Abstract
Although lesion size is widely considered to be the most reliable predictor of outcome after CNS injury, lesions of comparable size can produce vastly different magnitudes of functional impairment and subsequent recovery. This neuroanatomical–functional paradox is likely to contribute to the many failed attempts to independently replicate findings from animal models of neurotrauma. In humans, the analogous clinical–radiological paradox could explain why individuals with similar injuries can respond differently to rehabilitation. We describe the neuroanatomical–functional paradox in the context of traumatic spinal cord injury (SCI) and discuss the underlying mechanisms of the paradox, including the concepts of lesion-affected and recovery-related networks. We also consider the various secondary complications that further limit the accuracy of outcome prediction in SCI and provide suggestions for how to increase the predictive, translational value of preclinical SCI models.
Traumatic CNS injury has devastating and long-lasting consequences and poses a substantial medical need. After injury to the brain or spinal cord, the magnitude of functional recovery can vary considerably between individuals, even if the location and size of the primary lesion seems to be similar. Many factors can contribute to this ‘structure–function’ paradox, which is known as the ‘neuroanatomical–functional paradox’ by neurobiologists and as the ‘clinical–radiological paradox’ by physicians. Indeed, even similarly located lesions can have differing effects on circuit function, and the methods used to analyse function might not be sensitive enough to detect these subtle differences. Functional outcome can also be affected by the type or duration of activity performed after injury as well as by structural remodelling or functional plasticity within spared circuitry. The structure–function paradox can be observed in a range of CNS pathologies, including acute injury, such as stroke and traumatic brain injury, and inflammatory-degenerative diseases such as multiple sclerosis (BOX 1). In this Perspective, we explore the mechanisms of the paradox in the context of the injured spinal cord where, in contrast to the brain, structure–function relationships are relatively simple. We also introduce the concept of the ‘functionally eloquent’ spinal cord in analogy to the ‘eloquent brain’, which consists of regions that, if lesioned, will impair the recovery of function more so than other regions.
Box 1 |. The clinical–radiological paradox beyond spinal cord injury.
The mechanisms underlying recovery after traumatic spinal cord injury and their neuroanatomical requirements have rarely been definitively established113. Although this Review is focused on traumatic spinal cord injury, a non-linear relationship between anatomical lesion severity and functional outcome — a clinical–radiological paradox — also occurs following traumatic brain injury114 and ischaemic and inflammatory injury to the CNS115–118. For example, in multiple sclerosis (MS), a clinical–radiological paradox often exists whereby diffuse brain or spinal cord lesions are detected by MRI but do not correlate with the extent of neurological dysfunction119,120. Evidence indicates that, in patients with MS, on average, only the ninth lesion will result in a summation injury sufficient to induce a clinically detectable impairment. Even healthy individuals can be diagnosed with radiologically isolated syndrome or ‘asymptomatic MS’, in which radiological anomalies consistent with demyelination exist in the brain, but the individuals do not present with neurological impairment (clinically silent)121. A discrepancy between lesion burden and extent of disability also exists in experimental autoimmune encephalomyelitis, an animal model of MS122.
The structure–function paradox
An inability to predict functional outcome on the basis of anatomical or radiological data alone confounds prognosis for individuals with spinal cord injury (SCI). For example, evidence indicates that, despite lesion size remaining relatively stable, up to 25% of patients with motor and sensory complete SCI (category A on the American Spinal Injury Association Impairment Scale (AIS)) convert to incomplete SCI during the first year after injury1,2. Conversely, partial recovery of function can be followed by relapse or further functional decline, the mechanisms of which are also poorly understood.
In animal models of thoracic and cervical SCI, a non-linear relationship often exists between the magnitude of lesion pathology and functional outcome3,4 (FIG. 1). A major challenge of interventional SCI research is to create clinically relevant experimental lesions with predictable functional outcomes5–7. Animals within a single experimental cohort often show identical levels of functional recovery despite having remarkably differently sized lesions3–5 (FIG. 2). For example, the intent of all thoracic dorsal spinal hemisection injuries in rodents, whether they are created using a blade or scissors, is to ablate the entire dorsal spinal cord. However, this lesion paradigm spares varying amounts of ventral and lateral white matter and almost complete recovery of locomotor function invariably occurs8,9. This recovery can be attributed to relatively small amounts of unilateral sparing of axons located in the ventro-lateral funiculus (for example, reticulospinal axons3,10–12) that activate self-contained neuronal networks (for example, central pattern generators) in the lumbar spinal cord9. Spared reticulospinal fibres can also form appositions onto propriospinal projections, forming a reticulospinal detour that bypasses the primary spinal cord lesion13.
Fig. 1 |. Non-linear relationship between anatomical lesion severity and outcome.

After spinal cord injury, the severity of neurological deficit does not homogenously correlate with the size of the lesion (blue line). Thus, functional recovery cannot be predicted by assuming a linear correlation with lesion size (red line). This non-linear correlation results in ranges of lesion size that are ‘sensitive’ or ‘insensitive’ to experimental intervention. Diverging injury models produce different lesions with likewise diverging sensitivity to detect outcome modifying effects. The varying degree of functional relevance of a lesion is referred to as functional eloquence. Axes represent arbitrary scales from 0–100%.
Fig. 2 |. The neuroanatomical–functional paradox of recovery after spinal cord injury.

An example of mismatch between lesion size and functional outcome is illustrated for spinal cord lesions located at thoracic level Th8 in two rats. a | In this animal, the lesion (shown in grey) covered 54% of the cross-sectional area of the spinal cord. b | In this animal, the lesion covered 84% of the cross-sectional area of the spinal cord. A similar degree of locomotor recovery, measured with the Basso, Beattie and Bresnahan (BBB) open-field locomotor scale109 or grid-walking test, was achieved by both animals (a and b), despite the substantial differences in lesion size. Figure adapted with permission from REF.3, Elsevier.
In animal models of SCI, ex vivo histological measures of injury are often considered to correlate with the magnitude of functional impairment. However, even robust histological techniques and high-resolution microscopic analysis can yield misleading information on structure–function correlations. For example, in response to trauma or inflammation, the focal accumulation of amyloid precursor protein in damaged or ‘stressed’ axons can indicate impending axonal degeneration, but these pathological features are often transient or reversible14,15. Ignoring or underestimating the contribution of reversible forms of axonal injury will lead to an overestimation of lesion size and functional loss (FIG. 3).
Fig. 3 |. Reversible axonal injury and transient recovery.
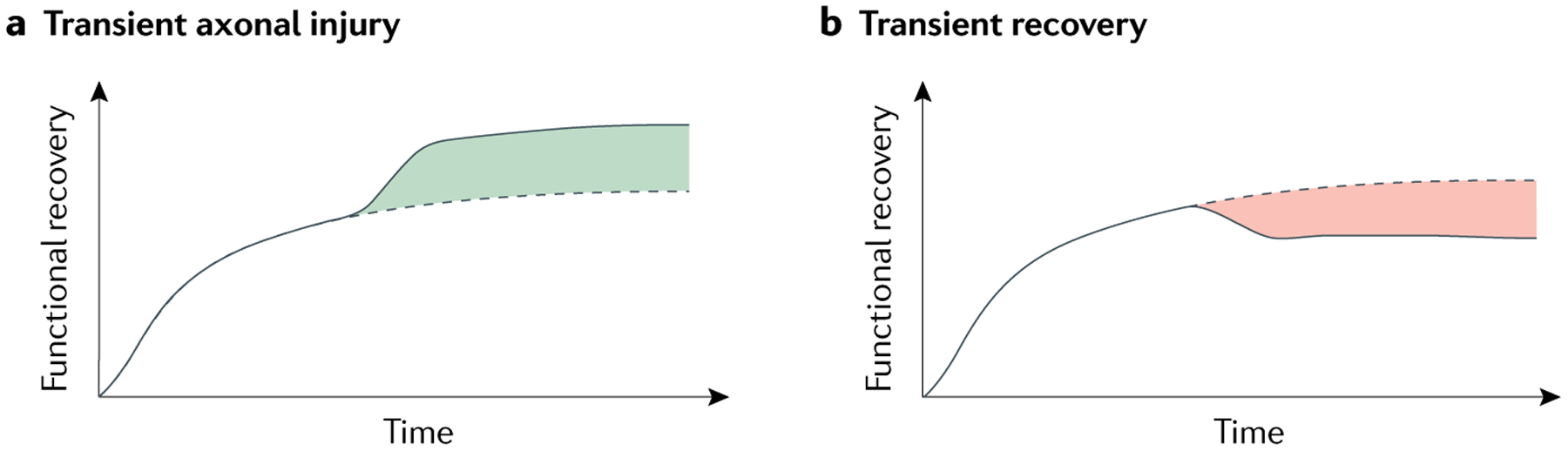
The underlying causes of dissociated longitudinal outcomes after spinal cord injury (SCI) include reversible (transient) axonal injury and transient recovery. Predicted functional recovery based on lesion size is indicated by the dotted line, whereas actual observed recovery is indicated by the solid line. a | The observed functional recovery can be greater than the predicted functional recovery. This mismatch (green) can be accounted for by the presence of reversible (transient) axonal injury that resolves during the early part of recovery14,15. b | Transient recovery can result in a mismatch (red) between the degree of recovery predicted from the initial lesion size and the final degree of recovery observed both after experimental SCI and human SCI48,54,55. This transient recovery can be caused by the delayed emergence of systemic disease modifiers48,54,55.
Similarly, quantitative histological measures of ‘white matter sparing’ in rodents often correlate with the recovery of locomotor function. The degree to which demyelination and remyelination contribute to the recovery of function after SCI is controversial and might vary depending on the specific axonal tract affected, the severity of injury and the type of remyelination (for example, oligodendrocyte-mediated or Schwann cell-mediated)3,10,11,16–18. The analysis of spared white matter also represents an imperfect proxy for predicting neurological recovery because evidence indicates that white matter can be devoid of axons19 — intact myelin sheaths or ‘myelin ghosts’ have been found to persist at or close to sites of injury, even after damaged axons have degenerated19. Large, space-filling lesions, such as those created by experimental and clinical syringomyelia, occupy most of the cross-sectional area of the spinal cord across several spinal segments, but these histologically obvious lesions are not always associated with marked functional impairment20–22. Conversely, small focal lesions in the spinal cord can cause almost a complete loss of function10,11,23. Therefore, as histological measurements of lesion size, axon pathology and/or myelin pathology cannot reliably predict functional outcome, additional criteria are required to refine the meaning of structure–function correlations and to help understand their limitations.
The eloquent spinal cord
Analogous to the ‘eloquent brain’, which consists of critical brain regions that control speech, motor functions and sensation24, the eloquent spinal cord consists of anatomical regions that are critical for the maintenance of normal motor, sensory and autonomic functions. A region of high functional eloquence in the CNS is defined as an area of injured CNS tissue in which a small amount of injury causes pronounced deficits. Therefore, propagated repair in these regions would theoretically produce substantial gains in function. A region of low functional eloquence is defined as an area of CNS tissue in which a small amount of injury remains silent or causes only minor deficits. In these regions, even profound repair would theoretically restore only small amounts of function. Therefore, lesions located in areas of low eloquence are associated with a lower likelihood of detecting the effect of an intervention on functional recovery than lesions in areas of high eloquence (FIG. 1).
One example of elevated eloquence in the spinal cord is the unique neural network that controls complex movements or ‘motor synergies’25. Neurons that comprise this network, referred to as motor synergy encoding (MSE) neurons, are located in the intermediate and ventral laminae of the spinal grey matter in the lumbar enlargement. MSE neurons receive multi-modal input and are pivotal in coordinating the neural input needed for complex multi-joint movements25. Therefore, a lesion that compromises MSE neurons would be expected to have more substantial effects on motor recovery than a lesion of a similar or even larger size that spares MSE neurons. In addition to lesion size, the 3D shape of the lesion is especially important. The functional role of grey matter changes across the rostrocaudal axis of the spinal cord with enlargements containing premotor interneuronal networks and motor neuron populations26. Lesions of similar size can have very different implications depending on whether they are located inside or outside of the enlargements.
Lesion size and shape within and between spinal segments are also an important consideration given the heterogeneous distribution of interneurons and propriospinal neurons within grey matter, their relative susceptibility to SCI and their varied contributions to recovery-associated networks27.
In addition to grey matter regions containing MSE neurons, other eloquent zones of grey matter include the dorsal horn and spinal lamina X, which is the location of medial partition neurons and any crossing fibres arising from spared contralateral axon tracts28,29. The dorsal horn is a key region for sensory–motor integration and is important for successful neurorehabilitation30. Therefore, the location and shape of the lesion is as or more important than lesion size for the understanding of structure–function relationships.
The relative amount of injury or sparing of eloquent spinal tissue is not only relevant for motor function. Indeed, rehabilitative training is less likely to sustain spontaneous recovery when lesions are located in motor areas that overlap with eloquent regions of the spinal cord containing sensory and autonomic neurons. Lesions affecting overlapping functional modalities are likely to cause various systemic pathologies and post-injury comorbidities that will influence outcome (discussed in more detail below). Furthermore, within eloquent regions — regardless of functional modality — the relative magnitude of sparing within ‘lesion-affected’ and ‘recovery-related’ networks will dictate the magnitude of functional impairment and, ultimately, the recovery of function (FIG. 4).
Fig. 4 |. Distinguishing between lesion-affected and recovery-related networks after spinal cord injury.
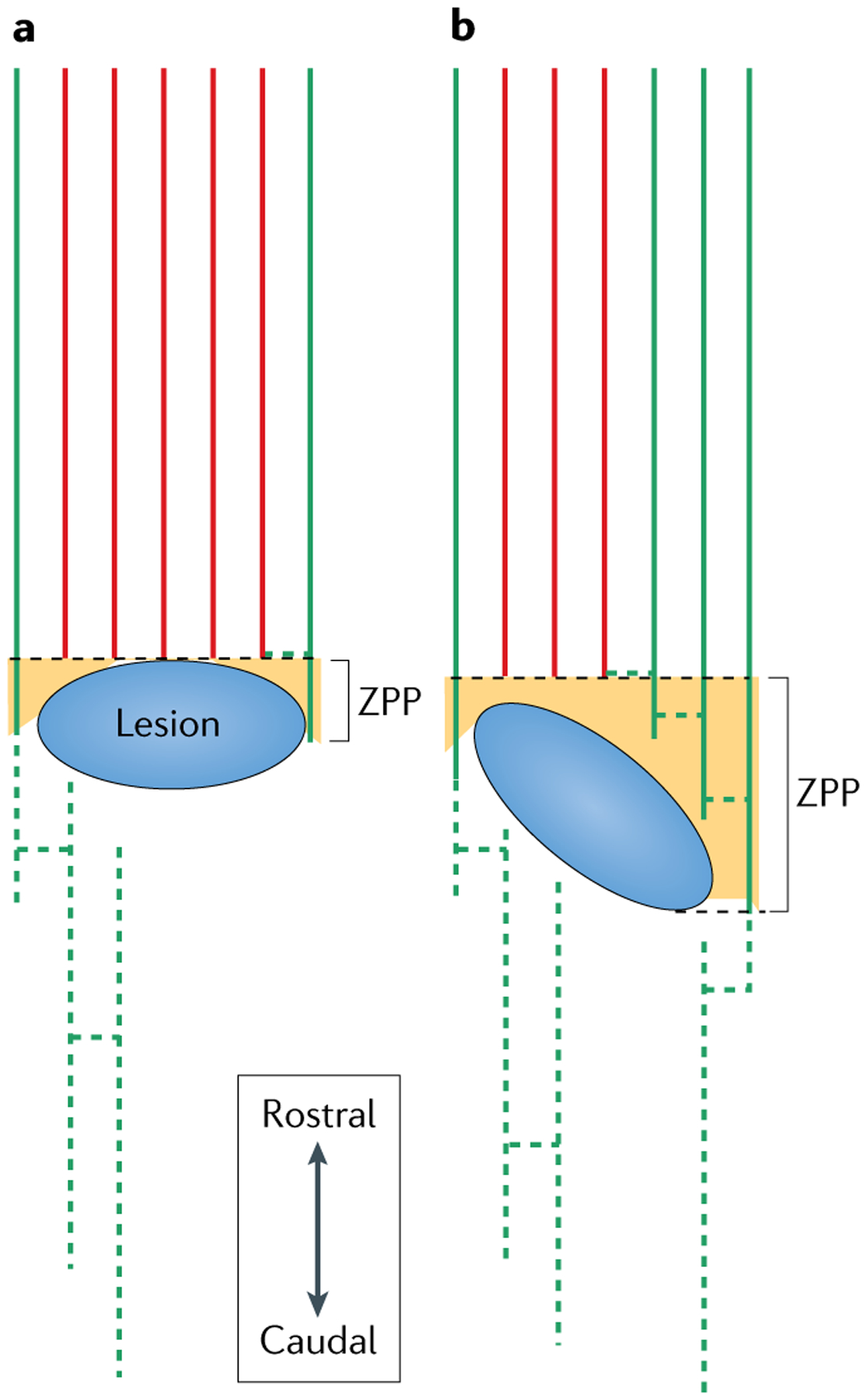
a | A horizontal lesion (blue) in the spinal cord damages central tracts (lesion-affected tracts, red solid lines) and spares some lateral tracts (green solid lines) that extend into the zone of partial preservation (ZPP; orange), and are able to sprout and re-connect with denervated circuitry (green dotted lines). b | A larger lesion but of a different orientation is associated with a better chance of recovery than the lesion illustrated in part a. The same tracts are affected in both scenarios; however, the ZPP in part b spares the segemental innervation of associated muscles and facilitates greater connectivity between the lesion-affected and recovery-related network (green solid and dotted lines) than is possible in the scenario illustrated in part a. Compared with lesions that leave no ZPPs, the presence of a ZPP after SCI is associated with a higher degree of motor score gain and a higher rate of conversion to less severe levels on the American Spinal Injury Association impairment scale31,32. Despite measuring the number of lesion-affected tracts or recovery-related networks as solitary entities, the degree of connectivity and integration between both will determine the potential for recovery.
Zones of partial preservation.
Lesion-affected and recovery-related networks in the spinal cord are distinct but interrelated determinants of recovery (FIG. 4). A lesion-affected network is a network that has undergone direct loss or deafferentation of somatotopic innervation as a result of variable amounts of damage to first-order (axon tracts above and/or below injury) and second-order (grey matter at injury level) motor neurons. The recovery-related circuitry comprises plasticity responses that are generated by neurite outgrowth and changes in synaptic strength, and can extend from spared axonal fibres (compensatory plasticity) or injured axonal fibres (restorative plasticity). The extent of this recovery-related circuitry depends on residual plasticity reservoirs, including corticospinal and rubrospinal reserve capacity, and their excitability.
The relative contribution of lesion-affected and recovery-related networks to recovery can vary even when lesion size is similar. For example, after SCI, individuals with similar lesion sizes and neurological impairment scores will have variable degrees of tissue sparing, either below or above the region of maximal tissue damage (FIG. 5). Sparing in these regions has been associated with substantial improvements in functional recovery31,32. The reason for these improvements is unclear, but a likely explanation relates to the observation that differences in lesion shape create variable zones of partial preservation (ZPPs) (FIG. 4b). These ZPPs represent the anatomical substrates allowing for connectivity between lesion-affected and recovery-related networks.
Fig. 5 |. Spared axonal fibres as outcome denominators independent of lesion size.

Spinal cord lesions of the same size can produce remarkably varied outcomes depending on the specific lesion-affected and recovery-related networks involved, as illustrated here in schematized coronal sections. Rodent spinal cord lesions that damage the corticospinal tract and reticulospinal tract (lesion 2, red) are more severe than lesions of the same size that spare reticulospinal fibres (lesion 1, blue) (left). In patients, sparing of the lateral rim of the cord would result in sacral sparing, as illustrated by lesion 1 (grey) (right). If the dorsolateral rim is spared, this will enable sacral motor sparing (voluntary anal contraction). If the ventrolateral rim of the cord is spared, this will enable sacral sensory sparing (feeling of deep anal pressure and/or sensation in S4/5 dermatomes110). In humans, lesions of the same size can differ substantially in the amount of tissue from functionally relevant networks that is spared; either sensory or motor sparing are associated with better odds for recovery31,110. These differences will not be mirrored in either serum or cerebrospinal fluid biomarker studies that assess volume of injured spinal cord tissue by measuring neuronal (for example, neurofilament) and glial (for example, GFAP) tissue damage, as the anatomical context of the injured tissue with regards to spared circuity (for example, the recovery-associated network) will be not reflected. In humans, the corticospinal tract is essential for fine motor control and has a more important role in locomotor control than in rodents111. To date, rodent models are rarely used to assess changes in myotomes or dermatomes as indicators of sensory or motor level and, therefore, zones of partial preservation are not objectively quantified in rodent spinal cord injury models.
As the corticospinal tract (CST) and other descending motor tracts project bilaterally, uneven or non-symmetrical lesions will produce diverging ZPPs composed of spared axons and neurons localized at a certain spinal cord segment (transverse plane) that can compensate for a unilateral loss of innervation. Indeed, within the CST, the term ‘corticospinal reserve capacity’ is used to describe recovery-related networks that can become engaged to compensate for lost innervation33. Dependent on the degree of injury, this concept allows for different states of compensation, which are defined by variable degrees of preserved circuitry, recruitment of secondary motor areas (for example, cortical M2 region), excitability of the corticospinal system (an indirect measure of axonal integrity), and intracortical and corticospinal inhibition. This compensatory plasticity is also influenced by the time that has passed since injury and the type of rehabilitation that has been performed1,2.
Plasticity responses.
Although the structure–function paradox in acute SCI relates mostly to features of the injury-affected network, in chronic SCI, both the injury-affected and recovery-associated networks can contribute to functional outcomes that deviate from lesion size-based predictions. As mentioned above, plasticity responses following SCI have been categorized as either compensatory or regenerative (restorative)8,28,34.
Compensatory plasticity can be illustrated by considering the CST and rubrospinal tracts (RST) in rodents. These tracts are located in the dorsolateral spinal cord and are the main tracts controlling paw motor function (FIG. 5). The overlap in function between the CST and RST and the ability of one to compensate for the loss of the other after injury is substantial and might explain why rats with small cervical lesions (up to 20% of total cord diameter) that affect only the RST or CST can regain the ability to reach for food pellets within days after injury35,36. By contrast, small lesions that severely damage both the CST and RST cause severe deficits in grasping that are largely refractory to rehabilitative training (FIG. 5). In these animals, any recovery that occurs can be attributed mostly to adaptive strategies such as scooping4,36. These observations could explain why lesions of different sizes can lead to similar degrees of functional recovery (FIG. 1).
Adaptive strategies can improve function in animal models and in individuals with SCI. However, these strategies train residual muscle forces to support movement and are not necessarily associated with improvements in spinal connectivity or axonal conduction22,37. The recovery of practical motor functions including movements that help individuals manage their daily lives (for example, those assessed by the SCI independence measure) can continue even after neurological recovery (assessed by the International Standards for Neurological Classification of SCI (ISNCSCI) motor score) has plateaued22.
In addition to compensatory plasticity, regenerative plasticity of injured axons, which consists of short distances of axonal regrowth and arborization and the formation of new connections, can contribute to recovery28,38. Regenerative plasticity in the CST has been associated with recovery of forelimb function39,40 and of some hindlimb locomotion41. However, robust axon outgrowth or regeneration and synapse formation can also occur without any notable functional improvements42 or even with impairment of spontaneous functional recovery43,44. This observation illustrates that the anatomical–functional paradox applies not only to lesion-induced impairment but also to anatomical indices of recovery. In this regard, when attempting to correlate anatomical measurements of regenerative growth or plasticity with outcome, it is important to ensure that the tract or circuitry being scrutinized can support the function that is being measured. For example, it would not be helpful to correlate anatomical changes in anterogradely labelled CST axons with overground locomotion in a rodent model of thoracic dorsal hemisection injury45. Instead, a more refined behavioural measure like grid or ladder walking would be needed to assess the functional integrity of spared or regenerating CST axons.
Ultimately, determining which axons promote the recovery of function and distinguishing them from those that are functionally silent or have unwanted functions, such as pain and spasticity, represents a substantial gap in our current knowledge that must be filled if anatomical–functional correlates are to be reliable metrics for the evaluation of loss or recovery of function after SCI.
Impact on the replication and translation of preclinical data.
The concept of variable functional eloquence in spinal cord tissue poses obvious challenges for the successful replication and translation of experimental data. Indeed, many attempts to replicate the positive effects of novel therapeutic interventions using slightly different animal models of SCI might have failed because the specific injury models being used in different labs created a distinct primary or secondary pathology within functionally eloquent areas of the spinal cord (FIG. 1). Lesions occupying areas of low functional eloquence result in a low sensitivity to detect a putative treatment effect, creating a ‘blind spot’ for interventional testing (FIG. 1). Thus, evaluating the efficacy of any intervention is challenged by the risk of obtaining either false-positive results, that is, an ineffective intervention seems to work owing to an effect on model-specific pathology, or false-negative results, where the intervention is effective, but the effect cannot be detected because the injury primarily affects regions of low functional eloquence.
As an example, in one preclinical study, a false-negative result was obtained during an attempt to independently replicate the positive results of previous a study of a pharmacological intervention for SCI46,47. After making adjustments to control for differences in injury mechanics and the location of primary trauma between the two studies, the neuroprotective efficacy of the drug was verified in follow-up experiments46,47. In retrospect, the differential effects of the drug in the two injury models could be explained by the presence of a larger ZPP and protection of recovery-associated networks in the original experimental lesion compared to the lesion produced in the attempted replication study (FIG. 4). After adjusting the model to produce a similar ZPP to the original study, the original finding was reproduced46,47.
The use of Egger regression and funnel-blot modelling to re-analyse data from a large number of preclinical studies indicated that the efficacy of specific experimental treatments in animal models of SCI is often overestimated48. This observation explains why reproducing and translating published experimental data is difficult6,49. Recent efforts to encourage the publication of all experimental SCI data, regardless of study outcome, might counteract these problems50,51.
Meta-inflammation
SCI is known to damage axons that control motor and sensory functions. However, SCI also damages axons and neurons that control autonomic (sympathetic) reflexes, creating a permanent state of dysautonomia characterized by slow but consistent pathological changes in various organs throughout the body. Much of the pathology resulting from dysautonomia can be attributed to the impairment of metabolically driven inflammatory states, known as immunometabolism or ‘meta-inflammation’52,53. In animal models of SCI and in humans, meta-inflammation is a result of the loss of control of sympathetic tone transmitted to the organs that direct metabolism (for example, liver, adrenal gland, muscle, adipose tissue and gut) or mediate immune responses (for example, spleen). As immune and metabolic processes are essential and normally tightly coupled52,53, we hypothesize that all or most comorbidities that affect individuals with SCI, for example, spontaneous infections in lung or skin, impaired wound healing, metabolic disease, chronic depression, atherosclerosis, fatigue and anxiety, are caused or propagated by meta-inflammation. In turn, the many downstream consequences of meta-inflammation could serve as modifiers of functional outcome, which — alone or together — cause the neurological outcome to deviate from predictions made from experimental or clinical indices of injury severity (FIGS 3,6).
Fig. 6 |. Outcome variability and underlying causes.
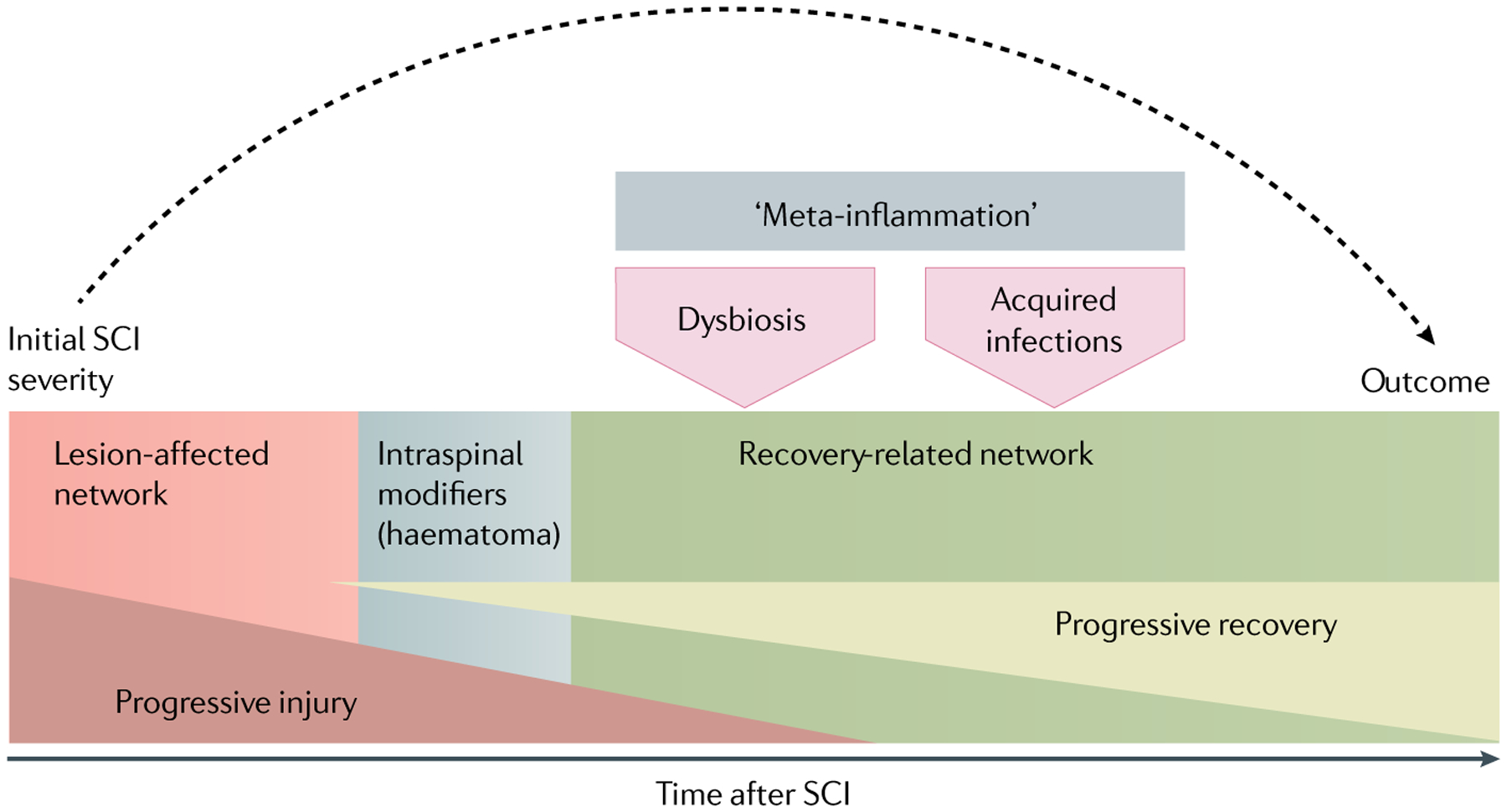
The prediction of functional outcome of spinal cord injury (SCI) on the basis of initial injury severity only (dashed arrow) ignores the variability that is introduced after initial injury and can affect the lesion-affected (red) and recovery-related networks (green). This variability can be caused by transient functional modifiers that do not affect neuroanatomy, for example, variable diaschisis. Intraspinal modifiers (shaded grey box) can impair recovery via an effect on the neuroarchitecture, for example, haematoma formation or delayed haemorrhagic transformation is associated with increased intraspinal pressure, and thereby with progressive injury to the lesion-affected network, as well as with iron toxification that affects the recovery-related network89,112. Post-SCI hyperglycaemia59, hypotensive and hypertensive episodes60,61 also impair the capacity of the injured cord to repair. Additional outcome confounders are acquired infections and fever episodes54–57 that trigger sustained metabolic derangement, that is, a shifted glucose to lactate ratio, at the lesion site57. SCI-associated gut dysbiosis and autoimmunity can also negatively affect functional outcome63–66.
Acquired infections are perhaps the best studied of the post-injury modifiers that can be linked to meta-inflammation (FIG. 6). Post-injury immune suppression and subsequent infection, for example, pneumonia or wound infection, are associated with inferior neurological and functional outcomes in patients for up to 5 years after SCI54,55. The ability of infections to modify neurological outcome for extended time periods post-infection has been independently verified using different clinical databases56. Mechanistically, an altered immunometabolism can explain why infections influence neurological function. Fever, which develops subsequent to infection, is associated with deranged metabolism at the injury site and impaired neurological improvement57. Infection is also a metabolic stressor and can raise blood sugar levels58. In acute SCI, hyperglycaemia is associated with the exacerbation of spinal cord damage and reduced functional improvement as measured by the AIS59.
Evidence indicates that other consequences of dysautonomia, including hypotensive and hypertensive episodes and altered homeostasis of gut microbiota (that is, gut dysbiosis), can further impair function of the injured cord60–64. The development of post-traumatic autoimmunity63 has also been associated with inferior motor recovery after SCI65,66 and with learning and memory deficits after stroke67,68. B lymphocyte/plasma cell-derived autoantibodies can cause cell lysis or block saltatory nerve conduction in seemingly intact myelinated axons69. Exuberant uncontrolled immunity may in part explain the atrophy and microstructural changes that occur in the brain after SCI70 as well as the delayed loss of saltatory nerve conduction that develops in anatomically intact spinal axons after SCI71.
Fatigue, depression and acquired severe pain syndromes are also prevalent after SCI and can reduce activity levels, thus restricting participation in rehabilitation, which is necessary for the maintenance of newly established circuitry72,73. Notably, pain and affective conditions are dynamic, and their effects on recovery can be amplified by acquired infections74.
In addition to affecting recovery, post-injury systemic modifiers can also undermine already established recovery. For example, functional deterioration — here defined by conversion to a lower AIS score — occurs in 17–31% of individuals with SCI75 (FIG. 3). In most individuals, these downward conversions cannot be explained by overt changes in neuroanatomy (for example, radiological evidence of syringomyelia)74,76, indicating the presence of additional mechanisms that deconstruct or impair function in established circuitry54. This phenomenon has previously been referred to as physiological instability of the spinal cord after injury76. The various comorbidities described above are likely to be responsible for neuro-worsening after SCI. However, systemic modifiers might also be able to positively affect neuroplasticity and recovery. For example, in animal models of chronic SCI, the induction of systemic inflammation can increase the benefits of rehabilitative training34,77.
Lesion-remote effects
Dynamic changes in parts of the CNS that are remote from but connected to the injury site could contribute to the observed anatomical–functional paradox70. Competitive, undirected compensatory plasticity78 and sustained oxygen deprivation (hypoperfusion) below the level of the injury can restrict locomotor capacity79 and might contribute to ongoing tissue damage. The observation that remote changes can interfere with functional recovery was first made in 1914 by Constantin von Monakow80,81, who termed the process diaschisis (“separated at a distance”). von Monakow’s observations have since been supported by the results of neuroimaging studies, where it was shown that motor recovery after hemiparetic brain damage is influenced by brain structures in locations remote from the CNS lesion82. Several types of diaschisis have been defined, for example, interference with functional connectivity between the brain and spinal cord, known as diaschisis corticospinalis, or between brain regions (within or across hemispheres), known as diaschisis associativa.
In one study, altered functional connectivity patterns in structurally normal-appearing CNS tissue were associated with behavioural deficits in individuals with ischaemic brain injury83. Similarly, the disturbance of connectivity between primary somatosensory cortex and secondary somatosensory cortex has been demonstrated in a subpopulation of patients after chronic SCI84. The ability to recruit neuronal activity from secondary motor cortex areas constitutes corticospinal reserve capacity33. After SCI, neuroaxonal architecture and function degrades in regions of deprived supraspinal input, enabling the formation of aberrant connectivity78,85.
Refined imaging biomarkers will be essential for the identification of factors that contribute to dynamic changes at the lesion site and in lesion-remote areas and to disentangle the structure–function paradox. Opportunities exist to perform neuroimaging in cohorts of patients with SCI that differ in measurable ways, including lesion site, lesion size and functional outcome. Although these multivariate investigations are complex, particularly when the contributions of different variables interact with one another, they have facilitated steps forward, for example, in our understanding of the effect of preserved tissue bridges on outcome after SCI86–88. Zones of preserved tissue bridges are neuroanatomical substrates for a ZPP.
Using data from validated cohorts of patients with SCI to relate imaging findings to specific recovery profiles is feasible and worthwhile. Besides the identification of imaging categorizations associated with AIS conversion rates89 (BASIC score) after acute cervical SCI, quantitative MRI assessment in chronic SCI has revealed surprisingly dynamic processes also associated with neurological outcome, for example, emerging neurodegeneration70. Multi-centre studies comparing data obtained from different scanners and vendors are ongoing and have the potential to provide further detail on the clinical course of patients with SCI. The use of advanced imaging techniques that enable the acquisition of high resolution images and the minimization of imaging artefacts, such as those caused by instrumentation (for example, implanted devices to stabilize the spine) or movement of the spinal column during imaging, will also improve our understanding of the structure–function relationship in SCI90,91. In conclusion, the combination of advanced imaging or electrophysical approaches to improve our ability to link the presence of spared and/or recovered circuitry with function will be another important step in experimental and human SCI.
Improving the translational potential
The neuroanatomical–functional paradox in animal models and the analogous clinical–radiological paradox in humans confounds accurate outcome predictions after SCI. Below, we outline several strategies or opportunities for improving the predictive or translational value of experimental models of SCI. We recognize that these strategies are unlikely to solve the structure–function paradox; however, they will reduce experimental ‘noise’, which will ultimately help achieve a better understanding of the factors that influence functional recovery after SCI.
Embracing variable data.
More comprehensive analysis and consideration of variable data, including outliers, will not only improve the predictive value of our experimental models of SCI but could also reveal modifiable factors that influence outcome and could therefore serve as novel therapeutic targets. Just as humans exhibit variable responses to drugs, not all rodents respond identically to therapeutics, even when syngeneic strains receive identical injuries92. A better understanding of variable recovery rates and responder characteristics would improve the reproduction and clinical translation of preclinical findings22,48,93,94.
Characterizing lesion-affected and regeneration-associated networks.
To resolve the structure–function paradox, it would be useful to incorporate additional anatomical and functional analyses that would complement the isolated histopathological assessments that are generally performed. Experimental plans could include analyses of myelin sparing at the lesion epicentre in conjunction with the quantification of the number or length of axons that grow beyond the lesion site95,96. For example, 3D reconstructions65 could be routinely used to quantify and visualize the preservation of essential motor neuron pools or premotor networks — that is, eloquent zones of the spinal cord — relative to the lesion epicentre or to traced axons that can serve as landmarks. This approach would enable the observation of the rewiring of spared and injured axons with neurons above, below and adjacent to the injury. Defining these zones of preserved tissue would provide a more complete picture of the lesion and would shed light on the role of spared neuronal circuitry in supporting functional recovery. Ideally, preclinical studies would also incorporate longitudinal electrophysiological and behavioural analyses to more fully capture nuances in functional recovery. This integrative analysis of function could provide more accurate predictions of the functional significance of anatomical measures than the current methods with the focus on lesion size and level.
Improving data analysis.
Incorporating data analysis methods that are already routine in randomized clinical trials into preclinical studies could provide more accurate data on the relationship between lesion characteristics and functional recovery. For example, a ‘per protocol analysis’ would require the results from all animals that were randomized into a treatment group to be reported as opposed to only reporting results from animals still in the study at the end of the follow-up period97. Unbiased recursive partitioning provides a data-driven rationale for the early stratification of cohorts based on simple experimental readouts, which can improve the predictive value of experimental endpoints98. For example, published data indicate that rats receiving calibrated spinal contusion injuries of identical force can be separated into functionally distinct cohorts99. This stratification was performed by analysing and correlating post-injury biomechanics, in particular the original magnitude of spinal cord displacement, with locomotor recovery scores at ~4 weeks post-injury99. In the future, these data could be used to prospectively stratify animals based on the early analysis of injury biomechanics, which could be performed before animals are randomized into experimental groups. This stratification would enable the comparison of like-for-like injuries.
Increasing the internal validity of animal models.
The ability to detect an effect of treatment on functional outcome in an animal model of SCI depends on the specific test being used45. Tests need to be tailored to injury location and severity to avoid floor and ceiling effects. For example, crossing a horizontal ladder offers a sensitive readout only for animals that can perform weight-supported stepping, whereas reaching for food pellets is almost impossible for rodents with severe injuries such as hemisection. Similarly, the ability to assess the tract-specific eloquence of a lesion will largely depend on the appropriateness and sensitivity of the selected functional test. In summary, the use of an appropriate test to detect the outcome of a specific lesion paradigm will enhance internal validity by increasing testing sensitivity, providing reliable readouts and reducing false-positive and false-negative findings.
Increasing the external validity of animal models.
Inherent in all preclinical modelling of SCI in animals is the exquisite control of variables, including sex, genetics, temperature, environment, age, weight and diet. This level of control limits variability and improves the resolution of hypothesis-driven experimentation. However, few or none of these factors can be controlled in clinical research. Incorporating all or even several of the above variables into the design of preclinical experiments would be impractical but specific opportunities for expanding the external validity of preclinical studies might exist. For example, in the clinic, patients with SCI receive antibiotics only after infections are acquired. Conversely, in most animal models of SCI, prophylactic antibiotic protocols are used to prevent systemic infections100. This use of antibiotics in preclinical models could exacerbate the effects of SCI-induced ‘meta-inflammation’ or preclude our ability to study the effects of comorbidities, such as infection, on recovery after SCI. Indeed, antibiotics disrupt the intestinal microbiome and host metabolism63,101,102. Microbiome alterations also affect immune function and several can influence neural function63,103,104.
Accounting for pre-injury and post-injury activity.
In neural development, activity drives the wiring of functionally related axons into a network105. Similarly, activity is important for meaningful plasticity and recovery after injury to the brain or spinal cord34. Task-specific activation of neuronal networks is required for the functional rewiring and integration of newly formed axonal sprouts (and possibly regenerating axons)106. Given the importance of activity in regulating CNS structure and function, baseline (pre-injury) functional activity in experimental animal cohorts should be considered as a variable capable of influencing functional outcome107. Between-group differences in cage activity levels — either overall locomotion or fine motor control — can significantly affect the recovery of motor function after SCI108. Therefore, it would be prudent to control for pre-injury and post-injury activity levels in the design of preclinical studies to ensure that the resulting data are meaningful and reproducible. Rehabilitative training is part of all treatments for patients with SCI in the clinic. Therefore, one recommendation for experimental animal studies is the addition of regular post-injury training, with the intention that animals activate neuronal circuitry (for example, locomotor training following thoracic SCI or reaching for food pellets following cervical SCI5), or the inclusion of at least one behaviour-related outcome measure even if the primary experimental end points of the study are anatomical or molecular readouts.
Conclusions
The anatomical–functional paradox in SCI is a powerful but poorly understood biological concept that affects the interpretation of experimental data, whether from preclinical models or clinical trials. In this Perspective, we discussed several key features and the potential underlying mechanisms of this paradox. We also presented solutions that, if implemented, would help us to better understand the factors that influence functional recovery. Our goal is to increase the recognition of the anatomical–functional paradox in the hope that researchers will be inspired to re-evaluate structure–function relationships after SCI. Clearly, there are logistical, economical and practical limitations that will preclude the development of ‘perfect’ translational models; however, rather than continue to rely exclusively on over-simplified anatomical measures as proxies for both damage and recovery, the contemplation of more comprehensive theories, for example, considering injury in terms of lesion-affected or recovery-associated networks, might lead to the development of new tools or experimental approaches that will reduce the impact of the paradox on study findings. Furthermore, simply being vigilant of and discussing the deficiencies of animal models of SCI and recognizing that structure does not always equal function will help scientists manage expectations of their data, for themselves, their peers and the public. This approach will help avoid the hype that often surrounds preclinical evidence that a given drug, gene, cell type or rehabilitation protocol promotes robust neuroprotection or axonal regeneration in SCI. Until the time when discrete neurobiological mechanisms can be reliably linked to changes in neurological recovery, the most robust and reliable indicator of whether a treatment is effective will be whether the treatment affects functional outcome.
Acknowledgements
The work of K.F. is supported by grants from the Canadian Health Research Council (CIHR), the Wings for Life Spinal Cord Research Foundation, the Craig H. Neilsen Foundation, the Canadian Research Chair Program. The work of P.G.P. is supported by the National Institutes of Neurological Disorders-N IH (Grants R01 NS083942, R01 NS099532 and R35 NS111582) and the Ray W. Poppleton Endowment. The work of J.M.S. is supported by the National Institute of Disability, Independent Living and Rehabilitation Research (NIDILRR Grant 90SI5020), the National Institutes of Neurological Disorders - NIH (Grant R01 NS118200), the European Union (EU Era Net - Neuron Program, SILENCE Grant 01EW170A), the Craig H. Neilsen Foundation (Grant 596764), the Wings for Life Spinal Cord Research Foundation and the William E. Hunt and Charlotte M. Curtis Endowment. J.M.S. is a Discovery Theme Initiative Scholar (Chronic Brain Injury) of Ohio State University.
Glossary
- Corticospinal tract
(CST). Comprises axons of the descending pyramidal tract, which controls motor function.
- External validity
The extent to which one can generalize the findings of an experimental study to reflect the situation in humans.
- Propriospinal projections
Axons that relay information on deep sensitivity and joint position. Constitute an essential pathway for the recovery of neurological function after spinal cord injury.
- Reticulospinal axons
Descending axons that relay information for extrapyramidal motor control.
- Syngeneic
Genetically similar or identical and immunologically compatible.
- Syringomyelia
Generic term referring to a fluid-formed cavity in the spinal cord that develops at the site of a lesion and can be present for a long time after the initial injury.
- Unbiased recursive partitioning
A regression analysis method that enables the binary analysis of non-parametric data.
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Neurology thanks E. Bradbury, P. Freund, P. Reier and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.Marino RJ, Ditunno JF Jr., Donovan WH & Maynard F Jr. Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch. Phys. Med. Rehabil 80, 1391–1396 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Fawcett JW et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45, 190–205 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Schucht P, Raineteau O, Schwab ME & Fouad K Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol 176, 143–153 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Hurd C, Weishaupt N & Fouad K Anatomical correlates of recovery in single pellet reaching in spinal cord injured rats. Exp. Neurol 247, 605–614 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Fouad K, Hurd C & Magnuson DS Functional testing in animal models of spinal cord injury: not as straight forward as one would think. Front. Integr. Neurosci 7, 85 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steward O, Popovich PG, Dietrich WD & Kleitman N Replication and reproducibility in spinal cord injury research. Exp. Neurol 233, 597–605 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Lam CJ, Assinck P, Liu J, Tetzlaff W & Oxland TR Impact depth and the interaction with impact speed affect the severity of contusion spinal cord injury in rats. J. Neurotrauma 31, 1985–1997 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballermann M & Fouad K Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur. J. Neurosci 23, 1988–1996 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Martinez M, Delivet-Mongrain H, Leblond H & Rossignol S Recovery of hindlimb locomotion after incomplete spinal cord injury in the cat involves spontaneous compensatory changes within the spinal locomotor circuitry. J. Neurophysiol 106, 1969–1984 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Loy DN et al. Functional redundancy of ventral spinal locomotor pathways. J. Neurosci 22, 315–323 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loy DN et al. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp. Neurol 177, 575–580 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Brustein E & Rossignol S Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. I. Deficits and adaptive mechanisms. J. Neurophysiol 80, 1245–1267 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Filli L et al. Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J. Neurosci 34, 13399–13410 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aboul-Enein F, Weiser P, Höftberger R, Lassmann H & Bradl M Transient axonal injury in the absence of demyelination: a correlate of clinical disease in acute experimental autoimmune encephalomyelitis. Acta Neuropathol. 111, 539–547 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Kerschensteiner M, Schwab ME, Lichtman JW & Misgeld T In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat. Med 11, 572–577 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Duncan GJ et al. Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nat. Commun 9, 3066 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartus K et al. Neuregulin-1 controls an endogenous repair mechanism after spinal cord injury. Brain 139, 1394–1416 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pukos N, Goodus MT, Sahinkaya FR & McTigue DM Myelin status and oligodendrocyte lineage cells over time after spinal cord injury: what do we know and what still needs to be unwrapped? Glia 67, 2178–2202 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen HS, Holmes N, Liu J, Tetzlaff W & Kozlowski P Validating myelin water imaging with transmission electron microscopy in a rat spinal cord injury model. Neuroimage 153, 122–130 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Goldstein B, Hammond MC, Stiens SA & Little JW Posttraumatic syringomyelia: profound neuronal loss, yet preserved function. Arch. Phys. Med. Rehabil 79, 107–112 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Dreizin D et al. Will the real SCIWORA please stand up? exploring clinicoradiologic mismatch in closed spinal cord injuries. AJR Am. J. Roentgenol 205, 853–860 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Curt A The translational dialogue in spinal cord injury research. Spinal Cord 50, 352–357 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Popovich PG et al. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J. Neuropathol. Exp. Neurol 61, 623–633 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Pouratian N & Bookheimer SY The reliability of neuroanatomy as a predictor of eloquence: a review. Neurosurg. Focus 28, E3 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Levine AJ et al. Identification of a cellular node for motor control pathways. Nat. Neurosci 17, 586–593 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stepien AE, Tripodi M & Arber S Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron 68, 456–472 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Conta AC & Stelzner DJ Differential vulnerability of propriospinal tract neurons to spinal cord contusion injury. J. Comp. Neurol 479, 347–359 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Filli L & Schwab ME Structural and functional reorganization of propriospinal connections promotes functional recovery after spinal cord injury. Neural Regen. Res 10, 509–513 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miles GB, Hartley R, Todd AJ & Brownstone RM Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc. Natl Acad. Sci. USA 104, 2448–2453 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y et al. Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature 561, 547–550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirshblum S et al. The impact of sacral sensory sparing in motor complete spinal cord injury. Arch. Phys. Med. Rehabil 92, 376–383 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waters RL, Adkins RH & Yakura JS Definition of complete spinal cord injury. Paraplegia 29, 573–581 (1991). [DOI] [PubMed] [Google Scholar]
- 33.Zdunczyk A et al. The corticospinal reserve capacity: reorganization of motor area and excitability as a novel pathophysiological concept in cervical myelopathy. Neurosurgery 83, 810–818 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Torres-Espín A et al. Eliciting inflammation enables successful rehabilitative training in chronic spinal cord injury. Brain 141, 1946–1962 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanagal SG & Muir GD Task-dependent compensation after pyramidal tract and dorsolateral spinal lesions in rats. Exp. Neurol 216, 193–206 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Whishaw IQ, Gorny B & Sarna J Paw and limb use in skilled and spontaneous reaching after pyramidal tract, red nucleus and combined lesions in the rat: behavioral and anatomical dissociations. Behav. Brain Res 93, 167–183 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Curt A, Van Hedel HJA, Klaus D & Dietz V, EM-SCI Study Group. Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J. Neurotrauma 25, 677–685 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Fouad K, Pedersen V, Schwab ME & Brösamle C Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr. Biol 11, 1766–1770 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Bareyre FM et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci 7, 269–277 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Krajacic A, Weishaupt N, Girgis J, Tetzlaff W & Fouad K Training-induced plasticity in rats with cervical spinal cord injury: effects and side effects. Behav. Brain Res 214, 323–331 (2010). [DOI] [PubMed] [Google Scholar]
- 41.van den Brand R et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336, 1182–1185 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Liu K et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci 13, 1075–1081 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Reynolds A, Kirry A, Nienhaus C & Blackmore MG Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J. Neurosci 35, 3139–3145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jayaprakash N et al. Optogenetic interrogation of functional synapse formation by corticospinal tract axons in the injured spinal cord. J. Neurosci 36, 5877–5890 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fouad K, Ng C & Basso DM Behavioral testing in animal models of spinal cord injury. Exp. Neurol 333, 113410 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popovich PG, Lemeshow S, Gensel JC & Tovar CA Independent evaluation of the effects of glibenclamide on reducing progressive hemorrhagic necrosis after cervical spinal cord injury. Exp. Neurol 233, 615–622 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simard JM, Popovich PG, Tsymbalyuk O & Gerzanich V Spinal cord injury with unilateral versus bilateral primary hemorrhage-effects of glibenclamide. Exp. Neurol 233, 829–835 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watzlawick R et al. Outcome heterogeneity and bias in acute experimental spinal cord injury: a meta-analysis. Neurology 93, e40–e51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Begley CG & Ioannidis JP Reproducibility in science: improving the standard for basic and preclinical research. Circ. Res 116, 116–126 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Callahan A et al. Developing a data sharing community for spinal cord injury research. Exp. Neurol 295, 135–143 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fouad K et al. FAIR SCI ahead: the evolution of the open data commons for pre-clinical spinal cord injury research. J. Neurotrauma 37, 831–838 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hotamisligil GS Foundations of immunometabolism and implications for metabolic health and disease. Immunity 47, 406–420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hotamisligil GS Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Failli V et al. Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain 135, 3238–3250 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Kopp MA et al. Long-term functional outcome in patients with acquired infections after acute spinal cord injury. Neurology 88, 892–900 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaja BNR et al. Association of pneumonia, wound infection, and sepsis with clinical outcomes after acute traumatic spinal cord injury. J. Neurotrauma 36, 3044–3050 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gallagher MJ et al. Markedly deranged injury site metabolism and impaired functional recovery in acute spinal cord injury patients with fever. Crit. Care Med 46, 1150–1157 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Marik PE & Bellomo R Stress hyperglycemia: an essential survival response! Crit. Care 17, 305 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kobayakawa K et al. Acute hyperglycemia impairs functional improvement after spinal cord injury in mice and humans. Sci. Transl Med 6, 256ra137 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Ryken TC et al. The acute cardiopulmonary management of patients with cervical spinal cord injuries. Neurosurgery 72 (Suppl. 2), 84–92 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Ehsanian R et al. Exploration of surgical blood pressure management and expected motor recovery in individuals with traumatic spinal cord injury. Spinal Cord 58, 377–386 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallagher MJ, Hogg FRA, Zoumprouli A, Papadopoulos MC & Saadoun S Spinal cord blood flow in patients with acute spinal cord injuries. J. Neurotrauma 36, 919–929 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Kigerl KA et al. Gut dysbiosis impairs recovery after spinal cord injury. J. Exp. Med 213, 2603–2620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmidt EKA et al. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLoS ONE 15, e0226128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ankeny DP, Guan Z & Popovich PG B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Invest 119, 2990–2999 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwab JM, Zhang Y, Kopp MA, Brommer B & Popovich PG The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp. Neurol 258, 121–129 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shibata D, Cain K, Tanzi P, Zierath D & Becker K Myelin basic protein autoantibodies, white matter disease and stroke outcome. J. Neuroimmunol 252, 106–112 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doyle KP et al. B-lymphocyte-mediated delayed cognitive impairment following stroke. J. Neurosci 35, 2133–2145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diamond B, Huerta PT, Mina-Osorio P, Kowal C & Volpe BT Losing your nerves? Maybe it’s the antibodies. Nat. Rev. Immunol 9, 449–456 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freund P et al. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. Lancet Neurol. 12, 873–881 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.James ND et al. Conduction failure following spinal cord injury: functional and anatomical changes from acute to chronic stages. J. Neurosci 31, 18543–18555 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Catalano SM & Shatz CJ Activity-dependent cortical target selection by thalamic axons. Science 281, 559–562 (1998). [DOI] [PubMed] [Google Scholar]
- 73.Zhang LI & Poo MM Electrical activity and development of neural circuits. Nat. Neurosci 4 (Suppl.), 1207–1214 (2001). [DOI] [PubMed] [Google Scholar]
- 74.Ditunno JF Jr & Formal CS Chronic spinal cord injury. N. Engl. J. Med 330, 550–556 (1994). [DOI] [PubMed] [Google Scholar]
- 75.Kirshblum S, Millis S, McKinley W & Tulsky D Late neurologic recovery after traumatic spinal cord injury. Arch. Phys. Med. Rehabil 85, 1811–1817 (2004). [DOI] [PubMed] [Google Scholar]
- 76.el Masry WS Physiological instability of the spinal cord following injury. Paraplegia 31, 273–275 (1993). [DOI] [PubMed] [Google Scholar]
- 77.Chen Q, Smith GM & Shine HD Immune activation is required for NT-3-induced axonal plasticity in chronic spinal cord injury. Exp. Neurol 209, 497–509 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beauparlant J et al. Undirected compensatory plasticity contributes to neuronal dysfunction after severe spinal cord injury. Brain 136, 3347–3361 (2013). [DOI] [PubMed] [Google Scholar]
- 79.Li Y et al. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat. Med 23, 733–741 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.von Monakow C Lokalisation im Gehirn und funktionelle Stoerungen induziert durch kortikale Laesionen. (Bergmann JF, 1914). [Google Scholar]
- 81.Carrera E & Tononi G Diaschisis: past, present, future. Brain 137, 2408–2422 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Seitz RJ et al. The role of diaschisis in stroke recovery. Stroke 30, 1844–1850 (1999). [DOI] [PubMed] [Google Scholar]
- 83.Baldassarre A et al. Dissociated functional connectivity profiles for motor and attention deficits in acute right-hemisphere stroke. Brain 139, 2024–2038 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Min YS et al. Alteration of resting-state brain sensorimotor connectivity following spinal cord injury: a resting-state functional magnetic resonance imaging study. J. Neurotrauma 32, 1422–1427 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Dietz V Behavior of spinal neurons deprived of supraspinal input. Nat. Rev. Neurol 6, 167–174 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Vallotton K et al. Width and neurophysiologic properties of tissue bridges predict recovery after cervical injury. Neurology 92, e2793–e2802 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huber E et al. Dorsal and ventral horn atrophy is associated with clinical outcome after spinal cord injury. Neurology 90, e1510–e1522 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pfyffer D, Huber E, Sutter R, Curt A & Freund P Tissue bridges predict recovery after traumatic and ischemic thoracic spinal cord injury. Neurology 93, e1550–e1560 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Talbott JF et al. The Brain and Spinal Injury Center score: a novel, simple, and reproducible method for assessing the severity of acute cervical spinal cord injury with axial T2-weighted MRI findings. J. Neurosurg. Spine 23, 495–504 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Wheeler-Kingshott CA et al. The current state-of-the-art of spinal cord imaging: applications. Neuroimage 84, 1082–1093 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stroman PW et al. The current state-of-the-art of spinal cord imaging: methods. Neuroimage 84, 1070–1081 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lang BT et al. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature 518, 404–408 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blesch A & Tuszynski MH Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 32, 41–47 (2009). [DOI] [PubMed] [Google Scholar]
- 94.Filli L & Schwab ME The rocky road to translation in spinal cord repair. Ann. Neurol 72, 491–501 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Freria CM et al. Deletion of the fractalkine receptor, CX3CR1, improves endogenous repair, axon sprouting, and synaptogenesis after spinal cord injury in mice. J. Neurosci 37, 3568–3587 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Church JS, Kigerl KA, Lerch JK, Popovich PG & McTigue DM TLR4 deficiency impairs oligodendrocyte formation in the injured spinal cord. J. Neurosci 36, 6352–6364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hansen CN et al. Elevated MMP-9 in the lumbar cord early after thoracic spinal cord injury impedes motor relearning in mice. J. Neurosci 33, 13101–13111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tanadini LG et al. Identifying homogeneous subgroups in neurological disorders: unbiased recursive partitioning in cervical complete spinal cord injury. Neurorehabil. Neural Repair 28, 507–515 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Ghasemlou N, Kerr BJ & David S Tissue displacement and impact force are important contributors to outcome after spinal cord contusion injury. Exp. Neurol 196, 9–17 (2005). [DOI] [PubMed] [Google Scholar]
- 100.Santos-Benito FF, Muñoz-Quiles C & Ramón-Cueto A Long-term care of paraplegic laboratory mammals. J. Neurotrauma 23, 521–536 (2006). [DOI] [PubMed] [Google Scholar]
- 101.Kigerl KA, Mostacada K & Popovich PG Gut microbiota are disease-modifying factors after traumatic spinal cord injury. Neurotherapeutics 15, 60–67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kigerl KA, Zane K, Adams K, Sullivan MB & Popovich PG The spinal cord-gut-immune axis as a master regulator of health and neurological function after spinal cord injury. Exp. Neurol 323, 113085 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Levy M, Kolodziejczyk AA, Thaiss CA & Elinav E Dysbiosis and the immune system. Nat. Rev. Immunol 17, 219–232 (2017). [DOI] [PubMed] [Google Scholar]
- 104.Berer K et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011). [DOI] [PubMed] [Google Scholar]
- 105.Wiesel TN & Hubel DH Extent of recovery from the effects of visual deprivation in kittens. J. Neurophysiol 28, 1060–1072 (1965). [DOI] [PubMed] [Google Scholar]
- 106.Raineteau O & Schwab ME Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci 2, 263–273 (2001). [DOI] [PubMed] [Google Scholar]
- 107.May Z, Fouad K, Shum-Siu A & Magnuson DS Challenges of animal models in SCI research: Effects of pre-injury task-specific training in adult rats before lesion. Behav. Brain Res 291, 26–35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caudle KL et al. Hindlimb immobilization in a wheelchair alters functional recovery following contusive spinal cord injury in the adult rat. Neurorehabil. Neural Repair 25, 729–739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Basso DM, Beattie MS & Bresnahan JC A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 (1995). [DOI] [PubMed] [Google Scholar]
- 110.Kirshblum SC et al. Patterns of sacral sparing components on neurologic recovery in newly injured persons with traumatic spinal cord injury. Arch. Phys. Med. Rehabil 97, 1647–1655 (2016). [DOI] [PubMed] [Google Scholar]
- 111.Courtine G et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med 13, 561–566 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farhadi HF et al. Impact of admission imaging findings on neurological outcomes in acute cervical traumatic spinal cord injury. J. Neurotrauma 35, 1398–1406 (2018). [DOI] [PubMed] [Google Scholar]
- 113.Bradbury EJ & McMahon SB Spinal cord repair strategies: why do they work? Nat. Rev. Neurosci 7, 644–653 (2006). [DOI] [PubMed] [Google Scholar]
- 114.Kapur N Paradoxes in rehabilitation. Disabil. Rehabil 42, 1495–1502 (2020). [DOI] [PubMed] [Google Scholar]
- 115.Page SJ, Gauthier LV & White S Size doesn’t matter: cortical stroke lesion volume is not associated with upper extremity motor impairment and function in mild, chronic hemiparesis. Arch. Phys. Med. Rehabil 94, 817–821 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Price CJ, Hope TM & Seghier ML Ten problems and solutions when predicting individual outcome from lesion site after stroke. Neuroimage 145, 200–208 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rorden C & Karnath HO Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat. Rev. Neurosci 5, 813–819 (2004). [DOI] [PubMed] [Google Scholar]
- 118.Inoue K, Madhyastha T, Rudrauf D, Mehta S & Grabowski T What affects detectability of lesion-deficit relationships in lesion studies? Neuroimage Clin. 6, 388–397 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barkhof F The clinico-radiological paradox in multiple sclerosis revisited. Curr. Opin. Neurol 15, 239–245 (2002). [DOI] [PubMed] [Google Scholar]
- 120.Barkhof F MRI in multiple sclerosis: correlation with expanded disability status scale (EDSS). Mult. Scler 5, 283–286 (1999). [DOI] [PubMed] [Google Scholar]
- 121.Okuda DT et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology 72, 800–805 (2009). [DOI] [PubMed] [Google Scholar]
- 122.Wuerfel J et al. Mouse model mimics multiple sclerosis in the clinico-radiological paradox. Eur. J. Neurosci 26, 190–198 (2007). [DOI] [PubMed] [Google Scholar]


