Abstract
A murine model of intratracheally induced histoplasmosis in immunocompromised B6C3F1 mice was used to evaluate a new triazole antifungal agent, posaconazole. This compound was previously shown to be comparable to amphotericin B and superior to itraconazole for the treatment of histoplasmosis in immunocompetent mice. The current study used mice that were depleted of T lymphocytes by intraperitoneal injection of anti-CD4 and anti-CD8 monoclonal antibodies beginning 2 days before infection and continuing at 5-day intervals until completion of the study. Groups of B6C3F1 mice that were depleted of CD4 and CD8 T cells were infected with an inoculum of 104 Histoplasma capsulatum yeasts. All mice receiving posaconazole at 1 or 0.1 mg/kg of body weight/day, amphotericin B at 2 mg/kg every other day (qod), or itraconazole at 75 mg/kg/day survived to day 29. Only 60% of mice receiving itraconazole at 10 mg/kg/day and none receiving amphotericin B at 0.2 mg/kg qod survived to that date. Fungal burdens were determined at day 14 of infection, 1 day after discontinuation of therapy. Quantitative colony counts and Histoplasma antigen levels in lung and spleen tissues declined following treatment with amphotericin B at 2 mg/kg qod, posaconazole at 5 and 1 mg/kg/day, and itraconazole at 75 mg/kg/day but not in mice treated with amphotericin B at 0.2 mg/kg qod or itraconazole at 10 mg/kg/day. Posaconazole at 0.1 mg/kg/day reduced fungal colony counts and antigen levels in spleens but not in lungs. This study shows posaconazole activity for the treatment of histoplasmosis in immunosuppressed animals.
Histoplasmosis is an important cause of progressive infection in immunocompromised individuals, including those with AIDS. In persons with AIDS, the course of disseminated histoplasmosis often is more severe than and the response to treatment is inferior (23, 24) to that in nonimmunocompromised individuals or those with other immunosuppressive disorders (19). Eighty-five percent of cases in patients with AIDS have occurred in those with CD4 cell counts below 200/μl (13, 20, 22), suggesting that CD4 cells play an important role in defense against Histoplasma capsulatum. Recent studies confirm the association of CD4 cell reduction with increased risk for disseminated histoplasmosis in persons with human immunodeficiency virus infection; case rates were 4.6 times higher in persons with CD4 cell counts below 150/μl than in those with higher counts (18). CD8 cell counts were below 200/μl in 35% of cases in AIDS patients enrolled in clinical trials evaluating the treatment of histoplasmosis (L. J. Wheat, unpublished data).
We have shown that dual depletion of CD4 and CD8 cells markedly alters the course of experimental histoplasmosis following pulmonary challenge (6; C. Schnizlein-Bick, M. Durkin, P. Connolly, S. Kohler, and J. Wheat, Program Abstr. 34th Annu. Meet. Infect. Dis. Soc. Am., abstr. 207, p. 74, 1996). While nonimmunosuppressed mice recovered uneventfully following intratracheal inoculation with 103 yeasts, CD4- and CD8-depleted mice experienced 100% mortality by day 28 of the infection. In an earlier study, posaconazole was superior to itraconazole and equivalent to amphotericin B for the treatment of intratracheally induced histoplasmosis in immunocompetent mice (3). The purpose of this project was to determine the effectiveness of posaconazole in the CD4- and CD8-depleted mouse model.
MATERIALS AND METHODS
Immunodepletion of CD4 and CD8 T lymphocytes.
Antibodies used for CD4 and CD8 cell depletion were from cell lines purchased from the American Type Culture Collection (ATCC) and were administered intraperitoneally as clarified ascitic fluid (14) every 5 days at a concentration that maintained CD4 and CD8 cell levels below 1% for at least 2 weeks. Ascitic fluid was clarified by centrifugation at 500 × g for 10 min and then filtration through a 0.45-μm-pore-diameter filter. Aliquots were stored at −40°C until needed. Clone 2.43, rat anti-mouse Lyt 2.2 (ATCC TIB-210), was used to deplete mice of CD8 lymphocytes, and clone GK1.5, rat anti-mouse L3T4 (ATCC TIB-207), was used to deplete mice of CD4 lymphocytes.
Preparation of H. capsulatum yeasts.
The yeast phase of a single clinical isolate of H. capsulatum, IU-CT, which is maintained in the Histoplasmosis Reference Laboratory specifically for antifungal and immune modulation studies, was one of a panel of isolates tested for susceptibility in vitro to several antifungal agents. For this isolate, the MIC was determined to be 0.0095 μg/ml with posaconazole, 0.5 μg/ml with amphotericin B, and 0.002 μg/ml with itraconazole (3). This isolate was grown in HMM medium (26) in a 37°C incubator with shaking at 150 rpm for 48 h. The yeast culture was centrifuged and washed with Hanks' balanced salt solution–20 mM HEPES. The inoculum cell count was determined using a hemacytometer and adjusted to 104 yeasts in 25 μl.
Mouse inoculation.
Six-week-old B6C3F1 mice (Harlan Sprague-Dawley) were anesthetized with 4.5% halothane at an oxygen flow rate of 0.9 liter/m. A 20-gauge, 1.25-in. Angiocath (Becton Dickinson) was passed through the mouth into the trachea, and 25 μl of the H. capsulatum inoculum was administered (7, 17).
Survival experiment.
Treatment began 4 days after infection with 104 yeasts and continued for 10 days. Mice received amphotericin B (Fungizone) at 2 or 0.2 mg/kg of body weight intraperitoneally every other day (qod). Itraconazole (10-mg/ml oral solution in hydroxypropyl-β-cyclodextrin; gift of Janssen Pharmaceutica, Beerse, Belgium) was given once daily by gavage at 75 or 10 mg/kg/day. Posaconazole (Schering-Plough Research Institute) was suspended in 0.4% methylcellulose (viscosity of a 2% aqueous solution at 25°C: 4,000 cP) and given by gavage at 1 or 0.1 mg/kg/day. When this study began, no trials had yet been conducted with humans; therefore, the dosages were selected based on pharmacokinetic and efficacy results from an earlier study with mice (3) and data from studies done by the sponsor. Subsequent human studies showed that a dose of 50 mg twice a day resulted in a maximum concentration similar to that in mice given 1 mg/kg once daily: 0.46 and 0.54 μg/ml, respectively. Clinical trials with humans have used doses of up to 400 mg twice daily, yielding peak blood drug levels of over 4 μg/ml. Control mice were treated with 0.4% methylcellulose alone. Mice were kept for 29 days, at which time survivors were sacrificed and fungal burden in the lungs and spleen was determined by measurement of antigen concentrations and quantitative culturing of tissue homogenates. A repeat survival experiment was conducted to assess reproducibility.
Fungal burden experiment.
Antigen levels and quantitative colony counts in animals that survived in the above experiment were measured at days 14 and 29. For the day-14 fungal burden experiment, the dosages used were the same as in the survival experiment, with the exception of the addition of a group treated with posaconazole at 5 mg/kg/day. Approximately 24 h after the last dose of antifungal drug, mice were sacrificed and lungs and spleen were removed aseptically. Organs were weighed and ground in Ten Broeck tissue grinders containing 2.0 ml of RPMI 1640 medium. Homogenates were diluted and plated on brain heart infusion agar containing 10% sheep blood. Plates were incubated for 10 days at 30°C, and colony counts were determined.
Of the 2.0 ml of undiluted organ homogenates, 0.1 ml (1/20) was cultured, representing a detection limit of 20 CFU/organ; therefore, plate counts were multiplied by 20 to obtain the counts for the entire organ. Quantitative culture data were expressed as CFU per gram by dividing the CFU per organ by the organ weights, ranging from about 0.159 to 0.328 g for lungs and about 0.077 to 0.245 g for spleens in treated and untreated mice, respectively. Histoplasma antigen in organ homogenates was measured by an enzyme immunoassay (EIA) (6). The organ homogenates were diluted (1:10 for spleen and 1:100 for lung) to yield results falling within the working range of the assay. The EIA results were obtained by dividing the mean value obtained for each organ by 1.5 times the mean value of the negative controls. Results of ≥1.0 were considered positive.
Statistical analysis.
A one-way analysis of variance was performed on the ranks of the antigen levels and colony counts (4). This nonparametric technique was chosen because of the heterogeneity of variances among the treatment groups. Pairwise comparisons of each treatment group to the control group were adjusted using Dunnett's multiple-comparison procedure. Comparisons of the therapeutic dose of posaconazole (1.0 mg/kg/day) to the therapeutic doses of amphotericin B (2 mg/kg qod) and itraconazole (10 mg/kg/day) were adjusted using Sidak's multiple-comparison method. A two-way factorial analysis of variance was performed on the ranks of the antigen levels and quantitative colony counts to compare the outcomes of the fungal burden experiment to those of the survival study. Pairwise comparisons of day 14 to day 29 within a treatment group were adjusted using Sidak's method. Survival times among the treatment groups were compared using Wilcoxon's test for survival analysis. An overall significance level of 0.05 was used to test all hypotheses.
RESULTS
Survival.
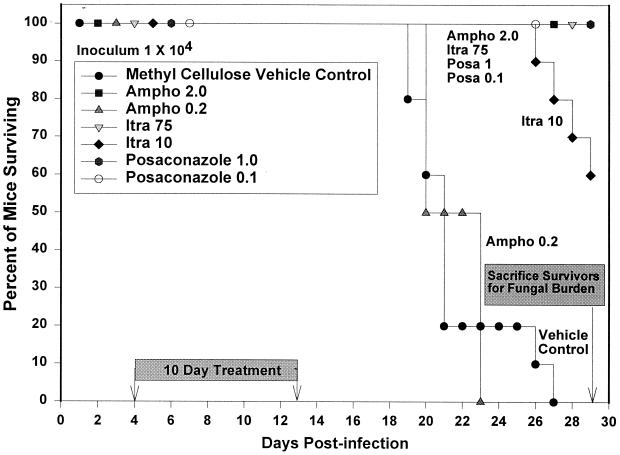
All mice receiving posaconazole at 1 and 0.1 mg/kg/day, itraconazole at 75 mg/kg/day, and amphotericin B at 2 mg/kg qod survived to day 29 (Fig. 1). At day 26, mice receiving itraconazole at 10 mg/kg/day began to die, and by the day of sacrifice (day 29), 40% had died. Mice receiving vehicle alone began to die at day 19, and mice receiving amphotericin B at 0.2 mg/kg qod began to die at day 20; no mice in either of these groups survived past day 27. Wilcoxon's test for survival analysis showed that there was a significant difference (P < 0.0001) among the survival curves. A second survival experiment to establish reproducibility had very similar results; all control and amphotericin B (0.2 mg/kg qod)-treated animals died before day 29. All mice treated with posaconazole at 1 mg/kg/day, itraconazole at 75 mg/kg/day, and amphotericin B at 2 mg/kg qod survived. Animals treated with itraconazole at 10 mg/kg/day showed similar results, with 38% dying between days 27 and 29. One mouse in the second survival experiment in the treatment group receiving posaconazole at 0.1 mg/kg/day died on the last day of the experiment, day 29.
FIG. 1.
Survival following intratracheal infection with 104 H. capsulatum yeasts. Therapy was given from day 4 to day 13, and anti-CD4 and anti-CD8 monoclonal antibodies were given at 5-day intervals beginning 2 days before infection. The control group received daily gavage with the methylcellulose vehicle used to dilute posaconazole. There were 10 animals in each group. Survivors were sacrificed at day 29. Ampho, amphotericin B; Itra, itraconazole; Posa, posaconazole.
Fungal burden at day 29.
Quantitative colony counts and antigen concentrations were measured in spleen and lung tissue homogenates of the mice that survived to day 29 in the first survival experiment (Table 1). Treatment with posaconazole at 1 mg/kg/day resulted in the lowest burden in comparison to those in groups treated with itraconazole and amphotericin B (P was <0.0001 for all measurements).
TABLE 1.
Quantitative culture at day 14 versus day 29
| Drug (dose)a | Median (range) CFU/g inb:
|
|||||
|---|---|---|---|---|---|---|
| Lungs
|
Spleen
|
|||||
| Day 14 | Day 29 | P | Day 14 | Day 29 | P | |
| Amphotericin B (2) | 1.26 × 103* (1.31 × 102–4.18 × 105) | 9.14 × 105 (9.85 × 104–2.40 × 106) | 0.0001 | 0* (0–5.88 × 102) | 5.75 × 106 (2.70 × 104–2.47 × 107) | 0.0001 |
| Amphotericin B (0.2) | 1.14 × 107 (5.33 × 106–1.47 × 107) | All died | 4.47 × 107 (1.42 × 107–6.78 × 107) | All died | ||
| Itraconazole (75) | 0* (0–3.23 × 102) | 8.19 × 105 (2.18 × 105–3.27 × 106) | 0.0001 | 0* (0–0) | 2.29 × 106 (7.00 × 105–5.36 × 106) | 0.0001 |
| Itraconazole (10) | 1.38 × 107 (6.52 × 106–2.12 × 107) | 8.06 × 108 (1.09 × 106–2.18 × 109) | 0.2953 | 1.15 × 107* (6.90 × 105–2.71 × 107) | 1.75 × 109 (1.66 × 107–2.96 × 109) | 0.0001 |
| Posaconazole (5) | 0* (0–1.41 × 102) | Not done | 0* (0–4.88 × 102) | Not done | ||
| Posaconazole (1) | 0* (0–2.11 × 102) | 2.25 × 104 (3.08 × 103–1.22 × 105) | 0.0001 | 0* (0–1.77 × 102) | 1.41 × 105 (8.55 × 103–2.77 × 105) | 0.0001 |
| Posaconazole (0.1) | 4.64 × 107 (1.21 × 107–1.65 × 108) | 2.03 × 106 (1.01 × 106–6.15 × 106) | 0.0001 | 3.02 × 106* (1.25 × 106–7.50 × 106 | 1.65 × 107 (2.99 × 106–3.29 × 107) | 0.0001 |
| None (control) | 1.13 × 107 (3.25 × 106–3.61 × 107) | All died | 4.25 × 107 (2.11 × 107–6.86 × 107) | All died | ||
Given in milligrams per kilogram per day for all drugs except amphotericin B, which was given in milligrams per kilogram qod.
Each group contained 7 to 10 animals. P values are for comparisons of day-14 and day-29 fungal burden data. Asterisks indicate significant differences (P < 0.0001) between the drug-treated animals and the vehicle-treated control animals. Comparisons were adjusted using Dunnett's multiple-comparison procedure.
Fungal burden at day 14.
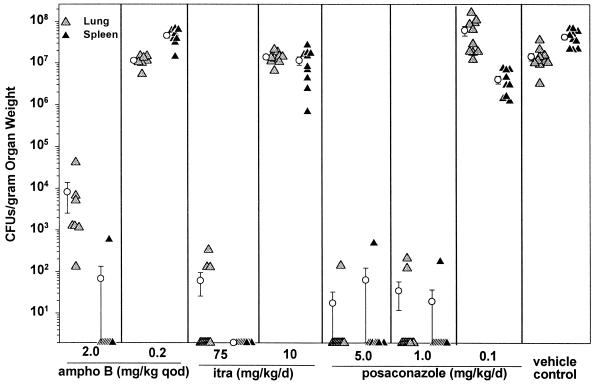
The mice treated with amphotericin B at 2 mg/kg qod, itraconazole at 75 mg/kg/day, or posaconazole at 5 or 1 mg/kg/day had reduced fungal burdens in the lungs and spleen at day 14 compared to vehicle-treated control mice (Fig. 2 and 3). The group receiving posaconazole at 5 mg/kg/day was added because the animals given 1 mg/kg/day had demonstrated a high fungal burden at day 29. In contrast to the results of the survival experiment at day 29, the numbers of CFU at day 14 were markedly suppressed compared to those in the vehicle-treated controls in the following groups, for lungs and spleen, as measured by antigen levels and colony counts: posaconazole at 5 mg/kg/day (P < 0.0001), posaconazole at 1 mg/kg/day (P < 0.0001), amphotericin B at 2 mg/kg qod (P < 0.0001), and itraconazole at 75 mg/kg/day (P < 0.0001) (Table 1). Amphotericin B at 0.2 mg/kg qod was not effective at reducing antigen levels in the lungs or spleen, while itraconazole at 10 mg/kg/day and posaconazole at 0.1 mg/kg/day had no effect in the lungs. Similar results were observed in a second experiment (data not shown).
FIG. 2.
Quantitative culture results for lung and spleen tissues at day 14, 24 h after the last dose of therapy. There were 7 to 10 mice in each study group. The minimum detection limit was 20 CFU/organ, representing about 60 to 260 CFU/g of tissue. Results of 0 indicate no growth in undiluted organ homogenates. ampho B, amphotericin B; itra, itraconazole.
FIG. 3.
Quantitative Histoplasma antigen levels in lung and spleen tissues of mice sacrificed on day 14, 24 h after the last dose of therapy. Antigen levels in homogenized tissues were measured by an EIA; results above 1.0 were considered positive. Lung homogenates were diluted 1:100, and spleen homogenates were diluted 1:10. There were 7 to 10 mice in each study group. ampho B, amphotericin B; itra, itraconazole.
In comparisons between treatment groups, posaconazole at 1 mg/kg/day significantly reduced CFU in the lung compared to amphotericin B at 2 mg/kg qod (P < 0.0001). Other comparisons between posaconazole at 1 mg/kg/day and amphotericin B at 2 mg/kg qod or itraconazole at 75 mg/kg/day in terms of antigen levels or colony counts were not significant.
Fungal burdens at day 14 versus day 29.
Of note, quantitative colony counts at day 29 were significantly higher than those at day 14 in the lungs and spleen in the following groups: posaconazole at 1 mg/kg/day, posaconazole at 0.1 mg/kg/day, amphotericin B at 2 mg/kg qod, itraconazole at 75 mg/kg/day, and itraconazole at 10 mg/kg/day (in the spleen only). (Table 1). Results of antigen testing paralleled those of culturing, also showing a rebound in antigen levels at day 29 compared to day 14 (data not shown).
DISCUSSION
This murine model of intratracheally induced histoplasmosis resembles natural infection following inhalation exposure. Animals develop a diffuse pulmonary infection followed by hematogenous dissemination to the liver and spleen (3, 7). The severity of the infection is determined by the size of the inoculum and the immune status of the host.
Immunosuppression by a variety of means has been shown to worsen the outcome of infection with H. capsulatum both in humans (19, 23, 24) and in animals. Immunosuppressed animal models have been established by administration of corticosteroids (15), cyclophosphamide (5, 15, 16), or antibodies to lymphocytes (1, 9), CD4 cells (8), interleukin 12 (27, 28), or gamma interferon (2, 27, 28). In addition, homozygous nude mice (10–12, 25), SCID mice (28), or gamma interferon knockout mice (2) have been used to create immunosuppressed animal models.
CD4 and CD8 depletion was chosen for this study to create an immunosuppressed state resembling that seen in persons with advanced AIDS. The level of CD4 and CD8 depletion used in this model may have been greater than that experienced in some AIDS patients with disseminated histoplasmosis, however, since CD4 counts may be above 200/μl in up to 15% of patients and CD8 counts are not uniformly or greatly suppressed (13, 20–22). In our model, CD4 depletion alone increased mortality and fungal burden but not to the extent observed with both CD4 and CD8 depletion (Schnizlein-Bick et al., Abstr. 34th Annu. Meet. Infect. Dis. Soc. Am.). The CD4- and CD8-depleted model was chosen to provide the most extreme challenge by which to evaluate antifungal therapy.
Posaconazole previously showed activity comparable to that of amphotericin B for the treatment of histoplasmosis in immunocompetent animals (3). In the current study, it was shown to be highly effective for the treatment of CD4- and CD8-depleted animals with disseminated histoplasmosis. Itraconazole at 10 mg/kg/day prolonged survival but failed to prevent deaths, which occurred in 40% of animals during week 4 of infection. Deaths in that group likely would have continued had the animals not been sacrificed at day 29. Posaconazole at 1.0 and 0.1 mg/kg/day was as effective as itraconazole at 75 mg/kg/day and amphotericin B at 2 mg/kg qod in preventing death. Amphotericin B was not effective when used at 0.2 mg/kg qod.
Posaconazole was also highly effective in reducing the fungal burden in tissues. Posaconazole at as low as 1 mg/kg/day markedly reduced fungal burden and sterilized lung and spleen tissues in 70% of mice sacrificed on day 14 of infection. Posaconazole at 0.1 mg/kg/day reduced fungal burden by 1 log unit in the spleen, while the results for the lungs were inconsistent. Itraconazole at 75 mg/kg/day also sterilized 70% of lung and spleen tissues but, at 10 mg/kg/day, failed to sterilize lung or spleen tissues from any animals. Amphotericin B at 2 mg/kg qod reduced fungal burden in comparison to that in untreated control animals but failed to sterilize lung tissues in any animals.
An interesting discrepancy was noted between the survival and fungal burden experiments. While posaconazole at 1 or 0.1 mg/kg/day, amphotericin B at 2 mg/kg qod, and itraconazole at 75 mg/kg/day all prevented death over the 29 days of observation and, except for posaconazole at 0.1 mg/kg/day, greatly reduced fungal burden at day 14, fungal burden at day 29 was high. At day 14, sterile cultures were observed in at least 80% of mice treated with the higher doses of amphotericin B, itraconazole, or posaconazole. No cultures in any group were sterile at day 29. Similar findings were reported for SCID mice treated with amphotericin B (28). Statistical comparisons of day-14 and day-29 data involved comparisons of groups that were infected at different times. Nevertheless, the differences observed between days 14 and 29 were sufficiently great to suggest that growth was suppressed only at day 14, despite negative culture results for the majority of animals, and that the infection recrudesced after discontinuation of therapy. These data suggest that a 2-week course of treatment is not curative for immunosuppressed animals. Our earlier report with immunocompetent animals did not show fungal burden rebound after discontinuation of therapy (3). These findings are consistent with the experience that treatment is usually curative in nonimmunocompromised human patients (19) but not in those with AIDS (23, 24).
In conclusion, posaconazole was at least as effective as amphotericin B and more effective than itraconazole in this model of intratracheally induced histoplasmosis in CD4- and CD8-depleted animals.
ACKNOWLEDGMENTS
This work was supported by a grant from Schering-Plough Research Institute on the basis of a model developed in a project sponsored by the Department of Veterans' Affairs.
REFERENCES
- 1.Adamson D M, Cozad G C. Effect of antilymphocyte serum on animals experimentally infected with Histoplasma capsulatum or Cryptococcus neoformans. J Bacteriol. 1969;100:1271–1276. doi: 10.1128/jb.100.3.1271-1276.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allendoerfer R, Deepe G S., Jr Intrapulmonary response to Histoplasma capsulatum in gamma interferon knockout mice. Infect Immun. 1997;65:2564–2569. doi: 10.1128/iai.65.7.2564-2569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connolly P, Wheat J, Schnizlein-Bick C, Durkin M, Kohler S, Smedema M, Goldberg J, Brizendine E, Loebenberg D. Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice. Antimicrob Agents Chemother. 1999;43:322–328. doi: 10.1128/aac.43.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conover W J, Iman R L. Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat. 1981;35:124–129. [Google Scholar]
- 5.Cozad G C, Lindsey T J. Effect of cyclophosphamide on Histoplasma capsulatum infections in mice. Infect Immun. 1974;9:261–265. doi: 10.1128/iai.9.2.261-265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durkin M M, Connolly P A, Wheat L J. Comparison of radioimmunoassay and enzyme-linked immunoassay methods for detection of Histoplasma capsulatum var. capsulatum antigen. J Clin Microbiol. 1997;35:2252–2255. doi: 10.1128/jcm.35.9.2252-2255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fojtasek M F, Sherman M R, Garringer T, Blair R, Wheat L J, Schnizlein-Bick C T. Local immunity in lung-associated lymph nodes in a murine model of pulmonary histoplasmosis. Infect Immun. 1993;61:4607–4614. doi: 10.1128/iai.61.11.4607-4614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez A M, Bullock W E, Taylor C L, Deepe G S., Jr Role of L3T4+ T cells in host defense against Histoplasma capsulatum. Infect Immun. 1988;56:1685–1691. doi: 10.1128/iai.56.7.1685-1691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodwin R A, Jr, des Prez R M. Histoplasmosis. Am Rev Respir Dis. 1978;117:929–956. doi: 10.1164/arrd.1978.117.5.929. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin R A, Jr, Shapiro J L, Thurman G H, Thurman S S, des Prez R M. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine. 1980;59:1–33. [PubMed] [Google Scholar]
- 11.Graybill J R, Palou E, Ahrens J. Treatment of murine histoplasmosis with UK 49,858 (fluconazole) Am Rev Respir Dis. 1986;134:768–770. doi: 10.1164/arrd.1986.134.4.768. [DOI] [PubMed] [Google Scholar]
- 12.Graybill J R, Williams D M, van Cutsem E, Drutz D J. Combination therapy of experimental histoplasmosis and cryptococcosis with amphotericin B and ketoconazole. Rev Infect Dis. 1980;2:551–558. doi: 10.1093/clinids/2.4.551. [DOI] [PubMed] [Google Scholar]
- 13.Hecht F M, Wheat J, Korzun A H, Hafner R, Skahan K J, Larsen R, Limjoco M T, Simpson M, Schneider D, Keefer M C, Clark R, Lai K K, Jacobson J M, Squires K, Bartlett J A, Powderly W. Itraconazole maintenance treatment for histoplasmosis in AIDS: a prospective, multicenter trial. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:100–107. doi: 10.1097/00042560-199710010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Jennings S R, Bonneau R H, Smith P M, Wolcott R M, Chervenak R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8 positive cytolic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol. 1991;133:234–252. doi: 10.1016/0008-8749(91)90194-g. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi G S, Travis S J, Medoff G. Comparison of fluconazole with amphotericin B in treatment of histoplasmosis in normal and immunosuppressed mice. Rev Infect Dis. 1990;12:s291–s293. doi: 10.1093/clinids/12.supplement_3.s291. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi G S, Travis S J, Rinaldi M G, Medoff G. In vitro and in vivo activities of SCH 39304, fluconazole, and amphotericin B against Histoplasma capsulatum. Antimicrob Agents Chemother. 1990;34:524–528. doi: 10.1128/aac.34.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohler S, Blair R, Schnizlein-Bick C, Fojtasek M, Connolly-Stringfield P, Wheat J. Clearance of Histoplasma capsulatum variety capsulatum antigen is useful for monitoring treatment of experimental histoplasmosis. J Clin Lab Anal. 1994;8:1–3. doi: 10.1002/jcla.1860080102. [DOI] [PubMed] [Google Scholar]
- 18.McKinsey D S, Spiegel R A, Hutwagner L, Stanford J, Driks M R, Brewer J, Gupta M R, Smith D L, O'Connor M C, Dall L. Prospective study of histoplasmosis in patients infected with human immunodeficiency virus: incidence, risk factors, and pathophysiology. Clin Infect Dis. 1997;24:1195–1203. doi: 10.1086/513653. [DOI] [PubMed] [Google Scholar]
- 19.Sathapatayavongs B, Batteiger B E, Wheat L J, Slama T G, Wass J L. Clinical and laboratory features of disseminated histoplasmosis during two large urban outbreaks. Medicine. 1983;62:263–270. doi: 10.1097/00005792-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Wheat J, Hafner R, Korzun A H, Limjoco M T, Spencer P, Larsen R A, Hecht F M, Powderly W AIDS Clinical Trial Group. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Med. 1995;98:336–342. doi: 10.1016/s0002-9343(99)80311-8. [DOI] [PubMed] [Google Scholar]
- 21.Wheat J, Hafner R, Wulfson M, Spencer P, Squires K, Powderly W, Wong B, Rinaldi M, Saag M, Hamill R, Murphy R, Connolly-Stringfield P, Briggs N, Owens S NIAID Clinical Trials & Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1993;118:610–616. doi: 10.7326/0003-4819-118-8-199304150-00006. [DOI] [PubMed] [Google Scholar]
- 22.Wheat J, MaWhinney S, Hafner R, McKinsey D, Chen D F, Korzun A, Skahan K J, Johnson P, Hamill R, Bamberger D, Pappas P, Stansell J, Koletar S, Squires K, Larsen R A, Cheung T, Hyslop N, Lai K K, Schneider D, Kauffman C, Saag M, Dismukes W, Powderly W. Treatment of histoplasmosis with fluconazole in patients with acquired immunodeficiency syndrome. Am J Med. 1997;103:223–232. doi: 10.1016/s0002-9343(97)00151-4. [DOI] [PubMed] [Google Scholar]
- 23.Wheat L. Histoplasmosis in the acquired immunodeficiency syndrome. Curr Top Med Mycol. 1996;7:7–18. [PubMed] [Google Scholar]
- 24.Wheat L J, Connolly-Stringfield P A, Baker R L, Curfman M F, Eads M E, Israel K S, Norris S A, Webb D H, Zeckel M L. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine. 1990;69:361–374. doi: 10.1097/00005792-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Williams D M, Graybill J R, Drutz D J. Histoplasma capsulatum infection in nude mice. Infect Immun. 1978;21:973–977. doi: 10.1128/iai.21.3.973-977.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worsham P L, Goldman W E. Quantitative plating of Histoplasma capsulatum without addition of conditioned medium or siderophores. J Med Vet Mycol. 1988;26:137–143. [PubMed] [Google Scholar]
- 27.Zhou P, Sieve M C, Bennett J, Kwon-Chung K J, Tewari R P, Gazzinelli R T, Sher A, Seder R A. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-gamma. J Immunol. 1995;155:785–795. [PubMed] [Google Scholar]
- 28.Zhou P, Sieve M C, Tewari R P, Seder R A. Interleukin-12 modulates the protective immune response in SCID mice infected with Histoplasma capsulatum. Infect Immun. 1997;65:936–942. doi: 10.1128/iai.65.3.936-942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]