Abstract
Although diagnostic criteria have been developed characterizing postural orthostatic tachycardia syndrome (POTS), no single set of criteria is universally accepted. Furthermore, there are gaps in the present criteria used to identify individuals who have this condition. The reproducibility of the physiological findings, the relationship of symptoms to physiological findings, the presence of symptoms alone without any physiological findings and the response to various interventions confuse rather than clarify this condition. As many disease entities can be confused with POTS, it becomes critical to identify what this syndrome is. What appears to be POTS may be an underlying condition that requires specific therapy. POTS is not simply orthostatic intolerance and symptoms or intermittent orthostatic tachycardia but the syndrome needs to be characterized over time and with reproducibility. Here we address critical issues regarding the pathophysiology and diagnosis of POTS in an attempt to arrive at a rational approach to categorize the syndrome with the hope that it may help both better identify individuals and better understand approaches to therapy.
Keywords: Tachycardia, Orthostatic intolerance, Postural orthostatic tachycardia
Introduction
Postural Orthostatic Tachycardia Syndrome (POTS), originally identified in 19821 and characterized further in 1993,2 was initially considered a condition in which symptoms were closely tied to, and caused by, orthostatic intolerance due to autonomic dysfunction. Clinical features of POTS resembled previously described syndromes including: neurasthenia, effort syndrome, soldier’s heart syndrome, Da Costa’s syndrome and, more recently (1970s), mitral valve prolapse syndrome.3–7 These ‘older’ syndromes have largely disappeared, whereas, POTS, at least in the manner it is currently employed, seems to more than fill the void.
Initially, POTS was thought to be due to autonomic dysfunction, albeit, of modest severity based on findings obtained during clinical laboratory studies (e.g., tilt-table test results, phase 2 Valsalva responses, quantitative sudomotor axon reflex tests, thermoregulatory sweat tests, peripheral nerve conduction, and excessive plasma catecholamines)2.8 Later, additional possibly responsible mechanisms were added including: neuropathy involving the distal vasculature sparing cardiac innervation, cardiovascular deconditioning, and cardiac beta-adrenoreceptor supersensitivity.9
This latter expansion of the syndrome’s causation inevitably broadened the range of associated conditions considered “POTS like” to include: volume depletion, inflammatory disorders and autoimmune diseases. Consequently, the original unified and meaningful initial POTS description of a select group of patients with orthostatic intolerance and sinus tachycardia has evolved to encompass a multitude of patients with a constellation of complaints. In many instances the presenting symptoms are nonspecific in nature, encompass multiple body systems and often are not even associated with orthostatic intolerance. There may not be any identifiable autonomic disorder at all.10–12
A further consequence of a less concrete POTS picture is that it is likely that clinical care may be affected adversely as clinicians are now contending with: 1) ill-defined diagnostic criteria, 2) symptoms unrelated to evident abnormal orthostatic or autonomic physiologic measurements, 3) inappropriate merging of POTS “pathophysiology” with underlying diseases, 4) attribution of syndrome-related complications in individual patients to a host of unproven etiologic theories and 5) inclusion of reversible conditions, such as, medication effects and drug use. Importantly, many treatable serious diseases present with tachycardia and these diagnoses can be missed if the patient is considered to have POTS as cause for the problem.
This communication was initiated to clarify the origins of the definition of POTS, to highlight gaps in definition that have resulted in diagnostic confusion and affected clinical care, to develop a direction for patient assessment and management and to consider areas of future research.
POTS background
The original POTS diagnostic criteria were derived primarily from findings during 80° head-up tilt-table testing2 in 16 symptomatic patients (of whom, 3 were men) selected from 188 individuals who presented to the Mayo Autonomic Disorders Clinic.2 The findings in these patients were compared to 21 men and 20 women controls and resulted in 2 diagnostic criteria: 1) sustained orthostatic heart rate increase >2 standard deviations above the control group, or 2) a baseline HR >110 bpm with further increase during tilt-table test of >20 bpm or to a level exceeding >140 bpm. While several small studies supported these findings,13–15 even the original description was problematic. Specifically, within the POTS cohort: 3 patients had substantial orthostatic hypotension and two patients did not have a heart rate increase >100 bpm on tilting.
Further uncertainty occurred in 2009 when, Low et al. expanded the POTS diagnostic criteria to include: hypovolemia, deconditioning and a “hyperadrenergic state”, among others.9 Thus, a “POTS” designation became more diagnostically sensitive, but even less specific. Broadened criteria allowed for several real and some poorly established illnesses, including suspected inflammatory diseases and connective tissue abnormalities (e.g., Ehlers Danlos syndrome), chronic fatigue syndrome, several autoimmune disorders (e.g. Sjögren’s syndrome), MAST cell disease, and primary gastroparesis, to be included as part of the POTS landscape16–20 (Table 1).
Table 1.
Conditions that can mimic POTS.
| Patients with suspected POTS may have: |
|---|
| Mast cell activation syndrome (MCAS) |
| Ehlers Danlos syndrome (EDS) |
| Autoimmune disease: often positive ANA, but does not meet specific diagnosis |
| Sjögren’s syndrome |
| Lupus erythematosus |
| Arthritis – rheumatoid, juvenile-onset rheumatoid arthritis |
| Autoimmune thyroid disease – Grave’s disease and Hashimoto’s thyroiditis |
| Gastroparesis and associated gastrointestinal diagnoses: small intestinal bacterial overgrowth (SIBO), irritable bowel syndrome (IBS), median arcuate ligament syndrome (MALS) |
| Migraine headaches |
| Sinus node disease |
| Chronic immune deficiency |
| Chronic regional pain syndromes |
| Small fiber neuropathy |
| Raynaud’s syndrome |
| Depression/anxiety |
| Urologic concerns: pelvic floor dysfunction, interstitial cystitis, endometriosis, polycystic ovary syndrome (PCOS) |
| Chiari malformation |
POTS diagnostic criteria were subsequently further modified in a Heart Rhythm Society (HRS) Expert Consensus Statement.21–22 Criteria included a heart rate increase ≥30 bpm (≥40 bpm those aged 12–19) when standing for several minutes. The HRS statement did not require tilt table testing for diagnosis but recommended use of findings derived by “moving from recumbent to standing” positions, even though tilt-testing and standing are not necessarily interchangeable.23 The criteria included symptoms due to orthostatic intolerance and symptoms unrelated to orthostatic intolerance.
Additionally, and importantly, all POTS criteria contain the condition that there is no significant orthostatic drop of systolic blood pressure (BP) (>20 mm Hg) but this criterion has led to consternation due to the possibility of “overlap syndromes” (e.g., POTS and orthostatic hypotension). In fact, criteria originally proposed by Schondorf and Low2 specifically distinguished POTS from orthostatic hypotension and vasovagal syncope with excessive tachycardia. In POTS, the time during which the BP remains stable is not defined and the timing of BP measurements was not expressly articulated. Furthermore, effects of factors such as food and hydration, reproducibility and chronicity of the “diagnostic” criteria, among others were not incorporated or addressed.
On the other hand, there has been a movement to reconsider POTS diagnostic standards. The result has further expanded the heterogeneity of conditions labeled “POTS”, in some cases, to not even include a criterion for positional heart rate change but, if that is accepted, then hypochondriasis, hypervigilance and anxiety may be mischaracterized as POTS. Some consider that POTS should not be defined by hemodynamic, heart rate or any physiological criteria at all. Then, the “syndrome” is simply defined by non-specific, non-reproducible and often vague complaints. In the end, discrepancies in diagnostic criteria in the literature and between clinicians and autonomic “experts” have resulted in a substantial increase in the number of individuals labeled as having “POTS” while diminishing the meaning or utility of such a diagnosis. In some instances, patients have defined their condition even before they see a physician by identifying symptoms reported on the internet. In others, clinicians may identify POTS to provide the frustrated patient with a “diagnosis”, albeit, an unsupported one. By so doing, serious underlying conditions may be missed.
Symptoms are the key that lead clinicians to propose a POTS diagnosis but these symptoms are non-specific. Commonly reported symptoms include orthostatic intolerance with lightheadedness, palpitations, tremor, weakness, blurred vision and exercise intolerance but also non-postural symptoms including bloating, nausea, diarrhea, and abdominal pain can also occur as well as systemic symptoms such as fatigue, sleep issues, migraines and “brain fog” (Table 2). However, most reported symptoms are nonspecific; a thorough search for underlying responsible causes should be the highest priority before assuming they indicate POTS.
Table 2.
Clinical presentation of POTS.
| Cardiovascular symptoms (pathognomonic) | |
|---|---|
| Cardiovascular system | Main: Orthostatic intolerance, orthostatic tachycardia, palpitations, dizziness, lightheadedness, (pre-) syncope, exercise intolerance. |
| Other frequent symptoms: dyspnea, chest pain/discomfort, acrocyanosis, Raynaud’s phenomenon, venous pooling, limb edema. | |
| Non-cardiovascular symptoms (accompanying) | |
| General symptoms | General deconditioning, chronic fatigue, exhaustion, heat intolerance, fever, debility, bedriddenness. |
| Nervous system | Headache/migraine, mental clouding (“brain fog”), cognitive impairment, concentration problems, anxiety, tremulousness, light and sound sensitivity, blurred/tunnel vision, neuropathic pain (regional), sleeping disorders, involuntary movements |
| Musculoskeletal system | Muscle fatigue, weakness, muscle pain |
| Gastrointestinal system | Nausea, dysmotility, gastroparesis, constipation, diarrhea, abdominal pain, weight loss. |
| Respiratory system | Hyperventilation, bronchial asthma, shortness of breath. |
| Urogenital system | Bladder dysfunction, nocturia, polyuria. |
| Skin | Petechiae, rashes, erythema, telangiectasias, abnormal sudomotor regulation, diaphoresis, pallor, flushing. |
From: Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Int Med 2019; 285: 352–66.32
Currently, a typical “POTS” population includes a large preponderance of females often having a range of symptoms without necessarily demonstrating marked postural hemodynamic intolerance or clearly defined autonomic abnormalities. This can be seen on multiple social media platforms often related to “dysautonomia” or “autonomic dysfunction”. As diagnostic criteria have become more vague, “POTS” has been increasingly applied to individuals with non-specific symptoms without demonstrable well-defined hemodynamic and heart rate criteria during upright posture.11
POTS diagnostic criteria: heart rate
In the landmark 1993 report by Schondorf and Low,2 heart rate increased by 20.1 ± 8.9 bpm in men and 14.8 ± 8.1 bpm in women between the second and third minute of tilt in the control population. The respective changes in systolic BPs were −2.4 ± 10.1 mm Hg in men and −6.1 ± 10.4 mm Hg in women. The final POTS designation in symptomatic patients was based on sustained orthostatic heart rate increase >2 standard deviations above that in the control group or a baseline HR >110 bpm with a further increase during tilt of >20 bpm or to >140 bpm presuming that these findings would otherwise be considered abnormal and unrelated to any other explanatory mechanism.
Whether heart rate increases noted above truly reflect an abnormal finding is uncertain. Unfortunately, broadening of the POTS inclusion criteria substantially undermined the issue of “unrelated to any other explanatory mechanism”. Several large studies report normal variations in heart rate response to upright tilt24–26 and point out that the observed response is not necessarily reproducible. Most recently, in a study of 252 individuals (aged 18–94 years) completing upright tilt-table testing24 the median heart rate increase for those aged 18–29 was 33.7 bpm with a 95% cut-off threshold of 50.9 bpm. Among individuals ages 30–59, the 95% threshold cut-off for the upper limit of heart rate change was 47.7 bpm, a value greater than findings seen in the control subjects noted previously2 and in the range of what some would consider POTS.
An additional aspect that should be a key to the POTS diagnostic criteria, that has not been addressed adequately, is the durability of the tachycardia response with position. A single isolated period of higher than expected heart rate (orthostatic trigger or not) should not be diagnosed as POTS or even deemed abnormal at all. Heart rate responses may vary due to a number of factors, and may occasionally meet current criteria for POTS. Nevertheless, to truly be considered POTS, there must be consistency in the orthostatic vital signs and testing indicating an ongoing susceptibility to excessive postural tachycardia24,25 (Table 3). In the 1993 Schondorf and Low2 report, durability was largely determined by referral delays. Currently, a persistence of symptoms, in association with definitive and reproducible heart rate responses for at least 3–6 months, is a reasonable minimum before considering POTS as the diagnosis.
Table 3.
Proposed criteria for Postural Orthostatic Tachycardia Syndrome (POTS).
| Reproducible orthostatic tachycardia (HR rise ≥ 30 bpm > age 19 and ≥ 40 bpm age ≤ 19) with symptoms of orthostatic intolerance |
|---|
| 1. A clear definition of orthostatic change in position and time in each position |
| 2. Orthostatic tachycardia within 3–10 min of standing and/or on a tilt table test |
| 3. No evidence for orthostatic hypotension at any time with standing |
| 4. A chronic condition present for at least six months |
| 5. No other explainable cause for orthostatic tachycardia or tachycardia |
| 6. Symptoms of orthostatic intolerance that include postural chest pain, exertional dyspnea, dependent acrocyanosis, dizziness, lightheadedness with associated heart rate response abnormalities. |
| 7. Orthostatic symptoms disappear when supine |
| 8. Extra orthostatic symptoms - chronic fatigue, “brain fog” |
| 9. Other autonomic symptoms – bloating, constipation, sweating abnormalities |
| 10. Syncope is not a criterion |
| 11. Symptoms alone do not make the diagnosis |
| 12. “Secondary” orthostatic tachycardia is not POTS |
POTS criteria: clinical features
Recently, the trend has been to include almost any symptom that occurs in a patient with postural tachycardia as being “POTS-related” and thus due to autonomic dysfunction rather than to consider such symptoms as non-specific or related to an undiagnosed comorbidity. Furthermore, postural tachycardia is not necessarily a sign of autonomic dysfunction. A large online survey of POTS patients reports a host of symptoms commonly including lightheadedness, tachycardia, presyncope, headache and difficulty concentrating.27 Although an important addition to the literature, the online survey cannot distinguish between individuals who have postural tachycardia for some other “non-POTS” medical reason, and those who have POTS.28 The same symptoms may occur in patients who have no evidence for either autonomic dysfunction or POTS.11,29 Symptoms alone may be due to a functional problem especially if symptoms are not associated with a verifiable autonomic disturbance.
A further diagnostic dilemma related to symptoms that are not associated with particular postures such as bloating, nausea, diarrhea and abdominal pain, and systemic symptoms including fatigue, sleep issues and migraine headaches, and cognitive issues, including the common, but poorly understood, symptom of “brain fog”.30 These symptoms are often considered “hallmarks” of POTS even if no other demonstrable autonomic dysfunction exists. Such non-specific symptoms may be associated with many clinical disorders or may even occur in the absence of any known medical affliction. The broad inclusion of poorly understood and often ill-defined symptoms have increased the apparent POTS population substantially but with an unfortunate lack of diagnostic focus and therapeutic utility.
Etiology and the concepts of primary and secondary POTS
If “POTS”, as originally described, is to be transformed into a descriptor of a multifaceted clinical picture, then, at a minimum, its association with a postural trigger should be retained. Further, the syndrome might reasonably be characterized as a primary condition (i.e., primary idiopathic POTS) or due to some other cause (i.e., secondary POTS). Considering POTS to be a single heterogeneous entity with a common underlying cause appears unlikely. For many, it is not even possible to demonstrate presence of an apparent autonomic disturbance.31
Regarding primary (idiopathic) POTS, two generally accepted forms exist: “partial dysautonomia” (neuropathic) and hyperadrenergic.32 The partial dysautonomic form appears due to inadequate peripheral and splanchnic vasoconstriction with orthostatic stress. The “hyperadrenergic” form, manifest by excess norepinephrine spillover, may be due to greater norepinephrine production and release at the synapse or reduction in norepinephrine re-uptake. Orthostatic hypertension and migraines are hallmarks of hyperadrenergic POTS. There is often a family history of tachycardia.
Multiple mechanisms have been considered to be causally linked to the development of primary idiopathic POTS including: viral illness (or any condition activating the innate immune response), peripheral denervation with supersensitivity, alpha-receptor hypersensitivity, acetylcholine and beta-receptor autoantibodies, a central hyperadrenergic state, norepinephrine transporter deficiency, decreased baroreceptor gain, idiopathic hypovolemia with associated altered aldosterone, renin and N-NOS and angiotensin II activity, an autoimmune response, mast cell activation and diminished cardiac size and mass, among others.2,8,16,33–46
The numerous proposed etiologies for primary POTS contribute to the current state of confusion, and likely do more harm than good especially since evidence reported is largely anecdotal. Recent reports postulate that specific events trigger POTS, including surgery, multiple sclerosis, vaccinations and concussion, although there is little supporting evidence at this time.47–50 For instance, the suggestion that head trauma51 may be a causative factor is an example of a poorly documented association. Similarly, gastric bypass, with a significant loss of weight and muscle mass might reasonably be considered a possible cause of secondary POTS but a causal relationship is not well established.52 Even syringomyelia and multiple sclerosis have been considered associated with POTS.53,54 Finally, viral illnesses (respiratory and gastrointestinal) have perhaps been most commonly reported in association with POTS. While initial information2 suggested an association, subsequent data from the same group were not confirmatory.9
It has been theorized that POTS is associated with multiple coexisting conditions including autoimmunity, fibromyalgia, functional gastrointestinal disorders, anxiety and hyper-vigilance, joint hypermobility, chronic fatigue syndrome, concussion, and migraine.49 However, no conclusive evidence points to a causal association between POTS and these conditions. Orthostatic intolerance and tachycardia, may mimic POTS but be a manifestation of a far more insidious and potentially treatable condition.
It has been postulated that cardiovascular deconditioning with decreased blood volume, decreased stroke volume and cardiac atrophy leads to sympathetic activation and parasympathetic withdrawal in the upright position.49 While this scenario may have existed (primarily as a transient occurrence in the early days of space flight with zero gravity when exercise was not possible), it was scrupulously excluded from consideration in the initial description of POTS.55 Including deconditioned individuals (other than as part of the differential diagnosis) makes little sense; it is an expected physiologic adaptation to inactivity and resolves with increased activity.56–58 One might even argue that primary POTS can be excluded if symptoms resolve rapidly after a short period of well-documented consistent prescribed exercise. Furthermore, if one were to postulate that deconditioning were the critical element a huge percentage of the population would fit into this category and yet do not have POTS.
Clinical features of POTS: diagnostic challenges and considerations
Marked dilution of the POTS designation has had important clinical consequences that complicated matters for clinicians. The presence of several key chronic clinical features seem crucial to even consider the diagnosis (Table 3).
Orthostatic intolerance
Despite the symptoms that patients with POTS report,9,11,21,27,59 symptoms alone are not diagnostic. In any event, symptoms of orthostatic intolerance should disappear when the patient is recumbent. This important diagnostic feature must be considered especially since so many symptoms are currently ascribed to POTS. Loss of postural intolerance as the crucial unique feature of POTS has undermined the key pathophysiologic feature that leads to a therapeutic strategy.
Chronicity
POTS is a chronic condition. In the initial report,60 latency from symptom onset to diagnosis was 13.6 ± 3 months. An appropriate duration of persistent, reproducible symptoms, while not carefully defined, is probably 3–6 months.49 Symptoms of shorter duration can frequently be reported in any number of transient conditions (e.g. vestibular dysfunction, viral illness, medication use or adjustment). In essence, this would mean that diagnostic criteria should be consistently measurable during the time that a patient has consistent complaints.
Syncope
Occasionally, patients with presumed vasovagal syncope (who often have an initial tachycardia response before culminating in hypotension and bradycardia) are labeled as having POTS. This is particularly vexing as it leads to a misdiagnosis of the problem (i.e., reflex vasovagal syncope). A close association of syncope to POTS should not be expected based on the orthostatic hemodynamic response characteristic of POTS, i.e. absence of blood pressure fall with upright posture due to a substantial reflex tachycardia response. While non-specific lightheadedness is common, and despite a dissenting opinion,61 most reports suggest that syncope (specifically, vasovagal syncope) is no more common in POTS patients than in the general population.
Diagnostic uncertainties
The present diagnosis of POTS is fraught with uncertainties that include: 1) whether testing is undertaken on tilt table or by active standing stand, 2) how to factor expected age-related heart rate changes, 3) how to eliminate confounders such as comorbid diseases, and 4) what to advise with respect to concomitant medications that could cause or suppress tachycardia.
There is an urgent need for robust studies on sensitivity, specificity and reproducibility of the measurements. A major challenge is an acceptable definition of what constitutes the “POTS” gold standard. It is also critical to define the potential participation of ancillary conditions since their recognition may better focus treatment strategy.
Challenges of a POTS diagnosis
Given the many vagaries, it is currently unclear who actually has POTS. The range of heart rates in otherwise healthy humans is wide and nearly 20% of healthy individuals of younger ages have heart rates that meet criteria for POTS.24,25 Thus, a significant proportion of asymptomatic individuals may fall into the “POTS” range of orthostatic heart rate change.
Specifics of the time course of heart rate changes in the upright position are not standardized. The first 30 s of an 80° upright posture have been excluded by some and, while avoiding the instability of initial orthostatic hypotension, the nature of this early evolution may be very important in the subsequent alteration of orthostatic vital signs. The basis for exclusion of the first 30 s was the expectation that an initial physiologic tachycardia associated with transition to upright posture occurs during the first 30–45 s and is finalized first after 3 min of continued orthostatic challenge.62
Although the tilt-table test was used in the initial description of POTS, the possibility of any method for testing transition of BP and heart rate from supine to the upright posture may be acceptable.63 However, tilt-table testing and active standing may not provide the same results.23 Further, even in health, the tilt-table test response may not be reproducible. In one report of tilt-table testing in 40 individuals, in which each individual had 10 upright tilt measurements, the heart rate response was not reproducible.64
Therefore, there is no reason to assume that a solitary or any tilt-test is valid64 or represents the “gold standard” to secure a diagnosis – or not. The repeatability of an active standing test may be even worse. Factors that may critically influence the outcome include how long the patient was supine before either the tilt or active standing test, and the patient’s volume status at the time. Using impedance methods it can take hours for an older adult to reach a steady state supine, or about 30 min in youngsters.65 Evaluation by active stand or tilt-test or both needs careful evaluation as it has for elderly patients.66
The problem with the tilt-test as a diagnostic tool in POTS also raises questions about the validity of any one measurement of heart rate response to standing to secure a POTS diagnosis. Therefore, it is important to evaluate at least several orthostatic heart rate measurements over time and at various times of the day; it may even be necessary to repeat testing on the same day (with comparison to normal controls). If heart rate changes with upright posture are consistent, then it can be concluded that they are independent of any specific external influence (e.g., food or water intervention). This proposed strategy is similar to diagnosing hypertension, where an isolated reading is not grounds for therapeutic intervention. Repeated measurements by a reliable pulse and blood pressure measuring device at home may be helpful. Problems related to time of day and hydration are potentially critical since water ingestion can simply eliminate the orthostatic tachycardia response purported to be due to POTS.67,68
The HRS consensus statement definitions of POTS excludes patients with orthostatic hypotension.21 However, little work has been done regarding the first few seconds and minutes of standing regarding transient drop in blood pressure with prompt recovery during, or preceding, tachycardia. Little is known about beat-to-beat heart rate and blood pressure changes during movement to upright posture in normal individuals and in those deemed to have POTS.
The issue of reproducibility of hemodynamic responses has not been evaluated carefully. While it seems unlikely that POTS patients will have reproducible heart rate elevations with every position change, nevertheless, if POTS is a chronic condition and symptoms are deemed to be associated with the physiological changes, then reproducibility of the heart rate change should be expected most of the time (i.e. similar to the measurement of hypertension, which may be variable, but should be reproducible enough to ascertain a diagnosis). In general, variations in response will depend on fluid loading and changes based on food and diet, but this should also correlate with an associated improvement in orthostatic symptoms. Fluid intake, salt intake, and glucose loads will have an effect on physiological heart rate and blood pressure response to orthostatic stress.69–71
Treatment dilemmas
There is little evidence to indicate that currently available pharmacologic intervention is effective for reversing or shortening the course of primary idiopathic POTS or even improving outcomes aside from a placebo effect. The initial step is to remove offending medications and consider the possibility that an underlying undiagnosed medical problem (including, for instance, inflammatory disease and salt and water depletion) is the cause of the “POTS-like” picture.
Unfortunately, many patients are exposed to various medications and often without symptomatic benefit and without altering the trajectory of the condition. These commonly include: fludrocortisone, midodrine, modafinil, vasopressin, serotonin reuptake inhibitors, yo-himbine, methylphenidate, alpha methyl-dopa, clonidine, phenobarbital, octreotide, and beta-blockers. The use of such drugs has been reported72–83 but prospective controlled studies supporting long-term benefit are lacking. Perhaps the therapeutic intervention with the most supportive data is low-dose propranolol84 since this has been relatively well-tested versus placebo although it did not perform as well as cardiovascular exercise.58 Without having an adequate control population and without properly defining the patients with the condition, the literature does not provide a clear picture of therapeutic responses to medical therapy.
Recently, use of drugs, such as, ivabradine and droxidopa have been reported but studies supporting long-term benefit are lacking.85,86 Some data suggest benefit of ivabradine in patients with vasovagal syncope with features mimicking POTS.87 In one single center experience of ivabradine, postural orthostatic tachycardia syndrome was not even defined so it is not clear what was being treated and for which patients.88
Given the unproven efficacy and the potential adverse effects of drugs currently used in POTS patients, it is prudent for clinicians to always start with more conservative strategies. Endurance and cardiovascular training (beginning in the semi-recumbant position, such as, with rowing),89 increased water intake and other relatively harmless strategies are easy to implement and may help. Involvement in a rigorous cardiovascular exercise program has shown clear, and sustained, benefits in patients with POTS.56,58
Trends in management of POTS
A concerning trend in the management of POTS is that patients have been given (or have given themselves) a diagnosis of POTS based on vague clinical symptoms alone following a self-triggered cursory internet search.27 Not uncommonly, clinicians meet patients for the first time already carrying a self-diagnosis of POTS or that have been given that diagnosis elsewhere based on insubstantial clinical evidence; the patient now has a personal agenda for therapeutic interventions. Medical clinics making a POTS diagnosis should take the responsibility for subsequent care, as only then can the diagnosis be substantiated or other bases for the POTS-like symptoms be properly identified. Further, there is a critical need for medical centers and professional societies to determine if the excessively inclusive diagnostic criteria for POTS has created a problem due to unduly ‘high’ diagnostic sensitivity but very low diagnostic specificity.
Conclusion
Identification and management of patients with POTS is a growing problem. There is a critical unmet medical need to define POTS clearly and search for an etiology of the syndrome. Symptoms ascribed to POTS are nonspecific and may not even be triggered primarily by postural change. Clinical symptoms alone cannot assure an appropriate diagnosis. These symptoms may be due to other treatable medical problems. Such symptoms when caused by an identifiable underlying medical condition(s) should be assigned to that condition(s) and not considered to be POTS (see: Fig. 1).
Fig. 1.
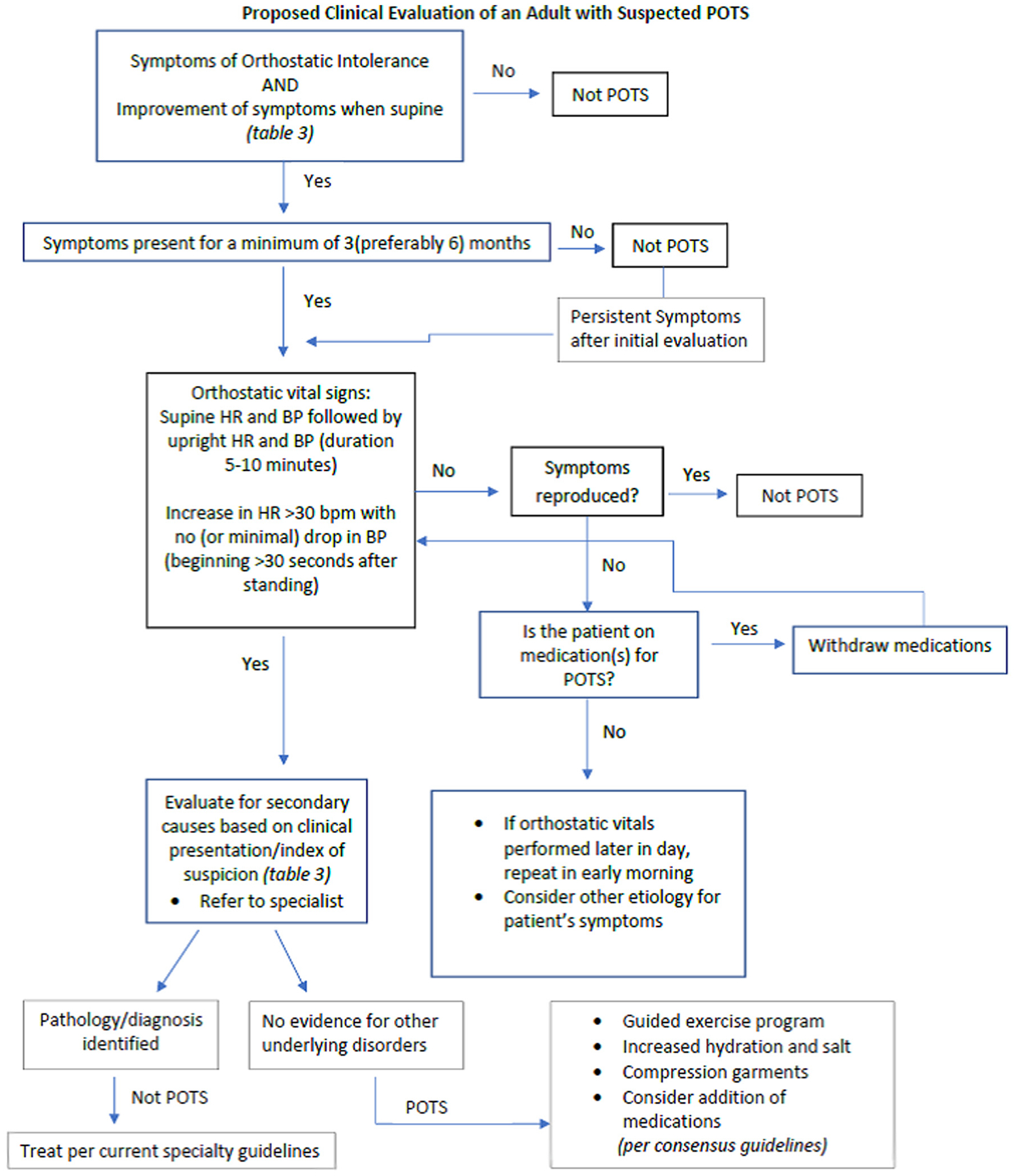
Proposed diagnostic flowchart in suspected POTS.
POTS is a syndrome comprising a chronic, reproducible, orthostatic heart rate increase with associated symptoms and relief by recumbent posture and accompanied by evidence of autonomic dysfunction. We encourage careful classification of disease entities and conditions responsible for a POTS-like picture since, without better understanding the problems and consequences, improvement in patient care is unlikely (Fig. 1). We do not support classification of diffuse and nonspecific symptoms as “POTS” if there is no uniform, reproducible and measurable physiologic, autonomic or hemodynamic identifying characteristics. A well-defined POTS population is an irrevocable prerequisite for further studies on a syndrome that may affect many people world-wide (Table 4).
Table 4.
Next steps.
| Define reproducible |
| Define length of symptoms with associated evidence for orthostatic tachycardia |
| Association of symptoms with tachycardia |
| Define normalcy–on tilt and standing at various ages and by sex |
| Define autonomic dysfunction |
Disclosures
Brian Olshansky MD - Lundbeck, Amarin, Sanofi-Aventis, Respicardia; David S. Cannom MD - Medtronic, Boston Scientific and Lundbeck; Artur Fedorowski MD, PhD - Medtronic and Biotronik; Christopher Gibbons MD, MMSc - Theravance, Lundbeck. Research Support: Grifols. Cutaneous NeuroDiagnostics; Richard Sutton MB, BS, DSc – none; Win-Kuang Shen, MD – none; James A. S. Muldowney III MD – Lundbeck; Tae Hwan Chung MD – none; Suzanne Feigofsky MD – Pacemate; Hemal M. Nayak, MD – none; Hugh Calkins MD – none; David Benditt MD - Medtronic Inc., Abbott Labs; Consultant: Zoll Corp.
Abbreviations:
- BP
blood pressure
- EDS
Ehlers Danlos syndrome
- HR
heart rate
- HRS
Heart Rhythm Society
- IBS
irritable bowel syndrome
- MALS
median arcuate ligament syndrome
- MCAS
mast cell activation syndrome
- SIBO
small intestinal bacterial overgrowth
- PCOS
polycystic ovary syndrome
- POTS
postural orthostatic tachycardia syndrome
References
- 1.Rosen SG, Cryer PE. Postural tachycardia syndrome. Reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading. Am J Med 1982;72:847–850. [DOI] [PubMed] [Google Scholar]
- 2.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 1993;43:132–137. [DOI] [PubMed] [Google Scholar]
- 3.Wood P. Da Costa’s syndrome (or effort syndrome). Lecture I. Br Med J 1941;1:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wooley CF. Where are the diseases of yesteryear? DaCosta’s syndrome, soldiers heart, the effort syndrome, neurocirculatory asthenia–and the mitral valve prolapse syndrome. Circulation 1976;53:749–751. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AA, Davies AO, Mares A, et al. Spectrum of dysautonomia in mitral valvular prolapse. Am J Med 1989;86:267–274. [DOI] [PubMed] [Google Scholar]
- 6.Lewis T. The soldier’s heart and the effort syndrome. JAMA 1919;73:441. [Google Scholar]
- 7.Weissman NJ, Shear MK, Kramer-Fox R, Devereux RB. Contrasting patterns of autonomic dysfunction in patients with mitral valve prolapse and panic attacks. Am J Med 1987;82:880–888. [DOI] [PubMed] [Google Scholar]
- 8.Noval V, Novak P, Opfer-Gehrking TL, Low PA. Postural tachycardia syndrome: time frequency mapping. Autonom Nerv Syst 1996;61:313–320. [DOI] [PubMed] [Google Scholar]
- 9.Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol 2009;20:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boris JR. Postural orthostatic tachycardia syndrome in children and adolescents. Auton Neurosci 2018;215:97–101. [DOI] [PubMed] [Google Scholar]
- 11.Boris JR, Huang J, Bernadzikowski T. Orthostatic heart rate does not predict symptomatic burden in pediatric patients with chronic orthostatic intolerance. Clin Auton Res 2020;30:19–28. [DOI] [PubMed] [Google Scholar]
- 12.Chelimsky G, Chelimsky T. The gastrointestinal symptoms present in patients with postural tachycardia syndrome: a review of the literature and overview of treatment. Auton Neurosci 2018;215:70–77. [DOI] [PubMed] [Google Scholar]
- 13.Bonyhay I, Freeman R. Sympathetic neural activity, sex dimorphism, and postural tachycardia syndrome. AnnNeurol 2007;61:332–339. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons CH, Bonyhay I, Benson A, Wang N, Freeman R. Structural and functional small fiber abnormalities in the neuropathic postural tachycardia syndrome. PLoS One 2013;8, e84716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. NEnglJMed 2000;343:1008–1014. [DOI] [PubMed] [Google Scholar]
- 16.Arnold AC, Ng J, Raj SR. Postural tachycardia syndrome - diagnosis, physiology, and prognosis. Auton Neurosci 2018;15:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vernino S, Stiles LE. Autoimmunity in postural orthostatic tachycardia syndrome: current understanding. Auton Neurosci 2018;215:78–82. [DOI] [PubMed] [Google Scholar]
- 18.Skufca J, Ollgren J, Ruokokoski E, Lyytikainen O, Nohynek H. Incidence rates of Guillain Barre (GBS), chronic fatigue/systemic exertion intolerance disease (CFS/SEID) and postural orthostatic tachycardia syndrome (POTS) prior to introduction of human papilloma virus (HPV) vaccination among adolescent girls in Finland, 2002–2012. Papillomavirus Res 2017;3:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arana J, Mba-Jonas A, Jankosky C, et al. Reports of postural orthostatic tachycardia syndrome after human papillomavirus vaccination in the vaccine adverse event reporting system. J Adolesc Health 2017;61:577–582. [DOI] [PubMed] [Google Scholar]
- 20.Blitshteyn S. Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS). Lupus 2015;24:1364–1369. [DOI] [PubMed] [Google Scholar]
- 21.Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc 2007;82:308–313. [DOI] [PubMed] [Google Scholar]
- 23.Plash WB, Diedrich A, Biaggioni I, et al. Diagnosing postural tachycardia syndrome: comparison of tilt testing compared with standing haemodynamics. Clin Sci (Lond) 2013;124:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker J, Kimpinski K. Normal versus abnormal: what normative data tells us about the utility of heart rate in postural tachycardia. Autonomic Neuroscience: Basic & Clinical 2019;221:102578. [DOI] [PubMed] [Google Scholar]
- 25.Ives CT, Kimpinski K. Higher postural heart rate increments on head-up tilt correlate with younger age but not orthostatic symptoms. J Appl Physiol (1985) 2013;115: 525–528. [DOI] [PubMed] [Google Scholar]
- 26.Petersen ME, Williams TR, Gordon C, Chamberlain-Webber R, Sutton R. The normal response to prolonged passive head up tilt testing. Heart 2000;84:509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw BH, Stiles LE, Bourne K, et al. The face of postural tachycardia syndrome - insights from a large cross-sectional online community-based survey. J Intern Med 2019;286:438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohn A, Chang C. The relationship between hypermobile Ehlers-Danlos syndrome (hEDS), postural orthostatic tachycardia syndrome (POTS), and mast cell activation syndrome (MCAS). Clin Rev Allergy Immunol 2019. in press. [DOI] [PubMed] [Google Scholar]
- 29.Chelimsky G, Kovacic K, Nugent M, Mueller A, Simpson P, Chelimsky TC. Comorbid conditions do not differ in children and young adults with functional disorders with or without postural tachycardia syndrome. J Pediatr 2015;167:120–124. [DOI] [PubMed] [Google Scholar]
- 30.Kharraziha I, Holm H, Bachus E, et al. Monitoring of cerebral oximetry in patients with postural orthostatic tachycardia syndrome. Europace 2019;21:1575–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsaik AK, Singer W, Allison TG, et al. Orthostatic intolerance without postural tachycardia: how much dysautonomia? Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society 2013;23:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med 2019;285:352–366. [DOI] [PubMed] [Google Scholar]
- 33.Fedorowski A, Li H, Yu X, et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace 2017;19:1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Zhang Q, Liao Y, Zhang C, Hao H, Du J. The value of acetylcholine receptor antibody in children with postural tachycardia syndrome. Pediatr Cardiol 2015;36:165–170. [DOI] [PubMed] [Google Scholar]
- 35.Gunning WT 3rd, Kvale H, Kramer PM, Karabin BL, Grubb BP. Postural orthostatic tachycardia syndrome is associated with elevated G-protein coupled receptor auto-antibodies. J Am Heart Assoc 2019;8, e013602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc 2014;3, e000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brady PA, Low PA, Shen WK. Inappropriate sinus tachycardia, postural orthostatic tachycardia syndrome, and overlapping syndromes. Pacing Clin Electrophysiol 2005;28:1112–1121. [DOI] [PubMed] [Google Scholar]
- 38.Garland EM, Winker R, Williams SM, et al. Endothelial NO synthase polymorphisms and postural tachycardia syndrome. Hypertension 2005;46:1103–1110. [DOI] [PubMed] [Google Scholar]
- 39.Shibao C, Arzubiaga C, Roberts LJ, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension 2005;45:385–390. [DOI] [PubMed] [Google Scholar]
- 40.Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 2005;111:1574–1582. [DOI] [PubMed] [Google Scholar]
- 41.Bonyhay I, Freeman R. Sympathetic nerve activity in response to hypotensive stress in the postural tachycardia syndrome. Circulation 2004;110:3193–3198. [DOI] [PubMed] [Google Scholar]
- 42.Stewart JM, Munoz J, Weldon A. Clinical and physiological effects of an acute alpha-1 adrenergic agonist and a beta-1 adrenergic antagonist in chronic orthostatic intolerance. Circulation 2002;106:2946–2954. [DOI] [PubMed] [Google Scholar]
- 43.Schondorf R, Benoit J, Stein R. Cerebral autoregulation in orthostatic intolerance. AnnNYAcadSci 2001;940:514–526. [DOI] [PubMed] [Google Scholar]
- 44.Stewart JM. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. PediatrRes 2000;48:218–226. [DOI] [PubMed] [Google Scholar]
- 45.Sandroni P, Novak V, Opfer-Gehrking TL, Huck CA, Low PA. Mechanisms of blood pressure alterations in response to the Valsalva maneuver in postural tachycardia syndrome. ClinAutonRes 2000;10:1–5. [DOI] [PubMed] [Google Scholar]
- 46.Spahic JM, Ricci F, Aung N, et al. Proconvertase furin is downregulated in postural orthostatic tachycardia syndrome. Front Neurosci 2019;13:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tripathi R, Bernitsas E. From postural orthostatic tachycardia syndrome to radiologically isolated syndrome. Case Rep Neurol Med 2018;2018:2956387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miranda NA, Boris JR, Kouvel KM, Stiles L. Activity and exercise intolerance after concussion: identification and management of postural orthostatic tachycardia syndrome. J Neurol Phys Ther 2018;42:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bryarly M, Phillips LT, Fu Q, Vernino S, Levine BD. Postural orthostatic tachycardia syndrome: JACC focus seminar. J Am Coll Cardiol 2019;73:1207–1228. [DOI] [PubMed] [Google Scholar]
- 50.Thomsen RW, Ozturk B, Pedersen L, et al. Hospital records of pain, fatigue, or circulatory symptoms in girls exposed to human papillomavirus vaccination: cohort, self-controlled case series, and population time trend studies. Am J Epidemiol 2020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanjwal K, Karabin B, Kanjwal Y, Grubb BP. Autonomic dysfunction presenting as postural tachycardia syndrome following traumatic brain injury. Cardiol J 2010;17: 482–487. [PubMed] [Google Scholar]
- 52.Ponnusamy V, Owens AP, Purkayastha S, Iodice V, Mathias CJ. Orthostatic intolerance and autonomic dysfunction following bariatric surgery: a retrospective study and review of the literature. Auton Neurosci 2016;198:1–7. [DOI] [PubMed] [Google Scholar]
- 53.Nogues M, Delorme R, Saadia D, Heidel K, Benarroch E. Postural tachycardia syndrome in syringomyelia: response to fludrocortisone and beta-blockers. Clin Auton Res 2001;11:265–267. [DOI] [PubMed] [Google Scholar]
- 54.Habek M, Krbot Skoric M, Crnosija L, Gabelic T, Barun B, Adamec I. Postural orthostatic tachycardia predicts early conversion to multiple sclerosis after clinically isolated syndrome. Eur Neurol 2017;77:253–257. [DOI] [PubMed] [Google Scholar]
- 55.Robertson D, Shannon JR, Biaggioni I, et al. Orthostatic intolerance and the postural tachycardia syndrome: genetic and environment pathophysiologies. Neurolab Autonomic Team. Pflugers Arch 2000;441:R48–R51. [DOI] [PubMed] [Google Scholar]
- 56.George SA, Bivens TB, Howden EJ, et al. The international POTS registry: evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm: The Official Journal of the Heart Rhythm Society 2016;13:943–950. [DOI] [PubMed] [Google Scholar]
- 57.Galbreath MM, Shibata S, VanGundy TB, Okazaki K, Fu Q, Levine BD. Effects of exercise training on arterial-cardiac baroreflex function in POTS. Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society 2011;21:73–80. [DOI] [PubMed] [Google Scholar]
- 58.Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension 2011;58:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011;21:69–72. [DOI] [PubMed] [Google Scholar]
- 60.Schondorf R, Benoit J, Stein R. Cerebral autoregulation is preserved in postural tachycardia syndrome. J Appl Physiol (1985) 2005;99:828–835. [DOI] [PubMed] [Google Scholar]
- 61.Kanjwal K, Sheikh M, Karabin B, Kanjwal Y, Grubb BP. Neurocardiogenic syncope coexisting with postural orthostatic tachycardia syndrome in patients suffering from orthostatic intolerance: a combined form of autonomic dysfunction. Pacing Clin Electrophysiol 2011;34:549–554. [DOI] [PubMed] [Google Scholar]
- 62.Low PA. Testing the autonomic nervous system. Semin Neurol 2003;23:407–421. [DOI] [PubMed] [Google Scholar]
- 63.Finucane C, van Wijnen VK, Fan CW, et al. A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin Auton Res 2019;29:427–441. [DOI] [PubMed] [Google Scholar]
- 64.Kaiser T, Jost WH, Konig J, Schimrigk K. Intra- and interindividual reproducibility of heart rate variations in the tilt-table test. Wien Klin Wochenschr 2000;112:322–328. [PubMed] [Google Scholar]
- 65.Stewart JM, Shaban MA, Fialkoff T, et al. Mechanisms of tilt-induced vasovagal syncope in healthy volunteers and postural tachycardia syndrome patients without past history of syncope. Physiol Rep 2019;7, e14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCrory C, Berkman LF, Nolan H, O’Leary N, Foley M, Kenny RA. Speed of heart rate recovery in response to orthostatic challenge. Circ Res 2016;119:666–675. [DOI] [PubMed] [Google Scholar]
- 67.Mathias CJ, Young TM. Water drinking in the management of orthostatic intolerance due to orthostatic hypotension, vasovagal syncope and the postural tachycardia syndrome. Eur J Neurol 2004;11:613–619. [DOI] [PubMed] [Google Scholar]
- 68.Ziffra J, Olshansky B. Acute water ingestion is a treatment for postural orthostatic tachycardia syndrome. Journal of Innovations in Cardiac Rhythm Management 2019;10:3541–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.el-Sayed H, Hainsworth R. Salt supplement increases plasma volume and orthostatic tolerance in patients with unexplained syncope. see comments. Heart 1996;75:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frey MA, Lathers C, Davis J, Fortney S, Charles JB. Cardiovascular responses to standing: effect of hydration. JournalofClinicalPharmacology 1994;34:387–393. [DOI] [PubMed] [Google Scholar]
- 71.Bungo MW, Charles JB, Johnson PC Jr. Cardiovascular deconditioning during space flight and the use of saline as a countermeasure to orthostatic intolerance. AviatSpaceEnvironMed 1985;56:985–990. [PubMed] [Google Scholar]
- 72.Kpaeyeh J Jr, Mar PL, Raj V, et al. Hemodynamic profiles and tolerability of modafinil in the treatment of postural tachycardia syndrome: a randomized, placebo-controlled trial. J Clin Psychopharmacol 2014;34:738–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordon VM, Opfer-Gehrking TL, Novak V, Low PA. Hemodynamic and symptomatic effects of acute interventions on tilt in patients with postural tachycardia syndrome. Clin Auton Res 2000;10:29–33. [DOI] [PubMed] [Google Scholar]
- 74.Cutsforth-Gregory JK, Sandroni P. Clinical neurophysiology of postural tachycardia syndrome. Handb Clin Neurol 2019;161:429–445. [DOI] [PubMed] [Google Scholar]
- 75.Zhao J, Tang C, Jin H, Du J. Plasma copeptin and therapeutic effectiveness of midodrine hydrochloride on postural tachycardia syndrome in children. J Pediatr 2014;165:290–294 e1. [DOI] [PubMed] [Google Scholar]
- 76.Chen L, Wang L, Sun J, et al. Midodrine hydrochloride is effective in the treatment of children with postural orthostatic tachycardia syndrome. Circ J 2011;75:927–931. [DOI] [PubMed] [Google Scholar]
- 77.Mar PL, Raj V, Black BK, et al. Acute hemodynamic effects of a selective serotonin reuptake inhibitor in postural tachycardia syndrome: a randomized, crossover trial. J Psychopharmacol 2014;28:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arnold AC, Okamoto LE, Diedrich A, et al. Low-dose propranolol and exercise capacity in postural tachycardia syndrome: a randomized study. Neurology 2013;80:1927–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moon J, Kim DY, Lee WJ, et al. Efficacy of propranolol, bisoprolol, and pyridostigmine for postural tachycardia syndrome: a randomized clinical trial. Neurotherapeutics 2018;15:785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coffin ST, Black BK, Biaggioni I, et al. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm 2012;9: 1484–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Use of methylphenidate in the treatment of patients suffering from refractory postural tachycardia syndrome. Am J Ther 2012;19:2–6. [DOI] [PubMed] [Google Scholar]
- 82.Hoeldtke RD, Bryner KD, Hoeldtke ME, Hobbs G. Treatment of postural tachycardia syndrome: a comparison of octreotide and midodrine. Clin Auton Res 2006;16:390–395. [DOI] [PubMed] [Google Scholar]
- 83.Goldstein DS, Holmes C, Frank SM, et al. Cardiac sympathetic dysautonomia in chronic orthostatic intolerance syndromes. Circulation 2002;106:2358–2365. [DOI] [PubMed] [Google Scholar]
- 84.Raj SR, Black BK, Biaggioni I, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation 2009;120:725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ewan V, Norton M, Newton JL. Symptom improvement in postural orthostatic tachycardia syndrome with the sinus node blocker ivabradine. Europace 2007;9:1202. [DOI] [PubMed] [Google Scholar]
- 86.Ruzieh M, Dasa O, Pacenta A, Karabin B, Grubb B. Droxidopa in the treatment of postural orthostatic tachycardia syndrome. Am J Ther 2017;24:e157–e161. [DOI] [PubMed] [Google Scholar]
- 87.Sutton R, Salukhe TV, Franzen-McManus AC, Collins A, Lim PB, Francis DP. Ivabradine in treatment of sinus tachycardia mediated vasovagal syncope. Europace 2014;16: 284–288. [DOI] [PubMed] [Google Scholar]
- 88.McDonald C, Frith J, Newton JL. Single centre experience of ivabradine in postural orthostatic tachycardia syndrome. Europace 2011;13:427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fu Q, Levine BD. Exercise in the postural orthostatic tachycardia syndrome. Auton Neurosci 2015;188:86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]


