Abstract
Background
Undifferentiated carcinoma of pancreas with osteoclast-like giant cells is an extremely rare tumor in pancreas. It is relatively difficult to have preoperative diagnosis due to the lack of specific tumor markers and pre-operative images.
Methods
In the present study, database of the pancreas center in the First Affiliated Hospital of Nanjing Medical University was retrospectively screened. A total of thirteen cases diagnosed as undifferentiated carcinoma of pancreas with osteoclast-like giant cells were included. Their clinical data and treatments were collected.
Results
Thirteen patients include eight males and five females, and the median age was 67 (60–72) years old. The lesions were found in more than half patients through health examination with no symptoms. NSE was elevated in eight cases (66%). CT scan revealed that cystic and solid lesions often had thick (4/5), contrast-enhanced (5/5) wall with smooth edges (5/5) and the boundary of lesions mainly with solid composition (4/10) is not well demarcated with normal pancreatic parenchyma. All patients received surgical resection. Eight patients had adjuvant chemotherapy and only one patient had adjuvant radiotherapy. The median survival time was 13 months. Five patients had postoperative metastasis or recurrence of tumor and four of them had died of this disease during follow-up.
Conclusion
Our data showed that elevated level of NSE and characteristic pre-operative images might provide aid with the pre-operative diagnosis for undifferentiated carcinoma of pancreas with osteoclast-like giant cells. Patients with suspected diagnosis should receive surgical intervention as soon as possible, supplemented with postoperative chemotherapy, in order to prolong the survival of patients.
Keywords: pancreatic cancer, undifferentiated carcinoma of pancreas, osteoclast-like giant cells, retrospective, pre-operative image
Introduction
Undifferentiated carcinoma of pancreas with osteoclast-like giant cells (UCOGCP) is an extremely rare tumor in pancreas, accounting for less than 1% in all pancreatic tumors. It is considered as a subtype of the pancreatic ductal adenocarcinoma according to the World Health Organization classification.1 The tumor is consisted of undifferentiated mononuclear tumor cells and CD68 and vimentin positive multinucleated osteoclast like giant cells. Previous studies of UCOGCP were mainly based on individual cases and clinicians have limited knowledge of it. Here we present the cases of 13 UCOGCP from our center to help clinicians better understand this rare disease.
Methods
Patients
We screened all patients with UCOGCP confirmed by pathological examination in our hospital from Jan, 2009 to July, 2021. Demographic information, laboratory examination, imaging features and pathological report of patients undergoing surgery in our center were retrospectively collected. Follow up data, including survival status and management of other treatments, were obtained through hospital records or regular telephone interviews with patients. Informed consent was signed at the time of admission. The study was complied with the Declaration of Helsinki and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University.
Surgical Procedures
The surgical procedure was determined by preoperative diagnosis and intraoperative tumor location, including pancreaticoduodenectomy with modified one-layer duct-to-mucosa pancreaticojejunostomy,2 left pancreatectomy with proximal pancreatic remnant oversewn after ligating the main pancreatic duct,3 and central pancreatomy.4 Standard and extended surgical approaches have been developed for resectable and borderline resectable pancreatic cancer.5–7 Heidelberg Triangle Operation was applied in one patient.8
Pathology Examination
The pathological diagnosis was based on the WHO diagnostic criteria. Undifferentiated carcinoma characterized by mononuclear tumor cells with significant nuclear atypia and heterochromatin and multinucleated osteoclast-like giant cells in HE staining are decisive feature for the diagnosis. Positive epithelial markers and osteoclastoid giant cells stained with positive CD68 and vimentin in immunohistochemistry staining are important reference for the diagnosis. TMN staging is based on the 8th edition of AJCC cancer staging manual.9
Complications
Surgical complications, including post-operative pancreatic fistula (POPF), delayed gastric empty (DGE), post-operative hemorrhage (POPH) and chylous leakage were assessed and graded based on criteria established by International Study Group of Pancreatic Surgery (ISGPS).10–13
Statistical Analysis
The Kaplan-Meier method was used for the univariate disease-free survival analysis, and the Log rank test was used to compare the survival time between groups separated by age, serum level of CA19-9, CEA, AFP, NSE, location of tumor, nerve invasion, vascular invasion, margin status and element of ductal adenocarcinoma respectively. All statistical analyses were performed using SPSS 22.0 software.
Results
Clinical Presentation
Sixteen UCOGCP cases with pathological confirmation were screened out from our hospital. Two patients were confirmed by biopsy of liver metastases. One patient was identified by endoscopy and refused surgical treatment. The remaining thirteen patients were treated surgically in our single center. Thirteen patients include eight males and five females, and the median age was 67 (60–72) years (Table 1). Patients’ symptoms were presented in Table 1. The lesions were found through health examination more than half patients with no symptoms. Five patients had epigastric pain or abdominal pain before diagnosis. One patient had jaundice as the first symptom due to the obstruction of bile duct system. Other nonspecific symptoms included poor appetite and weight loss. In addition, cases were concentrated in recent years.
Table 1.
Demographic Information and Laboratory Examination of Patients
| n | ||
|---|---|---|
| Sex | Male | 8 |
| Female | 5 | |
| Year of diagnosis | Before 2018 | 2 |
| 2018-Now | 11 | |
| Age at diagnosis | 67(60–72) | |
| Symptoms at diagnosis | Health examination | 7 |
| Abdominal pain | 5 | |
| Jaundice | 1 | |
| Alb(g/L) | 39.0(35.8–42.1) | |
| Tumor Marker | ||
| CA199(U/mL) | Normal | 8 |
| Abnormal | 4 | |
| Median(IQR) | 34.0(11.5–62.0) | |
| CEA(ng/mL) | Normal | 7 |
| Abnormal | 6 | |
| Median(IQR) | 3.0(2.4–11.8) | |
| AFP(ng/mL) | Normal | 12 |
| Abnormal | 0 | |
| Median(IQR) | 2.0(1.2–2.3) | |
| NSE(ng/mL) | Normal | 2 |
| Abnormal | 8 | |
| Median(IQR) | 23.0(21.5–26.0) |
Abbreviations: CA19-9, normal < 39 U/mL; CEA, normal < 4.7ng/mL; AFP, normal < 20ng/mL; NSE, neuron-specific enolase, normal <16.3ng/mL.
Laboratory Examinations
As shown in Table 1, the average preoperative albumin level was slightly lower than normal. The level of CA19-9 increased in four patients. Six patients had increased level of CEA. All of patients has normal level of AFP. It’s worth noting that NSE was elevated in eight cases.
CECT Characteristics (Table 2)
Table 2.
CECT Characteristics
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Body and tail | Neck | NA | NA | Head | NA | Body and Tail | Body and Tail | Body and Tail | Head | Head | Head | Head |
| Tumor size (cm) | 9.2 | 1.2 | NA | NA | 2.7 | NA | 5.0 | 11.0 | 17.2 | 2.2 | 3.5 | 4.9 | 4.3 |
| Radiological T stage | 3 | 1 | NA | NA | 2 | NA | 3 | 4 | 4 | 2 | 1 | 4 | 3 |
| Cystic or/and solid | Cystic and solid | Solid | NA | NA | Cystic | NA | Cystic and solid | Cystic and solid | Cystic and solid | Solid | Solid | Cystic and solid | Solid |
| Density | Low | Low | NA | NA | Low | NA | Low | Low | Low | Low | Low | Equal and low | Low |
| Calcification | N | N | NA | NA | N | NA | N | N | Y | N | N | N | N |
| Contrast-enhanced status of solid part | Y | Y | NA | NA | N | NA | Y | Y | Y | Y | Y | Y | Y |
| Artery-invasion | N | N | NA | NA | N | NA | N | Y | N | N | N | Y | N |
| Pancreatic duct dilation | N | Y | NA | NA | Y | NA | N | N | N | Y | N | Y | Y |
| Wall | |||||||||||||
| Thick/thin | Thin | NC | NA | NA | Thin | NA | Thick | Thick | Thick | NC | NC | Thick | NA |
| Contrast-enhanced status | Y | NC | NA | NA | Y | NA | Y | Y | Y | NC | NC | Y | NA |
| Edges | Smooth | Fuzzy | NA | NA | Smooth | NA | Smooth | Smooth | Smooth | Fuzzy | Fuzzy | Smooth | NA |
Abbreviations: Y, yes; N, no; NA, not available; NC, not clear.
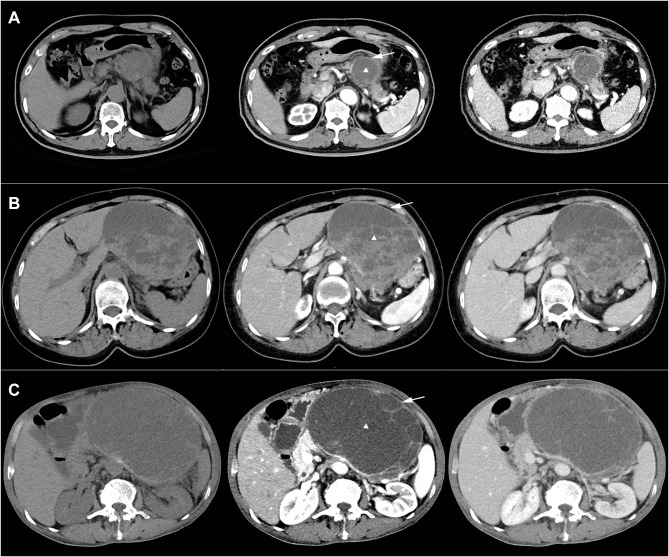
Based on enhanced CT examination done before surgery, there was no significant difference in tumor location and the median size of lesion was 4.6cm. It was rare to observe tumor calcification (1/10) and artery invasion (2/10). Pancreatic duct dilation appeared (5/10) when the lesion compressed the pancreatic duct. We found the lesions were cystic or cystic and solid and had hypodense or isodense compared to normal pancreatic parenchyma. In addition, as shown in Figure 1, we found that cystic and solid lesions often had thick (4/5), contrast-enhanced (5/5) wall with smooth edges (5/5). For lesions mainly with solid composition (4/10), the boundary is not well demarcated with normal pancreatic parenchyma.
Figure 1.
Characteristic pre-operative images of undifferentiated carcinoma of pancreas with osteoclast-like giant cells. (A) Female, 77-year-old (B) female, 70-year-old (C). Male, 72-year-old, intraoperative colon invasion. White triangle marks the cystic and solid lesion. White solid arrow marks the thick, contrast-enhanced wall with smooth edges.
Therapy
Surgical conditions and postoperative complications were listed in Table 3. The surgical procedure is determined by the preoperative diagnosis and location of the tumor. Six patients underwent Whipple procedure (PD or PPPD), six patients underwent distal pancreatectomy (DP) and one patients underwent middle segment pancreatectomy (MSP). Partial colectomy was performed in one patients due to tumor invasion. Extended lymph node dissection was performed in three patients and extended pancreatectomy was carried in two patients. The median operation time was 191(169–220) minutes. The median intraoperative bleeding is about 100 mL. Grade B pancreatic fistula appeared in four patients and there was no Grade C pancreatic fistula. Delayed gastric empty occurred in one patient. All patients had no postoperative hemorrhage expect one case of gastrointestinal bleeding. None of the patients underwent a second operation in perioperative period.
Table 3.
Operation and Surgical Complication
| n | ||
|---|---|---|
| Operation | PD/PPPD | 6 |
| MSP | 1 | |
| DP | 6 | |
| Extended LN dissection | Present | 3/12 |
| Extended pancreatectomy | Present | 2/12 |
| Additional organ resections | Present | 1/12 |
| Time of operation (min) (median, IQR) | 191(169–220) | |
| Estimated blood loss (mL) (median, IQR) | 100(63–200) | |
| Complication | ||
| Fistula | Grade A/ absent | 8 |
| Grade B | 4 | |
| Grade C | 0 | |
| DGE | Grade A/ absent | 11 |
| Grade B | 0 | |
| Grade C | 1 | |
| POPH | Present | 0/12 |
| Re-operation | Present | 0/13 |
Pathological Characteristics
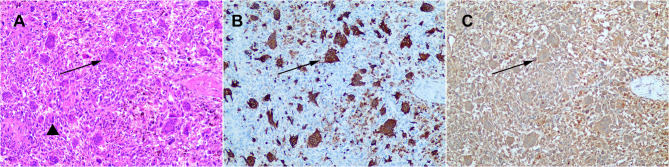
All specimen from surgery were examined pathologically. Mononuclear tumor cells with nuclear atypia and multinucleated osteoclast-like giant cells stained with positive CD68 and vimentin could be detected (Figure 2). As shown in Table 4, the tumor was present in the head, neck, body and tail of the pancreas, with no difference in distribution. The median value of the longest diameter of the tumor was 3.15 cm in our cases. No lymph node metastases was detected in most cases, with the lower quartile 0% and the upper quartile 9%. One patient had part of colon removed due to tumor invasion. Among all cases, tumor involved adjacent vital arteriovenous in one patient, in which portal vein was excised and reconstructed. Besides, tumor had vascular invasion in four patients and nerve invasion in three patients. Three patients had at least one positive margin and the other ten patients had negative margin or the tumor is less than 1mm from the surgical margin. In addition, elements of ductal adenocarcinoma were also found in the specimens of three patients.
Figure 2.
HE staining immunohistochemical staining of UCOGCP (A) HE staining (B) Immunohistochemical staining for CD68 (C) Immunohistochemical staining for vimentin. Triangle marks tumor cells solid arrow marks osteoclast-like giant cells.
Table 4.
Pathology Examination
| n | ||
|---|---|---|
| Pancreatic tumor location | Head | 6 |
| Neck and body | 1 | |
| Body and tail | 6 | |
| Tumor longest size (cm) median(IQR) | 3.15 (2.48–3.58) | |
| T stage | T1 | 2 |
| T2 | 5 | |
| T3 | 6 | |
| Positive lymph node ratio (median, IQR) | 0%(0%-9%) | |
| TMN stage | I | 6 |
| II | 4 | |
| III | 3 | |
| Combined organ resection | Part of colon | 1 |
| No combined organ resection | 12 | |
| Vascular invasion | 5/10(50%) | |
| Nerve invasion | 3/10(30%) | |
| Margin | Negative | 6 |
| <1mm | 4 | |
| Positive | 3 | |
| With PDAC | Present | 3 |
| Absent | 10 |
Follow-Up and Survival
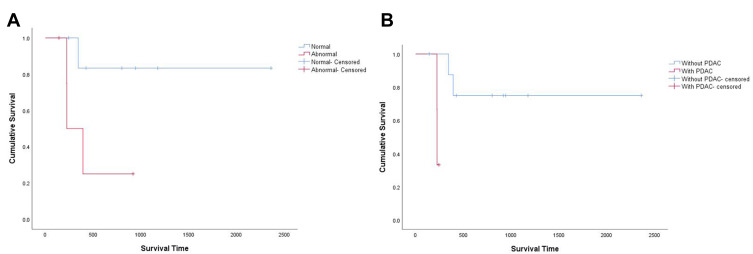
By the time of follow-up in September 2021, except for one patient lost follow-up, five of twelve patients had postoperative metastasis or recurrence of tumor and four of them had died of this disease (Table 5). The median survival time was 13 months. Eight patients had adjuvant chemotherapy and only one patient had adjuvant radiotherapy. According to Figure 3, We found that CEA (p=0.049) and elements of ductal adenocarcinoma under microscope (p=0.011) were significantly correlated to overall survival.
Table 5.
Follow Up and Survival
| n | ||
|---|---|---|
| Survival state | Alive | 8 |
| Dead | 4 | |
| Survival time(mo) (median, IQR) | 13.0(7.8–30.3) | |
| Adjuvant therapy | Radiotherapy | 1 |
| Chemo | 8 | |
| N | 3 | |
| Recurrence/metastasis | Recurrence | 2 |
| Metastasis | 3 | |
| N | 7 |
Figure 3.
Kaplan–Meier overall survival curves according to (A) CEA (P = 0.049) and (B) elements of ductal adenocarcinoma under microscope (P = 0.011).
Discussion
Rosai is widely believed to first describe the presence of osteoclast-like giant cells in pancreatic tumors in 1968.14 In the two cases reported, the microscopic composition of the pancreatic tumor is presented as osteoclast-like multinucleated giant cells (cytoplasm containing eosinophilic granules, sometimes vesicular) scattered between small mononuclear cells (sparse cytoplasm, round or oval nuclei). The fine structure of giant cells showed less endoplasmic reticulum and more prominent granular endoplasmic reticulum, with microvilli on the surface and no amorphous regions below. These differences from normal osteoclasts and giant cell tumors of bone rule out distant metastasis of bone tumors and extrasseous osteoclasts. Although the term “undifferentiated carcinoma with osteoclast-like giant cells” is not mentioned in Rosai’s paper, these two cases are believed to be the first reporting UCOGCP because of the description and identification of this component. Subsequent cases of pancreatic malignancies containing osteoclast-like giant cells have been reported. Since the incidence of pancreatic tumors is less than 1%, the number of cases reported in English literature retrieved by PubMed is only around one hundred.
At first, UCOGCP was thought to be the origin of pancreatic acinar cells. With the application of sequencing technology, some scholars found that KRAS mutations in tumor cells in UCOGCP were consistent with pancreatic ductal adenocarcinoma or intraductal tumors.15,16 Therefore, UCOGCP has gradually been recognized as a ductal epithelial origin. UCOGCP was classified as a special subtype of pancreatic ductal adenocarcinoma by WHO in 2010,1,17 and its cell components mainly included undifferentiated mononuclear tumor cells and CD68 and vimentin positive multinucleated osteoclast-like giant cells. It is worth noting that OGC may be present in mucus-producing tumors of the pancreas, which are outside the scope of UCOGCP.
The age of onset of UCOGCP is mainly elderly. Most of patients in the existing literature are over 60 years old, which is the similar age as most of our patients. There is no obvious gender tendency in the onset of UCOGCP. The minimum age of onset of UCOGCP reported in literature is 26 years old.18 The clinical manifestations are similar to those of other pancreatic tumors without specific clinical manifestations. The main manifestations are abdominal discomfort and pain, low back pain or poor appetite and weight loss. In our cases, more than half patients had no obvious symptoms or only nonspecific abdominal pain and was diagnosed through health examination. Tumor obstruction of the biliary tract may cause jaundice,15,19–21 which occurred in one of our cases. Tumor compression of the pancreatic duct may cause pancreatitis22 or dilation of the pancreatic duct,23 and there may be complaints of discomfort due to elevated tumor markers24 or incidental detection of other diseases during examination.25,26
The preoperative diagnosis of UCOGCP mainly depends on CT and MRI. PETCT can provide useful information about the metabolic status of the tumor and the presence or absence of distant metastasis.23,27,28 ERCP also plays a role in the diagnosis of UCOGCP involving the papilla of duodenum and pancreatic bile duct. Enhanced CT could be used to differentiate UCOGCP from other tumors, such as PDAC or SPT. It is reported that enhanced CT scans of the pancreas and parenchyma revealed smooth edges,19,20,29 low-density solid tumors with intratumoral hemorrhage, calcification, and cystic changes, as well as duct dilation and truncation involving the pancreatic duct system. PDAC, lack of blood supply tumor, often appears as no obvious enhancement and forms “double duct sign”, with the disappearance of the fat layer outside the pancreas. SPT is more common in young women and usually manifests as a solid-based, soft texture mass without pancreatic duct expansion. Based on our patient’s image, we found the lesions of UCOGCP cases in CT examination could be divided into solid lesion which only shows solid performance, cystic lesion which only shows cystic performance and cystic and solid lesion which shows both cystic and solid performance. When the tumor shows only solid performance or cystic performance, the image has no distinguished features and is similar to PDAC or MCN. However, when the tumor shows both cystic and solid performance, the tumor often has thick, contrast-enhanced wall with smooth edges and has no calcification. When these performances show up in a cystic and solid tumor at the same time, physicians have to be highly suspicious a diagnosis of UCOGCP. The T2-weighted phase of MRI shows low signal and the diffuse-weighted phase shows relatively low or isosignal region.30 However, due to the influence of common and frequently occurring diseases, UCOGCP may be wrongly diagnosed as pancreatic ductal adenocarcinoma, mucinous cystadenoma, mucinous carcinoma,31 solid pseudopapillary tumor,32 neuroendocrine tumor,23 pancreatic pseudocyst,33 and other rare types of pancreatic tumors. Tumor markers are of little help to diagnosis. Tumor markers CA199 and CEA of more than half of UCOGCP patients are in the normal range, and there are also reports of significant increase in CA19-9 and CEA.24,31,34,35 And it’s worth noting, based on what we found, the elevated level of NSE might be found in the majority of cases and suggestive for diagnosis of UCOGCP. In addition, there are relatively few tests for CA125 and other tumor markers.36 The preoperative tissues obtained by EUS or intraoperative examination of gross specimens as well as microscopic HE staining and immunohistochemical results are necessary conditions for diagnosis. EUS may not be able to make an effective diagnosis due to the lack of tissue volume.
The longest diameter span of UCOGCP in gross specimens ranged from 1cm to 28cm,24,27 with frequent occurrence of old bleeding and necrosis, low rate of lymph node metastasis. There are occasional cases of lymph node metastasis, with no more than five positive lymph node metastasis.37–41 In our cases, the positive rate of lymph nodes was also not relatively high. HE staining sections often show pleomorphic, spindle shaped cells and scattered osteoclastoid giant cells. Immunohistochemistry showed that undifferentiated carcinoma expressed a variety of epithelial tissue markers, and characteristic OGC markers were CD68 and vimentin positive.26
Radical surgical resection is the preferred treatment for UCOGCP, and the surgical method depends on tumor location and intraoperative exploration. Whipple procedure and distal pancreatectomy were performed in almost all our patients. However, middle-segment pancreatectomy was performed in one patient due to intraoperative exploration. We will continue to follow up to determine whether the central pancreatectomy, a function-preserving procedure, could benefit this patient. UCOGCP may be highly invasive and may locally invade adjacent organs such as stomach, jejunum, colon, left kidney and left adrenal gland.24,31,36,39,42–44 Combined with the corresponding organ resection could achieve the radical effect. Arteriovenous invasions such as celiac trunk and superior mesenteric vein are rarely reported.35,45 The portal vein was excised and reconstructed in one of our patients. The need for postoperative chemoradiotherapy is currently controversial, mainly due to the low incidence and lack of clinical studies. The chemotherapy regimen was mainly gemcitabine, and the others including capecitabine, Tegafur, which are commonly used chemotherapy for pancreatic ductal adenocarcinoma. In two cases with significantly above-average survival,22,43 10 years and 5.5 years respectively, patients were treated with gemcitabine. Portal vein tumor thrombus shrank after gemcitabine chemotherapy in a patient in Japan.29 We found patients with postoperative adjuvant therapy might have longer survival time than patients without postoperative adjuvant therapy based on our data, but it’s not statistically significant, which may be caused due to the small sample size. Chemotherapy might have a certain effect, but whether chemotherapy should be combined with the patient’s real situation and will. The therapeutic effects of radiotherapy in reported cases vary greatly.37,44 More data are needed to unify and standardize the treatment plan.
The rate of recurrence and metastasis after surgery is high. The survival time of patients varies, and the five-year survival rate in case report is extremely low. For comparison, the latest data showed the five-year survival rate of PDAC is 11%. Our data showed the median survival time is about 13 months, which is shorter than that of PDAC in our center. Patients with UCOGCP might have a poor prognosis than patients with PDAC, which is still not clearly established due to lack of valid large sample data. Metastatic lesions can be detected clinically only two months after surgery. Metastatic sites are common in liver and lung,20,21,24,28,34,35 as well as greater omentum,46 thoracic spine and pelvis.39 Some of our patient also developed liver or lung metastases after surgery. Some scholars have compared the patients with long (> 2 years) and short survival (< 1 year) and found that older age, male, larger tumor, positive lymph nodes, and features associated with ductal adenocarcinoma suggested worse prognosis and the survival of less than one year.22
UCOGCP is rare clinically, with low preoperative accurate diagnosis rate and poor prognosis. Patients with suspected diagnosis should receive surgical intervention as soon as possible, in order to prolong the survival period of patients. Postoperative chemotherapy may prolong survival to some extent, but more valid data are needed.
Acknowledgments
This work was supported by the grant from the National Natural Science Foundation of China (82173206).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. doi: 10.1111/his.13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei J, Liu X, Wu J, et al. Modified one-layer duct-to-mucosa pancreaticojejunostomy reduces pancreatic fistula after pancreaticoduodenectomy. Int Surg. 2015. doi: 10.9738/INTSURG-D-15-00094.1 [DOI] [PubMed] [Google Scholar]
- 3.Miao Y, Lu Z, Yeo CJ, et al. Management of the pancreatic transection plane after left (distal) pancreatectomy: expert consensus guidelines by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2020;168(1):72–84. doi: 10.1016/j.surg.2020.02.018 [DOI] [PubMed] [Google Scholar]
- 4.Gao H, Liu T, Wang G, et al. Central pancreatectomy for early-stage pancreatic ductal adenocarcinoma: a single-center case-control study. Langenbecks Archiv Surg. 2019;404(2):175–182. doi: 10.1007/s00423-019-01766-1 [DOI] [PubMed] [Google Scholar]
- 5.Hartwig W, Vollmer CM, Fingerhut A, et al. Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery. 2014;156(1):1–14. doi: 10.1016/j.surg.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 6.Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 2014;156(3):591–600. doi: 10.1016/j.surg.2014.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014;155(6):977–988. doi: 10.1016/j.surg.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 8.Hackert T, Strobel O, Michalski CW, et al. The TRIANGLE operation - radical surgery after neoadjuvant treatment for advanced pancreatic cancer: a single arm observational study. HPB. 2017;19(11):1001–1007. doi: 10.1016/j.surg.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 9.Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 10.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20–25. doi: 10.1016/j.surg.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 11.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761–768. doi: 10.1016/j.surg.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 12.Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161(3):584–591. doi: 10.1016/j.surg.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 13.Besselink MG, van Rijssen LB, Bassi C, et al. Definition and classification of chyle leak after pancreatic operation: a consensus statement by the International Study Group on Pancreatic Surgery. Surgery. 2017;161(2):365–372. doi: 10.1016/j.surg.2016.06.058 [DOI] [PubMed] [Google Scholar]
- 14.Rosai J. Carcinoma of pancreas simulating giant cell tumor of bone. Electron-microscopic evidence of its acinar cell origin. Cancer. 1968;22(2):333–344. doi: [DOI] [PubMed] [Google Scholar]
- 15.Carvounis EE, Smyrniotis V, Chatziioannou A, Paphitis A. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. Int J Gastrointest Cancer. 2003;33(2–3):103–106. doi: 10.1385/IJGC:33:2-3:103 [DOI] [PubMed] [Google Scholar]
- 16.Verbeke CS, Menon KV. Osteoclast-like giant cell tumour of the pancreas: an undifferentiated carcinoma of duct epithelial origin. Pancreatology. 2006;6(3):254. doi: 10.1159/000091963 [DOI] [PubMed] [Google Scholar]
- 17.Hanayneh W, Parekh H, Fitzpatrick G, Feely M, George TJ, Starr JS. Two cases of rare pancreatic malignancies. J Pancreatic Cancer. 2019;5(1):26–33. doi: 10.1089/pancan.2019.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano H, Morita K, Tachibana S, et al. Undifferentiated carcinoma with osteoclast-like giant cells arising in a mucinous cystic neoplasm of the pancreas. Pathol Int. 2008;58(6):383–389. doi: 10.1111/j.1440-1827.2008.02240.x [DOI] [PubMed] [Google Scholar]
- 19.Maksymov V, Khalifa MA, Bussey A, Carter B, Hogan M. Undifferentiated (anaplastic) carcinoma of the pancreas with osteoclast-like giant cells showing various degree of pancreas duct involvement. A case report and literature review. JOP. 2011;12(2):170–176. [PubMed] [Google Scholar]
- 20.Manduch M, Dexter DF, Jalink DW, Vanner SJ, Hurlbut DJ. Undifferentiated pancreatic carcinoma with osteoclast-like giant cells: report of a case with osteochondroid differentiation. Pathol Res Pract. 2009;205(5):353–359. doi: 10.1016/j.prp.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 21.Nai GA, Amico E, Gimenez VR, Guilmar M. Osteoclast-like giant cell tumor of the pancreas associated with mucus-secreting adenocarcinoma. Case report and discussion of the histogenesis. Pancreatology. 2005;5(2–3):279–284. doi: 10.1159/000085283 [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi S, Nakano H, Ooike N, Oohashi M, Koizumi S, Otsubo T. Long-term survivor of a resected undifferentiated pancreatic carcinoma with osteoclast-like giant cells who underwent a second curative resection: a case report and review of the literature. Oncol Lett. 2014;8(4):1499–1504. doi: 10.3892/ol.2014.2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato K, Urakawa H, Sakamoto K, Ito E, Hamada Y, Yoshimitsu K. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells showing intraductal growth and intratumoral hemorrhage: MRI features. Radiol Case Rep. 2019;14(10):1283–1287. doi: 10.1016/j.radcr.2019.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yazawa T, Watanabe A, Araki K, et al. Complete resection of a huge pancreatic undifferentiated carcinoma with osteoclast-like giant cells. Int Cancer Conf J. 2017;6(4):193–196. doi: 10.1007/s13691-017-0305-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah A, Khurana T, Freid L, Siddiqui AA. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas in a patient with new diagnosis of follicular Non-Hodgkin’s lymphoma. ACG Case Rep J. 2014;1(2):109–111. doi: 10.14309/crj.2014.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Togawa Y, Tonouchi A, Chiku T, et al. A case report of undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and literature review. Clin J Gastroenterol. 2010;3(4):195–203. doi: 10.1007/s12328-010-0160-2 [DOI] [PubMed] [Google Scholar]
- 27.Fu LP, Cheng AP, Wang XG, Fu JL, Jin L. 18F-FDG PET/CT in the detection of undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. Clin Nucl Med. 2017;42(8):615–616. doi: 10.1097/RLU.0000000000001719 [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Lee JM, Yoon JH, et al. Huge and recurrent undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. Quant Imaging Med Surg. 2018;8(4):457–460. doi: 10.21037/qims.2018.05.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshioka M, Uchinami H, Watanabe G, et al. Effective use of gemcitabine in the treatment of undifferentiated carcinoma with osteoclast-like giant cells of the pancreas with portal vein tumor thrombus. Intern Med. 2012;51(16):2145–2150. doi: 10.2169/internalmedicine.51.7670 [DOI] [PubMed] [Google Scholar]
- 30.Fukukura Y, Kumagae Y, Hirahara M, et al. CT and MRI features of undifferentiated carcinomas with osteoclast-like giant cells of the pancreas: a case series. Abdom Radiol. 2019;44(4):1246–1255. doi: 10.1007/s00261-019-01958-9 [DOI] [PubMed] [Google Scholar]
- 31.Wada T, Itano O, Oshima G, et al. A male case of an undifferentiated carcinoma with osteoclast-like giant cells originating in an indeterminate mucin-producing cystic neoplasm of the pancreas. A case report and review of the literature. World J Surg Oncol. 2011;9:100. doi: 10.1186/1477-7819-9-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang KY, Choi JI, Choi MH, et al. Magnetic resonance imaging findings of undifferentiated carcinoma with osteoclast-like giant cells of pancreas. Clin Imaging. 2016;40(1):148–151. doi: 10.1016/j.clinimag.2015.09.013 [DOI] [PubMed] [Google Scholar]
- 33.Oehler U, Jurs M, Kloppel G, Helpap B. Osteoclast-like giant cell tumour of the pancreas presenting as a pseudocyst-like lesion. Virchows Archiv. 1997;431(3):215–218. doi: 10.1007/s004280050091 [DOI] [PubMed] [Google Scholar]
- 34.Chiarelli M, Guttadauro A, Gerosa M, et al. An indeterminate mucin-producing cystic neoplasm containing an undifferentiated carcinoma with osteoclast-like giant cells: a case report of a rare association of pancreatic tumors. BMC Gastroenterol. 2015;15:161. doi: 10.1186/s12876-015-0391-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Georgiou G, Balasi E, Siozopoulou V, Tsili A, Fatouros M, Glantzounis G. Undifferentiated carcinoma of the head of pancreas with osteoclast-like giant cells presenting as a symptomatic cystic mass, following acute pancreatitis: case report and review of the literature. Int J Surg Case Rep. 2016;19:106–108. doi: 10.1016/j.ijscr.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hur YH, Kim HH, Seoung JS, et al. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. J Korean Surg Soc. 2011;81(2):146–150. doi: 10.4174/jkss.2011.81.2.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo YL, Ruan LT, Wang QP, Lian J. Undifferentiated carcinoma with osteoclast-like giant cells of pancreas: a case report with review of the computed tomography findings. Medicine. 2018;97(48):e13516. doi: 10.1097/MD.0000000000013516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeffrey I, Crow J, Ellis BW. Osteoclast-type giant cell tumour of the pancreas. J Clin Pathol. 1983;36(10):1165–1170. doi: 10.1136/jcp.36.10.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jo S. Huge undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. World J Gastroenterol. 2014;20(10):2725–2730. doi: 10.3748/wjg.v20.i10.2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Layfield LJ, Bentz J. Giant-cell containin g neoplasms of the pancreas: an aspiration cytology study. Diagn Cytopathol. 2008;36(4):238–244. doi: 10.1002/dc.20785 [DOI] [PubMed] [Google Scholar]
- 41.Nojima T, Nakamura F, Ishikura M, Inoue K, Nagashima K, Kato H. Pleomorphic carcinoma of the pancreas with osteoclast-like giant cells. Int J Pancreatol. 1993;14(3):275–281. doi: 10.1007/BF02784937 [DOI] [PubMed] [Google Scholar]
- 42.Abid H, Gnanajothy R. Osteoclast giant cell tumor of pancreas: a case report and literature review. Cureus. 2019;11(5):e4710. doi: 10.7759/cureus.4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao HQ, Yang YM, Zhuang Y, Liu P. Locally advanced undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. World J Gastroenterol. 2015;21(2):694–698. doi: 10.3748/wjg.v21.i2.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Temesgen WM, Wachtel M, Dissanaike S. Osteoclastic giant cell tumor of the pancreas. Int J Surg Case Rep. 2014;5(4):175–179. doi: 10.1016/j.ijscr.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakhi R, Hamza A, Khurram MS, Ibrar W, Mazzara P. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells reported in an asymptomatic patient: a rare case and literature review. Autopsy Case Rep. 2017;7(4):51–57. doi: 10.4322/acr.2017.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott R, Jersky J, Hariparsad G. Case report: malignant giant cell tumour of the pancreas presenting as a large pancreatic cyst. Br J Radiol. 1993;66(791):1055–1057. doi: 10.1259/0007-1285-66-791-1055 [DOI] [PubMed] [Google Scholar]