Abstract
Objective
We aimed to investigate the immunogenicity and safety of SpikoGen®, a subunit COVID-19 vaccine composed of a recombinant prefusion-stabilized SARS-CoV-2 spike protein combined with the Advax-CpG55.2™ adjuvant, in seronegative and seropositive populations as primary vaccination.
Methods
This randomized, placebo-controlled, double-blind phase 2 trial was conducted on 400 participants randomized 3:1 to receive two doses of 25 μg of SpikoGen® 3 weeks apart or the placebo. The primary safety outcomes were the incidence of solicited adverse events up to 7 days after each dose and unsolicited adverse events up to 28 days after the second dose. The primary immunogenicity outcomes were seroconversion against the S1 protein and the geometric mean concentration of S1 antibodies by days 21 and 35.
Results
The SpikoGen® vaccine was well tolerated and no serious adverse events were recorded. The most common solicited adverse events were injection site pain and fatigue, largely graded as mild and transient. By day 35 (2 weeks post second dose), the seroconversion rate against S1 was 63.55 (95% CI: 57.81–69.01) in the SpikoGen® group versus 7.23 (95% CI: 2.7–15.07) in the placebo group. The geometric mean concentration of S1 antibodies was 29.12 (95% CI: 24.32–34.87) in the SpikoGen® group versus 5.53 (95% CI: 4.39–6.97) in the placebo group. Previously infected seropositive volunteers showed a large SARS-CoV-2 humoral response after a single SpikoGen® dose.
Discussion
SpikoGen® had an acceptable safety profile and induced promising humoral and cellular immune responses against SARS-CoV-2.
Keywords: SpikoGen, Phase 2, Subunit protein vaccine, SARS-CoV-2, COVID-19, Advax-CpG
Introduction
Recombinant subunit protein vaccines are generally safe and well tolerated but typically require an adjuvant to increase the magnitude, quality, and persistence of the vaccine responses [1]. Alum adjuvants have largely been used for stimulation of immune response successfully. However, there are some concerns in the literature regarding the use of these adjuvants including T helper 2 (Th2) polarized immune responses [2] rather than the Th1 responses. Consequently, a variety of adjuvants have been used in COVID-19 vaccines under development including the traditional aluminum salts (alum) formulated with CpG [3,4] and saponin-based adjuvants [5]. NVX-CoV2373 containing Matrix-M saponin-based adjuvant showed efficacy against mild to severe COVID-19 in clinical trials [5,6]. Another trimeric spike protein vaccine namely SCB 2019 combined with CPG-Alum adjuvant has recently finished its phase 3 clinical trial. It was shown that vaccine had considerable protection against COVID-19 as well as acceptable safety profile [7].
Advax-CpG adjuvant is a combination of delta inulin polysaccharide [8] formulated with CpG oligonucleotide, a toll-like receptor 9 (TLR9) agonist that has been shown to enhance humoral and T cell responses in a broad range of animal species as well as humans, having low reactogenicity and a strong safety profile [[9], [10], [11]]. It is effective and well-tolerated in humans and has been shown to be highly effective and safe in newborn [12,13] and pregnant mice [14,15]. CpG55.2 is a proprietary 24mer class B CpG oligonucleotide that is a potent activator of both human and mouse TLR9.
The S protein extracellular domain antigen in SpikoGen® vaccine was designed using 3-D computer modeling [11] plus experience from SARS [16] and MERS [17] coronavirus vaccines. In mice, SpikoGen® induced high titers of neutralizing antibodies and memory CD4+ and CD8+ T cell responses [11]. An Australian Phase 1 trial in July 2020 involving 40 participants confirmed a satisfactory safety profile with induction of anti-S1 antibodies. The current phase 2 study was undertaken to provide additional safety and immunogenicity data on SpikoGen® vaccine in a larger population to support its advance to phase 3 clinical trial.
Methods
Study design
This study was a phase 2, parallel, randomized, double-blind, placebo-controlled trial on 400 participants with a 3:1 allocation ratio and a follow-up duration of 6 months after the second dose. The study was conducted at the Grand Hall of Espinas Palace Hotel, Tehran, Iran. This place was converted into a custom-built large clinical trial site for the duration of the study. A link for video of the clinical trial site and the inclusion and exclusion criteria are provided in the supplementary material. Written informed consent was obtained from all participants before enrollment. Vital signs including heart rate, respiratory rate, blood pressure, temperature, and O2 saturation were assessed before each dose.
The study was done in compliance with the International Conference on Harmonization's guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki with funding provided by CinnaGen Co. The study was approved by the ethics committee of the Iran National Committee for Ethics in Biomedical Research (ethics code number: IR.NREC.1400.002). The trial was registered at ClinicalTrials.gov with the registration code NCT04944368 and with the Iranian Registry of Clinical Trials registration code IRCT20150303021315N23.
Randomization and intervention
Eligible participants were randomly assigned to each group using a stratified randomization with 3:1 allocation ratio by R-CRAN-version 4.0.1. Participants were stratified by age and comorbidities, including respiratory disorders, cardiovascular disorders, body mass index of equal or greater than 40 kg/m2, diabetes, and liver disorders. Three strata were designed based on these factors: age <65 years without comorbidities, age <65 years with comorbidities, and age ≥65 years. Once the randomization was made, each patient was given an identification code throughout the study.
Consecutive allocation of the randomization code to the participants was concealed by use of the unblinded pharmacists who prepared the investigational product in a secure location that was not accessible or visible to other study staff. The vaccine preparer was unmasked but played no role in the data assessment, and the vaccine administrators, participants, and outcome assessors were blinded.
SpikoGen® vaccine comprised 25 μg recombinant spike protein extracellular domain with Advax-CpG55.2 adjuvant (15.5 mg Advax, 171 μg CPG) and was administered in two doses, 21 days apart. The full details are provided in the supplementary material.
Outcomes
The primary safety outcomes were the incidence of local and systemic solicited adverse events for 7 days after each dose and the incidence of unsolicited adverse events up to 28 days after the second dose. The primary immunogenicity outcomes were seroconversion rate against S1 protein and the geometric mean concentration (GMC) of S1 IgG in the two groups on days 21 and 35. The secondary immunogenicity outcomes included seroconversion rate, GMC, and geometric mean fold rise (GMFR) of antibodies against receptor binding domain (RBD) and neutralizing antibodies on days 21 and 35. GMC of IgA against S1 and T-cell responses were also assessed as secondary outcomes.
Follow-up and outcome assessment
Participants were instructed to use the electronic diaries at Visit 1. They completed the electronic diaries for any solicited adverse events (local and systemic reactions) on a daily basis for 7 days after each dose. Unsolicited adverse events were collected for 28 days after the second injection. Follow-up telephone calls were continued through day 201. Serious adverse events (SAEs) and suspected unexpected serious adverse reactions (SUSARs) were collected from day 0 (Visit 1) through day 201 (6 months after the second injection).
Blood samples were taken to evaluate any potential abnormalities in blood biochemistries at baseline and day 35. The adverse events reporting system was based on an electronic application. Safety outcomes were reported based on Medical Dictionary for Regulatory Activities classification. Each participant's severity score was assessed based on the Food and Drug Administration (FDA) toxicity grading scale [18]. Causality of the adverse events was also assessed.
Seronegative population was defined as being negative in the whole antibodies including IgG against S1, RBD, nucleocapsid protein, surrogate neutralizing antibodies, and IgA against S1 and RBD at baseline. Seropositivity was defined as being positive for at least a specific antibody class (IgG or IgA) against S1, RBD, nucleocapsid, or surrogate neutralizing antibodies at baseline. In the seronegative population, seroconversion was defined as a change in the status of antibody levels from negative to positive based on the prespecified threshold. In the seropositive population, seroconversion was defined as a fourfold increase in the antibody levels in comparison to baseline. Neutralizing antibodies were assessed using two methods: a surrogate virus neutralization test (sVNT) and a conventional virus neutralization test (cVNT). The details are provided in the supplementary material.
The whole blood samples of 75 study participants (56 from the SpikoGen® group and 19 from the placebo group) were collected randomly in the four blood collection tubes of the QuantiFERON SARS-CoV-2 RUO (Qiagen, Germany) toolset for assessing interferon-gamma release assay in the T cell response. Peripheral blood mononuclear cells were obtained by the Ficoll gradient method from whole blood samples taken from 18 study participants (14 from the SpikoGen® group and 4 from the placebo group) to assess the percentages of proliferating CD4+ and CD8+ cells. The details are provided in the supplementary material.
Statistical analysis
Sample size was not calculated based on the statistical power. Four hundred participants were chosen based on the FDA guidelines for number of participants needed for different phases of the vaccine trials [19]. Participants who received at least one dose of the vaccine were considered in the safety population. Safety was presented as counts and percentages of participants who had experienced solicited (local and systemic events), unsolicited, and follow-up adverse events. The immunogenicity objectives were reported based on the per-protocol set in which all randomly assigned participants received at least one dose of the vaccine and did not present any major protocol deviation. Participants who became infected with SARS-CoV-2 were excluded from the per-protocol population.
Continuous data were compared using t-test, and categorical data were assessed using chi-square or Fisher exact test. Hypothesis testing was two-sided, and we considered p values of less than 0.05 as significant. Participants with seroconversion due to vaccination were provided with two-sided 95% CI using the Clopper-Pearson method. The 95% CIs for GMC and GMFR were calculated based on the t-distribution of the log-transformed values and then back transformed into the original scale at each time point for presentation.
Wilcoxon tests were used to compare the paired samples to evaluate the percentages of CD4+/CD8+ cells proliferation in both groups. An ANCOVA model was performed to compare interferon-gamma concentrations between the two groups 21 and 35 days after first dose. The ANCOVA model adjusted for groups and baseline values of interferon-gamma. The Mann-Whitney U test was used to compare the increases of interferon-gamma between the two groups on days 21 and 35. A subgroup analysis of seronegative and seropositive participants at baseline was also performed. We used R (version 3.6.0) and STATA 14 for all statistical analyses.
Results
Study participants
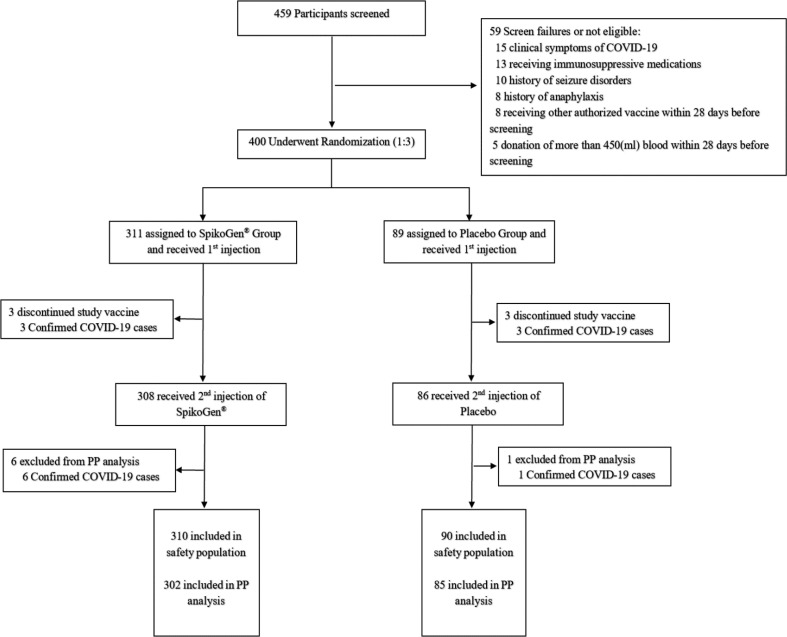
The trial was initiated on 30 May 2021. In total, 311 participants were randomized to the SpikoGen® group and 89 participants to the placebo group. Screening and randomization process of the participants are provided in the CONSORT diagram in Fig. 1 . Demographic data and past medical history of the participants are presented in Table 1 .
Fig. 1.
Flowchart of screening, randomization, and analysis of the participants. There was an individual who received an incorrect injection during the study. This volunteer in the SpikoGen® group received a single dose of placebo by error and did not receive the vaccine. The participant was included in the placebo safety population. This participant also developed COVID-19 and therefore was excluded from the Per Protocol analysis.
Table 1.
Demographic and clinical characteristics of the participants at baseline
| Characteristics | SpikoGen® n = 311 | Placebo n = 89 |
|---|---|---|
| Sex — n (%) | ||
| Male | 165 (53) | 50 (56) |
| Female | 146 (47) | 39 (44) |
| Body-mass index (kg/m2)a — mean ± SD | 25.94 ± 4.22 | 25.81 ± 4.12 |
| Age (y) — mean ± SD | 35.69 ± 9.65 | 35.69 ± 8.85 |
| Age category and risk for severe Covid-19 — n (%) | ||
| <65 years without comorbiditiesb | 249 (80) | 70 (79) |
| <65 years with comorbidities | 56 (18) | 18 (20) |
| ≥65 years | 6 (2) | 1 (1) |
| Baseline SARS-CoV-2 statusc — n (%) | ||
| Negative | 167 (54) | 52 (58) |
| Positive | 144 (46) | 37 (42) |
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Comorbidities included: respiratory disorders, cardiovascular disorders, body mass index of equal or greater than 40 kg/m2 diabetes and liver disorders.
Baseline SARS-CoV-2 status was positive if there was immunologic evidence of previous illness with COVID-19, as defined by a positive Antibody (IgG or IgA) status against SARS-CoV-2 nucleocapsid, S1, RBD, or surrogate neutralizing antibodies at baseline. Baseline SARS-CoV-2 status was negative if there was a negative immunogenicity test against these whole proteins
Safety outcomes
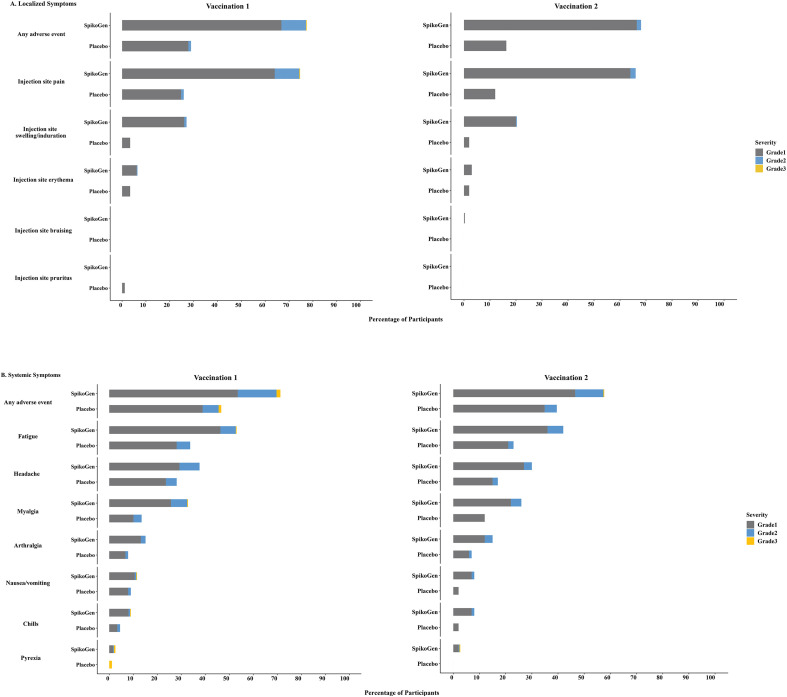
Overall, the vaccine was well tolerated with the majority of adverse events being graded as mild, although occurring at a higher rate in the SpikoGen® group when compared to the placebo group. Fig. 2 shows the overall incidence of local and systemic solicited AEs between the participants of each group.
Fig. 2.
Solicited local and systemic AEs. The percentage of participants in each group (SpikoGen®, Placebo) with Adverse Events (AEs) according to the maximum Food and Drug Administration (FDA) toxicity grading scale during the 7 days after each vaccination is plotted for solicited local (Panel A) and systemic (Panel B) adverse events. There was no grade 4 (life-threatening) event.
No serious AEs or hospitalization due to AEs were reported. The full data regarding the incidence of unsolicited AEs, adverse events reported in the follow-up period, grading, and the causality assessments of the AEs are provided in the supplementary material (Tables S1, S2, S3, and S4). There were no abnormalities in the blood biochemistries between days 0 and 35 in either group. (Table S5 in the supplementary material).
Immunogenicity outcomes
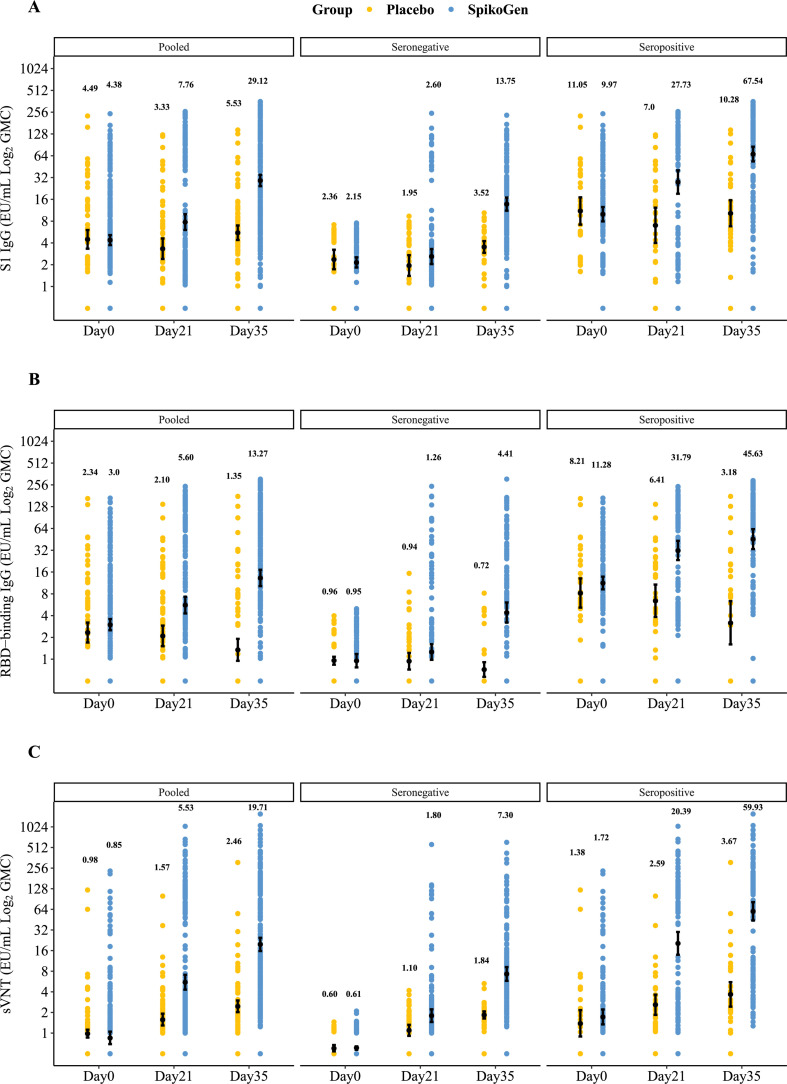
Spike antibody levels including S1, RBD, and neutralizing antibodies (sVNT) rose strongly in the SpikoGen® group 14 days after the second injection (Fig. 3 ). Baseline seropositive participants showed around a two-to threefold higher seroconversion rate to S1, RBD, and neutralizing antibodies (sVNT) after the first vaccination as compared to the seronegative population (Table 2 ).
Fig. 3.
Shown are geometric mean concentrations (GMCs) in the per-protocol set for S1 IgG responses (Panel A), receptor binding domain IgG responses (Panel B), and surrogate virus neutralization test responses (Panel C) at day 21 (day of the second injection) and day 35 (14 days after the second injection). Antibody values below the lower limit of quantification (LLOQ) were replaced by 0.5 × LLOQ. The 95% CI was calculated based on the t-distribution of the log-transformed values for GMC levels, then back transformed to the original scale for presentation. For each group, geometric means are depicted above the scatterplot.
Table 2.
Seroconversion rate of S1 IgG, RBD IgG, surrogate neutralizing IgG and S1 IgA in the participants
| Pooled |
Seronegative |
Seropositive |
||||
|---|---|---|---|---|---|---|
| SpikoGen® | Placebo | SpikoGen® | Placebo | SpikoGen® | Placebo | |
| sVNT | ||||||
| Day 21 SCR 95% CI | 128/307 (41.69) (36.12–47.43) | 15/86 (17.44) (10.10–27.13) |
40/165 (24.24) (17.92–31.52) |
5/50 (10) (3.33–21.81) |
88/142 (61.97) (53.45–69.98) |
10/36 (27.78) (14.20–45.19) |
| Day 35 SCR 95% CI |
233/299 (77.93) (72.79–82.50) |
17/83 (20.48) (12.41–30.76) |
111/158 (70.25) (62.47–77.25) |
4/48 (8.33) (2.32–19.98) |
122/141 (86.52) (79.76–91.69) |
13/35 (37.14) (21.47–55.08) |
| S1-IgG | ||||||
| Day 21 SCR 95% CI |
89/307 (28.99) (23.98–34.41) |
2/86 (2.33) (0.28–8.15) |
20/165 (12.12) (7.56–18.10) |
1/50 (2) (0.05–10.65) |
69/142 (48.59) (40.13–57.12) |
1/36 (2.78) (0.07–14.53) |
| Day 35 SCR 95% CI |
190/299 (63.55) (57.81–69.01) |
6/83 (7.23) (2.70–15.07) |
91/158 (57.59) (49.49–65.41) |
4/48 (8.33) (2.32–19.98) |
99/141 (70.21) (61.94–77.62) |
2/35 (5.71) (0.70–19.16) |
| RBD-IgG | ||||||
| Day 21 SCR 95% CI |
92/307 (29.97) (24.90–35.43) |
6/86 (6.98) (2.60–14.57) |
26/165 (15.76) (10.56–22.23) |
3/50 (6) (1.25–16.55) |
66/142 (46.48) (38.07–55.03) |
3/36 (8.33) (1.75–22.47) |
| Day 35 SCR 95% CI |
162/299 (54.18) (48.35–59.93) |
4/83 (4.82) (1.33–11.88) |
81/158 (51.27) (43.20–59.29) |
2/48 (4.17) (0.51–14.25) |
81/141 (57.45) (48.85–65.73) |
2/35 (5.71) (0.70–19.16) |
| S1-IgA | ||||||
| Day 21 SCR 95% CI |
71/307 (23.13) (18.52–28.25) |
1/86 (1.16) (0.03–6.31) |
13/165 (7.88) (4.26–13.10) |
0/50 (0) (0.03–7.11) |
58/142 (40.85) (32.68–49.40) |
1/36 (3) (0.07–14.53) |
| Day 35 SCR 95% CI |
92/299 (30.77) (25.58–36.34) |
1/83 (1.20) (0.03–6.53) |
24/158 (15.19) (9.98–21.75) |
0/48 (0) (0–7.40) |
68/141 (48.23) (39.74–56.79) |
1/35 (2.86) (0.07–14.92) |
CI, confidence interval; cVNT, conventional virus neutralization test; SCR, seroconversion rate; sVNT, surrogate virus neutralization test; RBD, receptor binding domain.
Percentages for seroconversion rate (SCR) were calculated as the number of participants who reported the event divided by the number of participants in the Per-Protocol Set within each visit multiply 100. The 95% confidence interval (CI) for SCR was calculated using the exact Clopper-Pearson method.
The full results of GMFRs and GMCs of each measured antibody are provided in the supplementary material (Tables S6 and S7).
The results of the conventional VNT are shown in Table 3 . The absolute effect of SpikoGen® on cVNT responses was calculated to be 82% in the pooled population.
Table 3.
cVNT seroconversion rate in the participants
| Pooled |
Seronegative |
|||
|---|---|---|---|---|
| SpikoGen® | Placebo | SpikoGen® | Placebo | |
| Day 21 SCR 95% CI | 115/303 (37.95) (32.47–43.68) |
23/84 (27.38) (18.21–38.20) |
52/232 (22) (17–28) |
14/72 (19) (11–30) |
| Day 35 SCR 95% CI | 256/295 (86.78) (82.37–90.43) |
25/83 (30.12) (20.53–41.18) |
187/225 (83.11) (77.56–87.76) |
16/71 (22.53) (13.46–34) |
CI, confidence interval; cVNT, conventional virus neutralization test; SCR, seroconversion rate.
The SARS-CoV-2 neutralization test results. The serum samples were first heat inactivated at 56ºC for 30 minutes, then serial dilutions were prepared and 100 μL of diluted samples incubated with 100-fold cell culture infectious dose 50% (CCID50) of SARS-CoV-2 virus for 60 minutes at 37ºC. The serum and virus mixtures were transferred into individual wells containing 2 × 104 African green monkey (Vero-E6) cells and incubated for 72 hours at 37ºC. The highest serum dilution that protected the Vero-E6 cells from the cytopathic effect (CPE) of SARS-CoV-2 was assigned as the neutralization titer. The cVNT results were interpreted as negative for titers ≤16 and positive for titers >16. Percentages for seroconversion rate (SCR) were calculated as the number of participants who reported the event divided by the number of participants in the Per-Protocol Set within each visit multiplied by 100. The 95% confidence interval (CI) for SCR was calculated using the exact Clopper-Pearson method.
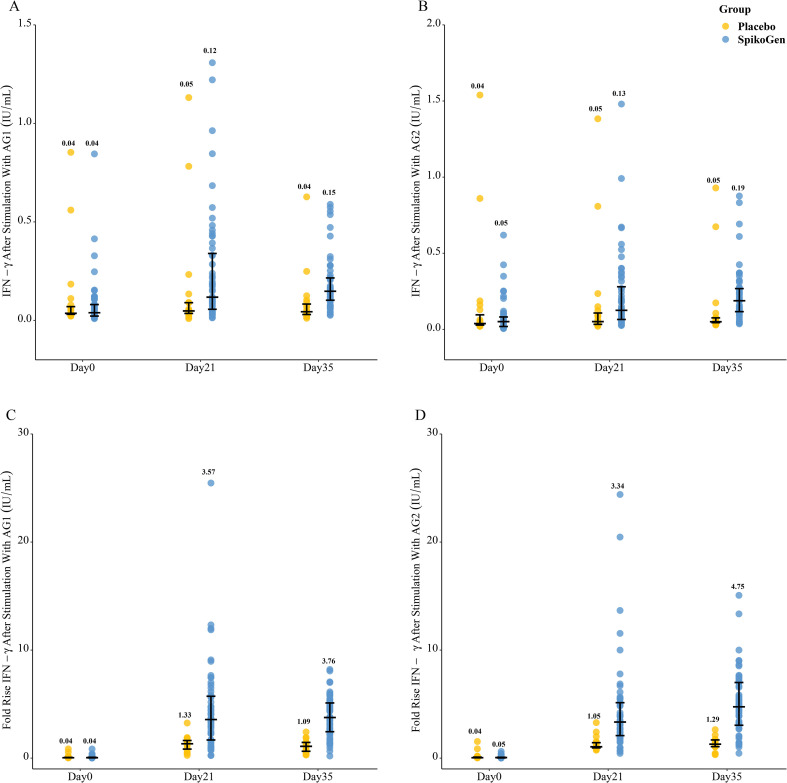
The results of the T-cell interferon gamma response are shown in Fig. 4 . Spike-stimulated interferon gamma production increased from day 0 to 35 in the SpikoGen® group compared to the placebo group. This increase was evident for both CD4+ (AG1) and combined CD4+ and CD8+ (AG2) peptide pools.
Fig. 4.
T cell response after stimulation with spike peptide pools. Shown are interferon-γ release after stimulation with AG1 (Panel A), and AG2 (Panel B), fold rise in Interferon-γ concentrations after stimulation with AG1 (Panel C) and AG2 (Panel D). Data are presented as median and interquartile range.
By day 35, the percentage of CD4+ and CD8+ T cells in the SpikoGen® group that proliferated in response to the spike protein had increased to 1.7% and 0.9%, respectively. The full results of the T cell response are provided in the supplementary material (Tables S8 to S10).
Discussion
The SpikoGen® vaccine exhibited acceptable safety and tolerability profile with no serious adverse events, and just typical vaccine-related solicited reactions including injection site pain, fatigue, and headache that were graded as mild and typically lasted for less than 2 days before resolving. These local and systemic reactions appear acceptable on the basis of a favourable comparison to the frequency and severity of solicited reactions reported for Nuvaxovid adjuvanted protein vaccine which has recently been EU-approved [20].
It is noteworthy that in this study, to increase the ability to detect those with past SARS-CoV-2 infection, seronegativity was defined as being seronegative across a panel of spike and nucleocapsid antibodies. SpikoGen® vaccine stimulated a strong humoral immune response in both seronegative and seropositive populations.
This study was also able to address the safety and immunogenicity of SpikoGen® vaccine when administered to those with prior SARS-CoV-2 infection. Notably, those participants who were seropositive at baseline showed strong increases in S1 IgG, RBD IgG, and neutralizing antibodies after just a single dose of SpikoGen® that was further boosted after the second dose. These results are consistent with studies of mRNA vaccines in SARS-CoV-2 seropositive and seronegative individuals where a single dose similarly induced a higher response in the seropositive participants [21].
SARS-CoV-2 infects and also transmits via the respiratory mucosa, making mucosal immunity of potential major importance alongside systemic immunity for protection. Normally a parenterally administered vaccine would not be expected to induce mucosal immunity. However, in our study SpikoGen® generated a significant seroconversion rate of S1 IgA compared to the placebo group, although this was largely restricted to the baseline seropositive group who had presumably already undergone a mucosal infection with SARS-CoV-2. Samples were not available for measurement of secretory IgA in this study, so we do not know the significance of SpikoGen® boosting of serum IgA in the previously infected participants. Interestingly, SpikoGen®-immunized ferrets had no recoverable virus in their nasal secretions 3 days after SARS-CoV-2 challenge [11]. If SpikoGen® could similarly be shown to reduce SARS-CoV-2 transmission in humans, this would represent a major breakthrough and is an area we are planning further studies.
While the neutralizing antibodies are thought to play a dominant role in protection, cellular immunity also contributes. The T cell response may be more durable and have a shorter onset of action than the humoral response [22]. A phase 2 study of Sputnik V adenovirus vector vaccine reported CD4+ (1.3%) and CD8+ (1.1%) proliferative T cells responses for the lyophilized formulation [23], broadly comparable to our own results. However, these assays are difficult to compare between studies given potential different methodologies used and the general lack of standardization. This positive finding of T cell response may be attributable to the Advax-CpG adjuvant, which was shown to induce a high frequency of memory CD8+ T cells in monkeys when combined with a cytomegalovirus vaccine antigen [24]. The ability of SpikoGen® vaccine to induce memory CD8+ T cell responses to spike protein has also been seen in mouse immunogenicity studies [11]. Notably, CpG55.2 is a TLR9 agonist that is involved in activating cellular immunity [25,26].
A limitation of this trial is that the duration of immunity induced by SpikoGen® vaccine was not assessed. These assessments are planned to be done in the future.
These results confirm that two intramuscular injections of 25 μg SpikoGen® 3 weeks apart induce robust humoral and T cell responses against SARS-CoV-2. A single dose of SpikoGen® was able to strongly enhance immunity in previously infected volunteers. SpikoGen® had an acceptable safety profile, with solicited adverse events being predominantly mild and transient in nature with no serious adverse events being reported among the trial participants. Based on these promising phase 2 results, a decision was taken to advance SpikoGen® to a phase 3 trial.
Transparency declaration
NA, RSH, AS, BY, BB, AT, and SB are members of the Orchid Pharmed medical department, which is in collaboration with CinnaGen company with respect to conducting clinical trials. KhR is in CinnaGen Medical Biotechnology Research Center. NP and LL are members of the Vaxine Pty Ltd. The remaining authors have no other relevant affiliations. This study was funded by CinnaGen Co. Anonymous participant data will be available upon a reasonable request to the corresponding author.
Author contributions
PT performed the research and coordinated the study. NA coordinated the study and collected data. RSH performed statistical analysis and designed tables and figures. MM, AS, BY, KhR, BB, AT, NP, and LL were involved in organization, coordination, conduct, and technical support of the study. SB was involved in the trial design, interpretation of the results, and drafted the manuscript. All authors critically reviewed the manuscript and approved the final version. All authors had full access to all data in the studies and had final responsibility for the decision to submit for publication. All authors are responsible for the accuracy and completeness of the data and fidelity of the trial to the protocol.
Acknowledgements
This study was funded by CinnaGen Co. The development of Advax-CpG55.2 adjuvant was supported by funding from National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Contract HHS-N272201400053C, HHSN272201800044C, and HHSN272201800024C. The authors are thankful to study participants, site research staff and members of the steering and DSMB committee.
Editor: A. Huttner
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.04.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Nascimento I.P., Leite L.C.C. Recombinant vaccines and the development of new vaccine strategies. Braz J Med Biol Res. 2012;45:1102–1111. doi: 10.1590/S0100-879X2012007500142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richmond P., Hatchuel L., Dong M., Ma B., Hu B., Smolenov I., et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–694. doi: 10.1016/S0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang Z., Zhu H., Wang X., Jing B., Li Z., Xia X., et al. Adjuvants for coronavirus vaccines. Front Immunol. 2020;11:589833. doi: 10.3389/fimmu.2020.589833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:117–183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bravo L., Smolenov I., Han H.H., Li P., Hosain R., Rockhold F., et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2022;399:461–472. doi: 10.1016/S0140-6736(22)00055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon D.L., Sajkov D., Woodman R.J., Honda-Okubo Y., Cox M.M.J., Heinzel S., et al. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax™ polysaccharide adjuvant. Vaccine. 2012;30:5407–5416. doi: 10.1016/j.vaccine.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon D., Kelley P., Heinzel S., Cooper P., Petrovsky N. Immunogenicity and safety of Advax™, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: a randomized controlled Phase 1 study. Vaccine. 2014;32:6469–6477. doi: 10.1016/j.vaccine.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda-Okubo Y., Saade F., Petrovsky N. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012;30:5373–5381. doi: 10.1016/j.vaccine.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L., Honda-Okubo Y., Huang Y., Jang H., Carlock M.A., Baldwin J., et al. Immunisation of ferrets and mice with recombinant SARS-CoV-2 spike protein formulated with Advax-SM adjuvant protects against COVID-19 infection. Vaccine. 2021;39:5940–5953. doi: 10.1016/j.vaccine.2021.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honda-Okubo Y., Ong C.H., Petrovsky N. Advax delta inulin adjuvant overcomes immune immaturity in neonatal mice thereby allowing single-dose influenza vaccine protection. Vaccine. 2015;33:4892–4900. doi: 10.1016/j.vaccine.2015.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakala I.G., Honda-Okubo Y., Li L., Baldwin J., Petrovsky N. A M2 protein-based universal influenza vaccine containing Advax-SM adjuvant provides newborn protection via maternal or neonatal immunization. Vaccine. 2021;39:5162–5172. doi: 10.1016/j.vaccine.2021.07.037. [DOI] [PubMed] [Google Scholar]
- 14.Honda-Okubo Y., Kolpe A., Li L., Petrovsky N. A single immunization with inactivated H1N1 influenza vaccine formulated with delta inulin adjuvant (Advax™) overcomes pregnancy-associated immune suppression and enhances passive neonatal protection. Vaccine. 2014;32:4651–4659. doi: 10.1016/j.vaccine.2014.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichinger K.M., Kosanovich J.L., Lipp M.A., Perkins T.N., Petrovsky N., Marshall C., et al. Maternal immunization with adjuvanted RSV prefusion F protein effectively protects offspring from RSV challenge and alters innate and T cell immunity. Vaccine. 2020;38:7885–7891. doi: 10.1016/j.vaccine.2020.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honda-Okubo Y., Barnard D., Ong C.H., Peng B.-H., Tseng C.-T.K., Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol. 2015;89:2995–2997. doi: 10.1128/JVI.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adney D.R., Wang L., van Doremalen N., Shi W., Zhang Y., Kong W.-P., et al. Efficacy of an adjuvanted Middle East Respiratory Syndrome Coronavirus spike protein vaccine in dromedary camels and alpacas. Viruses. 2019;11:212. doi: 10.3390/v11030212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popmihajlov Z., Pang L., Brown E., Joshi A., Su S.-C., Kaplan S.S., et al. A post hoc analysis utilizing the FDA toxicity grading scale to assess injection site adverse events following immunization with the live attenuated Zoster Vaccine (ZVL) Hum Vaccin Immunother. 2018;14:2916–2920. doi: 10.1080/21645515.2018.1502517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FDA . 2020. Development and licensure of vaccines to prevent COVID-19.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-and-licensure-vaccines-prevent-covid-19 Available from: [Google Scholar]
- 20.Dunkle L.M., Kotloff K.L., Gay C.L., Anez G., Adelglass J.M., Hernandez A.Q.B., et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022;386:531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaganathan S., Stieber F., Rao S.N., Nikolayevskyy V., Manissero D., Allen N., et al. Preliminary evaluation of QuantiFERON SARS-CoV-2 and QIAreach Anti-SARS-CoV-2 total test in recently vaccinated individuals. Infect Dis Ther. 2021;10:2765–2776. doi: 10.1007/s40121-021-00521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatulin A.I., Shcheblyakov D.V., Dzharullaeva A.S., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H., Monslow M.A., Freed D.C., Chang D., Li F., Gindy M., et al. Novel adjuvants enhance immune responses elicited by a replication-defective human cytomegalovirus vaccine in nonhuman primates. Vaccine. 2021;39:7446–7456. doi: 10.1016/j.vaccine.2021.10.075. [DOI] [PubMed] [Google Scholar]
- 25.Chu R.S., Targoni O.S., Krieg A.M., Lehmann P.V., Harding C.V. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murad Y.M., Clay T.M. CpG oligodeoxynucleotides as TLR9 agonists: therapeutic applications in cancer. BioDrugs. 2009;23:361–375. doi: 10.2165/11316930-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.