Abstract
We have previously demonstrated that Porphyromonas gingivalis is susceptible to killing by toluidine blue O (TBO) when irradiated with light from a helium-neon (HeNe) laser. The aim of this study was to determine whether a TBO-antibody conjugate (Ab-TBO) could be used to specifically target P. gingivalis to lethal photosensitization in the presence of Streptococcus sanguis or human gingival fibroblasts (HGFs). When a mixture of P. gingivalis and S. sanguis was exposed to 4 μg of TBO/ml and irradiated with HeNe laser light, there were 1.5- and 4.0-log10-unit reductions in the viable counts, respectively. In contrast, when TBO was conjugated with a murine monoclonal antibody against P. gingivalis lipopolysaccharide, the reductions in viable counts of P. gingivalis and S. sanguis amounted to 5.0 and 0.1 log10 units, respectively. Lethal photosensitization of P. gingivalis in the presence of HGFs using unconjugated TBO resulted in a 0.7-log10-unit reduction in P. gingivalis viable counts and a 99% reduction in the incorporation of tritiated thymidine ([3H]Tdr) by the HGFs. In contrast, when the Ab-TBO conjugate was used, there was a 100% reduction in P. gingivalis viable counts but no significant reduction in the incorporation of [3H]Tdr by HGFs. These results demonstrate that specific targeting of P. gingivalis can be achieved using TBO conjugated to a monoclonal antibody raised against a cell surface component of this organism.
Lethal photosensitization is a process by which a photosensitizer is activated by light of an appropriate wavelength resulting in the production of cytotoxic species which then kill the target cell (9). Our previous work has demonstrated that lethal photosensitization of Porphyromonas gingivalis using toluidine blue O (TBO) in combination with helium-neon (HeNe) laser light is possible (3). Bacteria are killed as a result of membrane and DNA damage due mainly to the production of singlet oxygen on irradiation of the dye (4). Lethal photosensitization is not a specific modality and has been shown to be effective against a variety of cells such as those in neoplasms (8), fungi (16–18, 24), viruses (21), and bacteria (2, 11, 13, 14, 23). Work carried out by Dobson and Wilson (7) using TBO as a photosensitizer in combination with HeNe laser light showed that, in dental plaque samples containing P. gingivalis, Fusobacterium nucleatum, streptococci, black-pigmented anaerobes, and Actinobacillus actinomycetemcomitans, all bacteria were susceptible to lethal photosensitization. The fact that lethal photosensitization is not specific is advantageous in one respect: it is possible to kill all the bacteria present in a mixed infection. However, this also means that commensal bacteria and host tissues could be adversely affected. Many therapeutic regimens used for oral infections eliminate both pathogenic and commensal organisms indiscriminately, thereby disrupting the natural ecosystem of the oral cavity (23). Therefore, it is important to develop a treatment that could specifically target the pathogenic organism without causing any adverse effects on the commensal oral flora or the host tissue. For photodynamic therapy, one method of achieving this is to conjugate the photosensitizer to an antibody raised against the target organism or cell. Photosensitizers that have been conjugated to antibodies include porphyrins (19), sulfonated aluminum phthalocyanine (20), and hematoporphyrin (10). The target cells have mainly been neoplasms; however, Berthiaume et al. (1) have shown that an antibody against Pseudomonas aeruginosa (which they used to infect the dorsal skin of mice) conjugated with tin(IV) chlorin e6 achieved a 95% reduction in viable bacteria when exposed to light with a wavelength of 630 nm. The aim of this study was to specifically target P. gingivalis by lethal photosensitization when in the presence of Streptococcus sanguis (a member of the normal oral microflora) or human gingival fibroblasts (HGFs) using TBO conjugated to an antibody (Ab-TBO conjugate) against P. gingivalis lipopolysaccharide (LPS).
MATERIALS AND METHODS
Laser and photosensitizer.
The laser used in the study was a HeNe gas laser (NEC Corporation, Tokyo, Japan) with a measured output of 7.3 mW, which emits light in a collimated beam (diameter, 1.3 mm) with a wavelength of 632.8 nm. The photosensitizer used in the experiments was TBO (Sigma Ltd., Poole, United Kingdom).
P. gingivalis W50 was maintained by twice-weekly subculture on Wilkins-Chalgren agar (Oxoid Ltd., Basingstoke, United Kingdom), and S. sanguis was maintained by twice-weekly subculture on tryptone soya agar (Oxoid Ltd.). The bacteria were both incubated at 37°C in an anaerobic cabinet (10% carbon dioxide, 10% hydrogen, and 80% nitrogen [Don Whitley Scientific Ltd., Yorkshire, United Kingdom]). For experimental purposes, a few colonies of P. gingivalis were inoculated into Bacteroides medium, which consisted of 10 g of tryptone soya broth, 10 g of proteose peptone, 5 g of yeast extract, 5 g of glucose, 5 g of sodium chloride, and 0.75 g of cysteine-HCl per liter of distilled water. The pH was adjusted to 7.5, and the broth was autoclaved at 121°C for 15 min. The medium was supplemented with hemin (Sigma Ltd.) and menadione (Sigma Ltd.) prior to use so that the final concentrations were 5 μg/ml and 0.5 μg/ml, respectively. The culture was incubated in an anaerobic chamber until it reached stationary phase (approximately 24 h).
For experimental purposes a few colonies of S. sanguis were inoculated into tryptone soya broth (Oxoid Ltd.) supplemented with 0.5% yeast extract (Oxoid Ltd.) and incubated until they reached stationary phase (approximately 15 h).
Preparation of Ab-TBO conjugate.
Murine monoclonal antibody (2 mg) against P. gingivalis LPS (prepared and characterized by P. Shepherd, and J. C. Cridland, Guy's Hospital [5]) was added to 0.4 mg of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (Pierce Ltd.), which reacts with the carboxyl groups of the antibody molecule. Sulfo-N-hydroxysuccinimide (Pierce Ltd.) (1.1 mg) was added, and the solution was mixed continuously for 30 min at room temperature. TBO (5 mg) was added, and the solution was mixed for a further 5 h at room temperature. The reaction was stopped by adding 20 mM ethanolamine. The solution was dialyzed against phosphate-buffered saline (pH 8.2) containing 0.15 M NaCl–2.96 mM Na2HPO4–1.0 mM KHPO4 until the dialysis solution was no longer blue. The Ab-TBO conjugate was concentrated using polyethylene glycol (Pierce Ltd.). In order to further remove any unconjugated TBO, the Ab-TBO solution was added to a filter unit (molecular mass cutoff = 3,000 Da; Sigma Ltd.) and centrifuged at 1,000 × g for 15 min. Dialysis buffer was added to the solution, and the centrifugation was repeated. This procedure was carried out until the filtrate was no longer blue. The absorbance of the TBO present in the conjugate was measured at 633 nm, and it was found that 4 μg (determined by using a standard curve) of TBO was conjugated to 2 mg of antibody.
Antibody-targeted lethal photosensitization of P. gingivalis and S. sanguis.
Stationary-phase P. gingivalis and S. sanguis cells were harvested and washed in sterile saline (0.85% [wt/vol]). The cells were resuspended in Ab-TBO conjugate, and 100 μl of the suspension was aliquoted into a 96-well microtiter plate. Triplicate wells were exposed to laser light at a dose of 4.4 J. The same procedure was carried out using free TBO (not conjugated to antibody) at a concentration of 4 μg/ml. Control wells were neither sensitized nor exposed to laser light. The same procedures were carried out on a suspension containing both P. gingivalis and S. sanguis. Aliquots (100 μl) were removed from each well and added to 900 μl of Wilkins-Chalgren broth (Oxoid Ltd.). Serial dilutions were made, and 50-μl aliquots from each dilution were removed and plated out on Fastidious anaerobe agar (Lab M Ltd., Bury, United Kingdom) containing 5% (wt/vol) horse blood for the enumeration of P. gingivalis colonies (distinguishable from S. sanguis colonies by their black color) and/or on tryptone soya agar (for enumeration of S. sanguis colonies). Survivors were enumerated after 24 h (for S. sanguis) and 4 to 7 days (for P. gingivalis). All experiments were carried out at least three times.
Antibody-targeted lethal photosensitization of P. gingivalis in the presence of HGFs.
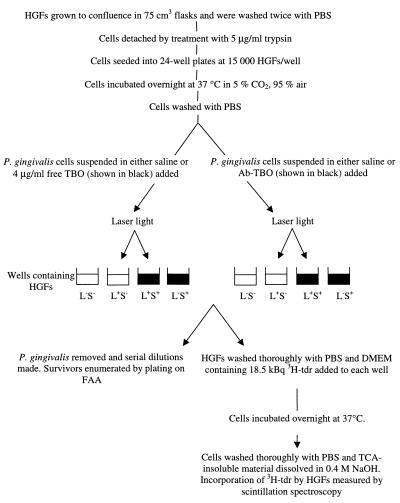
HGFs were kindly provided by Sajeda Meghji (Department of Oral and Maxillofacial Surgery, Eastman Dental Institute). The HGFs were maintained in Dulbecco's minimal Eagle's medium (Gibco, Paisley, United Kingdom) supplemented with 10% fetal calf serum (Sigma Ltd.), 100 IU of penicillin/ml, and 100 μg of streptomycin (Sigma Ltd.) per ml and incubated at 37°C in 5% CO2–air and were used at early passage. Antibody-targeted lethal photosensitization of HGFs was carried out using the method shown in Fig. 1. The cells were seeded at 15,000 per well and used the next day when confluent. The HGFs were monitored by microscopy to ascertain whether any P. gingivalis cells had adhered to them, and DNA synthesis in the HGFs was determined by measuring uptake of tritiated thymidine ([3H]Tdr) as outlined in Fig. 1. Experiments were carried out on at least three separate occasions.
FIG. 1.
Outline of the method used for determining the effects of TBO and Ab-TBO (in both the presence and absence of laser light) on HGFs and P. gingivalis in cultures containing both cell types. PBS, phosphate-buffered saline; FAA, Fastidious anaerobe agar; DMEM, Dulbecco's minimal Eagle's medium; TCA, trichloroacetic acid; L+, treatment with laser light; L−, no laser light treatment; S+, treatment with sensitizer; S−, no sensitizer treatment.
All statistical analyses were carried out using single-factor analysis of variance. Data are means ± standard deviations of four values. Differences were considered to be statistically significant when the value of P was less than 0.05. Bacterial viability was expressed as CFU per milliliter.
RESULTS
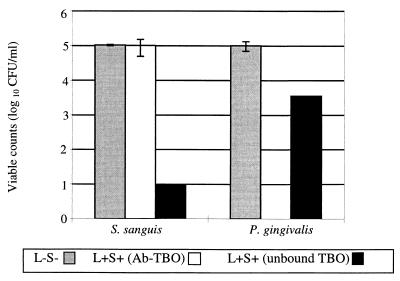
With unconjugated TBO and 4.4 J of light, there was a statistically significant 99.99% (4-log10-unit) reduction in the viable count for S. sanguis compared to a 97.5% (1.5-log10-unit) reduction for P. gingivalis (Fig. 2), although the latter reduction was also statistically significant. However, when the TBO was bound to an antibody (Ab-TBO) against P. gingivalis LPS, there was a 2% (0.1-log10-unit) reduction in viable counts of S. sanguis, which was not statistically significant, and a 100% (5-log10-unit) reduction in the number of viable P. gingivalis. Irradiation with the same dose of laser light in the absence of TBO had no effect on the viability of either P. gingivalis or S. sanguis. The viability of neither organism was affected by exposure to TBO or Ab-TBO in the dark.
FIG. 2.
Effect of irradiation with 4.4 J of HeNe laser light on the viability of P. gingivalis and S. sanguis (in a suspension containing both organisms) when in the presence of 4 μg of either unconjugated TBO or Ab-TBO/ml. L−S−, cells were not sensitized or exposed to laser light; L+S+, cells were sensitized and exposed to laser light.
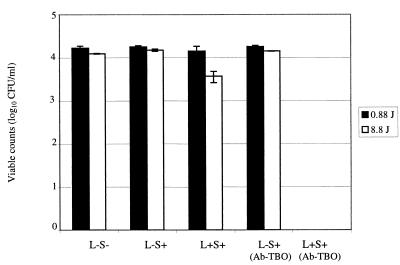
Figure 3 shows the reduction in viable counts of P. gingivalis when irradiated with laser light and treated with either TBO or Ab-TBO in the presence of HGFs. Microscopic monitoring of the HGFs revealed that P. gingivalis did not adhere to these cells. At a free-TBO concentration of 4 μg/ml and a light dose of 0.88 J, there was no reduction in bacterial counts, whereas at a light dose of 8.8 J there was a statistically significant 0.7-log10-unit reduction. In contrast, when the Ab-TBO conjugate was used, there was a 100% reduction in the viable count when either 0.88 or 8.8 J of light was used.
FIG. 3.
Viable counts of P. gingivalis when sensitized with 4 μg of unconjugated TBO or Ab-TBO/ml and exposed to 0.88 and 8.8 J of laser light in the presence of HGFs. (L−S−, no laser light and no TBO; L−S+, no laser light, but cells were sensitized with TBO or Ab-TBO; L+S+, cells were exposed to laser light and sensitized with TBO or Ab-TBO.
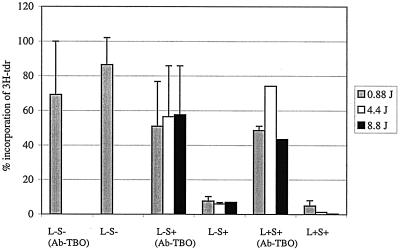
Figure 4 shows that in the presence of P. gingivalis, at a TBO concentration of 4 μg/ml, there were statistically significant reductions (compared to results for no sensitization or exposure to laser light) in the incorporation of [3H]Tdr into HGFs when exposed to increasing doses of laser light. These amounted to reductions of 94, 98, and 99%, respectively, when light doses of 0.88, 4.4, and 8.8 J were used. Similar reductions in [3H]Tdr incorporation were obtained when the HGFs were exposed to the unconjugated TBO in the dark. In contrast, when Ab-TBO was used, there was no significant reduction in the incorporation of [3H]Tdr into HGFs; this was true for cultures which had been irradiated with laser light as well as for those which had not.
FIG. 4.
The incorporation of [3H]Tdr into HGFs sensitized with 4 μg of TBO or Ab-TBO/ml and exposed to increasing laser light doses in the presence of P. gingivalis. Abbreviations are as defined in the legend for Fig. 3.
DISCUSSION
Photodynamic therapy for bacterial infections could be both rapid and effective. However, the nonspecific binding of photosensitizer could be a bar to the use of such treatment due to light-induced damage to commensal bacteria and to host tissues. In this study we report that conjugation of the photosensitizer TBO to a monoclonal antibody to P. gingivalis LPS may overcome this potential limitation.
When conjugating any photosensitizer to an antibody, it is desirable that, on exposure to laser light, the yield of cytotoxic species should be the same as that of the unconjugated photosensitizer (22). Furthermore, as photosensitizers are sensitive to alterations in their structure, conjugation of a photosensitizer to an antibody may alter the activity of the photosensitizer and/or the specificity of the antibody (6). The results of this study have shown that TBO conjugated to an antibody against P. gingivalis is an effective photosensitizer of this organism. Indeed, the kills achieved with the Ab-TBO conjugate were greater than those achieved with the unconjugated TBO.
The monoclonal antibody used in these experiments was raised to P. gingivalis LPS, and it has been shown by Ní Eidhin and Mouton (12) that these types of antibodies are highly specific for the organism. They found that an antibody to P. gingivalis LPS recognized 10 different P. gingivalis strains but recognized none of the 34 non-P. gingivalis strains of bacteria (22 Prevotella and 12 Bacteroides strains tested). This specificity is supported by the results of the present study, as S. sanguis was more prone to lethal photosensitization than P. gingivalis when free TBO was used; however when Ab-TBO was used, there was a 100% reduction in viable counts of P. gingivalis compared to a 2% reduction of S. sanguis viable counts on exposure to laser light.
The reduction in the viable counts of P. gingivalis in pure culture at a light dose of 8.8 J was 100% (15). However, when lethal photosensitization of P. gingivalis was carried out in the presence of HGFs using unconjugated TBO, there was only a 0.7-log10-unit reduction in P. gingivalis viable counts but a 99% reduction in the incorporation of [3H]Tdr by human cells, a measure of DNA synthesis. This situation was completely turned around when TBO was conjugated with the anti-P. gingivalis monoclonal antibody. This resulted in complete killing of the bacteria with minimal inhibition of DNA synthesis.
The data obtained can also give some insight into the mechanism of the bactericidal effect. The fact that the TBO is bound to the antibody and therefore cannot enter the bacterial cell suggests that the photosensitizer does not necessarily have to be present intracellularly in order to exert a bactericidal effect. Our previous work has shown that membrane proteins may be affected by lethal photosensitization, and one group of proteins affected may be the proteases of this organism. Work carried out by Packer et al. showed that lethal photosensitization using TBO and HeNe laser light does indeed cause a decrease in the proteolytic activity of P. gingivalis (15). This could lead to a reduction in bacterial colonization of the oral cavity (as these proteases are involved in adhesion to host tissues) and less degradation of antibodies, cytokines, and extracellular matrix polymers.
In conclusion, the results of this study have demonstrated that specific targeting of P. gingivalis to lethal photosensitization can be achieved by linking TBO to an antibody against the organism. Such an approach, if used in vivo, could enable the killing of this important periodontopathogen without collateral damage either to host tissues or to the normal oral microflora. This could form the basis of a new approach to the treatment of periodontitis, the most prevalent chronic infectious disease of humans. The antibody-photosensitizer conjugate could be applied to the disease lesion (the periodontal pocket, a gap formed between the tooth and gum) using a blunt syringe and the conjugate could be activated by irradiating with light via an optical fiber inserted into the pocket. Use of the particular antibody-photosensitizer conjugate employed in this study would, of course, be of value only to individuals suffering from periodontitis due solely to P. gingivalis. Periodontitis due to other periodontopathogenic species (e.g., A. actinomycetemcomitans) would obviously require antibody-photosensitizer conjugates with different specificities. We are currently investigating the efficacy of such conjugates in an animal model prior to clinical evaluation.
ACKNOWLEDGMENTS
We acknowledge the Charles Wolfson Charitable Trust for providing funding for this work.
REFERENCES
- 1.Berthiaume F S, Reiken R, Toner M, Tompkins R G, Yarmush M L. Antibody-targeted photolysis of bacteria in vivo. Bio/Technology. 1994;12:703–705. doi: 10.1038/nbt0794-703. [DOI] [PubMed] [Google Scholar]
- 2.Bertoloni G, Rossi F, Valduga G, Jori G, Lier J V. Photosensitising activity of water- and lipid-soluble phthalocyanine in E. coli. FEMS Microbiol Lett. 1990;71:149–156. doi: 10.1111/j.1574-6968.1990.tb03814.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M. Influence of dosimetric and physiological conditions on the lethal photosensitisation of Porphyromonas gingivalis. Photochem Photobiol. 1997;66:1026–1031. doi: 10.1111/j.1751-1097.1997.tb07964.x. [DOI] [PubMed] [Google Scholar]
- 4.Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M. A study of the uptake of toluidine blue O by Porphyromonas gingivalis and the mechanism of lethal photosensitisation. Photochem Photobiol. 1998;68:370–376. [PubMed] [Google Scholar]
- 5.Cridland J C, Booth V, Ashley F P, Curtis M A, Wilson R F, Shepherd P. Preliminary characterisation of antigens recognised by monoclonal antibodies raised to Porphyromonas gingivalis and by sera from patients with periodontitis. J Periodontol Res. 1994;29:339–347. doi: 10.1111/j.1600-0765.1994.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 6.Dahl T A. Antibody-targeted photosensitization. The Spectrum newsletter. 1992. pp. 11–16. [Google Scholar]
- 7.Dobson J, Wilson M. Sensitization of oral bacteria in biofilms to killing by light from a low-power laser. Arch Oral Biol. 1992;37:883–887. doi: 10.1016/0003-9969(92)90058-g. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty T J, Potter W R, Bellinier D. Photodynamic therapy for the treatment of cancer: current status and advances. In: Kessel D, editor. Photodynamic therapy of neoplastic disease. Boca Raton, Fla: CRC Press; 1990. pp. 1–19. [Google Scholar]
- 9.Dougherty T J, Gomer C J, Henderson B W, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamblin M R, Newman E L. Photosensitiser targeting in photodynamic therapy. I. Conjugates of haematoporphyrin with albumin and transferrin. J Photochem Photobiol B. 1994;26:45–56. doi: 10.1016/1011-1344(94)85035-6. [DOI] [PubMed] [Google Scholar]
- 11.Merchat M, Bertolini G, Giacomini P, Villanueva A, Jori G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J Photochem Photobiol B. 1996;32:153–157. doi: 10.1016/1011-1344(95)07147-4. [DOI] [PubMed] [Google Scholar]
- 12.Ní Eidhin D, Mouton C. The lipopolysaccharide of Porphyromonas gingivalis is not antigenically cross-reactive with that of other species. J Dent Res. 1994;73:661–670. doi: 10.1177/00220345940730031201. [DOI] [PubMed] [Google Scholar]
- 13.Nitzan Y, Dror R, Ladan H, Malik Z, Kimel S, Gottfried V. Structure-activity relationship of porphyrines for photoinactivation of bacteria. Photochem Photobiol. 1995;62:342–347. doi: 10.1111/j.1751-1097.1995.tb05279.x. [DOI] [PubMed] [Google Scholar]
- 14.Orenstein A, Klein D, Kopolovic J, Winkler E, Malik Z, Keller N, Nitzan Y. The use of porphyrins for eradication of Staphylococcus aureus in burn wound infections. FEMS Immunol Med Microbiol. 1997;19:307–314. doi: 10.1111/j.1574-695X.1997.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 15.Packer S, Bhatti M, Burns T, Wilson W. Inactivation of proteolytic enzymes from Porphyromonas gingivalis using light-activated agents. Lasers Med Sci. 2000;15:24–30. doi: 10.1007/s101030050043. [DOI] [PubMed] [Google Scholar]
- 16.Paardekooper M, van den Broek P J, De Bruijne A W, Elferink J G, Dubbelman T M, van Steveninck J. Photodynamic treatment of yeast cells with the dye toluidine blue: all-or-none loss of plasma membrane barrier properties. Biochim Biophys Acta. 1992;1108:86–90. doi: 10.1016/0005-2736(92)90117-5. [DOI] [PubMed] [Google Scholar]
- 17.Paardekooper M, De Bruijne A W, Van Steveninck J, van den Broek P J. Inhibition of transport systems in yeast by photodynamic treatment with toluidine blue. Biochim Biophys Acta. 1993;1151:143–148. doi: 10.1016/0005-2736(93)90097-j. [DOI] [PubMed] [Google Scholar]
- 18.Paardekooper M, De Bruijne A W, van Steveninck J, van den Broek P J. Intracellular damage in yeast cells caused by photodynamic treatment with toluidine blue. Photochem Photobiol. 1995;61:84–89. doi: 10.1111/j.1751-1097.1995.tb09247.x. [DOI] [PubMed] [Google Scholar]
- 19.Papkovskii D B, Savitskii A P, Egorova S G, Sukhin G M, Chissov V I, Krasnovskii A A, Yu Egorov S, Ponomarev G V, Kirillova G V. Photodestruction in vitro of tumour cells sensitised by porphyrins and their conjugates with specific antibodies. Biomed Sci. 1990;1:401–407. [PubMed] [Google Scholar]
- 20.Savitsky A P, Lopatin K V. pH dependence of fluorescence and absorbance spectra of free sulphonated aluminum phthalocyanine and its conjugate with monoclonal antibodies. J Photochem Photobiol B. 1992;13:327–333. doi: 10.1016/1011-1344(92)85073-4. [DOI] [PubMed] [Google Scholar]
- 21.Smetana Z, Ben-Hur E, Mendelson E, Salzberg S, Wagner P, Malik Z. Herpes simplex virus proteins are damaged following photodynamic inactivation with phthalocyanines. J Photochem Photobiol B. 1998;44:77–83. doi: 10.1016/S1011-1344(98)00124-9. [DOI] [PubMed] [Google Scholar]
- 22.Strong L, Yarmush D M, Yarmush M L. Antibody-targeted photolysis. Photophysical, biochemical and pharmacokinetic properties of antibacterial conjugates. Ann N Y Acad Sci. 1994;1994:297–320. [PubMed] [Google Scholar]
- 23.Wilson M. Bactericidal effect of laser light and its potential use in the treatment of plaque-related diseases. Int Dent J. 1994;44:181–189. [PubMed] [Google Scholar]
- 24.Wilson M, Mia N. Effect of environmental factors on the lethal photosensitisation of Candida albicans in vitro. Lasers Med Sci. 1994;9:105–109. [Google Scholar]