Abstract
Cutaneous malignancies including melanomas and keratinocyte carcinomas (KC) are the most common types of cancer, occurring at a rate of over one million per year in the United States. KC, which include both basal cell carcinomas and squamous cell carcinomas, are substantially more common than melanomas and form the subject of this chapter. Ultraviolet radiation (UVR), both UVB and UVA, as occurs with sunlight exposure is generally regarded as causal for these malignancies, but UVB is also required for vitamin D synthesis in the skin. Keratinocytes are the major cell in the epidermis. These cells not only produce vitamin D but contain the enzymatic machinery to metabolize vitamin D to its active metabolite, 1,25(OH)2D, and express the receptor for this metabolite, the vitamin D receptor (VDR). This allows the cell to respond to the 1,25(OH)2D that it produces. Based on our own data and that reported in the literature, we conclude that vitamin D signaling in the skin suppresses UVR-induced epidermal tumor formation. In this chapter we focus on four mechanisms by which vitamin D signaling suppresses tumor formation. They are inhibition of proliferation/stimulation of differentiation with discussion of the roles of hedgehog, Wnt/β-catenin, and hyaluronan/CD44 pathways in mediating vitamin D regulation of proliferation/differentiation, regulation of the balance between oncogenic and tumor suppressor long noncoding RNAs, immune regulation, and promotion of DNA damage repair (DDR).
Keywords: 1,25-Dihydroxyvitamin D; 6,4 Photoproducts; Adaptive immunity; Basal cell carcinoma; Cancer; Cathelicidin; Cyclobutane pyrimidine dimers; CYP27B1; Differentiation; DNA damage repair; Epidermis; Gli 1; Gli 2; Hedgehog; Immune function; Innate immunity; LEF/TCF; p53; Patched 1; Proliferation; Smoothened; Squamous cell carcinoma; Toll-like receptors; UV radiation; Vitamin D; Vitamin D analogs; Vitamin D receptor; β-catenin
Introduction
Over one million skin cancers occur annually in the United States, 80% of which are basal cell carcinomas (BCC), 16% squamous cell carcinomas (SCC), and 4% melanomas, making skin cancer by far the most common cancer afflicting humankind [1]. Ultraviolet radiation (UVR) from the sun is the major etiologic agent for these cancers. The highest energy UVR, UVC (below 280 nm), does not penetrate the atmosphere. Of the solar radiation that does reach the earth, 95% is UVA and 5% UVB. UVB (280–320 nm), although it does not penetrate past the epidermis, is absorbed by DNA in the epidermal cells creating characteristic mutations identified as cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6–4) pyrimidone photoproducts (6–4PP), which if not repaired result in C to T or CC to TT mutations, the UVB “signature” lesion [2, 3]. UV wavelengths between 320 and 400 nm (UVA) are capable of penetrating into the dermis and do their DNA damage (e.g., 8 hydroxy 2′ deoxyguanosine and peroxynitrite production) primarily by oxidative processes [4], although at high enough dose levels UVA can produce CPDs [5]. On the other hand, UVB is required to convert 7-dehydrocholesterol in the skin to previtamin D3, which then isomerizes to vitamin D3. Moreover, the skin is capable of converting the vitamin D produced to its active metabolite 1,25(OH)2D [6], and this conversion is potentiated by UVR at least in part by cytokines such as TNF-α [7] which are increased by UVR in the epidermis [8]. Both melanocytes [9] and keratinocytes [10] express the vitamin D receptor (VDR) and respond to 1,25(OH)2D with reduced proliferation and increased differentiation [11, 12]. Sun avoidance may reduce one’s risk of developing skin cancer, but this practice could result in suboptimal levels of vitamin D in the body. Vitamin D supplementation can compensate, but the skin remains the major source of vitamin D availability for most of the world’s population. Moreover, low-dose UVR may be protective against skin cancer via the vitamin D signaling mechanisms that will be reviewed in this article, and some epidemiologic evidence is consistent with a potential benefit of low-dose UVR. In a recent report, an international panel of experts in endocrinology, dermatology, photobiology, epidemiology, and anthropology [13, 14] concluded that sunscreens could be protective against the harmful effects of solar radiation while still enabling vitamin D production. In this chapter, after a review of vitamin D metabolism and VDR function, I will examine potential mechanisms that have been proposed for vitamin D-induced antitumor mechanisms in general and then focus on those mechanisms that have been shown to be operative in the epidermis.
Vitamin D Metabolism
Vitamin D3 is produced from 7-dehydrocholesterol (7-DHC). UVB breaks the B ring of the 7-DHC to produce previtamin D3, which subsequently undergoes a temperature-dependent rearrangement of the triene structure to form vitamin D3, lumisterol, and tachysterol. This process is relatively rapid and reaches a maximum within hours [13, 15, 16], although both the amount of epidermal pigmentation and the intensity of exposure influence the time required. With continued UV exposure the biologically inactive lumisterol and tachysterol accumulate eliminating the risk of excessive production of vitamin D. Sunlight exposure increases melanin production, which can absorb UVB, and so provides another mechanism by which excess vitamin D3 production can be prevented. The intensity of UVR is dependent on latitude and season. In Edmonton, Canada (52°N), very little vitamin D3 is produced in exposed skin from mid-October to mid-April, while in San Juan (18°N) the skin is able to produce vitamin D3 all year long [17]. Clothing and sunscreen effectively prevent vitamin D3 production in the covered areas. Vitamin D3 produced in the skin can be carried to the liver and other tissues for further metabolism to 25-hydroxyvitamin D (25OHD) and then to the kidney to produce 1,25(OH)2D by the enzyme CYP27B1. The major 25-hydroxylase in the liver is CYP2R1, but the main 25-hydroxylase in keratinocytes appears to be CYP27A1 [18]. However, CYP2R1 has been found in dermal fibroblasts [19]. The expression of CYP27A1 like that of CYP27B1 is mitochondrial. Its expression is increased by vitamin D and UVB irradiation [18], but otherwise its regulation is unclear. The microsomal CYP2R1 is a more specific 25-hydroxylase, but as of yet its expression in keratinocytes has not been reported. The production of 1,25(OH)2D in the skin is under quite different regulation compared to its production by the kidney, although the same enzyme, CYP27B1, is involved. In the kidney parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23), and 1,25(OH)2D itself are the principal hormonal regulators: PTH stimulates, whereas FGF23 and 1,25(OH)2D inhibit 1,25(OH)2D production. Keratinocytes respond to PTH with increased 1,25(OH)2D production, but these cells do not have the classic PTH receptor and do not respond to cyclic AMP [6] unlike the kidney. The effect of FGF23 on keratinocyte CYP27B1 expression or function has not been reported. Furthermore, unlike the kidney, 1,25(OH)2D does not directly affect CYP27B1 expression in keratinocytes. Rather, 1,25(OH)2D regulates its own levels in the keratinocyte by inducing CYP24A1, the catabolic enzyme for 1,25(OH)2D3 [20]. In the keratinocyte the major regulators of 1,25(OH)2D production are cytokines such as tumor necrosis factor-α (TNF) [7] and interferon-γ (IFN) [21]. These cytokines are activated in the skin by UVB, which of course also increases the substrate via increased vitamin D production. The differences in regulation of CYP27B1 in kidney vs nonrenal cells such as macrophages and most likely keratinocytes have recently been shown to involve different regions of the CYP27B1 promoter that are accessible to regulatory factors in a tissue-specific manner [22].
Vitamin D Receptor: Mechanism of Action
The VDR is a member of the nuclear hormone receptor superfamily [23]. These members are characterized by a highly conserved DNA-binding domain characterized by two zinc fingers and a structurally conserved ligand-binding domain that has at its C-terminal end the AF2 domain to which coactivator complexes bind [24]. The ligand-binding domain also serves as the region to which VDR binds to its transcriptional partners, RXR being the major one. In general ligand (i.e., 1,25(OH)2D) binding is required for the VDR/RXR heterodimer to form and bind to those regions on the DNA called vitamin D response elements (VDRE). Ligand binding also alters the structure of the VDR with major movement of helix 12 (C-terminus) into position to enclose the ligand while exposing sites on the VDR in helices 3, 5, and 12 to which coactivators bind. These coactivators can in turn recruit chromatin-modifying enzymes such as histone acetyl transferases (SRC, CBP/p300, pCAF) and DNA demethylases or proteins that bridge the gap between the VDRE and the transcription machinery (Mediator complex) including TATA-associated factors, TFIIb, and RNA polymerases (primarily RNA pol II). In the absence of ligand binding, sites for corepressors are exposed. These corepressors recruit another set of chromatin-modifying enzymes such as histone deacetylases and DNA methyl transferases [25]. The most common VDRE is comprised of two head-to-tail half sites of hexanucleotides separated by three nucleotides, referred to as DR3 VDREs. The sequence of these DR3 half sites is heterogeneous, with a consensus approximated by RGKTSA where R = A or G, K = G or T, and S=C or G. Moreover, many VDREs are not DR3s, although DR3s tend to have the highest affinity for VDR/RXR heterodimers.
The VDREs can be quite distant from the transcription start site of the gene being regulated, occurring in introns, between genes, and in either a 5′ or 3′ relationship to the coding region [26]. Moreover, putative VDREs as demonstrated by techniques such as ChIP-seq, in which binding sites to the genome by VDR are identified using a combination of chromatin immunoprecipitation of the VDR to DNA followed by high-throughput sequencing of those binding regions, number in the thousands, with a substantial degree of cell/tissue specificity [27]. Most genes have several VDREs. In a review of two such ChIP-seq studies, Carlberg et al. [28] noted that the study in a lymphoblastoid line identified 2776 VDREs for 232 genes, whereas the study in THP-1 monocytes identified 1820 VDREs for 638 genes. In the latter study 408 of the genes were upregulated and 230 downregulated. Most of the VDREs for the upregulated genes were within 400kbp of the transcription start site; this was less true for the downregulated genes. Only 93 of the upregulated genes had VDREs within 30kbp of the transcription start site. Moreover, only 31.7% of the VDREs were DR3s. These two studies had only 18% overlap of the VDREs identified but differed not only in cell line but dose and time after 1,25(OH)2D was administered before the cells were analyzed. Earlier microarray studies had likewise demonstrated the many genes regulated by 1,25 (OH)2D and the surprising lack of consensus from one study to the next perhaps due to tissue specificity and/or differences in dose and time of 1,25(OH)2D exposure. These studies demonstrate the diversity of vitamin D-regulated genes and diversity in type and location of VDREs. Such studies have revolutionized our concepts of the scope and means of vitamin D signaling and reveal many potential mechanisms by which vitamin D signaling can regulate cancer formation. In this regard an unbiased systems biology approach mapping genetic loci underlying susceptibility to skin cancer puts the VDR in the center of a complex set of networks linking regulation of barrier function, inflammation, and tumor formation [28].
The VDR is essential for most actions of 1,25 (OH)2D and its analogs. Tumors that are unresponsive to vitamin D have either lost their ability to produce 1,25(OH)2D (i.e., decreased CYP27B1) [29, 30], increased their metabolism of 1,25(OH)2D via upregulation of CYP24A1 [31], lost VDR transcriptional activity through posttranslational alterations in RXR [32], or decreased their VDR expression. The latter may be secondary to increased activity in tumors of inhibitors of VDR expression such as SNAIL [33] and SLUG [34], increased methylation of the VDR promoter [35], or increased expression of miRNA125b, an inhibitor of VDR expression [36]. In melanoma cell lines, the administration of 5-aza cytidine (to inhibit DNA methyltransferase) and trichostatin (to inhibit HDAC activity) could restore responsiveness to 1,25(OH)2D by increasing VDR levels and reducing miR125b expression [37]. P53 cooperates with VDR in its antitumor functions [38]. The nuclear levels of p53 are increased by 1,25(OH)2D by nongenomic mechanisms [39]. Nevertheless p53 mutations in tumors can also contribute to resistance to vitamin D in tumors. The E3 ubiquitin ligase and transcriptional regulator murine double minute (MDM2) is also regulated by 1,25(OH)2D [40], and unlike p53 it inhibits VDR levels and transcriptional activity [41] as well as that of p53.
However, not all actions of 1,25(OH)2D involve the VDR, and not all actions of VDR are genomic. Nongenomic actions of 1,25 (OH)2D are rapid and include a number of signaling pathways such as the opening of chloride and calcium channels, activation of mitogen-activated protein kinases, protein kinase C, phosphatidylinositol 3-kinase, and phospholipase C (review in [42]. As will be noted subsequently, these nongenomic actions contribute to the protective effect of topical administration of 1,25(OH)2D and its nongenomic analogs on UVB-induced CPD formation in the skin [43]. These rapid effects of 1,25(OH)2D are mediated by either or both VDR acting in the membrane and an unrelated receptor variably known as membrane-associated rapid response steroid-binding protein (MARRS), ERp57, GRp58, ERp60, or protein disulfide isomerase family A, member 3 (PDIA3). In a study with fibroblasts from patients with variable mutations in the VDR, Sequeira et al. [39] demonstrated that both receptors including VDR lacking the DNA-binding domain mediated the protective effect of 1,25(OH)2D on UVB-induced CPD formation. That said, most attention to VDR as a tumor suppressor has focused on its genomic actions.
Mechanisms of Tumor Suppression by Vitamin D: General
The demonstration that many genes and pathways are influenced by vitamin D signaling has suggested a large number of potential means by which vitamin D signaling can control tumor growth (recent reviews in [44, 45]).
Cell Cycle Regulation
Regulation by 1,25(OH)2D of the cell cycle in a number of cells, normal and malignant, has been demonstrated. This results from an upregulation of cell cycle inhibitors such as p21cip and p27kip (cyclin-dependent kinase inhibitors) [46] and retinoblastoma-like protein 2 and retinoblastoma-binding protein 6 [47] and decreased expression of cyclins [48] and cyclin-dependent kinases [49]. In addition 1,25(OH)2D increases the interaction of FoxO proteins (tumor suppressors controlling proliferation [50]) with VDR and FoxO regulators Sirt1 and protein phosphatase 1 that maintain FoxO in the nucleus by blocking MAPK phosphorylation [51]. Increased c-MYC expression and activity are frequently found in cancer [52]. c-MYC induces the expression of a number of cell cycle regulatory genes such as cyclin D2 and cdk4. 1,25(OH)2D inhibits the expression of c-MYC [53], and c-MYC expression is increased in the skin and gut of VDR null mice [54].
Growth Factors
1,25(OH)2D and its analogs regulate a number of growth factor pathways. Insulin-like growth factor (IGF)-stimulated proliferation of the breast and prostate cells is reduced by 1,25(OH)2D via its induction of IGF-I-binding protein 3 [55, 56]. TGFβ2 exerts antiproliferative actions in epithelial cells. 1,25(OH)2D and its analogs increase the expression of TGFβ2 and TGFβ receptors in breast and prostate cancer cells [47, 49, 57] while suppressing the expression of the latent TGFβ-binding protein [49, 58]. GDF15 (growth differentiation factor 15) is a member of the TGFβ superfamily and like TGFβ is antiproliferative in prostate cancer cells. Its expression is increased by 1,25(OH)2D [59, 60]. Bone morphogenic proteins (BMPs) are also members of the TGFβ superfamily that have been found to be dysregulated in certain cancers [61]. The expression of several BMPs is regulated by 1,25(OH)2D and its analogs in a number of malignant cell lines [48, 49, 62]. Wnt/β-catenin signaling will be dealt with in depth when we focus on vitamin D-regulated pathways in the skin, but this pathway has been extensively studied in the colon based on the frequency of mutations in the adenomatous polyposis coli (APC) gene in colon cancer [63]. In the canonical pathway of Wnt/β-catenin signaling, the APC complex that would otherwise bind and phosphorylate β-catenin, targeting it for proteasomal degradation, is inactivated, allowing β-catenin to move to the nucleus where it binds to LEF/TCF leading to transcription of genes involved with proliferation. 1,25(OH)2D/VDR binds to β-catenin, preventing its movement into the nucleus and/or binding to LEF/TCF [64, 65]. Moreover, by increasing the levels of E-cadherin, which binds β-catenin in the plasma membrane, 1,25(OH)2D can further reduce the translocation of β-catenin into the nucleus [64, 65]. Furthermore, 1,25 (OH)2D can suppress Wnt signaling by stimulating the expression of the Wnt antagonist DKK-1 [66]. Cystatin D, an inhibitor of several cysteine proteases of the cathepsin family that appear to be involved in Wnt signaling, has likewise been shown to be a target gene of 1,25 (OH)2D [67]. The induction of cystatin D and other 1,25(OH)2D target genes such as E-cadherin appears to involve a nongenomic action requiring calcium activation of RhoA-ROCK-p38MAPK-MSK in colon cancer cells [68]. We have shown that this pathway requires the 1,25(OH)2D-induced calcium receptor in keratinocytes [69]. These and other studies point to the interaction between calcium and vitamin D signaling in the regulation of tumor formation [70], an interaction that to date has received little attention, but which is supported by our observations that mice lacking both VDR and the calcium-sensing receptor (CaSR) develop KC spontaneously [71, 72].
Apoptosis
In addition to inhibiting proliferation, 1,25 (OH)2D promotes apoptosis in a number of malignant cell lines in part by downregulation of anti-apoptotic genes Bcl-2 and Bcl-XL [73, 74] and upregulation of the proapoptotic gene GOS2 (G0G1 switch gene 2) [48, 75]. Transcripts of other pro-apoptotic genes increased by 1,25 (OH)2D include death-associated protein-3, caspase 8 apoptosis-related cysteine peptidase, and fas-associated death domain-like apoptosis regulator as well as a number of caspases [47]. Telomerase is a mechanism that enables cancer cells to escape apoptosis. 1,25(OH)2D suppresses telomerase expression by inducing miRNA498, a transcript in the complementary strand of CTC-360P6 [76]. Of interest is this miRNA has its own VDRE [76]. On the other hand, a mutant form of p53 has been shown to reverse the anti-apoptotic effect of 1,25(OH) 2D [77].
Oxidative Stress
As noted previously in the discussion of UVA-induced effects on the epidermis, oxidative stress can lead to oxidative DNA damage, marked by 8 hydroxy 2′-deoxyguanosine and reactive nitrogen species. In VDR knockout mice, 8 hydroxy 2′-deoxyguanosine levels are increased in the colon [78] and reduced by vitamin D supplementation in humans [79]. 1,25(OH)2D induces several antioxidant enzymes in cancer cells including thioredoxin reductase 1 [47, 49], superoxide dismutase [49, 59], and glucose-6 phosphate dehydrogenase [58]. The induction of genes associated with DNA repair will be discussed at greater length when we focus on UVB damage to the epidermis, but the induction by 1,25(OH)2D of GADD45α (growth arrest and DNA-damage inducible α), p53, RAD23B, PCNA, and DAP-1α may all contribute to this aspect of tumor suppression by 1,25(OH)2D/VDR [47, 75, 80].
Prostaglandins
Prostaglandins have been shown to stimulate cancer cell growth [81]. 1,25(OH)2D blocks prostaglandin signaling by inhibiting COX2 expression and that of prostaglandin receptors while increasing the expression of hydroxyprostaglandin dehydrogenase 15-NAD, the prostaglandin-inactivating enzyme [60, 82].
Angiogenesis
Growing tumors require a blood supply. 1,25 (OH)2D inhibits angiogenesis by blocking the expression and function of VEGF (vascular endothelial growth factor) and other proangiogenic factors [83–85]. Mice lacking VDR had larger and more vascular tumors when implanted with prostate cells from TRAMP mice [86].
Immune System
The immune system plays an important protective role in cancer protection [87] as evidenced by the increased numbers of malignancies in immunosuppressed hosts including SCCs in immunosuppressed renal transplant patients [88]. UVB results in immunosuppression [89], which can either be ameliorated [43] or enhanced [90] by 1,25(OH)2D as will be dealt with in more depth when we focus on the skin, as it has not received much study in the context of tumor protection in general by 1,25 (OH)2D.
Mechanisms of Tumor Suppression by 1,25(OH)2D/VDR in the Epidermis
The potential for vitamin D signaling as protection against epidermal tumor formation was demonstrated when Zinser et al. [91] observed that 85% of the VDR null mice but none of the controls developed skin tumors within 2 months following 7,12 dimethylbenzanthracene (DMBA) administration. These were primarily papillomas. These results have been confirmed using topical administration of DMBA/TPA [92]. However, although only papillomas were seen in the VDR null mice, RXRα null mice developed both BCC and SCC [92]. Subsequently, Ellison et al. [93] and our own group [94] demonstrated that VDR null mice were also more susceptible to tumor formation following UVB and many of the tumors were SCC and BCC. Moreover, as mentioned previously, deletion of both VDR and CaSR resulted in spontaneous tumor formation albeit in 1-year-old mice [72]. The appearance of BCC in these studies is surprising since the typical malignancy induced in mouse skin by UVR, ionizing radiation, or chemical carcinogens is SCC not BCC [95]. Given that BCC generally result from increased hedgehog (Hh) signaling [96], and that lack of VDR results in BCC when β-catenin signaling is increased [97], we became interested in the relationship between vitamin D, Hh, and β-catenin signaling in tumor suppression. Along with these studies, we noted that UVR increased the breakdown of hyaluronan to shorter forms that are known to promote inflammation and tumor progression stimulating our examination of this pathway [98]. We also discovered that VDR regulated the expression of long noncoding RNAs (lncRNAs) such that in VDR null mouse epidermis the balance between oncogenic and tumor suppressor lncRNAs was shifted to oncogenic species [99]. The lack of a normal innate immune response in CYP27B1 null mice to wounding [100] or infection [101] and the increased numbers of SCC in immunocompromised patients [88] suggested that disruption of the immune system might contribute to the increased susceptibility to tumor formation when vitamin D signaling was impaired. Moreover, we [94] noted a reduction in clearance in CPDs following UVB exposure of the skin of VDR null mice, suggesting that disruption of DNA damage repair was playing a role in tumor susceptibility in these mice. In what follows I will examine potential mechanisms and pathways within those mechanisms for their contribution to the role of VDR as a tumor suppressor including regulation of proliferation and differentiation with particular attention to the hedgehog (Hh), Wnt/β-catenin, hyaluronan/CD44 pathways, long noncoding RNAs, immunoregulation, and DNA damage repair (Fig. 14.1).
Fig. 14.1. Multiple mechanisms by which 1,25(OH)2D/VDR suppresses tumor formation.
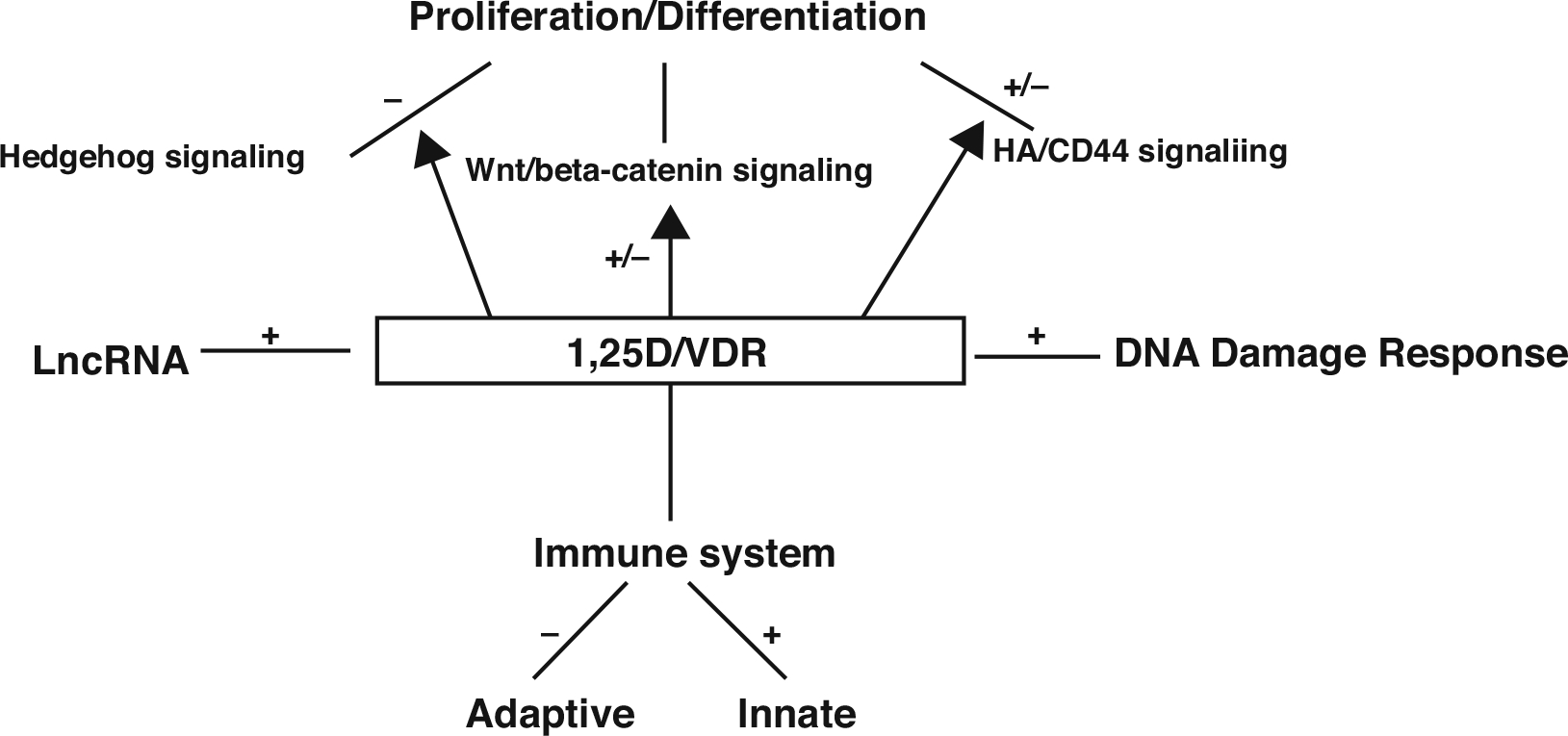
Vitamin D signaling has the potential to suppress tumor formation by affecting a number of pathways. As depicted in this figure and discussed in the text, there are four major mechanisms: regulation of proliferation and differentiation via three pathways: hedgehog signaling, Wnt/b-catenin signaling, and HA/CD44 signaling; regulation of long noncoding RNAs by increasing the balance of tumor suppressors to oncogenic lncRNAs; immunity by suppressive adaptive and stimulating innate immunity; and promoting DNA damage response
Vitamin D Regulation of Epidermal Proliferation and Differentiation
The epidermis is composed of four layers of keratinocytes at different stages of differentiation (reviewed in [11]). The basal layer (stratum basale, SB) rests on the basal lamina separating the dermis and epidermis. Within this layer are the stem cells. These cells proliferate, providing the cells for the upper differentiating layers. The basal cells are characterized by keratins K5 and K14 as well as the stem cell marker K15 and integrin α6β4. These cells have the highest expression of CYP27B1 and VDR in the epidermis. As the cells migrate upward from this basal layer into the spinous layer (stratum spinosum, SS), they initiate the production of the keratins K1 and K10, the keratins characteristic of the more differentiated layers of the epidermis. Cornified envelope precursors such as involucrin also appear in the spinous layer as does the enzyme transglutaminase K, responsible for the ε-(γ-glutamyl)lysine cross-linking of these substrates into the insoluble cornified envelope. Migrating further into the granular layer (stratum granulosum {SG}), lying above the spinous layer, the cells acquire the electron-dense keratohyalin granules containing profilaggrin and loricrin that give the SG its name. Loricrin is a major component of the cornified envelope. Filaggrin serves to bundle the keratin filaments, but also when proteolyzed is thought to contribute to the hydration of the outer layers. The granular layer also contains lamellar bodies – lipid, enzyme, and antimicrobial peptide-filled structures that fuse with the plasma membrane, divesting their contents into the extracellular space between the SG and stratum corneum (SC). The secreted enzymes process the lipids that contribute to the permeability barrier of the epidermis in conjunction with the keratin bundles and cornified envelope. The antimicrobial peptides provide a barrier against infectious organisms in the SC.
1,25(OH)2D increases essentially every step of this differentiation process [102–107] while inhibiting proliferation at least at concentrations above 1 nM. These actions complement those of calcium [69]; the response to which is enhanced by 1,25(OH)2D via its induction of the CaSR [108, 109] and the phospholipase C enzymes [110–112] that regulate intracellular calcium and other signaling molecules critical for the differentiation process. The antiproliferative effects are accompanied by a reduction in the expression of c-myc [113] and cyclin D1 [114] and an increase in the cell cycle inhibitors p21cip and p27kip. In addition, 1,25(OH)2D and its receptor regulate the processing of the long-chain glycosylceramides that are critical for permeability barrier formation [115] and induce the receptors, toll-like receptor 2 (TLR2) and its coreceptor CD14, that initiate the innate immune response in skin [100]. Activation of these receptors leads to the induction of CYP27B1 (the enzyme that produces 1,25(OH)2D), which in turn induces cathelicidin resulting in the killing of invasive organisms [100, 116]. Deletion of either VDR [117, 118] or CYP27B1 [119] results in defects in the differentiation process leading to an abnormal barrier and increased proliferation of the epidermis with a defective innate immune response [100]. Three pathways that appear to be important in vitamin D signaling in the epidermis with respect to proliferation and differentiation that we believe underlie the predisposition of the VDR null mouse to tumor formation are the Hh, Wnt/β-catenin, and hyaluronan/CD44 pathways, any and all of which could be influenced by the changes in long noncoding RNAs altered in their expression by the VDR.
The Hedgehog (Hh) Pathway
In the skin sonic hedgehog (Shh) is the ligand for patched (Ptch) 1, a 12-transmembrane domain protein that in the absence of Shh inhibits the function of another membrane protein smoothened (Smo). Smo in turn maintains a family of transcription factors, Gli1 and Gli2 in particular, in the cytoplasm bound to suppressor of fused (Sufu) [120, 121]. When Shh binds to Ptch 1, the inhibition of Smo is relaxed and Gli1 and 2 are released from Sufu and move into the nucleus where they initiate transcription of a number of factors including each other as well as Ptch 1, the antiapoptotic factor bcl2, cyclins D1 and D2, E2F1, and cdc45 (all of which promote proliferation), while suppressing genes associated with keratinocyte differentiation such as K1, K10, involucrin, loricrin, and the VDR [122–126].
The appearance of BCC is characteristic of tumors formed when Hh signaling is disrupted [127], although activation of Hh signaling also predisposes to UVR-induced SCC formation [128]. VDR null animals overexpress elements of the Hh signaling pathway in their epidermis and the epidermal portion (utricles) of the hair follicles [94]. Moreover, 1,25(OH)2D suppresses the expression of all elements of the Hh pathway in a dose-dependent fashion that requires the VDR [94, 129] and reduces tumor growth in Ptch 1 null mice. The promoters of Shh and Gli1 have binding sites for VDR [130] suggesting that the effects of 1,25(OH)2D on these genes are direct. However, vitamin D has also been shown to bind to and inhibit the actions of smoothened (Smo) directly without seeming to require further metabolism to 1,25(OH)2D [131, 132].
The Wnt/β-Catenin Pathway
Wnt signaling via activation of β-catenin has a complex role in VDR function as discussed briefly earlier. In the canonical pathway, the receptor for Wnt ligands is a family of seven-transmembrane Frizzled receptors and an LRP5 or LRP6 coreceptor. When Wnt binds to this complex, disheveled (Dvl) is phosphorylated resulting in disruption of the axin/APC complex and inhibition of glycogen synthase kinase 3β ((GSK-3β)). In the basal state, GSK-3β phosphorylates the serine(s) within exon 3 of β-catenin resulting in its degradation by the E3 ubiquitin ligase. Wnt signaling, by blocking this phosphorylation, increases the availability of β-catenin in the nucleus, where it binds to transcription factors of the T-cell factor (TCF) and lymphoid enhancer factor (LEF) families to promote expression of genes such as cyclin D1 and c-myc [133] important for proliferation. β-catenin also forms part of the adherens junction complex with E-cadherin where it may play an important role in keratinocyte differentiation [134]. Tyrosine phosphorylation of E-cadherin, as occurs after calcium administration to keratinocytes, promotes the binding of β-catenin and other catenins to the adherens junction complex [134, 135] making it less available for transcriptional activity. 1,25(OH)2D increases E-cadherin expression and its membrane localization [136]. Overexpression and/or activating mutations in the β-catenin pathway lead to skin tumors, in this case pilomatricomas or trichofolliculomas (hair follicle tumors) [137–139]. As noted earlier VDR binds to β-catenin and reduces the transcriptional activity of β-catenin in a 1,25 (OH)2D-dependent fashion [64]. On the other hand binding of β-catenin to VDR in its AF-2 domain enhances the 1,25(OH)2D-dependent transcriptional activity of VDR [65]. Palmer et al. [97] evaluated the interaction between VDR and β-catenin in transcriptional regulation in keratinocytes and identified putative response elements for VDR and β-catenin/LEF in a number of genes. These interactions were either positive or negative, depending on the gene being evaluated. The hypothesis put forward is that genes in which the interaction was positive (i.e., stimulated transcription) benefited from β-catenin acting as a coactivator for VDR on VDREs, whereas in situations where the interaction was negative (i.e., suppression of transcription), VDR prevented β-catenin from binding to TCF/LEF required for transcription in those genes. We [114] have found in keratinocytes that knockdown of VDR reduces E-cadherin expression and formation of the β-catenin/E-cadherin membrane complex resulting in increased β-catenin transcriptional activity, whereas 1,25 (OH)2D administration has the opposite effect. This was associated with increased (with VDR knockdown) or decreased (with 1,25(OH)2D administration) keratinocyte proliferation and cyclin D1 expression. On the other hand Cianferotti et al. [140] found a reduction in proliferation of keratinocytes in the dermal portion of the hair follicle (below the bulge) in VDR null mice, and no stimulation of proliferation when β-catenin was overexpressed in these cells in contrast to the stimulation of proliferation in control animals. Moreover, when we examined mice lacking VDR specifically in their keratinocytes, we observed a reduction in both epidermal and hair follicle stem cells and a reduction in β-catenin signaling opposite to our original observations in keratinocyte cultures [141]. Thus VDR/β-catenin interactions can be positive or negative, depending on the gene/cell/function being evaluated and the cellular context, but disruption of this pathway does appear to affect stem cell numbers and their differentiation.
The β-catenin and Shh pathways interact [55] [97]. Both are required for normal hair follicle development and cycling. Putative β-catenin/LEF response elements have been found in a number of Hh pathway genes [97]. Conditional deletion of β-catenin eliminates Shh expression from the hair follicle [142] and tongue [62], whereas Shh inhibits β-catenin transcriptional activity [143]. However, the degree to which β-catenin/Shh interactions occur in the formation of skin cancer has not been carefully examined.
The Hyaluronan/CD44 Pathway
Hyaluronan (HA), a major glycosaminoglycan within the extracellular matrix, binds to CD44 [144], a functionally important membrane receptor found in most cells including keratinocytes. CD44 is encoded by 19 exons of which 12 can be alternatively spliced, generally involving exons 6–14 to form variants (v1–10) [145]. Differentiated keratinocytes express epican, CD44v3–10 (i.e., CD44 containing exons 9–14 in addition to exons 1–5, 15–17,19), whereas undifferentiated keratinocytes, mouse skin after chronic UVR, and SCCs express a variety of shorter CD44s with variable numbers of exons between the first 5 and last 5 [146]. These different isoforms of CD44 appear to signal differently [146]. HA is synthesized by different HA synthases, and its size is further modified by hyaluronidases. UVR alters both HA synthesis and its degradation [147]. Large HA predominates in normal mouse skin, whereas small HA predominates in cancer tissue [148]. Large HA promotes transcriptional activation and differentiation, whereas small HA induces the expression of proinflammatory cytokines/chemokines as well as cell proliferation and migration [149]. We have proposed that large HA/CD44 epican promotes keratinocyte differentiation and DNA repair through Rac/PKNγ and p38MAPK signaling, whereas small HA/CD44 variant promotes proliferation and inflammation through RhoA/ROK-dependent NFκB/Stat-3 signaling [98]. 1,25(OH)2D blocks small HA/CD44-mediated RhoA/ROK activation and NFkB-p65 signaling as well as inflammatory gene expression and proliferation in transformed keratinocytes and SCC [98].
Long Noncoding RNAs (LncRNAs)
Only about 2% of the genome is actively transcribed and translated into proteins, while a much larger percentage of the genome is actively transcribed without protein coding potential [150]. These noncoding transcripts can be broadly categorized into short and long noncoding RNAs. The arbitrary size delineation is at 200 bases in length: small noncoding RNAs are less than 200 bases, whereas lncRNAs are endogenous cellular RNAs larger than 200 bases and can even be greater than 100 kb in length [151]. LncRNAs account for 80% of the transcriptome [150]; they are spliced and contain polyadenylation signals, much like messenger RNAs [152]. LncRNAs are expressed across all mammalian genomes and have emerged as master regulators of embryonic pluripotency, differentiation, and body axis patterning, promoting developmental transitions [152, 153] and regulating histone modifications hence influencing the epigenetic programs of the transcriptome [154]. A number of these lncRNAs when aberrantly expressed are associated with cancers. We explored the potential role of lncRNAs in VDR protection against skin tumor formation by profiling 90 well-annotated mouse lncRNAs from mouse keratinocytes cultured in vitro and mouse epidermis from epidermal-specific VDR null mice and their normal littermates [99, 155]. We found that several well-known oncogenes, including H19, HOTTIP, and Nespas, are significantly increased, whereas tumor suppressor lncRNAs (Kcnq1ot1, lincRNA-p21) were attenuated in VDR-deleted keratinocytes. A similar pattern of lncRNA-expression profiling was observed in the epidermis of epidermal-specific VDR null mice vs. control littermates. In addition to the altered lncRNAs (H19, HOTTIP, Nespas, Kcnq1ot1, lincRNA-p21) in VDR-deleted cultured keratinocytes, there was an increase in other oncogenes (mHOTAIR, Malat1, and SRA) and a decrease in other tumor suppressors (Foxn2-as, Gtl2-as, H19-as) in VDR null mouse epidermis. This study reveals a novel mechanism for protection by VDR against skin cancer formation by maintaining the balance of oncogenic to tumor-suppressing lncRNAs, although the relevant pathways involved require further investigation.
Vitamin D Regulation of Immune Function in the Skin
VDR and CYP27B1 are found in professional immune cells, namely, dendritic cells, macrophages, and lymphocytes [156, 157], responsible for both innate and adaptive immune responses as well as in epithelial cells expressing the components of the innate immune response. 1,25(OH)2D regulates the proliferation and function [158] of these cells. Although it is not clear the extent to which dysregulated immune function contributes to cancer development in the skin, a link between inflammation and cancer susceptibility in the skin involving VDR has been established [28].
Adaptive Immunity
The adaptive immune response involves the ability of T and B lymphocytes to produce cytokines and immunoglobulins, respectively, in response to antigens presented to them by cells such as macrophages and dendritic cells. 1,25(OH)2D suppresses the adaptive immune response by inhibiting proliferation, immunoglobulin production, and differentiation of B-cell precursors into plasma cells [157]. 1,25(OH)2D inhibits T cell proliferation [159] and the differentiation of CD4 cells into Th1 cells capable of producing IFN-γ and IL-2 and activating macrophages [160] and Th17 cells capable of producing IL17 and IL22 [161, 162]. On the other hand, 1,25(OH)2D stimulates IL-4, IL-5, and IL10 production [163] by increasing CD4 cell differentiation into Th2 and regulatory T cells (Treg) [164]. The IL-10 so produced is one means by which Treg block Th1 and Th17 development. Part of these effects is mediated by the negative impact of 1,25(OH)2D on the maturation and antigen presenting capability of dendritic cells [165]. It is unclear if this suppression of the adaptive immune system alters tumor surveillance in the skin.
Innate Immunity
The innate immune response involves the activation of toll-like receptors (TLRs) [166] that serve as transmembrane pathogen-recognition receptors detecting specific membrane patterns (PAMP) shed by a wide variety of infectious agents [167]. Activation of TLRs leads to the induction of antimicrobial peptides and reactive oxygen species, which kill the organism. Cathelicidin is the best studied of these antimicrobial peptides. The expression of cathelicidin is induced by 1,25(OH)2D in both myeloid and epithelial cells [168, 169], cells that also express CYP27B1 and so are capable of producing 1,25(OH)2D needed for this induction. Stimulation of TLR2 in macrophages [170] or keratinocytes [100] results in increased CYP27B1 expression, which in the presence of adequate substrate (25OHD) induces cathelicidin expression. Lack of substrate (25OHD), VDR, or CYP27B1 blunts the ability of these cells to respond with respect to cathelicidin production [100, 169, 170].
The major cells involved in adaptive immunity in the skin include the Langerhans cells, dendritic cells, and T cells. The Langerhans cells are dendritic-like cells within the epidermis that when activated by invading organisms migrate to the lymph nodes serving the skin where they present the antigens to the T cells, initiating the adaptive immune response [171]. Keratinocytes, on the other hand, are equipped with toll-like receptors that enable them to respond to invading organisms with elaboration of antimicrobial peptides such as cathelicidin [116]. However, cathelicidin also induces an inflammatory response [172]. UVB leads to a reduction in Langerhans cells and blunts their antigen presenting activity [173–175] but stimulates the innate immune function of keratinocytes perhaps as a consequence of UVB-induced vitamin D/1,25(OH)2D production in the skin [176, 177].
The potential role of altered skin immunity by UVB with respect to skin carcinogenesis was suggested by Kripke and Fisher [178]. They found that skin tumors originally induced in mice by chronic UVR would grow when transplanted into mice that had been UV irradiated but not when transplanted into control mice. The role of 1,25(OH)2D production in UVR immunosuppression is not clear. Topical application of high concentrations of 1,25(OH)2D protected against UVR induced suppression of contact hypersensitivity in the mouse [179], but a study in humans by the same group showed suppression of delayed hypersensitivity (Mantoux test) by topical 1,25(OH)2D [180]. These data are limited but raise some concern about the balance between innate and adaptive immunity in tumor surveillance and how that balance is affected by vitamin D.
Vitamin D Regulation of the DNA Damage Response
DNA damage response (DDR) is the means by which UVR- and chemical-induced DNA damage is prevented from producing fixed DNA mutations [181]. DDR involves a cascade of damage recognition, repair, and signal transduction that coordinates the response of the cell to DNA damage. DDR activates checkpoints that delay the cell cycle, provides time for repair, and directs damaged cells into senescent or apoptotic pathways. DDR involves a number of components and is well orchestrated, tightly controlled, and highly accurate in normal primary cells such that the spontaneous mutation rate is very low, and changes in copy number are negligible [182–184]. As noted earlier UVB causes CPD and 6–4PP formation, which are bulky adducts that block the movement of replicative DNA polymerase, a high-fidelity enzyme, with a shift to translesion synthesis by lower-fidelity DNA polymerases [185]. Moreover, CPDs if they occur in promoter regions can block the binding of transcription factors [186]. With malignant transformation DDR becomes less controlled, and mutation rates and copy number abnormalities increase by orders of magnitude [182, 183, 187, 188]. Nucleotide excision repair (NER) is the principal means by which UVR damage is repaired, enabling repair before DNA replication begins. This is important as NER plays a major role in reducing the amount of damage that becomes fixed as mutations during replication [189–191]. During NER, the DNA damage is recognized by XPC acting in a complex with hRAD23B supported in some cases by the DNA damage-binding protein DDB1 and 2 [192, 193], the DNA is unwound around the lesion, and 30 base pair portions of DNA containing the lesion are excised by endonucleases such as XPF and XPG followed by fill in with DNA polymerases such as Pol δ,ε,κ.
The NER process has two main branches involving different mechanisms for the initial recognition of DNA damage [194]: transcription-coupled repair (TCR) during which DNA polymerases stop replication at the site of the lesion until it is repaired [195–199] and global genomic repair (GGR), during which non-transcribed regions of the genome are repaired [200]. Keratinocytes in the epidermis of mice lacking VDR are deficient in DDR as demonstrated by a reduced rate of clearing CPDs and 6–4PPs following UVB [201]. Moreover, 1,25(OH)2D increases CPD clearance in VDR intact mice [202, 203]. These actions have been demonstrated with 1,25(OH)2D analogs that are not thought to have genomic activity [202]. However, at least part of this enhancement of CPD clearance is due to the upregulation of two genes important for DDR: XPC (xeroderma pigmentosum complementation group C) and DDB2 (damage-specific DNA-binding protein 2 also known as XPE) [201, 204]. Furthermore, 1,25(OH)2D has been shown to increase the levels of p53, which could enhance apoptosis in those cells bearing excess DNA damage [203], and reduce UVR-induced oxidative stress contributing to the DNA damage [203]. As such these actions of vitamin D signaling on DDR contribute to the reduced susceptibility of normal skin to UVB-induced tumor formation.
Summary
The VDR is present in nearly every cell in the body. Moreover, the enzyme, CYP27B1, required for the production of the VDR ligand, 1,25(OH)2D, is likewise widely distributed. Because of its abundance of 7-DHC, the epidermis is unique in its capability to produce vitamin D, metabolize it to 1,25(OH)2D, and respond to 1,25(OH)2D in a number of ways. Recent data from RNA-seq and ChIP-seq studies have demonstrated hundreds, perhaps thousands, of genes regulated by 1,25(OH)2D/VDR via VDREs which are located throughout the gene. The selection of the genes regulated by 1,25(OH)2D/VDR at any one time is cell specific and most likely dose and time specific with respect to exposure to 1,25(OH)2D. As a result of these studies, numerous pathways have been discovered by which 1,25(OH)2D/VDR may prevent cancer. In the skin UVB is critical for vitamin D production, but UVB is also the major cause of skin cancer. This chapter examines the question of whether the beneficial effects of UVB on vitamin D production can counter the harmful effects on carcinogenesis. Epidemiologic data suggest that there may be a threshold below which UVR is not carcinogenic, a threshold that would suffice for adequate vitamin D production. Conceivably, vitamin D production at such levels of UVB exposure might even be protective. Four mechanisms for such protection were examined. The first mechanism focuses on the role of vitamin D signaling in keratinocyte proliferation and differentiation. Three pathways affecting proliferation and differentiation, namely, the hedgehog, Wnt/β-catenin, and hyaluronan/CD44 pathways, were evaluated. Mice lacking the VDR have increased expression of the hedgehog pathway, a key pathway involved in BCC formation. The role and regulation of the Wnt/β-catenin pathway in tumor formation is less clear as it is reduced in stem cells of the hair follicle and epidermis in VDR null mice in vivo but increased in keratinocytes lacking VDR when evaluated in vitro. Hair follicle tumors occur when Wnt/β-catenin signaling is excessive, but only in mice lacking VDR are malignant tumors (BCC) found. VDR/1,25(OH)2D inhibits expression of the components of the Hh pathway, but the interaction of VDR with β-catenin can be inhibitory or stimulatory with respect to gene expression depending on the gene. Overexpression of the hedgehog and Wnt/β-catenin pathways leads to increased proliferation and decreased differentiation associated with tumor development. The impact of hyaluronan/CD44 signaling on keratinocyte proliferation and differentiation depends both on the ligand (short HA vs long HA) and receptor (variant CD44 vs epican CD44). The short HA/CD44variant promotes proliferation and inflammation, whereas the long HA/CD44Epican promotes differentiation. VDR/1,25(OH)2D inhibits the short HA/CD44 variant pathway. The second mechanism discussed is VDR/1,25(OH)2D regulation of the expression of long noncoding RNAs. The loss of VDR results in an increased ratio of oncogenic to tumor suppressor lncRNAs. The third mechanism involves the immune system, although the role of the immune system in epidermal carcinogenesis is not clear. However, an unbiased genomic examination of pathways associated with tumor susceptibility, inflammation, keratinocyte differentiation, and tumor formation linked these events with the VDR. 1,25(OH)2D/VDR promotes innate immunity but suppresses adaptive immunity. Whether this is beneficial regarding tumor development requires further study. The fourth mechanism is DNA damage response (DDR). The epidermis of VDR null mice shows impaired DDR following UVR. 1,25(OH)2D accelerates DDR by what appears to be genomic and nongenomic actions. Thus the skin has developed mechanisms to protect itself from the harmful effects of UVR. Vitamin D production, metabolism, and regulation of the processes described in this chapter are likely to play key roles in this protection.
Acknowledgments
I would like to acknowledge the collaborators whose work I have referenced, namely, Drs. Zhongjian Xie, Chialing Tu, Arnaud Teichert, Kumar Pillai, Yuko Oda, Yan Jiang, Dennis Oh, and James Cleaver. Current support includes funding from the VA R&D program IO1 BX003814.
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. [DOI] [PubMed] [Google Scholar]
- 2.Freeman SE, Hacham H, Gange RW, Maytum DJ, Sutherland JC, Sutherland BM. Wavelength dependence of pyrimidine dimer formation in DNA of human skin irradiated in situ with ultraviolet light. Proc Natl Acad Sci USA. 1989;86:5605–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussein MR. Ultraviolet radiation and skin cancer: molecular mechanisms. J Cutan Pathol. 2005;32:191–205. [DOI] [PubMed] [Google Scholar]
- 4.Mason RS, Reichrath J. Sunlight vitamin D and skin cancer. Anti Cancer Agents Med Chem. 2013;13:83–97. [PubMed] [Google Scholar]
- 5.Besaratinia A, Synold TW, Chen HH, Chang C, Xi B, Riggs AD, Pfeifer GP. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci USA. 2005;102:10058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikle DD, Nemanic MK, Gee E, Elias P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J Clin Invest. 1986;78:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikle DD, Pillai S, Gee E, Hincenbergs M. Tumor necrosis factor-alpha regulation of 1,25-dihydroxyvitamin D production by human keratinocytes. Endocrinology. 1991;129:33–8. [DOI] [PubMed] [Google Scholar]
- 8.Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch Dermatol Res. 2009;302:5–17. [DOI] [PubMed] [Google Scholar]
- 9.Colston K, Colston MJ, Fieldsteel AH, Feldman D. 1,25-dihydroxyvitamin D3 receptors in human epithelial cancer cell lines. Cancer Res. 1982;42:856–9. [PubMed] [Google Scholar]
- 10.Pillai S, Bikle DD, Elias PM. 1,25-Dihydroxyvitamin D production and receptor binding in human keratinocytes varies with differentiation. J Biol Chem. 1988;263:5390–5. [PubMed] [Google Scholar]
- 11.Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–6. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF, McLaughlin JA, Clark MB, Doppelt SH. Factors that influence the cutaneous photosynthesis of previtamin D3. Science. 1981;211:590–3. [DOI] [PubMed] [Google Scholar]
- 14.Passeron T, Bouillon R, Callender V, Cestari T, Diepgen TL, Green AC, Pols JC, Bernard BA, Ly F, Bernerd F, Marrot L, Nielsen M, Verschoore M, Jablonski NG, Young AR, (2019) Sunscreen photoprotection and vitamin D status. British Journal of Dermatology 181 (5):916–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holick MF, McLaughlin JA, Clark MB, Holick SA, J.T., ARR PJ, Blank IH, Parrish JA. Photosynthesis of previtamin D3 in human and the physiologic consequences. Science. 1980;210:203–5. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF, Richtand NM, McNeill SC, Holick SA, Henley JW, Potts JT. Isolation and identification of previtamin D3 from the skin of exposed to ultraviolet irradiation. Biochemistry. 1979;18:1003–8. [DOI] [PubMed] [Google Scholar]
- 17.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann B, Tiebel O, Meurer M. Expression of vitamin D3 25-hydroxylase (CYP27) mRNA after induction by vitamin D3 or UVB radiation in keratinocytes of human skin equivalents–a preliminary study. Arch Dermatol Res. 1999;291:507–10. [DOI] [PubMed] [Google Scholar]
- 19.Vantieghem K, De Haes P, Bouillon R, Segaert S. Dermal fibroblasts pretreated with a sterol Delta7-reductase inhibitor produce 25-hydroxyvitamin D3 upon UVB irradiation. J Photochem Photobiol B. 2006;85:72–8. [DOI] [PubMed] [Google Scholar]
- 20.Xie Z, Munson SJ, Huang N, Portale AA, Miller WL, Bikle DD. The mechanism of 1,25-dihydroxyvitamin D(3) autoregulation in keratinocytes. J Biol Chem. 2002;277:36987–90. [DOI] [PubMed] [Google Scholar]
- 21.Bikle DD, Pillai S, Gee E, Hincenbergs M. Regulation of 1,25-dihydroxyvitamin D production in human keratinocytes by interferon-gamma. Endocrinology. 1989;124:655–60. [DOI] [PubMed] [Google Scholar]
- 22.Meyer MB, Benkusky NA, Kaufmann M, Lee SM, Onal M, Jones G, Pike JW. A kidney-specific genetic control module in mice governs endocrine regulation of the cytochrome P450 gene Cyp27b1 essential for vitamin D3 activation. J Biol Chem. 2017;292:17541–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): genomic and non-genomic mechanisms. Best Pract Res. 2011;25:543–59. [DOI] [PubMed] [Google Scholar]
- 25.Battaglia S, Maguire O, Campbell MJ. Transcription factor co-repressors in cancer biology: roles and targeting. Int J Cancer. 2010;126:2511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). Endocrinol Metab Clin N Am. 2010;39:255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlberg C, Seuter S, Heikkinen S. The first genome-wide view of vitamin D receptor locations and their mechanistic implications. Anticancer Res. 2012;32:271–82. [PubMed] [Google Scholar]
- 28.Quigley DA, To MD, Perez-Losada J, Pelorosso FG, Mao JH, Nagase H, Ginzinger DG, Balmain A. Genetic architecture of mouse skin inflammation and tumour susceptibility. Nature. 2009;458:505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu JY, Feldman D, McNeal JE, Peehl DM. Reduced 1alpha-hydroxylase activity in human prostate cancer cells correlates with decreased susceptibility to 25-hydroxyvitamin D3-induced growth inhibition. Cancer Res. 2001;61:2852–6. [PubMed] [Google Scholar]
- 30.Brozyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Expression of the vitamin D-activating enzyme 1alpha-hydroxylase (CYP27B1) decreases during melanoma progression. Hum Pathol. 2012;44:374–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother Pharmacol. 2006;57:234–40. [DOI] [PubMed] [Google Scholar]
- 32.Solomon C, White JH, Kremer R. Mitogen-activated protein kinase inhibits 1,25-dihydroxyvitamin D3-dependent signal transduction by phosphorylating human retinoid X receptor alpha. J Clin Invest. 1999;103:1729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larriba MJ, Martin-Villar E, Garcia JM, Pereira F, Pena C, de Herreros AG, Bonilla F, Munoz A. Snail2 cooperates with Snail1 in the repression of vitamin D receptor in colon cancer. Carcinogenesis. 2009;30:1459–68. [DOI] [PubMed] [Google Scholar]
- 34.Mittal MK, Myers JN, Misra S, Bailey CK, Chaudhuri G. In vivo binding to and functional repression of the VDR gene promoter by SLUG in human breast cells. Biochem Biophys Res Commun. 2008;372:30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marik R, Fackler M, Gabrielson E, Zeiger MA, Sukumar S, Stearns V, Umbricht CB. DNA methylation-related vitamin D receptor insensitivity in breast cancer. Cancer Biol Ther. 2010;10:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohri T, Nakajima M, Takagi S, Komagata S, Yokoi T. MicroRNA regulates human vitamin D receptor. Int J Cancer. 2009;125:1328–33. [DOI] [PubMed] [Google Scholar]
- 37.Essa S, Reichrath S, Mahlknecht U, Montenarh M, Vogt T, Reichrath J. Signature of VDR miRNAs and epigenetic modulation of vitamin D signaling in melanoma cell lines. Anticancer Res. 2012;32:383–9. [PubMed] [Google Scholar]
- 38.Reichrath J, Reichrath S, Heyne K, Vogt T, Roemer K. Tumor suppression in skin and other tissues via cross-talk between vitamin D- and p53-signaling. Front Physiol. 2014;5:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sequeira VB, Rybchyn MS, Tongkao-On W, Gordon-Thomson C, Malloy PJ, Nemere I, Norman AW, Reeve VE, Halliday GM, Feldman D, Mason RS. The role of the vitamin D receptor and ERp57 in photoprotection by 1alpha,25-dihydroxyvitamin D3. Mol Endocrinol (Baltimore, Md). 2012;26:574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Reed G, Guardia J, Lakhan S, Couture O, Hays E, Chandar N. Vitamin D directly regulates Mdm2 gene expression in osteoblasts. Biochem Biophys Res Commun. 2013;430:370–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heyne K, Heil TC, Bette B, Reichrath J, Roemer K. MDM2 binds and inhibits vitamin D receptor. Cell Cycle. 2015;14:2003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tongkao-On W, Gordon-Thomson C, Dixon KM, Song EJ, Luu T, Carter SE, Sequeira VB, Reeve VE, Mason RS. Novel vitamin D compounds and skin cancer prevention. Dermato-Endocrinol. 2013;5:20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dixon KM, Norman AW, Sequeira VB, Mohan R, Rybchyn MS, Reeve VE, Halliday GM, Mason RS. 1alpha,25(OH)(2)-vitamin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer Prev Res (Philadelphia, Pa). 2011;4:1485–94. [DOI] [PubMed] [Google Scholar]
- 44.Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 2012;441:61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cellular Welsh J. and molecular effects of vitamin D on carcinogenesis. Arch Biochem Biophys. 2012;523:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hager G, Formanek M, Gedlicka C, Thurnher D, Knerer B, Kornfehl J. 1,25(OH)2 vitamin D3 induces elevated expression of the cell cycle-regulating genes P21 and P27 in squamous carcinoma cell lines of the head and neck. Acta Otolaryngol. 2001;121:103–9. [DOI] [PubMed] [Google Scholar]
- 47.Swami S, Raghavachari N, Muller UR, Bao YP, Feldman D. Vitamin D growth inhibition of breast cancer cells: gene expression patterns assessed by cDNA microarray. Breast Cancer Res Treat. 2003;80:49–62. [DOI] [PubMed] [Google Scholar]
- 48.Lin R, Nagai Y, Sladek R, Bastien Y, Ho J, Petrecca K, Sotiropoulou G, Diamandis EP, Hudson TJ, White JH. Expression profiling in squamous carcinoma cells reveals pleiotropic effects of vitamin D3 analog EB1089 signaling on cell proliferation, differentiation, and immune system regulation. Mol Endocrinol (Baltimore, Md). 2002;16:1243–56. [DOI] [PubMed] [Google Scholar]
- 49.Peehl DM, Shinghal R, Nonn L, Seto E, Krishnan AV, Brooks JD, Feldman D. Molecular activity of 1,25-dihydroxyvitamin D3 in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. J Steroid Biochem Mol Biol. 2004;92:131–41. [DOI] [PubMed] [Google Scholar]
- 50.Burgering BM. A brief introduction to FOXOlogy. Oncogene. 2008;27:2258–62. [DOI] [PubMed] [Google Scholar]
- 51.An BS, Tavera-Mendoza LE, Dimitrov V, Wang X, Calderon MR, Wang HJ, White JH. Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor. Mol Cell Biol. 2010;30:4890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrish F, Isern N, Sadilek M, Jeffrey M, Hockenbery DM. c-Myc activates multiple metabolic networks to generate substrates for cell-cycle entry. Oncogene. 2009;28:2485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rohan JN, Weigel NL. 1Alpha,25-dihydroxyvitamin D3 reduces c-Myc expression, inhibiting proliferation and causing G1 accumulation in C4–2 prostate cancer cells. Endocrinology. 2009;150:2046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salehi-Tabar R, Nguyen-Yamamoto L, Tavera-Mendoza LE, Quail T, Dimitrov V, An BS, Glass L, Goltzman D, White JH. Vitamin D receptor as a master regulator of the c-MYC/MXD1 network. Proc Natl Acad Sci USA. 2012;109:18827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colston KW, Perks CM, Xie SP, Holly JM. Growth inhibition of both MCF-7 and Hs578T human breast cancer cell lines by vitamin D analogues is associated with increased expression of insulin-like growth factor binding protein-3. J Mol Endocrinol. 1998;20:157–62. [DOI] [PubMed] [Google Scholar]
- 56.Sprenger CC, Peterson A, Lance R, Ware JL, Drivdahl RH, Plymate SR. Regulation of proliferation of prostate epithelial cells by 1,25-dihydroxyvitamin D3 is accompanied by an increase in insulin-like growth factor binding protein-3. J Endocrinol. 2001;170:609–18. [DOI] [PubMed] [Google Scholar]
- 57.Yang L, Yang J, Venkateswarlu S, Ko T, Brattain MG. Autocrine TGFbeta signaling mediates vitamin D3 analog-induced growth inhibition in breast cells. J Cell Physiol. 2001;188:383–93. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, Li P, Bao J, Nicosia SV, Wang H, Enkemann SA, Bai W. Suppression of death receptor-mediated apoptosis by 1,25-dihydroxyvitamin D3 revealed by microarray analysis. J Biol Chem. 2005;280:35458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lambert JR, Kelly JA, Shim M, Huffer WE, Nordeen SK, Baek SJ, Eling TE, Lucia MS. Prostate derived factor in human prostate cancer cells: gene induction by vitamin D via a p53-dependent mechanism and inhibition of prostate cancer cell growth. J Cell Physiol. 2006;208:566–74. [DOI] [PubMed] [Google Scholar]
- 60.Krishnan AV, Shinghal R, Raghavachari N, Brooks JD, Peehl DM, Feldman D. Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using cDNA microarrays. Prostate. 2004;59:243–51. [DOI] [PubMed] [Google Scholar]
- 61.Kodach LL, Wiercinska E, de Miranda NF, Bleuming SA, Musler AR, Peppelenbosch MP, Dekker E, van den Brink GR, van Noesel CJ, Morreau H, Hommes DW, Ten Dijke P, Offerhaus GJ, Hardwick JC. The bone morphogenetic protein pathway is inactivated in the majority of sporadic colorectal cancers. Gastroenterology. 2008;134:1332–41. [DOI] [PubMed] [Google Scholar]
- 62.Lee HJ, Liu H, Goodman C, Ji Y, Maehr H, Uskokovic M, Notterman D, Reiss M, Suh N. Gene expression profiling changes induced by a novel Gemini Vitamin D derivative during the progression of breast cancer. Biochem Pharmacol. 2006;72:332–43. [DOI] [PubMed] [Google Scholar]
- 63.Schneikert J, Behrens J. The canonical Wnt signalling pathway and its APC partner in colon cancer development. Gut. 2007;56:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, Zinser G, Valrance M, Aranda A, Moras D, Norman A, Welsh J, Byers SW. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell. 2006;21:799–809. [DOI] [PubMed] [Google Scholar]
- 66.Aguilera O, Pena C, Garcia JM, Larriba MJ, Ordonez-Moran P, Navarro D, Barbachano A, Lopez de Silanes I, Ballestar E, Fraga MF, Esteller M, Gamallo C, Bonilla F, Gonzalez-Sancho JM, Munoz A. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28:1877–84. [DOI] [PubMed] [Google Scholar]
- 67.Alvarez-Diaz S, Larriba MJ, Lopez-Otin C, Munoz A. Vitamin D: proteases, protease inhibitors and cancer. Cell Cycle (Georgetown, Tex). 2010;9:32–7. [DOI] [PubMed] [Google Scholar]
- 68.Ordonez-Moran P, Larriba MJ, Palmer HG, Valero RA, Barbachano A, Dunach M, de Herreros AG, Villalobos C, Berciano MT, Lafarga M, Munoz A. RhoA-ROCK and p38MAPK-MSK1 mediate vitamin D effects on gene expression, phenotype, and Wnt pathway in colon cancer cells. J Cell Biol. 2008;183:697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bikle DD, Xie Z, Tu CL. Calcium regulation of keratinocyte differentiation. Expert Rev Endocrinol Metabo. 2012;7:461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res. 2009;29:3687–98. [PubMed] [Google Scholar]
- 71.Bikle DD, Oda Y, Tu CL, Jiang Y. Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer. J Steroid Biochem Mol Biol. 2015;148:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bikle DD, Jiang Y, Nguyen T, Oda Y, Tu CL. Disruption of vitamin D and calcium signaling in keratinocytes predisposes to skin cancer. Front Physiol. 2016;7:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diaz GD, Paraskeva C, Thomas MG, Binderup L, Hague A. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res. 2000;60:2304–12. [PubMed] [Google Scholar]
- 74.Blutt SE, McDonnell TJ, Polek TC, Weigel NL. Calcitriol-induced apoptosis in LNCaP cells is blocked by overexpression of Bcl-2. Endocrinology. 2000;141:10–7. [DOI] [PubMed] [Google Scholar]
- 75.Palmer HG, Sanchez-Carbayo M, Ordonez-Moran P, Larriba MJ, Cordon-Cardo C, Munoz A. Genetic signatures of differentiation induced by 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 2003;63:7799–806. [PubMed] [Google Scholar]
- 76.Kasiappan R, Shen Z, Tse AK, Jinwal U, Tang J, Lungchukiet P, Sun Y, Kruk P, Nicosia SV, Zhang X, Bai W. 1,25-Dihydroxyvitamin D3 suppresses telomerase expression and human cancer growth through microRNA-498. J Biol Chem. 287:41297–41309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stambolsky P, Tabach Y, Fontemaggi G, Weisz L, Maor-Aloni R, Siegfried Z, Shiff I, Kogan I, Shay M, Kalo E, Blandino G, Simon I, Oren M, Rotter V. Modulation of the vitamin D3 response by cancer-associated mutant p53. Cancer Cell. 2010;17:273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kallay E, Pietschmann P, Toyokuni S, Bajna E, Hahn P, Mazzucco K, Bieglmayer C, Kato S, Cross HS. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis. 2001;22:1429–35. [DOI] [PubMed] [Google Scholar]
- 79.Fedirko V, Bostick RM, Long Q, Flanders WD, McCullough ML, Sidelnikov E, Daniel CR, Rutherford RE, Shaukat A. Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: a randomized clinical trial. Cancer Epidemiol Biomark Prev. 2010;19:280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang F, Li P, Fornace AJ Jr, Nicosia SV, Bai W. G2/M arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through the induction of GADD45 via an exonic enhancer. J Biol Chem. 2003;278:48030–40. [DOI] [PubMed] [Google Scholar]
- 81.Muller-Decker K, Furstenberger G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol Carcinog. 2007;46:705–10. [DOI] [PubMed] [Google Scholar]
- 82.Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65:7917–25. [DOI] [PubMed] [Google Scholar]
- 83.Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1 alpha,25-dihydroxyvitamin D (3) inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214–20. [DOI] [PubMed] [Google Scholar]
- 84.Campbell CL, Savarese DM, Quesenberry PJ, Savarese TM. Expression of multiple angiogenic cytokines in cultured normal human prostate epithelial cells: predominance of vascular endothelial growth factor. Int J Cancer. 1999;80:868–74. [DOI] [PubMed] [Google Scholar]
- 85.Ben-Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mabjeesh NJ. 1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther. 2007;6:1433–9. [DOI] [PubMed] [Google Scholar]
- 86.Chung I, Han G, Seshadri M, Gillard BM, Yu WD, Foster BA, Trump DL, Johnson CS. Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res. 2009;69:967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (New York, NY). 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- 88.Zavos G, Karidis NP, Tsourouflis G, Bokos J, Diles K, Sotirchos G, Theodoropoulou E, Kostakis A. Nonmelanoma skin cancer after renal transplantation: a single-center experience in 1736 transplantations. Int J Dermatol. 2011;50:1496–500. [DOI] [PubMed] [Google Scholar]
- 89.Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53:1333–6. [DOI] [PubMed] [Google Scholar]
- 90.Gorman S, Kuritzky LA, Judge MA, Dixon KM, McGlade JP, Mason RS, Finlay-Jones JJ, Hart PH. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–83. [DOI] [PubMed] [Google Scholar]
- 91.Zinser GM, Sundberg JP, Welsh J. Vitamin D (3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis. 2002;23:2103–9. [DOI] [PubMed] [Google Scholar]
- 92.Indra AK, Castaneda E, Antal MC, Jiang M, Messaddeq N, Meng X, Loehr CV, Gariglio P, Kato S, Wahli W, Desvergne B, Metzger D, Chambon P. Malignant transformation of DMBA/TPA-induced papillomas and nevi in the skin of mice selectively lacking retinoid-X-receptor alpha in epidermal keratinocytes. J Invest Dermatol. 2007;127:1250–60. [DOI] [PubMed] [Google Scholar]
- 93.Ellison TI, Smith MK, Gilliam AC, Macdonald PN. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008;128:2508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teichert AE, Elalieh H, Elias PM, Welsh J, Bikle DD. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J Invest Dermatol. 2011;131:2289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Daya-Grosjean L, Sarasin A. The role of UV induced lesions in skin carcinogenesis: an overview of oncogene and tumor suppressor gene modifications in xeroderma pigmentosum skin tumors. Mutat Res. 2005;571:43–56. [DOI] [PubMed] [Google Scholar]
- 96.Aszterbaum M, Rothman A, Johnson RL, Fisher M, Xie J, Bonifas JM, Zhang X, Scott MP, Epstein EH Jr. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885–8. [DOI] [PubMed] [Google Scholar]
- 97.Palmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS One. 2008;3:e1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bourguignon LY, Bikle D. Selective Hyaluronan-CD44 Signaling promotes miRNA-21 expression and interacts with vitamin D function during cutaneous squamous cell carcinomas progression following UV irradiation. Front Immunol. 2015;6:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang YJ, Bikle DD. LncRNA profiling reveals new mechanism for VDR protection against skin cancer formation. J Steroid Biochem Mol Biol. 2014;144 (Pt A):87–90. [DOI] [PubMed] [Google Scholar]
- 100.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muehleisen B, Bikle DD, Aguilera C, Burton DW, Sen GL, Deftos LJ, Gallo RL. PTH/PTHrP and Vitamin D Control Antimicrobial Peptide Expression and Susceptibility to Bacterial Skin Infection. Sci Transl Med. 2012;4:135ra166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pillai S, Bikle DD. Role of intracellular-free calcium in the cornified envelope formation of keratinocytes: differences in the mode of action of extracellular calcium and 1,25 dihydroxyvitamin D3. J Cell Physiol. 1991;146:94–100. [DOI] [PubMed] [Google Scholar]
- 103.Bikle DD, Pillai S, Gee E. Squamous carcinoma cell lines produce 1,25 dihydroxyvitamin D, but fail to respond to its prodifferentiating effect. J Invest Dermatol. 1991;97:435–41. [DOI] [PubMed] [Google Scholar]
- 104.Hosomi J, Hosoi J, Abe E, Suda T, Kuroki T. Regulation of terminal differentiation of cultured mouse epidermal cells by 1 alpha,25-dihydroxyvitamin D3. Endocrinology. 1983;113:1950–7. [DOI] [PubMed] [Google Scholar]
- 105.Smith EL, Walworth NC, Holick MF. Effect of 1 alpha,25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J Invest Dermatol. 1986;86:709–14. [DOI] [PubMed] [Google Scholar]
- 106.McLane JA, Katz M, Abdelkader N. Effect of 1,25-dihydroxyvitamin D3 on human keratinocytes grown under different culture conditions. In Vitro Cell Dev Biol. 1990;26:379–87. [DOI] [PubMed] [Google Scholar]
- 107.Hawker NP, Pennypacker SD, Chang SM, Bikle DD. Regulation of human epidermal keratinocyte differentiation by the vitamin D receptor and its coactivators DRIP205, SRC2, and SRC3. J Invest Dermatol. 2007;127:874. [DOI] [PubMed] [Google Scholar]
- 108.Tu CL, Chang W, Xie Z, Bikle DD. Inactivation of the calcium sensing receptor inhibits E-cadherin-mediated cell-cell adhesion and calcium-induced differentiation in human epidermal keratinocytes. J Biol Chem. 2008;283:3519–28. [DOI] [PubMed] [Google Scholar]
- 109.Tu CL, Oda Y, Komuves L, Bikle DD. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 2004;35:265–73. [DOI] [PubMed] [Google Scholar]
- 110.Xie Z, Bikle DD. Cloning of the human phospholipase C-gamma1 promoter and identification of a DR6-type vitamin D-responsive element. J Biol Chem. 1997;272:6573–7. [DOI] [PubMed] [Google Scholar]
- 111.Xie Z, Bikle DD. Phospholipase C-gamma1 is required for calcium-induced keratinocyte differentiation. J Biol Chem. 1999;274:20421–4. [DOI] [PubMed] [Google Scholar]
- 112.Xie Z, Bikle DD. Inhibition of 1,25-Dihydroxyvitamin-D-induced keratinocyte differentiation by blocking the expression of phospholipase C-gamma1. J Invest Dermatol. 2001;117:1250–4. [DOI] [PubMed] [Google Scholar]
- 113.Matsumoto K, Hashimoto K, Nishida Y, Hashiro M, Yoshikawa K. Growth-inhibitory effects of 1,25-dihydroxyvitamin D3 on normal human keratinocytes cultured in serum-free medium. Biochem Biophys Res Commun. 1990;166:916–23. [DOI] [PubMed] [Google Scholar]
- 114.Bikle DD. The vitamin D receptor: a tumor suppressor in skin. Discov Med. 2011;11:7–17. [PMC free article] [PubMed] [Google Scholar]
- 115.Oda Y, Uchida Y, Moradian S, Crumrine D, Elias P, Bikle D. Vitamin D receptor and coactivators SRC 2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J Invest Dermatol. 2009;129:1367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, Chang S, Crumrine D, Yoshizawa T, Kato S, Bikle DD. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11–6. [DOI] [PubMed] [Google Scholar]
- 118.Bikle DD, Elalieh H, Chang S, Xie Z, Sundberg JP. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340–53. [DOI] [PubMed] [Google Scholar]
- 119.Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol. 2004;122:984–92. [DOI] [PubMed] [Google Scholar]
- 120.Barnfield PC, Zhang X, Thanabalasingham V, Yoshida M, Hui CC. Negative regulation of Gli1 and Gli2 activator function by Suppressor of fused through multiple mechanisms. Differentiation. 2005;73:397–405. [DOI] [PubMed] [Google Scholar]
- 121.Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, Ericson J, Toftgard R, Teglund S. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell. 2006;10:187–97. [DOI] [PubMed] [Google Scholar]
- 122.Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Ikram MS, Quinn AG, Philpott MP, Frischauf AM, Aberger F. The zinc-finger transcription factor GLI2 antagonizes contact inhibition and differentiation of human epidermal cells. Oncogene. 2004;23:1263–74. [DOI] [PubMed] [Google Scholar]
- 123.Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Philpott MP, Esterbauer H, Hauser-Kronberger-C, Frischauf AM, Aberger F. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004;64:7724–31. [DOI] [PubMed] [Google Scholar]
- 124.Regl G, Neill GW, Eichberger T, Kasper M, Ikram MS, Koller J, Hintner H, Quinn AG, Frischauf AM, Aberger F. Human GLI2 and GLI1 are part of a positive feedback mechanism in Basal Cell Carcinoma. Oncogene. 2002;21:5529–39. [DOI] [PubMed] [Google Scholar]
- 125.Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, Dlugosz AA. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet. 2000;24:216–7. [DOI] [PubMed] [Google Scholar]
- 126.Nilsson M, Unden AB, Krause D, Malmqwist U, Raza K, Zaphiropoulos PG, Toftgard R. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci USA. 2000;97:3438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, Negus K, Smyth I, Pressman C, Leffell DJ, Gerrard B, Goldstein AM, Dean M, Toftgard R, Chenevix-Trench G, Wainwright B, Bale AE. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–51. [DOI] [PubMed] [Google Scholar]
- 128.Ping XL, Ratner D, Zhang H, Wu XL, Zhang MJ, Chen FF, Silvers DN, Peacocke M, Tsou HC. PTCH mutations in squamous cell carcinoma of the skin. J Invest Dermatol. 2001;116:614–6. [DOI] [PubMed] [Google Scholar]
- 129.Uhmann A, Niemann H, Lammering B, Henkel C, Hess I, Nitzki F, Fritsch A, Prufer N, Rosenberger A, Dullin C, Schraepler A, Reifenberger J, Schweyer S, Pietsch T, Strutz F, Schulz-Schaeffer W, Hahn H. Antitumoral effects of calcitriol in basal cell carcinomas involve inhibition of hedgehog signaling and induction of vitamin D receptor signaling and differentiation. Mol Cancer Ther. 2012;10:2179–88. [DOI] [PubMed] [Google Scholar]
- 130.Luderer HF, Gori F, Demay MB. Lymphoid enhancer-binding factor-1 (LEF1) interacts with the DNA-binding domain of the vitamin D receptor. J Biol Chem. 2011;286:18444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4:e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tang JY, Xiao TZ, Oda Y, Chang KS, Shpall E, Wu A, So PL, Hebert J, Bikle D, Epstein EH Jr. Vitamin D3 inhibits hedgehog signaling and proliferation in murine Basal cell carcinomas. Cancer Prev Res (Philadelphia, Pa). 2011;4:744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. [DOI] [PubMed] [Google Scholar]
- 134.Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282:8695–703. [DOI] [PubMed] [Google Scholar]
- 135.Bienz M Beta-catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol. 2005;15:R64–7. [DOI] [PubMed] [Google Scholar]
- 136.Shah S, Hecht A, Pestell R, Byers SW. Transrepression of beta-catenin activity by nuclear receptors. J Biol Chem. 2003;278:48137–45. [DOI] [PubMed] [Google Scholar]
- 137.Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999;21:410–3. [DOI] [PubMed] [Google Scholar]
- 138.Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–14. [DOI] [PubMed] [Google Scholar]
- 139.Xia J, Urabe K, Moroi Y, Koga T, Duan H, Li Y, Furue M. beta-Catenin mutation and its nuclear localization are confirmed to be frequent causes of Wnt signaling pathway activation in pilomatricomas. J Dermatol Sci. 2006;41:67–75. [DOI] [PubMed] [Google Scholar]
- 140.Cianferotti L, Cox M, Skorija K, Demay MB. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci USA. 2007;104:9428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oda Y, Hu L, Nguyen T, Fong C, Zhang J, Guo P, Bikle DD. Vitamin D receptor is required for proliferation, migration, and differentiation of epidermal stem cells and progeny during cutaneous wound repair. J Invest Dermatol. 2018;138:2423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. Beta-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–45. [DOI] [PubMed] [Google Scholar]
- 143.Iwatsuki K, Liu HX, Gronder A, Singer MA, Lane TF, Grosschedl R, Mistretta CM, Margolskee RF. Wnt signaling interacts with Shh to regulate taste papilla development. Proc Natl Acad Sci USA. 2007;104:2253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Underhill C CD44: the hyaluronan receptor. J Cell Sci. 1992;103(Pt 2):293–8. [DOI] [PubMed] [Google Scholar]
- 145.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA. 1992;89:12160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haggerty JG, Bretton RH, Milstone LM. Identification and characterization of a cell surface proteoglycan on keratinocytes. J Invest Dermatol. 1992;99:374–80. [DOI] [PubMed] [Google Scholar]
- 147.Averbeck M, Gebhardt CA, Voigt S, Beilharz S, Anderegg U, Termeer CC, Sleeman JP, Simon JC. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J Invest Dermatol. 2007;127:687–97. [DOI] [PubMed] [Google Scholar]
- 148.Stern R Complicated hyaluronan patterns in skin: enlightenment by UVB? J Invest Dermatol. 2007;127:512–3. [DOI] [PubMed] [Google Scholar]
- 149.Bourguignon LY, Wong G, Xia W, Man MQ, Holleran WM, Elias PM. Selective matrix (hyaluronan) interaction with CD44 and RhoGTPase signaling promotes keratinocyte functions and overcomes age-related epidermal dysfunction. J Dermatol Sci. 2013;72:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–9. [DOI] [PubMed] [Google Scholar]
- 151.Gibb EA, Vucic EA, Enfield KS, Stewart GL, Lonergan KM, Kennett JY, Becker-Santos DD, MacAulay CE, Lam S, Brown CJ, Lam WL. Human cancer long non-coding RNA transcriptomes. PLoS One. 2011;6:e25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mattick JS. Long noncoding RNAs in cell and developmental biology. Semin Cell Dev Biol. 2011;22:327. [DOI] [PubMed] [Google Scholar]
- 153.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spitale RC, Crisalli P, Flynn RA, Torre EA, Kool ET, Chang HY. RNA SHAPE analysis in living cells. Nat Chem Biol. 2013;9:18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jiang YJ, Bikle DD. LncRNA: a new player in 1alpha, 25(OH)(2) vitamin D(3) /VDR protection against skin cancer formation. Exp Dermatol. 2014;23:147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–93. [DOI] [PubMed] [Google Scholar]
- 157.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–47. [DOI] [PubMed] [Google Scholar]
- 158.Bikle D Vitamin D and immune function: understanding common pathways. Curr Osteoporos Rep. 2009;7:58–63. [DOI] [PubMed] [Google Scholar]
- 159.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol). J Clin Invest. 1984;74:1451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125:1704S–8S. [DOI] [PubMed] [Google Scholar]
- 161.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. [DOI] [PubMed] [Google Scholar]
- 162.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. [DOI] [PubMed] [Google Scholar]
- 164.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. [DOI] [PubMed] [Google Scholar]
- 165.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–45. [DOI] [PubMed] [Google Scholar]
- 166.Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends Mol Med. 2007;13:117–24. [DOI] [PubMed] [Google Scholar]
- 167.Medzhitov R Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. [DOI] [PubMed] [Google Scholar]
- 168.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–77. [DOI] [PubMed] [Google Scholar]
- 169.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. [DOI] [PubMed] [Google Scholar]
- 170.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. [DOI] [PubMed] [Google Scholar]
- 171.Kripke ML, Munn CG, Jeevan A, Tang JM, Bucana C. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J Immunol. 1990;145:2833–8. [PubMed] [Google Scholar]
- 172.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. [DOI] [PubMed] [Google Scholar]
- 173.Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–53. [PubMed] [Google Scholar]
- 174.Simon JC, Cruz PD Jr, Tigelaar RE, Sontheimer RD, Bergstresser PR. Adhesion molecules CD11a, CD18, and ICAM-1 on human epidermal Langerhans cells serve a functional role in the activation of alloreactive T cells. J Invest Dermatol. 1991;96:148–51. [DOI] [PubMed] [Google Scholar]
- 175.Tang A, Udey MC. Inhibition of epidermal Langerhans cell function by low dose ultraviolet B radiation. Ultraviolet B radiation selectively modulates ICAM-1 (CD54) expression by murine Langerhans cells. J Immunol. 1991;146:3347–55. [PubMed] [Google Scholar]
- 176.Glaser R, Navid F, Schuller W, Jantschitsch C, Harder J, Schroder JM, Schwarz A, Schwarz T. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J Allergy Clin Immunol. 2009;123:1117–23. [DOI] [PubMed] [Google Scholar]
- 177.Mallbris L, Edstrom DW, Sundblad L, Granath F, Stahle M. UVB upregulates the antimicrobial protein hCAP18 mRNA in human skin. J Invest Dermatol. 2005;125:1072–4. [DOI] [PubMed] [Google Scholar]
- 178.Kripke ML, Fisher MS. Immunologic parameters of ultraviolet carcinogenesis. J Natl Cancer Inst. 1976;57:211–5. [DOI] [PubMed] [Google Scholar]
- 179.Dixon KM, Deo SS, Norman AW, Bishop JE, Halliday GM, Reeve VE, Mason RS. In vivo relevance for photoprotection by the vitamin D rapid response pathway. J Steroid Biochem Mol Biol. 2007;103:451–6. [DOI] [PubMed] [Google Scholar]
- 180.Damian DL, Kim YJ, Dixon KM, Halliday GM, Javeri A, Mason RS. Topical calcitriol protects from UV-induced genetic damage but suppresses cutaneous immunity in humans. Exp Dermatol. 2010;19: e23–30. [DOI] [PubMed] [Google Scholar]
- 181.Li G, Mitchell DL, Ho VC, Reed JC, Tron VA. Decreased DNA repair but normal apoptosis in ultraviolet-irradiated skin of p53-transgenic mice. Am J Pathol. 1996;148:1113–23. [PMC free article] [PubMed] [Google Scholar]
- 182.Tlsty TD, Margolin BH, Lum K. Differences in the rates of gene amplification in nontumorigenic and tumorigenic cell lines as measured by Luria-Delbruck fluctuation analysis. Proc Natl Acad Sci USA. 1989;86:9441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tlsty TD. Normal diploid human and rodent cells lack a detectable frequency of gene amplification. Proc Natl Acad Sci USA. 1990;87:3132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Maher VM, Dorney DJ, Mendrala AL, Konze-Thomas B, McCormick JJ. DNA excision-repair processes in human cells can eliminate the cytotoxic and mutagenic consequences of ultraviolet irradiation. Mutat Res. 1979;62:311–23. [DOI] [PubMed] [Google Scholar]
- 185.Sinha RP, Hader DP. UV-induced DNA damage and repair: a review. Photochem Photobiol scie. 2002;1:225–36. [DOI] [PubMed] [Google Scholar]
- 186.Tommasi S, Swiderski PM, Tu Y, Kaplan BE, Pfeifer GP. Inhibition of transcription factor binding by ultraviolet-induced pyrimidine dimers. Biochemistry. 1996;35:15693–703. [DOI] [PubMed] [Google Scholar]
- 187.Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–7. [DOI] [PubMed] [Google Scholar]
- 188.Bielas JH, Loeb KR, Rubin BP, True LD, Loeb LA. Human cancers express a mutator phenotype. Proc Natl Acad Sci USA. 2006;103:18238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chen RH, Maher VM, McCormick JJ. Effect of excision repair by diploid human fibroblasts on the kinds and locations of mutations induced by (+/−)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene in the coding region of the HPRT gene. Proc Natl Acad Sci USA. 1990;87:8680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wood RD. DNA damage recognition during nucleotide excision repair in mammalian cells. Biochimie. 1999;81:39–44. [DOI] [PubMed] [Google Scholar]
- 191.Sertic S, Pizzi S, Lazzaro F, Plevani P, Muzi-Falconi M. NER and DDR: classical music with new instruments. Cell Cycle. 2012;11:668–74. [DOI] [PubMed] [Google Scholar]
- 192.Sugasawa K, Okamoto T, Shimizu Y, Masutani C, Iwai S, Hanaoka F. A multistep damage recognition mechanism for global genomic nucleotide excision repair. Genes Dev. 2001;15:507–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fitch ME, Nakajima S, Yasui A, Ford JM. In vivo recruitment of XPC to UV-induced cyclobutane pyrimidine dimers by the DDB2 gene product. J Biol Chem. 2003;278:46906–10. [DOI] [PubMed] [Google Scholar]
- 194.Mellon I, Bohr VA, Smith CA, Hanawalt PC. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci USA. 1986;83:8878–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mellon I, Spivak G, Hanawalt PC. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987;51:241–9. [DOI] [PubMed] [Google Scholar]
- 196.Mellon I, Rajpal DK, Koi M, Boland CR, Champe GN. Transcription-coupled repair deficiency and mutations in human mismatch repair genes. Science. 1996;272:557–60. [DOI] [PubMed] [Google Scholar]
- 197.Bohr VA. Gene specific DNA repair. Carcinogenesis. 1991;12:1983–92. [DOI] [PubMed] [Google Scholar]
- 198.Hanawalt PC. Transcription-coupled repair and human disease. Science. 1994;266:1957–8. [DOI] [PubMed] [Google Scholar]
- 199.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–74. [DOI] [PubMed] [Google Scholar]
- 200.Wood RD, Mitchell M, Sgouros J, Lindahl T. Human DNA repair genes. Science. 2001;291:1284–9. [DOI] [PubMed] [Google Scholar]
- 201.Demetriou SK, Ona-Vu K, Teichert AE, Cleaver JE, Bikle DD, Oh DH. Vitamin D receptor mediates DNA repair and Is UV inducible in intact epidermis but not in cultured keratinocytes. J Invest Dermatol. 2012;132:2097–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dixon KM, Deo SS, Wong G, Slater M, Norman AW, Bishop JE, Posner GH, Ishizuka S, Halliday GM, Reeve VE, Mason RS. Skin cancer prevention: a possible role of 1,25dihydroxyvitamin D3 and its analogs. J Steroid Biochem Mol Biol. 2005;97:137–43. [DOI] [PubMed] [Google Scholar]
- 203.Gupta R, Dixon KM, Deo SS, Holliday CJ, Slater M, Halliday GM, Reeve VE, Mason RS. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J Invest Dermatol. 2007;127:707–15. [DOI] [PubMed] [Google Scholar]
- 204.Moll PR, Sander V, Frischauf AM, Richter K. Expression profiling of vitamin D treated primary human keratinocytes. J Cell Biochem. 2007;100:574–92. [DOI] [PubMed] [Google Scholar]


