Abstract
Human monocytes transport and accumulate ciprofloxacin and other fluoroquinolones. Although little is known about the mechanisms of transport, we expected monocytes to be similar to other cells of myeloid lineage. In the present study, monocyte fluoroquinolone transport was characterized and compared to the corresponding transport pathways of human polymorphonuclear leukocytes (PMNs) and HL-60 cells. Ciprofloxacin transport by monocytes was saturable, temperature dependent, sodium independent, and relatively insensitive to pH. Quiescent monocytes transported ciprofloxacin with a Km of 171 μg/ml and a Vmax of 32.7 ng/min/106 cells. Adenine competitively inhibited ciprofloxacin transport by quiescent monocytes (Ki = 3.8 mM), but nucleosides had no significant inhibitory effect. In all of these respects, transport by monocytes was similar to that observed for quiescent PMNs and immature HL-60 cells. Unlike PMNs, however, monocytes and immature HL-60 cells did not exhibit dramatically enhanced ciprofloxacin transport when activated by phorbol myristate acetate (PMA). Consistent with this finding, HL-60 cells committed to granulocytic differentiation exhibited a significant component of PMA-inducible ciprofloxacin transport activity, while HL-60 cells committed to monocytic differentiation did not. In PMNs, the PMA-inducible component of transport appeared to be mobilized from a granule compartment, since its activity could be modulated by agents that enhance or inhibit stimulated degranulation. Thus, quiescent monocytes, PMNs, and HL-60 cells take up ciprofloxacin via similar energy-dependent transport mechanisms. Unlike granulocytes, monocytes do not express a second, higher-affinity pathway for ciprofloxacin accumulation when they are activated by PMA.
Phagocytic killing by polymorphonuclear leukocytes (PMNs), monocytes, and macrophages is the primary host defense against bacterial infections. Despite the effectiveness of this defense, Salmonella and other intracellular pathogens can invade phagocytes and survive inside them, avoiding the lysosomal compartment (5, 9). Cellular invasion is an important step in the progression of many serious bacterial infections because it allows pathogens to evade host defense mechanisms and benefit from a rich nutrient supply.
Cell-permeating antimicrobial agents can potentially play an important role in eliminating infections by intracellular pathogens. Unfortunately, beta-lactams and cephalosporins do not penetrate the plasma membrane effectively. In contrast, fluoroquinolones accumulate inside phagocytes and are highly active against most aerobic and facultative gram-negative bacteria (11). Resting PMNs and mononuclear phagocytes take up ciprofloxacin so effectively that the intracellular levels of this agent are routinely several times higher than the plasma levels (7, 8, 10, 20). When activated by phorbol esters, PMNs can accumulate ciprofloxacin even more avidly (18). PMNs loaded with ciprofloxacin exhibit enhanced intracellular killing of bacteria relative to control cells that contain no antimicrobial agent (7, 25). The mechanisms by which PMNs accumulate fluoroquinolones are energy dependent and were recently characterized (26). Differences in observed kinetic properties, pH dependence, and response to inhibitors suggest that PMNs possess at least two systems for taking up fluoroquinolones. One is a relatively low-affinity system that operates continuously, while the other is a higher-affinity, higher-velocity system that operates in phorbol myristate acetate (PMA)-activated cells. The low-affinity transport system can be competitively inhibited by adenine, while the high-affinity system is competitively inhibited by a broad range of neutral and cationic amino acids (26).
Comparatively little is known about the mechanisms by which mononuclear phagocytes transport fluoroquinolones. Since monocytes and PMNs share a myeloid lineage, it is reasonable to hypothesize that their fluoroquinolone transport systems are similar. To test this hypothesis, we characterized the transport of ciprofloxacin and other fluoroquinolones in monocytes. In addition, human promyelocytic leukemia (HL-60) cells, which can be committed to granulocytic or monocytic pathways (6, 12), were used as a model system to study changes in fluoroquinolone transport activity during myelomonocytic development. Although quiescent monocytes, PMNs, and HL-60 cells exhibited many similarities, our results suggest that activated PMNs possess a mechanism for high-affinity fluoroquinolone transport that is acquired during granulocytic maturation and is not expressed in activated monocytes or HL-60 cells.
MATERIALS AND METHODS
Isolation of monocytes and PMNs.
Human monocytes and PMNs were isolated from citrated whole blood obtained from healthy volunteers using Ficoll-Hypaque density gradient centrifugation (4). The mononuclear cell layer was aspirated, washed three times with phosphate-buffered saline containing 0.02% EDTA, and resuspended in Hanks' balanced salt solution (HBSS). Monocytes were purified by adherence to sterile plastic tissue culture flasks during 1 h of incubation at 37°C. Nonadherent cells were decanted, and the flasks were washed twice with HBSS. The monocytes were gently scaped into suspension in HBSS. After the Ficoll-Hypaque separation, PMNs were further purified by dextran sedimentation (4). Residual erythrocytes were eliminated by hypotonic lysis, and the remaining PMNs were washed three times with phosphate-buffered saline.
HL-60 cell culture.
Human promyelocytic leukemia cells (HL-60; American Type Culture Collection) were cultured at 37°C in 5% CO2 in RPMI 1640 medium supplemented with 15% heat-inactivated fetal bovine serum. Immature HL-60 cells were differentiated into granulocytic HL-60 cells by culturing with 1.3% dimethyl sulfoxide for 7 days (19). Monocytic differentiation was induced by culturing immature cells with 0.4 mM sodium butyrate for 4 days as previously described (3).
Fluoroquinolone transport.
Transport of ciprofloxacin and other fluoroquinolones was assayed by measuring cell-associated fluorescence as previously described (26). Cells were suspended in HBSS at a density of 5 × 106 cells/ml, warmed to 37°C, and incubated with a fluoroquinolone. The assay was terminated before the end of the linear phase of transport. Aliquots of the cell suspension were rapidly withdrawn, layered over 0.3 ml of canola oil-dibutyl phthalate (3:10), and centrifuged for 45 s at 15,000 × g in a microcentrifuge. The aqueous and oil layers were removed, and the pellet was recovered by cutting off the end of the microcentrifuge tube. The pellet was dispersed in 1.5 ml of 100 mM glycine-HCl (pH 3.0) by agitation at room temperature. The samples were centrifuged at 5,600 × g for 5 min, and the fluorescence of the supernatant was measured with a fluorescence spectrometer. For quantitation of ciprofloxacin, excitation and emission wavelengths of 278 and 445 nm, respectively, were used. For norfloxacin, ofloxacin, and lomefloxacin, the excitation and emission wavelengths were 280 and 440 nm, 292 and 496 nm, and 330 and 452 nm, respectively. The sensitivity of detection of these fluoroquinolones was approximately 1 ng/ml, and their recovery from cell pellets was essentially quantitative. Lineweaver-Burk analysis was used to determine the Km and Vmax of fluoroquinolone transport.
Adenine transport.
The transport of adenine was assayed by measuring cell-associated radioactivity. Experimental conditions were identical to those used to assay fluoroquinolone transport, except that [8-3H]adenine (24 Ci/mmol; Amersham Pharmacia Biotech) was used as the substrate. Liquid scintillation counting was used to measure radioactivity.
Lactoferrin assay.
Specific granule release by PMNs was determined by assaying the amount of lactoferrin released into cell-free supernatants using an established enzyme-linked immunosorbent assay (14). These data were expressed as a percentage of the total cell content of lactoferrin released from cells lysed with 0.1% Triton X-100.
RESULTS
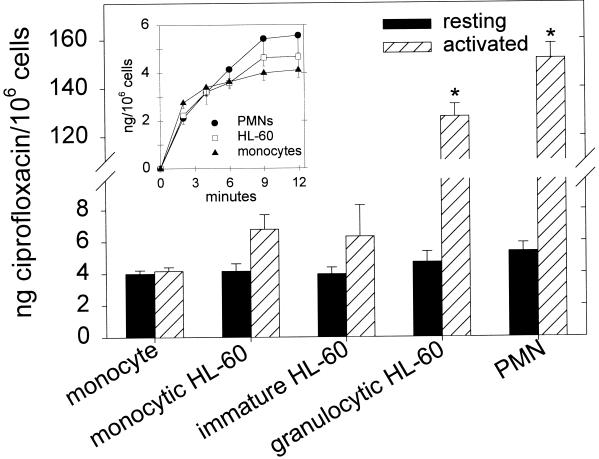
Like PMNs, quiescent human monocytes accumulated ciprofloxacin (5 μg/ml) rapidly, reaching steady-state levels within 12 min (Fig. 1, inset). When normalized to cell number, the steady-state ciprofloxacin content of monocytes was not significantly different from those of resting PMNs, immature HL-60 cells, or HL-60 cells committed to monocytic or granulocytic maturation (P > 0.2, analysis of variance [ANOVA]) (Fig. 1). Like immature HL-60 cells and monocytic HL-60 cells, monocytes activated by PMA exhibited no significant changes in ciprofloxacin accumulation (P > 0.05, paired t test). Likewise, neither tumor necrosis factor alpha (20 ng/ml) nor fMet-Leu-Phe (fMLP, 100 nM) altered monocyte ciprofloxacin accumulation (P > 0.05) (data not shown). In contrast, PMA-activated PMNs and granulocytic HL-60 cells accumulated significantly more ciprofloxacin than their corresponding quiescent controls (P ≤ 0.002). Moreover, fMLP-activated PMNs exhibited transient enhancement of ciprofloxacin accumulation (Fig. 2).
FIG. 1.
Ciprofloxacin accumulation by resting and PMA-activated human monocytes, HL-60 cells, and PMNs. Cells were isolated as described in Materials and Methods and incubated with 5 μg of ciprofloxacin per ml at 37°C until the cellular ciprofloxacin content reached steady state (approximately 12 min). Where indicated, cells were pretreated with 100 nM PMA at the beginning of incubation with ciprofloxacin. The data are presented as the mean and standard error of the mean. There were no significant differences in ciprofloxacin content among the five types of resting myeloid cells tested (P = 0.26, ANOVA). Asterisks indicate that PMA induced a significant increase in cell ciprofloxacin content (P ≤ 0.002, paired t test). (Inset) Time course of ciprofloxacin accumulation by resting monocytes, immature HL-60 cells, and PMNs.
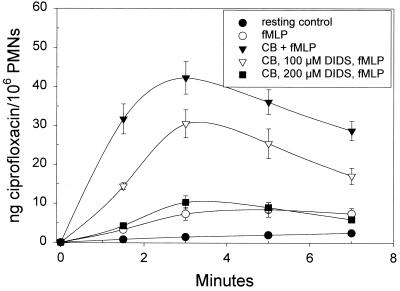
FIG. 2.
Modulation of inducible PMN ciprofloxacin uptake by agents that enhance or inhibit degranulation. Where indicated, PMNs were pretreated for 10 min with 5 μg of cytochalasin B (CB) per ml. In some experiments, PMNs were treated for 3 min with DIDS, an inhibitor of PMN degranulation. The assay was initiated by adding 5 μg of ciprofloxacin per ml and fMLP (or, in the resting controls, ciprofloxacin only). Ciprofloxacin content was assayed as outlined in Materials and Methods and is expressed as the mean and standard error of the mean of three experiments. Ciprofloxacin accumulation by fMLP-activated PMNs was significantly enhanced at all time points when the cells were pretreated with 5 μg of cytochalasin B per ml (P < 0.05, Dunnett's test). When PMNs were exposed to 200 μM DIDS, cytochalasin B had no significant effect on the accumulation of ciprofloxacin by fMLP-activated PMNs (P > 0.05, Dunnett's test).
As shown in Table 1, the Km and Vmax values for monocyte ciprofloxacin transport (171 μg/ml and 32.7 ng/min/106 cells, respectively) were not significantly different from those observed for resting HL-60 monocytes, immature HL-60 cells, HL-60 granulocytes, and PMNs (P > 0.05, ANOVA). When activated by PMA, however, HL-60 granulocytes and PMNs transported ciprofloxacin with a higher affinity than any of the resting cells (P < 0.05). To determine whether this PMA-inducible component of transport might be mobilized from a granule compartment, we examined the effects of agents that modulate PMN degranulation (Fig. 2). Pretreatment with cytochalasin B, which enhances degranulation in response to fMLP, significantly enhanced ciprofloxacin accumulation by fMLP-stimulated PMNs (P < 0.05, Dunnett's test). Under these conditions, the selective protein kinase C inhibitor Go6976 (BioMol Research Laboratories) blocked the activation of ciprofloxacin uptake by fMLP (50% inhibitory concentration, 150 nM) (data not shown). Moreover, treatment with 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), an anion channel blocker that inhibits PMN degranulation (23), profoundly inhibited ciprofloxacin accumulation by cytochalasin B-treated, fMLP-activated PMNs. As the DIDS concentration was incrementally increased from 50 to 200 μM under these conditions, inhibition of ciprofloxacin accumulation was correlated with an inhibition of lactoferrin release from specific granules (r = 0.91). DIDS also inhibited ciprofloxacin accumulation and lactoferrin release in PMA-activated PMNs, and these inhibitory effects were correlated (r = 0.92).
TABLE 1.
Kinetics of ciprofloxacin transport by myeloid cellsa
| Cells | Km (μg/ml) | Vmax (ng/min/106 cells) |
|---|---|---|
| Monocytes | 171* ± 8.27 | 32.7* ± 4.68 |
| Monocytic HL-60 | 169* ± 15.0 | 41.2* ± 2.26 |
| Immature HL-60 | 150* ± 20.4 | 34.4* ± 4.19 |
| Granulocytic HL-60 (resting) | 187* ± 10.7 | 39.8* ± 1.86 |
| Granulocytic HL-60 (PMA activated) | 13.6† ± 1.35 | 38.5* ± 2.51 |
| PMNs (resting) | 167* ± 12.2 | 25.2* ± 2.64 |
| PMNs (PMA activated) | 13.1† ± 1.37 | 70.6 ± 5.33 |
Resting cells were suspended in HBSS and incubated with various concentrations of ciprofloxacin for 4 min. Activated cells were pretreated with 100 nM PMA for 90 s and then incubated with ciprofloxacin for 5 min. The assays were terminated by pelleting the cells through an oil cushion. Km and Vmax values were determined by Lineweaver-Burk analysis. Results are expressed as the mean and standard error of the mean of four determinations. The differences in mean Km and Vmax values were greater than would be expected by chance (P < 0.05, ANOVA). Identical superscripts within a column denote comparisons that were not significantly different (P > 0.05, Tukey's test), while a difference in superscripts denotes a significant difference (P < 0.05).
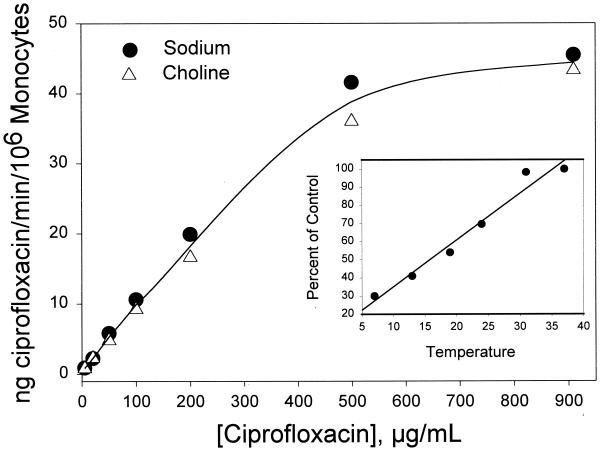
In resting monocytes, ciprofloxacin transport was found to be sodium independent and strongly temperature dependent (r = 0.98) (Fig. 3). There were no significant differences in ciprofloxacin transport velocity regardless of whether the cells were suspended in HBSS or a sodium-free modification of HBSS in which choline chloride was substituted for NaCl. In both buffers, ciprofloxacin transport was saturable at high substrate concentrations. Immature HL-60 cells exhibited a similar pattern of saturation and sodium independence (data not shown).
FIG. 3.
Sodium independence of ciprofloxacin transport by monocytes. Cells were suspended in HBSS containing NaCl or an Na+-free modification of HBSS containing choline chloride. Ciprofloxacin was added to the indicated final concentrations, and the velocity of transport was assayed for 4 min at 37°C. The data are presented as the mean of three experiments. Under all of the indicated conditions, Na+ had no significant effect on transport (P > 0.10, paired t test). Similar results were observed with immature HL-60 cells. (Inset) Temperature dependence of monocyte ciprofloxacin transport (r = 0.982). The transport activity observed in the 37°C control was 3.01 ng/min/106 PMNs.
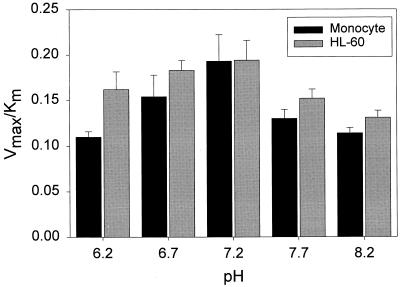
To determine the effect of pH on ciprofloxacin transport, transport kinetics were analyzed over the pH range of 6.2 to 8.2 (Fig. 4). The efficiency of transport (as assessed by Vmax/Km) and its pattern of sensitivity to pH were similar in monocytes and immature HL-60 cells. In monocytes, the Vmax/Km ratio was highest at pH 7.2 and was approximately 40% lower at pHs 6.2 and 8.2. Ciprofloxacin transport by HL-60 cells was also most efficient at pH 7.2. Between pH 6.2 and pH 8.2, there were no significant differences in the efficiency of transport by these two types of cells (P ≥ 0.1, t test).
FIG. 4.
pH dependence of ciprofloxacin transport by monocytes and immature HL-60 cells. Cells were suspended at 37°C in HBSS adjusted to the indicated pHs for kinetic analysis of ciprofloxacin transport. The results are presented as the mean and standard error of the mean of at least three experiments. The efficiency of transport (Vmax/Km ratio) was not significantly different in monocytes and HL-60 cells over the pH range illustrated (P ≥ 0.10, t test).
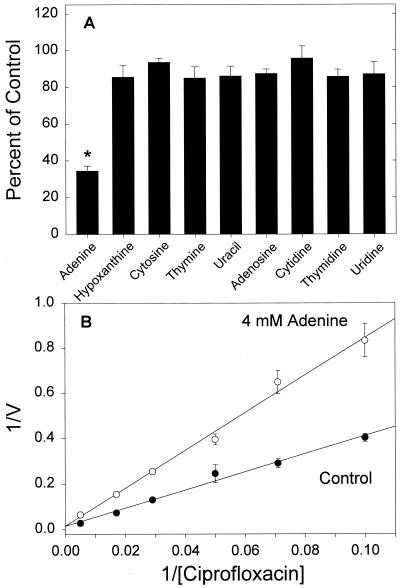
Recent work has shown that fluoroquinolone transport by resting PMNs can be competitively inhibited by purine nucleobases (26). Several nucleobases and nucleosides were examined to determine whether these agents compete with ciprofloxacin in monocytes (Fig. 5A). Of these, adenine was the only agent to significantly inhibit ciprofloxacin transport (P < 0.05, Dunnett's test). As shown in Fig. 5B, the mechanism of inhibition was competitive (Ki = 3.8 ± 1.1 mM, n = 4). Parallel experiments with HL-60 cells yielded a similar pattern of competitive inhibition (Ki = 4.1 ± 0.42 mM, n = 4), and confirmatory experiments with monocytes revealed that ciprofloxacin competitively inhibited [3H]adenine transport (Ki = 2.32 ± 0.3 mg/ml). Moreover, papaverine, a benzylisoquinoline known to inhibit nucleobase transport (16), strongly inhibited monocyte ciprofloxacin transport. In kinetic studies, papaverine acted as a competitive inhibitor (Ki = 1.44 ± 0.093 mM, n = 3).
FIG. 5.
Inhibition of monocyte ciprofloxacin transport by adenine. (A) Effect of nucleobases and nucleosides on transport. Monocytes were incubated with 5 μg of ciprofloxacin per ml and 5 mM indicated compound. Transport was monitored as previously described. The data are expressed as the mean and standard error of the mean of three experiments, with the asterisk denoting a significant difference from the control (P < 0.05, Dunnett's test). (B) Mechanism of inhibition of ciprofloxacin transport by adenine. Ciprofloxacin transport was assayed for 4 min in the presence and absence of 4 mM adenine. The mechanism of inhibition was competitive, with an observed Ki of 3.8 ± 1.1 mM. V, transport velocity in nanograms per minute per 106 cells. The data are means with standard errors of the means for four experiments.
To assess the influence of fluoroquinolone structure on transport, we compared the kinetics of ciprofloxacin transport by quiescent monocytes with those of three other structurally distinct fluoroquinolones (Table 2). During the initial linear phase of transport, the affinity and maximum velocity of norfloxacin transport were not significantly different from those of ciprofloxacin (P > 0.05, Tukey's test). However, lomefloxacin and ofloxacin were transported with significantly lower affinity and higher velocity than ciprofloxacin or norfloxacin (P < 0.05).
TABLE 2.
Kinetics of monocyte fluoroquinolone transporta
| Fluoroquinolone | Km (μg/ml) | Vmax (ng/min/106 cells) | Vmax/Km |
|---|---|---|---|
| Ciprofloxacin | 171* ± 8.27 | 32.7† ± 4.68 | 0.190 ± 0.034 |
| Norfloxacin | 174* ± 16.4 | 33.8† ± 1.64 | 0.198 ± 0.015 |
| Ofloxacin | 297 ± 21.8 | 84.6* ± 10.8 | 0.286 ± 0.040 |
| Lomefloxacin | 537 ± 26.8 | 92.3* ± 10.0 | 0.173 ± 0.018 |
Monocytes were suspended in HBSS and incubated with various concentrations of ciprofloxacin for 4 min. The assays were terminated by pelleting the cells through an oil cushion. Km and Vmax values were determined by Lineweaver-Burk analysis. Results are expressed as the mean and standard error of the mean of four determinations. The differences in mean Km and Vmax values were greater than would be expected by chance (P < 0.05, ANOVA). Identical superscripts within a column denote pairwise comparisons that were not significantly different (P > 0.05, Tukey's test). Significant differences were noted in all other comparisons.
DISCUSSION
The effectiveness of an antimicrobial agent depends not only on its activity but also on its ability to reach sites of infection. Previous studies have shown that fluoroquinolones accumulate and remain active inside human phagocytes, eventually reaching concentrations that are several times higher than extracellular concentrations (7, 8, 10, 20). The activity and intracellular accumulation of fluoroquinolones make them useful for eliminating facultative intracellular pathogens that resist phagocytic killing. Mononuclear phagocytes loaded with fluoroquinolones exhibit enhanced intracellular killing of certain bacteria, including Mycobacterium avium (which causes disseminated infections in patients with advanced AIDS) and Mycobacterium tuberculosis (21, 22). The objective of the present study was to characterize the mechanism by which monocytes accumulate ciprofloxacin and other fluoroquinolones, focusing on differences between monocytes and other cells of myeloid lineage. The human promyelocytic leukemia (HL-60) cell line, which can mature into granulocytes as well as monocytes (3, 19), was used as a model of myeloid maturation.
Our results show that monocyte ciprofloxacin transport is saturable, temperature dependent, sodium independent, and relatively insensitive to pH. Resting monocytes transport ciprofloxacin with a relatively low affinity (Km = 171 μg/ml) and a maximum velocity of approximately 32.7 ng/min/106 cells. This process can be competitively inhibited by adenine, suggesting that ciprofloxacin and adenine both serve as substrates for this transporter. In all of these respects, the characteristics of ciprofloxacin transport by monocytes are similar to those observed in parallel studies of immature HL-60 cells and in our previous studies of quiescent PMNs (26). Adenine transport is essential for normal phagocyte function, since it is the biosynthetic precursor of ATP. Phagocytes consume large amounts of ATP during phagocytosis but have a limited capability for de novo adenine synthesis (1, 15). They meet their energy requirements by scavenging adenine from the extracellular environment and converting it to ATP (13, 17). Since ciprofloxacin competitively inhibits adenine transport, it could potentially impair phagocyte function by constraining the ability of phagocytes to produce sufficient amounts of ATP.
Previous work has shown that PMN fluoroquinolone transport is up-regulated when these cells are activated by PMA, fMLP, or other agents that induce the activation of protein kinase C (18, 26). This enhanced activity is mediated by a high-affinity, sodium-independent transport system that also transports a variety of neutral and cationic amino acids (26). In the present study, treatment with PMA failed to significantly enhance ciprofloxacin accumulation by monocytes or immature HL-60 cells. When HL-60 cells were induced toward granulocytic maturation, however, they exhibited a significant component of PMA-inducible ciprofloxacin transport activity. Conversely, HL-60 cells committed to monocytic differentiation failed to exhibit this activity. Since granulocytes expressed this activity while monocytes and HL-60 cells (which lack specific granules) (19, 24) did not, we investigated the possibility that the high-affinity, PMA-inducible fluoroquinolone transport activity might be granule associated. Mobilization of membrane proteins from granules to the cell surface is known to play an important role in PMN activation (reviewed in references 2 and 24). Treatment with cytochalasin B, which enhances degranulation in response to the chemoattractant fMLP (24), significantly enhanced ciprofloxacin accumulation by fMLP-activated PMNs. DIDS, which inhibits the mobilization of several types of PMN granules (23), blocked the enhancement of ciprofloxacin accumulation normally observed in PMNs activated by fMLP and PMA. In the DIDS studies, there was a strong correlation between inhibition of lactoferrin mobilization from specific granules and inhibition of ciprofloxacin accumulation. Our results suggest that the high-affinity ciprofloxacin transport system is mobilized from a granule compartment. They also suggest that protein kinase C plays a role in regulating its activity.
As previously noted for PMNs, certain structural features of fluoroquinolones influence the kinetics of their transport by monocytes. Ofloxacin and lomefloxacin, which have substituents at positions 7 and 8 that make them bulkier than ciprofloxacin or norfloxacin, were transported with significantly lower affinity and higher velocity than the latter two agents. On the other hand, ciprofloxacin and norfloxacin were transported with similar affinities and velocities despite differences in their N-1 substituents. The overall efficiencies of transport (as judged by the Vmax/Km ratio) were not significantly different for these four fluoroquinolones, and the Km and Vmax values observed in monocytes were very similar to those reported for quiescent PMNs (26).
In summary, monocytes and PMNs, which mature from a common precursor, use a similar low-affinity mechanism to transport and accumulate fluoroquinolones. This system, which also transports adenine, is the main mechanism for fluoroquinolone accumulation by monocytes and resting PMNs. Monocytes do not appear to express a second, higher-affinity transport system that PMNs acquire during granulocytic maturation. Although activated PMNs have the potential to accumulate fluoroquinolones more avidly than activated monocytes, it is uncertain whether this activity occurs in vivo. Phagocytosis, cytokines, and chemoattractants are the main stimuli for PMN activation at infection sites, and these stimuli appear to up-regulate PMN ciprofloxacin transport in a transient, less profound manner than phorbol esters do. When PMNs and monocytes are circulating in a quiescent state or are in the early stages of chemotaxis toward an infection site, they are likely to take up fluoroquinolones with similar efficiencies.
ACKNOWLEDGMENTS
We thank Fanjie Zhang and Gillian Levy for technical assistance.
This work was supported by Public Health Service grants DE00338 and DE12601 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Investig. 1982;70:550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borregaard N, Lollike K, Kjeldsen L, Sengelov H, Bastholm L, Nielsen M H, Bainton D F. Human neutrophil granules and secretory vesicles. Eur J Haematol. 1993;51:187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 3.Boyd A W, Metcalf D. Induction of differentiation in HL60 leukemic cells: a cell cycle dependent all-or-none event. Leuk Res. 1984;8:27–43. doi: 10.1016/0145-2126(84)90029-8. [DOI] [PubMed] [Google Scholar]
- 4.Boyum A. Isolation of mononuclear cells and granulocytes from human peripheral blood. J Clin Lab Investig. 1968;21:77–89. [PubMed] [Google Scholar]
- 5.Buchmeier N A, Heffron F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect Immun. 1991;59:2232–2238. doi: 10.1128/iai.59.7.2232-2238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins S J. The HL-60 premyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987;70:1233–1244. [PubMed] [Google Scholar]
- 7.Easmon C S, Crane J P. Uptake of ciprofloxacin by human neutrophils. J Antimicrob Chemother. 1985;16:67–73. doi: 10.1093/jac/16.1.67. [DOI] [PubMed] [Google Scholar]
- 8.Easmon C S, Crane J P. Uptake of ciprofloxacin by macrophages. J Clin Pathol. 1985;38:442–444. doi: 10.1136/jcp.38.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkow S, Isberg R R, Portnoy D A. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- 10.Garraffo R, Jambou D, Chichmanian R, Ravoire S, Lapalus P. In vitro and in vivo ciprofloxacin pharmakinetics in human neutrophils. Antimicrob Agents Chemother. 1991;35:2215–2218. doi: 10.1128/aac.35.11.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hand W L, King-Thompson N L. The entry of antibiotics into human monocytes. J Antimicrob Chemother. 1989;16:681–689. doi: 10.1093/jac/23.5.681. [DOI] [PubMed] [Google Scholar]
- 12.Harris P, Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and J937 cell lines. J Leukoc Biol. 1985;37:407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins R A, Berlin R D. Purine transport in polymorphonuclear leukocytes. Biochim Biophys Acta. 1969;173:324–337. doi: 10.1016/0005-2736(69)90115-1. [DOI] [PubMed] [Google Scholar]
- 14.Hetherington S V, Spitznagel J K, Quie P G. An enzyme-linked immunoassay for measurement of lactoferrin. J Immunol Methods. 1983;65:183–190. doi: 10.1016/0022-1759(83)90314-9. [DOI] [PubMed] [Google Scholar]
- 15.Karnovsky M, Lazdins J, Simmons S. Metabolism of activated mononuclear phagocytes at rest and during phagocytosis. In: van Furth R, editor. Mononuclear phagocytes in immunity, infection and pathology. Oxford, England: Blackwell Scientific Publishing Co. Ltd.; 1975. pp. 423–439. [Google Scholar]
- 16.Kraupp M, Paskutti B, Schon S, Marz R. Inhibition of purine nucleobase transport in human erythrocytes and cell lines by papaverine. Biochem Pharmacol. 1994;48:41–47. doi: 10.1016/0006-2952(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 17.Loike J D, Kozler V E, Silverstein S C. Increased ATP and creatine phosphate turnover in phagocytosing mouse peritoneal macrophages. J Biol Chem. 1979;254:9558–9564. [PubMed] [Google Scholar]
- 18.Loo K C, Cario A C, Zhang F, Walters J D. Regulation of ciprofloxacin uptake in human promyelocytic leukemia cells and polymorphonuclear leukocytes. J Leukoc Biol. 1997;61:619–623. doi: 10.1002/jlb.61.5.619. [DOI] [PubMed] [Google Scholar]
- 19.Newburger P E, Chovaniec M E, Greenberger J S, Cohen H J. Functional changes in human leukemic cell line HL-60. A model for myeloid differentiation. J Cell Biol. 1979;82:315–322. doi: 10.1083/jcb.82.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perea E J, Garcia I, Pascual A. Comparative penetration of lomefloxacin and other quinolones into human phagocytes. Am J Med. 1992;92(Suppl. 4A):48–51. doi: 10.1016/0002-9343(92)90309-y. [DOI] [PubMed] [Google Scholar]
- 21.Shiratsuchi H, Jacobs M R, Pearson A J, Vankataprasad N, Klopman G, Ellner J J. Comparison of the activity of fluoroquinolones against Mycobacterium avium in cell-free systems and a human monocyte in-vitro infection model. J Antimicrob Chemother. 1996;37:491–500. doi: 10.1093/jac/37.3.491. [DOI] [PubMed] [Google Scholar]
- 22.Skinner P S, Furney S K, Kleinert D A, Orme I M. Comparison of activities of fluoroquinolones in murine macrophages infected with Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1995;39:750–753. doi: 10.1128/AAC.39.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suchard S J, Burton M J, Stoehr S J. Thrombospondin receptor expression in human neutrophils coincides with release of a subpopulation of specific granules. Biochem J. 1992;284:513–520. doi: 10.1042/bj2840513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tapper H. The secretion of preformed granules by macrophages and neutrophils. J Leukoc Biol. 1996;59:613–622. doi: 10.1002/jlb.59.5.613. [DOI] [PubMed] [Google Scholar]
- 25.van Rensburg C E J, Joone G, Anderson R. Interactions of the oxygen-dependent antimicrobial system of the human neutrophil with difloxacin, ciprofloxacin, perfloxacin, and fleroxacin in the intraphagocytic eradication of Staphylococcus aureus. J Med Microbiol. 1990;32:15–17. doi: 10.1099/00222615-32-1-15. [DOI] [PubMed] [Google Scholar]
- 26.Walters J D, Nakkula R, Zhang F. Mechanisms of fluoroquinolone transport by human neutrophils. Antimicrob Agents Chemother. 1999;43:2710–2715. doi: 10.1128/aac.43.11.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]