Abstract
The present study investigates the antiParkinsonian activity of dipeptidyl peptidase-4 (DPP-IV) inhibitor, linagliptin. The experimental Parkinson's disease (PD) was induced by administration of rotenone at a dose of 1.5 mg/kg at alternate day subcutaneously for 21 days. Standard drug (levodopa-200 mg/kg and carbidopa-50 mg/kg) and treatment drug (linagliptin-5 mg/kg, 10 mg/kg, and 20mg/kg) were administered orally daily 1 h before rotenone administration. In a rat rotenone model, linagliptin improved muscle coordination, motor performance, and corrected akinesia. Pretreatment with linagliptin showed significant higher levels of superoxide dismutase, catalase, and glutathione in brain homogenate of animals. Linagliptin significantly elevated the levels of striatal DA and active glucagon-like peptide 1 in brain homogenate of animals. Furthermore, linagliptin amended alterations induced by rotenone in the thiobarbituric acid reactive substances and inflammatory marker such as tumor necrosis factor-α level. The results of the present study indicate the neuroprotective potential of linagliptin for the management of PD might be due to remarkable improvement in motor functions, antioxidant, anti-inflammatory, anti-apoptotic, and neuroprotective mechanisms.
Keywords: Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1, linagliptin, Parkinson's disease, rotenone
Introduction
Parkinson's disease (PD) is the neurodegenerative disorder which affects movement in 1%–2% of the worldwide population above the age of 65.[1] The primary pathological finding in PD is damage to the dopaminergic projections in the nigrostriatal pathway of brain which causes loss of dopamine (DA) that results in extrapyramidal effects.[2] Mitochondrial dysfunction, oxidative stress, protein aggregation, neurotoxins, and neuroinflammation are some of the major pathological reasons linked with PD.[3]
Rotenone is used as a pesticide which is a risk factor for the advancement of PD. The animal rotenone model was used to find out multiple mechanisms such as aggregation of a-synuclein, microglia activation, and induction of apoptosis.[2]
Levodopa (L-dopa) is the main pharmacological treatment for PD which is combined with a drug that inhibits the metabolism of DA peripherally. L-dopa has adverse effects such as nausea/vomiting, involuntary movements, orthostatic hypotension, and psychiatric disturbance. Some drugs are used in combination with L-dopa, but efficacy decreases over time, in parallel with neuronal loss, and therefore, the durability of treatment is limited. Not a drug can prevent disease progression.[4]
Glucagon-like peptide-1 (GLP-1) is an incretin hormone which is associated with glucose homeostasis. There are some evidences that suggest GLP-1 has beneficial role in neurodegenerative diseases. GLP-1R is a G-protein coupled receptor for GLP-1 activity. Activation of adenyl cyclase leads to increment in intracellular Cyclic adenosine monophosphate that phosphorylates various downstream pathways ultimately leading to neuroprotection.[5]
Dipeptidyl peptidase-4 (DPP-IV) inhibitors are used for the treatment of type II diabetes mellitus. Many studies have shown that GLP-1 R stimulation has neuroprotective effects which result in improvements in motor and nonmotor deficits.[5]
However, it is observed that GLP-1 is readily degraded by DPP-IV into a metabolite that is inactive against GLP-1 R. Linagliptin is a DPP-IV inhibitor and it is reported to have anti-inflammatory and antioxidant properties that can be explored for neuroprotective effects in PD.[6]
Thus, the study was carried out to characterize the neuroprotective effects of linagliptin in PD models in rats.
Materials and Methods
Experimental animals
Experiment was carried out according to the CPCSEA guidelines. The research proposal was approved by the IAEC, and Protocol approval number was BKMGPC/IAEC20/RP33/2017. Adult male Wistar rats were used.
Experimental design
The normal control group was administered 0.5% Na CMC p.o. daily. Disease control group was administered with rotenone in 1% DMSO (1.5 mg/kg/48 h/11 doses, s.c.,) at alternate days for 3 weeks.[7] Levodopa (200 mg/kg) and carbidopa (50 mg/kg) were administered to animals of standard control group p.o. daily for 21 days and 1 h before the administration of Linagliptin (5, 10, and 20 mg/kg) was given p.o. daily to different treatment groups for 21 days and 1 h before the administration of rotenone.
Evaluation parameters
Screening of animals was done for motor diminishing effects using open field, catalepsy, rotarod, and postural instability test. After evaluation of the behavior parameters, animals were sacrificed. Brain was quarantined and was kept in ice-cold saline. Brain was kept in 10% formalin for histopathology. The remaining brain was frozen at −20°C for the estimation of biochemical parameters. A 10% tissue homogenate was prepared in phosphate buffer. Centrifugation of tissue homogenate was done at 4000 rpm for 10 min at 4°C. Clear supernatant was collected for use in analysis of antioxidant assays, DA level, active GLP-1 level, and tumor necrosis factor-alpha (TNF-α) level.[8,9,10,11,12,13,14]
Statistical analysis
All values were written as mean ± standard error of the mean. Statistically significant was considered by one-way analysis of variance if P =< 0.05.
Results
Effect of linagliptin in open field test
Pretreatment with linagliptin (10 mg/kg) showed a significant reduction in immobility (62.50 ± 0.76) as compared to those of disease control group (115.5 ± 3.42). Rearing frequency and activity index of rotenone treated animals were significantly decreased which showed significant increase after linagliptin treatment (10 mg/kg).
Effect of linagliptin in rotarod performance
Linagliptin treatment (10 mg/kg) showed significantly high latency of fall (34.66 ± 1.14) as compared to those of disease control group (15.33 ± 1.20) which indicates enhanced motor performance and balance as compared to disease control group. These effects were correlated by a 2.4-fold increment in striatal DA content [Figure 1].
Figure 1.
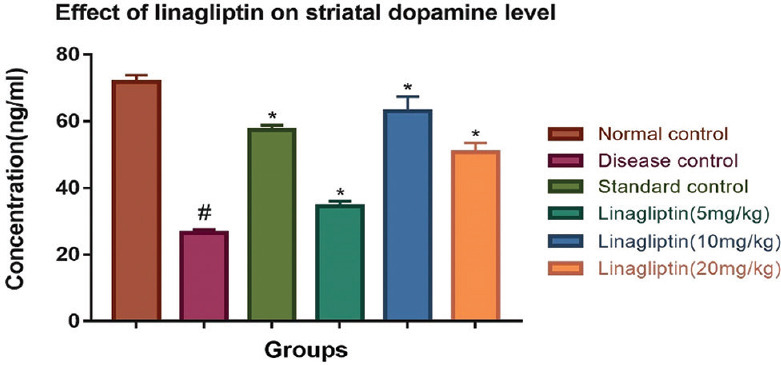
Effect of linagliptin on rotenone-induced alterations in striatal DA level in brain homogenate
Effect of linagliptin in catalepsy test
Catatonia in rotenone-administered animals was markedly raised (21.18 ± 0.57) as compared to normal control (3.01 ± 0.16) group of animals which was abolished by treatment with linagliptin (10 mg/kg) (9.93 ± 0.40) as compared with disease control group.
Effect of linagliptin on postural instability
Animals treated with rotenone showed prolongation (6.73 ± 0.12) in displacement needed to trigger a catchup step when compared with normal control group (3.58 ± 0.17) animals. Linagliptin (10 mg/kg) treatment significantly reduced (4.30 ± 0.12) the displacement needed to trigger a catchup step as compared with disease control group animals.
Effect of linagliptin on TBARS level of brain
Linagliptin treatment (10 mg/kg) to animals showed significant reduction (0.104 ± 0.019) in TBARS level as compared to disease control group animals.
Effect of linagliptin on rotenone-induced alterations on antioxidant assays in brain homogenate
Treatment with linagliptin (10 mg/kg) showed significant elevation in the superoxide dismutase level, catalase level, and GSH level of brain homogenate as compared to disease control group animals as shown in Table 1.
Table 1.
Effect of linagliptin on rotenone-induced alterations on antioxidant assays in brain homogenate
| Groups | SOD (reduced NBT pM/min/mg) | Catalase (µM of H2 O2 oxidized/minute/mg protein) | GSH (µmol/mg protein) |
|---|---|---|---|
| Normal control | 0.035±0.0010 | 5.97±0.95 | 3.10±0.46 |
| Disease control | 0.010±0.0010# | 0.65±0.28# | 1.14±0.11# |
| Standard control | 0.019±0.0023* | 4.10±0.59* | 2.40±0.37* |
| Linagliptin (5 mg/kg) | 0.013±0.0020* | 4.41±1.26* | 2.57±0.22* |
| Linagliptin (10 mg/kg) | 0.026±0.0020* | 4.86±0.71* | 3.06±0.46* |
| Linagliptin (20 mg/kg) | 0.018±0.0010* | 4.60±0.86* | 2.61±0.14* |
H2 O2=Hydrogen peroxide, SOD=Superoxide dismutase, NBT=Nitroblue tetrazolium, GSH=Glutathione. All values are expressed as Mean±SEM. # p< 0.05 and *p< 0.05
Effect of linagliptin on striatal DA level of brain
Linagliptin (10 mg/kg) treatment significantly elevated (63.06 ± 4.34) the level of dopamine in animals as compared to disease control group animals (26.59 ± 0.89) as shown in Figure 1.
Effect of linagliptin on active glucagon-like peptide-1 level of brain
Treatment with linagliptin (10 mg/kg) showed significant elevation (2671.32 ± 22.63) in active GLP-1 concentrations as when compared with disease control group (2150.41 ± 19.87) [Figure 2].
Figure 2.
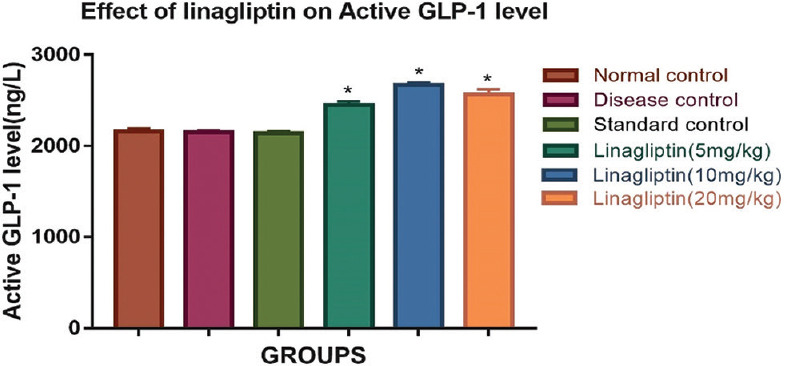
Effect of linagliptin on rotenone-induced alterations in active glucagon-like peptide-1 level in brain homogenate
Effect of linagliptin on tumor necrosis factor-alpha level of brain
Linagliptin (10 mg/kg) treatment significantly lowers (310.58 ± 44.56) the level of TNF-alpha in animals as compared with disease control group animals (1415.58 ± 34.48) [Figure 3].
Figure 3.

Effect of linagliptin on rotenone-induced alterations in tumor necrosis factor-alpha level in brain homogenate
Histopathological examination
Nigral tissues of disease control group of animals showed shrinkage of neurons, disrupted cell structure, and inflammatory cell infiltrate when compared with normal control group of animals. Linagliptin (10 mg/kg) resulted in a normalization of cell structure. In addition to that inflammatory cell infiltrate is significantly decreased compared to disease control group.
Discussion
In this study, we have evaluated the effects of linagliptin (DPP-IV inhibitor) in rotenone-induced PD in animals at three different doses, i.e., 5 mg/kg, 10 mg/kg, and 20 mg/kg. The deficits in the motor coordination and balance were observed in disease control animals by rotarod test, catalepsy test, postural instability test, and open field test. Present research work evaluated the latency to fall time in rotarod, cataleptic behavior, postural instability, activity index, and rearing behavior in the Parkinsonian animals. Rotenone produces PD-like behavioral symptoms by reducing the dopaminergic neurons.[15] Treatment with linagliptin (10 mg/kg) significantly attenuated the latency to fall time, cataleptic behavior, postural instability, activity index, and decreased rearing behavior induced by rotenone in animals. Significantly, lower level of DA in disease control animals was attenuated by the treatment with linagliptin (10 mg/kg). The depletion of DA level has been considered as main factor contributing to PD-like behavioral symptoms. The restoration of DA level by linagliptin may be the main reason for attenuation of rotenone-induced behavioral deficits.
Rotenone exposure to animals is a significant model for studying the mechanism of oxidative stress damaging the dopaminergic neurons.[16] Lipid peroxidation is the process in which polyunsaturated fatty acids are converted to lipid peroxides. The presence of metal complexes enhances their breakdown into reactive species and increases in lipid peroxidation. If continued, it causes gradual loss in membrane potential, membrane integrity, and membrane fluidity.[17] Linagliptin (10 mg/kg) significantly lowered the level of lipid peroxidase products in the treated animals.
Superoxide dismutase is an antioxidant enzyme that protects cell components from reactive oxygen species (ROS). It catalyzes the dismutation of superoxide into hydrogen peroxide and oxygen.[12] Catalase is another antioxidant enzyme that decomposes H2O2 produced in the natural cellular processes. Although H2O2 alone is not reactive species enough to produce chain of lipid peroxidation products, it reacts with superoxide radical which produces hydroxyl ion and that is very reactive. Catalase converts H2O2 to water and nonROS. GSH is an important antioxidant defense mechanism that is altered in the patients of PD. GSH helps in clearance of H2O2 and suppression of hydroxyl free radical generation.[18] Treatment with linagliptin (10 mg/kg) significantly raised the level of antioxidant defense mechanisms in the animals of linagliptin treated group compared to disease control group animals. These effects are observed because linagliptin activates GLP1-R receptor that leads to restoration of mitochondrial function by inhibiting the glycogen synthase kinase-3 pathway.[5]
Rotenone is an inhibitor of mitochondrial complex I that leads to adenosine triphosphate depletion, this causes activation of microglia. Further, it leads to increase in reactive oxidative species that trigger the mitogen-activated protein kinase pathway, which increases the pro-inflammatory factors like TNF-α.[19] Animals treated with linagliptin (10 mg/kg) significantly reduced the level of TNF-α in the brain compared to disease group. The activation of GLP-1 signals the AKT pathway which regulates the inflammatory mediators like TNF-α through nuclear factor kappa B (NF-кb) signaling pathway. NF-кB signaling pathway is attenuated by the linagliptin which hampers the production of inflammatory mediators like TNF-α.[5]
Treatment with linagliptin (10 mg/kg) significantly attenuated the lowering of striatal DA level imposed by rotenone in the animals. These effects may be due to anti-apoptotic activity of the GLP-1 activation. The present investigation showed significantly higher level of GLP-1 in brains of animals treated with linagliptin (10 mg/kg) that may be due to the DPP-IV inhibitory action of linagliptin. This increase in GLP-1 concentration in brain showed significant neuroprotection against rotenone.
Moreover, the evaluation of nigral tissue using H and E stain showed many histopathological alterations which are supported by of apoptotic signs such as increase in inflammatory cell infiltrates, shrinked neurons, and disruption of normal cell structure in rotenone administered animals. Pro-inflammatory mediators like TNF-α are observed to be higher and may be responsible for the apoptosis of neurons in the PD.[20] Animals treated with linagliptin (10 mg/kg) significantly normalized the cell structure and decreased the presence of inflammatory cell infiltrates.
Conclusion
From the present investigation, it was concluded that linagliptin (10 mg/kg) significantly attenuates the development of PD induced by rotenone administration through GLP-1 activity. The neuroprotective effect of linagliptin (10 mg/kg) might be related to its antioxidant, anti-inflammatory, and anti-apoptotic activity mediated by GLP-1R which has a beneficial role in PD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are thankful to the B.K. Mody Government Pharmacy College, Rajkot, for providing basic research facilities and resources for the completion of the research work.
References
- 1.Montie HL, Durcan TM. The cell and molecular biology of neurodegenerative diseases: An overview. Front Neurol. 2013;4:194. doi: 10.3389/fneur.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badawi GA, Abd El Fattah MA, Zaki HF, El Sayed MI. Sitagliptin and liraglutide reversed nigrostriatal degeneration of rodent brain in rotenone-induced Parkinson's disease. Inflammopharmacology. 2017;25:369–82. doi: 10.1007/s10787-017-0331-6. [DOI] [PubMed] [Google Scholar]
- 3.Huang Z, Fuente-Fernández Rd, Stoessl AJ. Etiology of Parkinson's disease. Can J Neurol Sci. 2003;30(Suppl 1):S10–8. doi: 10.1017/s031716710000319x. [DOI] [PubMed] [Google Scholar]
- 4.Holst JJ, Burcelin R, Nathanson E. Neuroprotective properties of GLP-1: Theoretical and practical applications. Curr Med Res Opin. 2011;27:547–58. doi: 10.1185/03007995.2010.549466. [DOI] [PubMed] [Google Scholar]
- 5.Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson's disease: Mechanisms of action. Drug Discov Today. 2016;21:802–18. doi: 10.1016/j.drudis.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Ma M, Hasegawa Y, Koibuchi N, Toyama K, Uekawa K, Nakagawa T, et al. DPP-4 inhibition with linagliptin ameliorates cognitive impairment and brain atrophy induced by transient cerebral ischemia in type 2 diabetic mice. Cardiovasc Diabetol. 2015;14:54. doi: 10.1186/s12933-015-0218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Gawad HA, Abdallah D, El-Abhar H. Rotenone-induced Parkinson's like disease: Modulating role of coenzyme Q10. J Biol Sci. 2004;4:568–74. [Google Scholar]
- 8.Khorevin VI, Levchouk TE, Gorkovenko AV, Mishunina TM. Effect of L-DOPA on the behavioral activity of wistar and spontaneously hypertensive (SHR) rats in the open-field test. Neurophysiology. 2004;36:116–25. [Google Scholar]
- 9.Abdelsalam RM, Safar MM. Neuroprotective effects of vildagliptin in rat rotenone Parkinson's disease model: Role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. J Neurochem. 2015;133:700–7. doi: 10.1111/jnc.13087. [DOI] [PubMed] [Google Scholar]
- 10.Costall B, Naylor RJ. On catalepsy and catatonia and the predictability of the catalepsy test for neuroleptic activity. Psychopharmacologia. 1974;34:233–41. doi: 10.1007/BF00421964. [DOI] [PubMed] [Google Scholar]
- 11.Woodlee MT, Kane JR, Chang J, Cormack LK, Schallert T. Enhanced function in the good forelimb of hemi-parkinson rats: Compensatory adaptation for contralateral postural instability? Exp Neurol. 2008;211:511–7. doi: 10.1016/j.expneurol.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma N, Bafna P. Effect of Cynodon dactylon on rotenone induced Parkinson's disease. Orient Pharm Exp Med. 2012;12:167–75. [Google Scholar]
- 13.Aksoy D, Solmaz V, Çavuşoğlu T, Meral A, Ateş U, Erbaş O. Neuroprotective effects of eexenatide in a rotenone-induced rat model of Parkinson's disease. Am J Med Sci. 2017;354:319–24. doi: 10.1016/j.amjms.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson's disease. Neurobiol Dis. 2009;34:279–90. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, et al. Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci. 2003;23:10756–64. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamilselvam K, Braidy N, Manivasagam T, Essa MM, Prasad NR, Karthikeyan S, et al. Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson's disease. Oxid Med Cell Longev. 2013;45:1–11. doi: 10.1155/2013/102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halliwell B, Gutteridge JM. Oxygen radicals and the nervous system. Trends Neurosci. 1985;8:22–6. [Google Scholar]
- 18.Knight TR, Fariss MW, Farhood A, Jaeschke H. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol Sci. 2003;76:229–36. doi: 10.1093/toxsci/kfg220. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Wu JY, Zhou F, Sun XL, Yao HH, Yang Y, et al. The regulation of rotenone-induced inflammatory factor production by ATP-sensitive potassium channel expressed in BV-2 cells. Neurosci Lett. 2006;394:131–5. doi: 10.1016/j.neulet.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Nagatsu T. Parkinson's disease: Changes in apoptosis-related factors suggesting possible gene therapy. J Neural Transm (Vienna) 2002;109:731–45. doi: 10.1007/s007020200061. [DOI] [PubMed] [Google Scholar]


