akin to current strategies for measuring peripapillary retinal nerve fibre layer thickness [67]. The thickness of BMO-MRW has been combined with pNFL atrophy [68].
The GCL in the macula or the GC-IPL has been shown to be impaired by the process of neurodegeneration in earlier stages than the NFL [66], and the most involved areas seem to be the papillomacular bundle and the inferonasal area: the atrophy of these layers could be a pathological biomarker of the early stages of the disease [65]. The GC-IPL includes retinal ganglion cell (RGC) bodies and retinal astrocytes, which are often measured together because of the low contrast between these two components in OCT scans. A close correlation of GC-IPL thickness with both macular volume and NFL thickness has been demonstrated, all three reduced compared with the healthy control group. Macular metrics changes between MS patients with or without prior optic neuritis are controversial in different studies [69, 70]. Another layer under investigation is the inner nuclear layer (INL): some studies have shown that it is not affected by atrophy in MS, but rather by thickening, which is a possible sign of inflammation activity and response to treatment [65]. It may be involved by the presence of microcystic macular oedema (MMO), characterized by cysts confined to this layer, an occurrence much more frequent in patients who developed optic neuritis. In addition, the onset of MMO seems to be related to the severity of the disease without treatment with fingolimod [71, 72]. In patients treated with fingolimod, macular oedema has also been reported but this observation may represent an associated, and not causal, finding since macular cysts occur in adults with MS [63]. It has been shown that MMO is not typical of demyelinating diseases as MS and NMO, but it has also been described in glaucoma, autosomal dominant optic atrophy, Leber’s optic neuropathy, Tanzanian endemic optic neuropathy and compressive neuropathy. Its onset and manifestation are similar regardless of the cause of the optic atrophy: this evidence supposes the same etiopathogenetic origin [73]. The most accredited hypotheses relating to its aetiology are neuroinflammation, retrograde degeneration and vitreous traction. Concerning neuroinflammation, Gelfand et al. suggested that pseudocysts are a consequence of focal blood-retinal barrier disruption that generates release of proinflammatory agents and microglial activation with fluid accumulation [71]. Instead, Abegg et al. postulated that retrograde degeneration leads to the development of MMO in patients affected by optic neuropathy. Indeed, thinning of GCL and INL in areas affected by pseudocysts and the prolonged time delay between neuronal damage and the onset of MMO in their patients is more likely to be due to degeneration than inflammation. Based on these results, the authors postulated that Muller cells degenerate and play a key role in the formation of MMO due to lack of retinal fluid absorption [74]. Regarding the vitreous traction, MMO would develop as a schisis: this hypothesis is based on the location of pseudocysts only in macular areas affected by vitreous traction in patients with optic atrophy of non-inflammatory aetiology [75]. This one was a suggestive proposition, however other studies have not confirmed this correlation [76, 77]. Nevertheless, these mechanisms are not yet totally understood and they are still under investigation; however, the knowledge of this sign linked to optic neuropathy is crucial to make differential diagnosis between MMO and macular oedema associated with retinal vein occlusion, diabetes, uveitis and age-related macular degeneration.
In contrast to INL, the outer nuclear and outer plexiform layer (ON-OPL) currently does not appear to be significantly altered in this pathology, although according to some researchers it may be crucial in the future in the differential diagnosis with other neurodegenerative diseases [44].
A recent study documented a higher number of HRS in the inner retinal layers on OCT images in patients with relapse-onset MS at the time of diagnosis compared to healthy controls [49]. These clusters of microglia cells could be an important biomarker of neuroinflammation.
As for the choroid, no alterations have been found in the early stages of MS, but some researchers reported a peripapillary thinning in the late stages: the hypothesis is a reduction of vascular flow resulting from fibres degeneration and then optic nerve atrophy [78].
In summary, pNFL thickness has the potential to be a reliable marker of long-term axonal degeneration, whereas macular GCL-GCIPL thickness is considered an early index of neuronal integrity [65]. Given these evidences, OCT has the potential to become a mainstay in monitoring patients with MS as NFL and macular GC-IPL are affected by the disease and their changes are associated with clinical and paraclinical parameters: physical disability, cognitive impairments as well as brain atrophy of different areas visible in MRI [79]. All these results suggest that retinal changes assessed by OCT are a marker of global axonal loss and neurodegeneration [80] (Table 1).
The OCT findings in MS in paediatric population will be addressed separately in the section on paediatric disorders.
Optic neuromyelitis and neuromyelitis optica spectrum disorders
OCT has also proved useful in studying retinal changes in Devic’s disease, also known as optic neuromyelitis (NMO), and in the neuromyelitis optica spectrum disorders (NMOSD) in general. NMOSD consist in a group of inflammatory neurodegenerative diseases of autoimmune aetiology distinct from MS [81, 82]. It has been shown that the average thickness of pNFL is significantly reduced in NMOSD compared to healthy controls, even in patients not affected by optic neuritis (ON) [83]. Moreover, this index is also decreased even when related to MS patients and this discrepancy is higher in eyes with history of ON [70, 84]. This would appear to be due to greater ON-dependent axonal damage and a higher frequency of these attacks in NMOSD; this could be a marker to distinguish MS from the latter [85], but further studies will be required to validate this theory. Another interesting finding is that, in case of ON, macular GCC (mNFL + GC-IPL) and pNFL are also thinned in the unaffected eye, although to a lesser extent than in the affected one [86]. On the other hand, as far as macular thickness is concerned, no significant differences in macular volume and GC-IPL were identified between the two conditions, while the thickness of INL and macular NFL seems to be reduced in MNOSD compared to MS [85]. Even in NMOSD cases of MMO have been observed, much more frequently than in MS, and the necessary prerequisite for it to occur would be ON [87], although the etiopathogenesis is not yet known. Microcystic oedema, thinner GCL and NFL thickness were reported more frequently in patients with NMOSD and optic neuritis than in patients with MS and optic neuritis [10].
In recent years, some researchers have also investigated the presence of retinal vascular alterations in NMOSD patients: they reported that there is a lower peripapillary and parafoveal vascular density compared to healthy subjects on OCT-A scans and in addition this is associated with NFL and GCPIL thinning on OCT images [88]. Such vascular alterations are linked to a decline in visual acuity [89] and could be a predictive marker of visual impairment [90]. As with OCT parameters, the vascular density of the SCP and DCP also appears to be lower in MNOSD than in MS, probably due to the greater inflammatory involvement of the optic nerve [91] (Table 1).
Longitudinal extensive transverse myelitis
Longitudinal extensive transverse myelitis (LETM) is described as an inflammation of the spinal cord involving at least three vertebral segments in patients without MS and who did not have episodes of ON. Few studies have been performed to identify OCT alterations in this disorder: in some of these it has been shown a reduction in NFL thickness in patients with LETM compared to healthy controls [92], but elsewhere not [93]. Finally, Fernandes et al. reported an increase in INL thickness in NMO and LETM patients in comparison with MS patients and healthy subjects in their cross-sectional study [84].
Neurocognitive and neurodegenerative disorders
Research in Alzheimer and Parkinson disease evaluated thinning of the peripapillary NFL or macular GCC, macular foveal thinning and reduced macular volume as potential OCT biomarkers in diagnosis and screening [7]. Some studies have attempted to establish the clinical significance of OCT features and studied associations between OCT retinal findings and visual function [5, 8, 63, 94] and cognitive, motor, and neurologic impairment [11, 12, 95–103].
Neurocognitive disorders are characterized by an acquired cognitive decline rather than it being present from birth. The most common cause of dementia is Alzheimer’s disease (AD), accounting for between 60 to 80% of cases [104].
AD is characterized by the accumulation of extracellular amyloid-beta plaques and intracellular neurofibrillary tangles of hyperphosphorylated tau within the brain [104]. These proteins initiate inflammation, drive synaptic and neuronal loss, processes which ultimately result in cerebral atrophy and global systemic decline. Aβ deposition is seen in the retinas of patients affected by AD, the plaques are smaller in size than those found intracerebrally, and typically distributed in the superior and inferior retinal quadrants [105, 106]. Deposition of amyloid in the retina has been shown to precede amyloid plaque formation in the brain in AD transgenic mice [105].
Within the continuum of AD, there are three broad phases: preclinical AD, mild cognitive impairment (MCI) and dementia. The preclinical phase of AD is an insidious process which may extend to a period of 20 years [107]. Current gold standard biomarkers for detection of preclinical AD include Aβ positron emission tomography (PET) imaging, MRI analysis and cerebrospinal fluid (CSF) assays [104].
The NFL comprises the axons of retinal GC which directly project to the lateral geniculate nucleus. In 1986 Hinton et al. demonstrated a reduction in the NFL and GC number within the optic nerves of AD [108]. These findings have been confirmed by many studies using OCT imaging. Chan’s meta-analysis demonstrated that SD-OCT measurements of the inner retina were consistently thinner in AD patients in comparison to controls [104]. The firmest associations were seen when measuring the GC-IPL thickness, GCC thickness, and peripapillary NFL thickness. Chan et al. suggested that assessing the thickness of the GC-IPL rather than that of the NFL may be a more sensitive indicator. They suggested that the retinal GC was affected in a similar manner to the cerebral neuron [104]. Cheung noted that individuals with AD not only show a reduction in the GC-IPL but also overall macular volume [109]. Alber concluded that the mNFL is not a useful biomarker in patients with either MCI or AD, as not only is it thin, but it is also influenced by pathology at the vitreoretinal interface [110].
Peripapillary NFL thinning occurs in patients with MCI and/or AD [111]. Cheung et Ong found that the IT sector was the most affected region in patients with MCI but in patients with AD the inferior sector was most involved. The prognostic sign of a thin NFL in the development of cognitive decline and AD has been investigated by two large population-based analyses [112]. The UK Biobank study concluded that a thinner NFL was not only associated with lower cognitive testing scores, but patients with thinner measurements were more likely to show reduced cognitive function three years later [104]. The Rotterdam Study advised that a thinner NFL was associated with an increased risk of developing dementia, highlighting its potential role as a biomarker of early dementia [104].
The choroid of patients with AD has been shown to thin faster than in matched controls [113]. Bulut et al. also showed a significantly reduced choroidal thickness in patients with either MCI or AD [114].
Rifai et al. performed a systematic review of the application of OCT angiography in AD. They concluded that although OCT-A metrics may have the potential to serve as biomarkers for AD, they recommended using the ETDRS grid along with standardizing terminology based on segmentation between the various OCT devices [115].
It is important to acknowledge that many patients may be misclassified as AD whereas they have a mixed dementia with a significant vascular component. This will negatively impact on the accuracy of elucidating AD biomarkers. Longitudinal studies are also needed, where the patient is followed over their disease course with serial imaging.
Kim et al. studied patients with Frontotemporal Dementia and found a thinning of the outer retina which correlated with patients’ cognitive function. The thinning preferentially affected the ONL and ellipsoid zone differing from the findings in AD [47] (Table 1).
Parkinson’s disease
Parkinson’s disease (PD) is a progressive neurodegenerative disorder of the CNS. It occurs secondary to depletion of dopaminergic neurons in the substantia nigra of the midbrain. A subpopulation of amacrine cells in the retina are dopamine-releasing neurons. A reduced concentration of retinal dopamine is seen in PD [116]. Loss of the amacrine cells leads to thinning of retinal GC [117]. Alpha-synuclein has been identified in the inner retina of patients with PD [118].
There has been a heterogeneity of OCT findings in PD, necessitating meta-analysis studies. Meta-analysis of time domain-OCT and spectral domain-OCT imaging demonstrated the mean pNFL to be thinner in eyes of patients with PD compared with control eyes in 2014. Chrysou and Huang’s meta-analyses looked at spectral-domain OCT studies. It was concluded that patients with PD have significantly thinner retinas compared with controls, with the inner retinal layers being predominantly affected [119]. Both the GC-IPL and the NFL are thinned in PD [120]. There was no significant difference in the thickness of the INL, OPL and ONL between eyes of PD and controls [120].
In PD there is a marked neuronal loss in the inferior disc quadrant with relative sparing of the nasal sector. Comparisons have been drawn between the relative sparing of the nasal sector of the pNFL in PD and the neuronal changes observed in patients with primary open angle glaucoma (POAG) [119]. There is an increased incidence of PD in patients with POAG, with a hazard ratio of 1.28 (95% CI; 1.05 to 1.46) in comparison to healthy controls [121]. There has also been a higher incidence of glaucoma (16.3% vs 6.6% in healthy controls) reported in patients with PD.
This findings of Chrysou’s meta-analysis differs from prior studies which suggested preferential involvement of the temporal quadrant of the optic disc [122].
The Hoehn and Yahr scale (H-Y) was the first rating scale used to describe the progression of PD. It consists of five stages. There is no change in central macular thickness demonstrable until stage H-Y III is reached [120]. When examining macular volume there was a statistical difference between H-Y II and H-Y III suggesting that this may be a more sensitive parameter to detect disease progression.
Enhanced depth imaging SD-OCT has demonstrated a reduction in choroidal thickness in PD [123]. Choroidal thinning was shown in the inner and outer quadrants as well as subfoveally (also feature of AD).
As well as neurodegeneration, vascular impairment has been identified as an associated factor in PD. Kwapong et al. demonstrated that there is a reduced retinal microvascular density exhibited in patients with PD [124]. The reduction of density in the superficial retinal capillary plexus (SRCP) was more significant than that seen in the deep plexus. In the superficial retinal capillary plexus Kwapong saw a reduction in all four quadrants both individually, as well as the total annular zone (TAZ). In PD patients there was a positive correlation between the average ganglion cell layer and inner plexiform (GCIP) thickness and the SCP microvascular density of the 2.5 mm total annular zone around the FAZ (159). There was no relationship found between either disease duration or disease severity with retinal microvascular densities or intraretinal layer thickness. Their results demonstrated that the retinal capillary densities were significantly decreased in the early stages of PD when compared with healthy matched controls, suggesting that the retinal capillary impairment occurs early in PD before the manifestation of clinically apparent motor disorders.
Some patients with PD who have E46K mutation in the α‐synuclein gene (E46K‐SNCA) will also have Lewy body disease (LBD). LBD also includes aggressive forms of idiopathic Parkinson’s disease and dementia with Lewy bodies. Parafoveal thinning of GC-IPL is a sensitive and clinically relevant imaging biomarker for Lewy body diseases, specifically for Parkinson’s disease [125].
Amyotrophic lateral sclerosis, Huntington’s disease and Schizophrenia
Amyotrophic lateral sclerosis (ALS) is the most common degenerative disease affecting motor neurons. It lacks both an effective treatment and a biomarker. OCT studies in patients with ALS, have found a significant thinning of the NFL [126].
In Huntington’s disease the temporal NFL is preferentially thinned and there is a reduction in macular volume [127].
Schizophrenia is a chronic and progressive neurocognitive disorder characterized by a reduction in brain volume. OCT imaging on patients with Schizophrenia show significant peripapillary NFL thinning, macular thinning and reduction of macular volume when compared to controls. These OCT features correlate with the duration of Schizophrenia [117] (Table 1).
Paediatric neuroinflammatory/neurodegenerative disorders
Neuroinflammatory/neurodegenerative disorders have been extensively studied during adulthood and aging [1, 2]. In contrast, the body of research in childhood is relatively sparse. Although inflammation also plays a major role in neurologic paediatric pathology [4], clinical manifestations of diseases in children can differ from adults [5]. This section focuses on OCT retinal features in paediatric neuroinflammatory/neurodegenerative disorders that serve as potential biomarkers of visual function or can be correlated with changes in the CNS. It should be noted that many of the reports reviewed have few cases because of the rarity of the conditions. More research is needed, but it is useful to review the studies to date.
Paediatric neuroinflammatory/neurodegenerative disorders encompass a heterogeneous group of diseases with variability in epidemiology, pathophysiology, clinical manifestations, diagnosis and treatment [4, 5, 103, 128–130]. The number of disorders has increased over the last decade and new entities are continually being discovered [4, 103, 128–130]. A comprehensive list of neuroinflammatory/neurodegenerative conditions in childhood, noted at this time, is in Table 2. As a result of focal and polyfocal neurologic deficits, clinical presentations vary from single to recurrent symptoms and signs, including seizures, cognitive, sensitive and motor/tone/coordination impairments, psychiatric disorders, and vision loss [4, 103, 128, 129]. In addition, optic neuritis, optic atrophy, pigmentary retinopathy, and retinal dystrophies can occur [4, 103, 129]. Serum, tissue and cerebrospinal fluid tests along with genetic and immunohistochemical studies are performed for the diagnostic workup [4, 103, 128, 129]. In addition, neuropsychological testing (e.g. electroencephalography, visual evoked potential, electroretinogram), neuroimaging of the brain and spine using magnetic resonance imaging (MRI) [103, 128, 129] and, more recently, OCT of the retina are performed. A summary of neurologic tests discussed in this section is in Table 3.
Table 2.
Paediatric neuroinflammatory/neurodegenerative disorders.
| I. ACQUIRED DISEASES: |
|---|
| I.I. Acquired demyelinating syndromes: |
| Acute Disseminated Encephalomyelitis (ADEM)* |
| Associated with optic neuritis |
| Without optic neuritis |
| paediatric multiple sclerosis (MS)* |
| Associated with optic neuritis |
| Without optic neuritis |
| Clinically isolated syndrome (CIS)* |
| Transverse myelitis (TM)* |
| Antibody-mediated demyelinating diseases |
| Neuromyelitis optica spectrum disorder (NMOSD)* |
| NMOSD aquaporine-4 (AQP)-antibody-seropositive |
| NMOSD aquaporine-4 (AQP)-antibody-seronegative |
| Serum antibodies to myelin oligodendrocyte glycoprotein (MOG)-associated demyelination* |
| Associated with optic neuritis |
| Without optic neuritis |
| I.II. Other immune-mediated encephalopathies and epilepsies: |
| Autoimmune (antibody-mediated) encephalitis associated with paraneoplastic and non-paraneoplastic syndromes |
| Antibodies targeting neuronal cell-surface antigens (antibody/complement-mediated immune response) |
| Anti N-methyl D-Aspartate (Anti-NMDA) Receptor Encephalitis* |
| Associated with ovarian teratoma |
| Post-Herpes virus simplex (HSV) anti-NMDAR encephalitis |
| Encephalitis with leucine-rich glioma-inactivated 1 (LGI1) and contactin-associated protein-like 2 (Caspr2) antibodies |
| Anti-glycine receptor encephalitis |
| Anti-gamma-aminobutyric acid type A (GABAA) receptor encephalitis |
| Anti-gamma-aminobutyric acid type B (GABAB) receptor encephalitis |
| Anti-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) encephalitis |
| Ophelia syndrome in Hodgkin’s lymphoma |
| Anti-Dopamine Receptor 2 Antibody-Positive Encephalitis |
| Antibodies targeting intracellular antigens (T-cell-mediated neuronal cytotoxicity) |
| Antineuronal nuclear antibody type 1 (Anti-Hu) limbic encephalitis |
| Anti-Ma2 encephalitis |
| Anti-glutamic acid decarboxylase (GAD) encephalitis |
| T cell-mediated encephalopathies |
| Rasmussen encephalitis |
| Hemophagocytic lymphohistiocytosis (HLH) |
| Acute necrotizing encephalopathy of childhood (ANEC) |
| Other autoimmune encephalopathies |
| Hashimoto encephalopathy |
| Opsoclonus myoclonus ataxia syndrome (OMA) |
| Acute encephalopathy with biphasic seizures and reduced diffusion (AESD) |
| Febrile Infection-related epilepsy syndrome (FIRES) |
| Guillain–Barré syndrome |
| Bickerstaff brainstem encephalitis |
| I.III. Paediatric stroke: |
| Childhood primary angiitis of the SNC (cPACNS) |
| Non-progressive (NP) medium-large vessel cPACNS (angiography positive) or focal cerebral arteriopathy (FCA) |
| Progressive (P) medium-large vessel cPACNS (angiography positive) |
| Small-vessel (SV) cPACNS (angiography negative) |
| Secondary paediatric stroke |
| Post-infective paediatric stroke |
| Post-varicella arteriopathy |
| Tuberculosis |
| Vascular dissection |
| Cardioembolic |
| Moyamoya syndrome |
| Early-Onset Stroke and Vasculopathy Associated with Mutations in ADA2 (congenital) |
| Hypoxic-ischaemic encephalopathy (HIE) or arterial ischaemic stroke in the paediatric population* |
| I.IV. Systemic vasculitis and other rheumatologic diseases: |
| Neuropsychiatric systemic lupus erythematosus (SLE) syndromes (NPSLE) |
| Behçet’s syndrome |
| Granulomatous |
| Childhood-onset neurosarcoidosis |
| Sjögren’s Syndrome |
| Childhood-onset granulomatosis with polyangiitis |
| Microscopic polyangiitis |
| Henoch-Schönlein Purpura |
| Kawasaki disease |
| Juvenile dermatomyositis |
| Childhood morphea |
| Inflammatory bowel disease |
| Antiphospholipid antibody syndrome |
| Celiac disease |
| Mixed connective tissue diseases |
| Macrophage activation syndrome related to juvenile idiopathic arthritis |
| Susac syndrome |
| Drug induced vasculitis |
| Paraneoplastic vasculitis |
| Autoinflammatory diseases |
| Interferonopathies |
| Hemophagocytic lymphohistiocytosis |
| I.V. Infections: |
| Postinfectious encephalopathies |
| Subacute sclerosing panencephalitis |
| Postmycoplasma basal ganglia encephalitis |
| Post-Herpes simplex virus encephalitis movement disorder |
| Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS) |
| Paediatric acute-onset neuropsychiatric syndrome |
| Encephalitis lethargica |
| Progressive rubella syndrome |
| Progressive multifocal leukoencephalopathy |
| Sydenham Chorea after a group A beta-haemolytic streptococcal infection |
| Postinfection cerebellitis |
| Whipple disease |
| Bacteria |
| Borrelia burgdorferi |
| Listeria monocytogenes |
| Mycoplasma pneumonia |
| Mycobacterium tuberculosis |
| Treponema pallidum – Neurosyphilis |
| Viruses |
| Adenovirus |
| Enterovirus |
| Epstein-Barr virus |
| Human immunodeficiency virus* |
| Human T-cell lymphotropic virus type 1 |
| Influenza |
| SARS COVID-19 |
| Measles |
| Rabies |
| Rubella |
| Varicella zoster virus |
| West Nile virus |
| Parasites |
| Malaria |
| Neurocysticercosis |
| I.VI. Neoplastic: |
| Primary SNC neoplasms |
| Astrocytoma |
| Lymphoma |
| Medulloblastoma |
| Systemic neoplasms affecting SNC |
| Paraneoplastic syndromes |
| Metastatic syndromes |
| Langerhans cell histiocytosis |
| Secondary neoplasms and other disorders related to radiation/chemotherapy treatment |
| I.VII. Toxic and medication-associated disorders: |
| Medications |
| Metronidazole |
| Cyclosporine |
| Antiepileptic drugs |
| Recreational drugs |
| Alcohol |
| Marijuana |
| Synthetic cannabinoids |
| Cocaine |
| Opioids |
| Methamphetamines |
| Poisoning/accidental ingestions |
| Ethylene glycol |
| Methanol |
| Inhalants |
| Chronic lead poisoning |
| Radiation |
| Chemotherapy |
| e.g. Methotrexate, cyclosporine, cytosine-arabinoside |
| I. VIII. Acquired metabolic, endocrine and nutritional disorders: |
| Vitamin B12, vitamin E or folate deficiency |
| Hepatic encephalopathy |
| Wernicke–Korsakoff syndrome |
| Hypothyroidism |
| Extrapontine myelinolysis |
| I.IX. Child abuse and trauma |
| II. CONGENITAL DISORDERS OR GENETICALLY DETERMINED DISORDERS |
| II.I Disorders that affect white matter predominantly (Leukodystrophies or leukoencephalopathies):* |
| Myelin disorders |
| Pelizaeus-Merzbacher disease |
| Waardenburg-Hirschsprung |
| Metachromatic leukodystrophy |
| Multiple sulfatase deficiency |
| Krabbe disease |
| X-Linked adrenoleukodystrophy |
| Phenylketonuria and other aminoacidopathies |
| Canavan disease |
| Cx32-related Charcot-Marie-Tooth disease |
| Astrocytopathies |
| Alexander disease |
| Megalencephalic leukoencephalopathy |
| CIC-2 related disease |
| Vanishing white matter disease |
| Aicardi-Goutières syndrome |
| Cx-43-related oculodentogenesis dysplasia |
| Leuko-axonopathies |
| Hypomyelination with atrophy of basal ganglia and cerebellum |
| Hypomyelination with congenital cataract |
| GM1 and GM2 gangliosidosis |
| AGC1 related diseases |
| AIMP1-related diseases |
| HSPD1-related disease |
| Pol-III-related leukodystrophies |
| Leukoencephalopathy with brainstem and spinal cord involvement and high lactacte |
| Giant axonal neuropathy |
| Microgliopathies |
| CSD1R-related disorders |
| Nasau-Hakola disease |
| Leukovasculopathies |
| Cerebral arteriopathy with subcortical infarcts and leukoencelopathy |
| Cerebral amyloid angiopathy |
| Capthesin A-related arteriopathy with strokes and leukoencephalopathy |
| Leukoencephalopathy with calcifications and cysts |
| II.II Disorders that affect grey matter predominantly (Poliodystrophies): |
| Monogenic early onset epileptic encephalopathies and progressive myoclonus epilepsies |
| Dravet syndrome |
| Unverricht/Lundborg disease |
| Lafora disease |
| KCTV7-related epilepsies |
| Rett syndrome |
| Lysosomal storage diseases |
| Neuronal ceroid lipofuscinosis group* |
| CLN1, CLN2, CLN3, CLN5, CLN6, CLN7, CLN10, CLN14 |
| Nieman Pick type C disease* |
| Sialidosis type 1 |
| Fabry disease |
| Gaucher’s disease |
| Mucopolysaccaridoses |
| Alsin-related disorders |
| Infantile ascending hereditary spastic paraplegia |
| Juvenile primary lateral sclerosis |
| Autosomal recessive juvenile amyotrophic lateral sclerosis |
| Hereditary spastic paraplegia |
| Menkes disease |
| II.III. Disorders with prominent involvement of basal ganglia: |
| Neurodegeneration with brain iron accumulation disorders (NBIA) |
| Pantothenate kinase-associated neurodegeneration (PKAN) |
| Phospholipase-associated neurodegeneration (PLAN) |
| Infantile neuroaxonal dystrophy (INAD) |
| Atypical infantile neuroaxonal dystrophy (aINAD) (Karak syndrome) |
| Neuroferritinopathy (NFT) |
| Mitochondrial membrane protein–associated neurodegeneration (MPAN) |
| β propeller protein–associated neurodegeneration (BPAN) |
| COASY protein–associated neurodegeneration (CoPAN) |
| Aceruloplasminemia |
| Fatty acid dehydroxylase–associated neurodegeneration (FAHN) |
| Woodhouse–Sataki syndrome |
| Kufor–Rakeb disease (PARK 9) |
| Biotin-thiamine-responsive basal ganglia disease |
| Monogenic heredodegenerative dystonia |
| Inherited disorders presenting with chorea |
| Huntington disease |
| VPS 13A-Choreo-acanthocytosis |
| Infantile onset striatal necrosis |
| Organic acidurias |
| Glutaric aciduria type 1 |
| Homocystinuria |
| Propionic aciduria |
| Hydroxybutyric aciduria |
| Methylmalonic aciduria |
| Glut-1 deficiency syndrome |
| Wilson disease |
| Lesch-Nyhan disease |
| II.IV. Disorders with prominent involvement of brainstem and cerebellum or paediatric movement disorders: |
| Infantile-onset Spinocerebellar ataxia (IOSCA)* |
| e.g. SCA2 (CAG repeat occurs over 200), SCA3 (Larger expansion), SCA7, SCA12, SCA13, SCA14, SCA27 |
| Ponto-cerebellar hypoplasia |
| Friedreich ataxia |
| Ataxia telangiectasia |
| Marinesco-Sjögren Syndrome |
| II.V. Disorders with prominent involvement of spinal cord, cranial and peripheral nerves: |
| Spinal muscular atrophy |
| Hereditary motor and sensory neuropathies |
| Refsum disease |
| Alstrom syndrome |
| II.VI. Disorders with prominent involvement of skeletal muscles: |
| Dystrophinopathies |
| Duchenne muscular dystrophy |
| Becker muscular dystrophy |
| Congenital myotonia |
| Neonatal onset myotonic dystrophy |
| II.VII. Mitochondrial encephalopathies: |
| Alpers disease |
| Mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes MELAS |
| DNA polymerase gamma (POLG)-related disorders |
| Leigh syndrome |
| Dystonia-deafness syndrome |
| Myoclonic epilepsy with ragged red fibres (MERRF) |
| Leber hereditary optic neuropathy (LHON) |
| Kearns-Sayre syndrome |
| II.VIII. Other inherited disorders: |
| Sickle cell disease |
Highlighted conditions indicate articles, reviewed for this paper, which described OCTs.
Table 3.
Ophthalmic and neurologic tests used in paediatric neuroinflammatory/neurodegenerative disorders.
| Test name | Objective | Parameters measured by test | Ranking score | Interpretation of test | OCT features related to ophthalmic or neurological impairment measured by test | ||
|---|---|---|---|---|---|---|---|
| Hamburg CLN3 ophthalmic rating scale | To monitor progression of retinal degeneration in CLN3 disease | VA | BCVA measured in Snellen. | Grade 0 (unaffected) | Score: 14 points. BCVA ≥ 20/25. No retinal degeneration. No EZ or ONL loss. | VA < = 20/160, fundoscopic abnormalities and loss of EZ and ONL by OCT reflect structural and functional retinal damage in patients with CLN3 disease. | Loss of EZ and ONL are present in grade 2 and 3 of Hamburg CLN3 scale. In addition, loss of EZ area is strongly associated with VA loss. |
| Fundoscopy abnormalities | Optic pallor, macular striae, macular orange pigment, peripheral bony spicules, vessel loss. | Grade 1 (affected) | Score between 10–13 points. Subtle retinal degeneration. No EZ or ONL loss. | ||||
| Grade 2 (severely affected) | Score between 5–9 points. Evident retinal degeneration. EZ and ONL loss. | ||||||
| OCT assessment | EZ and ONL integrity. | Grade 3 (end stage) | Score between 0–4 points. BCVA < = 20/160. Optic pallor, macular atrophy with orange pigment deposition, vessel loss and peripheral bone spicules. EZ and ONL loss. | ||||
| modified Disability Rating Scale (mDRS) | To assess severity of disease and monitor treatment effect | Ambulation | Clumsiness, autonomous ataxia gait, outdoor or indoor assisted ambulation, wheelchair-bound. | 1 | Mild disability | The maximum score that a patient can get is 25, representing severe disability in overall neurological impairment. | Thinning of OPL is associated with severe disability in mDRS in patients with Nieman Pick type C disease. |
| Motor response | Tremor, dysmetria/dystonia, seizures. | 2–3 | Partial disability | ||||
| Language | Delayed acquisition, dysarthria, non-verbal communication, absence of communication. | 4–6 | Moderate disability | ||||
| Swallowing | Abnormal chewing, occasional dysphagia, daily dysphagia, nasogastric/gastric button feeding. | 7–11 | Moderately severe | ||||
| Seizures | Occasional seizures, seizures with antiepileptic drugs, seizures resistant to antiepileptic drugs. | 12–16 | Severe | ||||
| Ocular movements | Slow ocular pursuit, vertical ophthalmoplegia, complete ophthalmoplegia. | 17–25 | Extremely severe | ||||
| Assessment and Rating of Ataxia (SARA) | To assess severity of clinical impairment in cerebellar ataxia | Gait | Walking, turning and walking tandem. | 0 | No ataxia | Each domain (gait, stance, sitting, speech, finger chase, nose-finger test, fast alternating hand movements, heel-shin slide) ranked from 0 to 8. SARA score is sum of all domain scores. | The thinning of combined GCL with IPL thickness is associated with worse SARA score in Nieman Pick disease. |
| Stance | Ability to stand in natural position, with feet together in parallel and in tandem. | ||||||
| Sitting | To sit on an examination bed without support of feet, eyes open and arms outstretched to the front. | ||||||
| Speech disturbance | Speech in assessed during normal conversation. | ||||||
| Finger chase | Presence of dysmetria, under/overshooting target (<5 cm, < 15 cm, > 15 cm). | 40 | Most severe ataxia | ||||
| Nose-finger test | Presence of tremor and amplitude of it (< 2 cm, < 5 cm, > 5 cm). | ||||||
| Fast alternating hand movements | Irregularities doing the task. | ||||||
| Heel-shin slide | Abnormalities doing the task. | ||||||
| Expanded Disability Status Scale (EDSS) | To qualify disability and disease progression in patients with MS and other diseases | Ambulation | Ability to walk, requirement of assistance, use of wheelchair or to be restricted to bed. | 0 | Normal neurological status | The lower scale values of test measure impairments based on neurological examination; upper range of scale (> EDSS 6) measures handicaps of patients with MS. Scores from 0 to 3.5 are determined by neurological deficit without impairment of ambulation. | Thinning of peripapillary RNFL is strongly correlated with upper range score in EDSS scale. |
| Pyramidal | Paraparesis, hemiparesis, monoparesis, quadriparesis, monoplegia, paraplegia, hemiplegia, quadriplegia. | 1 | No disability with only minimal signs | ||||
| Cerebellar | Ataxia. | 2 | Minimal disability | ||||
| Brainstem | Nystagmus, extraocular weakness, dysarthria, inability to swallow or speak. | 3 | Moderate disability | ||||
| Sensory | Decrease in touch, pain and position sense. | 4 | Relatively severe disability | ||||
| 5 | Disability affects full daily activities | ||||||
| Bowel and bladder | Urinary hesitancy, urgency or retention. Urinary incontinence. | 6 | Assistance required walking and working | ||||
| 7 | Essentially restricted to walk and work | ||||||
| Visual | VA measured in Snellen. | 8 | Restricted to bed or wheelchair | ||||
| Cerebral | Mood alteration, decrease in mentation, dementia or chronic brain syndrome. | 9 | Bedridden and unable to communicate effectively or eat/swallow | ||||
| 10 | Death | ||||||
CLN3 juvenile ceroid-lipofuscinosis, neuronal 3, VA visual acuity, BCVA best corrected visual acuity, OCT Optical coherence tomography, EZ Ellipsoid zone, ONL outer nuclear layer, OPL outer plexiform layer, cm centimetres, GLC ganglion cell layer, IPL inner plexiform layer, MS multiple sclerosis.
A multidisciplinary approach is often important, including the participation of neurologists, paediatricians, rheumatologists, radiologists, geneticists, psychiatrists, infectious disease and immunology experts, and ophthalmologists [129]. Treatment varies depending on the diagnosis and underlying symptoms [129]. Emerging therapies seek to improve the clinical outcomes in these disorders [103, 130].
OCT features related to visual function
Demyelinating Diseases (acute disseminated encephalomyelitis, multiple sclerosis and transverse myelitis)
The most common demyelinating disease in children is acute disseminated encephalomyelitis (ADEM) with an incidence of 0.3–.6 per 100,000 per year [129]. OCT has revealed thinning of the NFL in patients with ADEM compared to healthy controls [5, 8], suggesting anterior optic pathway dysfunction [5]. In a cross-sectional study that evaluated ADEM, multiple sclerosis (MS) and transverse myelitis [8], NFL thinning was associated with reduced Snellen visual acuity (VA converted to LogMAR) (R = − 0.47, P < 0.001) [8]. Correlations between NFL thinning and prolonged P100 latency (>110 ms) in visual evoked potential (R = − 0.51, P < 0.001) and a lower number of letters read correctly in low-contrast letter acuity (LCLA) charts (R = 0.42, P = 0.002) were also identified [8]. The LCLA score was markedly lower in children with MS (22 ± 10 letters) compared to controls (31 ± 6 letters) [8]. The LCLA test is a way to assess contrast sensitivity and is based on the number of letters identified correctly, with a maximum score of 70 (75 letters) [131]. The test has been used widely in patients with MS [131]. Correlation analyses between NFL thickness and VA, visual evoked potential, and LCLA test, were not done for each disease [8].
In another study, only children with MS were evaluated [9]. The cohort was divided into patients with or without a history of optic neuritis. NFL thinning was only identified in patients with a history of optic neuritis. In all MS patients with and without optic neuritis, there was not a significant relationship between NFL thickness and LCLA score (P = 0.103) [9]. The sample size of MS patients with optic neuritis was small; therefore, statistical analysis was not performed between LCLA and NFL thickness in the subgroup of MS patients with optic neuritis.
Although there is not yet enough evidence in the literature to determine if NFL thickness is a biomarker related to visual function in paediatric MS, much knowledge is known from adults who are more likely than children to be diagnosed with MS. Part of this observation may be because of the infrequency of MS in children compared to adults or because the duration children have MS is shorter than in adults.
Lysosomal storage diseases
The loss of outer retinal layers, ellipsoid zone (EZ) and outer nuclear layer (ONL) is associated with visual dysfunction in the lysosomal storage disease, juvenile ceroid-lipofuscinosis, neuronal 3 (CLN3). In a retrospective, cross-sectional study in juvenile CLN3 disease, VA, optic pallor, macular striae, macular orange pigment, peripheral bony spicules, vessel loss, macular atrophy, loss of EZ and the ONL were used to develop a scale of ophthalmic features to monitor progression of retinal disease [94] called the “Hamburg CLN3 ophthalmic rating scale”. The loss of the EZ and ONL were associated with grade 2, indicating severe disease, and grade 3, end stage disease, and were strongly correlated with reduced visual acuity (R = 0.83, P < 0.001) [94].
Association between OCT features and clinical changes within CNS
Nieman Pick type C disease
In a case-control study, the relationship between OCT features and clinical scales of neurologic impairment were studied in patients with symptomatic Nieman Pick disease (NPC), those without symptoms but who carried mutations of NPC, and healthy controls. In symptomatic NPC patients, the macular NFL and the combined GCL with IPL were statistically significantly thinner compared to healthy controls [96]. Asymptomatic patients who carried mutations did not have statistically significant OCT changes compared to healthy controls. Thinning of the mean combined outer plexiform layer (OPL) and ONL was associated with worse neurologic impairment using a modified Disability Rating Scale (mDRS) (ρ =− 0.617, P < 0.05) [96]. The mDRS is a way to measure functional neurologic performance based on the impairment of ambulation, motor response, language, swallowing, seizures and ocular movements [97]. A strong correlation was also observed between thinning of the combined GCL with IPL and major motor disability using the Assessment and Rating of Ataxia (SARA) score (ρ =− 0.622, P < 0.05) [96]. The SARA score assesses the impairment of gait, speech, coordination, and other cerebellar functions in patients with ataxia [98]. Other clinical parameters, including lower amplitude of upward vertical saccades and slower velocity horizontal saccades, were related to thinning of the peripapillary NFL (ρ = 0.645, P < 0.05) and of the combined GCL with IPL (ρ = 0.609, P < 0.05). The evaluation of saccadic movements is one of the ways to assess cerebellar function. Focal, degenerative, or systemic/immune deficits affecting the cerebellum in neuroinflammatory/neurodegenerative disorders can be associated with slow saccadic velocities and low amplitudes [99].
Hypoxic Ischaemic encephalopathy
Several studies of infants with hypoxic ischaemic encephalopathy (HIE) sought to find noninvasive methods to assess neurologic damage from ischaemia and to predict the association with future neurologic dysfunction or abnormalities in structural CNS features. Although few studies have been done, non-invasive OCT does not require sedation and can be done at the bedside [13, 14], most recently demonstrated in a hypothermia treatment trial [14].
Human immunodeficiency virus (HIV)
Retinal thinning has been associated with microstructural white matter injury in children infected with human immunodeficiency virus (HIV). Blokhuis et al. studied retinal features of 29 children with perinatal HIV-infection treated with combination antiretroviral therapy. Thinning of the total retina at the fovea, as well as, all individual retinal layers including GCL, IPL, inner nuclear layer (INL) and ONL was associated with higher mean diffusivity and radial diffusivity on brain MRI scans [100].
Leukodystrophies
Retinal neurodegeneration detected by OCT has been shown to be a surrogate outcome measure for the progression of other childhood-onset degenerative diseases, such as the myelopathy in adrenoleukodystrophy [11]. A strong correlation has been found between clinical parameters like the Expanded Disability Status Scale (EDSS) and thinning of peripapillary RNFL (Spearman’s rho = 0.51, P = 0.02) [11]. EDSS is a method to measure the impairment of neurologic functions, such as motor functions, coordination, sensory functions, and visual function, among others [101]. Although this scale has mostly been used in MS [101], it has also been employed in other diseases such as adrenoleukodystrophy [11].
Spinocerebellar ataxia (SCA)
Spinocerebellar ataxia (SCA) comprises a number of progressive degenerative hereditary diseases that present at different ages. Alvarez et al. evaluated a series of cases of seven adults in Spain genetically confirmed with SCA-3 and found NFL thinning associated with severe neurologic impairment measured by SARA score (R = −0.64, P = 0.012) [12].
In another study performed in Brazil, OCT retinal features and clinical neurologic impairment measured by the SARA score were compared between patients with SCA-3 and SCA-10. The NFL was thinner in SCA-3 than in SCA-10 but the difference was not statistically significant. Furthermore, in SCA-3, thinning of the RNFL in the nasal region was associated with severe neurologic impairment as measured by the SARA score (R = − 0.695, P = 0.025); in contrast, RNFL thickness in SCA-10 was not related to the SARA score [95].
In conclusion, OCT has been used to identify retinal features, including thinning of the NFL, GCL, IPL, INL, OPL, as well as loss of ONL and EZ in paediatric neuroinflammatory/neurodegenerative disorders. These features have been described as markers of axonal loss, and their clinical significance relies on their relationship with some clinical parameters such as vision, motor/coordination and sensory functions. OCT can be considered a method to predict visual function and may be helpful to clinicians and researchers working with children. In addition, OCT might predict progression of systemic findings and be a valuable tool to assess and monitor anterior optic pathway dysfunction. Although some differences in OCT retinal features have been found among distinct neuroinflammatory/neurodegenerative disorders, the clinical diagnosis of CNS diseases cannot be replaced by retinal imaging. At present, OCT imaging has not been found to differentiate among different neuroinflammatory/neurodegenerative disorders.
OCT is a non-invasive way to assess neurological changes in neuroinflammatory/neurodegenerative disorders, but more study is needed to increase sample sizes in many diseases and controls, in relation to the duration of the disease and the age of the child.
Conclusions
The novel biomarkers of inflammation and neurodegeneration in the retina, easily visualized by the OCT have the potential to increase our knowledge on the systemic inflammatory and neurodegenerative condition, and thus provide a non-invasive way to monitor the CNS status and facilitate the multidisciplinary interaction when dealing with the patients. Further study of OCT retinal biomarkers as well as its automatic evaluation and validation is warranted in both adults and in children.
SUMMARY
OCT offers a unique opportunity to evaluate specific retinal changes in systemic neuroinflammatory/neurodegenerative disorders in both adults and paediatric population.
These OCT features act as biomarkers of neuroinflammation/neurodegeneration and include hyper-reflective retinal spots/dots/foci, subfoveal neuroretinal detachment (SND), changes in thickness of specific retinal layers and modification of integrity of both the inner and the outer retinal layers.
Evaluation of OCT biomarkers of neuroinflammation/neurodegeneration can help in monitoring the status of the CNS and facilitate a multidisciplinary approach necessary for accurate diagnostic and therapeutic management of these disorders.
Author contributions
SV: study concept or design. SV, MMP, MEH, LO’T, AN, CL: data collection, data analysis or interpretation, writing the paper. PN, EV, MEH, SV: Critical revision of the article.
Competing interests
SV: Abbvie-Allergan, Apellis, Bayer, Novartis, Roche SPA: Advisory Board participation MMP: none MEH: This work was supported by the National Institutes of Health EY014800 and an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah; and the National Institutes of Health R01EY015130 and R01EY017011 to MEH. LO’T: Bayer and Novartis: Advisory Board participation. AN: none. Celeste Limoli: none. EV: Allergan: Consultant/Advisor, Lecture fees, Grant support (AAO Financial Disclosure and First-slide Policy 2021); FB Vision and Santen: Consultant/Advisor, Lecture fees; Bruschettini, Oftagest, Sooft, Thea and Visufarma: Lecture fees; Alfa intes, OffHealth, Servimed and Shire: Grant support (AAO Financial Disclosure and First-slide Policy 2021). PN: none.
Footnotes
The original online version of this article was revised: Affiliation 2 was corrected to Eye Clinic, IRCCS MultiMedica, Milan, Italy.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/12/2022
A Correction to this paper has been published: 10.1038/s41433-022-02083-6
References
- 1.Chen WW, Zhang X, Huang WJ. Role of neuroinflammation in neurodegenerative diseases (Review) Mol Med Rep. 2016;13:3391–6. doi: 10.3892/mmr.2016.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–69. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakhtadze S, Geladze N, Khachapuridze N. Inflammation in childhood epilepsy syndromes. Georgian Med N. 2021;312:88–92. [PubMed] [Google Scholar]
- 4.Sudhakar SV, Muthusamy K, Shroff M. Imaging of childhood inflammatory brain diseases. Top Magn Reson Imaging. 2018;27:409–31.. doi: 10.1097/RMR.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 5.Borchert M, Liu GT, Pineles S, Waldman AT. Pediatric optic neuritis: what is new. J Neuroophthalmol. 2017;37:S14–s22. doi: 10.1097/WNO.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5:463–6. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou WC, Tao JX, Li J. Optical coherence tomography measurements as potential imaging biomarkers for Parkinson’s disease: A systematic review and meta-analysis. Eur J Neurol. 2021;28:763–74.. doi: 10.1111/ene.14613. [DOI] [PubMed] [Google Scholar]
- 8.Yeh EA, Weinstock-Guttman B, Lincoff N, Reynolds J, Weinstock A, Madurai N, et al. Retinal nerve fiber thickness in inflammatory demyelinating diseases of childhood onset. Mult Scler (Houndmills, Basingstoke, Engl) 2009;15:802–10. doi: 10.1177/1352458509104586. [DOI] [PubMed] [Google Scholar]
- 9.Waldman AT, Hiremath G, Avery RA, Conger A, Pineles SL, Loguidice MJ, et al. Monocular and binocular low-contrast visual acuity and optical coherence tomography in pediatric multiple sclerosis. Mult Scler Relat Disord. 2013;3:326–34.. doi: 10.1016/j.msard.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett JL, de Seze J, Lana-Peixoto M, Palace J, Waldman A, Schippling S, et al. Neuromyelitis optica and multiple sclerosis: Seeing differences through optical coherence tomography. Mult Scler (Houndmills, Basingstoke, Engl) 2015;21:678–88. doi: 10.1177/1352458514567216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Ballegoij WJC, Huffnagel IC, van de Stadt SIW, Weinstein HC, Bennebroek CAM, Engelen M, et al. Optical coherence tomography to measure the progression of myelopathy in adrenoleukodystrophy. Ann Clin Transl Neurol. 2021;8:1064–72. doi: 10.1002/acn3.51349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez G, Rey A, Sanchez-Dalmau FB, Muñoz E, Ríos J, Adán A. Optical coherence tomography findings in spinocerebellar ataxia-3. Eye (Lond, Engl) 2013;27:1376–81. doi: 10.1038/eye.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran-Viet D, Wong BM, Mangalesh S, Maldonado R, Cotten CM, Toth CA, et al. Hanheld spectral domain optical coherence tomography imaging through the undilated pupil in infants born preterm or with hipoxic injury or hydrocephalus. Retina. 2017;38:1588–94. doi: 10.1097/IAE.0000000000001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grego L, Pignatto S, Busolini E, Rassu N, Samassa F, Prosperi R, et al. Spectral-domain OCT changes in retina and optic nerve in children with hypoxic-ischaemic encephalopathy. Graefe’s Arch Clin Exp Ophthalmol = Albrecht von Graefes Arch fur klinische und experimentelle Ophthalmologie. 2021;259:1343–55.. doi: 10.1007/s00417-020-04996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 16.Lynch MA. The multifaceted profile of activated microglia. Mol Neurobiol. 2009;40:139–56. doi: 10.1007/s12035-009-8077-9. [DOI] [PubMed] [Google Scholar]
- 17.Vujosevic S, Simó R. Local and systemic inflammatory biomarkers of diabetic retinopathy: an integrative approach. Invest Ophthalmol Vis Sci. 2017;58:Bio68–bio75. doi: 10.1167/iovs.17-21769. [DOI] [PubMed] [Google Scholar]
- 18.Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–11. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 19.Vujosevic S, Micera A, Bini S, Berton M, Esposito G, Midena E. Aqueous humor biomarkers of müller cell activation in diabetic eyes. Investigative Ophthalmol Vis Sci. 2015;56:3913–8. doi: 10.1167/iovs.15-16554. [DOI] [PubMed] [Google Scholar]
- 20.Vujosevic S, Torresin T, Bini S, Convento E, Pilotto E, Parrozzani R, et al. Imaging retinal inflammatory biomarkers after intravitreal steroid and anti-VEGF treatment in diabetic macular oedema. Acta Ophthalmol. 2017;95:464–71. doi: 10.1111/aos.13294. [DOI] [PubMed] [Google Scholar]
- 21.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 22.Vujosevic S, Midena E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Müller cells alterations. J Diabetes Res. 2013;2013:905058. doi: 10.1155/2013/905058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y, Chen H, Su SB. Neuroinflammatory responses in diabetic retinopathy. J Neuroinflammation. 2015;12:141. doi: 10.1186/s12974-015-0368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vujosevic S, Bini S, Torresin T, Berton M, Midena G, Parrozzani R, et al. Hyperreflective retinal spots in normal and diabetic eyes: b-scan and en face spectral domain optical coherence tomography evaluation. Retina. 2017;37:1092–103.. doi: 10.1097/IAE.0000000000001304. [DOI] [PubMed] [Google Scholar]
- 25.Vujosevic S, Bini S, Midena G, Berton M, Pilotto E, Midena E. Hyperreflective intraretinal spots in diabetics without and with nonproliferative diabetic retinopathy: an in vivo study using spectral domain OCT. J Diabetes Res. 2013;2013:491835. doi: 10.1155/2013/491835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogino K, Murakami T, Tsujikawa A, Miyamoto K, Sakamoto A, Ota M, et al. Characteristics of optical coherence tomographic hyperreflective foci in retinal vein occlusion. Retina. 2012;32:77–85. doi: 10.1097/IAE.0b013e318217ffc7. [DOI] [PubMed] [Google Scholar]
- 27.Coscas GCF, Vismara S, Zourdanic A, Li Calzi CI. Optical coherence tomography in age-related macular degeneration: OCT in AMD. Publishing S, editor 2009 1–389 p.
- 28.Curcio CA, Zanzottera EC, Ach T, Balaratnasingam C, Freund KB. Activated retinal pigment epithelium, an optical coherence tomography biomarker for progression in age-related macular degeneration. Investig Ophthalmol Vis Sci. 2017;58:BIO211–BIO26. doi: 10.1167/iovs.17-21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vujosevic S, Berton M, Bini S, Casciano M, Cavarzeran F, Midena E. Hyperreflective retinal spots and visual function after anti-vascular endothelial growth factor treatment in center-involving diabetic macular edema. Retina. 2016;36:1298–308. doi: 10.1097/IAE.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 30.Pilotto E, Miante S, Torresin T, Puthenparampil M, Frizziero L, Federle L, et al. Hyperreflective foci in the retina of active relapse-onset multiple sclerosis. Ophthalmology. 2020;127:1774–6. doi: 10.1016/j.ophtha.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Vujosevic S, Toma C, Villani E, Muraca A, Torti E, Florimbi G, et al. Diabetic macular edema with neuroretinal detachment: OCT and OCT-angiography biomarkers of treatment response to anti-VEGF and steroids. Acta Diabetol. 2020;57:287–96. doi: 10.1007/s00592-019-01424-4. [DOI] [PubMed] [Google Scholar]
- 32.Chen KC, Jung JJ, Curcio CA, Balaratnasingam C, Gallego-Pinazo R, Dolz-Marco R, et al. Intraretinal hyperreflective foci in acquired vitelliform lesions of the macula: clinical and histologic study. Am J Ophthalmol. 2016;164:89–98. doi: 10.1016/j.ajo.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Pang CE, Messinger JD, Zanzottera EC, Freund KB, Curcio CA. The onion sign in neovascular age-related macular degeneration represents cholesterol crystals. Ophthalmology. 2015;122:2316–26. doi: 10.1016/j.ophtha.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao D, Leong B, Messinger JD, Kar D, Ach T, Yannuzzi LA, et al. Hyperreflective foci, optical coherence tomography progression indicators in age-related macular degeneration, include transdifferentiated retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2021;62:34. doi: 10.1167/iovs.62.10.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee H, Jang H, Choi YA, Kim HC, Chung H. Association between soluble CD14 in the aqueous humor and hyperreflective foci on optical coherence tomography in patients with diabetic macular edema. Investigative Ophthalmol Vis Sci. 2018;59:715–21. doi: 10.1167/iovs.17-23042. [DOI] [PubMed] [Google Scholar]
- 36.Deák GG, Bolz M, Kriechbaum K, Prager S, Mylonas G, Scholda C, et al. Effect of retinal photocoagulation on intraretinal lipid exudates in diabetic macular edema documented by optical coherence tomography. Ophthalmology. 2010;117:773–9. doi: 10.1016/j.ophtha.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 37.Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Otsuka H, Sonoda Y. Retinal morphologic changes and concentrations of cytokines in eyes with diabetic macular edema. Retina. 2014;34:741–8. doi: 10.1097/IAE.0b013e3182a48917. [DOI] [PubMed] [Google Scholar]
- 38.Coscas G, Gaudric A. Natural course of nonaphakic cystoid macular edema. Surv Ophthalmol. 1984;28:471–84. doi: 10.1016/0039-6257(84)90229-7. [DOI] [PubMed] [Google Scholar]
- 39.van Dijk HW, Verbraak FD, Kok PH, Garvin MK, Sonka M, Lee K, et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010;51:3660–5. doi: 10.1167/iovs.09-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Dijk HW, Verbraak FD, Kok PH, Stehouwer M, Garvin MK, Sonka M, et al. Early neurodegeneration in the retina of type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2012;53:2715–9. doi: 10.1167/iovs.11-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:1156–63. doi: 10.1167/iovs.10-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynch SK, Abràmoff MD. Diabetic retinopathy is a neurodegenerative disorder. Vis Res. 2017;139:101–7. doi: 10.1016/j.visres.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knier B, Schmidt P, Aly L, Buck D, Berthele A, Mühlau M, et al. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain. 2016;139:2855–63. doi: 10.1093/brain/aww219. [DOI] [PubMed] [Google Scholar]
- 44.Martinez-Lapiscina EH, Sepulveda M, Torres-Torres R, Alba-Arbalat S, Llufriu S, Blanco Y, et al. Usefulness of optical coherence tomography to distinguish optic neuritis associated with AQP4 or MOG in neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord. 2016;9:436–40. doi: 10.1177/1756285616655264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zafar S, Sachdeva M, Frankfort BJ, Channa R. Retinal neurodegeneration as an early manifestation of diabetic eye disease and potential neuroprotective therapies. Curr Diabetes Rep. 2019;19:17. doi: 10.1007/s11892-019-1134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maheshwary AS, Oster SF, Yuson RM, Cheng L, Mojana F, Freeman WR. The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol. 2010;150:63–7. doi: 10.1016/j.ajo.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim BJ, Irwin DJ, Song D, Daniel E, Leveque JD, Raquib AR, et al. Optical coherence tomography identifies outer retina thinning in frontotemporal degeneration. Neurology. 2017;89:1604–11. doi: 10.1212/WNL.0000000000004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun JK, Lin MM, Lammer J, Prager S, Sarangi R, Silva PS, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132:1309–16. doi: 10.1001/jamaophthalmol.2014.2350. [DOI] [PubMed] [Google Scholar]
- 49.Radwan SH, Soliman AZ, Tokarev J, Zhang L, van Kuijk FJ, Koozekanani DD. Association of disorganization of retinal inner layers with vision after resolution of center-involved diabetic macular edema. JAMA Ophthalmol. 2015;133:820–5. doi: 10.1001/jamaophthalmol.2015.0972. [DOI] [PubMed] [Google Scholar]
- 50.Rübsam A, Parikh S, Fort PE. Role of inflammation in diabetic retinopathy. Int J Mol Sci. 2018;19:942. doi: 10.3390/ijms19040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nian S, Lo ACY, Mi Y, Ren K, Yang D. Neurovascular unit in diabetic retinopathy: pathophysiological roles and potential therapeutical targets. Eye Vis. 2021;8:15. doi: 10.1186/s40662-021-00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simó R, Hernández C. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol Metab. 2014;25:23–33. doi: 10.1016/j.tem.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Santos AR, Ribeiro L, Bandello F, Lattanzio R, Egan C, Frydkjaer-Olsen U, et al. Functional and structural findings of neurodegeneration in early stages of diabetic retinopathy: cross-sectional analyses of baseline data of the EUROCONDOR Project. Diabetes. 2017;66:2503–10. doi: 10.2337/db16-1453. [DOI] [PubMed] [Google Scholar]
- 54.Montesano G, Ometto G, Higgins BE, Das R, Graham KW, Chakravarthy U, et al. Evidence for structural and functional damage of the inner retina in diabetes with no diabetic retinopathy. Investigative Ophthalmol Vis Sci. 2021;62:35. doi: 10.1167/iovs.62.3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soni D, Sagar P, Takkar B. Diabetic retinal neurodegeneration as a form of diabetic retinopathy. Int Ophthalmol. 2021;41:3223–48. doi: 10.1007/s10792-021-01864-4. [DOI] [PubMed] [Google Scholar]
- 56.van de Kreeke JA, Darma S, Chan Pin Yin JMPL, Tan HS, Abramoff MD, Twisk JWR, et al. The spatial relation of diabetic retinal neurodegeneration with diabetic retinopathy. PLOS ONE. 2020;15:e0231552. doi: 10.1371/journal.pone.0231552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vujosevic S, Muraca A, Alkabes M, Villani E, Cavarzeran F, Rossetti L, et al. Early microvascular and neural changes in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy. Retina. 2019;39:435–45. doi: 10.1097/IAE.0000000000001990. [DOI] [PubMed] [Google Scholar]
- 58.Gallina D, Zelinka CP, Cebulla CM, Fischer AJ. Activation of glucocorticoid receptors in Müller glia is protective to retinal neurons and suppresses microglial reactivity. Exp Neurol. 2015;273:114–25. doi: 10.1016/j.expneurol.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scarinci F, Varano M, Parravano M. Retinal sensitivity loss correlates with deep capillary plexus impairment in diabetic macular ischemia. J Ophthalmol. 2019;2019:7589841. doi: 10.1155/2019/7589841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campos A, Campos EJ, Martins J, Rodrigues FSC, Silva R, Ambrósio AF. Inflammatory cells proliferate in the choroid and retina without choroidal thickness change in early Type 1 diabetes. Exp Eye Res. 2020;199:108195. doi: 10.1016/j.exer.2020.108195. [DOI] [PubMed] [Google Scholar]
- 61.Gupta C, Tan R, Mishra C, Khandelwal N, Raman R, Kim R, et al. Choroidal structural analysis in eyes with diabetic retinopathy and diabetic macular edema—A novel OCT based imaging biomarker. PLOS ONE. 2018;13:e0207435. doi: 10.1371/journal.pone.0207435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abadia B, Suñen I, Calvo P, Bartol F, Verdes G, Ferreras A. Choroidal thickness measured using swept-source optical coherence tomography is reduced in patients with type 2 diabetes. PLOS ONE. 2018;13:e0191977. doi: 10.1371/journal.pone.0191977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang-Link YM, Fredrikson M, Link H. Benign Multiple Sclerosis is Associated with Reduced Thinning of the Retinal Nerve Fiber and Ganglion Cell Layers in Non-Optic-Neuritis Eyes. J Clin Neurol. 2015;11:241–7. doi: 10.3988/jcn.2015.11.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birkeldh U, Manouchehrinia A, Hietala MA, Hillert J, Olsson T, Piehl F, et al. The temporal retinal nerve fiber layer thickness is the most important optical coherence tomography estimate in multiple sclerosis. Front Neurol. 2017;1–8. [DOI] [PMC free article] [PubMed]
- 65.Petzold A, Balcer LJ, Calabresi PA, Costello F, Frohman TC, Frohman EM, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16:797–812. doi: 10.1016/S1474-4422(17)30278-8. [DOI] [PubMed] [Google Scholar]
- 66.Pietroboni AM, Carandini T, Dell’Arti L, Bovis F, Colombi A, De Riz MA, et al. Evidence of retinal anterograde neurodegeneration in the very early stages of multiple sclerosis: a longitudinal OCT study. Neurol Sci. 2020;41:3175–83.. doi: 10.1007/s10072-020-04431-4. [DOI] [PubMed] [Google Scholar]
- 67.Reis AS, O’Leary N, Yang H, Sharpe GP, Nicolela MT, Burgoyne CF, et al. Influence of clinically invisible, but optical coherence tomography detected, optic disc margin anatomy on neuroretinal rim evaluation. Invest Ophthalmol Vis Sci. 2012;53:1852–60. doi: 10.1167/iovs.11-9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen J, Rothman A, Gonzalez N, Avornu A, Ogbuokiri E, Balcer LJ, et al. Macular ganglion cell and inner plexiform layer thickness is more strongly associated with visual function in multiple sclerosis than bruch membrane opening-minimum rim width or peripapillary retinal nerve fiber layer thicknesses. J Neuroophthalmol. 2019;39:444–50. doi: 10.1097/WNO.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walter SD, Ishikawa H, Galetta KM, Sakai RE, Feller DJ, Henderson SB, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119:1250–7. doi: 10.1016/j.ophtha.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ashtari F, Ataei A, Kafieh R, Khodabandeh Z, Barzegar M, Raei M, et al. Optical coherence tomography in neuromyelitis optica spectrum disorder and multiple sclerosis: a population-based study. Mult Scler Relat Disord. 2021;47:102625. doi: 10.1016/j.msard.2020.102625. [DOI] [PubMed] [Google Scholar]
- 71.Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135:1786–93.. doi: 10.1093/brain/aws098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saidha S, Sotirchos ES, Ibrahim MA, Crainiceanu CM, Gelfand JM, Sepah YJ, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11:963–72.. doi: 10.1016/S1474-4422(12)70213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kessel L, Hamann S, Wegener M, Tong J, Fraser CL. Microcystic macular oedema in optic neuropathy: case series and literature review. Clin Exp Ophthalmol. 2018;46:1075–86. doi: 10.1111/ceo.13327. [DOI] [PubMed] [Google Scholar]
- 74.Abegg M, Dysli M, Wolf S, Kowal J, Dufour P, Zinkernagel M. Microcystic macular edema: retrograde maculopathy caused by optic neuropathy. Ophthalmology. 2014;121:142–9. doi: 10.1016/j.ophtha.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 75.Barboni P, Carelli V, Savini G, Carbonelli M, La Morgia C, Sadun AA. Microcystic macular degeneration from optic neuropathy: not inflammatory, not trans-synaptic degeneration. Brain. 2013;136:e239. doi: 10.1093/brain/awt014. [DOI] [PubMed] [Google Scholar]
- 76.Lujan BJ, Horton JC. Microcysts in the inner nuclear layer from optic atrophy are caused by retrograde trans-synaptic degeneration combined with vitreous traction on the retinal surface. Brain. 2013;136:e260. doi: 10.1093/brain/awt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brandt AU, Oberwahrenbrock T, Kadas EM, Lagrèze WA, Paul F. Dynamic formation of macular microcysts independent of vitreous traction changes. Neurology. 2014;83:73. doi: 10.1212/WNL.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 78.Garcia-Martin E, Jarauta L, Pablo LE, Bambo MP, Ara JR, Martin J, et al. Changes in peripapillary choroidal thickness in patients with multiple sclerosis. Acta Ophthalmol. 2019;97:e77–e83. doi: 10.1111/aos.13807. [DOI] [PubMed] [Google Scholar]
- 79.Barreiro-González A, Sanz MT, Carratalà-Boscà S, Pérez-Miralles F, Alcalá C, Carreres-Polo J, et al. Magnetic resonance imaging and optical coherence tomography correlations in multiple sclerosis beyond anatomical landmarks. J Neurol Sci. 2020;419:117180. doi: 10.1016/j.jns.2020.117180. [DOI] [PubMed] [Google Scholar]
- 80.Alonso R, Gonzalez-Moron D, Garcea O. Optical coherence tomography as a biomarker of neurodegeneration in multiple sclerosis: a review. Mult Scler Relat Disord. 2018;22:77–82. doi: 10.1016/j.msard.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Weinshenker BG, Wingerchuk DM. Neuromyelitis spectrum disorders. Mayo Clin Proc. 2017;92:663–79. doi: 10.1016/j.mayocp.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 82.Jarius S, Wildemann B, Paul F. Neuromyelitis optica: clinical features, immunopathogenesis and treatment. Clin Exp Immunol. 2014;176:149–64. doi: 10.1111/cei.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, Ford ET, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2011;135:521–33. doi: 10.1093/brain/awr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fernandes DB, Raza AS, Nogueira RGF, Wang D, Callegaro D, Hood DC, et al. Evaluation of inner retinal layers in patients with multiple sclerosis or neuromyelitis optica using optical coherence tomography. Ophthalmology. 2013;120:387–94. doi: 10.1016/j.ophtha.2012.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peng A, Qiu X, Zhang L, Zhu X, He S, Lai W, et al. Evaluation of the retinal nerve fiber layer in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. J Neurol Sci. 2017;383:108–13. doi: 10.1016/j.jns.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 86.Akaishi T, Kaneko K, Himori N, Takeshita T, Takahashi T, Nakazawa T, et al. Subclinical retinal atrophy in the unaffected fellow eyes of multiple sclerosis and neuromyelitis optica. J Neuroimmunol. 2017;313:10–5. doi: 10.1016/j.jneuroim.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Gelfand JM, Cree BA, Nolan R, Arnow S, Green AJ. Microcystic inner nuclear layer abnormalities and neuromyelitis optica. JAMA Neurol. 2013;70:629–33. doi: 10.1001/jamaneurol.2013.1832. [DOI] [PubMed] [Google Scholar]
- 88.Oertel FC, Kuchling J, Zimmermann H, Chien C, Schmidt F, Knier B, et al. Microstructural visual system changes in AQP4-antibody-seropositive NMOSD. Neurol Neuroimmunol Neuroinflamm. 2017;4:e334. doi: 10.1212/NXI.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwapong WR, Peng C, He Z, Zhuang X, Shen M, Lu F. Altered macular microvasculature in neuromyelitis optica spectrum disorders. Am J Ophthalmol. 2018;192:47–55. doi: 10.1016/j.ajo.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 90.Yap TE, Balendra SI, Almonte MT, Cordeiro MF. Retinal correlates of neurological disorders. Ther Adv Chronic Dis. 2019;10:2040622319882205. doi: 10.1177/2040622319882205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee GI, Park KA, Oh SY, Min JH, Kim BJ. Differential patterns of parafoveal and peripapillary vessel density in multiple sclerosis and neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. 2021;49:102780. doi: 10.1016/j.msard.2021.102780. [DOI] [PubMed] [Google Scholar]
- 92.Monteiro ML, Fernandes DB, Apóstolos-Pereira SL, Callegaro D. Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:3959–66. doi: 10.1167/iovs.11-9324. [DOI] [PubMed] [Google Scholar]
- 93.Ratchford JN, Quigg ME, Conger A, Frohman T, Frohman E, Balcer LJ, et al. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009;73:302–8. doi: 10.1212/WNL.0b013e3181af78b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dulz S, Atiskova Y, Wibbeler E, Wildner J, Wagenfeld L, Schwering C, et al. An ophthalmic rating scale to assess ocular involvement in juvenile CLN3 disease. Am J Ophthalmol. 2020;220:64–71. doi: 10.1016/j.ajo.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 95.Spina Tensini F, Sato MT, Shiokawa N, Ashizawa T, Teive HAG. A comparative optical coherence tomography study of spinocerebellar ataxia types 3 and 10. Cerebellum. 2017;16:797–801. doi: 10.1007/s12311-017-0856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Havla J, Moser M, Sztatecsny C, Lotz-Havla AS, Maier EM, Hizli B, et al. Retinal axonal degeneration in Niemann-Pick type C disease. J Neurol. 2020;267:2070–82.. doi: 10.1007/s00415-020-09796-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pineda M, Perez-Poyato MS, O’Callaghan M, Vilaseca MA, Pocovi M, Domingo R, et al. Clinical experience with miglustat therapy in pediatric patients with Niemann-Pick disease type C: a case series. Mol Genet Metab. 2010;99:358–66. doi: 10.1016/j.ymgme.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 98.Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia. Neurology. 2006;66:1717. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- 99.Jensen K, Beylergil SB, Shaikh AG. Slow saccades in cerebellar disease. Cerebellum Ataxias. 2019;6:1. doi: 10.1186/s40673-018-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blokhuis C, Demirkaya N, Cohen S, Wit FWNM, Scherpbier HJ, Reiss P, et al. The eye as a window to the brain: neuroretinal thickness is associated with microstructural white matter injury in HIV-infected children. Investigative Ophthalmol Vis Sci. 2016;57:3864–71. doi: 10.1167/iovs.16-19716. [DOI] [PubMed] [Google Scholar]
- 101.Meyer-Moock S, Feng Y-S, Maeurer M, Dippel F-W, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14:58. doi: 10.1186/1471-2377-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ashizawa T, Öz G, Paulson HL. Spinocerebellar ataxias: prospects and challenges for therapy development. Nat Rev Neurol. 2018;14:590–605. doi: 10.1038/s41582-018-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mastrangelo M. Clinical approach to neurodegenerative disorders in childhood: an updated overview. Acta neurologica Belgica. 2019;119:511–21. doi: 10.1007/s13760-019-01160-0. [DOI] [PubMed] [Google Scholar]
- 104.Chan VTT, Sun Z, Tang S, Chen LJ, Wong A, Tham CC, et al. Spectral-domain OCT measurements in alzheimer’s disease: a systematic review and meta-analysis. Ophthalmology. 2019;126:497–510. doi: 10.1016/j.ophtha.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54:S204–17. doi: 10.1016/j.neuroimage.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koronyo Y, Biggs D, Barron E, Boyer DS, Pearlman JA, Au WJ, et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight. 2017;2:e93621. [DOI] [PMC free article] [PubMed]
- 107.Scharre DW. Preclinical, prodromal, and dementia stages of alzheimer’s disease. Pract Neurol. 2019.
- 108.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N. Engl J Med. 1986;315:485–7. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 109.Cheung CY, Chan VTT, Mok VC, Chen C, Wong TY. Potential retinal biomarkers for dementia: what is new? Curr Opin Neurol. 2019;32:82–91. doi: 10.1097/WCO.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 110.Alber J, Goldfarb D, Thompson LI, Arthur E, Hernandez K, Cheng D, et al. Developing retinal biomarkers for the earliest stages of Alzheimer’s disease: What we know, what we don’t, and how to move forward. Alzheimers Dement. 2020;16:229–43. doi: 10.1002/alz.12006. [DOI] [PubMed] [Google Scholar]
- 111.den Haan J, Verbraak FD, Visser PJ, Bouwman FH. Retinal thickness in Alzheimer’s disease: A systematic review and meta-analysis. Alzheimers Dement (Amst) 2017;6:162–70. doi: 10.1016/j.dadm.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cheung CY, Ong YT, Hilal S, Ikram MK, Low S, Ong YL, et al. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2015;45:45–56. doi: 10.3233/JAD-141659. [DOI] [PubMed] [Google Scholar]
- 113.Trebbastoni A, Marcelli M, Mallone F, D’Antonio F, Imbriano L, Campanelli A, et al. Attenuation of Choroidal Thickness in Patients With Alzheimer Disease: Evidence From an Italian Prospective Study. Alzheimer Dis Assoc Disord. 2017;31:128–34. doi: 10.1097/WAD.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 114.Bulut M, Yaman A, Erol MK, Kurtuluş F, Toslak D, Doğan B, et al. Choroidal Thickness in Patients with Mild Cognitive Impairment and Alzheimer’s Type Dementia. J Ophthalmol. 2016;2016:7291257. doi: 10.1155/2016/7291257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rifai OM, McGrory S, Robbins CB, Grewal DS, Liu A, Fekrat S, et al. The application of optical coherence tomography angiography in Alzheimer’s disease: A systematic review. Alzheimers Dement (Amst) 2021;13:e12149. doi: 10.1002/dad2.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harnois C, Di Paolo T. Decreased dopamine in the retinas of patients with Parkinson’s disease. Invest Ophthalmol Vis Sci. 1990;31:2473–5. [PubMed] [Google Scholar]
- 117.Chhablani PP, Ambiya V, Nair AG, Bondalapati S, Chhablani J. Retinal Findings on OCT in Systemic Conditions. Semin Ophthalmol. 2018;33:525–46. doi: 10.1080/08820538.2017.1332233. [DOI] [PubMed] [Google Scholar]
- 118.Bodis-Wollner I, Kozlowski PB, Glazman S, Miri S. α-synuclein in the inner retina in parkinson disease. Ann Neurol. 2014;75:964–6. doi: 10.1002/ana.24182. [DOI] [PubMed] [Google Scholar]
- 119.Chrysou A, Jansonius NM, van Laar T. Retinal layers in Parkinson’s disease: A meta-analysis of spectral-domain optical coherence tomography studies. Parkinsonism Relat Disord. 2019;64:40–9. doi: 10.1016/j.parkreldis.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 120.Huang J, Wang Q, Li K, Zhang Q, Xu G, Liu J, et al. Spectral domain OCT can differentiate the retinal morphological changes of patients with Parkinson’s disease in clinical middle stages. Neurol Sci. 2020;41:1909–12. doi: 10.1007/s10072-020-04266-z. [DOI] [PubMed] [Google Scholar]
- 121.Bayer AU, Keller ON, Ferrari F, Maag KP. Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer’s disease and Parkinson’s disease. Am J Ophthalmol. 2002;133:135–7. doi: 10.1016/S0002-9394(01)01196-5. [DOI] [PubMed] [Google Scholar]
- 122.La Morgia C, Barboni P, Rizzo G, Carbonelli M, Savini G, Scaglione C, et al. Loss of temporal retinal nerve fibers in Parkinson disease: a mitochondrial pattern? Eur J Neurol. 2013;20:198–201. doi: 10.1111/j.1468-1331.2012.03701.x. [DOI] [PubMed] [Google Scholar]
- 123.Moschos MM, Chatziralli IP. Evaluation of Choroidal and Retinal Thickness Changes in Parkinson’s Disease Using Spectral Domain Optical Coherence Tomography. Semin Ophthalmol. 2018;33:494–7. doi: 10.1080/08820538.2017.1307423. [DOI] [PubMed] [Google Scholar]
- 124.Kwapong WR, Ye H, Peng C, Zhuang X, Wang J, Shen M, et al. Retinal Microvascular Impairment in the Early Stages of Parkinson’s Disease. Invest Ophthalmol Vis Sci. 2018;59:4115–22. doi: 10.1167/iovs.17-23230. [DOI] [PubMed] [Google Scholar]
- 125.Murueta-Goyena A, Del Pino R, Reyero P, Galdós M, Arana B, Lucas-Jiménez O, et al. Parafoveal thinning of inner retina is associated with visual dysfunction in Lewy body diseases. Mov Disord. 2019;34:1315–24. doi: 10.1002/mds.27728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cerveró A, Casado A, Riancho J. Retinal changes in amyotrophic lateral sclerosis: looking at the disease through a new window. J Neurol. 2021;268:2083–9. doi: 10.1007/s00415-019-09654-w. [DOI] [PubMed] [Google Scholar]
- 127.Kersten HM, Danesh-Meyer HV, Kilfoyle DH, Roxburgh RH. Optical coherence tomography findings in Huntington’s disease: a potential biomarker of disease progression. J Neurol. 2015;262:2457–65.. doi: 10.1007/s00415-015-7869-2. [DOI] [PubMed] [Google Scholar]
- 128.Nouri MN, Yeh EA. Neuroinflammatory and demyelinating disorders of childhood. Clin Child Neurol. 2020:651–77.
- 129.Malani Shukla N, Lotze TE, Muscal E. Inflammatory diseases of the central nervous system. Neurol Clin. 2021;39:811–28. doi: 10.1016/j.ncl.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 130.Twilt M, Benseler SM. Childhood inflammatory brain diseases: pathogenesis, diagnosis and therapy. Rheumatol. 2014;53:1359–68. doi: 10.1093/rheumatology/ket398. [DOI] [PubMed] [Google Scholar]
- 131.Balcer LJ, Raynowska J, Nolan R, Galetta SL, Kapoor R, Benedict R, et al. Validity of low-contrast letter acuity as a visual performance outcome measure for multiple sclerosis. Mult Scler. 2017;23:734–47. doi: 10.1177/1352458517690822. [DOI] [PMC free article] [PubMed] [Google Scholar]