Abstract
Cancer is the second leading cause of death in the world. Given that cancer is a highly individualized disease, predicting the best chemotherapeutic treatment for individual patients can be difficult. Ex vivo models such as mouse patient-derived xenografts (PDX) and organoids are being developed to predict patient-specific chemosensitivity profiles before treatment in the clinic. Although promising, these models have significant disadvantages including long growth times that introduce genetic and epigenetic changes to the tumor. The zebrafish xenograft assay is ideal for personalized medicine. Imaging of the small, transparent fry is unparalleled among vertebrate organisms. In addition, the speed (5–7 days) and small patient tissue requirements (100–200 cells per animal) are unique features of the zebrafish xenograft model that enable patient-specific chemosensitivity analyses.
Zebrafish Have Become a Leading Animal Model for Human Disease and Personalized Medicine
Fifty years ago, George Streisinger took a leap of faith. He chose a fish for his vertebrate genetic model organism (see Glossary). Many of his contemporaries believed that a fish would be too evolutionarily distant from mammals to have any relevance for human health, and that he would only learn about the genetics of fish [1]. We have since learned that zebrafish and humans have more in common than not. For example, zebrafish retain most of the same organs (‘two eyes, mouth, brain, spinal cord, intestine, pancreas, liver, bile ducts, kidney, esophagus, heart, ear, nose, muscle, blood, bone, cartilage, and teeth’) but notably lack lungs. Zebrafish would therefore be unsuitable for the development of lung disease models (although they would make an excellent lung cancer metastasis model) [2]. Publication of the complete zebrafish genome in 2013 showed that 70% of the genes in zebrafish have human orthologs, and cross-comparison of disease-related genes annotated in the Online Mendelian Inheritance in Man (OMIM) database reveals that 82% of those genes have at least one zebrafish ortholog [2,3].
Tumorigenesis and metastasis occur in a broad range of vertebrate animals. Some mammals such as mice are capable of modeling aspects of the human disease. Likewise, zebrafish are capable of modeling aspects of human tumorigenesis and metastasis. Both mice and zebrafish are simply animal models. Results obtained using both models are more likely to be generalizable and therefore relevant to humans. Only those processes that directly relate to differences in the natural history of a species need change [4]. The result is that zebrafish make excellent human disease models, and their experimental advantages far outweigh the slight increase in relevance gained by choosing a mammalian model. An overview of zebrafish in biomedical research is provided in an award-winning short movie for an educated lay audience (www.zebrafishfilm.org).
Streisinger selected zebrafish (Danio rerio) as a vertebrate model organism for genetic studies because they are small, hearty, fecund fish that regularly lay hundreds of externally fertilized, transparent eggs. The transparent embryos allow straightforward identification of mutant phenotypes and enable exquisite analysis of development. Large-scale genetic screens have subsequently identified hundreds of mutant genes that influence nearly every process of animal development [1]. As the mutations were being identified, it became apparent that many were homologous to human disease-causing mutations, launching zebrafish as a human disease model [5] (Box 1). In parallel, the development of high-throughput chemical screens in zebrafish resulted in the identification of novel indications for previously known chemicals, as well as new classes of compounds, some of which have led to human clinical trials (for a detailed review of chemical screens using zebrafish see [6]).
Box 1. Zebrafish Model Helps to Save a Boy.
Detailed molecular and cellular characterization of tissue and organ systems in zebrafish has revealed striking similarities between fish and mammals [2]. Even systems long thought to be absent in fish are clearly present upon close examination. For example, the lymphatic system was thought to be a mammalian invention, until a transgenic zebrafish demonstrated an extensive lymphatic system in this organism. Ironically, the lymphatic transgenic zebrafish has recently been credited with helping to save the life of a 10 year old boy with a rare lymphatic disorder [84] (https://abc30.com/health/health-watch-how-zebrafish-helped-save-the-life-of-a-10-year-old-boy/5761154/).
Given the similarities in genes, molecular genetic pathways, and drug responses, it is unsurprising that zebrafish have proven to be excellent models of human cancer biology. Like humans, chemical carcinogen exposure in zebrafish induces a wide array of tumors in many different organs [7]. Likewise, mutations in oncogenes or tumor-suppressor genes result in an increased incidence of tumor formation as well as in heightened sensitivity to carcinogen exposure. In addition, the engineering of transgenic cancer models has proven to be highly informative, as have allograft and xenograft models. Importantly, these zebrafish cancer models display a spectrum of cancer types, histological presentation, and molecular features that are similar to those of cancers in mammals [7]. Zebrafish cancer models have led to a better understanding of cancer development, interaction with the microenvironment, metastasis, and drug resistance. Further, these models are helping to identify drug targets and are being utilized as platforms for cancer drug discovery [8–10]. The positive impact that zebrafish models are having on cancer research and drug development is supported by N350 reported case studies of cancer drug discovery projects using this organism [11].
An exciting new use of zebrafish xenograft models is for personalized medicine. The promising vision is that a tumor biopsy could be transplanted into hundreds of zebrafish embryos to identify a patient-specific chemosensitivity profile [12]. This profile would arm oncologists with the information to guide treatment decisions, and perhaps identify alternative chemotherapeutic and targeted compounds (Figure 1, Key Figure). In the worst-case scenario, where no treatment exists, xenografts of biopsy tissue could enable extensive drug screening to identify new, patient-specific, targeted agents. The zebrafish xenograft assay is unparalleled among cancer models for personalized medicine. The small, transparent fry are ideal for imaging, and new state-of-the-art imaging techniques are revealing cancer cellular behaviors at unprecedented resolution [13]. These imaging capabilities combined with the speed of assays (5–7 days) and small patient tissue requirements (100–200 cells per animal) are unique features of the zebrafish xenograft model that enable patient-specific chemosensitivity analyses [8–10]. In this review we focus on recent progress using zebrafish xenografts for the identification of new drug targets, drug discovery, and personalized medicine.
Figure 1.
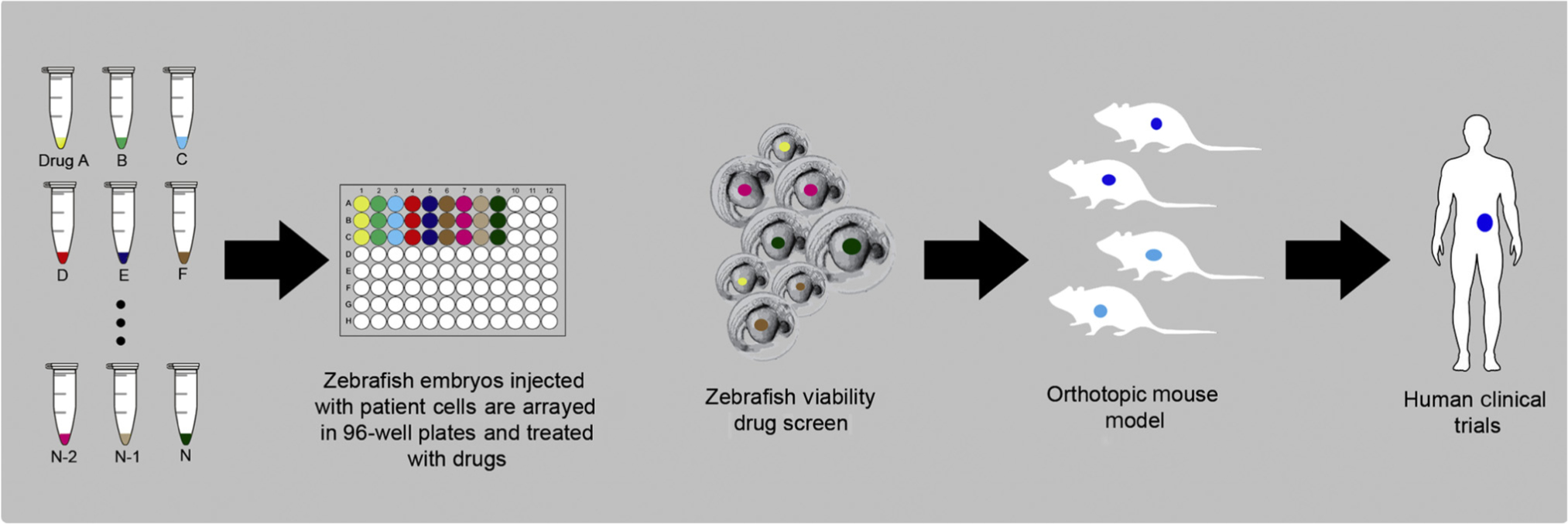
Key Figure Personalized Zebrafish Xenografting
Tumor-bearing zebrafish embryos are arrayed in 96-well plates where they are imaged and then treated with a battery of drugs selected as potential chemotherapeutics based on molecular profiling, tumor board input, and the judgment of the treating oncologist. Tumor cell behaviors including proliferation, angiogenesis, migration, and metastases are evaluated over a 3–5 day period. Based on the effects of the tested drugs, or drug combinations, on the selected behaviors, a chemosensitivity profile for the tumor of each patient can be developed. The drug sensitivity assay must first be validated in mouse orthotopic models to ensure similar sensitivity to the drug(s). In the future, in less than 7 days, the oncologist will be armed with potentially actionable information to guide treatment decisions.
Zebrafish Xenografts Model Multiple Steps of Cancer Progression at Single-Cell Resolution
The first zebrafish xenografts were largely designed to offer an alternative to mouse xenografts [14]. Since then, multiple variations of zebrafish xenograft assays have been reported. The most significant alternative assays are adult versus larval stage xenografts. Owing to the powerful imaging opportunities in zebrafish, the primary underlying biological phenomena being interrogated using zebrafish xenografts assays are tumor growth, angiogenesis, immune/stroma interaction, and metastasis.
Adult Zebrafish Xenografts
The advantage of the adult zebrafish xenograft is that mature tissues more closely resemble the tissue environment that is likely to be encountered by cancer cells in patient or mouse xenografts. Until recently, adult xenografts have been limited by the need to suppress the adult immune system, either by γ-irradiation or by dexamethasone treatment [15,16]. Nonetheless, excellent imaging has been obtained using adult xenografts, particularly by using a transparent zebrafish mutant named Casper [15–17]. The Casper line was derived from a cross between the nacre mutant, which completely lacks melanocytes, and the roy mutant, which completely lacks iridophores [18]. Recently, a transparent immunocompromised zebrafish line has been generated, the prkdcfb103/fb103 il2rgafb104/fb104 Casper-strain zebrafish, which can be engrafted with human tumor cells that subsequently respond to drug treatments [19]. Parallel rhabdomyosarcoma xenografts into these fish and mice demonstrated similar dose–response and kinetics in both organisms to combinatorial therapy with temozolomide (TMZ) and olaparib [20]. Notably, the histological and molecular features of the xenografts were comparable in both organisms. Therefore, the adult xenograft assay will likely be comparable to mouse xenografts (Box 2). However, zebrafish xenografts have some notable experimental advantages over the mouse system. Fish are smaller and less expensive to house and maintain. Most importantly, zebrafish xenografts can be imaged at cellular resolution, allowing tracking of individual cell behaviors, such as changes in morphology, migration, and cell-cycle dynamics, over time [20]. In addition, the creation of humanized zebrafish, by replacing the fish hematopoietic system with human hematopoietic stem and progenitor cells, has recently been accomplished [21]. Finally, the imaging possibilities offered by the combination of xenografts and fluorescently labeled transgenic fish will ultimately make this system tremendously informative, particularly for detailed studies of tumor cell–tumor microenvironment interactions [13].
Box 2. Mouse Xenografts, the ‘Gold Standard’.
Xenografts are a crucial element in the toolbox of the cancer researcher, and permit the study of human cancers in vivo. Immunodeficient ‘nude’ mice have long been considered to be the gold standard for xenografts because (i) they are small and, compared to large mammals, require less infrastructure and consequently lower costs; (ii) they are evolutionarily close to humans; and (iii) they are amenable to manipulation in laboratory settings. Unfortunately, current mouse xenograft models suffer from several shortcomings that hinder the development of new medical interventions for cancer. Typical xenograft experiments require large numbers of cells (approximately 106 cells/injection in mice), limiting the number of studies that can be performed from a single tissue biopsy specimen. In addition, transplanted mice must be monitored for as long as 3–6 months before euthanasia and specimen collection can be performed. This process is lengthy, expensive, and is hindered by a lack of easily accessible real-time monitoring of cells within the mouse. However, the most crucial disappointment with mouse xenograft models has been the consistent failure, in human clinical trials, of drugs selected based on preclinical studies on these models [85]. Therefore, alternatives to the mouse xenograft model have been sought. One such model is the zebrafish.
Larval Zebrafish Xenograft
In contrast to adult zebrafish xenografts, larval zebrafish xenografts fundamentally differ from mouse xenografts in several important aspects. A key difference is that larval xenografts measure tumor cell behaviors at cellular to subcellular resolution from the time of transplant to ~5 days post-transplant. This time-frame limits the number of cell divisions between injection and analysis. Thus, the larval xenograft assay assesses tumor cells as soon as they are transplanted, whereas the mouse xenograft measures what the tumors become after some weeks to months of growing inside the mouse. How these differences contribute to the ultimate usefulness of these models remains to be determined. However, there are significant experimental advantages of the larval xenograft, including high-resolution intravital imaging, high-throughput 96-well format drug screening, small tissue sample requirements, and overall assay speed. In addition, there is no need for immunosuppression because larval zebrafish do not develop a functional adaptive immune system until ~10 days post-fertilization, which occurs outside the time-frame of most assays [22]. Undoubtedly, these advantages have contributed to the exponential rise in publications using larval zebrafish in the past few years, and >270 papers report xenograft assays of various types to study cancer biology, drug target identification, drug discovery, and personalized medicine. For highly aggressive cancers, where patient survival may only be a matter of months, the larval xenograft assay may be the only viable assay.
Source of Tumor Cells for Xenografting
The source of tumor cells to be implanted has significant consequences for interpretation of the results. Cancer cell lines (CCLs) are the workhorses of cancer biology; however, their response to drug treatments is not a dependable prediction of response in patients. It is plausible that extended in vitro conditions alters drug sensitivity in many CCLs. Similarly, most tumors consist of a highly heterogeneous population of cells, and each patient carries unique mutations and characteristics, a phenomenon that cannot be replicated in CCLs [23].
An alternative to CCLs is patient-derived primary cancer cell cultures that retain tumor heterogeneity [24–26]. These have been successfully used in zebrafish, and show similar drug sensitivity to the mouse model [27]. Consequently, larval and adult zebrafish xenografts generated from patient-derived primary cancer cell cultures (zPDX) have been developed for various types of cancers, including adenoid cystic carcinoma [27], neuroendocrine cancers [28,29], breast cancer [30], head and neck tumors [31], pancreatic cancer [32], and melanoma [20,33]. In zPDX models, patient-derived cells are cultured under short-term conditions and then transplanted into zebrafish. Further development of zPDXs could engraft fragments of tumor directly, thus completely eliminating the intermediary steps. This is frequently done in mouse PDX models, and only requires differences in the size of tumor fragment for transplantation. In both scenarios, zPDX models benefit by minimizing the genetic and physiological alterations that occur during long-term cell culture.
Regardless of the xenograft source material, zebrafish models have largely allowed robust and consistent engraftment of cancers.
Zebrafish Xenografts Clarify the Mechanistic Underpinnings of Tumor Cell Behaviors and Their Interactions with the Cancer-Associated Microenvironment
Xenograft assays enable investigation of the mechanisms of cancer biology, particularly characterization of the effects of mutations or altered gene expression in cancer cells. Better understanding of cancer mechanisms often leads to potential therapeutics. Many facets of cancer biology have recently been investigated using zebrafish adult and larval xenografts; these include molecular genetic pathways, cancer stem cells, the role of cancer cell heterogeneity in cancer biology, exosomes and cancer–stroma communication, the ability of cancer cells to attract new blood vessels, cancer-associated fibroblasts, immune–cancer interactions, and cancer cell interactions with their physical environment.
Zebrafish xenografts assays have been frequently incorporated into studies on the control of cancer growth, invasion, and migration. Components of cancer-related pathways have been altered by gene mutations [34,35], gene overexpression or knockdown [35–42], protein inactivation by antibody binding [41], pharmacological inhibition of protein function [34,36,42,43], and cell culture selection for drug resistance [32,43]. In many of these studies, zebrafish xenograft models were run in parallel with mouse models, which reported comparable results [34,36–38,41–43].
The influence of the stem cell marker CD133 on tumor cell proliferation and metastasis was investigated by overexpression and knockdown of CD133 in melanoma cells. By labeling control and experimentally manipulated cells with differently colored fluorophores, and cotransplantation into zebrafish embryos, altered growth and metastasis have been directly compared [44]. In addition, by isolating the stem cell fraction of tumor cells, drugs could be tested that specifically inhibited stem cell-driven proliferation and metastasis in zebrafish xenograft assays [45]. Zebrafish xenografts also enable studies of cancer cell heterogeneity owing to the high-resolution imaging that is possible with this system [46,47].
In zebrafish xenografts, new blood vessels can be observed growing into the tumor. The cellular and subcellular resolution of zebrafish provides an unprecedented view of tumor angiogenesis [48]. Specifically, Britto et al. came to this conclusion following a series of cohesive steps beginning with the discovery of differential angiogenesis based on the injected cell type (B16-F1 mouse melanoma vs MDA-MB-231 human breast carcinoma vs HEK293 human embryonic kidney cells vs abiotic beads) [48]. Furthermore, in cellular ablation experiments, removal of zebrafish neutrophils does not affect angiogenesis; interestingly, however, removal of zebrafish macrophages significantly hinders angiogenesis in mouse and human cell lines injected into zebrafish [49–52]. The ability to precisely mix tumor cells and cancer-associated fibroblasts (CAFs) and image their interactions at high resolution makes zebrafish xenografts well suited for studying tumor–CAF interactions and for testing potential therapeutic compounds [49]. In addition, the role of exosome–stroma communication in tumor metastasis has been studied using zebrafish xenografts. The development of transgenic zebrafish lines with fluorescent protein-expressing macrophages and neutrophils further revealed significant conservation of function between mammals and zebrafish [50]. These models enable high-resolution imaging of detailed tumor–immune cell interactions in zebrafish xenografts [48,51]. Alternatively, immune cells isolated from human tumors, bone marrow, or peripheral blood have been labeled and cotransplanted into zebrafish embryos [52].
As cancer progresses toward metastasis, circulating tumor cells must navigate the vascular system, localize within an organ or tissue, and extravasate into surrounding tissues before forming secondary metastatic tumors. These processes are modeled in zebrafish by injecting tumor cells directly into the circulation at the yolk sinus or common cardinal vein. Advances in imaging technologies coupled with the optical transparency of the zebrafish embryo have resulted in progress in understanding metastasis though direct imaging of cell behavior in zebrafish xenografts [53,54]. These studies demonstrate the utility of zebrafish xenograft models for investigating a wide variety of cancer mechanisms, which often leads to the identification of drug targets for anticancer treatments (Table 1).
Table 1.
Selected Publications Testina Drugs in Zebrafish Modelsa
| Cancer | Drug studied | Zebrafish results | Refs |
|---|---|---|---|
| Glioma | IR + TMZ | Combinatorial IR + TMZ resulted in a synergistic decrease in human glioma growth in zebrafish embryos, and complete elimination in 21% of embryos. | [65] |
| Glioma | Unspecified MMP-9 inhibitor | After MMP-9 inhibitor treatment, there was a 66.7% drop in embryos with invasive glioma cells. | [72] |
| Melanoma | Dasatinib | In vitro treatment with dasatinib revealed 20% growth inhibition. At concentrations >1 μM, dasatinib was toxic after 5 days exposure in zebrafish embryos. | [33] |
| Quisinostat/MLN-4924 | In vitro treatment with experimental anticancer drugs quisinostat and MLN-4924 resulted in >50% growth inhibition. Both were nontoxic at all concentrations tested in zebrafish embryos. | ||
| NSCLC | Bosutinib | Bosutinib pretreated cell lines injected into zebrafish embryos affected migration in SRC siRNA-transfected cells, but not ACK1 siRNA-transfected cells, suggesting a dependence on ACK1 in KRAS-mutant NSCLC cell lines for migration. | [35] |
| Melanoma | Crizotinib | Crizotinib treatment significantly reduced cell migration in zebrafish of cell lines with high c-Met expression, but had no effect in low c-Met expression. | [69] |
| NSCLC | BPIQ | BPIQ caused a dose-dependent decrease in viability and proliferation in vivo. | [67] |
| Breast cancer | GLPG0187 (av integrin antagonist) | Treatment of MDA-MB-231 cells with GLPG0187 resulted in ~70% reduction in migration in zebrafish. | [42] |
| PDAC | U0126 (MEK inhibitor) | U0126 had antiproliferative effects in vivo in KRAS-mutant cell lines. | [64] |
| Breast cancer | Visfatin | Treatment of MDA-MB-231 cells with visfatin resulted in ~8% increase in invasion in zebrafish. | [61] |
| Breast cancer | IT1t (CXCR4 antagonist) | CXCR4 inhibition with IT1t led to a 39–60% decrease in tumor burden at 4 dpi. | [55] |
| Glioblastoma | LY294002 (PI3K inhibitor) | LY2940002 treatment significantly reduced glioblastoma proliferation and migration in vivo. | [71] |
| Breast cancer | SKLB646 (SRC/RAF/VEGFR2 inhibitor) | Treatment with SKLB646 resulted in reduced angiogenesis and tumor growth in a tumor-induced neovascularization zebrafish model. | [57] |
| HCC | Indeno[1,2-b]quinoxaline derivatives | Compound 10a reduced tumor growth in vivo in a dose-dependent manner. | [62] |
| Breast cancer | TAT-NLS-BLBD-6 (synthetic β-catenin/LEF-1 peptide inhibitor) | MCF-7 and MDA-MB-23 GFP-labeled cells treated with TAT-NLS-BLBD-6 resulted in decreased fluorescence at 2 dpi compared with untreated counterparts in a zebrafish xenograft model. | [58] |
| Glioblastoma | Onalespib, TMZ | Combinatorial onalespib and TMZ showed a synergistic decrease in tumor burden as well as extended survival in zebrafish models. | [86] |
| Glioblastoma | TMZ | TMZ treatment for 5–10 dpi resulted in significantly increased zebrafish survival as well as decreased tumor burden. A subpopulation of undifferentiated GBM cells survived following TMZ treatment, causing recurrence after cessation of treatment. | [47] |
| CRC | FOLFOXorFOLFIRI | KRAS mutant lines displayed higher response to FOLFOX than FOLFIRI, and various cell lines tested displayed varying chemosensitivities in zebrafish. | [83] |
| NSCLC | Osimertinib | Osimertinib displayed antiproliferative effects at 1 μM and antiangiogenic effects at 0.25 μM in NSCLC-xenografted zebrafish. | [68] |
| Breast cancer | Abnormal cannabidiol (Abn-CBD) and analog O-1602 | 2 μM of Abn-CBD or O-1602 reduced the viability of MDA-MB-231 xenografted cells in zebrafish by 50%. | [63] |
Abbreviations: CRC, colorectal cancer; dpi, days post injection; HCC, hepatocellular carcinoma; IR, ionizing radiation; MMP-9, matrix metallopeptidase 9; NSCLC, non-small cell lung cancer; PDAC, pancreatic ductal adenocarcinoma; PI3K, phosphatidylinositol-3-kinase; TMZ, temozolomide.
Zebrafish Xenografts Facilitate the Identification of New Drug Targets for Metastatic Cancers
Zebrafish xenografts are particularly useful for identifying drug targets for inhibiting metastases because they provide an inherently multiscale model. Although the imaging resolution of the zebrafish larva is cellular to subcellular, the cells are seen in the context of tissues, organs, and the whole animal. Transplanted cells injected into the yolk sac migrate and intravasate into the circulation where they travel throughout the larval body until they find a favorable place to stop and then extravasate from the circulation. The cells may then invade surrounding tissues and form secondary tumors. Because a favored place for cells to stop is the ventral caudal vasculature, a simple measure of migration/metastasis is to count the number of embryos with cells in the tail. This assay has been used to investigate the mechanisms of metastasis in several cancer types and has identified several potential drugs and drug targets for cancer therapy. These include linking the YAP–CXCR2 [40,41] and PIT1–CXCR4 [39,55] pathways to increased metastatic potential of breast cancer cells. Several clinical studies of inhibitors of the CXCR2 and CXCR4 pathways are currently underway [56].
In addition, zebrafish xenografts have enabled the study of several novel therapies, including ectopic overexpression of protein deacetylases, epigenetic modulators, VEGFR inhibitors, and inhibition of SMYD3, CDCA7, MTH1, and p38b/CK2/SET [34,42,57–59].
These studies used the straightforward migration/metastasis assay of counting larva with cells in the tail. However, these assays potentially provide much more information. Evaluation by imaging at subcellular resolution has been demonstrated for multiple steps of metastasis, including primary tumor growth, migration and invasion, interaction with the tumor microenvironment, extracellular vesicle (exosome) secretion, vascular trafficking, tumor cell arrest, extravasation, extracellular vesicle uptake into the premetastatic niche, and metastatic colonization [13]. Future studies may leverage these imaging capabilities to investigate the mechanisms of metastasis and identify new drug targets for potential therapeutic interventions in this deadliest facet of cancer.
Drug-resistant cells typically become trapped in the tail vasculature (metastatic cells) of the larva or at the injection site (yolk sac). Although not yet reported, future studies could theoretically isolate drug-resistant cells from the tails and yolk sac of zebrafish xenografts to investigate potential drug targets. Furthermore, in drug-treated larva, drug-resistant metastatic populations may also persist. These enriched populations can provide rich material for identifying potential drug targets for drug-resistant cancers (Figure 2).
Figure 2. Drug Resistance Modeling in Zebrafish.
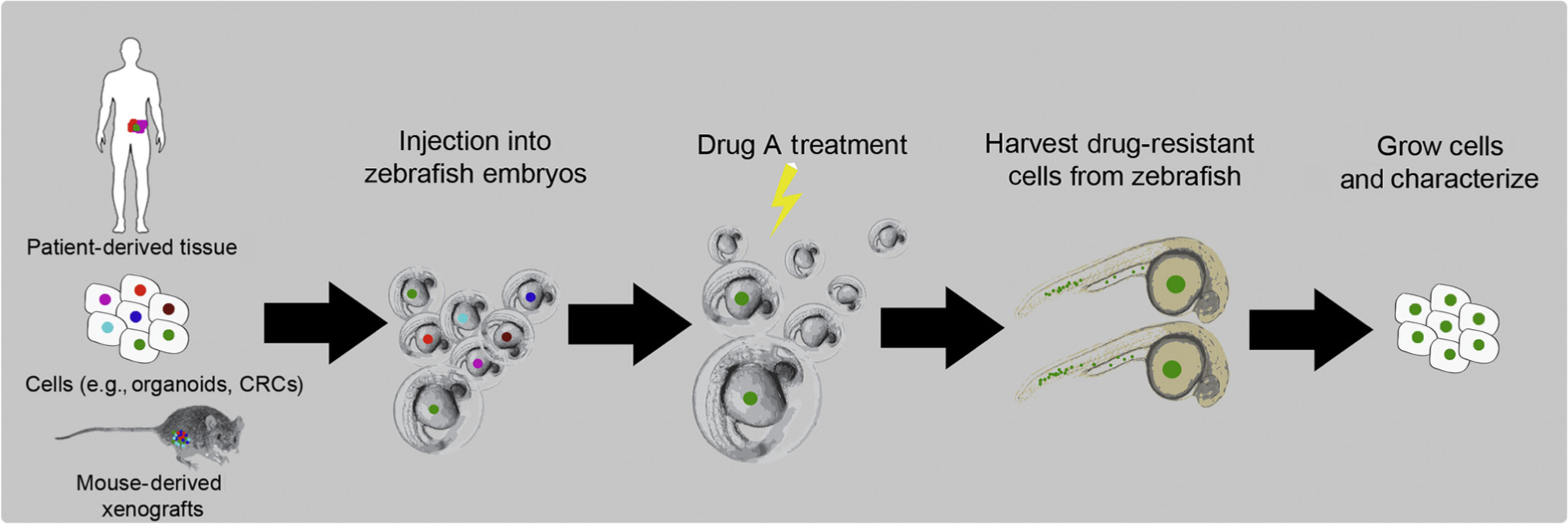
Tissue or cells from a tumor biopsy, or patient-derived cell culture, organoids, or mouse patient-derived xenografts (PDX), maintain tumor heterogeneity and are therefore likely to be representative of the tumor of the patient when transplanted into hundreds of zebrafish embryos. The tumor-bearing embryos are treated with a drug of interest, for example, the chemotherapy the patient is receiving. After 3–5 days, some embryos will have cells that have intravasated and migrated to the tail in a process that has similarities to metastasis, and some cells will remain viable at the injection site in the yolk sac. The subset of cells that are resistant to drug treatment can then potentially be isolated, and subsequent mutation and gene expression profiling of these cells can identify potential drug targets. These resistant mutations then can be engineered into cells and validated in mouse orthotopic models, and new drug(s) identified for these resistant cells can then be used in clinical trials. Abbreviation: CRCs, conditionally reprogrammed cells.
Zebrafish Xenografts Enable Drug Discovery Validation and Screening
The zebrafish xenograft assay has frequently been used as an in vivo reporter for validation of anticancer drug efficacy (Table 1). In addition, the scalable nature of this assay with potential 96-well format screening makes it a potential platform for the identification of new drugs through chemical compound screens (Figure 1). Several recent studies use zebrafish xenografts for in vivo validation of drug efficacy, either alone or as a complementary in vivo assay to mouse xenografts [29,35,59–65]. In addition to validating drug efficacy, zebrafish xenografts have validated the effectiveness of drug delivery platforms, particularly for nanomedicine formulations [66].
Some compounds have progressed to testing in clinical trials. For example, BPIQ, a novel synthetic quinolone derivative whose analogs are topotecan and irinotecan, was identified to have dose-dependent antiproliferative and antimigratory effects using in vivo zebrafish non-small cell lung cancer (NSCLC) xenograft models [67]. More recently, zebrafish NSCLC xenograft models have been used to examine osimertinib resistance, and have identified a subpopulation of NSCLCs with EGFR and T790M mutations that displayed increased sensitivity to osimertinib [68]. Human uveal melanoma (UM) toxicity and migration activity in zebrafish was studied following treatment with a Src tyrosine kinase inhibitor, dasatinib, as well as two experimental anticancer drugs (quisinostat and MLN-4924). Treatment with these drugs resulted in reduced tumor growth and migration in zebrafish xenograft models of Src overactivation cell lines [33]. A similar zebrafish UM xenograft was used to study the c-Met inhibitor crizotinib, also with promising results. In this study, crizotinib treatment of zebrafish engrafted with UM overexpressing c-Met significantly reduced tumor cell burden and migration [69]. Based on the results of this and other studies, crizotinib is now under clinical trials as adjuvant therapy in the treatment of UM [70].
Zebrafish drug assays have also been leveraged for the study of relatively rare cancers. Adenoid cystic carcinomas (ACCs) of the salivary gland are a rare but highly metastatic cancer. Zebrafish transplanted with both mouse PDX tissue and conditionally reprogrammed cells from patient tissue demonstrated rapid intravasation of cells within 48 h [27]. Treatment of zebrafish with regorafenib, an FDA-approved VEGFR2 and TIE2 tyrosine kinase receptor inhibitor, resulted in significantly reduced invasion. This was the first study to report the use of regorafenib in ACCs. A Phase II trial (NCT04119453) investigating the use of rivoceranib, another VEGFR inhibitor, in recurrent or metastatic ACC is currently recruiting patients.
Zebrafish larva are particularly well suited for medium-throughput chemical screens because they can be screened in 96-well format with automated imaging and analysis [9,11,71,72]. Small screens for inhibitors of leukemia stem cells and highly metastatic melanoma have produced novel therapeutics [73,74]. In a liver cancer study, the novel multiple kinase inhibitors, 419S1 and 420S1, were tested for efficacy on 293T/EDN1 cells and 15 patient hepatocellular carcinoma resections using zebrafish xenograft assays for proliferation and metastasis. In addition, the compounds were further tested in a battery of different zebrafish assays, including angiogenesis, liver toxicity, embryo toxicity, a zebrafish transgenic liver cancer model, and a zebrafish transgenic obesity model [75]. This study demonstrates the ability to use zebrafish to rapidly test a large number of drug properties in a personalized drug-screening context, giving us a glimpse into the future.
Zebrafish Xenografts Are a Unique Ex Vivo Assay for Rapidly Assaying Drug Sensitivity of Patient Biopsies
Mutations specific to an individual patient, within the same type of cancer, can result in drastically different responses to chemotherapy [69]. Consequently, therapeutic interventions based on population-level trials result in individual patients undergoing multiple rounds of trial and error. Thus, over the past decade there has been a steady movement toward personalized, precision medicine, and a move from large, population-based clinical trials to individual n = 1 trials [76].
Given the realization that the tumor of every patient may be unique, and that patients might benefit from a targeted set of therapeutics based on their unique cancer, tremendous effort has gone into growing patient biopsy samples ex vivo. The primary methods have been (i) to grow the cells of the patient in culture using an immortalization technique, such as chemical reprogramming [24], (ii) to generate patient-derived organoids in 3D culture conditions [77], and (iii) to generate PDX in mice, which have been called avatars [78]. Next-generation sequencing has opened the possibility of identifying actionable mutations that, when validated with ex vivo models, could inform clinical decisions. Given advances in adult zebrafish xenografts, it is likely that zebrafish avatars will soon join these models. Larval zebrafish xenografts have been used to test drug sensitivity of cells generated with all of these ex vivo platforms, namely patient-derived cell culture, organoids, and mouse PDX. The results from zebrafish assays generally show concordance with these assays [8,9,12].
The extensive use of zebrafish xenografts for mechanistic and drug discovery experiments lends support to the idea that this platform will be useful for testing the drug sensitivity of patient cells. Multiple studies have validated the zebrafish xenograft assay using patient-derived cells from other ex vivo models, thus extending the usefulness of these platforms [20,27–33,79]. For cell culture-based platforms, zebrafish xenografts provide a fast whole-animal assay, and zebrafish could extend the speed and drug screening capacity of mouse PDX. In essence, zebrafish provide a complementary assay for a multiplex preclinical approach to cancer care [27].
Perhaps the most impactful use of zebrafish for clinical cancer care will be for testing drug sensitivity directly from patient biopsies. It was originally shown in 2009 that patient tumor tissue can be implanted into zebrafish embryos [80]. These techniques were technically challenging, and it was unclear whether zebrafish xenografts would reliably identify drug sensitivities. After a decade of experience using zebrafish xenografts, abundant data now support the use of these assays. Recently, several groups have reported the use of zebrafish xenografts for measuring the drug sensitivity of patient tumor cells. Importantly, these studies show accurate prediction of patient responses [31,75,79,81–83]. Although these studies are promising, the numbers of patients are still very low. Larger clinical studies incorporating zebrafish xenografts will need to be carried out to fully validate this model.
Concluding Remarks and Future Perspectives
In the near term, zebrafish xenografts will be used to identify the best treatment option based on the response of the biopsy tissue of a patient. Although still requiring optimization, from a research perspective there is convincing evidence that zebrafish xenografts can report the chemotherapeutic sensitivity of patient tissue. Therefore, we believe that their use in a personalized clinical setting on an experimental basis is warranted. It is also time for zebrafish xenografts to be integrated into clinical trials to evaluate their predictive efficacy for selecting the most appropriate therapy, as well as for identifying potential responders to new therapeutic agents. A second fruitful area for zebrafish xenografts is in identifying new drugs on a personalized basis. Armed with the molecular profile data of a patient and input from the appropriate tumor board, a promising set of compounds could be selected for testing before commencement of treatment. Finally, zebrafish xenografts may offer hope when no treatment options are available. Thousands of embryos could be implanted with patient biopsy tissue, allowing screening of large numbers of compounds. Although much work will be necessary to demonstrate that zebrafish xenografts are reliable and broadly applicable diagnostic platforms (see Outstanding Questions), it is possible that, one day, a fish could help to save your life.
Outstanding Questions.
Do zebrafish xenografts reliably predict patient response to therapy? Recent studies are promising, but the patient numbers are low. Larger clinical studies incorporating zebrafish xenografts need to be carried out to fully validate this model.
Can information from zebrafish intravital imaging assays be used to reinforce the predictive value of personalized medicine?
An adult humanized zebrafish model has been created for blood cancer studies. Can a larval humanized model be made? Will it be useful for immunotherapy testing, particularly in a personalized medicine setting?
Highlights.
Zebrafish xenografts are proven models of human cancer biology that provide rapid in vivo validation of anticancer drugs and drug targets.
Zebrafish have emerged as unsurpassed models for imaging tumor metastasis.
Adult zebrafish xenografts are similar to mouse xenografts but have significant experimental advantages.
The larval zebrafish xenograft assay has unique characteristics that make it unlike all other ex vivo assays and a top choice for in vivo drug screening.
Zebrafish xenograft assays are ideal for testing drug sensitivity directly from patient biopsies in a personalized medicine context.
Glossary
- Allograft
transplantation of cells or tissue from one individual to another individual of the same species
- Conditionally reprogrammed cells
cells cultured using a technique that enables the robust and efficient propagation of patient-derived primary tissue in vitro
- Exosomes
membrane-bound extracellular vesicles that often carry protein or nucleic acid messengers
- Extravasation
the movement of cancer cells out of the blood or lymphatic vessels into surrounding tissues
- Humanized zebrafish
zebrafish carrying a functional human hematopoietic system
- Intravasation
invasion of cancer cells through the basement membrane into a blood or lymphatic vessel
- Intravital imaging
microscopy in living organisms
- Metastasis
growth of a secondary malignant tumor at a distance from the site of the primary cancer lesion
- Microenvironment
the surrounding tissues; these comprise stromal cell types, infiltrating immune cells, and extracellular matrix
- Model organism
an organism chosen for in-depth studies on biological phenomena that are expected to be generalizable to other organisms, most often people
- Nanomedicine
the use of nanoscale materials for improving drug stability, targeting, and delivery
- Personalized medicine
medical treatment tailored to the characteristics, such as the molecular profile, of an individual patient. Also called precision medicine, stratified medicine, P4 (predictive, preventive, personalized, participatory) medicine, and theranostics
- Xenograft
transplantation of cells or tissue from one species into a different species
References
- 1.Grunwald DJ and Eisen JS (2002) Headwaters of the zebrafish – emergence of a new model vertebrate. Nat. Rev. Genet. 3, 717–724 [DOI] [PubMed] [Google Scholar]
- 2.Gut P et al. (2017) Little fish, big data: zebrafish as a model for cardiovascular and metabolic disease. Physiol. Rev. 97, 889–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howe K et al. (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spikol ED et al. (2016) Zebrafish models of Prader–Willi syndrome: fast track to pharmacotherapeutics. Diseases 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santoriello C and Zon LI (2012) Hooked! Modeling human disease in zebrafish. J. Clin. Invest. 122, 2337–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rennekamp AJ and Peterson RT (2015) 15 years of zebrafish chemical screening. Curr. Opin. Chem. Biol. 24, 58–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoletov K and Klemke R (2008) Catch of the day: zebrafish as a human cancer model. Oncogene 27, 4509–4520 [DOI] [PubMed] [Google Scholar]
- 8.Baxendale S et al. (2017) The power of zebrafish in personalised medicine. Adv. Exp. Med. Biol. 100, 179–197 [DOI] [PubMed] [Google Scholar]
- 9.Astone M et al. (2017) Fishing for cures: the alLURE of using zebrafish to develop precision oncology therapies. NPJ Precis. Oncol. 1, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirchberger S et al. (2017) Quo natas, Danio – recent progress in modeling cancer in zebrafish. Front. Oncol. 7, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letrado P et al. (2018) Zebrafish: speeding up the cancer drug discovery process. Cancer Res. 78, 6048–6058 [DOI] [PubMed] [Google Scholar]
- 12.Fazio M and Zon LI (2017) Fishing for answers in precision cancer medicine. Proc. Natl. Acad. Sci. U. S. A. 114, 10306–10308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osmani N and Goetz JG (2019) Multiscale imaging of metastasis in zebrafish. Trends Cancer 5, 766–778 [DOI] [PubMed] [Google Scholar]
- 14.Haldi M et al. (2006) Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 9, 139–151 [DOI] [PubMed] [Google Scholar]
- 15.Stoletov K et al. (2007) High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc. Natl. Acad. Sci. U. S. A. 104, 17406–17411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heilmann S et al. (2015) A quantitative system for studying metastasis using transparent zebrafish. Cancer Res. 75, 4272–4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Q et al. (2016) Imaging tumour cell heterogeneity following cell transplantation into optically clear immune-deficient zebrafish. Nat. Commun. 7, 10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White RM et al. (2008) Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2, 183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore JC et al. (2016) Single-cell imaging of normal and malignant cell engraftment into optically clear prkdc-null SCID zebrafish. J. Exp. Med. 213, 2575–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan C et al. (2019) Visualizing engrafted human cancer and therapy responses in immunodeficient zebrafish. Cell 177, 1903–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajan V et al. (2019) Humanized zebrafish enhance human hematopoietic stem cell survival and promote acute myeloid leukemia clonal diversity. Haematologica Published online December 29, 2019. 10.3324/haematol.2019.223040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novoa B and Figueras A (2012) Zebrafish: model for the study of inflammation and the innate immune response to infectious diseases. Adv. Exp. Med. Biol. 946, 253–275 [DOI] [PubMed] [Google Scholar]
- 23.Dagogo-Jack I and Shaw AT (2018) Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 15, 81–94 [DOI] [PubMed] [Google Scholar]
- 24.Liu X et al. (2017) Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat. Protoc. 12, 439–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Correa BRS et al. (2018) Patient-derived conditionally reprogrammed cells maintain intra-tumor genetic heterogeneity. Sci. Rep. 8, 4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal S et al. (2015) Identification of different classes of luminal progenitor cells within prostate tumors. Cell Rep. 13, 2147–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C et al. (2017) A multiplex preclinical model for adenoid cystic carcinoma of the salivary gland identifies regorafenib as a potential therapeutic drug. Sci. Rep. 7, 11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaudenzi G et al. (2017) Patient-derived xenograft in zebrafish embryos: a new platform for translational research in neuroendocrine tumors. Endocrine 57, 214–219 [DOI] [PubMed] [Google Scholar]
- 29.Gianoncelli A et al. (2019) Adrenocortical carcinoma xenograft in zebrafish embryos as a model to study the in vivo cytotoxicity of abiraterone acetate. Endocrinology 160, 2620–2629 [DOI] [PubMed] [Google Scholar]
- 30.Mercatali L et al. (2016) Development of a patient-derived xenograft (PDX) of breast cancer bone metastasis in a zebrafish model. Int. J. Mol. Sci. 17, 1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Samadi A et al. (2019) PCR-based zebrafish model for personalised medicine in head and neck cancer. J. Transl. Med. 17, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parasido E et al. (2019) The sustained induction of c-MYC drives Nab-paclitaxel resistance in primary pancreatic ductal carcinoma cells. Mol. Cancer Res. 17, 1815–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Ent W et al. (2014) Modeling of human uveal melanoma in zebrafish xenograft embryos. Invest. Ophthalmol. Vis. Sci. 55, 6612–6622 [DOI] [PubMed] [Google Scholar]
- 34.Das I et al. (2020) AXL and CAV-1 play a role for MTH1 inhibitor TH1579 sensitivity in cutaneous malignant melanoma. Cell Death Differ. Published online January 9, 2020. 10.1038/s41418-019-0488-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan DS et al. (2014) Bosutinib inhibits migration and invasion via ACK1 in KRAS mutant non-small cell lung cancer. Mol. Cancer 13, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S (2020) Deubiquitinase activity profiling identifies UCHL1 as a candidate oncoprotein that promotes TGFbeta-induced breast cancer metastasis. Clin. Cancer Res. 26, 1460–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berens EB et al. (2017) Keratin-associated protein 5–5 controls cytoskeletal function and cancer cell vascular invasion. Oncogene 36, 593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.AlHossiny M et al. (2016) Ly6E/K signaling to TGFbeta promotes breast cancer progression, immune escape, and drug resistance. Cancer Res. 76, 3376–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Ordonez A et al. (2018) Breast cancer metastasis to liver and lung is facilitated by Pit-1–CXCL12–CXCR4 axis. Oncogene 37, 1430–1444 [DOI] [PubMed] [Google Scholar]
- 40.Arnandis T et al. (2018) Oxidative stress in cells with extra centrosomes drives non-cell-autonomous invasion. Dev. Cell 47, 409–424.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharif GM et al. (2015) Cell growth density modulates cancer cell vascular invasion via Hippo pathway activity and CXCR2 signaling. Oncogene 34, 5879–5889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y et al. (2015) Genetic depletion and pharmacological targeting of alphav integrin in breast cancer cells impairs metastasis in zebrafish and mouse xenograft models. Breast Cancer Res. 17, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tiek DM et al. (2019) Estrogen-related receptor beta activation and isoform shifting by cdc2-like kinase inhibition restricts migration and intracranial tumor growth in glioblastoma. FASEB J. 33, 13476–13491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simbulan-Rosenthal CM et al. (2019) CRISPR-Cas9 knockdown and induced expression of CD133 reveal essential roles in melanoma invasion and metastasis. Cancers (Basel) 11, E1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peverelli E et al. (2017) Dopamine receptor type 2 (DRD2) and somatostatin receptor type 2 (SSTR2) agonists are effective in inhibiting proliferation of progenitor/stem-like cells isolated from nonfunctioning pituitary tumors. Int. J. Cancer 140, 1870–1880 [DOI] [PubMed] [Google Scholar]
- 46.Franzetti GA et al. (2017) Cell-to-cell heterogeneity of EWSR1-FLI1 activity determines proliferation/migration choices in Ewing sarcoma cells. Oncogene 36, 3505–3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welker AM et al. (2017) Changes in tumor cell heterogeneity after chemotherapy treatment in a xenograft model of glioblastoma. Neuroscience 356, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Britto DD et al. (2018) Macrophages enhance Vegfa-driven angiogenesis in an embryonic zebrafish tumour xenograft model. Dis. Model. Mech. 11, dmm035998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun DY et al. (2019) Cancer-associated fibroblast regulate proliferation and migration of prostate cancer cells through TGF-beta signaling pathway. Life Sci. 235, 116791. [DOI] [PubMed] [Google Scholar]
- 50.Renshaw SA et al. (2006) A transgenic zebrafish model of neutrophilic inflammation. Blood 108, 3976–3978 [DOI] [PubMed] [Google Scholar]
- 51.Vazquez Rodriguez G et al. (2018) Adipocytes promote early steps of breast cancer cell dissemination via interleukin-8. Front. Immunol. 9, 1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J et al. (2015) Novel mechanism of macrophage-mediated metastasis revealed in a zebrafish model of tumor development. Cancer Res. 75, 306–315 [DOI] [PubMed] [Google Scholar]
- 53.Paul CD et al. (2019) Tissue architectural cues drive organ targeting of tumor cells in zebrafish. Cell Syst. 9, 187–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Follain G et al. (2018) Hemodynamic forces tune the arrest, adhesion, and extravasation of circulating tumor cells. Dev. Cell 45, 33–52 [DOI] [PubMed] [Google Scholar]
- 55.Tulotta C et al. (2016) Inhibition of signaling between human CXCR4 and zebrafish ligands by the small molecule IT1t impairs the formation of triple-negative breast cancer early metastases in a zebrafish xenograft model. Dis. Model. Mech. 9, 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng Y et al. (2019) Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim. Biophys. Acta Rev. Cancer 1871, 289–312 [DOI] [PubMed] [Google Scholar]
- 57.Zheng MW et al. (2016) Preclinical evaluation of a novel orally available SRC/Raf/VEGFR2 inhibitor, SKLB646, in the treatment of triple-negative breast cancer. Mol. Cancer Ther. 15, 366–378 [DOI] [PubMed] [Google Scholar]
- 58.Hsieh TH et al. (2016) A novel cell-penetrating peptide suppresses breast tumorigenesis by inhibiting beta-catenin/LEF-1 signaling. Sci. Rep. 6, 19156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fenizia C et al. (2019) SMYD3 promotes the epithelial–mesenchymal transition in breast cancer. Nucleic Acids Res. 47, 1278–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrovic J et al. (2020) Lectin from Laetiporus sulphureus effectively inhibits angiogenesis and tumor development in the zebrafish xenograft models of colorectal carcinoma and melanoma. Int. J. Biol. Macromol. 148, 129–139 [DOI] [PubMed] [Google Scholar]
- 61.Hung AC et al. (2016) Extracellular visfatin-promoted malignant behavior in breast cancer is mediated through c-Abl and STAT3 activation. Clin. Cancer Res. 22, 4478–4490 [DOI] [PubMed] [Google Scholar]
- 62.Tseng CH et al. (2016) Discovery of indeno[1,2-b]quinoxaline derivatives as potential anticancer agents. Eur. J. Med. Chem. 108, 258–273 [DOI] [PubMed] [Google Scholar]
- 63.Tomko A et al. (2019) Antitumor activity of abnormal cannabidiol and its analog O-1602 in taxol-resistant preclinical models of breast cancer. Front. Pharmacol. 10, 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo M et al. (2015) U0126 inhibits pancreatic cancer progression via the KRAS signaling pathway in a zebrafish xenotransplantation model. Oncol. Rep. 34, 699–706 [DOI] [PubMed] [Google Scholar]
- 65.Geiger GA et al. (2008) Temozolomide-mediated radiosensitization of human glioma cells in a zebrafish embryonic system. Cancer Res. 68, 3396–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tu WM et al. (2020) Longitudinal and quantitative assessment platform for concurrent analysis of anti-tumor efficacy and cardiotoxicity of nano-formulated medication in vivo. Anal. Chim. Acta 1095, 129–137 [DOI] [PubMed] [Google Scholar]
- 67.Chiu CC et al. (2015) BPIQ, a novel synthetic quinoline derivative, inhibits growth and induces mitochondrial apoptosis of lung cancer cells in vitro and in zebrafish xenograft model. BMC Cancer 15, 962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li XY et al. (2019) Zebrafish xenograft model of human lung cancer for evaluating osimertinib resistance. Biomed. Res. Int. 2019, 3129748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Ent W et al. (2015) Embryonic zebrafish: different phenotypes after injection of human uveal melanoma cells. Ocul. Oncol. Pathol. 1, 170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang J et al. (2018) Treatment of uveal melanoma: where are we now? Ther. Adv. Med. Oncol. 10, 1758834018757175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wehmas LC et al. (2016) Developing a novel embryo–larval zebrafish xenograft assay to prioritize human glioblastoma therapeutics. Zebrafish 13, 317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang XJ et al. (2013) A novel zebrafish xenotransplantation model for study of glioma stem cell invasion. PLoS One 8, e61801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang B et al. (2014) Quantitative phenotyping-based in vivo chemical screening in a zebrafish model of leukemia stem cell xenotransplantation. PLoS One 9, e85439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu A et al. (2018) Rapid identification of antimicrometastases drugs using integrated model systems with two dimensional monolayer, three dimensional spheroids, and zebrafish xenotransplantation tumors. Biotechnol. Bioeng. 115, 2828–2843 [DOI] [PubMed] [Google Scholar]
- 75.Lin HS et al. (2019) Identification of novel anti-liver cancer small molecules with better therapeutic index than sorafenib via zebrafish drug screening platform. Cancers (Basel) 11, E739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schork NJ (2015) Personalized medicine: time for one-person trials. Nature 520, 609–611 [DOI] [PubMed] [Google Scholar]
- 77.Hwang CI et al. (2016) Preclinical models of pancreatic ductal adenocarcinoma. J. Pathol. 238, 197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malaney P et al. (2014) One mouse, one patient paradigm: new avatars of personalized cancer therapy. Cancer Lett. 344, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin J et al. (2016) A clinically relevant in vivo zebrafish model of human multiple myeloma to study preclinical therapeutic efficacy. Blood 128, 249–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weiss FU et al. (2009) Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology 137, 2136–2145 [DOI] [PubMed] [Google Scholar]
- 81.Costa B et al. (2019) Developments in zebrafish avatars as radiotherapy sensitivity reporters – towards personalized medicine. EBioMedicine 102578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu JQ et al. (2017) Patient-derived xenograft in zebrafish embryos: a new platform for translational research in gastric cancer. J. Exp. Clin. Cancer Res. 36, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fior R et al. (2017) Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts. Proc. Natl. Acad. Sci. U. S. A. 114, E8234–E8243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li D et al. (2019) ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nat. Med. 25, 1116–1122 [DOI] [PubMed] [Google Scholar]
- 85.Voskoglou-Nomikos T et al. (2003) Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin. Cancer Res. 9, 4227–4239 [PubMed] [Google Scholar]
- 86.Canella A et al. (2017) Efficacy of onalespib, a long-acting second-generation HSP90 inhibitor, as a single agent and in combination with temozolomide against malignant gliomas. Clin. Cancer Res. 23, 6215–6226 [DOI] [PMC free article] [PubMed] [Google Scholar]


