Abstract
The combination of immune checkpoint inhibitors (ICIs) and other anticancer agents is the standard of care for various cancers. Bevacizumab, an anti-angiogenesis inhibitor, causes serious adverse events such as pulmonary hemorrhage (PH). Here, we present a case of drug-induced diffuse alveolar hemorrhage (DAH), an adverse event, in a patient with hepatocellular carcinoma who was treated with a combination of ICIs and anti-angiogenesis inhibitors after long-term use of lenvatinib, which inhibits vascular endothelial growth factor (VEGF). An 85-year-old man with hepatocellular carcinoma initially received lenvatinib, a multi-kinase inhibitor, but the drug was later switched to bevacizumab-atezolizumab combination therapy owing to disease progression. After five cycles, he developed dyspnea and diffuse ground-glass opacities, which improved with discontinuation of the combination therapy and initiation of steroid pulse therapy. Our case findings indicate that both ICIs and anti-angiogenesis inhibitors cause drug-induced DAH, and their combination may increase the severity of DAH. Moreover, long-term VEGF inhibition may induce the development of DAH. Clinicians need to be aware that long-term VEGF inhibition may be associated with DAH and should consider the risk management of such adverse events while using this combination therapy.
Keywords: long-term vegf inhibition, adverse events, diffuse alveolar hemorrhage, combination therapy, anti-angiogenesis inhibitor, immune checkpoint inhibitor
Introduction
Recently, the use of immune checkpoint inhibitors (ICIs) together with other anticancer agents (e.g., cytotoxic anticancer agents, angiogenesis inhibitors) has become the standard of care for various cancers [1-4]. Atezolizumab, an ICI, interferes with the binding of programmed cell death 1 ligand 1 (PD-L1) to its two receptors, programmed cell death 1 (PD-1) and B7.1 [5]. Atezolizumab inhibits immunosuppressive signals by blocking the PD-L1/PD-1 immune checkpoint within the tumor microenvironment and consequently, increases T-cell-mediated immunity against the tumor [5]. Adverse events associated with the use of ICIs include lung failure, particularly diffuse alveolar hemorrhage (DAH), which can sometimes be fatal. Therefore, it is important to manage these life-threatening conditions [6,7].
Pulmonary hemorrhage (PH) is a serious adverse event associated with bevacizumab, a monoclonal antibody that targets vascular endothelial growth factor (VEGF) [8]. Tumor infiltration into the mediastinum and major vessels and tumor cavitation in the lung can be the underlying causes for the occurrence of PH [8]. Treatment with bevacizumab causes alveolar hemorrhage even in the absence of mediastinal invasion, macrovascular invasion, or tumor vacuolation. Alveolar hemorrhage may occur because of fragile pulmonary capillary walls, resulting from VEGF inhibition [9,10]. Lenvatinib is a multi-kinase inhibitor that inhibits VEGF.
Herein, we report a case of alveolar hemorrhage that occurred as an adverse effect of the concomitant use of an angiogenesis inhibitor and ICI after the long-term use of lenvatinib.
Case presentation
An 85-year-old man regularly visited our hospital for chronic hepatitis C treatment since 2004. There was no indication of respiratory disease, but he had a smoking history of 40 pack-years. In 2011, he was diagnosed with hepatocellular carcinoma T2N0M0 Stage II after undergoing partial hepatectomy to resect a mass in hepatic segments 5 and 8. In 2016, the mass recurred in hepatic segment 8, and he, therefore, underwent radiofrequency ablation. In September 2019, the tumor recurred in hepatic segment 8. Therefore, treatment with lenvatinib, a multi-kinase inhibitor, was started. In September 2020, trans-catheter arterial chemoembolization was performed.
Lenvatinib was continued until January 2021. Owing to the disease's progression, the drug was switched to bevacizumab-atezolizumab combination therapy. Thereafter, an enlarged mass was found in hepatic segment 8 (Figure 1). The best response to the combination therapy was stable disease. However, after receiving five cycles of the combination therapy within one week, the patient developed dyspnea. Chest computed tomography revealed the presence of diffuse ground-glass opacities (Figure 2). On admission, his oxygen saturation was 92%, which was achieved with a mask providing 5 L oxygen/min, and his respiratory rate was 24 breaths/min. Respiratory function test could not be performed due to his poor respiratory condition.
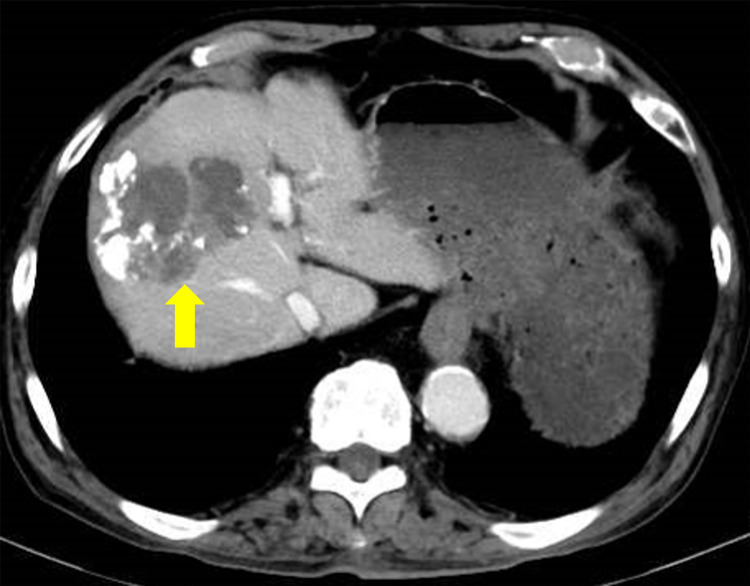
Figure 1. Abdominal computed tomography findings.
Abdominal computed tomography findings of hepatocellular carcinoma. An enlarged mass was then found in hepatic segment 8 (arrow).
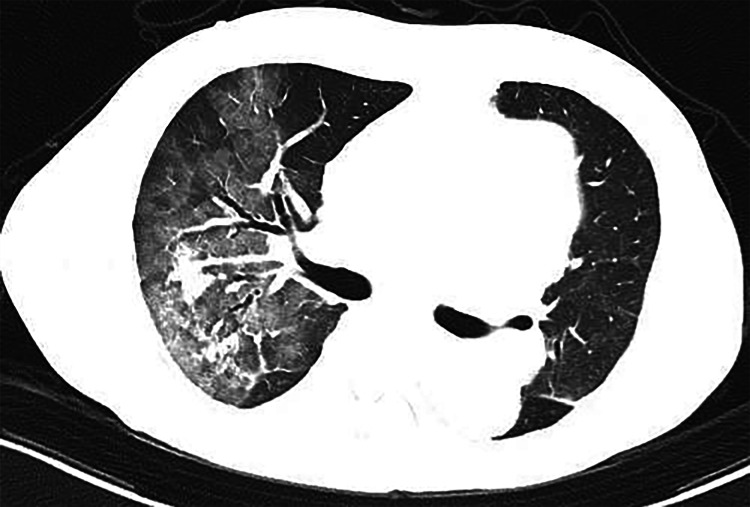
Figure 2. Chest computed tomography findings on admission.
Chest computed tomography revealed the presence of diffuse ground-glass opacities.
Echocardiography revealed normal cardiac function. The polymerase chain reaction (PCR) analysis results for coronavirus disease-19 (COVID-19), using nasal swab fluid, was negative. The prothrombin time was 16.1 (reference value: 10.5-13.5) s, and the D-dimer level was 2.2 (reference value: <1.0) μg/mL. Only mild coagulation abnormalities were noted. The Krebs von den Lunge-6 and surfactant protein-D levels were within the normal limits. The levels of antinuclear antibody, proteinase-3 anti-neutrophil cytoplasmic antibodies (ANCA), myeloperoxidase ANCA, and anti-glomerular basement membrane antibodies were also within the normal limits. Bronchoalveolar lavage (BAL) was performed in segment 3 of the right upper segment bronchus, and the BAL fluid (BALF) was found to contain blood (Figure 3).
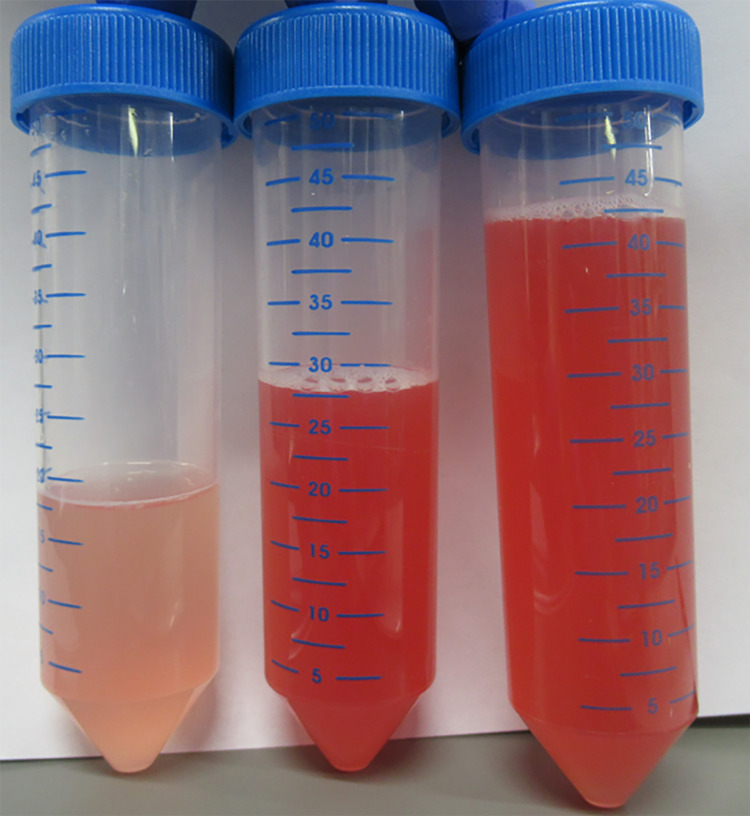
Figure 3. Image showing bronchoalveolar lavage (BAL).
Image showing bronchoalveolar lavage (BAL) performed via the right B3 segment; blood-stained lavage fluid was observed.
An analysis of the BALF cell count revealed neutrophilic inflammation (64% of the volume recovered; total cell count: 7.6 × 105 cells/mL, neutrophil count: 70.4%, eosinophil count: 0%, lymphocyte count: 4.6%, macrophage count: 25%). Cytological examination showed no malignant cells, fungal elements, or viral cytopathic changes; only hemosiderin-phagocytosing macrophages were observed (Figure 4). There were no notable bacterial, mycobacterial, or fungal pathogens in the BALF cultures. A PCR analysis of BALF showed no Pneumocystis jiroveci infection.
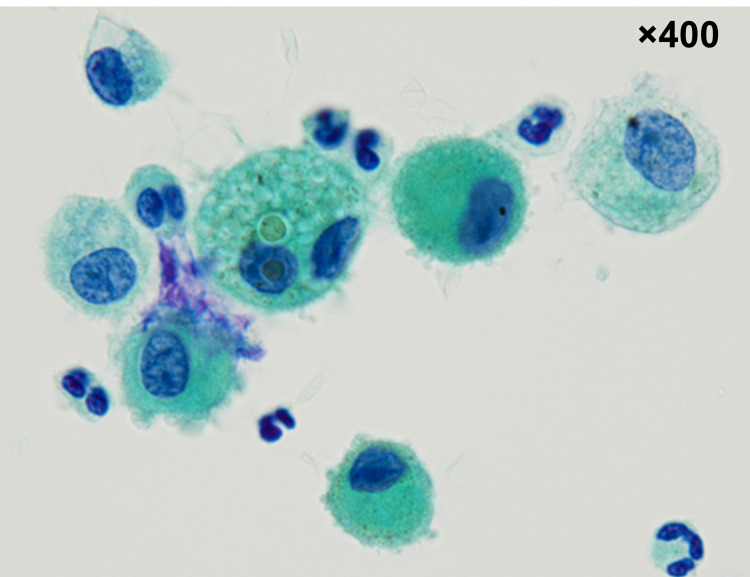
Figure 4. Cytological examination of the bronchoalveolar lavage fluid (BALF).
Cytological examination of the bronchoalveolar lavage fluid (BALF) using Papanicolaou-stained smears showing hemosiderin-laden macrophages.
Based on the BALF findings and the exclusion of diseases causing DAH, we diagnosed the patient with drug-induced DAH. Therefore, the combination therapy was discontinued, and steroid pulse therapy (methylprednisolone, 1 g daily) was initiated, after which the ground-glass opacities improved on Day 4 of admission.
Discussion
We report a case of alveolar hemorrhage that occurred as an adverse effect of the concomitant use of an angiogenesis inhibitor and ICI after the long-term use of lenvatinib, which inhibits VEGF. The condition improved on discontinuing the combination therapy and initiating steroid pulse therapy.
DAH may be caused by various diseases, such as congestive heart failure, infections, coagulation disorders, collagen diseases, and vasculitis [10]. Most cases of DAH are due to the breakdown of the alveolar capillary barrier resulting from immunological factors, such as vasculitis or specific drug reactions [11]. Here, the patient was diagnosed with drug-induced DAH by excluding other diseases causing DAH. The mechanism underlying the pathogenesis of DAH-associated immune-related adverse events is not fully understood, but it is hypothesized that cytotoxic T lymphocytes damaged endothelial cells in the small blood vessels of the lung [6].
In the present case, although the patient was diagnosed with DAH, we could not determine whether it was caused by ICIs or angiogenesis inhibitors. Most cases of DAH are considered to be caused by the disruption of the alveolar capillary wall [12]. The VEGF not only enhances endothelial cell proliferation but is also important for maintaining vascular integrity; VEGF inhibition by bevacizumab administration may increase the friability of the capillaries [13]. Lenvatinib is a multi-kinase inhibitor that inhibits VEGF and, having been administered before the combination therapy and the underlying long-term VEGF inhibition, may have been associated with DAH.
Additionally, PD-1 and PD-L1 inhibitors may cause hemorrhagic complications due to immune activation, which may disrupt the balance of the coagulation-fibrinolysis system [14]. Although risk factors for predicting the development of DAH have not been identified, older age and smoking history are known risk factors for the development of acute lung injury and acute respiratory distress syndrome [15,16]. Our patient was elderly and had a history of smoking, which could have contributed to the development of DAH.
Conclusions
Long-term VEGF inhibition may increase the friability of the capillaries. Thus, clinicians need to be aware that long-term VEGF inhibition may be associated with DAH. The combined use of angiogenesis inhibitors and ICIs could increase the severity of alveolar hemorrhage. Therefore, clinicians should pay more attention to the management of adverse effects when such a combination therapy is administered.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Updated analysis of KEYNOTE- 024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. Reck M, Rodríguez-Abreu D, Robinson AG, et al. J Clin Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 2.Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Lancet Oncol. 2018;19:1480–1492. doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 3.Anti-angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Song Y, Fu Y, Xie Q, Zhu B, Wang J, Zhang B. Front Immunol. 2020;11:1956. doi: 10.3389/fimmu.2020.01956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Nat Rev Clin Oncol. 2020;17:725–741. doi: 10.1038/s41571-020-0413-z. [DOI] [PubMed] [Google Scholar]
- 5.Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitor. Deng R, Bumbaca D, Pastuskovas CV, et al. mAbs. 2016;8:593–603. doi: 10.1080/19420862.2015.1136043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diffuse alveolar hemorrhage with nivolumab monotherapy. Shannon VR, Subudhi SK, Huo L, Faiz SA. Respir Med Case Rep. 2020;30:101131. doi: 10.1016/j.rmcr.2020.101131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durvalumab-induced diffuse alveolar hemorrhage: an autopsy case report. Kanaoka K, Ikebe S, Ihara S, Tsuji H, Yasuoka H, Minami S. Case Rep Oncol. 2020;13:696–701. doi: 10.1159/000507848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Predicting and managing the risk of pulmonary haemorrhage in patients with NSCLC treated with bevacizumab: a consensus report from a panel of experts. Reck M, Barlesi F, Crinò L, Henschke CI, Isla D, Stiebeler S, Spigel DR. Ann Oncol. 2012;23:1111–1120. doi: 10.1093/annonc/mdr463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diffuse alveolar haemorrhage may be associated with intravitreal injection of bevacizumab in a patient with systemic risk factors. Seto R, Yamada H, Wada H, Osawa M, Nagao T, Nakano Y. BMJ Case Rep. 2011:820103224. doi: 10.1136/bcr.08.2010.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diffuse alveolar hemorrhage as a fatal adverse effect of bevacizumab: an autopsy case. Ikeda S, Sekine A, Kato T, et al. Jpn J Clin Oncol. 2014;44:497–500. doi: 10.1093/jjco/hyu023. [DOI] [PubMed] [Google Scholar]
- 11.Pulmonary capillaritis and alveolar hemorrhage. Update on diagnosis and management. Green RJ, Ruoss SJ, Kraft SA, Duncan SR, Berry GJ, Raffin TA. Chest. 1996;110:1305–1316. doi: 10.1378/chest.110.5.1305. [DOI] [PubMed] [Google Scholar]
- 12.Severe respiratory failure due to diffuse alveolar hemorrhage: clinical characteristics and outcome of intensive care. Rabe C, Appenrodt B, Hoff C, et al. J Crit Care. 2010;25:230–235. doi: 10.1016/j.jcrc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Bevacizumab, bleeding, thrombosis, and warfarin. Kilickap S, Abali H, Celik I. J Clin Oncol. 2003;21:3542–3543. doi: 10.1200/JCO.2003.99.046. [DOI] [PubMed] [Google Scholar]
- 14.Disorder of coagulation-fibrinolysis system: an emerging toxicity of anti-PD-1/PD-L1 monoclonal antibodies. Sato R, Imamura K, Sakata S, et al. J Clin Med. 2019;8:762. doi: 10.3390/jcm8060762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cigarette smoking, alcohol consumption, and risk of ARDS: a 15-year cohort study in a managed care setting. Iribarren C, Jacobs DR Jr, Sidney S, Gross MD, Eisner MD. Chest. 2000;117:163–168. doi: 10.1378/chest.117.1.163. [DOI] [PubMed] [Google Scholar]
- 16.Incidence and outcomes of acute lung injury. Rubenfeld GD, Caldwell E, Peabody E, et al. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]