Abstract
Resistance to clarithromycin in breakthrough Mycobacterium avium complex (MAC) isolates typically occurs 3 to 4 months after the initiation of monotherapy in bacteremic AIDS patients. It has been suggested that continuation of clarithromycin therapy still results in clinical and microbiological improvement. To study this paradox, C57BL/6 beige mice were infected with a clarithromycin-resistant (MIC, ≥128 μg/ml) strain of MAC 101 (CLA-R MAC 101) and treated with 200 mg of clarithromycin per kg of body weight/day alone or in combination with ethambutol (100 mg/kg/day) for 2 weeks. Mice infected with a clarithromycin-susceptible strain of MAC 101 had bacterial loads reduced by 90% in the liver and 91% in the spleen (P < 0.05, compared with the control). Clarithromycin treatment of CLA-R MAC 101 resulted in a 65% reduction of bacterial loads in the liver (P = 0.009) and a 71% reduction in the spleen (P = 0.009), compared with the results for the untreated control. CLA-R MAC 101 and MAC 101 (isogenic strains) had comparable growth rates in murine tissue, ruling out a loss of virulence of CLA-R MAC 101. Strains of MAC currently defined as macrolide resistant may still respond to treatment with an agent such as clarithromycin within infected tissues.
Disseminated Mycobacterium avium complex (MAC) infection is the most common bacterial infection in patients with advanced stages of AIDS (8, 9). Prior to the advent of MAC prophylaxis and the highly active antiretroviral treatment regimen, 50 or 60% of patients with CD4 lymphocyte counts of <50/mm3 ultimately developed MAC disease (14). Earlier studies showed that MAC infection is associated with increased morbidity and more rapid mortality (4). However, more recent studies showed that when patients receive prophylactic antibiotics, there is a significant increase in survival (3, 6, 15). However, only a few antimicrobial agents, such as macrolides, rifabutin, and ethambutol, have shown activity either as prophylaxis or as therapy for MAC infection (reviewed in reference 11).
Macrolides (clarithromycin, azithromycin, and roxithromycin) are very active against MAC, but clinical trials demonstrated that the use of clarithromycin for 3 to 4 months led to the selection of clarithromycin-resistant MAC strains in the blood in a large percentage of patients (3). Experimental studies with mice have shown that the frequency of resistance of MAC strains to clarithromycin can reach approximately 10−3 after 12 weeks of monotherapy (2), although resistance to azithromycin takes longer to emerge than resistance to clarithromycin (2).
Intriguing, however, is a recent observation by Dube and colleagues (5) that suggests that treatment of clarithromycin-resistant MAC disease with clarithromycin alone or in combination with other agents, such as ethambutol or rifabutin, appears to be clinically and microbiologically effective.
In this study, we sought to investigate the activity of clarithromycin in vivo against a genotypically characterized clarithromycin-resistant strain of MAC. In addition, we compared the virulence of clarithromycin-resistant and -susceptible isotypic strains of MAC as well as strains with the most common mutations in the 23S rRNA gene both in a macrophage system and in vivo.
MATERIALS AND METHODS
Mycobacteria.
MAC 101 (serovar 1), MAC JJL, and MAC JWT were originally isolated from the blood of an AIDS patient with disseminated MAC infection. MAC organisms for animal inoculation were grown at 37°C for 10 days on Middlebrook 7H11 agar (Difco Laboratories, Detroit, Mich.) supplemented with oleic acid, albumin, dextrose, and catalase (OADC; Difco). The MAC cultures were examined for purity, and transparent colonies were harvested and suspended in Hanks' balanced salt solution (HBSS). The suspension was adjusted to 3 × 108 CFU/ml using a McFarland turbidity standard, and the number of CFU per milliliter of the final inoculum was confirmed by plating serial dilutions on 7H11 agar (2). The clarithromycin-resistant MAC 101 strain used here (CLA-R MAC 101) has been characterized previously (2). It was obtained from mice infected with MAC 101 and treated with clarithromycin. It has a single-base mutation in the 23S rRNA gene (A2275 → C2275) that confers resistance to clarithromycin (MIC, ≥128 μg/ml). CLA-R MAC 101 was grown on 7H11 agar containing 32 μg of clarithromycin per ml but otherwise was prepared in the same manner as MAC 101.
Macrolide (clarithromycin and azithromycin)-resistant strains were isolated from AIDS patients receiving macrolide therapy for disseminated MAC disease (strains JJL-R and JWT-R). These strains have mutations in the 23S rRNA that confer resistance to clarithromycin; the mutation in strain JJL-R is A2274 → T, and that in strain JWT-R is A2274 → C. These strains were cultured for 10 days on Middlebrook 7H11 agar.
Human macrophage studies.
Macrophage studies were carried out as previously described (1). The source of macrophages was the human monocyte cell line U937 cultured in RPMI 1640 medium (pH 7.2) (Sigma Chemical Co., St. Louis, Mo.) supplemented with 5% heat-inactivated fetal bovine serum and 2 mM l-glutamine. Cells were grown to a density of 5 × 108 cells per ml and then centrifuged, washed, and resuspended in fresh medium. The concentration of cells was adjusted to 106 cells per ml, and 1 ml of the cell suspension was added to each well of a 24-well tissue culture plate (Costar, Cambridge, Mass.). Monolayers were treated with 0.5 μg of phorbol myristate acetate per ml for 24 h to stimulate maturation of the monocytes. Monolayers were monitored for the numbers of cells, and no difference was observed in the extent of cell detachment among the experimental groups (1).
On the day of the experiment, bacteria were harvested, washed twice in HBSS, resuspended in HBSS, and sonicated for 5 s to disperse clumps. The turbidity of the suspension was adjusted to be equivalent to a McFarland no. 1 standard, and the suspension was diluted to approximately 5 × 107 CFU/ml. Each monolayer was infected with 100 μl of final suspension, and the actual number of CFU per milliliter of the final suspension was determined by quantitative plate counts. At 4 h and 4 days after infection, the mycobacterial CFU/ml were determined by lysing macrophage monolayers. Cold sterile water (0.5 ml) was added to each well, and the mixture was allowed to stand for 10 min at room temperature. Then, 0.5 ml of a lysing solution (1.1 ml of 7H9 broth plus 0.4 ml of 0.25% sodium dodecyl sulfate in phosphate buffer) was added to each well, and the mixture was allowed to stand for an additional 10 min. The sodium dodecyl sulfate was then neutralized with 20% bovine albumin, the mixture was serially diluted, and 0.1 ml was plated on 7H10 agar plates. The plates were allowed to dry at room temperature for 15 min and were incubated for 2 weeks. Duplicate plates were prepared for each well, and the results were reported as mean CFU per milliliter of macrophage lysate. Each experiment was performed three times.
Mice.
Female beige mice (C57BL/6 bg/bg) were purchased from Jackson Laboratories (Bar Harbor, Maine) and housed for 2 weeks before the experiments. The mice (8 to 10 weeks old and weighing 14 to 18 g) were infected intravenously (i.v.).
Antimicrobial agents.
Clarithromycin was provided by the manufacturer (Abbott Laboratories, Abbott Park, Ill.) and prepared as a suspension in a sterile saturated sucrose solution as previously described (2). Ethambutol was purchased from Sigma and dissolved in sterile water.
Treatment of mice infected with CLA-R MAC 101.
Experiments were designed to evaluate a clinical observation that individuals with clarithromycin-resistant MAC still benefit from the use of clarithromycin (5). Mice were infected i.v. with either CLA-R MAC 101 (4 × 107 CFU) or MAC 101 (6 × 107 CFU); 7 days after infection, several mice were killed and examined to establish the baseline level of infection. Then, drugs (clarithromycin, 200 mg/kg of body weight; ethambutol, 100 mg/kg; or a combination of both) were administered by gavage without sedation over the entire period of the experiment (2 weeks of treatment). Control mice received an equivalent volume of sterile saturated sucrose solution. Mice were killed after 2 weeks of treatment (week 3 of the experiment), and spleens and livers were removed and cultured quantitatively as reported previously (1, 2). To decrease the possibility of drug carryover, mice were killed 48 h after the last dose of antibiotic.
Comparative evaluation of the virulence of MAC 101 and CLA-R MAC 101 in mice.
To examine comparatively the abilities of strains MAC 101 and CLA-R MAC 101 (4 × 107) to replicate in mice, C57BL/6 beige mice were infected i.v. and the loads of bacteria in the liver and in the spleen were determined at 1, 3, and 5 weeks postinfection.
Quantitative culturing of organs.
Mice were sacrificed, and the livers and spleens were removed by aseptic dissection. The organs were weighed, suspended and homogenized in 7H9 broth, and then serially diluted in 7H9 broth. Aliquots of the suspensions were plated on 7H11 agar with OADC. Organs from CLA-R MAC 101-infected mice were plated on 7H11 agar with 32 μg of clarithromycin per ml. The plates were incubated for 8 to 10 days at 37°C, and the colonies were counted as previously described and expressed as CFU per gram of tissue and standard error (1, 2).
Statistical analysis.
The differences between experimental groups at the same time point were analyzed by Student's t test and analysis of variance. A P value of <0.05 was considered statistically significant. The number of mice per group and the expected differences in observations were based on an analysis of variance model that provided 0.90 power for each experiment.
RESULTS
Table 1 shows the number of mice used in the experiments reported. This number provided 0.90 power for each experiment.
TABLE 1.
Number of mice used per experimenta
| Efficacy expt
|
Virulence expt
|
||
|---|---|---|---|
| Treatment | No. of mice | Infecting organism | No. of mice |
| Clarithromycin | 16 | MAC 101 | 35 |
| Ethambutol | 13 | CLA-R MAC 101 | 38 |
| Clarithromycin + ethambutol | 15 | ||
| Control (3 wk) | 14 | ||
| 1-wk control | 6 | ||
The number of mice per group and the expected differences in observations were based on an analysis of variance model that provided 0.90 power for each experiment.
Clarithromycin treatment.
To investigate if CLA-R MAC 101 still responds to treatment with clarithromycin, mice were infected with either MAC 101 or CLA-R MAC 101 and then treated with clarithromycin. Table 2 shows that both strains were affected, with a significant reduction in the bacterial load in the spleen. The achieved reduction in the number of MAC in the spleen was half the reduction observed in mice infected with a clarithromycin-susceptible strain.
TABLE 2.
Effect of treatment of mice infected with either clarithromycin-susceptible or clarithromycin-resistant MAC with clarithromycina
| Strain | Therapy | Decrease in log10 CFUb | Mean ± SE CFU/g in spleen |
|---|---|---|---|
| MAC 101 | Clarithromycin | (2.3 ± 0.3) × 107 | |
| Control (3 wk) | 1.5* | (7.7 ± 0.4) × 108 | |
| Control (1 wk) | 1.02* | (2.5 ± 0.4) × 108 | |
| CLA-R MAC 101 | Clarithromycin | (5.3 ± 2.1) × 107 | |
| Control (3 wk) | 0.65* | (1.8 ± 0.4) × 108 | |
| Control (1 wk) | 0.32† | (8.5 ± 0.5) × 107 |
Mice were infected with MAC strains for 1 week, and then the number of bacteria in the spleen was quantitated. Then, mice were treated with clarithromycin at 200 mg/kg/day for 2 weeks. At the end of week 3, mice were killed.
Decrease occurring between the indicated control group and the clarithromycin-treated group as a result of drug treatment. Differences between the clarithromycin-treated group and the control groups had P values of <0.05 (∗) and >0.05 (†).
Clarithromycin-ethambutol treatment.
Table 3 shows that the addition of ethambutol to clarithromycin as the regimen to treat MAC disseminated infection in mice did not increase the reduction in the number of bacteria in the spleen compared with clarithromycin alone.
TABLE 3.
Effect of treatment of mice infected with CLA-R MAC 101 with clarithromycin alone or in combination with ethambutola
| Therapy | Mean ± SE CFU/g inb:
|
|
|---|---|---|
| Liver | Spleen | |
| Clarithromycin (200 mg/kg/day) | (3.1 ± 1.2) × 107* | (5.3 ± 2.1) × 107* |
| Ethambutol (100 mg/kg/day) | (4.4 ± 1.5) × 107 | (7.2 ± 2.2) × 107* |
| Clarithromycin + ethambutol | (2.4 ± 0.5) × 107*† | (7.2 ± 2.5) × 107*† |
| Control (3 wk) | (8.8 ± 1.7) × 107 | (1.8 ± 0.4) × 108 |
| Control (1 wk) | (5.4 ± 0.8) × 107 | (8.5 ± 0.5) × 107 |
Mice were infected with CLA-R MAC 101 and received 2 weeks of treatment with clarithromycin and/or ethambutol.
P < 0.05 for comparison with the 3-week control (∗), and P > 0.05 for comparison with clarithromycin alone (†).
MAC growth.
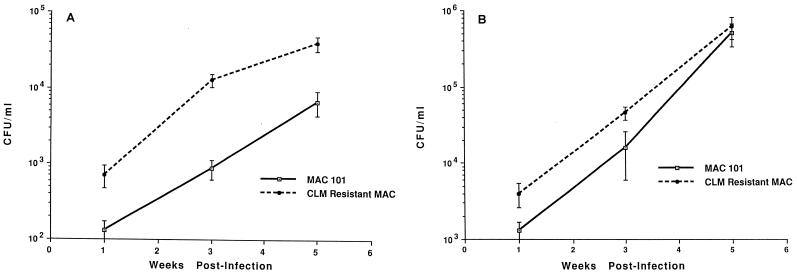
As one possible explanation for the finding that CLA-R MAC 101 still responds to therapy with clarithromycin, we hypothesized that CLA-R MAC 101 was less virulent than MAC 101. To address this question, we compared the growth of both strains in mice. As shown in Fig. 1, the growth of clarithromycin-susceptible and clarithromycin-resistant strains was comparable in both the liver and the spleen for 4 weeks. Figure 1 and Tables 2 and 3 differ regarding the bacterial burden, probably because the inoculum used in the “virulence” experiments was significantly lower. The intent was to avoid overwhelming the system with a large inoculum.
FIG. 1.
Comparative growth of MAC 101 and CLA-R MAC 101 in the liver (A) and spleen (B). Mice were infected with MAC strains as described in Materials and Methods, and infection was monitored for 5 weeks. Quantitation of the number of bacteria in the liver and spleen was done after 1, 3, and 5 weeks. CLM, clarithromycin. Bars indicate standard errors.
Since it is plausible that point mutations in different nucleotides may make some but not all strains of CLA-R MAC 101 less virulent than clarithromycin-susceptible MAC 101, we examined the growth of three different isotype strains containing various point mutations in U937 human macrophages. As shown in Table 4, strains MAC 101, CLA-R MAC 101, JWT, JWT-R, JJL, and JJL-R showed similar growth rates in macrophages.
TABLE 4.
Comparative growth in macrophages of clarithromycin-susceptible and -resistant M. avium strains containing the most common mutation observed in the 23S rRNA gene in AIDS isolatesa
| Strain | Mean ± SE CFU/g on the following day after infectionb:
|
||
|---|---|---|---|
| 0 | 2 | 4 | |
| MAC 101 | (7.9 ± 0.4) × 104* | (2.5 ± 0.3) × 105* | (8.6 ± 0.4) × 105* |
| CLA-R MAC 101 | (8.0 ± 0.3) × 104 | (3.0 ± 0.1) × 105 | (9.5 ± 0.3) × 105 |
| JWT | (8.6 ± 0.3) × 104* | (1.3 ± 1.2) × 105* | (9.8 ± 0.8) × 105* |
| JWT-R | (9.1 ± 0.7) × 104 | (1.5 ± 0.5) × 105 | (9.5 ± 1.9) × 105 |
| JJL | (8.6 ± 0.4) × 104* | (1.5 ± 0.4) × 105* | (8.4 ± 0.3) × 105* |
| JJL-R | (8.6 ± 0.3) × 104 | (1.6 ± 0.5) × 105 | (8.8 ± 0.2) × 105 |
U937 macrophages were infected as described in Materials and Methods, and intracellular bacterial replication was monitored for 4 days. Monolayers were then harvested, and intracellular bacteria were quantitated. Strains MAC 101, JWT, and JJL are clarithromycin susceptible, and strains CLA-R MAC 101, JWT-R, and JJL-R are clarithromycin resistant.
The P value for comparisons between resistant and susceptible strains was >0.05.
DISCUSSION
The introduction of an effective prophylactic regimen against MAC infections in AIDS patients significantly changed the prognosis of the disease (3, 6). Macrolides (both clarithromycin and azithromycin) are very active prophylactically for preventing MAC disease, but the use of clarithromycin and, to a lesser degree, azithromycin is associated with breakthrough MAC infection (3, 6). Since studies have shown that when resistance develops, it is cross-reactive between azithromycin and clarithromycin (7), and because macrolides constitute the core of anti-MAC therapy, it is assumed that not many alternatives are available at this time for MAC disease.
A recent study by Dube and colleagues, however, demonstrated that in patients receiving clarithromycin prophylaxis and who developed breakthrough infection, therapy with a macrolide in combination with another antimicrobial agent, such as ethambutol, was still effective in reducing the number of bacteria in the blood (5). Our results agree with these clinical findings. Although a clarithromycin-resistant strain was less responsive to clarithromycin therapy than a clarithromycin-susceptible strain, the response was still significant. It is important to mention that mortality during the experiment was not a factor in the results. These findings raise questions about the mechanisms of clarithromycin resistance in MAC, the tissue clarithromycin level during therapy, and the virulence of clarithromycin-resistant MAC strains.
Clarithromycin resistance in MAC is assumed to be due to a single base mutation in the 23S rRNA gene (12, 13). Three different base-pair substitutions have been identified for adenine-2058 and for adenine-2059 thus far in MAC (12, 13). Since MAC has only one copy of the 23S rRNA gene, in contrast to Escherichia coli, which has five copies of the 23S rRNA gene, it was concluded that a base mutation in the 23S rRNA gene was sufficient to induce the resistant phenotype. Other groups, however, have proposed that mutations in ribosome proteins could also translate in CLA-R MAC 101 (F. Doucet-Populaire, R. Goldman, J. Grosset, and V. Jarlier, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., p. 56, 1995). Therefore, the mechanism of resistance or the sites of mutation in the 23S rRNA gene that confer resistance to clarithromycin in vitro may differ from those in vivo.
The second possibility is that the tissue level achieved by clarithromycin is significantly higher than has been thought. Previous studies have shown that clarithromycin can be concentrated in monocytes and macrophages 10- to 20-fold (10), but it is plausible that in vivo it achieves even higher intracellular concentrations. If this hypothesis is correct, treatment with azithromycin should result in a greater reduction in bacterial load than clarithromycin.
Finally, there is the possibility that the clarithromycin-resistant strain is less virulent than the clarithromycin-susceptible strain. For comparison, isoniazid-resistant M. tuberculosis is less virulent than isoniazid-susceptible M. tuberculosis, due to the absence of the katG gene (16). Our results, however, make this hypothesis unlikely, since in both human macrophages and mice MAC strains representing all clinically relevant mutations showed neither a decrease in the growth rate nor an impairment of virulence, respectively.
Although the mechanism of resistance to macrolides is known, the reason for a clinical response in infections with clarithromycin-resistant strains of MAC is currently unknown. Our data raise the possibility that infections with clarithromycin-resistant strains may be still treated with clarithromycin and that other mechanisms of resistance may be possible.
ACKNOWLEDGMENTS
We thank Karen Allen for preparing the manuscript and Abbott Laboratories for providing clarithromycin.
This work was supported by contract AI-25140 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Bermudez L E, Inderlied C B, Kolonoski P, Wu M, Barbara-Burnham L, Young L S. Activities of bay Y 3118, levofloxacin, and ofloxacin alone or in combination with ethambutol against Mycobacterium avium complex in vitro, in human macrophages, and in beige mice. Antimicrob Agents Chemother. 1996;40:546–551. doi: 10.1128/aac.40.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez L E, Nash K A, Petrofsky M, Young L S, Inderlied C B. Effect of ethambutol on emergence of clarithromycin-resistant Mycobacterium avium complex in the beige mouse model. J Infect Dis. 1996;174:1218–1222. doi: 10.1093/infdis/174.6.1218. [DOI] [PubMed] [Google Scholar]
- 3.Chaisson R E, Benson C A, Dube M P, Heifets L B, Korvick J A, Elkin S, Smith T, Craft J C, Sattler F R. Clarithromycin therapy for bacteremic Mycobacterium avium complex disease. A randomized, double-blind, dose-ranging study in patients with AIDS. AIDS Clinical Trials Group Protocol 157 Study Team. Ann Intern Med. 1994;121:905–911. doi: 10.7326/0003-4819-121-12-199412150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Chin D P, Reingold A L, Stone E N. The impact of Mycobacterium avium complex bacteremia and its treatment on survival of AIDS patients: a prospective study. J Infect Dis. 1994;170:578–584. doi: 10.1093/infdis/170.3.578. [DOI] [PubMed] [Google Scholar]
- 5.Dube M P, Sattler F R, Torriani F J, See D, Havlir D V, Kemper C A, Dezfuli M G, Bozzette S A, Bartok A E, Leedom J M, Tilles J G, McCutchan J A. A randomized evaluation of ethambutol for prevention of relapse and drug resistance during treatment of Mycobacterium avium complex bacteremia with clarithromycin-based combination therapy. California Collaborative Treatment Group. J Infect Dis. 1997;176:1225–1232. doi: 10.1086/514116. [DOI] [PubMed] [Google Scholar]
- 6.Havlir D V, Dube M P, Sattler F R, Forthal D N, Kemper C A, Dunne M W, Parenti D M, Lavelle J P, White A C, Jr, Witt M D, Bozzette S A, McCutchan J A. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. California Collaborative Treatment Group. N Engl J Med. 1996;335:392–398. doi: 10.1056/NEJM199608083350604. [DOI] [PubMed] [Google Scholar]
- 7.Heifets L, Mor N, Vanderkolk J. Mycobacterium avium strains resistant to clarithromycin and azithromycin. Antimicrob Agents Chemother. 1993;37:2364–2370. doi: 10.1128/aac.37.11.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horsburgh C R., Jr Mycobacterium avium complex in the acquired immunodeficiency syndrome (AIDS) N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 9.Inderlied C B, Kemper C A, Bermudez L E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohno Y, Yoshida H, Suwa T, Buga T. Comparative pharmacokinetics of clarithromycin, a new macrolide antibiotic, and erythromycin in rats. Antimicrob Agents Chemother. 1989;33:751–756. doi: 10.1128/aac.33.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korvick J, Benson C. Advances in the treatment and prophylaxis of Mycobacterium avium complex in individuals infected with human immunodeficiency virus. In: Korvick J, Benson C, editors. Mycobacterium avium complex infection: progress in research and treatment. New York, N.Y: Marcel Dekker, Inc.; 1996. pp. 241–262. [Google Scholar]
- 12.Meier A, Kirschner P, Springer B, Steingrube V A, Brown B A, Wallace R J, Jr, Bottger E C. Identification of mutations in 23S rRNA gene of clarithromycin-resistant Mycobacterium intracellulare. Antimicrob Agents Chemother. 1994;38:381–384. doi: 10.1128/aac.38.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nash K A, Inderlied C B. The genetic basis of macrolide resistance in Mycobacterium avium. Antimicrob Agents Chemother. 1995;3:2625–2630. doi: 10.1128/aac.39.12.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nightingale S D, Byrd L T, Southern P M, Jockusch J D, Cal S X, Wynne B. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165:1082–1085. doi: 10.1093/infdis/165.6.1082. [DOI] [PubMed] [Google Scholar]
- 15.Nightingale S D, Cameron D W, Gordin F M, Sullam P M, Cohn D L, Chaisson R, Eron L J, Sparti P D, Bihari B, Kaufman D L, Stern J J, Pearce D D, Winberg W G, LaMarca A, Siegal F P. Two placebo controlled trials of rifabutin prophylaxis against Mycobacterium avium complex infection in AIDS. N Engl J Med. 1993;329:828–833. doi: 10.1056/NEJM199309163291202. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Young D. Molecular mechanisms of isoniazid: a drug at the front line of tuberculosis control. Trends Microbiol. 1993;1:109–113. doi: 10.1016/0966-842x(93)90117-a. [DOI] [PubMed] [Google Scholar]