ABSTRACT
Introduction
Children’s maximal muscle strength is consistently lower than adults’, even when normalized to body size. Lower volitional muscle activation (VA) in children is often considered one of the main reasons for age-related differences in muscular performance. However, some recent studies have reported similar VA in children and adults, bringing into question whether there is indeed an age-related increase in VA. The purpose of this review was to determine the effect of age on VA during maximal isometric contractions.
Methods
Literature examining VA differences, using twitch interpolation in children (7–14 yr) and adults (16–28 yr), was systematically reviewed. Of the 1915 studies initially identified, 19 data sets were eligible for inclusion in the qualitative analysis and 14 in the quantitative meta-analysis (comprising 207 children and 193 adults).
Results
Significantly lower VA in children was reported in 9/19 (47%) studies. A random-effects meta-analysis found a strong effect of age on VA, supporting lower VA in children compared with adults (Hedges’ g = 1.55; confidence interval: 0.9–2.13). Moderator analysis included muscle group, sex, children’s age, stimulation number (singlet, multiple), type (electric, magnetic), and location (muscle, nerve), of which only muscle group was significant (P < 0.001). A significant Egger’s regression test and asymmetrical funnel plot suggest that publication bias may be present.
Conclusions
Overall, these findings suggest that compared with adults, children activate their motor-unit pool less compared with adults. Moreover, that the degree of VA increase with age may be influenced by the muscle examined (upper vs lower extremity). However, more research is needed to elucidate the influence of this possible factor, as the current review contains limited data from upper body muscles. The developmental mechanism responsible for children’s lower VA requires further research.
Key Words: VOLUNTARY ACTIVATION, INTERPOLATED TWITCH, MUSCLE ACTIVATION, MAXIMAL VOLUNTARY CONTRACTION, MATURATION
Children’s muscle performance is consistently lower than adults’ (e.g., lower maximal strength and rate of force development), even after accounting for differences in body size (1–4). Based on differences in growth rate versus the rate of strength gain, Asmussen and Heeboll-Nielsen (5) suggested, already in 1955, that beyond body size, lower volitional muscle activation (VA) in children can explain their lower muscular performance. This notion has been supported by subsequent studies demonstrating lower size-normalized maximal strength and lower rate of force development (2,5–7), as well as differences in the electromyographic (EMG) pattern during various contraction tasks (8,9). Several studies have also demonstrated lower VA in children, compared with adults, during maximal volitional contractions (MVC) of various muscle groups (e.g., quadriceps, biceps bracii, adductor pollicis) (4,10–14). However, several recent studies have failed to identify child–adult VA differences (15–21). These inconsistent findings have clouded our understanding of the maturational changes in muscle performance and the influence that possible changes in VA may have on performance, specifically maximal strength.
VA is typically assessed and quantified using twitch interpolation (interpolated twitch technique, or ITT), where an electric or magnetic pulse is applied to the muscle or motor nerve during MVC. This technique was first introduced by Merton (22), who argued that when an electrical stimulus is applied to the muscle or associated motor nerve during an MVC and additional force is evoked (superimposed twitch), there is incomplete volitional activation of the muscle (i.e., activation deficit). Neural mechanisms resulting in activation deficit include submaximal motor-unit recruitment and/or suboptimal firing rates during volitional contractions.
Using twitch interpolation, there are currently two approaches for calculating VA. The more traditional approach is the “central activation ratio” (VACAR), which quantifies VA as percentage fraction: MVC/(MVC + superimposed twitch) × 100 (22). At present, a more commonly used variant of VACAR adds a resting twitch after the MVC, to account for peripheral phenomena such as potentiation, and is often termed “VA” or “VAITT” to distinguish it from VACAR. VAITT is calculated as follows: (1 − superimposed twitch/resting twitch) × 100 (23). Both calculations have been used to quantify VA mainly during maximal isometric contractions in various populations (e.g., children, adults, elderly, and those with chronic conditions).
There are several methodological and physiological factors that may affect VA determination by affecting the muscle’s mechanical output. For instance, stimulation intensity, number of stimuli (single vs multiple stimuli), stimulation frequency (when multiple stimuli are used), musculotendinous stiffness, muscle potentiation, and coactivation, are all factors found to affect VA in adults (24–26). More specifically, these factors influence the accuracy and reliability of VA determination. For the purpose of this review, it is also important to note that some of these factors are also affected by maturation. For example, coactivation is often found to be greater in children than in adults, whereas musculotendinous stiffness and muscle potentiation may be lower in children (27,28). Nevertheless, most studies have used either the VAITT or VACAR approach similarly in children and adults.
Therefore, the purpose of this review was to systematically examine child–adult differences in VA during maximal isometric contractions and possible related mediators. The published research, which examined VA (by estimating VAITT or VACAR) in both children and adults, was systematically reviewed and integrated in a meta-analysis. We hypothesized that VA in children would be lower than in adults. We also hypothesized that factors such as stimulation methods (stimulation number, type, and location), muscle group examined (upper or lower limb), and age of the child participants would influence the observed age effect.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used to guide our investigation and present the systematic review (http://www.prisma-statement.org/). Furthermore, before data extraction, the methods and analysis procedures of the systematic review were disclosed publicly on the International Prospective Register of Systematic Reviews (209907).
Literature search
An initial search of MEDLINE (OVID), MBASE (OVID), SPORTDiscus (EBSCO), and Web of Science databases was performed on August 11, 2020, to identify relevant studies. The combination of keywords and/or phrases (mp), and MeSH terms (/) pertinent to “children” (Child/, child*mp, adolesc*mp, youth*mp, boy*mp, girl*mp), “adults” (Adult/, *adult/, *middle age/, *young adult/, Adult*mp, men mp, man mp, women mp, woman mp, female*mp, male*mp) and “voluntary muscle activation” (voluntary activation mp, muscle activation mp, central fatigue mp, central activation mp, volitional activation mp, motor unit activation mp) were used to search for relevant articles. This search was repeated on April 22, 2021, to check for additional articles published in the intervening period. One additional article was found (23).
Once the search was complete, all the identified publications were uploaded into screening and citation management software (Covidence and Zotero, respectively). After duplicates were removed, three reviewers (B.F., J.M., C.O.) independently screened titles and abstracts for relevant articles. Conflicts among reviewers were resolved by an additional reviewer (R.D.). Next, article eligibility was further assessed by two reviewers (B.F., S.W.), independently, by screening the full text of all the remaining articles. Also, at this stage, the reference lists were screened to identify any relevant articles that were missed in the search. Conflicts between reviewers were resolved by an additional reviewer (R.D.). See Figure 1 for a PRISMA flowchart of the articles included and excluded throughout the screening process.
FIGURE 1.
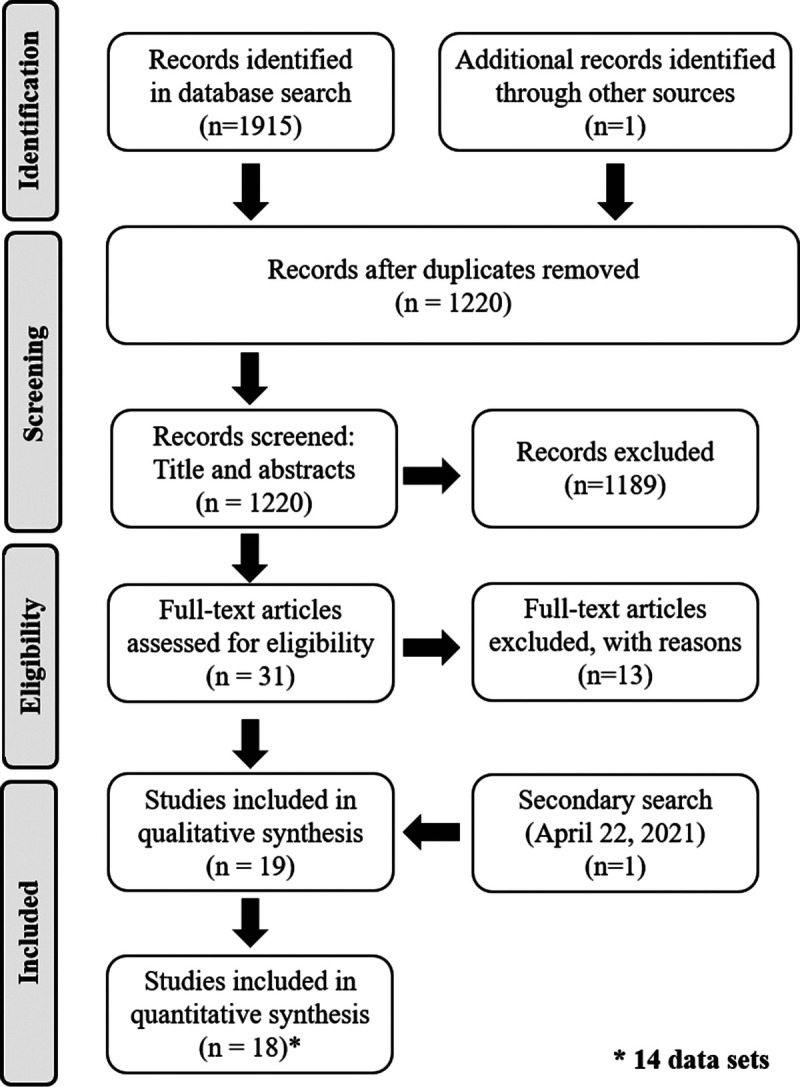
PRISMA flowchart of study selection.
Identification and selection of studies
Studies were included in the review if they assessed VA in healthy children and adolescents or young adults during maximal isometric contractions, using VAITT or VACAR. If an intervention (e.g., fatiguing contractions) was part of the study design, baseline values were extracted. If data were presented in the article as figures, authors were contacted, and the relevant data requested. If data could not be provided, group means and SDs were estimated using WebPlotDigitizer (29), which is well accepted and has been shown to be a valid tool for extracting data from figures (30). No limits were placed on the year of publication, and only full-text articles published in English were identified. Studies were excluded from the meta-analysis if effect sizes (ES) could not be calculated (10).
Quality assessment
Risk of bias was assessed using a combination of the Appraisal tool for Cross-Sectional Studies (AXIS tool) (31) and Quality Assessment Tool for Quantitative Studies (32). Some items were removed, as they were not relevant for cross-sectional studies with no intervention. The assessment tools were used to evaluate the following qualities: 1) sampling/target population, 2) design, 3) procedures, 4) statistical analysis, 5) reporting of findings, 6) reporting withdrawals/nonresponders, and 7) possible bias from funding sources. The risk of bias assessment was completed for all studies by two researchers independently (B.F., S.W.), and disagreements were resolved by consensus. Publication bias was also assessed using a funnel plot and Egger’s regression test.
Data extraction and analysis
From the included studies, participant characteristics (sex, age, and pubertal stage or maturational status for the children), muscle group examined, calculation method (VAITT or VACAR) for estimating VA, stimulation number (singlet, doublet, train), technique (electric or magnetic), location (muscle or nerve), and estimated VA (group mean and SD) were extracted by two authors independently (B.F., S.W.). Conflicts were resolved by consensus.
Narrative synthesis
For the narrative synthesis, extracted data were compiled into a table where each comparison is listed separately (Table 1). Comparisons are organized so that those who report a significant difference in VA between children and adults are listed first, then by muscle examined, and lastly, in alphabetical order.
TABLE 1.
Studies reporting VA in both children and adults.
| Reference | Sex | Participant Number and Age, n (Age in Years) | Muscle | Stim Type/Location |
VA Calculation | Stim No. | VA Results (%) | Significant Difference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Children | Adults | Children | Adults | |||||||
| Blimkie (10) | Male | 25 (~10) | 10 (~16) | KE | ES/M | VAITT | Single | 77.7 | 95.3 | Yes |
| Kluka et al. (14) | Male | 13 (10.2 ± 1.1) | 10 (23.9 ± 2.9) | KE | MS/N | VAITT | Single | 88.0 ± 8.0 | 94.0 ± 4.0 | Yes |
| O’Brien et al. (12)a | Female | 10 (9.3 ± 0.8) | 10 (27.4 ± 4.2) | KE | MS/M | VAITT | Doublet | 66.9 ± 13.0 | 86.6 ± 6.6 | Yes |
| O’Brien et al. (4)a | Female | 10 (9.3 ± 0.8) | 10 (27.4 ± 4.2) | KE | MS/M | VAITT | Doublet | 68.0 ± 11.6 | 86.6 ± 6.6 | Yes |
| Streckis et al. (33)b | Female | 7 (13.6 ± 0.2) | 7 (20.8 ± 0.5) | KE | ES/M | VAITT | Train (500 ms) | 83.2 ± 2.6 | 94.4 ± 1.1 | Yes |
| Grosset et al. (13), 7 yr | Male | 6 (7) | 9 (21.0 ± 2.3) | PF | ES/N | VACAR | Single | 87.0 ± 4.7 | 98.5 ± 0.2 | Yes |
| Kluka et al. (34) | Male | 14 (10.0 ± 1.0) | 15 (24.6 ± 4.2) | PF | MS/N | VAITT | Single | 87.6 ± 1.6 | 92.4 ± 1.7 | Yes |
| Gillen et al. (35) | Male | 10 (9.8 ± 0.5) | 10 (17.6 ± 0.8) | EF | ES/M | VAITT | Doublet | 72.6 ± 4.9 | 89.8 ± 5.5 | Yes |
| Gillen et al. (35) | Female | 10 (9.8 ± 0.6) | 10 (16.9 ± 1.0) | EF | ES/M | VAITT | Doublet | 67.2 ± 6.7 | 90.3 ± 3.5 | Yes |
| Martin et al. (11) | Male | 13 (11.6 ± 0.1) | 8 (25.6 ± 1.5) | ADP | MS/N | VAITT | Single | 85.0 ± 2.7 | 94.8 ± 1.4 | Yes |
| Bontemps et al. (15) | Male | 18 (10.4 ± 0.8) | 19 (21.7 ± 3.4) | KE | MS/N | VAITT | Single | 90.1 ± 6.3 | 92.8 ± 3.9 | No |
| Chalchat et al. (16) | Male | 11 (10.3 ± 0.7) | 13 (23.8 ± 3.1) | KE | MS/N | VAITT | Single | 93.2 ± 2.9 | 94.9 ± 2.4 | No |
| Gorianovas et al. (36)b | Male | 11 (11.8 ± 0.9) | 11 (20.8 ± 1.9) | KE | ES/M | VAITT | Train (250 ms) | 87.5 ± 7.8 | 93.3 ± 6.2 | No |
| O’Brien et al. (12)a | Male | 10 (8.9 ± 0.7) | 10 (28.2 ± 3.6) | KE | MS/M | VAITT | Doublet | 75.1 ± 12.8 | 85.6 ± 8.5 | No |
| O’Brien et al. (4)a | Male | 10 (8.9 ± 0.7) | 10 (28.2 ± 3.6) | KE | MS/M | VAITT | Doublet | 75.1 ± 12.8 | 86.7 ± 9.3 | No |
| Piponnier et al. (17)c | Male | 21 (10.4 ± 0.7) | 24 (21.4 ± 3.2) | KE | MS/N | VAITT | Single | 90.4 ± 6.0 | 92.7 ± 4.1 | No |
| Piponnier et al. (18)c | Male | 22 (10.3 ± 0.7) | 22 (21.6 ± 3.3) | KE | MS/N | VAITT | Single | 90.9 ± 5.6 | 92.6 ± 4.3 | No |
| Piponnier et al. (19)d | Male | 9 (9.9 ± 1.3) | 11 (23.6 ± 3.0) | KE | MS/N | VAITT | Single | 87.1 ± 7.6 | 92.2 ± 3.2 | No |
| Streckis et al. (33)b | Male | 7 (13.9 ± 0.3) | 7 (22.2 ± 0.9) | KE | ES/M | VAITT | Train (500 ms) | 91.5 ± 2.4 | 92.8 ± 1.3 | No |
| Ratel et al. (21)d | Male | 11 (9.9 ± 1.2) | 12 (23.9 ± 3.5) | KE | MS/N | VAITT | Single | 86.9 ± 7.6 | 91.2 ± 2.6 | No |
| Belanger and McComas (37) | Male | 10 (11.0 ± 2.3) | 8 (16.5 ± 0.9) | PF | ES/N | VAITT | Single | 94.0 ± 11.3 | 99.4 ± 1.8 | No |
| Grosset et al. (13), 10 yr | Male | 11 (10) | 9 (21.0 ± 2.3) | PF | ES/N | VACAR | Single | 95.6 ± 1.0 | 98.5 ± 0.2 | No |
| Grosset et al. (13), 11 yr | Male | 5 (11) | 9 (21.0 ± 2.3) | PF | ES/N | VACAR | Single | 96.7 ± 0.3 | 98.5 ± 0.2 | No |
| Hatzikotoulas et al. (38) | Male | 11 (10.7 ± 0.2) | 11 (26.4 ± 0.7) | PF | ES/N | VACAR | Single | 98.1 ± 0.4 | 98.5 ± 0.5 | No |
| Piponnier et al. (17)c | Male | 21 (10.4 ± 0.7) | 24 (21.4 ± 3.2) | PF | MS/N | VAITT | Single | 95.2 ± 3.9 | 95.4 ± 4.5 | No |
| Piponnier et al. (20)c | Male | 19 (10.2 ± 0.6) | 23 (21.5 ± 3.3) | PF | MS/N | VAITT | Single | 93.6 ± 5.1 | 94.4 ± 3.9 | No |
| Belanger and McComas (37) | Male | 10 (11.0 ± 2.3) | 8 (16.5 ± 0.9) | DF | ES/M | VAITT | Single | 100 ± 0 | 100 ± 0 | No |
| Blimkie (10) | Male | 26 (10) | 10 (16) | EF | ES/M | VAITT | Single | 89.4 | 89.9 | No |
Note that some studies report volitional activation of multiple groups (age groups, male/female). Each comparison is listed separately.
bVA data estimated from a figure using WebPlotDigitizer (35).
dData reported in Piponnier et al. (19) and Ratel et al. (21) come from the same participants’ data set.
ES, electrical stimulation; M, muscle; MS, magnetic stimulation; N, nerve; VACAR, volitional activation, calculated the central activation ratio approach; VAITT, volitional activation, calculated using the traditional ITT.
Meta-analysis of pooled data
A meta-analysis of the pooled data was used to further examine age-related differences in VA between children and adults. We used Campbell’s Collaboration calculator (www.campbellcollaboration.org/resources) to compute Hedges’ g ES. A mixed-effects model was used to examine main effect of age on VA. This model was appropriate, as ES values vary considerably because of between-study differences (e.g., methodological procedures, participants’ demographic characteristics), and we aimed to generalize our findings beyond the sampled studies (39). After the calculation of confidence intervals, Cochran’s Q statistic was used to test the heterogeneity of the pooled distribution. Since the Q statistic maintained a low power (limited studies in meta-analysis), the I2average was also computed (40). Moreover, computation of I2 is required to assist with the interpretation of the Q test, because the Q test does not indicate the extent of true heterogeneity, but rather just that the effect is significant. To further explore the origins of heterogeneity, a moderator analysis was conducted, with muscle group, sex, stimulus type, number and location, and age of child participants. All statistical procedures were performed in Comprehensive Meta-Analysis program (version 3.0). Significance of ES was determined at P < 0.05.
RESULTS
Study Selection
A total of 1915 studies were identified in the initial search (Fig. 1). An additional study was identified in paper records, and another study was identified in the secondary search (see Literature search). After the removal of duplicates (n = 695), 1189 additional studies were excluded after title and abstract screening. Full-text screening was conducted on 32 studies, and 19 were included in the final qualitative analysis. Of the 19 studies, 18 were included in the meta-analysis (see Selection criteria). After communication with some of the authors, it was determined that some of the studies reported results from the same participant data set. These studies were pooled, leaving a total of 14 separate participant sets in the final meta-analysis.
Study Characteristics: Narrative Synthesis
Table 1 summarizes the 19 studies included in the qualitative review. The studies included were published between 1989 and 2021 and comprised a total of 233 children (7–14 yr) and 203 young adults (16–28 yr). Some participants were included in more than one study (see Table 1 footnotes). The mean sample sizes were 16 (range, 7–37) and 14 (range, 7–24) for children and adults, respectively. All but four studies (79%) examined males only. A statistically significant lower VA in children was reported in 47% (9/19) of the studies. However, in all but one case, children’s reported VA values were lower than in adults. In this single case, dorsiflexor VA was identical in children and adults (37).
Eighty-four percent (16/19) of the studies examined VA only in lower-extremity muscles. Some of these studies examined two lower-extremity muscle groups (10,17,37) (one tested dorsiflexors (DF), 6 tested plantar flexors (PF), 12 tested knee extensors (KE)). One of 19 studies examined both lower and upper extremity muscles (elbow flexors (EF) and KE) (10), and 2 of 19 studies examined only an upper extremity muscle (adductor pollicis (ADP) or EF) (11,35). In the lower extremity muscles, VA was significantly lower in children in 7/17 (41%) of the studies (4,10,12–14,33,34). Although motor units are typically classified along a spectrum from low to high threshold, it is presumed that high-threshold motor units are primarily composed of fibers with type II metabolic and mechanical characteristics (41,42). It is hypothesized that children activate their high-threshold motor units to a lesser extent, during maximal contractions, compared with adults (43). Thus, child–adult differences in VA may be influenced by the relative type-II composition of the tested muscle. The PF muscle group, which plays a chronic postural role, is predominantly composed of endurance-oriented muscle fibers of the type-I fiber characteristics. This group was examined in 32% (6/19) of the studies included in the qualitative analysis (13,17,20,34,37,38). Of these studies, a significant child–adult VA difference was reported in two (33%) of the studies (13,14). Fifteen of the studies examined muscles, which could be classified as having a “mixed” fiber type, containing similar proportion of type-I and type-II muscle fibers (4,10–12,14–19,21,33,35–37). Of these studies, 47% (7/15) reported VA to be significantly lower in children than in adults (4,10–12,14,33,35).
The age range of the children who participated in the included studies was 7–14 yr. In 32% (6/19) of studies, the mean age of the children was less than 10 yr (4,12,13,19,21,35). Sixty-seven percent (4/6) of these studies reported lower VA in children compared with adults (4,12,13,35). Of the studies investigating “older” children (>10 yr, n = 14), 35% (5/14) reported significantly lower VA in children compared with adults (10,11,14,33,34).
Most of the studies (74%) used a single stimulus (singlet) when evoking a muscle twitch for the assessment of VA. Five studies (26%) used multiple stimuli, where 60% (3/5) utilized doublet stimuli (4,12,35). The other two studies using stimuli trains of 250–500 ms (33,36). Of the studies using multiple stimuli, 80% (4/5) reported significantly lower VA in children compared with adults (4,12,33,35).
Thirty-seven percent (7/19) of studies evoked twitches using electrical stimulation (10,13,33,35–38), where the remaining used magnetic stimulation. Of the studies using electrical stimulation, 57% (4/7) reported significant differences in VA between children and adults (10,13,33,35). Of the studies using magnetic stimulation, 42% (5/12) reported significant child–adult differences in VA.
Thirty-two percent (6/19) of studies applied the stimulus (electrical or magnetic) directly to the muscle (4,10,12,33,36,37). Of these studies, 67% (4/6) reported significantly lower VA in children compared with adults (4,10,12,33). Of the studies that applied the stimulus (electrical or magnetic) to the nerve, 36% (5/14) reported significantly lower VA in children than in adults (11,13,14,34,35).
Risk of bias
A subset of questions from two validated questionnaires were used to assess risk of bias among the studies included (31,32). For the Thomas et al. (32) assessment, studies were ranked “strong,” “moderate,” or “weak.” For the Downes et al. (31) assessment, studies were classified as either “meeting” the criteria (“yes”) or “not meeting” the criteria (“no”).
Using Thomas et al.’s (32) assessment tool, 18 studies were classified as “strong” and 1 “moderate” in terms of the validity of the data-collection method. Selection bias was rated as “strong” for 1 study, “moderate” for 15 studies, and “weak” for the remaining 3. Seven studies were rated as “strong,” 8 as “moderate,” and 4 as “weak,” in terms of controlling for confounding variables (e.g., training status). All studies but one were rated as “weak” for reporting of participants who withdrew from the study. Lastly, all studies were given a “weak” rating for “blinding” the data reduction and analysis.
Quality ratings using the Downes et al.’s (31) assessment tool are as follows: Only two studies provided justification for sample size. Sixteen of the studies clearly defined the target population, and it is likely that the selection processes resulted in recruiting representative participants. However, in most studies, little information was provided about recruitment strategy, which made the representativeness of the population difficult to evaluate. Eighteen studies defined the procedures, protocols, and statistical procedures clearly enough for them to be repeated. None of the studies had funding where a conflict of interest would be of concern. Finally, none of the studies disclosed whether there were “nonresponders” or any participants where VA could not be assessed.
Meta-Analysis of Pooled Data
Main analyses
The meta-analysis was performed on 14 data sets, including 207 children and 193 young adults. Hedges’ g, 95% confidence intervals, and Q statistics for the studies included in the meta-analysis are presented in Table 2 and illustrated in Figure 2. The test of heterogeneity Q revealed that the observed ES across the studies was large (Hedges’ g = 1.55; confidence interval: 0.96–2.13) and that approximately 85% of the variance observed is a true effect rather than sampling error (I2average = 85.20).
TABLE 2.
Random-effects meta-analysis results.
| Reference | Statistics for Each Study | ||||||
|---|---|---|---|---|---|---|---|
| Hedges’ g | SE | Variance | Lower Limit | Upper Limit | Z | P | |
| Belanger and McComas (37) | 0.425 | 0.457 | 0.209 | −0.471 | 1.321 | 0.929 | 0.353 |
| Bontemps et al. (15) | 0.507 | 0.327 | 0.107 | −0.134 | 1.149 | 1.551 | 0.121 |
| Chalchat et al. (16) | 0.622 | 0.406 | 0.165 | −0.173 | 1.417 | 1.533 | 0.125 |
| Gillen et al. (35), | 3.709 | 0.518 | 0.268 | 2.694 | 4.723 | 7.164 | <0.001 |
| Grosset et al. (13) | 2.403 | 0.471 | 0.222 | 1.480 | 3.326 | 5.102 | <0.001 |
| Gorianovas et al. (36) | 0.816 | 0.428 | 0.183 | −0.024 | 1.655 | 1.905 | 0.057 |
| Hatzikotoulas et al. (38) | 0.962 | 0.445 | 0.198 | 0.090 | 1.834 | 2.161 | 0.031 |
| Kluka et al. (14) | 0.878 | 0.426 | 0.181 | 0.043 | 1.712 | 2.062 | 0.039 |
| Kluka et al. (34) | 2.823 | 0.518 | 0.268 | 1.809 | 3.837 | 5.455 | <0.001 |
| Martin et al. (11) | 4.076 | 0.763 | 0.582 | 2.582 | 5.571 | 5.345 | <0.001 |
| O’Brien et al. (4,12) | 1.431 | 0.349 | 0.122 | 0.747 | 2.114 | 4.102 | <0.001 |
| Piponnier et al. (17,18,20) | 0.260 | 0.298 | 0.089 | −0.323 | 0.844 | 0.874 | 0.382 |
| Piponnier et al. (19) and Ratel et al. (21) | 0.817 | 0.430 | 0.185 | −0.026 | 1.660 | 1.901 | 0.057 |
| Streckis et al. (33) | 3.075 | 0.551 | 0.303 | 1.995 | 4.155 | 5.581 | <0.001 |
| Overall | 1.545 | 0.300 | 0.090 | 0.958 | 2.133 | 5.156 | <0.001 |
FIGURE 2.
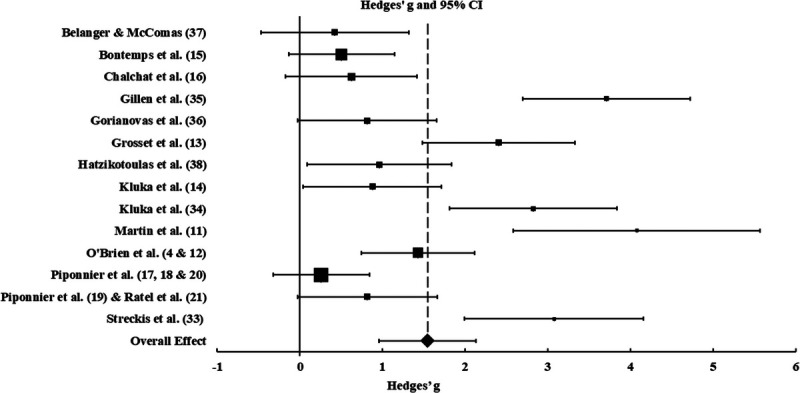
Forest plot of mean, overall, and individual study effects. Diamond and dashed line indicate the mean over all random effect for differences in VA between children and adults. Squares indicate the individual study effect, with the size indicating the weighting, and 95% confidence intervals are indicated by horizontal lines. Effects to the right of the 0 indicate VA greater in adults than in children.
A funnel plot based on the Hedges’ g ES (x axis) and standard errors (y axis) for each study is presented in Figure 3. Because the funnel plot is asymmetrical, the possibility of publication bias was further explored using Egger’s regression test. The intercept of Egger’s test was significant (t = 4.41, P < 0.001), suggesting presence of publication bias. However, Rosenthal’s fail-safe N test revealed that 529 studies would be needed to nullify (i.e., nonsignificant result; P > 0.05) these findings, and Owrin’s fail-safe N test revealed that 159 additional studies with a null effect (g = 0) would be needed to bring the observed ES values to a trivial value of Hedges’ g = 0.10. Overall, these results suggest that there is an effect of age on VA. That is, VA is lower in children compared with adults.
FIGURE 3.
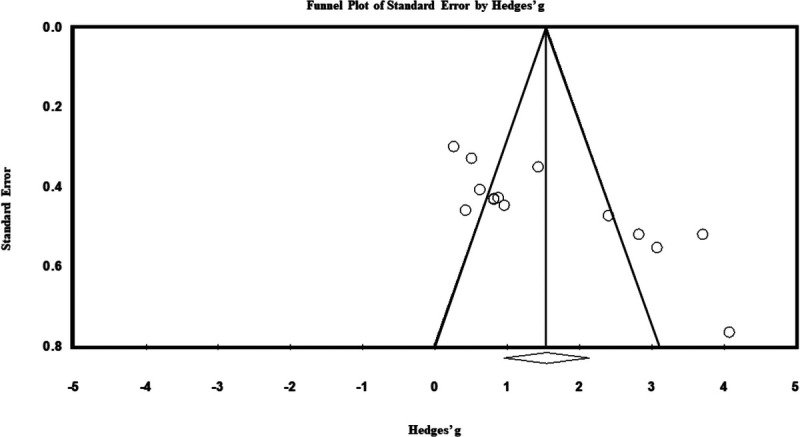
Funnel plot of standard error by Hedges’s g.
Moderator analyses
Six moderators were examined for their contribution in accounting for the ES heterogeneity, namely, muscle group (upper or lower body), sex (male or female), age category (young or older children), stimulation type (electric or magnetic), stimulation location (muscle or nerve) and number of stimulations (single or doublet/train). The only moderator found to be significant was muscle group (upper vs lower extremity; Table 3).
TABLE 3.
Results of moderator analysis.
| Moderator | No. Data Sets | Point Estimate | SE | Lower Limit | Upper Limit | Q | P |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| Young children <10 yr | 4 | 2.056 | 0.583 | 0.913 | 3.199 | ||
| Older children >10 yr | 10 | 1.328 | 0.336 | 0.670 | 1.986 | ||
| Total between | 1.169 | 0.280 | |||||
| Stimulation type | |||||||
| Muscle | 6 | 1.873 | 0.537 | 0.821 | 2.925 | ||
| Nerve | 8 | 1.283 | 0.344 | 0.609 | 1.957 | ||
| Total between | 0.857 | 0.354 | |||||
| Stimulation number | |||||||
| Single | 10 | 1.262 | 0.315 | 0.645 | 1.880 | ||
| Multiple | 4 | 2.217 | 0.652 | 0.940 | 3.495 | ||
| Total between | 1.741 | 0.187 | |||||
| Stimulation locationa | |||||||
| Muscle | 3 | 1.719 | 0.583 | 0.576 | 2.862 | ||
| Nerve | 10 | 1.617 | 0.386 | 0.859 | 2.374 | ||
| Total between | 4.802 | 0.091 | |||||
| Muscle group | |||||||
| Lower body | 12 | 1.196 | 0.250 | 0.707 | 1.686 | ||
| Upper body | 2 | 3.825 | 0.428 | 2.985 | 4.664 | ||
| Total between | 28.106 | <0.001 | |||||
| Sex | |||||||
| Male | 11 | 1.271 | 0.304 | 0.675 | 1.866 | ||
| Male and female | 3 | 2.472 | 0.664 | 1.170 | 3.775 | ||
| Total between | 2.705 | 0.100 |
aBelanger and McComas (37) was not included because stimulus was evoked to the muscle (DF) and nerve (PF).
DISCUSSION
This meta-analysis is the first to review differences in VA between children and adults. Overall, less than half the individual studies reported significantly lower VA in children. However, once pooled, the meta-analysis of 14 data sets, including 207 children and 193 young adults, showed that VA is lower in children compared with adults, regardless of sex. This effect was also independent of the stimulation methodology used (i.e., magnetic vs electrical, single vs multiple impulses) and age of the child participants. Overall, these findings suggest that children activate their motor-unit pool to a lesser extent than adults. Lower VA in children can explain children’s lower body size–normalized maximal and explosive strength (4,44).
Although the pooled analysis showed a strong effect of age on VA, many of the individual data sets included in the meta-analysis concluded that VA did not differ between children and adults, based on lack of statistical significance (64% of comparisons; Table 1). The apparent discrepancy between the findings of some individual study comparisons and the current meta-analysis may have resulted from the small, convenience samples used in most studies (mean sample sizes of studies included in the meta-analysis, respectively). With such sample sizes and given the modest between-group differences and potentially large within-group variability, the individual studies may not have had the statistical power to detect a significant age effect, as was demonstrated in the present meta-analysis (45,46). Moreover, this sampling strategy can be problematic as the participants assessed may not be completely representative of the target population (47,48). For example, it is not clear whether participants in many of these studies were sedentary, physically active, or highly trained. In studies involving exercise, there may be a selection bias, as volunteers are likely to favor exercise or sports training. Assuming training increases VA, specifically in children (49–51), such a bias may decrease the likelihood of detecting a true difference between samples. Furthermore, small sample sizes may exaggerate this problem (52). Thus, studies with small sample sizes should be interpreted with caution. For this reason, meta-analyses are essential to compile findings from quality research and allow for a more robust examination of the research question.
The funnel plot and Egger’s regression test revealed that the meta-analysis may have been impacted by publication bias. Given our meta-analysis involved only 14 data sets from relatively small studies, publication bias may not be reliably detected by statistical tests (53). Moreover, an asymmetrical funnel plot and significant Egger’s test are attributed to “true heterogeneity” rather than publication bias (54,55). True heterogeneity among studies may have resulted from the different study designs, participant characteristics, or techniques used. More specifically, these factors could cause the precision of measurement to be dissimilar among studies, which consequently leads to the underlying effect examined by studies to be different. In the present review, we pooled data from cross-sectional studies comparing children and adults where participants’ age, training history, habitual activity level, testing protocols and techniques, as well as the muscles evaluated among studies were different. Therefore, we suggest that the observed funnel plot asymmetry and significant Egger’s regression test reflect a true difference among studies (i.e., true heterogeneity), rather than publication bias.
Factors that may affect child–adult VA differences
Based on the current literature, VA in adults seems to be >90%, whereas it varies widely in children (67%–100%). For example, O’Brien et al. (4,12) reported boys’ KE VA to be 75.1%, whereas Piponnier et al. (17) reported it to be 90.4%. In an attempt to provide some insight into this observation, we conducted a moderator analysis. The discussion hereinafter highlights our findings and discusses factors that may influence child–adult differences in VA.
Of the moderators included in the analysis, muscle group was the only one that was statistically significant (Table 3), suggesting that the effect of age on VA may differ between upper and lower extremity muscles. However, only two studies examined upper extremity muscles (ADP and EF) (11,35). These studies also exhibited the largest ES (g = 4.076 and 3.709). Therefore, the finding of greater child–adult VA differences in upper extremity muscles should be treated with caution. More studies are needed to elucidate whether child–adult VA differences vary between upper and lower extremity muscles and those with functional differences.
Child–adult differences in VA may also depend on the functionality and composition of the examined muscle or muscle group. It has been suggested that children activate their high-threshold motor units to a lesser extent compared with adults (43). We hypothesized that, under such conditions, child–adult VA differences would be smaller in muscle groups known to have a high percentage of low-threshold, type-I fibers (e.g., PF) (53,54). Such age-related differences are not clearly observed in the present review, although they are in the expected direction. That is, child–adult VA differences in the pooled data were ~4% (~93% vs 97%) in the PF and ~7% in mixed-composition muscles (~86% vs 93%). Although the observed VA values and child–adult differences thereof might not reflect the true values, it should be highlighted that these findings are congruent with children’s proposed lower capacity to activate higher-threshold motor units. Indeed, it is also possible that differences in muscle functionality (e.g., muscles used extensively vs those used irregularly), or in the muscles’ motor-unit recruitment range, may contribute to differing VA between muscles. Thus, it is possible that VA response may be muscle-specific, and that future studies should further examine how muscle characteristics, such as muscle composition, function, or recruitment range, affect VA in general and child–adult differences thereof, in particular.
Processes (e.g., myelination and synaptic pruning) involved in neuromotor maturation (e.g., myelination and synaptic pruning) seem to become heightened before and during puberty (11–14 yr; (36,44,45); see discussion hereinafter for more details). Implications of such changes can be observed by Grosset et al. (13), who examined PF VA and reported a linear decrease in activation deficit with increasing age (7–11 yr), with a significant child–adult difference only between 7-yr-old children and adults. These finding suggests that child–adult VA differences are age-dependent, with larger differences in studies of younger children. Thus, in the present analysis, we categorized studies that examined “younger” (<10 yr) or “older” children (≥10 yr). The qualitative narrative synthesis of studies (Table 1) suggests that child–adult differences in VA may be greater when younger children are examined. Specifically, child–adult differences seem to be more than twice as large in studies that examined “younger” than in those that tested “older” children (~14% vs ~4%, respectively). Nevertheless, the moderator analysis revealed that age group was not statistically significant (Table 3), indicating that children’s age did not influence the main effect of age (child vs adult) on VA. It is possible that statistical significance was not reached because there were only four data sets that included younger children. Thus, although examining VA in young children may be technically difficult, studies examining cohorts of multiple maturity levels (including young children) are necessary to further explain the nature of VA changes with maturation.
There is a lack of research regarding sex-related differences in neuromotor performance in adults and more so in children. Indeed, only 4/19 studies included in this review had a female participant group. During growth, sex-related differences in muscle performance are typically not observed until puberty (e.g., anaerobic power, muscular strength) (1,56–58). Moreover, age-related changes in muscle performance may be different in females compared with males and with a different timeline (2,7). Likewise, Long et al. (59) found cycling EMG threshold difference between girls and women (5.4%) to be smaller than the difference observed between boys and men (11.5%) (60). On the other hand, sex-related differences were not observed in isometrically determined EMG threshold (61). Furthermore, O’Brien et al. (4,12), Streckis et al. (33), and Gillen et al. (35) reported larger child–adult VA differences in females compared with males. Although the data are limited, it is important to note that in all cases in which VA was examined in females (4,12,33,35), it was found to be significantly lower in girls than in women. In the present analysis, sex was not found to be a statistically significant moderator (Table 3), suggesting that sex of the participants did not influence the observed child–adult differences in VA. In view of the limited data in females, more female–male comparative research is needed to understand whether there are sex-related differences in neuromuscular function, specifically during maturation.
Twitch interpolation (i.e., VAITT and VACAR) is a commonly used technique. Its validity and reliability in estimating VA have recently been questioned (26,62), because its results may be influenced by factors such as muscle length, composition, potentiation, or technical application. Specifically, these factors seem to influence the mechanical properties or output of the muscle and consequently affect superimposed-to-resting twitch ratios used to estimate VA (26) Notably, children have lower musculotendinous stiffness compared with adults (27,63), which can affect the resting twitch force (i.e., length–tension relationship). As was demonstrated by Hill (64), lower stiffness (or greater compliance) will result in dampened twitch torque and a delay in twitch onset. Potentially, this could result in a larger superimposed-to-resting twitch ratio in children and an underestimation of VA. More studies are needed to investigate how age-related differences in the muscle’s mechanical properties influence the superimposed-to-resting twitch ratio and therefore child–adult differences in VA. Possibly a child-specific, or a more sensitive VA test should be developed.
Methodological factors related to the stimulation can also influence the estimation of VA when using twitch interpolation. The studies within this review differ widely with respect to stimuli number, type, location, frequency, and timing. These differences make it difficult to directly compare studies and may explain the high inter-study variability in reported VA. In adults, it has been argued that using doublets or train stimulations may result in a more accurate estimate of VA (62,65), as such stimulation reduces the slack of series elastic components, resulting in a larger, better detectable superimposed twitch (66,67). As mentioned previously, children have lower musculotendinous stiffness than adults (27,63). Therefore, multiple stimulations may be required to estimate VA more accurately in children. Among adults, doublet stimulations have been shown to result in greater superimposed twitch torques than singlet stimulations (68), resulting in larger superimposed-to-resting twitch ratios and lower VAs. Although this seems congruent with greater motor-unit activation by doublet versus singlet stimulation, the notion has never been proven or refuted in adults. This may also be the case in children. Based on the pooled data, child–adult VA differences were larger (~8%) in studies using multiple stimulations than in those using singlets. Moreover, studies using multiple stimulations reported lower VA in both children and adults (by ~12% and ~5%, respectively) than studies using singlet stimulation. Considering children’s suggested greater activation deficit, stimulation number seems to be an important factor explaining why VA is overestimated by singlet stimulations. Although the use of doublets or train stimulation may be advantageous, we and others (15) have found that it is uncomfortable and not well tolerated by children. As observed in adults (69), this discomfort and anticipated pain may result in underperformance during MVC, thus affecting the SItw, resulting in an underestimation of VA. In children, the effect of the anticipated pain and discomfort may be further accentuated, explaining the apparent reliance on singlet stimulation in the studies reviewed (14/19 studies). The moderator analysis revealed that technical factors (e.g., stimulation number) were nonsignificant (Table 3), suggesting that child–adult differences in VA were not influenced by the stimulation procedures. However, it is possible that statistical significance was not reached because of the small number of studies and the large variability in VA. Future studies should examine the effects of different stimulation methodology on VA determination in children. Moreover, other techniques that do not involve electrical stimulation (e.g., magnetic resonance imaging) should be explored for the assessment of VA in children (70).
Possible mechanisms underlying child–adult VA differences
Much of the literature examining child–adult differences in VA focuses on peripheral factors that may affect VA, as reflected in the moderator analyses within this study. However, age-related increases in VA may be attributed to maturational changes occurring within the central nervous system. Cortical white matter (myelin) allows for fast and saltatory conduction of action potentials. Total cortical white matter volume increases from birth until ~30 yr of age (71–74), with accelerated myelination occurring in specific regions at different stages of development (75,76). Increased myelination, essential for high-quality interregional cortical communication, is observed with progressing maturation (77). Motor evoked potential thresholds (MEPTh) provide insight into the degree of cortical connectivity (e.g., myelination) and excitability of the corticospinal tracts, with a high MEPTh indicating lesser development (78). Several studies report MEPTh decreases with age during childhood, reaching adult levels by adolescence (78,79). Therefore, it is possible that enhanced myelination and connectivity lead to greater muscle activation, specifically of higher-threshold motor units.
Synaptic pruning is another process in the maturing cortex in which unused or “weak” synaptic connections are eliminated to create more efficient neural networks (80). Pruning may increase the strength of neuronal connections sufficiently to facilitate the depolarization of neurons (particularly neurons of higher-threshold motor units), which previously could not be depolarized, or allow them to depolarize at more optimal rates (81). As reflected from autopsy and magnetic resonance imaging studies, pruning seems to peak around the transition from childhood to adolescence (82–84). This is in line with the suggestion that child–adult differences in VA are more prominent when children are <10 yr old (13). That is, the timing of myelination and synaptic pruning may be related to the increasing VA in maturing children.
CONCLUSIONS
The present review compiled data from 19 studies and performed a meta-analysis on 14 data sets. The qualitative synthesis of the data shows that in all the studies but one, VA is lower in children compared with adults. However, likely because of the small sample sizes and the inherent variability associated with VA determination, many of the comparisons were not found to be statistically different. The quantitative meta-analysis found a strong main effect of age on VA, with lower VA in children compared with adults. Lower VA in children can explain their lower body-size–normalized maximal and explosive strength (4,44). This may also partly explain children’s strength improvements after resistance training without concomitant hypertrophy (49,50), but more research is needed to elucidate the possible resistance training effect on children’s VA. The age effect was found independent of physiological or methodological factors. However, because of the small number of available studies, more research is needed to provide better insights into these factors. Most of the studies to date examined mainly lower extremity muscles in males only. To attain a more comprehensive understanding of VA and maturation, future studies should compare lower versus upper extremity muscles, as well as males versus females. Mechanisms underlying an age-related increase in VA are unclear, but centrally regulated processes such as myelination and synaptic pruning are likely involved.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC grant to B. Falk). S. Woods holds an Ontario Graduate Scholarship, and C. O’Mahoney received an NSERC Undergraduate Student Research Award. The authors would like to thank Brock University Librarians Evelyn Feldman and Elizabeth Yates providing invaluable assistance with the systematic data search.
The authors declare no conflict of interest. The results of the present study do not constitute endorsement by the American College of Sports Medicine. All results presented here are done so clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Contributor Information
STACEY WOODS, Email: sw16hl@brocku.ca.
CARAGH O’MAHONEY, Email: co17xd@brocku.ca.
JAMES MAYNARD, Email: jm15mc@brocku.ca.
RAFFY DOTAN, Email: rdotan@brocku.ca.
GERSHON TENENBAUM, Email: gtenenbaum@admin.fsu.edu.
EDSON FILHO, Email: soares_medeiros@yahoo.com.br.
REFERENCES
- 1.De Ste Croix MBA, Armstrong N, Welsman J. Concentric isokinetic leg strength in pre-teen, teenage and adult males and females. Artic Biol Sport. 1999;16(2):75–86. [Google Scholar]
- 2.Falk B Usselman C Dotan R, et al. Child–adult differences in muscle strength and activation pattern during isometric elbow flexion and extension. Appl Physiol Nutr Metab. 2009;34(4):609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebestreit H, Mimura K, Bar-Or O. Recovery of muscle power after high-intensity short-term exercise: comparing boys and men. J Appl Physiol (1985). 1993;74(6):2875–80. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. In vivo measurements of muscle specific tension in adults and children. Exp Physiol. 2010;95(1):202–10. [DOI] [PubMed] [Google Scholar]
- 5.Asmussen E, Heeboll-Nielsen KR. A dimensional analysis of physical performance and growth in boys. J Appl Physiol. 1955;7(6):593–603. [DOI] [PubMed] [Google Scholar]
- 6.Asai H, Aoki J. Force development of dynamic and static contractions in children and adults. Int J Sports Med. 1996;17(3):170–4. [DOI] [PubMed] [Google Scholar]
- 7.Falk B, Brunton L, Dotan R, Usselman C, Klentrou P, Gabriel D. Muscle strength and contractile kinetics of isometric elbow flexion in girls and women. Pediatr Exerc Sci. 2009;21(3):354–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halin R, Germain P, Bercier S, Kapitaniak B, Buttelli O. Neuromuscular response of young boys versus men during sustained maximal contraction. Med Sci Sports Exerc. 2003;35(6):1042–8. [DOI] [PubMed] [Google Scholar]
- 9.Parra ME, Miller JD, Sterczala AJ, Trevino MA, Dimmick HL, Herda TJ. Differences in the firing rate versus recruitment threshold relationships of the vastus lateralis in children ages 7–10 years and adults. Hum Mov Sci. 2020;72:102650. [DOI] [PubMed] [Google Scholar]
- 10.Blimkie CJR. Age- and sex-associated variation in strength during childhood: anthropometric, morphologic, neurological, biomechanical, genetic and physical activity correlates. In: Gisolfi C, Lamb D, editors. Perspectives in Exercise Science and Sports. Indianapolis (IN): Benchmark; 1989. pp. 99–157. [Google Scholar]
- 11.Martin V, Kluka V, Garcia Vicencio S, Maso F, Ratel S. Children have a reduced maximal voluntary activation level of the adductor pollicis muscle compared to adults. Eur J Appl Physiol. 2015;115(7):1485–91. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. The effects of agonist and antagonist muscle activation on the knee extension moment–angle relationship in adults and children. Eur J Appl Physiol. 2009;106(6):849–56. [DOI] [PubMed] [Google Scholar]
- 13.Grosset JF, Mora I, Lambertz D, Pérot C. Voluntary activation of the triceps surae in prepubertal children. J Electromyogr Kinesiol. 2008;18(3):455–65. [DOI] [PubMed] [Google Scholar]
- 14.Kluka V Martin V Vicencio S, et al. Effect of muscle length on voluntary activation level in children and adults. Med Sci Sports Exerc. 2015;47(4):718–24. [DOI] [PubMed] [Google Scholar]
- 15.Bontemps B Piponnier E Chalchat E, et al. Children exhibit a more comparable neuromuscular fatigue profile to endurance athletes than untrained adults. Front Physiol. 2019;10:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalchat E Piponnier E Bontemps B, et al. Characteristics of motor unit recruitment in boys and men at maximal and submaximal force levels. Exp Brain Res. 2019;237(5):1289–302. [DOI] [PubMed] [Google Scholar]
- 17.Piponnier E Martin V Bontemps B, et al. Child–adult differences in neuromuscular fatigue are muscle dependent. J Appl Physiol. 2018;125(4):1246–56. [DOI] [PubMed] [Google Scholar]
- 18.Piponnier E Martin V Chalchat E, et al. Effect of muscle–tendon unit length on child–adult difference in neuromuscular fatigue. Med Sci Sports Exerc. 2019;51(9):1961–70. [DOI] [PubMed] [Google Scholar]
- 19.Piponnier E Martin V Bourdier P, et al. Maturation-related changes in the development and etiology of neuromuscular fatigue. Eur J Appl Physiol. 2019;119(11):2545–55. [DOI] [PubMed] [Google Scholar]
- 20.Piponnier E Ratel S Chalchat E, et al. Plantar flexor muscle–tendon unit length and stiffness do not influence neuromuscular fatigue in boys and men. Eur J Appl Physiol. 2020;120(3):653–64. [DOI] [PubMed] [Google Scholar]
- 21.Ratel S Kluka V Vicencio SG, et al. Insights into the mechanisms of neuromuscular fatigue in boys and men. Med Sci Sports Exerc. 2015;47(11):2319–28. [DOI] [PubMed] [Google Scholar]
- 22.Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123(3):553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas CK, Woods JJ, Bigland-Ritchie B. Impulse propagation and muscle activation in long maximal voluntary contractions. J Appl Physiol. 1989;67(5):1835–42. [DOI] [PubMed] [Google Scholar]
- 24.Folland JP, Williams AG. Methodological issues with the interpolated twitch technique. J Electromyogr Kinesiol. 2007;17(3):317–27. [DOI] [PubMed] [Google Scholar]
- 25.Gandevia SC, McNeil CJ, Carroll TJ, Taylor JL. Twitch interpolation: superimposed twitches decline progressively during a tetanic contraction of human adductor pollicis. J Physiol. 2013;591(5):1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dotan R, Woods S, Contessa P. On the reliability and validity of central fatigue determination. Eur J Appl Physiol. 2021;121(9):2393–411. [DOI] [PubMed] [Google Scholar]
- 27.Lambertz D, Mora I, Grosset J-F, Perot C. Evaluation of musculotendinous stiffness in prepubertal children and adults, taking into account muscle activity. J Appl Physiol. 2003;95(1):64–72. [DOI] [PubMed] [Google Scholar]
- 28.Arabatzi F Patikas D Zafeiridis A, et al. The post-activation potentiation effect on squat jump performance: age and sex effect. Pediatr Exerc Sci. 2014;26(2):187–94. [DOI] [PubMed] [Google Scholar]
- 29.Rohatgi A. WebPlotDigitizer: Version 4.4. 2020. Available from: https://automeris.io/WebPlotDigitizer. Accessed January 13, 2022.
- 30.Drevon D, Fursa SR, Malcolm AL. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif. 2017;41(2):323–39. [DOI] [PubMed] [Google Scholar]
- 31.Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). Br Med J Open. 2016;6(12):e011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004;1(3):176–84. [DOI] [PubMed] [Google Scholar]
- 33.Streckis V, Skurvydas A, Ratkevicius A. Children are more susceptible to central fatigue than adults. Muscle and Nerve. 2007;36(3):357–63. [DOI] [PubMed] [Google Scholar]
- 34.Kluka V Martin V Vicencio SG, et al. Effect of muscle length on voluntary activation of the plantar flexors in boys and men. Eur J Appl Physiol. 2016;116(5):1043–51. [DOI] [PubMed] [Google Scholar]
- 35.Gillen ZM Housh TJ Schmidt RJ, et al. Comparisons of muscle strength, size, and voluntary activation in pre- and post-pubescent males and females. Eur J Appl Physiol. 2021;121(9):2487–97. [DOI] [PubMed] [Google Scholar]
- 36.Gorianovas G, Skurvydas A, Streckis V, Brazaitis M, Kamandulis S, McHugh MP. Repeated bout effect was more expressed in young adult males than in elderly males and boys. Biomed Res Int. 2013;2013:218970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belanger AY, Mccomas AJ. Contractile properties of human skeletal muscle in childhood and adolescence. Eur J Appl Physiol Occup Physiol. 1989;58(6):563–7. [DOI] [PubMed] [Google Scholar]
- 38.Hatzikotoulas K, Patikas D, Ratel S, Bassa E, Kotzamanidis C. Central and peripheral fatigability in boys and men during maximal contraction. Med Sci Sports Exerc. 2014;46(7):1326–33. [DOI] [PubMed] [Google Scholar]
- 39.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-analysis. 1st ed. West Sussex (UK): Wiley; 2009. [Google Scholar]
- 40.Gavaghan DJ, Moore AR, McQuay HJ. An evaluation of homogeneity tests in meta-analyses in pain using simulations of individual patient data. Pain. 2000;85(3):415–24. [DOI] [PubMed] [Google Scholar]
- 41.Vollestad NK, Vaage O, Hermansen L. Muscle glycogen depletion patterns in type I and subgroups of type II fibres during prolonged severe exercise in man. Acta Physiol Scand. 1984;122(4):433–41. [DOI] [PubMed] [Google Scholar]
- 42.Burke RE. Motor units: anatomy, physiology, and functional organization. Handb Phys Ther. 1981;2:345–422. [Google Scholar]
- 43.Dotan R, Mitchell C, Cohen R, Klentrou P, Gabriel D, Falk B. Child–adult differences in muscle activation—a review. Pediatr Exerc Sci. 2012;24(1):2–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dotan R, Mitchell C, Cohen R, Gabriel D, Klentrou P, Falk B. Child–adult differences in the kinetics of torque development. J Sports Sci. 2013;31(9):945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matt G, Cook T. Threats to the validity of generalized inferences from research sysntheses. In: Cooper H, Hedges LV, Valentine JC, editors. The Handbook of Research Synthesis. New York (NY): Russel Sage Foundation; 2019. p. 489–516. [Google Scholar]
- 46.Schmidt FL, Hunter JE. Integrating research findings across studies. In: Methods of Meta-Analysis: Correcting Error and Bias in Research Findings. Thousand Oaks (CA): SAGE Publications Ltd; 2015. pp. 3–37. [Google Scholar]
- 47.Williamson GR. Misrepresenting random sampling? A systematic review of research papers in the journal of advanced nursing. J Adv Nurs. 2004;44(3):278–88. [DOI] [PubMed] [Google Scholar]
- 48.Creswell JW. Research Design. 4th ed. Los Angeles (CA): SAGE Publications Ltd; 2014. pp. 1–265. [Google Scholar]
- 49.Ozmun JC, Mikesky AE, Surburg PR. Neuromuscular adaptations following prepubescent strength training. Med Sci Sports Exerc. 1994;26(4):510–4. [PubMed] [Google Scholar]
- 50.Ramsay JA, Blimkie CJ, Smith K, Garner S, Macdougall JD, Sale DG. Strength training effects in prepubescent boys. Med Sci Sports Exerc. 1990;22(5):605–14. [DOI] [PubMed] [Google Scholar]
- 51.Waugh CM, Korff T, Fath F, Blazevich AJ. Effects of resistance training on tendon mechanical properties and rapid force production in prepubertal children. J Appl Physiol. 2014;117(3):257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.VanVoorhis C, Morgan B. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43–50. [Google Scholar]
- 53.Rothstein HR, Sutton AJ, Borenstein M. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Chichester (UK): John Wiley & Sons Ltd; 2006. 1–356 p. [Google Scholar]
- 54.Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ. 1998;316(7):469; author reply 470-1. [PMC free article] [PubMed] [Google Scholar]
- 55.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(16):597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doré E, Martin R, Ratel S, Duché P, Bedu M, Van Praagh E. Gender differences in peak muscle performance during growth. Int J Sports Med. 2005;26(4):274–80. [DOI] [PubMed] [Google Scholar]
- 57.Martin RJF, Dore E, Twisk J, Van Praagh E, Hautier CA, Bedu M. Longitudinal changes of maximal short-term peak power in girls and boys during growth. Med Sci Sports Exerc. 2004;36(3):498–503. [DOI] [PubMed] [Google Scholar]
- 58.Round JM, Jones DA, Honour JW, Nevill AM. Hormonal factors in the development of differences in strength between boys and girls during adolescence: a longitudinal study. Ann Hum Biol. 1999;26(1):49–62. [DOI] [PubMed] [Google Scholar]
- 59.Long D Dotan R Pitt B, et al. The electromyographic threshold in girls and women. Pediatr Exerc Sci. 2017;29(1):84–93. [DOI] [PubMed] [Google Scholar]
- 60.Pitt B Dotan R Millar J, et al. The electromyographic threshold in boys and men. Eur J Appl Physiol. 2015;115(6):1273–81. [DOI] [PubMed] [Google Scholar]
- 61.Woods S, Dotan R, Jenicek N, Falk B. Isometric-based EMG threshold in girls and women. Eur J Appl Physiol. 2020;120(4):907–14. [DOI] [PubMed] [Google Scholar]
- 62.de Haan A, Gerrits KH, de Ruiter CJ. Counterpoint: the interpolated twitch does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol (1985). 2009;107:355–6. [DOI] [PubMed] [Google Scholar]
- 63.Kubo K, Kanehisa H, Kawakami Y, Fukanaga T. Growth changes in the elastic properties of human tendon structures. Int J Sports Med. 2001;22(2):138–43. [DOI] [PubMed] [Google Scholar]
- 64.Hill AV. The effect of series compliance on the tension developed in a muscle twitch. Proc R Soc London Ser B Biol Sci. 1951;138(892):325–9. [Google Scholar]
- 65.Shield A, Zhou S. Assessing voluntary muscle activation with the twitch interpolation technique. Sports Med. 2004;34(4):253–67. [DOI] [PubMed] [Google Scholar]
- 66.Loring SH, Hershenson MB. Effects of series compliance on twitches superimposed on voluntary contractions. J Appl Physiol. 1992;73(2):516–21. [DOI] [PubMed] [Google Scholar]
- 67.Suter E, Herzog W. Effect of number of stimuli and timing of twitch application on variability in interpolated twitch torque. J Appl Physiol. 2001;90(3):1036–40. [DOI] [PubMed] [Google Scholar]
- 68.Freeman SR, Durfee WK. Twitch response of intact human tibialis anterior muscle to doublet stimulation at graded strengths. Conf Proc IEEE Eng Med Biol Soc. 2006;Suppl:6757–60. [DOI] [PubMed] [Google Scholar]
- 69.Button DC, Behm DG. The effect of stimulus anticipation on the interpolated twitch technique. J Sports Sci Med. 2008;7(4):520–4. [PMC free article] [PubMed] [Google Scholar]
- 70.Segal RL. Use of imaging to assess normal and adaptive muscle function. Phys Ther. 2007;87(6):704–18. [DOI] [PubMed] [Google Scholar]
- 71.Wilke M, Krägeloh-Mann I, Holland SK. Global and local development of gray and white matter volume in normal children and adolescents. Exp Brain Res. 2007;178(3):296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infance to late adulthood. Arch Neurol. 1994;51(9):874–87. [DOI] [PubMed] [Google Scholar]
- 73.Giedd JN Blumenthal J Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–3. [DOI] [PubMed] [Google Scholar]
- 74.Paus T Zijdenbos A Worsley K, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283(5409):1908–11. [DOI] [PubMed] [Google Scholar]
- 75.Miller DJ Duka T Stimpson CD, et al. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci. 2012;109(41):16480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grydeland H Vértes PE Váša F, et al. Waves of maturation and senescence in micro-structural MRI markers of human cortical myelination over the lifespan. Cereb Cortex. 2019;29(3):1369–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sporns O. The human connectome: origins and challenges. Neuroimage. 2013;80:53–61. [DOI] [PubMed] [Google Scholar]
- 78.Garvey MA, Ziemann U, Bartko JJ, Denckla MB, Barker CA, Wassermann EM. Cortical correlates of neuromotor development in healthy children. Clin Neurophysiol. 2003;114(9):1662–70. [DOI] [PubMed] [Google Scholar]
- 79.Säisänen L, Julkunen P, Lakka T, Lindi V, Könönen M, Määttä S. Development of corticospinal motor excitability and cortical silent period from mid-childhood to adulthood—a navigated TMS study. Clin Neurophysiol. 2018;48(2):65–75. [DOI] [PubMed] [Google Scholar]
- 80.Kolb B, Whishaw I. Fundamentals of Human Neuropsychology. 7th ed. NewYork (NY): Worth Publishers; 2015. [Google Scholar]
- 81.Lieberman OJ, McGuirt AF, Tang G, Sulzer D. Roles for neuronal and glial autophagy in synaptic pruning during development. Neurobiol Dis. 2019;122:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Groeschel S, Vollmer B, King MD, Connelly A. Developmental changes in cerebral grey and white matter volume from infancy to adulthood. Int J Dev Neurosci. 2010;28(6):481–9. [DOI] [PubMed] [Google Scholar]
- 83.Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. [DOI] [PubMed] [Google Scholar]
- 84.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–78. [DOI] [PubMed] [Google Scholar]


