Abstract
Study objective
A multi-institutional interdisciplinary team was created to develop a research group focused on leveraging artificial intelligence and informatics for cardio-oncology patients. Cardio-oncology is an emerging medical field dedicated to prevention, screening, and management of adverse cardiovascular effects of cancer/cancer therapies. Cardiovascular disease is a leading cause of death in cancer survivors. Cardiovascular risk in these patients is higher than in the general population. However, prediction and prevention of adverse cardiovascular events in individuals with a history of cancer/cancer treatment is challenging. Thus, establishing an interdisciplinary team to create cardiovascular risk stratification clinical decision aids for integration into electronic health records for oncology patients was considered crucial.
Design/setting/participants
Core team members from the Medical College of Wisconsin (MCW), University of Wisconsin-Milwaukee (UWM), and Milwaukee School of Engineering (MSOE), and additional members from Cleveland Clinic, Mayo Clinic, and other institutions have joined forces to apply high-performance computing in cardio-oncology.
Results
The team is comprised of clinicians and researchers from relevant complementary and synergistic fields relevant to this work. The team has built an epidemiological cohort of ~5000 cancer survivors that will serve as a database for interdisciplinary multi-institutional artificial intelligence projects.
Conclusion
Lessons learned from establishing this team, as well as initial findings from the epidemiology cohort, are presented. Barriers have been broken down to form a multi-institutional interdisciplinary team for health informatics research in cardio-oncology. A database of cancer survivors has been created collaboratively by the team and provides initial insight into cardiovascular outcomes and comorbidities in this population.
Keywords: Team science, Cardio-oncology, Cancer survivorship, Artificial intelligence, Informatics
1. Introduction
A team may be defined as a set of individuals with complementary skills who are committed to a common goal, set of performance objectives, and approach for which they hold one another accountable [1]. Although organizational specialists proposed this definition to describe workgroups in business, it is equally applicable to interdisciplinary healthcare teams [2], including research teams. In fact, an American Heart Association Scientific Statement advocates for an interdisciplinary team approach to maximize scientific discoveries [3]. To apply this in research practice, how can an interdisciplinary multi-institutional team be established that encompasses a variety of expertise and includes diverse individuals at various stages of training and career, to chart a path for the application of artificial intelligence in cardio-oncology? How can team science be applied in the setting of informatics and a learning health system to predict and optimize the best care and management for cancer survivors at risk for cardiovascular events? A learning healthcare system applies scientific knowledge during clinical care while also eliciting insights from that care in order to spur innovation in the delivery of optimal healthcare and inspire new research areas [4]. What should an interdisciplinary team look like in this setting? Who should be on the team? How should the team be structured and why? How should team members across disciplines communicate to foster and optimize collaboration? Should there be one comprehensive team, or smaller teamlets? What is the value of operating across disciplines within and across institutions? Is there value in diversity of perspectives, leveraging variety in training backgrounds and current practice methods and patterns, sharing experiences across institutions, pooling versus comparing patient cohorts, testing and validating algorithms, or exchanging ideas that may work across institutions versus focusing on only one? While the answers to some of these questions may seem self-evident, success in building interdisciplinary teams to develop and execute projects designed to promote equity, improve risk stratification, prevent adverse cardiovascular events, and improve health outcomes for cancer survivors will depend upon deep and unflinching consideration of such questions and taking the sometimes difficult or non-obvious actions required to clear the hurdles they suggest.
Why is it necessary to ask these questions in the setting of cardio-oncology? Cardio-oncology itself is an interdisciplinary field, involving a range of health professionals including physicians, advanced practice providers, pharmacists, and trainees within and across many disciplines such as cardiology, hematology, medical oncology, radiation and surgical oncology, radiology, and pharmacy [5]. In the same way that an interdisciplinary team is required for a cardio-oncology patient care, an interdisciplinary research team is also needed to study this complex cohort. Heart disease is the leading cause of death in the United States with over 655,000 deaths per year, followed closely by cancer [6], [7]. In fact, cardiovascular disease is a leading cause of death in cancer survivors, second only to cancer recurrence or development of secondary cancer [8], [9], [10], [11]. Almost 2 million new cancer diagnoses and more than 600,000 new cancer deaths are estimated each year [12]. Currently, almost 17 million Americans are cancer survivors [13], and this is expected to increase to more than 22 million by 2030 [14]. Among cancer survivors, higher rates of cardiovascular disease have been reported in African Americans than in Caucasians, a disparity associated with socioeconomic inequities [15], [16], [17], [18], [19]. Health disparities in African Americans are particularly relevant to southeastern Wisconsin (SE WI). Milwaukee is a metropolitan city in the heart of SE WI with substantial disparities in health and socioeconomic status [20], [21]. Milwaukee has a population of nearly 1 million and is the fifth poorest city in America. Demographically, Milwaukee County is predominantly white (50.7%), followed by African American (25.9%), and Hispanic or Latino (15.4%) [21]. As the largest city in the state, Milwaukee has the state's most concentrated health resources. Despite this fact, health disparities are rampant, with rates of cancer (breast, cervical, lung, and prostate), cardiovascular disease, and mortality that are higher than the state average [21]. Additionally, Milwaukee County has one of the most segregated urban communities in the nation. African American residents primarily live on the city's North Side. These neighborhoods experience higher occurrences of unemployment, incarceration, and poverty, with substandard housing. This often leads to limited access to healthcare and a predisposition to health disparities [21], such as in cardio-oncology [15], [16], [17], [18], [19], [22], [23], [24].
With the millions of cancer survivors who are cared for in primary care, cardiology, oncology, surgery, and other areas within medicine, it is important to recognize that cardiovascular disease in cancer survivors is an urgent public health issue for which the best solution would be informed by population science. To address cardio-oncology care and research across a population, it would be beneficial to adopt the philosophy of a learning health system, so that as the interdisciplinary team develops its research approach, this can be tested across a learning health system. To achieve this, the research team should leverage informatics in order to use predictive analytics, artificial intelligence, and data from the electronic health record to identify cancer survivors at highest risk for cardiovascular disease. Once these individuals are identified, oncology/hematology and cardiology clinicians across the institution should be notified or alerted to cancer patients or survivors who are at high or very high risk for cardiovascular disease. These alerts can be in the form of best practice alerts [25], and clinical decision aids can then potentially be used by oncologists and cardiologists to optimize care for these patients [5]. Such informatics-based personalized approaches may enable precision cardiovascular risk prediction in these survivors and could be developed via interdisciplinary research teams. However, there is a gap in knowledge about how to develop an interdisciplinary multi-institutional research team to pursue informatics research in cardio-oncology within a learning health system. Specifically, there is no current research on building such teams to facilitate the development and use of machine learning artificial intelligence algorithms in cardio-oncology or to address health disparities in SE WI or nationwide. In such a new and emerging field as cardio-oncology, how can such an interdisciplinary team be put together? With so few individuals trained in cardio-oncology patient care or research, how can groups of individuals be convened that can effectively learn together and apply team science approaches for cardio-oncology? Why are team science and a learning healthcare system perspective important for cardio-oncology?
Artificial intelligence, informatics, and learning health systems are relatively newer techniques in cardio-oncology that, in the setting of team science, have the potential to transform care for patients. For optimal success in creating an informatics-rich atmosphere for the benefit of patients in cardio-oncology, an interdisciplinary team comprised of data scientists, clinicians, and patient advocates needs to be formed and evolve to operate seamlessly within a learning healthcare system environment. To approach this process with any less diversity or precision would be charting a suboptimal course forward for the future of cardio-oncology. This article describes the process of building a well-functioning interdisciplinary research team across institutions and presents initial findings and output from the team. In a companion article titled “Team Principles for Successful Interdisciplinary Research Teams”, a more comprehensive literature review on team science and team building is provided, with a more expansive description of lessons learned from this process (Brown et al., Team Principles for Successful Interdisciplinary Research Team Science, In Review, AHJ Plus; provided as Supplemental Material for concurrent review).
2. Materials and methods
2.1. Establishing team constituents and dynamics
In order to build this interdisciplinary and multi-institutional team, first the kinds of expertise needed for the comprehensive team were delineated. After determining the kinds of expertise needed, where to find the varying expertise in professional circles and networks and in relatively close proximity to facilitate collaboration was then determined. The value of reaching out to individuals at the team's core institution (MCW), as well as partner institutions in the SE WI Clinical and Translational Science Institute (CTSI), in addition to individuals at Mayo Clinic, Cleveland Clinic, and University of Ottawa, with whom the team leader and convener at MCW had collaborated, and who were already working to apply their expertise in cardiology, was recognized. The team was therefore built in a stepwise fashion, starting with individuals at Froedtert and Medical College of Wisconsin (F&MCW), then branching out to SE WI CTSI institution members (MSOE and UWM), before expanding to the broader professional multi-institutional network (Mayo Clinic, Cleveland Clinic, and University of Ottawa). In this way, a range of stakeholders was carefully considered, to build a collaborative, interdisciplinary, and multi-institutional team. Recruited team members made suggestions on additional key collaborations to include, consistent with a snowball sampling recruitment techniques in which participants are asked to assist in identifying other potential participants. Once the team was formed, the process of establishing team meetings was then tackled. In-person versus virtual meetings were considered, and the option best suited to the needs of the team was selected.
The team leader facilitated team discussions. Team leadership was designed to capture the overall vision of the team's projects and output long-term, with subgroups of the team being led organically by individuals with appropriate expertise. As a team, ideas for research directions, publications, and relative contributions of each participant to the team were discussed. Each team member's voice was valued and each team member was offered opportunities for active participation and collaboration. Career development and team science training were provided, for collective training of the team on well-established methodologies for growth and advancement. Consensus building was made a critical part of team discussions each week to ensure addressing project steps and funding initiatives from various angles and deciding how to proceed cohesively. A variety of team members from multiple institutions participated in planning budgets for personnel, time, and equipment. Additionally, support was obtained from institutional grants and contracts office, particularly regarding assistance with grant preparation, budgeting, submission, and execution. Performance markers and deliverables were determined early on and throughout the team's tenure together, in order to measure progress for internal and external reports, and to be able to build on milestones and plan for new ones. A patient advocate, or more aptly described as a “patient with lived experience”, was considered a key member of the team to help shape the team's research efforts to complement patient-centered care.
The team performed a needs assessment, development of an infrastructure, and initial creation and plans for research, career, and team development [26]. A needs and interest assessment was pursued to determine topic areas that should be studied and would be embraced by team members. The content and knowledge base of team members was also assessed to determine the variety of input that could be gained as the team discussed potential projects and their components. Microsoft Teams was used to host and record meetings and store documents, to facilitate asynchronous connection with and follow-up of documents, discussions, and supplemental chatbox content, and follow along with discussions even if asynchronously. Knowledge sharing was made a routine part of team dynamics early on, to recognize the valuable input from members of the team. Interactive lectures or workshops were given by various members of the team. These were modeled after typical journal clubs in which published papers crucial to team projects would be dissected, discussed, explained, and used for project planning. The journal club-style sessions were administered by a variety of members of the team, including college or graduate students who would join the team for the summer or throughout the year and use administration of journal club for their learning process and important contributions to the team. Trainees and faculty both also contributed to knowledge translation into research project applications, as well as during presentations given internally and externally reporting on results from the team's collective work. As the team continued to work together overtime various levels of team building efforts were pursued in a graded fashion. Accordingly, the team enrolled in a team science and development course funded by the SE WI CTSI. This course allowed team members to develop even greater interdependency, cohesiveness, efficacy, and efficiency.
2.2. Epidemiological cohort
The team prepared deidentified data for ~5000 F&MCW-treated adult male/female cancer survivors (including ethnic/racial minorities) at varying levels of risk of developing cardiovascular disease and generated epidemiological data tables for this population. Database constituents were identified in the Clinical Research Data Warehouse (CRDW) using the Informatics for Integrating Biology & the Bedside (I2B2) cohort discovery toolset, which allows investigators to identify patients with certain characteristics for research purposes. The CRDW is a multi-sourced clinical data repository, with aggregation of electronic health record, billing, and 3rd party clinical systems data which provides a more complete longitudinal view of patient histories than any single data source. The CRDW includes important supplemental cancer data from the North American Association of Central Cancer Registries funded by the National Cancer Institute of the National Institutes of Health. The follow-up period for the cohort of ~5000 spans the entire available time period available in CRDW: from the year 2000 to the year 2020. The F&MCW CRDW was used as a resource to capture patient data from ~5000 individuals to build a database of cancer survivors for retrospective analysis and artificial intelligence algorithm training and validation in the F&MCW population, to match other populations in the multi-institutional team [27], [28]. The cohort data includes laboratory, physiology, demographics, outcomes, medications, imaging, and medical history information relevant to cardiovascular risk stratification in individuals with a history of cancer, similar to information used in the artificial intelligence algorithm published by our multi-institutional team members [27], [28]. This data “reservoir” was designed to include the following: males and females 18 years of age or older with a history of cancer; ascertainment of ejection fraction within three years prior to cancer diagnosis; individuals who have or have not developed cardiovascular disease; and the full available range of racial/ethnicity diversity.
2.2.1. Cardiovascular outcomes and comorbidities ICD codes
ICD-9 and ICD-10 codes for cardiovascular comorbidities and outcomes atrial fibrillation, coronary artery disease, cardiomegaly, cardiomyopathy, diabetes mellitus, heart failure, hypertension, peripheral artery disease, and stroke were used to develop the epidemiological patient cohort (Table 1). The ICD-9 codes 427.3, 411.1, 413, 411.0, 411.8, 414, 410, 429.7, 412, 429.3, 425, 425.4, 249, 250, 428, 272, 401, 402, 403, 404, 405, 440, 444, 443.9, 437.0, 430, 431, 432, 433, 434, 437.1, and 438 were utilized. The ICD-10 codes I48, I20, I25, I24, I21, I22, I23, I25.2, I51.7, I42.7, I42.9, I43, I42.0, I42.5, E08, E09, E10, E11, E13, I50, E78, I10, I11, I12, I13, I15, I16, I70, I74, I73.9, I67.2, I60, I61, I62, I63, I65, I66, I67.81, I67.9, I67.82, and I69 were utilized.
Table 1.
Frequency of cardiovascular outcomes and comorbidities at baseline or over 20 years of follow-up in the epidemiological cohort (n = 4626).
| Cardiovascular diagnosis | ICD-9 code | ICD-10 code | Present at any time n (%) | Present at baseline n (%) | Developed after cancer diagnosis n (%) |
|---|---|---|---|---|---|
| Atrial Fibrillation | 427.3 | I48 | 1514 (33) | 1027 (22) | 487 (11) |
| Coronary Artery Disease | 411.1, 413, 411.0, 411.8, 414, 410, 429.7, 412 | I20, I25, I24, I21, I22, I23, I25.2 | 2669 (58) | 2037 (44) | 632 (14) |
| Cardiomegaly | 429.3 | I51.7 | 1736 (36) | 1340 (27) | 396 (9) |
| Cardiomyopathy | 425, 425.4 | 42.7, I42.9, I43, I42.0, I42.5 | 3133 (68) | 2598 (56) | 535 (12) |
| Diabetes | 249, 250 | E08, E09, E10, E11, E13 | 1443 (31) | 1092 (24) | 351 (8) |
| Heart Failure | 428 | I50 | 2826 (61) | 2253 (49) | 573 (13) |
| Hyperlipidemia | 272 | E78 | 3273 (71) | 2788 (60) | 485 (11) |
| Hypertension | 401, 402, 403, 404, 405 | I10, I11, I12, I13, I15, I16 | 3987 (86) | 3567 (51) | 420 (9) |
| Peripheral Artery Disease | 440, 444, 443.9 | I70, I74, I73.9 | 1867 (40) | 1265 (27) | 602 (13) |
| Stroke | 437.0, 430, 431, 432, 433, 434, 437.1, 438 | I67.2, I60, I61, I62, I63, I65, I66, I67.81, I67.9, I67.82, I69 | 1311 (28) | 924 (20) | 387 (8) |
2.2.2. Cancer pharmacotherapy
Medi-Span pharmaceutical classes/sub-classes [29] for alkylating agents, various antibodies especially antibodies that target the human epidermal growth factor receptor 2 (HER2), antineoplastic antibodies (anthracyclines), antimetabolites, endocrine therapies, enzyme inhibitors, mitotic inhibitors, and others were used to determine the distribution of patients on these medications in these cancer drug classes.
2.2.3. Cancer radiation therapy
The MOSAIQ Radiation Oncology software system [30] was used to query radiation therapy in the cohort. Data was available for 2015 to 2020.
2.2.4. Data sharing and access
Local team SE WI CTSI core team members were given access to deidentified data to assist with cohort data gathering and quality checks. The UWM and MSOE IRBs both formally relied on the MCW IRB for team projects. A data use agreement was in place among the participating sites.
2.2.5. Statistics
Descriptive statistics were employed for comparing distributions. The Pearson's Chi-Squared test was used to determine statistical differences between cardiovascular outcomes developed after cancer diagnosis in those treated with cancer therapies compared to those who were not treated with cancer therapies. Statistical significance was defined as a p-value<0.005, due to multiple comparisons.
3. Results
3.1. Disciplines of interdisciplinary team constituents
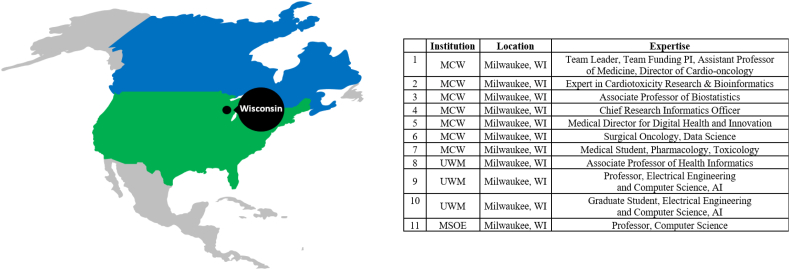
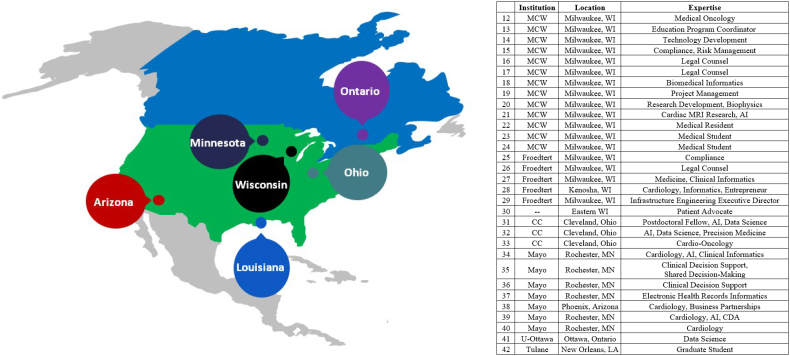
The following types of required expertise were determined for this interdisciplinary teamwork: cardio-oncology, health informatics, artificial intelligence (including machine learning and natural language processing), health/clinical/biomedical informatics, research-related information technology, biostatistics, shared decision-making, clinical decision support, community engagement, and health disparities. Consequently, individuals at MC W, then U WM and MSOE, then Mayo Clinic in Rochester MN, Cleveland Clinic in Cleveland OH, and University of Ottawa in Canada with these expertise were recruited to participate in the team (Fig. 1, Fig. 2).
Fig. 1.
Core team members' geographic and institutional distribution.
AI: Artificial Intelligence; MCW: Medical College of Wisconsin; MSOE: Milwaukee School of Engineering; PI: Principal Investigator; UWM: University of Wisconsin-Milwaukee; WI: Wisconsin.
Fig. 2.
Additional team members' geographic and institutional distribution.
AI: Artificial Intelligence; CC: Cleveland Clinic; CDA: Clinical Decision Aids; CTSI: Clinical & Translational Science Institute; Froedtert: Froedtert Hospital; LA: Louisiana; Mayo: Mayo Clinic; MCW: Medical College of Wisconsin; MN: Minnesota; MSOE: Milwaukee School of Engineering; Tulane: Tulane University; U-Ottawa: University of Ottawa; UWM: University of Wisconsin-Milwaukee; WI: Wisconsin.
3.2. Team science approach and scientific contributions
The team science approach embraced by the group takes advantage of multiple strengths within SE WI CTSI member institutions. Through this interdisciplinary team, MCW, UWM, and MSOE are establishing a multi-institutional center in SE WI for high-performance computing in healthcare. Team members from all three local institutions have been meeting weekly since mid-2020 using the Microsoft Teams platform to build collaborative analytics projects and interprofessional trust in the research team. This is in the context of a larger group of more than 40 individuals from at least five institutions (three of which are founding member institutions of the SE WI CTSI; the other team members are primarily from Mayo Clinic and Cleveland Clinic). During these meetings, the group discusses potential new projects, deadlines and timelines, and who will be responsible for accomplishing different tasks, to promote team cohesiveness and effectiveness. The team has been assembled to pursue collaborative multi-institutional projects on predictive analytics and application of personalized medicine tools in clinical practice for preventing and managing heart disease in cancer patients and survivors. Currently, the team is building a clinical decision aid based on data science and artificial intelligence principles to assist physicians in ascertaining risk level for cardiovascular toxicity in cancer patients. Part of the overarching project requires collaboration among MCW, MSOE, and UWM to bring together regional expertise in health informatics, natural language processing, machine learning, and supercomputing with the goal to reproduce and validate artificial intelligence algorithms that use deidentified patient data gathered from F&MCW databases and electronic health records to guide prediction. The team also collaborates with qualitative scientists external to the core team, for conduct and analyses of focus groups of clinicians and patients.
3.3. Team meetings and dynamics
The importance of team science was emphasized as the interdisciplinary team was built for artificial intelligence and informatics work in cardiology. A core team of approximately 10 individuals was gathered to meet on a weekly basis, and a broader team of ~45 individuals was brought together to meet on a monthly basis or remain connected through email discussions or ad hoc meetings (Fig. 1, Fig. 2). These meetings and team interactions have been ongoing for 18 months since project inception. While in-person meetings can carry tremendous benefit, virtual meetings have been the lifeline of the group. This was particularly useful as the team was established in the midst of the coronavirus disease 2019 (COVID-19) pandemic. Consequently, the team was able to socially distance and maintain safety of all team members through virtual meetings. This also facilitated scheduling meetings across disciplines, institutions, and state lines. Given the geographical distribution of team constituents, virtual meetings offered facile recurring communication opportunities and helped facilitate team success. Meetings were scheduled by the team's appointed project manager, who maintains and distributes the team calendar, agenda, minutes, and action items.
The core team involves institutions from the SE WI CTSI, including MCW, UWM, and MSOE and meets for one hour at the beginning of each week. The project manager facilitates each team meeting, in partnership with the team leader. The broader monthly group meets at the end of each month, with similar support. Frequent ad hoc meetings and working sessions are also pursued to focus on subgroups, such as the data teamlet, and compliance and legal discussions. The team is currently writing multiple manuscripts together describing the work with natural language processing and machine learning, and team members across various career stages and institutions are participating in manuscript writing. The team is currently enrolling in a team science course to take the understanding and implementation of team science to the next level.
The team serves as an example for other groups across team core and partner institutions, as the team advances institutional priorities. The team's core institution (MCW) has strategic priorities that include:
-
•
Preferred Choice – serve as the preferred choice for patient care [and clinical research] in the region;
-
•
Accelerate Discovery – advance efforts in research for biomedical discoveries with federal, society, and foundation funding;
-
•
Think Next Gen – train students, residents, and fellows to be excellent clinicians and innovative forward thinkers;
-
•
Health of Our Community - partner with community leaders inside and outside the institution to improve health patterns and combat disparities;
-
•
Health Starts from Within – support and encourage each employee or trainee to pursue and achieve optimal wellness goals.
The team is demonstrating success in all of these areas for the team core and partner institutions as a collective group, as follows. The team includes a member of the community in the region who serves as a patient advocate with both clinical and social experience with the topics of team projects. Additionally, the team partners with cardiology and oncology clinicians that provide excellent care for patients with medical conditions studied in team projects. The team's research projects help inform clinical practice and incorporation of informatics in cardiology, oncology, and cardio-oncology for patients. The research group has begun investigations using peer-reviewed institutional grants that are federally funded. Trainees are closely involved in the team, which includes undergraduate, graduate, and medical students, as well as medical residents. The research team also includes experts in population science and health disparities and emphasizes the inclusion of ethnical and racial minorities in the epidemiological patient study cohort. Each team meeting starts with informal conversations checking in on team members' wellbeing and recent recreational activities, to support and encourage each other towards optimal health from within.
3.4. Multi-institutional team leaders and participants
To pursue this and subsequent studies, a multi-institutional interdisciplinary research group has been assembled with expertise in all of the disciplines listed above, along with data science, integration of personalized care tools in the electronic health record, and innovation to achieve equitable health outcomes in racial and ethnic minorities. The junior faculty team leader and team funding principal investigator (PI) is an Assistant Professor of Medicine and the Director of Cardio-Oncology in the Division of Cardiovascular Medicine at MCW. In the data science realm, she has had various levels of training in and exposure to Bioinformatics, Biomedical Informatics, Computational Biology, and Clinical Informatics. The PI maintains a formal appointment as Research Collaborator at Mayo Clinic in Rochester MN, working with Mayo faculty board certified in both cardiology and clinical informatics. Her health services research focuses on precision and systems medicine, artificial intelligence/machine learning, and informatics. The team funding MCW co-PI is fellowship-trained and board-certified in clinical informatics and health services research. He is also the Medical Director for Digital Health and Innovation at Inception Health (owned by F&MCW) and executive faculty member of the Collaborative for Healthcare Delivery Science within the Center for Advancing Population Science at MCW. The team funding MSOE co-PI is a Professor of Computer Science at MSOE. His expertise is in software development for artificial intelligence and data science using supercomputers such as Rosie, the high-performance computing cluster at MSOE. The team funding UWM co-PI is a Professor in the Department of Electrical Engineering and Computer Science at UWM. He has over 30 years of research experience in signal processing, image processing, and machine learning and applications. For the past 10 years, his research has focused on applying machine learning to medical data analysis. In addition, the team co-investigators at Cleveland Clinic recently published research by the multi-institutional team partners at Cleveland Clinic in the Journal of the American Heart Association (JAHA) and PLoS Medicine has shown that cardiovascular disease outcomes in cancer survivors can be predicted using machine learning techniques [28], [31]. Working together, the collective team can produce translational research that is more innovative, generalizable, and impactful than they could working as individual researchers or institutions in isolation.
In addition to the team funding PI and co-PIs, the team funding co-investigators include the Chief Research Informatics Officer at the MCW CTSI. He leads the development of applied clinical research informatics for MCW system's clinicians, researchers and faculty. His team supports biomedical informatics multi-institutional collaborations across CTSI hubs. Another team funding co-investigator is an expert in cardio-oncology research and bioinformatics. She provides consultation on the collection and export of data from F&MCW databases for future artificial intelligence algorithm computations. A third team funding co-investigator is an Associate Professor of Biostatistics. He has research expertise in the application of artificial intelligence and machine learning to pertinent biostatistical questions. He assists with the development and execution of methods for team research proposals and guides data analyses. A UWM team funding co-investigator is an Associate Professor of Health Informatics and Administration and the Director of the Health Informatics graduate training program. His expertise is in health data science and predictive analysis for health outcomes using clinical data, such as modeling events in clinical trials and using clinical notes to predict disease. A Cleveland Clinic co-investigator is a lead author on a machine learning (artificial intelligence) publication in cardio-oncology with the team [31]. He developed and validated a cardio-oncology artificial intelligence algorithm to predict mortality and CVD outcomes in cancer survivors using the electronic health record, and the algorithm will be adapted and operationalized regionally in SE WI through the collaborative multi-institutional team efforts. A Mayo Clinic team funding co-investigator/consultant is an expert in the application of artificial intelligence in cardiology with ensuring health equity in algorithm training and predictions.
3.5. Team building
The research team includes clinicians, translational scientists, population scientists, data scientists, computer scientists, community engagement specialists, and patient advocates, among others with a commitment to the team building process. To build the cohesive partnership, the team is pursuing together the external team development course through MCW CTSI (funded by MCW CTSI mini-grants). The team is also dedicating portions of team meetings to team science journal/book clubs to continue to discuss and apply team science principles [32], [33]. All of the team's funding principal investigators routinely work and publish in teams throughout their research career using team science principles.
3.6. Team personnel
Together, the individuals in the team's project manager (provided by the SE WI CTSI) and research program manager (provided by the F&MCW Clinical Trials Office) roles work closely together with the weekly core team members to coordinate project components by tracking progress, managing deadlines and milestone targets, and facilitating communication among multiple project members and sites, including management of weekly and monthly team meetings, minutes, and action items. They assist with team reports and assessments, and data entry and analysis. Additionally, they help with the creation of the project's final report, outlining outcomes and planned submissions for external funding. Their positions help ensure the project team meets the appropriate milestones for the project's timeline. Both also assist in grant application creation, including tasks such as obtaining supplemental documents from appropriate investigators (i.e., NIH biosketches, Letters of Support, etc.), helping compile budgetary information, and ensuring all required components are in proper format for timely submission. Individual team members help with manuscript preparation and publication arrangement (i.e., proper formatting, submission, and payment coordination). The team members in the data analyst/scientist roles assist with advanced data management. They query, pull, map, format, validate, and confirm data from the CRDW. They also mine, clean, interpret, and validate structured and unstructured data from electronic medical records and other databases to ensure accurate, complete, and uniform data to produce actionable insights. They help acquire data from various sources and maintain project databases, with assurance of statistical quality and efficiency. They organize and analyze large amounts of data, using software specifically designed for the tasks. The data team members are using to varying extents high-performance supercomputing clusters at F&MCW, MSOE, and Cleveland Clinic.
3.7. Team funding
Initial team funding by the National Institutes of Health has supported the team to identify the epidemiological study population and build custom deidentified data extracts from the CRDW housed at F&MCW. The resultant database will serve as input for artificial intelligence algorithms developed and run at team partner institutions. The team also plans to seek additional future federal and foundation or society extramural funding. Extramural funding will support expansion of team efforts using machine learning for predictive analytics in the electronic health record to prevent cardiovascular disease in cancer patients and survivors. The long-term goal of the project is to build automated systems within the electronic health records to facilitate clinicians' identifying and mitigating the risk of cardiovascular events among cancer patients.
3.8. CTSI team support
The team leverages CTSI support in the following ways. The CTSI CRDW at F&MCW has been used to identify and extract the deidentified epidemiological cohort with study-specific variables for this population. Patient data may be stored in the CTSI REDCap database. A CTSI Project Manager coordinates project components and keeps the team milestones on track. In addition to the machine learning biostatistician on the team, the team has the opportunity if needed to apply for CTSI Biostatistics, Epidemiology and Research Design Mini-Grants, through which the team has access to additional limited assistance with data analysis plans, sample size calculations, short preliminary analyses related to study design, consultation for project aims and study design, and preliminary data analysis and interpretation of findings. The team also works closely with members of other related teams hosted by the SE WI CTSI. Further, CTSI mini-grants are being obtained for the team science course.
3.9. Team performance
Indicators of successful team performance include the number of publications and their impact factors, the number of citations and the relative citation ratio, the degree to which research teams are cross-disciplinary, the number of scientific presentations and variety of discipline-based venues; and the number and diversity of trainees [34]. The team so far is substantially cross-disciplinary (Fig. 1, Fig. 2), involves approximately 10 trainees (medical residents and medical and undergraduate/graduate students), and has led to an institutional presentation for a federally funded program, with additional novel publications in the works.
3.10. Epidemiological cohort
3.10.1. Demographics
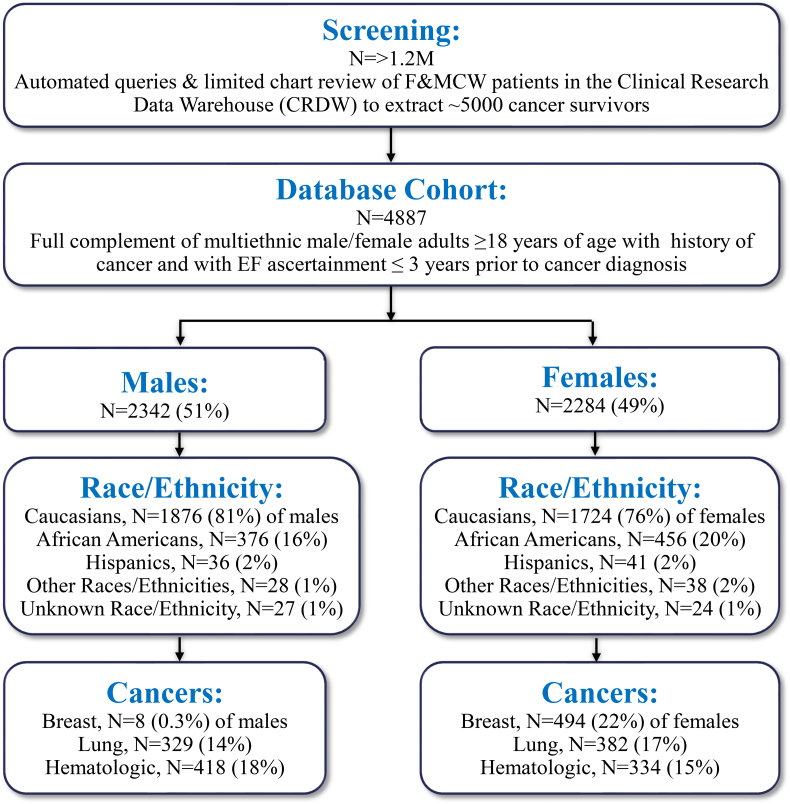
So far, the collaborative teamwork has been underway for 18 months throughout the COVID-19 pandemic. IRB approval for building the patient cohort has been granted, with the IRB approval process for imminent prospective portions of the study currently ongoing. The team has prepared deidentified data for ~5000 cancer survivors and has generated domain-specific data analyses for this population from the CRDW (Fig. 1, Table 1). In the longitudinal cohort (n = 4626) spanning 2000–2020, there were 2343 (51%) men, 2283 (49%) women, 3600 (78%) Caucasians, 834 (18%) African Americans, 77 (2%) Hispanics, 66 (1%) other races/ethnicities, and 51 (1%) unknown, with overall age range 18–90 years old (Fig. 3). Among Caucasians, 1876 (52%) were males and 1724 (48%) were females. Among African Americans, 376 (45%) were males and 456 (55%) were females. Among Hispanics, 36 (1%) were males and 41 (1%) were females. Among other races/ethnicities, 28 (42%) were males and 38 (58%) were females. Among those of unknown race/ethnicity, 27 (53%) were males and 24 (47%) were females. The majority of cancers represented were breast cancer (502, 11%), lung cancer (711, 16%), hematologic cancers (744, 16%), and prostate cancer (401, 9%). Among those with breast cancer, 8 (2%) of the entire cohort were men and 494 (98%) were women. Among those with lung cancer 329 (46%) were men and 382 (54%) were women. Among those with hematologic cancers, 418 (56%) were men and 334 (44%) were women.
Fig. 3.
Epidemiological cohort database flowchart.
3.10.2. Cardiovascular outcomes and comorbidities
Prior to cancer diagnosis in the overall cohort (n = 4626), 2253 (49%) individuals had baseline systolic or diastolic heart failure, 459 (10%) individuals had baseline cardiomyopathy, 1027 (22%) individuals had baseline atrial fibrillation, 2037 (44%) individuals had baseline coronary artery disease, 924 (20%) individuals had a baseline history of ischemic or hemorrhagic stroke, 1265 (27%) individuals had baseline peripheral artery disease, 1092 (24%) individuals had baseline diabetes mellitus, 3567 (77%) individuals had baseline hypertension, 2788 (60%) individuals had baseline hyperlipidemia (Table 1). Over the years after cancer diagnosis in the cohort, 573 (13%) individuals developed new systolic or diastolic heart failure, 352 (8%) individuals developed new cardiomyopathy, 487 (11%) individuals developed new atrial fibrillation, 632 (14%) individuals developed new coronary artery disease, 387 (8%) individuals experienced an ischemic or hemorrhagic stroke, 602 (13%) individuals developed new peripheral artery disease, 351 (8%) individuals developed new diabetes mellitus, 420 (9%) individuals developed new hypertension, 485 (11%) individuals developed new hyperlipidemia (Table 1).
3.10.3. Cancer therapies
In the overall cohort (n = 4626), 1845 (40%) individuals were treated with any pharmacologic cancer therapy, 980 (21%) with alkylating agents, 412 (9%) with various antibodies, 57 (1%) with HER2 agents, with antineoplastic antibodies (anthracyclines), 655 (14%) with endocrine therapies, 275 (6%) with enzyme inhibitors, 667 (14%) with mitotic inhibitors, and 1032 (22%) with other cancer drug classes (Table 2). Among those treated with any pharmacologic cancer therapy, 961 (52%) were men and 884 (48%) were women. Among those treated with alkylating agents, 447 (46%) were men and 533 (54%) were women. Among those treated with various antibodies, 224 (54%) were men and 191 (46%) were women. Among those treated with HER2 agents, 9 (16%) were men and 48 (84%) were women. Among those treated with endocrine therapies, 231 (35%) were men and 424 (65%) were women. Among those treated with enzyme inhibitors, 133 (48%) were men and 142 (52%) were women. Among those treated with mitotic inhibitors, 282 (42%) were men and 385 (58%) were women. Among those treated with other cancer agents, 567 (55%) were men and 465 (45%) were women. In the available cohort (n = 2300; 2015–2020) from the MOSAIQ Radiation Oncology database, 564 (25%) individuals received radiation therapy, of whom 275 (49%) were men, and of whom 289 (51%) were women.
Table 2.
Frequency of new development of cardiovascular outcomes over 20 years of follow-up in the epidemiological cohort (n = 4626).
| New cardiovascular diagnosis | Any N (%;p)a |
Alk N (%;p) |
Ab N (%;p) |
Antib N (%;p) |
Antim N (%;p) |
Endo N (%;p) |
Enz N (%;p) |
Mit N (%;p) |
Other N (%;p) |
|---|---|---|---|---|---|---|---|---|---|
| Atrial Fibrillation | 224 (12; 0.004) |
111 (11; 0.359) |
46 (12; 0.373) |
60 (12; 0.406) |
79 (11; 0.417) |
69 (11; 0.995) |
41 (15; 0.015) |
81 (12; 0.142) |
83 (12; 0.292) |
| Coronary Artery Disease | 267 (15; 0.192) |
136 (14; 0.825) |
58 (15; 0.441) |
83 (16; 0.097) |
101 (15; 0.448) |
105 (16; 0.0568) |
47 (17; 0.088) |
94 (14; 0.726) |
100 (14; 0.759) |
| Cardiomegaly | 254 (14; 0.0001) |
145 (15; 0.0004) |
65 (17; 0.0008) |
89 (17; <0.0001) |
110 (16; 0.0001) |
101 (16; 0.0009) |
47 (17; 0.0031) |
109 (16; <0.0001) |
100 (14; 0.026) |
| Cardiomyopathy | 190 (10; <0.0001) |
104 (11; 0.0001) |
53 (14; <0.0001) |
59 (11; 0.006) |
70 (10; 0.007) |
61 (9; 0.0759) |
51 (19; <0.0001) |
65 (10; 0.0245) |
85 (12; 0.0001) |
| Heart Failure | 281 (15; <0.0001) |
155 (16; 0.0002) |
71 (18; 0.0002) |
83 (16; 0.008) |
114 (17; 0.0004) |
111 (17; 0.0001) |
58 (21; <0.0001) |
105 (16; 0.0045) |
100 (14; 0.149) |
| Peripheral Artery Disease | 2594 (14; 0.092) |
134 (14; 0.489) |
60 (16; 0.134) |
68 (13; 0.935) |
94 (14; 0.640) |
114 (17; 0.0003) |
35 (13; 0.884) |
101 (15; 0.077) |
74 (10; 0.023) |
| Stroke | 166 (9; 0.206) |
89 (9; 0.362) |
41 (11; 0.102) |
58 (11; 0.0135) |
70 (10; 0.074) |
69 (11; 0.031) |
26 (10; 0.501) |
69 (10; 0.046) |
65 (9; 0.431) |
Ab: Antibodies; Alk: Alkylating agents; Antib: Antineoplastic antibodies (anthracyclines); Antim: Antimetabolites; Endo: Endocrine therapies; Enz: Enzyme inhibitors; Mitotic Inhibitors; Other: Other pharmacologic cancer therapies.
Percentage of individuals treated with cancer therapy who developed cardiovascular outcome or comorbidity after cancer diagnosis; compared to individuals treated with cancer therapy who did not develop cardiovascular outcome or comorbidity after cancer diagnosis; bold indicates statistical significance using a p-value<0.005, due to multiple comparisons.
3.10.4. Impact of cancer therapies on cardiovascular outcomes
In our preliminary analyses, the types of cancer therapies received in the cohort determined the development of cardiovascular outcomes (Table 2). As anticipated, the development of cardiomyopathy was particularly statistically significant for those treated with any cancer therapy, antibodies (including trastuzumab), and enzyme inhibitors (such as the tyrosine kinase inhibitor imatinib), as well as mitotic inhibitors (such as paclitaxel which is usually administered with anthracycline therapy for breast cancer). There was a trend towards the development of cardiomyopathy in those treated with antineoplastic antibiotics (anthracyclines), as well as those treated with mitotic inhibitors (such as paclitaxel which is usually administered with anthracycline therapy for breast cancer). Of note, the development of cardiomegaly was particularly statistically significant for those treated with any cancer therapy, antineoplastic antibiotics (anthracyclines) and mitotic inhibitors (such as paclitaxel which is usually administered with anthracycline therapy for breast cancer), as well as alkylating agents, antibodies (including trastuzumab), antimetabolites, endocrine therapy, and enzyme inhibitors (such as the tyrosine kinase inhibitor imatinib). The development of atrial fibrillation was significant in those treated with any cancer therapy, with a trend towards significance in those treated with enzyme inhibitors (such as the tyrosine kinase inhibitor imatinib or ibrutinib).
4. Discussion
Clinicians, researchers, and individuals with diverse scientific backgrounds and complementary interdisciplinary perspectives with a rich and wide knowledge base have been brought together to establish a team of Cardio-Oncology Artificial Intelligence and Precision (CAIP) research investigators (Fig. 1, Fig. 2, Table 3). The group is composed of members with expertise in wide-ranging disciplines. The team members bring key research skills allowing the team to approach the overall problem of cardiovascular health among cancer patients in new and innovative ways. Team principles evident in the group include a high diversity of membership, deep knowledge integration, and geographic dispersion of team members [32], [33], as well as high task interdependence. The members of the team work together and depend on each other to complete tasks. Individual tasks contribute to the larger goal of the team and the project. The weekly meetings promote this aspect. As is typical in other successful interdisciplinary teams [35], the team includes research and data scientists, statisticians, clinicians, entrepreneurs, and trainees – with many individuals wearing more than one of these hats.
Table 3.
Main components of multi-institutional interdisciplinary team development for collaborative pursuit of artificial intelligence and other health informatics tools in cardio-oncology.
| Component | Key considerations |
|---|---|
| Group Members | Interdisciplinary, multi-institutional, diverse expertise, different stages of training and career |
| Meeting Structure | One large group vs. smaller workgroups, open communication vs. hierarchical (within workgroups) |
| Ground Rules | Respect for diversity in perspectives & training, multiple formats for exchanging ideas |
| Data Management | Methodology needed for data sharing, pooling patient cohorts, and testing algorithms |
Team project steps are foundational for subsequently building clinical decision aids based on the artificial intelligence algorithm and other machine learning predictive analytics, regionally. The team has created a database of ~5000 cancer survivors, with laboratory, physiology, demographics, outcomes, medications, imaging, and medical history information relevant to cardiovascular risk ascertainment in cancer survivors. This information is consistent with the data needed for input to the artificial intelligence algorithm published by our multi-institutional team members [27], [28]. While the findings from initial analyses are provided here, more granular analyses are ongoing, including testing for interactions and adjusting for age and other potential confounders. Working closely with the machine learning biostatistician, the data team members will collectively apply the artificial intelligence algorithms to F&MCW patient data to ultimately build a clinical decision aid for physicians to use with patients to help prevent cardiovascular disease in cancer survivors. The primary long-term goal of team projects is to complete the creation and integration of clinical decision aids in electronic health records for use by cardiologists, oncologists, and other clinicians to improve outcomes for cardio-oncology patients using artificial intelligence models for more accurate prediction. As feedback from clinician users will be incorporated into future iterations of the clinical decision aids, this interdisciplinary clinical and translational research will thrive in the learning health system in this illustrative “bedside to bench to bedside” approach.
Another goal being achieved by the team is building a stronger medical tech community in SE WI, regarding artificial intelligence and data science. Expertise from CTSI member institutions in SE WI in the team has been combined to create a bridge between the use of high-performance supercomputing clusters at MCW and MSOE. This allows for running algorithms and analyses on secure patient data in controlled environments by the team in this newly developed informatics community in SE WI. The supercomputers are instrumental in accurately reproducing output of the artificial intelligence algorithm prediction models for outcomes of cancer patients with cardiovascular disease. Leveraging the state-of-the-art, high-performance supercomputing center at MSOE facilitates one of MSOE's strategic initiatives of exceptional learning and discovery. Partnering with the CTSI and other member institutions in SE WI on this community-engaged health informatics project embodies the MSOE strategic plan's byline, being Extraordinary Together [36].
An additional goal successfully being accomplished by the team is expanding collaborative research in advanced medical machine learning applications among MCW, MSOE, and UWM, combining strengths in medicine, advanced computing, and facilities, and strong PhD research programs at UWM in electrical engineering, computer sciences, and health sciences. The collaborative team will continue conducting research activities that will lead to future extramural grant funding and ultimately to tangible clinical improvements. The multi-institutional team will continue to integrate research into clinical workflows of the health systems, analyze data from electronic health records, and advance the applications of research-related information technology.
The team has parsed initial information regarding racial and ethnic minorities in the database of ~5000 cancer survivors. The existing and future models are built on databases that include African American cancer survivors. Application of these algorithms to data from diverse patient populations will hopefully continuously provide output to help assess and address health disparities in Milwaukee. Motivated by the core location in SE WI, team studies on subpopulations from among the established epidemiological cohort of ~5000 will intentionally oversample ethnic and racial minorities among the population, particularly African Americans, given the prevalence of related cardiovascular health disparities in cancer survivors [15], [16], [17], [18], [19]. Indeed, research proposals in the team will purposefully recruit sufficient representatives from this high-risk population to draw statistically significant conclusions and examine the ability of this approach to improve disparities research in cardio-oncology. This is the first of many collaborative team publications for the dissemination of findings to the broader medical and scientific community. With the publication of each collaborative study, the team will advance the establishment of this multi-institutional center for high-performance computing informatics to protect the hearts of cancer survivors, especially for racial and ethnic minorities. Publication of these findings could be ground-breaking for furthering efforts at applying artificial intelligence algorithms for optimal personalized patient care in cardio-oncology. The epidemiological cohort published by our team can serve as an external validation cohort for cross-institution collaborations and consortia. In the Cardiology Oncology Innovation Network (COIN; CardioOncCOIN.Org), for example, providing this large sample size for collaborating institutions in this consortium can advance applications of artificial intelligence and other health informatics methodologies to overcome current limitations in Cardio-Oncology regarding small sample sizes in this specialized field.
Lessons learned in the team building process include the following. Team building is necessary to build a foundation for successfully applying the artificial intelligence algorithms in cardio-oncology. Highly organized and well-structured team meetings help streamline and clarify team goals, vision, and output. Frequent participation and engagement by all team members in team communications, whether in the large group meeting and/or in the meetings of smaller teamlets, promotes unified forward in a singular direction. A variety of perspectives on the team is essential to help sort out obstacles and surmount or navigate roadblocks. Various backgrounds, experience, and expertise among team members informs the best ways in which to take on and eliminate these obstacles. Understanding differences among institutions regarding access to resources and managing of data and so on help generalize these practices for groups performing team science. It is key that the team as a whole creates and adheres to timelines and milestones in order to advance and make timely and meaningful progress together. This is especially important when the team has funding which requires periodic reports to the sponsoring group such as the National Institutes of Health to keep track of the utilization of funds and achievement of deliverables and milestone goals. Some team meetings are in the format of workshops as needed, to enhance the development, training, and knowledge acquisition and translation of the collective team relevant to the selected projects. Teaching of the team across education levels and areas of expertise from multiple team members over the course of the team's tenure together can be useful to share knowledge among the group, so that team members can better understand and participate in discussions of esoteric or specialized topics that are needed for the research projects together. Challenges from previous literature that have also been noted and overcome include scheduling conflicts for meetings albeit virtual, managing multiple points of view, enhancing project management, and addressing “language barriers” across disciplines [37], [38], [39]. In conclusion, interdisciplinary barriers have been overcome and a multi-institutional team has been established, bringing the disciplines and skills required to implement artificial intelligence algorithms in clinical decision aid for cardio-oncology that will ultimately be integrated into the electronic health records to promote health equity and improve health outcomes in individuals with cancer.
Funding
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Numbers UL1TR001436 and KL2TR001438. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
CRediT authorship contribution statement
Conception and design: SAB.
Drafting of the manuscript: SAB.
Interpretation of data: SAB, RS, KO, JZ, JB, FC, AH, MB, JC, AK, PC, PN, RHJ, KH, LYS, BC, YCC, JO.
Critical revision: SAB, KO, AH, MB, JC, AK, RHJ, KH, LYS, BC, JO.
Final approval of manuscript: All authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Katzenbach J., Smith D. Harvard Business School Press; 1993. The Wisdom of Teams: Creating the High-performance Organization. [Google Scholar]
- 2.Sandor M., Copeland D., Robinson S. Team-building interventions for interdisciplinary teams: a case study of a pediatric client. 1998;23(6) doi: 10.1002/j.2048-7940.1998.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 3.Foraker R.E., Benziger C.P., DeBarmore B.M., Cené C.W., Loustalot F., Khan Y., et al. Achieving optimal population cardiovascular health requires an interdisciplinary team and a learning healthcare system: a scientific statement from the American Heart Association. Circulation. 2021;143(2):e9–e18. doi: 10.1161/CIR.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maddox T.M., Albert N.M., Borden W.B., Curtis L.H., Ferguson T.B., Kao D.P., et al. The learning healthcare system and cardiovascular care: a scientific statement from the American Heart Association. Circulation. 2017;135(14):e826–e857. doi: 10.1161/CIR.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 5.Brown S.A., Patel S., Rayan D., Zaharova S., Lin M., Nafee T., et al. A virtual-hybrid approach to launching a cardio-oncology clinic during a pandemic. Cardiooncology. 2021;7(1):2. doi: 10.1186/s40959-020-00088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prevention CfDCa. Leading Causes for Death: National Center for Health Statistics, FastStats Service; [Available from: https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm.
- 7.Centers for Disease Control and Prevention Leading Causes for Death: National Center for Health Statistics, FastStats Service. 2018. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm Available from:
- 8.Mehta L.S., Watson K.E., Barac A., Beckie T.M., Bittner V., Cruz-Flores S., et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137(8):e30–e66. doi: 10.1161/CIR.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Squires R.W., Shultz A.M., Herrmann J. Exercise training and cardiovascular health in cancer patients. Curr. Oncol. Rep. 2018;20(3):27. doi: 10.1007/s11912-018-0681-2. [DOI] [PubMed] [Google Scholar]
- 10.Patnaik J.L., Byers T., DiGuiseppi C., Dabelea D., Denberg T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow E.J., Leger K.J., Bhatt N.S., Mulrooney D.A., Ross C.J., Aggarwal S., et al. Paediatric cardio-oncology: epidemiology, screening, prevention, and treatment. Cardiovasc. Res. 2019;115(5):922–934. doi: 10.1093/cvr/cvz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J. Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 13.HN Noone A.M., Krapcho M., Miller D., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., Chen H.S., Feuer E.J., Cronin K.A., editors. SEER Cancer Statistics Review, 1975-2015, Based on November 2017 SEER Data Submission, Posted to the SEER Web Site, April 2018. National Cancer Institute; Bethesda, MD: 2018. https://seer.cancer.gov/csr/1975_2015/ Available from: [Google Scholar]
- 14.Miller K.D., Nogueira L., Mariotto A.B., Rowland J.H., Yabroff K.R., Alfano C.M., et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 15.Hasan S., Dinh K., Lombardo F., Kark J. Doxorubicin cardiotoxicity in african americans. J. Natl. Med. Assoc. 2004;96(2):196–199. [PMC free article] [PubMed] [Google Scholar]
- 16.Lotrionte M., Biondi-Zoccai G., Abbate A., Lanzetta G., D'Ascenzo F., Malavasi V., et al. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am. J. Cardiol. 2013;112(12):1980–1984. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Finkelman B.S., Putt M., Wang T., Wang L., Narayan H., Domchek S., et al. Arginine-nitric oxide metabolites and cardiac dysfunction in patients with breast cancer. J. Am. Coll. Cardiol. 2017;70(2):152–162. doi: 10.1016/j.jacc.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litvak A., Batukbhai B., Russell S.D., Tsai H.L., Rosner G.L., Jeter S.C., et al. Racial disparities in the rate of cardiotoxicity of HER2-targeted therapies among women with early breast cancer. Cancer. 2018;124(9):1904–1911. doi: 10.1002/cncr.31260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baron K.B., Brown J.R., Heiss B.L., Marshall J., Tait N., Tkaczuk K.H., et al. Trastuzumab-induced cardiomyopathy: incidence and associated risk factors in an inner-city population. J. Card. Fail. 2014;20(8):555–559. doi: 10.1016/j.cardfail.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Greer D., Baumgardner D., Bridgewater F., Frazer D., Kessler C., et al. LeCounte E. Milwaukee Health Report 2013: Health Disparities in Milwaukee by Socioeconomic Status Milwaukee, WI: Center for Urban Population Health. 2013. https://institutionalrepository.aah.org/pop/1/ Available from:
- 21.City of Milwaukee Community Health Assessment: Understanding the Health Needs of Our Community Milwaukee, WI: City of Milwaukee. 2015-2016. https://city.milwaukee.gov/ImageLibrary/Groups/healthAuthors/ADMIN/PDFs/Reports/MKEHealthDeptCommunityHealthAssessment2015-2016.pdf Available from:
- 22.Prasad P., Branch M., Asemota D., Elsayed R., Addison D., Brown S.-A. Cardio-oncology preventive care: racial and ethnic disparities. Curr. Cardiovasc. Risk Rep. 2020;14(10):18. [Google Scholar]
- 23.Fazal M., Malisa J., Rhee J.W., Witteles R.M., Rodriguez F. Racial and ethnic disparities in cardio-oncology: a call to action. JACC CardioOncol. 2021;3(2):201–204. doi: 10.1016/j.jaccao.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohman R.Y.E., Abel M. Inequity in cardio-oncology: identifying disparities in cardiotoxicity and links to cardiac and cancer outcomes. JAHA. 2021;10 doi: 10.1161/JAHA.121.023852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundlöf D.W., Patel B.D., Schadler K.C., Biggs R.G., Silverstein Fadlon C.A., Corotto P.S., et al. Development of a cardio-oncology program in a community hospital. JACC CardioOncol. 2019;1(2):310–313. doi: 10.1016/j.jaccao.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silver S. A multidisciplinary allied health faculty team: formation and first year production of problem-based learning in gerontology/geriatrics. J. Allied Health. 1998;27(2) [PubMed] [Google Scholar]
- 27.Chan H.P., Hadjiiski L., Zhou C., Sahiner B. Computer-aided diagnosis of lung cancer and pulmonary embolism in computed tomography-a review. Acad. Radiol. 2008;15(5):535–555. doi: 10.1016/j.acra.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou Y., Zhou Y., Hussain M., Budd G.T., Tang W.H.W., Abraham J., et al. Cardiac risk stratification in cancer patients: a longitudinal patient-patient network analysis. PLoS Med. 2021;18(8) doi: 10.1371/journal.pmed.1003736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kluwer Wolters. Wolters Kluwer. 2021. https://www.wolterskluwer.com/en/solutions/medi-span/medi-span Available from:
- 30.Elekta MOSAIQ Radiation Oncology: Elekta. 2021. https://www.elekta.com/software-solutions/care-management/mosaiq-radiation-oncology/ Available from:
- 31.Zhou Y., Hou Y., Hussain M., Brown S.A., Budd T., Tang W.H.W., et al. Machine learning-based risk assessment for cancer therapy-related cardiac dysfunction in 4300 longitudinal oncology patients. J. Am. Heart Assoc. 2020;9(23) doi: 10.1161/JAHA.120.019628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett L.M., Gadlin H. Collaboration and team science: from theory to practice. J. Investig. Med. 2012;60(5):768–775. doi: 10.231/JIM.0b013e318250871d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooke N., Hilton M. The National Academies Press; Washington, D.C.: 2015. Enhancing the Effectiveness of Team Science. 280 p. [PubMed] [Google Scholar]
- 34.Vogel A., Knebel A., Faupel-Badger J., Portilla L., Simeonov A. A systems approach to enable effective team science from the internal research program of the National Center for Advancing Translational Sciences. J. Clin. Transl. Sci. 2021;5(1) doi: 10.1017/cts.2021.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel M., Moseley T., Nia E., Perez F., Kapoor M., Whitman G. Team science: a practical approach to starting collaborative projects. J.Breast Imaging. 2021;3(6) doi: 10.1093/jbi/wbab034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MSOE University Extraordinary Together MSOE University Board of Regents. 2018-2020. https://www.msoe.edu/about-msoe/strategicplan/ Available from:
- 37.Guise J.-M., Geller S., Regensteiner J.G., Raymond N., Nagel J. Team mentoring for interdisciplinary team science: lessons F... : academic medicine. Acad. Med. 2021;92(2):214–221. doi: 10.1097/ACM.0000000000001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karam E., Lévesque M., Jacquemin G., Delure A., Robidoux I., Laramée M., et al. Building a multidisciplinary team for burn treatment – lessons learned from the Montreal tendon transfer experience. Ann Burns Fire Disast. 2014;27(1):3–7. [PMC free article] [PubMed] [Google Scholar]
- 39.Wiltschko A.B. Building an interdisciplinary team set on bringing the sense of smell to computers: iScience. iScience. 2021;24(3) doi: 10.1016/j.isci.2021.102136. [DOI] [PMC free article] [PubMed] [Google Scholar]