Abstract
Purpose
The COVID-19 pandemic resulted in a partial to total shutdown of endoscopy in many healthcare centers. This study aims to quantify the impact of the reduction in colonoscopies on colorectal cancer (CRC) detection and screening.
Methods
After institutional ethics board approval, the endoscopy database at an academic tertiary-care center in Montreal, Canada, was searched for all colonoscopies performed from during the first wave locally (March–June 2020), and during the ramp up period where endoscopy service resumed (July to August 2020). We compared these periods to the same periods in 2019, the pre-pandemic periods. The indications, CRC and adenoma detection rates, as well as the prioritization of urgent procedures were compared.
Results
In the first wave, only 462 colonoscopies were performed, compared to 2515 in the same period in 2019, an 82% reduction. The ramp up period saw 843 colonoscopies performed compared to 1328 in 2019, a 35% reduction. Urgent and inpatient colonoscopies numbers increased (324 (24.8%) vs. 220 (5.7%)) while surveillance and high-risk screening colonoscopies fell (376 (28.8%) vs 1869 (48.6%)). Emergency access to colonoscopy was preserved with a median time to endoscopy of < 1 day (IQR 0,1) in both pandemic periods. During the pandemic periods, there was an absolute reduction in CRC diagnosis of 28, despite the CRC detection per colonoscopy rate increasing slightly in the first wave from 1.7% (44) to 3.9% (18), and in the ramp up period from 2.5% (33) to 3.6% (31). The rate of adenoma detection per colonoscopy did not increase significantly between the pre- and pandemic periods, resulting in reduction in adenoma removal in 723 patients.
Discussion
The restriction of access to colonoscopy resulted in a significant reduction in screening and surveillance of high-risk patients, adenomas removed, and CRCs diagnosed. Clinicians and patients will face the oncologic ramifications this the coming years.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00464-022-09211-z.
Keywords: COVID-19 pandemic, Colonoscopy, Colorectal cancer
In December 2019, a novel virus emerged quickly spreading worldwide. By March 2020, the Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) was declared a pandemic by the World Health Organization [1, 2]. Public health measures, including lockdowns and limitations on non-essential activities were introduced with the goal of slowing the spread of the virus [3]. Once it became clear that resources would be strained to their maximum capacity, limitations were placed on all non-urgent care with the suspension of the majority of elective, non-essential procedures to allow resources to be re-prioritized towards combating the pandemic [4, 5].
In response to local outbreaks, Quebec introduced some of the most severe lockdown restrictions in Canada at that time [6]. In Montreal, one of the central hotspots, the first wave began in March 2020, with case counts continuing to rise daily until finally falling at the beginning of July. During this first wave in Quebec, there were 59,845 COVID-19 cases confirmed, 7310 patients were hospitalized, and 5829 patients died [7]. Non-urgent care access was strictly limited during this period, including most non-emergent colonoscopy services, such as those performed as part of the colorectal cancer (CRC) screening and detection program in Canada.
CRC is the third most common cancer diagnosed in Canada, and represented an estimated 12% of new cancers diagnosed in 2020 [8]. Screening and surveillance programs are in place for prevention and early detection of CRC, and these programs use a variety of methods including guaiac smear fecal occult blood testing (gFOBT), fecal immunochemical testing (FIT), flexible sigmoidoscopy and colonoscopy [9]. The Canadian Task Force on Preventative care 2016 guidelines and the Quebec provincial guidelines recommend immunochemical testing every two years as the primary method of screening for all average risk Canadians over 50 years old, with preference for FIT where available [10, 11]. These recommendations explicitly exclude anyone with specific risk factors for CRC, at which point the screening method is left to the discretion of the healthcare professional and the patient. For high risk patients, colonoscopy-based screening and surveillance is recommended [11, 12]. These recommendations are similar to the US Preventive Services Task Force recommendations (USPST), however, the USPST makes no recommendations on the specific type of screening strategy to be used [13].
The overall CRC incidence and associated mortality have fallen over the last 20 years, largely attributed to effective screening. While it is difficult to conclusively demonstrate the direct impact of colonoscopy-based screening on this fall, there is a distinct trend of rising colonoscopy rates in the United States that parallel the reduction CRC incidence [14]. As with most non-emergent procedures, the number of colonoscopies performed during the COVID-19 pandemic was restricted as resources were redirected towards critical and emergent care [15, 16]. It remains to be seen what impact this sharp reduction in screening and prevention will lead to with regards to CRC incidence and mortality in the years to come.
To investigate the impact of COVID 19 on high-risk screening, surveillance, and diagnostic colonoscopies for CRC, we conducted a retrospective review at a single tertiary-care center. The goal of this study was to characterize the absolute reduction in number of colonoscopies, changes in indications and prioritization, and differences in cancer and polyp detection rates during the (i) first wave of the pandemic and (ii) ramp up period. The results of these two periods were then compared to those performed in the same months 1 year prior, the pre-pandemic periods. By studying the ramp up period, when endoscopy services resumed, we were able to characterize the success of this early ‘catch up’ period.
Methods
Population
After institutional review board approval, we performed a retrospective review of an institutional endoscopy database at an academic tertiary-care health care center in Montreal, Canada, covering four distinct periods. These periods were as follows: (1) the first wave, consisting of the four month shut down period associated with the first wave of the COVID-19 pandemic in Montreal (March to June 2020), (2) the ramp up period, consisting of the first 2 months following the first wave where endoscopy service resumed (July and August 2020), and finally the two comparison periods from the previous year:(3) March to June 2019 and (4) July and August 2019 (Fig. 1). The endoscopy database was queried to identify all colonoscopies which were performed during the periods of interest and an accompanying chart review was performed. This study was conducted and reported as per the STROBE guidelines [17].
Fig. 1.
Investigation periods
Patient demographics and indications for colonoscopy
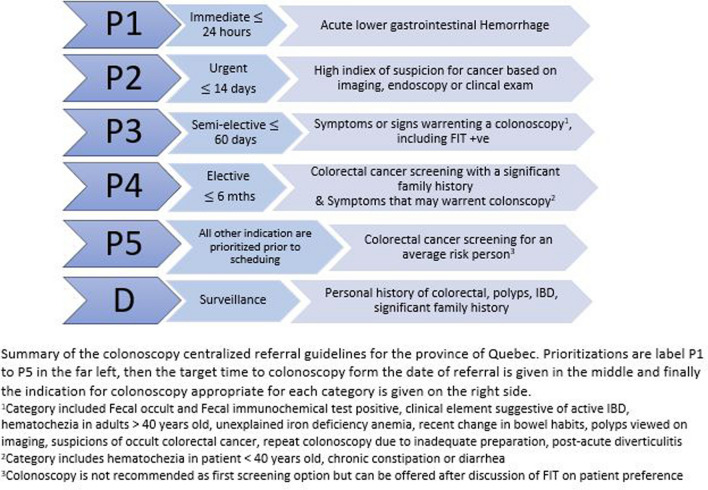
Patient demographics were retrieved through chart review and included age, gender, height, and weight of each patient. The referrals for colonoscopy were reviewed to identify the indication for the colonoscopy: diagnostic for symptomatic patients, screening in patient with specific risk factors, and surveillance in a patient with a personal history of adenomas or cancer. For diagnostic colonoscopies, symptoms were classified as bleeding, changes in bowel habits, abdominal pain, weight loss, FIT positive testing, multiple symptoms or other symptoms at the clinician’s discretion. The referring physician’s prioritization level as classified by the Quebec provincial health guidelines (Fig. 2) was also retrieved from the referral. Urgent indication for colonoscopies included referrals for P1, gastrointestinal hemorrhage, P2, high suspicion of CRC, and inpatient requests. Indications for high-risk screening (P4) and surveillance (C) endoscopy included personal history of CRC or previous polyps, family history of CRC or polyps, and syndromes or disease states resulting in a higher risk of CRC. P4 referrals could also include symptoms warranting a colonoscopy at the clinician’s discretion, these symptomatic patients were removed from calculation of high-risk screening rates. Average risk screening (P5) was defined as patients with no symptoms and none of the above risk factors. The time from referral to the date of colonoscopy was calculated. The rate of incomplete colonoscopies in each period were recorded as a quality indicator.
Fig. 2.
Sante et serivices sociaux colonoscopy referral guidelines for the province of Quebec
Colonoscopy findings
Results of the colonoscopy were retrieved from the endoscopy database, including the diagnosis of CRC, the number and location of any polyps found, quality of preparation and completeness of exam. For any polyps removed, they were classified on final pathology as: hyperplastic, tubular, tubulovillious, sessile serrated, inflammatory, or juvenile polyps. Any high-grade dysplasia (HGD) or in situ malignancy found was also recorded. Findings of CRC were confirmed by pathology review. For newly diagnosed CRC in the ramp up period, cancer stage following resection was determined through pathology and chart review. The performing endoscopist was also recorded, as either one of eight gastroenterologists or one of seven colorectal surgeons.
Statistical analysis
Descriptive statistics were used to examine the baseline characteristics of the individual periods using proportions for categorical variables and means with standard deviation (SD) for continuous variables. Polyp, adenoma and cancer detection rates were calculated as proportions of colonoscopies performed during that period. Crude univariate comparisons were performed between the pre-pandemic and pandemic periods using a two-sided t test for continuous variables and chi squared tests for categorical variables. Since we only performed crude analyses, we used pairwise deletion to address missing values, that is we eliminated information only when the particular data-point needed was missing. If there was missing data elsewhere in the data set, the existing values were used. This is a valid method to address missing values as it does not discard of any data, which is important for the study question [18]. Missing data was clearly denoted in all tables. All data analysis was performed in SPSS (version 26).
Results
Number and characteristics of colonoscopies
During the first wave of the pandemic, we observed an 81.6% reduction in the number of colonoscopies performed, with only 462 completed compared to 2515 in the same four-month period in 2019. During the ramp up period, 843 colonoscopies were performed in the two-month time period whereas 1328 had been performed in the same time period in the year prior, representing a 36.5% reduction. There were no significant differences in patient characteristics between colonoscopies performed in the covid periods compared to the respective periods in the prior year. Likewise, neither the number of incomplete scopes nor the training background of the endoscopist were significantly different between pre- and pandemic periods (Table 1).
Table 1.
Patient characteristics in the pre-pandemic (2019) vs COVID periods (2020)
| Pre-COVID 2019 | First wave | p-Value | Pre-COVID 2019 | Ramp up 2020 | p-Value | |
|---|---|---|---|---|---|---|
| Number of colonoscopies | 2515 | 462 | – | 1328 | 843 | – |
| Patient characteristics | ||||||
| Age (mean, SD) | 61 ± 14 | 59 ± 17 | 0.112 | 60 ± 15 | 60 ± 15 | 0.878 |
| Female, n (%) | 1282 (51.0%) | 226 (48.9%) | 0.416 | 702 (52.8%) | 441 (52.3%) | 0.489 |
| BMI, mean (SD) | 27 ± 5.2 | 27 ± 5.4 | 0.436 | 26.5 ±5.5 | 26.6 ± 5.4 | 0.634 |
| Missing date for BMII (no.) | 106 | 47 | – | 101 | 68 | – |
| Endoscopist, n (%) | 0.118 | 0.704 | ||||
| Gastroenterologist | 1811 (72.0) | 349 (75.5) | – | 974 (73.3%) | 566 (67.1%) | – |
| Colorectal Surgeon | 704 (28.0) | 113 (24.5) | – | 354 (26.7%) | 277 (32.9%) | – |
| Incomplete scopes, n (%) | 106 (4.2) | 19 (4.1) | 0.607 | 44 (3.3%) | 45 (5.3%) | 0.525 |
| Missing data (no.) | 0 | 2 | – | 7 | 6 | – |
Indications for colonoscopies
During the first wave, there was an increase in proportion of urgent colonoscopies, from 4.7% (117) to 26.4% (122), with a corresponding fall in the proportion of screening and surveillance colonoscopies, from 51.1% (1, 287) to 32.3% (149), when compared to the same period in 2019 (Table 2). For high-risk screening colonoscopies, there was an even greater reduction from 201 (8.3%) to 9 (1.9%). Furthermore, high-risk surveillance colonoscopies in asymptomatic patients decreased from 785 (31.2%) to 102 (22.1%) colonoscopies. The proportion of diagnostic scopes remained relatively constant from 42.8% (1076) in pre-pandemic to 40.5% (187) in the first wave of the pandemic, however, the proportion and absolute number of inpatient colonoscopies increased from 0.6% (15) to 13.6% (63). (Table 2).
Table 2.
Indication for and prioritization of colonoscopies in the pre-pandemic (2019) vs COVID periods (2020)
| Pre-COVID 2019 | First wave | p Value | Pre-COVID 2019 | Ramp up 2020 | p-Value | |
|---|---|---|---|---|---|---|
| Number of colonoscopies, n | 2515 | 462 | – | 1328 | 843 | – |
| Indication, n (%) | ||||||
| Urgenta | 117 (4.7%) | 122 (26.4%) | < 0.0001 | 68 (5.1%) | 130 (15.4%) | 0.637 |
| Diagnostic | 1076 (42.8%) | 187 (40.5%) | – | 677 (51.0%) | 477 (56.6%) | – |
| Screening/surveillanceb | 1287 (51.1%) | 149 (32.3%) | < 0.0001 | 582 (43.8%) | 227 (26.9%) | 0.001 |
| Inpatient request | 15 (0.65%) | 63 (13.6%) | – | 20 (1.5%) | 9 (10.7%) | – |
| Pre-transplant screening | 13 (0.5%) | 3 (0.7%) | – | 1 (0.1%) | 2 (0.1%) | – |
| Unspecified | 19 (0.8%) | 1 (0.2%) | – | 0 (0%) | 7 (1.0%) | – |
| Symptoms, n (%) | ||||||
| Bleeding | 395 (15.7%) | 107 (23.2%) | – | 243 (18.3%) | 185 (21.9%) | – |
| Change in BM | 180 (7.2%) | 70 (15.2%) | – | 168 (12.7%) | 148 (17.6%) | – |
| Abdominal pain | 229 (9.1%) | 45 (9.7%) | – | 141 (10.6%) | 82 (9.7%) | – |
| Weight loss | 27 (1.1%) | 24 (5.2%) | – | 17 (1.3%) | 27 (3.2%) | – |
| Fit (+) | 137 (5.4%) | 19 (4.1%) | – | 93 (7.0%) | 45 (5.1%) | – |
| Other | 121 (4.8%) | 68 (14.7%) | – | 234 (17.6%) | 164 (19.5%) | – |
| Multiple symptoms | 146 (5.8%) | 66 (14.3%) | – | 135 (10.2%) | 110 (13.0%) | – |
| No symptoms reported | 1581 (62.9%) | 214 (46.3%) | – | 586 (44.1%) | 325 (38.6%) | |
| High-risk surveillance, n (%) | ||||||
| Personal history of CRC | 146 (5.8%) | 35 (7.6%) | 0.135 | 53 (4.0%) | 49 (5.8%) | 0.021 |
| Missing data | 2 | 3 | 0 | 0 | ||
| Personal Hx Polyps | 599 (23.8%) | 57 (12.3%) | < 0.0001 | 258 (19.4%) | 185 (21.9%) | 0.443 |
| Missing data | 2 | 2 | 0 | 0 | ||
| Family History of CRC | 372 (14.8%) | 50 (10.9%) | 0.026 | 187 (14.1%) | 102 (12.1%) | 0.525 |
| Missing data | 4 | 2 | 1 | 0 | ||
| Family history of polyp | 77 (3.1%) | 1 (0.2) | 0.431 | 54 (4.1%) | 28 (3.3%) | 0.827 |
| Missing data | 6 | 2 | 7 | 0 | ||
| IBD/FAP/Lynch | 0.673 | 0.013 | ||||
| IBD | 226 (9.0%) | 45 (9.8%) | – | 82 (6.7%) | 78 (9.3%) | – |
| FAP | 1 (0.04%) | 0 (0.0%) | – | 1 (0.1%) | 0 (0%) | – |
| Lynch | 16 (0.6%) | 1 (0.2) | – | 3 (0.2%) | 11 (1.3%) | - |
| Missing data for IBD/FAP/Lynch | 6 | 4 | – | 0 | 0 | – |
acorresponds to P1 & P2 and inpatient referrals as prioritized by the Quebec government provincial guidelines
bcorresponds to P4, P5 and surveillance colonoscopies
During the ramp up period, urgent colonoscopies were again more common with 130 (15.4%) performed, in comparison to 68 (5.1%) in the pre-pandemic period. (Table 2). There were fewer inpatient requests, 10 (1.1%) in the ramp up compared to 23 (1.7%) in the pre-pandemic period. Diagnostic colonoscopies accounted for a larger proportion of the colonoscopies performed, 56.6% (477) vs 677 (51.0%). There was a corresponding lower proportion of screening and surveillance colonoscopies performed in the ramp up, 26.9% (277) vs 43.8% (582) when compared to the same months in 2019. High-risk surveillance and high-risk screening in patient accounted for only 4.5% (38) vs 22.2% (296), and 1.9% (16) vs 7.5% (100) in ramp up compared to pre-pandemic months, respectively. (Table 2).
For diagnostic colonoscopies, the most common indication in symptomatic patients was bleeding (16.6% and 22.4%), followed by changes in bowel habits (9.1% and 18.2%) and abdominal pain (9.6% and 14.2%), in the pre- and pandemic periods, respectively. FIT positive colonoscopies, following the primary recommended non-invasive population screening tool in Quebec during these periods, accounted for 5.9% and 4.9% of colonoscopy indications in the pre- and pandemic periods, respectively. (Table 2).
Prioritization and time from referral to colonoscopy
The time to colonoscopy from date of referral was calculated for each priority level as per the Quebec provincial health guidelines from P1-5. Patients referred for P1 indications, urgent gastrointestinal hemorrhage, were scoped within 0–3 days in all periods (Table 3). P2 referrals resulted in a colonoscopy at a median of 3 days (IQR 0, 19) in the first wave period vs. 11 days (IQR 2,35) in the year prior. In the ramp up period, the P2 colonoscopies were performed within a median of 5 days (IQR 0, 10) vs 14 days (IQR 2, 21) in the year prior. These two urgent indications saw no delay from referral to colonoscopy as a result of the pandemic. The patients who received a colonoscopy during the first wave and the ramp up period for other indications (including P3, P4 and P5) had no significant difference in time from referral to colonoscopy. (Table 3).
Table 3.
Time from referral to colonoscopy and number of colonoscopies by indication
| Prioritization | Number of scopes by indication (n., %) | Delay (median in days, IQR) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-COVID (Mar–Jun 2019) | First wave (Mar–Jun 2020) | Pre-COVID (Mar–Jun 2019) | Missing data | First wave (Mar–Jun 2020) | Missing data | |||||
| P1 | 17 | 0.7% | 30 | 6.5% | 2 | 1,3 | 0 | 0 | 0,1 | 1 |
| P2 | 85 | 3.4% | 29 | 6.3% | 11 | 2, 35 | 2 | 3 | 0, 19 | 2 |
| P3 | 1076 | 42.8% | 187 | 40.5% | 56 | 23, 91 | 260 | 54 | 19, 132 | 2 |
| P4 | 303 | 12.0% | 17 | 3.7% | 73 | 27, 169 | 73 | 71 | 25, 274 | 0 |
| P5 | 136 | 5.4% | 18 | 3.9% | 70 | 43, 190 | 27 | 96 | 54, 210 | 0 |
| Surveillance | 848 | 33.7% | 114 | 24.7% | 99 | 49, 327 | 206 | 98 | 42, 376 | 11 |
| Inpatient request | 15 | 0.6% | 63 | 13.6% | 4 | 12, 123 | 3 | 0 | 0,0 | 1 |
| Transplant | 13 | 0.5% | 3 | 0.7% | 22 | 12, 123 | 4 | 87 | 0, 104 | 0 |
| Unspecified | 19 | 0.8% | 1 | 0.2% | 8 | 4, 47 | – | 18 | N/A | – |
| Pre-COVID (Jul–Aug 2019) | Ramp Up (Jul–Aug 2020) | Pre-COVID (Jul–Aug 2019) | Missing data | Ramp up (Jul–Aug 2020) | Missing data | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 21 | 2% | 31 | 3.7% | 1 | (1,2) | 0 | 0 | (0,1) | 0 |
| P2 | 24 | 2% | 89 | 10.7% | 14 | (2,21) | 0 | 5 | (0, 10) | 0 |
| P3 | 677 | 51% | 477 | 56.6% | 79 | (20,92) | 106 | 143 | (25, 235) | 1 |
| P4 | 140 | 11% | 58 | 6.9% | 166 | (43, 161) | 19 | 97 | (8, 306) | 0 |
| P5 | 92 | 7% | 7 | 0.1% | 246 | (23, 70) | 19 | 116 | (13, 483) | 1 |
| Surveillance | 350 | 26% | 162 | 19.2% | 261 | (42, 268) | 40 | 194 | (50, 411) | 2 |
| Inpatient request | 23 | 1.7% | 10 | 1.2% | 3 | (1, 5) | 0 | 1 | (0, 8) | 0 |
| Transplant | 1 | 0.1% | 2 | 0.02% | 0 | NA | 0 | 54 | N/A | 0 |
| Unspecified | 0 | 0% | 7 | 0.1% | 0 | NA | – | 146 | (138,234) | – |
Polyp and adenoma detection
Polyps were detected in 36.2% (167) of colonoscopies performed during the first wave period in comparison to 36.6% (929) during the same period in the previous year. A similar rate of polyp detection between the periods resulted in an absolute reduction of polyps removed in 762 patients. For adenoma detection rates specifically, it was 30.7% (142) in the first wave versus 30.5% (766) in the year prior, representing an absolute reduction in adenomas removed in 624 patients. In the ramp up period, polyps were removed in 350 (41.5%) and adenomas in 252 (30.0%) colonoscopies. In the same period in 2019, they were removed in 489 (36.8%) and 351 (26.6%) of colonoscopies, respectively. Again, a similar rate of adenoma detection resulted in adenomas being removed in 99 less patients in the ramp up period in comparison to the same period pre-pandemic. In total, 14 polyps with HGD were removed in pre-covid ear, compared to 42 in the pre-pandemic era.
CRC detection
The CRC detection rate increased in the colonoscopies during pandemic period, however, the absolute number of colon cancers detected decreased in both periods of the pandemic in comparison to the pre-pandemic year (Table 4). In the first wave period, 18 colon and rectal cancers were diagnosed, with 3.5% of colonoscopies exhibiting a cancer, whereas during the same period in 2019, 44 cancers were diagnosed, 1.7% of colonoscopies. This resulted in an absolute reduction in cancers diagnosed of 26. During the ramp up period, 31 cancers were diagnosed, representing 3.6% of colonoscopies, compared to 33 cancers in the prior year, representing 2.5% of colonoscopies. This results in an overall absolute reduction of 28 cancers diagnosed in the 6-month pandemic period compared to the same 6 months in the previous year.
Table 4.
Colonoscopy findings in colonoscopies during the pre-pandemic (2019) vs COVID periods (2020)
| Pre COVID2019 | First wave 2020 | Pre-COVID 2019 | Ramp up 2020 | |
|---|---|---|---|---|
| Number of cancers, n (%) | 44 (1.7) | 18 (3.9) | 33 (2.5%) | 31 (3.6%) |
| Location, n (%) | ||||
| Ileum | 0 (0%) | 1 (6%) | 3 (9.0%) | 3 (9.7%) |
| Cecum | 5 (11.4%) | 6 (33.3) | 7 (21.2%) | 3 (9.7%) |
| Ascending | 3 (6.8%) | 1 (5.6%) | 10 (30.3) | 5 (1.6%) |
| Transverse (includes flexures) | 6 (13.6%) | 1 (5.6%) | 2 (6.1%) | 1 (3.2%) |
| Descending | 3 (6.8%) | 1 (5.6%) | 5 (1.5%) | 4 (12.9%) |
| Sigmoid | 8 (18.2%) | 2 (11.1%) | 6 (1.8%) | 15 (58.4%) |
| Rectum | 18 (40.9%) | 5 (27.8%) | 0 (0%) | 0 (0%) |
| Other (inc. anastomosis) | 1 (2.3%) | 1 (5.6%) | 0 (0%) | 0 (0%) |
| Polyps removed, n (%)a | 929 (36.9%) | 167 (36.2%) | 489 (36.8%) | 350 (41.5%) |
| Adenoma removed, n (%)a | 766 (30.5%) | 142 (30.7%) | 351 (26.6%) | 252 (30.0%) |
| Type, n | ||||
| Hyperplastic | 210 | 35 | 46 | 23 |
| Tubular | 655 | 114 | 157 | 92 |
| Tubulovillous | 66 | 8 | 9 | 4 |
| Sessile serrated | 91 | 0 | 17 | 12 |
| High grade dysplasia | 27 | 0 | 15 | 14 |
| Inflammatory | 92 | 5 | 25 | 23 |
| Number of polyps, n (%)a | ||||
| 1 | 505 (20.1%) | 82 (17.8%) | 261 (19.6%) | 187 (22.2%) |
| 2 | 232 (9.2%) | 38 (8.2%) | 107 (8.1%) | 75 (8.9%) |
| 3 | 8 (3.5%) | 15 (3.3%) | 60 (4.5%) | 44 (5.2%) |
| 4 | 37 (1.5%) | 12 (2.6%) | 28 (21.1%) | 19 (2.3%) |
| 5–9 | 47 (10.2%) | 8 (1.7%) | 27 (20.3%) | 22 (2.6%) |
| > 10 | 18 (7.2%) | 8 (1.7%) | 6 (0.5%) | 3 (3.6%) |
| AJCC cancer stage | ||||
| 0 | – | – | 1 (3.0%) | 2 (6.5%) |
| I | – | – | 6 (18.2%) | 3 (9.6%) |
| 2 | – | – | 11 (33.3%) | 6 (20%) |
| 3 | – | – | 8 (24.3%) | 7 (22.6%) |
| 4 | – | – | 4 (12.1%) | 9 (29.0%) |
| Stage unavailable | – | – | 2 (6.1%) | 1 (3.4%) |
| Non-adenocarcinomas | – | – | 1 (3.0%) | 3 (9.6%) |
aPrecentage of positive finding per colonoscopies completed in each period
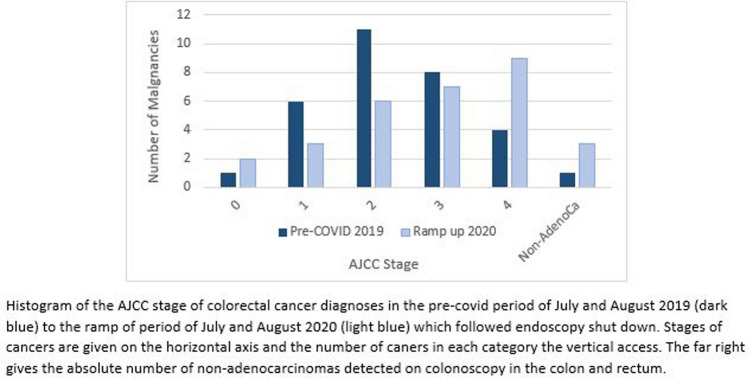
When comparing the ramp up era to the previous year, there were more metastatic cancer diagnoses amongst all the cancers detected (9 (29.0%) vs 4 (12.1%)). Most cancers detected in 2020 were stage IV, compared to stage II in 2019 (Fig. 3). In terms of location, there were 17 (54.8%) rectal and rectosigmoid cancer diagnosed in the ramp up period in comparison with 7 (21.2%) in this location the year prior. (Table 4).
Fig. 3.
Colorectal cancer stage in those diagnosed in July and August 2019, pre-COVID pandemic, to July and August 2020, Ramp up period
Discussion
This study provides timely clinical evidence of the direct impact of the COVID-19 pandemic on colonoscopy access, prioritization, and outcomes. We have seen a significant reduction in adenoma removal, CRC diagnosis, and a trend towards a more advanced CRC stage at diagnosis, all which are consistent with what has been seen in other parts of the world [19–22]. In our center, the first wave shut down resulted in a dramatic 82% reduction in colonoscopy numbers. Following this there was an attempt to return to pre-pandemic numbers but despite best intentions, there was a persistent, though lesser, reduction in colonoscopy numbers, 37%. This reflected the ongoing resource limitations, especially skilled nursing support, as well as patient reluctance to enter the hospital due to fears of contracting the virus. Access to colonoscopy was preserved for patients with the most urgent indications according to the current provincial guidelines. However, we observed an increase in the number of inpatient colonoscopy requests during the first wave likely as a result of limited outpatient access for non-emergent indications. The lack of access may have effectively driven patients into hospital at a time where hospital beds were at a premium. This is an unfortunate but predictable consequence of the re-prioritization for resources for the pandemic.
The benefit of colonoscopy screening is early detection of CRC and the opportunity to remove adenomas interrupting of the adenoma to adenocarcinoma pathway (22). The adenoma detection rate per colonoscopy remained relatively stable in all periods during this study, and as a result of the decreased in numbers of colonoscopies, adenomas were left in situ in an estimated 723 patients, including high-risk adenomas with HGD in 28. A similar stable adenoma detection rate was seen in a central Italian study by D’Ovidio et al., looking at colonoscopies performed during the pandemic for a selected high risk patient group. They did, however, have an increase in the ‘high risk’ adenoma detection rate from 25 to 47% (including adenomas > 10 mm, villous, serrated and HGD) in comparison to a previous year not seen in our study [5]. Prioritization of colonoscopies during the pandemic did increase the rate of CRC detection in our cohort, but it could not entirely offset the absolute reduction in colonoscopy numbers. There was also a troubling trend seen in the final pathology of the CRC diagnosed during the ramp up period with more advanced stages and more metastatic cancer was identified when compared to the same period in 2019. A similar finding of more advanced CRC at diagnosis was also seen in Australia and New Zealand, where the post-lockdown period saw an increase in urgent or emergency procedures for colorectal cancer, more stoma creation, and a proportionally higher rise in Stage II or III disease and decrease in stage I disease [23].
According to predictive modelling, the reduction in screening, adenoma removal and early CRC detection will likely result in an increased incidence of CRC and more advanced stage at diagnosis. Ricciardello et al. estimated for the Italian population that screening delays beyond 4–6 months would result in a significant increase in advanced CRCs (Stage III and IV) and that a delay of 12 months would increase the mortality associated with CRC [24]. Similar predictive modeling was done for the Canadian population using a validated mathematic model, which predicted a six-month suspension of primary screening could increase both the incidence and mortality associated with CRC [25]. Early data from our four-month shut down seems to support these predictions.
It has been suggested that non-invasive screening approaches such as FIT maybe be used as an alternative screening modality to reduce overall demands and as a way to prioritize colonoscopy access [26–28]. There are some challenges to this approach. In a Californian study, non-invasive testing, including FIT, decreased during the pandemic in parallel with the reduction in colonoscopies, although the authors noted an earlier return to pre-pandemic levels while colonoscopies lagged behind [29]. This reduction in FIT testing was also seen in the Taiwanese population, which similar to Canadian guidelines has a primarily FIT lead CRC screening program [30]. In this population there were also signs of patient reluctance to enter the health care environment during the pandemic even for diagnostic testing following positive FIT screening, with an overall rescheduling and cancellation of diagnostic colonoscopies following a positive FIT test of 10.9%, significantly higher than previous years. This reluctance was also seen in the D’Ovidio et al. study where only 43% of high-risk patients, who were FIT positive or in polypectomy surveillance programs, invited for colonoscopy underwent the procedure as the rest declined [5]. Non-invasive screening methods, like FIT, may well be the way to meet screening needs in the pandemic but to do so there needs to be a systematic approach to preserve access to these methods, especially where primary care services are disrupted. Patients’ reasonable concern about entering the health care environment during the pandemic will also need to be addressed if the necessary diagnostic colonoscopy following a positive FIT test are to be completed.
Our study specifically addresses the impact of restricting colonoscopy access in a population where alternative screening methods for CRC were in place and still available, and where urgent access to colonoscopy was preserved. In our study, average risk screening accounted for only 4% of colonoscopies performed in the first wave and 0.1% in the ramp up, down from 5 and 7% in the pre-pandemic period respectively. This is significantly less than the 18% estimated by the Gastrointestinal Endoscopy COVID working group for Ontario, Canada, which might reflect the benefit of a direct chart access to review the indications or better compliance with guidelines at our institution [31]. USPST guidelines do not mandate non-invasive screening as the primary tools for average risk screening, and a recent study in Chicago reported that 25% of colonoscopes are done for these patients, suggesting that in other populations prioritizing non-invasive methods may will decrease demands for colonoscopy in these populations [27]. Given adherence to provincial guidelines limited our average risk screening colonoscopies, the dramatic reduction in screening and surveillance colonoscopies seen in this study included reduced screening in patients with some of the highest risk for adenomas and CRC such as patients with inflammatory bowel disease, FAP and Lynch syndrome [32, 33]. Alternative methods to preserve colonoscopy access should be considered for these high-risk patients.
The strengths of this study include the detailed information, including indication and prioritization, available from the endoscopy database, referrals, and chart reviews. Furthermore, the availability of a suitable historical control facilitated the interpretation of observations made during the pandemic. However, the data in the study is from a single academic tertiary hospital and therefore may not be generalizable to all healthcare settings. Finally, it is difficult to make any conclusive statements on stage migration from this relatively small sample size over a limited time period, and the true outcomes of delayed colonoscopies are likely not yet apparent.
The current health care guidelines were not designed for use during the strict resource limitation of a global pandemic. When standard provincial guidelines were used during this pandemic, they resulted in a small increase in the rate of CRC diagnosis per colonoscopy but an overall reduction in CRC diagnoses and adenoma removal. High risk screening and surveillance colonoscopies were deprioritized and there were not enough resources to adequately meet the needs of this patient population. In the coming month and years, clinicians will have to manage the consequences of delayed colonoscopies, including missed opportunities for prevention and early detection with the resulting increase in the incidence of CRC and CRC stage at diagnoses. It is unlikely that the COVID-19 pandemic will remain an isolated occurrence. We owe it to our patients to evaluate our past response and plan for the future to minimize the secondary harms of resource re-allocation wherever possible.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
All authors contributed to the design of the study. JH, CW, GR and MB participated in data acquisition. JH, CW, CAV, AP, BM participated in data analysis and interpretation. JH, CW and MB prepared the first draft of the manuscript. All authors contributed to, and approved, the final version of the manuscript.
Funding
There is no specific grant support or funding for the study.
Disclosures
Jessica Holland, Michelle Cwintal, Georgia Rigas, Carol-Ann Vasilevsky, Nancy Morin, Gabriella Ghitulescu, Julio Fair and Allison Pang have no conflict of interest or financial ties to disclose. Marylise Boutros has received a teaching honorarium from Johnson and Johnson.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jessica Holland, Email: Jessica.holland@mail.mcgill.ca.
Michelle Cwintal, Email: michelle.cwintal@mail.mcgill.ca.
Georgia Rigas, Email: grigas@jgh.mcgill.ca.
Allison J. Pang, Email: allison.pang@mcgill.ca
Carol-Ann Vasilevsky, Email: gabriela.ghitulescu@mcgill.ca.
Nancy Morin, Email: nancy.morin@mcgill.ca.
Gabriela Ghitulescu, Email: gabriela.ghitulescu@mcgill.ca.
Julio Faria, Email: julio.faria@mcgill.ca.
Marylise Boutros, Email: marylise.boutros@mcgill.ca.
References
- 1.Park M, Cook AR, Lim JT, Sun Y, Dickens BL. A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med. 2020;9(4):967. doi: 10.3390/jcm9040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Director-General's opening remarks at the media briefing on COVID-19 [press release]. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-202011 March 2020
- 3.Yu A, Prasad S, Akande A, Murariu A, Yuan S, Kathirkamanathan S, et al. COVID-19 in Canada: a self-assessment and review of preparedness and response. J Glob Health. 2020;10(2):0203104. doi: 10.7189/jogh.10.0203104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Vecchio BG, Calabrese E, Biancone L, Monteleone G, Paoluzi OA. The impact of COVID-19 pandemic in the colorectal cancer prevention. Int J Colorectal Dis. 2020;35(10):1951–1954. doi: 10.1007/s00384-020-03635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Ovidio V, Lucidi C, Bruno G, Lisi D, Miglioresi L, Bazuro ME. Impact of COVID-19 pandemic on colorectal cancer screening program. Clin Colorectal Cancer. 2021;20(1):e5–e11. doi: 10.1016/j.clcc.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raynaulta MF, Lacroixb A, Quirionb R. Quebec's public health approach in response to the global COVID-19 pandemic. Bull Acad Natl Med. 2020;204(9):e157–e159. doi: 10.1016/j.banm.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(INESSS) UpdlIndeeseess. First wave of the COVID-19 pandemic in Québec: A look at the factors associated with hospitalization and death. Dec 2020
- 8.Committee aCSA. Canadian Cancer Statistics. Canadian Cancer Society 2019
- 9.Kornbluth A, Sachar DB, Amer CG. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology practice parameters committee. Am J Gastroenterol. 2010;105(3):501–523. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 10.Care CTFoPH Recommendations on screening for colorectal cancer in primary care. Can Med Assoc J. 2016;188(5):340–348. doi: 10.1503/cmaj.151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.cancérologie Dgd. Normes de pratique clinique relatives à la coloscopie In: sociaux LDdcdmdlSedS, editor. Bibliothèque et Archives nationales du Québec, 2018
- 12.Juul T, Battersby NJ, Christensen P, Janjua AZ, Branagan G, Laurberg S, et al. Validation of the English translation of the low anterior resection syndrome score. Colorectal Dis. 2015;17(10):908–916. doi: 10.1111/codi.12952. [DOI] [PubMed] [Google Scholar]
- 13.Force UPST. Screening for Colorectal Cancer: US preventive services task force recommendation statement. JAMA. 2021;325(19):1965–1977. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 14.Ladabaum U, Dominitz JA, Kahi C, Schoen RE. Strategies for colorectal cancer screening. Gastroenterology. 2020;158(2):418–432. doi: 10.1053/j.gastro.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Balzora S, Issaka RB, Anyane-Yeboa A, Gray DM, 2nd, May FP. Impact of COVID-19 on colorectal cancer disparities and the way forward. Gastrointest Endosc. 2020;92(4):946–950. doi: 10.1016/j.gie.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feletto E, Grogan P, Nickson C, Smith M, Canfell K. How has COVID-19 impacted cancer screening? Adaptation of services and the future outlook in Australia. Public Health Res Pract. 2020;30(4):14. doi: 10.17061/phrp3042026. [DOI] [PubMed] [Google Scholar]
- 17.Knottnerus A, Tugwell P. STROBE–a checklist to strengthen the reporting of observational studies in epidemiology. J Clin Epidemiol. 2008;61(4):323. doi: 10.1016/j.jclinepi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64(5):402–406. doi: 10.4097/kjae.2013.64.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris EJA, Goldacre R, Spata E, Mafham M, Finan PJ, Shelton J, et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol. 2021;6(3):199–208. doi: 10.1016/S2468-1253(21)00005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buscarini E, Benedetti A, Monica F, Pasquale L, Buttitta F, Cameletti M, et al. Changes in digestive cancer diagnosis during the SARS-CoV-2 pandemic in Italy: a nationwide survey. Dig Liver Dis. 2021;53(6):682–688. doi: 10.1016/j.dld.2021.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Patel S, Issaka RB, Chen E, Somsouk M. Colorectal cancer screening and COVID-19. Am J Gastroenterol. 2021;116(2):433–434. doi: 10.14309/ajg.0000000000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2021;14:1–15. doi: 10.1007/s12029-021-00679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams E, Kong JC, Singh P, Prabhakaran S, Warrier SK, Bell S. The impact of the COVID-19 pandemic on colorectal cancer diagnosis and management: a Binational Colorectal Cancer Audit study. ANZ J Surg. 2021;91(10):2091–2096. doi: 10.1111/ans.17071. [DOI] [PubMed] [Google Scholar]
- 24.Ricciardiello L, Ferrari C, Cameletti M, Gaianill F, Buttitta F, Bazzoli F, et al. Impact of SARS-CoV-2 Pandemic on Colorectal Cancer Screening Delay: Effect on Stage Shift and Increased Mortality Clinical gastroenterology and hepatology: the official clinical practice. J Am Gastroenterol Assoc. 2021;19(7):1410–7.e9. doi: 10.1016/j.cgh.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yong JH, Mainprize JG, Yaffe MJ, Ruan Y, Poirier AE, Coldman A, et al. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J Med Screen. 2021;28(2):100–107. doi: 10.1177/0969141320974711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loveday C, Sud A, Jones ME, Broggio J, Scott S, Gronthound F, et al. Prioritisation by FIT to mitigate the impact of delays in the 2-week wait colorectal cancer referral pathway during the COVID-19 pandemic: a UK modelling study. Gut. 2021;70(6):1053–1060. doi: 10.1136/gutjnl-2020-321650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao AH, Chang SY, Stevoff CG, Komanduri S, Pandolfino JE, Keswani RN. Adoption of multi-society guidelines facilitates value-based reduction in screening and surveillance colonoscopy volume during COVID-19 pandemic. Dig Dis Sci. 2021;66(8):2578–2584. doi: 10.1007/s10620-020-06539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Issaka RB, Taylor P, Baxi A, Inadomi JM, Ramsey SD, Roth J. Model-based estimation of colorectal cancer screening and outcomes during the COVID-19 pandemic. JAMA Netw Open. 2021;4(4):e216454. doi: 10.1001/jamanetworkopen.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myint AA. Noninvasive colorectal cancer screening tests help close screening gaps during coronavirus disease 2019 pandemic. Gastroenterology. 2021;161(2):712–714. doi: 10.1053/j.gastro.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng SY, Chen CF, He HC, Chang LC, Hsu WF, Wu MS, et al. Impact of COVID-19 pandemic on fecal immunochemical test screening uptake and compliance to diagnostic colonoscopy. J Gastroenterol Hepatol. 2021;36(6):1614–1619. doi: 10.1111/jgh.15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tinmouth J, Dong S, Stogios C, Rabeneck L, Rey M, Dubé C, et al. Estimating the backlog of colonoscopy due to Coronavirus disease 2019 and comparing strategies to recover in Ontario Canada. Gastroenterology. 2021;160(4):1400–2.e1. doi: 10.1053/j.gastro.2020.11.048. [DOI] [PubMed] [Google Scholar]
- 32.Kastrinos F, Syngal S. Inherited colorectal cancer syndromes. Cancer J. 2011;17(6):405–415. doi: 10.1097/PPO.0b013e318237e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(s2):1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.