Abstract
The Coronavirus-disease 2019 (COVID-19) was declared as a global pandemic on March 11, 2020 by the World Health Organization. Since then, the scientific community has been actively engaged in developing a vaccine against the dreaded disease. Considerable research has also been performed for drugs that can directly interfere with the viral replication pathway. However, the production of these vaccines and drugs demands a lot of time and effort which is undesirable considering the pace at which the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is spreading across the continents. For this reason, the possible role of dietary nutrients in reducing the risk of SARS-CoV-2 infection as well as mitigating the symptoms, may be explored. These natural substances are readily available, have negligible side effects and confer several benefits to the immune system. Macronutrients like proteins are vital for antibody production. Dietary constituents such as omega-3-fatty acids, vitamin C, vitamin E, phytochemicals such as carotenoids and polyphenols exhibit anti-inflammatory and antioxidant properties. This review highlights the significance of relevant nutrients in boosting the immune system.
Keywords: Macronutrients, Micronutrients, Phytochemicals, Anti-inflammatory, Antioxidant
Abbreviations: ACE2, Angiotensin Converting Enzyme 2; ALA, α-linolenic acid; COVID-19, Coronavirus-disease 2019; CRP, C-reactive protein; DHA, docosahexanoic acid; EPA, eicosapentanoic acid; RDA, Recommended Dietary Allowance; ROS, reactive oxygen species; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus-2
1. Introduction
In December, 2019 several pneumonia-like cases were reported in the city of Wuhan in China. Later it was found that these were caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [1]. The enormous transmission rate of this novel strain SARS-CoV-2 resulted in the global pandemic of coronavirus disease 2019 also known as COVID-19. The SARS-CoV-2 is an enveloped, β-coronavirus, with a positive-sense, single-stranded RNA genome. The four major structural proteins of this virus are Spike protein (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N) [2]. The spike protein interacts with host cell membrane receptor Angiotensin Converting Enzyme 2 (ACE2) to enable the entry of the virus during infection [3]. The clinical manifestations of a SARS-CoV-2 infection comprise of either moderate symptoms such as fever, mild coughing and shortness of breath or severe complications including acute respiratory distress syndrome, cardiac complications, multiple organ dysfunction syndrome, septic shock, and eventually death [4]. The acute complications are triggered by a cytokine storm [5].
It has always been a challenge to combat the newly emerging viruses [6]. However, there is growing evidence highlighting the integral role of nutrition in boosting the immunity against this pathogenic invasion [7]. As per the nutritional guidelines laid down by the World Health Organization for adults, eating a well-balanced diet everyday enriched in vitamins, minerals, dietary fiber, proteins and antioxidants helps to keep the disease at bay [8]. This review gives an in-depth insight into the various dietary constituents such as macronutrients, micronutrients and phytochemicals that strengthen the immune system. Macronutrients refer to those nutrients which are required in large amounts in the diet such as proteins, lipids and carbohydrates [9]. Micronutrients such as vitamins and minerals are required only in small amounts by the body [10]. The phytochemicals like polyphenols and carotenoids are chemicals derived from plants whose nutritional significance will be discussed in detail in this review [11].
2. The nutritional significance of macronutrients
2.1. Proteins
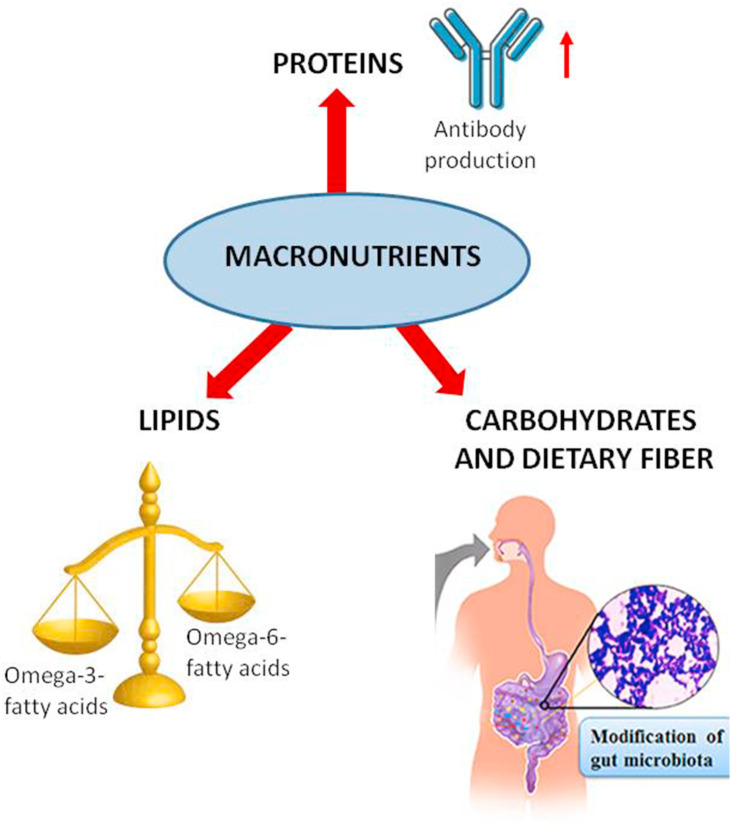
As per the recommended dietary allowance (RDA), on an average an individual needs 0.8 g/kg body weight. An adequate amount of protein intake is essential for antibody and complement production in the body [12] (Fig. 1 ). The protein consumed in the diet is broken down into amino acids which are then reassembled into antibodies and complement proteins that are the major players of the immune system. Amino acids play a major role in regulating the activation of macrophages, natural killer cells, B and T lymphocytes. They are important for the production of cytokines and cytotoxic substances [13]. Amino acids also regulate key metabolic pathways of the immune response against infectious pathogens. The normal sustainance of immunocompetence requires adequate dietary provision of all amino acids. A protein deficient diet leads to an impaired immunity which in turn is directly related to an increase in COVID-19 infection risk. Amino acids like arginine and glutamine are very important in stimulating the immune system. Proteins of high biological value which contain essential amino acids and from healthy dietary sources such as from eggs, poultry, meat and fish also constitute an essential component of an anti-inflammatory diet [14].
Fig. 1.
Nutritional significance of macronutrients.
2.2. Lipids
Fatty acids in the diet significantly influence the immune response. Omega-3 and omega-6 fatty acids are two essential fatty acids which need to be consumed in the diet as the human body cannot synthesize them. The omega-3 fatty acids largely comprise of α-linolenic acid (ALA) from plant sources and docosahexanoic acid (DHA) and eicosapentanoic acid (EPA) from fish and seafood sources. The dietary intake of these omega-3 fatty acids has been demonstrated to trigger anti-inflammatory reactions in the body [15]. On the other hand, omega-6 fatty acids such as arachidonic acid is a precursor of pro-inflammatory eicosanoids like prostaglandins and leukotrienes. High levels of omega-6 fats are found in refined vegetable oils and also in nuts and seeds. A healthy ratio of omega-6/omega-3 fatty acids is between 1:1–4:1. Omega-6 fats provide energy to the body but people should ideally consume more omega-3 fats [16] (Fig. 1). Omega-3 fatty acids change the composition of the phospholipid bilayer of the host cell membrane, thereby preventing viral entry. DHA and EPA get incorporated in the plasma membrane and affect the clumping of toll-like receptors. This results in prevention of signals that activate NF-κB, production of fewer pro-inflammatory mediators and eventually reduction in complications of COVID-19 infection. DHA and EPA also serve as precursors of resolvins D and E which reduce the production of pro-inflammatory mediators eventually resulting in a decrease in lung inflammation [17].
2.3. Carbohydrates and dietary fiber
Carbohydrate consumption has been reported to increase serotonin production, which in turn has a positive impact on a person's mood [18]. Thus, carbohydrate-rich foods have an anti-stress, self-medicating effect. However the amount of carbohydrates in the diet needs to be regulated as excess carbohydrates may lead to obesity, diabetes and heart diseases which may increase the complications due to COVID-19 infection [19].
Dietary fiber is the portion of plant-derived food or analogous carbohydrates that cannot be completely broken down by human digestive enzymes. The dietary fiber has been found to have a prebiotic effect such as fostering the growth of beneficial microbes like Bifidobacterium sp. and Lactobacillus sp. and inhibiting the pathogens like Clostridium sp [20] (Fig. 1). This is of considerable interest as recently COVID-19 has been associated with gastrointestinal disturbances in addition to respiratory symptoms [21].
3. The nutritional significance of micronutrients
3.1. Vitamins
Vitamin A plays a crucial role in maintaining the morphology of the epithelium as well as in formation of a healthy mucus layer of the respiratory and gastrointestinal tract [22] (Table 1 ). The two major forms of vitamin A in the diet include preformed vitamin A and provitamin A carotenoids. The active forms of vitamin A include retinal, retinol and retinoic acid. A low vitamin A status is correlated with a hampered function of macrophages, neutrophils, T-cells as well as B-cells [23]. The immunomodulatory role of vitamin A may play a crucial role in the fight against COVID-19 infection.
Table 1.
Nutritional significance of micronutrients.
| Name of vitamin/mineral | Function |
|---|---|
| Vitamin A | Maintains the lining of the respiratory and gastrointestinal tract thereby protecting against infection |
| Vitamin B | Important component of several coenzymes |
| Vitamin C | Classical antioxidant |
| Vitamin D | Regulator of innate and adaptive immune systems |
| Vitamin E | Modulates the TH1/TH2 balance |
| Zinc | Co-factor for various enzymes involved in antioxidant reactions |
| Selenium | Quenching of ROS |
| Iron | Important for T-cell proliferation and maturation |
B vitamins (B1, B2, B3, B5, B6, B7, B9, and B12) are water soluble vitamins which form an integral part of several coenzymes (Table 1). They play a crucial role in cell metabolism and participate in energy production [24]. Vitamin B plays a pivotal role in activation of both the innate and adaptive immune responses, down regulates the production of pro-inflammatory cytokines and inflammation and also improves respiratory function considerably [25]. B vitamins also reduce gastrointestinal problems, prevent hypercoagulability and reduce the duration of hospital stay for COVID-19 patients [24].
Vitamin C is a classical antioxidant which has a significant impact on the immune system. It influences the activity of phagocytes and T-lymphocytes (Table 1). Ascorbic acid in the diet has been reported to lower the concentration of C-reactive protein. Vitamin C has been postulated to exhibit antiviral activity by augmenting the production of interferon proteins [26]. Given the antioxidant and antiviral effect of vitamin C and its favorable safety profile it may be an effective choice for the treatment of COVID-19.
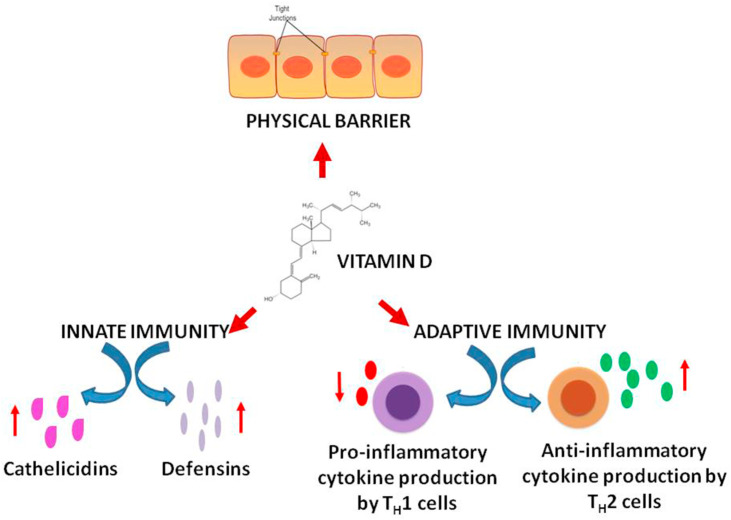
Vitamin D can be synthesized by the body in the presence of sunlight. The active form of vitamin D is calcitriol (1,25 dihydroxyvitamin D). It not only plays a regulatory role in calcium homeostasis and thus maintains bone health but also regulates the immune system. It has been proposed that vitamin D reduces the risk of microbial infections in three ways. Firstly, it augments the natural protective barriers by helping in the preservation of the tight junctions between epithelial cells. The disruption of these junctions is a pathogenic mechanism of the upper respiratory tract viruses. Secondly, vitamin D enhances the innate immunity by promoting the release of defensins and cathelicidins which have been demonstrated to exhibit antiviral eefects. Thirdly vitamin D boosts the adaptive immunity by reducing the production of pro-inflammatory cytokines by TH1 cells and increasing the production anti-inflammatory cytokines by TH2 cells [27] (Fig. 2 ). Low levels of vitamin D are reported in many adults at the end of the winter season due to a limited exposure to sunlight. There is an observed difference in mortality due to COVID-19 between Northern Hemisphere and Southern Hemisphere [28]. This indicates a possible role of vitamin D in governing the pathogenesis of COVID-19.
Fig. 2.
Role of vitamin D in boosting the immunity.
Vitamin E is a fat-soluble vitamin which includes both tocopherols and tocotrienols. It is a potent antioxidant. Vitamin E has been demonstrated to regulate the immune response by modulating the TH1/TH2 balance and initiation of T-lymphocyte signals [29] (Table 1). Vitamin E not only acts through anti-oxidant pathways to increase the number of T cells but it also increases mitogenic lymphocyte responses, IL-2 cytokine secretion, activity of natural killer cells and eventually decreases the risk of COVID-19 infection [30].
3.2. Minerals
3.2.1. Zinc
Zinc is an essential micronutrient which is required as a co-factor for many enzymes involved in antioxidant reactions (Table 1). The deficiency of zinc causes oxidative stress and also influences both the natural and acquired immunity. It has been reported that a low zinc status increases the risk of viral infections [31]. Several studies have shown that zinc interferes with the viral replication cycle by inhibiting viral uncoating, interfering with viral transcription, protein translation and polyprotein processing. It has been proposed that Zn+2 inhibits the replication of SARS-CoV by blocking the RNA synthesis via inhibition of the RNA-dependent RNA polymerase [32].
3.2.2. Selenium
Several antioxidant enzymes such as glutathione peroxidase, selenoprotein P, and thioredoxin reductase require the trace element selenium for their synthesis [33]. Thus, the primary role of selenium is its ability to act as an antioxidant and quench the reactive oxygen species (ROS) (Table 1). Dietary selenium deficiency has been associated with a high level of pathogenicity of several viruses [34,35]. The cure rate for COVID-19 was found to be significantly higher in patients with a high selenium intake in the diet [33]. Studies have proven that a supra nutritional levels of selenium might suppress not only the life cycle of SARS-CoV-2 but also its mutation to a more virulent form. This is because of the fact that both selenoproteins as well as redox-active selenium species in the selenium metabolic pool attenuate viral-induced oxidative stress, cytokine storm and organ damage. Another study has proven that sodium selenite oxidizes the thiol groups in the protein disulfide isomerase of the coronavirus, thereby rendering it incapable of penetrating the host cell membrane [35].
3.2.3. Iron
Iron homeostasis is tightly regulated during bacterial and viral infections. When there is an inflammatory response, iron absorption is decreased so as to limit the pool of iron available to the multiplying bacteria and viruses. However, some studies have demonstrated that during a prolonged period of iron deficiency, antibody production is considerably reduced. Iron also plays an important role in T-cell proliferation and maturation as well as regulation of cytokine production [36] (Table 1). A study has implicated the role of hyperferritinemia and an increased inflammatory state in COVID-19 pathogenesis [36].
3.3. Phytochemicals
3.3.1. Polyphenols
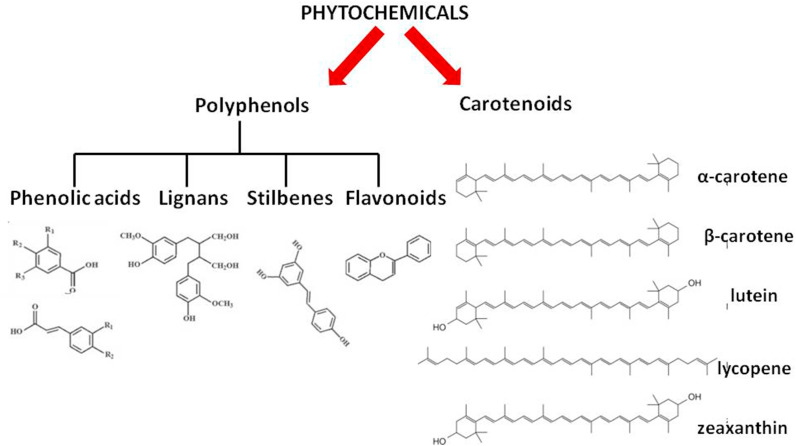
Polyphenols are phenolic compounds derived from plants which are enriched with antioxidant and anti-inflammatory properties. Dietary polyphenols are broadly classified into four groups which comprise of phenolic acids, lignans, stilbenes and flavonoids (Fig. 3 ). Several polyphenols, for example the flavonoid quercetin exhibits antiviral properties [37]. Quercetin has been reported to decrease viral infectivity and hampers intracellular viral replication [26]. There is a plethora of evidence highlighting the prebiotic effects of polyphenols on the gut. This may help to amend the dysbiosis of the gut microbiota reported to be triggered by SARS-CoV-2 infection. Curcuminoids have been shown to reduce the C-reactive protein (CRP) concentrations. Curcumin is the bioactive compound present in turmeric which has a multi-mechanistic mode of action. It can inhibit viral entry into the cell, encapsulation of the virus and viral proteases. It modulates various signaling pathways. Curcumin may play a beneficial role in treating the COVID-19 infection as it is capable of modulating various targets which are responsible for the attachment and internalization of SARS-CoV-2 in many organs such as kidney, liver and the cardiovascular system. It may also suppress fibrosis-associated pathways and pulmonary edema which are triggered in COVID-19 infection [38,39].
Fig. 3.
Classification of phytochemicals.
3.3.2. Carotenoids
Carotenoids are tetraterpenoids. They include orange, red and yellow organic pigments produced by plants, algae and bacteria. Some of the common carotenoids are α- and β-carotene, lutein, zeaxanthin and lycopene (Fig. 3). Carotenoids are well known for their antioxidant properties and quenching of ROS. Thus, low levels of carotenoids are associated with oxidative stress. Antiviral roles of lutein, carotene and zeaxanthin have been reported. Some carotenoids serve as a precursor of vitamin A which is directly associated with immunomodulating functions [40]. It has been reported that carotenoids alleviate the inflammatory responses that lead to lung damage during COVID-19 infection [41].
4. The nutritional significance of probiotics
Some patients with COVID-19 have been reported to show intestinal microbial dysbiosis which refers to an imbalance in the composition and function of the intestinal microorganisms. This highlights the involvement of the gut–lung axis in SARS-CoV-2 infection. Thus, for maintenance of health and prevention or treatment of the disease manipulation of the intestinal microbiota is often proposed as a potential approach [42]. This can be done by either stimulating the colonisation of the gastrointestinal tract by beneficial bacteria or by administration of probiotics. Probiotics are living microorganisms which confer health benefits to the host when administered in adequate amounts. They have been reported to boost the immune system. They also strengthen the integrity of the junction and maintain the morphology of the epithelium of the respiratory and gastrointestinal tract thereby reducing the risk of entry of SARS-CoV-2. Studies have reported a reduction in both upper and lower respiratory tract infections upon treatment with probiotics. Several probiotics such as lactic acid bacteria (LAB) have been shown to produce peptides which inhibit ACE2, the binding site of SARS-CoV-2 [43]. Thus, probiotics may serve as an adjunctive therapy in the alleviation of COVID-19.
5. Energy requirement in case of critically ill as well as non-critical patients suffering from COVID-19
Calories come from carbohydrates (1 g yields 4 calories), fats (1 g gives 9 calories) and proteins (1 g yields 4 calories) [12]. Non protein calorie refers to the combined energy from lipids and carbohydrates. As protein is the only macronutrient which provides nitrogen, non protein calorie-to-nitrogen ratio (NPC:N) may be used to assess whether nitrogen or protein intake is sufficient to maintain muscle tissue. It describes the balance between energy and protein. Proteins are composed of 16% nitrogen. A NPC:N ratio of 150:1 to 200:1 is adequate for stable patients. However for critically ill patients or those who are having difficulty in maintaining muscle mass, a higher protein intake is optimal and a NPC:N ratio of 100:1 or lesser is desirable [44].
About 5% of patients suffering from COVID-19 develop an acute respiratory distress syndrome (ARDS) and require respiratory support in critical care units. As per the clinical guidelines, nutritional support should be started within the first 48 h of admission to the intensive care unit (ICU) along with enteral nutrition (EN) [44]. By the end of the first week of admission to the ICU, the caloric and protein target should be reached. In case the patient is incompatible with EN, parenteral nutrition (PN) should be started after 3rd to 7th day of admission to the ICU. One such study was conducted in a tertiary hospital in Madrid which was severely hit by the pandemic in the first wave [45]. In that retrospective study, spanning a period from March to May, 2020, medical nutrition treatment (MNT) was given to critically ill patients with COVID-19 and the clinical outcome was monitored closely. The variables recorded comprised of age, sex, body mass index (BMI), comorbidities mainly like obesity, hypertension, diabetes and date of admission to the ICU [46]. The type of respiratory support being provided such as invasive mechanical ventilation (IMV), high flow nasal cannula (HFNC) or non invasive ventilation (non-IMV) was noted. The caloric or energy requirement was calculated by 25 kcal/kg adjusted body weight (ABW) and the protein requirement as 1.3 g/kg ABW/day. On the 4th and 7th day of admission to the ICU, energy and protein administered were calculated. This also took into account the calories provided in the form of propofol administration. The MNT type provided was either EN, PN or mixed EN + PN. Patients were also closely monitored for metabolic complications and acute kidney failure (AKF) [47]. Eventually, their length of the stay (LOS) at the hospital or the mortality was recorded. A total of 176 patients were included in the study. Majority of them met the energy and protein requirements during the first week of admission to the ICU by the use of PN or PN + EN. Thus, PN can be used safely alongwith EN in critically ill patients with COVID-19 by closely monitoring them for metabolic complications [47].
Malnutrition has harmful consequences on COVID-19 patients. Thus, nutritional care of these patients cannot be overlooked. Early nutritional supplementation of non-critically ill COVID-19 patients who have been hospitalized is also very crucial. The nutritional status of these patients should be enhanced at the time of admission itself [48]. Nutritional support, mainly protein intake should be provided in the form of oral nutritional supplements (ONS). COVID-19 patients should have an adequate protein intake of 1.5 g/d and calorie intake of 25–30 kcal/d. At the time of admission, a high calorie dense diet of different consistensies and which is readily digestible should be administered to the patient. This should be accompanied with oral supplementation of whey proteins as well as intravenous infusion of multivitamins and multimineral trace elements solutions in physiological saline [49]. If the patient exhibits hydroxy vitamin D deficiency, cholecalciferol should be administered. If the patient is unable to eat or incompatible with ONS then parenteral nutrition is prescribed [48].
6. Future directions
This review focuses on the interactions of the various macronutrients, micronutrients and phytochemicals with the immune system and its relevance during the COVID-19 crisis. Research indicates that a high protein diet facilitates antibody production [12]. A diet enriched in omega-3 fatty acids, vitamins and minerals is highly recommended during this pandemic situation (Table 2 ). In the present-day scenario where many research groups are actively engaged in developing vaccines and novel therapeutics against COVID-19, a balanced nutrient status which reduces inflammation and oxidative stress may strengthen the immune system and thus may be the key to tackle the current situation. Several studies have shown that nutraceuticals have the ability to boost immunity and exhibit antiviral, antioxidant and anti-inflammatory effects. These comprise of vitamin C and D, zinc, selenium, curcumin, cinnamaldehyde, quercetin, lactoferrin, probiotics and several other natural compounds. Some of these phytonutrients may be grouped in correct combination to formulate a food supplement which in turn can not only boost immunity but also prevent viral spread [11]. Thus, nutraceuticals provide both prophylactic as well as therapeutic support against COVID-19.
Table 2.
Important dietary constituents which boost the immune system and their food sources.
| Dietary constituent | Food source |
|---|---|
| A. Macronutrients | |
| 1. Proteins | Egg white, beef, chicken, milk, dairy products, yogurt, soybeans |
| 2. Lipids | Avocado, Tuna fish, Salmon |
| 3. Carbohydrates | Figs, blueberries, whole-wheat bread |
| 4. Dietary fiber | Lentils, chickpeas, orange |
| B. Micronutrients | |
| 1. Vitamins | |
| i) Vitamin A | Carrots, mango, Salmon, eggs |
| ii) B vitamins (B1, B2, B3, B5, B6, B7, B9, and B12) | Peanuts, plain yogurt, lentils, Tuna fish |
| iii) Vitamin C | Oranges, lemon, broccoli, cauliflower |
| iv) Vitamin D | Chicken, egg, low fat yogurt, Salmon |
| v) Vitamin E | Sunflower seeds, nuts, almonds, kiwi |
| 2. Minerals | |
| i) Zinc | Nuts, pumpkin seeds, beef, lamb |
| ii) Selenium | Sunflower seeds, salmon, ham |
| iii) Iron | Dried apricots, cherry tomatoes, peas |
| C. Phytochemicals | |
| 1. Polyphenols | Oranges, blueberries, strawberries |
| 2. Carotenoids | Tomatoes, spinach, cantaloupe |
Authorship statement
F. Calcuttawala conceptualized and drafted the original manuscript and also reviewed and edited the vinal version.
Funding
No funding was received to assist with the preparation of this manuscript.
Declaration of competing interest
The author has no conflicts of interest to declare that are relevant to the content of this article.
Acknowledgements
I would like to acknowledge Abdulkadir Raja for providing valuable assistance in writing this article.
References
- 1.Wu D., Wu T., Liu Q., Yang Z. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang K., Gheblawi M., Oudit G.Y. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020;142(5):426–428. doi: 10.1161/CIRCULATIONAHA.120.047049. [DOI] [PubMed] [Google Scholar]
- 4.Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., et al. Coronavirus disease 2019-COVID-19. Clin Microbiol Rev. 2020;33(4) doi: 10.1128/CMR.00028-20. e00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H., Zhou Y., Zhang M., Wang H., Zhao Q., Liu J. Updated approaches against SARS-CoV-2. Antimicrob Agents Chemother. 2020;64(6) doi: 10.1128/AAC.00483-20. e00483-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iddir M., Brito A., Dingeo G., Fernandez Del Campo S.S., Samouda H., La Frano M.R., et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12(6):1562. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.BourBour F., Mirzaei Dahka S., Gholamalizadeh M., Akbari M.E., Shadnoush M., Haghighi M., et al. Nutrients in prevention, treatment, and management of viral infections; special focus on Coronavirus. Arch Physiol Biochem. 2020:1–10. doi: 10.1080/13813455.2020.1791188. [DOI] [PubMed] [Google Scholar]
- 9.Nassar M.F. The macronutrients' interplay. Clin Nutr. 2019;38(6):2943–2944. doi: 10.1016/j.clnu.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Gasmi A., Tippairote T., Mujawdiya P.K., Peana M., Menzel A., Dadar M., et al. Micronutrients as immunomodulatory tools for COVID-19 management. Clin Immunol. 2020;220:108545. doi: 10.1016/j.clim.2020.108545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mrityunjaya M., Pavithra V., Neelam R., Janhavi P., Halami P.M., Ravindra P.V. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front Immunol. 2020;11:570122. doi: 10.3389/fimmu.2020.570122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amaral J.F., Foschetti D.A., Assis F.A., Menezes J.S., Vaz N.M., Faria A.M. Immunoglobulin production is impaired in protein-deprived mice and can be restored by dietary protein supplementation. Braz J Med Biol Res. 2006;39(12):1581–1586. doi: 10.1590/s0100-879x2006001200009. [DOI] [PubMed] [Google Scholar]
- 13.Li P., Yin Y.L., Li D., Kim S.W., Wu G. Amino acids and immune function. Br J Nutr. 2007;98(2):237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- 14.Kostovcikova K., Coufal S., Galanova N., Fajstova A., Hudcovic T., Kostovcik M., et al. Diet rich in animal protein promotes pro-inflammatory macrophage response and exacerbates colitis in mice. Front Immunol. 2019;10:919. doi: 10.3389/fimmu.2019.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahidi F., Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol. 2018;9:345–381. doi: 10.1146/annurev-food-111317-095850. [DOI] [PubMed] [Google Scholar]
- 16.Radzikowska U., Rinaldi A.O., ÇelebiSözener Z., Karaguzel D., Wojcik M., Cypryk K., et al. The influence of dietary fatty acids on immune responses. Nutrients. 2019;11(12):2990. doi: 10.3390/nu11122990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hathaway D., Pandav K., Patel M., Riva-Moscoso A., Singh B.M., Patel A., et al. Omega 3 fatty acids and COVID-19: a comprehensive review. Infect Chemother. 2020;52(4):478–495. doi: 10.3947/ic.2020.52.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wurtman R.J., Wurtman J.J., Regan M.M., McDermott J.M., Tsay R.H., Breu J.J. Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am J Clin Nutr. 2003;77(1):128–132. doi: 10.1093/ajcn/77.1.128. [DOI] [PubMed] [Google Scholar]
- 19.Muscogiuri G., Barrea L., Savastano S., Colao A. Nutritional recommendations for CoVID-19 quarantine. Eur J Clin Nutr. 2020;74(6):850–851. doi: 10.1038/s41430-020-0635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L., Tu L. Implications of gastrointestinal manifestations of COVID-19. Lancet Gastroenterol Hepatol. 2020;5(7):629–630. doi: 10.1016/S2468-1253(20)30132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCullough F.S., Northrop-Clewes C.A., Thurnham D.I. The effect of vitamin A on epithelial integrity. Proc Nutr Soc. 1999;58(2):289–293. doi: 10.1017/s0029665199000403. [DOI] [PubMed] [Google Scholar]
- 23.Stephensen C.B. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 24.Shakoor H., Feehan J., Mikkelsen K., Al Dhaheri A.S., Ali H.I., Platat C., et al. Be well: a potential role for vitamin B in COVID-19. Maturitas. 2020;144:108–111. doi: 10.1016/j.maturitas.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jovic T.H., Ali S.R., Ibrahim N., Jessop Z.M., Tarassoli S.P., Dobbs T.D., et al. Could vitamins help in the fight against COVID-19? Nutrients. 2020;12(9):2550. doi: 10.3390/nu12092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ColungaBiancatelli R.M.L., Berrill M., Catravas J.D., Marik P.E. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg M., Al-Ani A., Mitchell H., Hendy P., Christensen B. Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North-supports vitamin D as a factor determining severity. Authors' reply. Aliment Pharmacol Ther. 2020;51(12):1438–1439. doi: 10.1111/apt.15796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee G.Y., Han S.N. The role of vitamin E in immunity. Nutrients. 2018;10(11):1614. doi: 10.3390/nu10111614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shakoor H., Feehan J., Al Dhaheri A.S., Ali H.I., Platat C., Ismail L.C., et al. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19? Maturitas. 2021;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy A., Sarkar B., Celik C., Ghosh A., Basu U., Jana M., et al. Can concomitant use of zinc and curcumin with other immunity-boosting nutraceuticals be the arsenal against COVID-19? Phytother Res. 2020;34(10):2425–2428. doi: 10.1002/ptr.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander J., Tinkov A., Strand T.A., Alehagen U., Skalny A., Aaseth J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12(8):2358. doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J., Saad R., Taylor E.W., Rayman M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37:101715. doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guillin O.M., Vindry C., Ohlmann T., Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11(9):2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kieliszek M., Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19) Med Hypotheses. 2020;143:109878. doi: 10.1016/j.mehy.2020.109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edeas M., Saleh J., Peyssonnaux C. Iron: innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis. 2020;97:303–305. doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy E., Delvin E., Marcil V., Spahis S. Can phytotherapy with polyphenols serve as a powerful approach for the prevention and therapy tool of novel coronavirus disease 2019 (COVID-19)? Am J Physiol Endocrinol Metab. 2020;319(4):E689–E708. doi: 10.1152/ajpendo.00298.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocha F.A.C., de Assis M.R. Curcumin as a potential treatment for COVID-19. Phytother Res. 2020;34(9):2085–2087. doi: 10.1002/ptr.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zahedipour F., Hosseini S.A., Sathyapalan T., Majeed M., Jamialahmadi T., Al-Rasadi K., et al. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. 2020;34(11):2911–2920. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chew B.P., Park J.S. Carotenoid action on the immune response. J Nutr. 2004;134(1):257S–261S. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- 41.Khalil A., Tazeddinova D., Aljoumaa K., Kazhmukhanbetkyzy Z.A., Orazov A., Toshev A.D. Carotenoids: therapeutic strategy in the battle against viral emerging diseases, COVID-19: an overview. Prev Nutr Food Sci. 2021;26(3):241–261. doi: 10.3746/pnf.2021.26.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akour A. Probiotics and COVID-19: is there any link? Lett Appl Microbiol. 2020;71(3):229–234. doi: 10.1111/lam.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bottari B., Castellone V., Neviani E. Probiotics and covid-19. Int J Food Sci Nutr. 2021;72(3):293–299. doi: 10.1080/09637486.2020.1807475. [DOI] [PubMed] [Google Scholar]
- 44.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miguélez M., Velasco C., Camblor M., Cedeño J., Serrano C., Bretón I., et al. Nutritional management and clinical outcome of critically ill patients with COVID-19: a retrospective study in a tertiary hospital. Clin Nutr. 2021;S0261–5614(21):499–504. doi: 10.1016/j.clnu.2021.10.020. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomes F., Schuetz P., Bounoure L., Austin P., Ballesteros-Pomar M., Cederholm T., et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr. 2018;37(1):336–353. doi: 10.1016/j.clnu.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Doig G.S., Simpson F., Sweetman E.A., Finfer S.R., Cooper D.J., Heighes P.T., et al. Early PN Investigators of the ANZICS Clinical Trials Group. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA. 2013;309(20):2130–2138. doi: 10.1001/jama.2013.5124. [DOI] [PubMed] [Google Scholar]
- 48.Caccialanza R., Laviano A., Lobascio F., Montagna E., Bruno R., Ludovisi S., et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74:110835. doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng T.B., Cheung R.C., Wong J.H., Wang Y., Ip D.T., Wan D.C., et al. Antiviral activities of whey proteins. Appl Microbiol Biotechnol. 2015;99(17):6997–7008. doi: 10.1007/s00253-015-6818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]