Abstract
Pramipexole is a potent agonist of D3 and D2 dopamine receptors, currently approved for clinical use in Parkinson’s disease (PD) and restless leg syndrome. Several studies have shown that pramipexole significantly increases the risk of pathological gambling and impulse-control disorders. While these iatrogenic complications can impose a severe social and financial burden, their treatment poses serious clinical challenges. Our group previously reported that the steroidogenic inhibitor finasteride reduced pathological gambling severity in PD patients who developed this complication following pramipexole treatment. To study the mechanisms underlying these effects, here we tested the impact of finasteride in a rat model of pramipexole-induced alterations of probability discounting. We previously showed that, in rats exposed to low doses of the monoamine-depleting agent reserpine (1 mg/kg/day, SC), pramipexole (0.3 mg/kg/day, SC) increased the propensity to engage in disadvantageous choices. This effect was paralleled by a marked D3 receptor upregulation in the nucleus accumbens. First, we tested how finasteride (25–50 mg/kg, IP) intrinsically affects probability discounting. While the highest dose of finasteride produced a marked lack of interest in lever pressing (manifested as a significant increase in omissions), the 25 mg/kg (IP) dose did not intrinsically modify probability discounting. However, this finasteride regimen significantly reduced the adverse effects of reserpine and pramipexole in probability discounting by diminishing rats’ propensity to engage in highly disadvantageous probabilistic choices. The same regimen also reversed the upregulation of D3 receptors in the nucleus accumbens induced by reserpine and pramipexole. These findings confirm that finasteride opposes the impulsivity caused by pramipexole and suggest that this effect may be underpinned by a normalizing effect on D3 receptor expression in the nucleus accumbens.
Keywords: Pramipexole, probability discounting, finasteride, D3 dopamine receptors, nucleus accumbens
1. INTRODUCTION
Pramipexole (pramipexole) is a potent non-ergot dopaminergic agonist with high selectivity for D3 and, to a lesser extent, D2 receptors (Mierau et al., 1995). This drug is currently approved to manage motor symptoms in Parkinson’s disease (PD) (Lieberman et al., 1997; Montplaisir et al., 1999). Over the past decade, cogent evidence has documented that a subset of patients treated with pramipexole develops pathological gambling (Driver-Dunckley et al., 2003; Dodd et al., 2005; Etminan et al., 2017) as well as impulse-control disorders, such as compulsive shopping and hypersexuality (Weintraub et al., 2006; Weintraub et al., 2010; Moore et al., 2014).
Impulse-control disorders often compound the severe social and financial burden experienced by PD patients (Weintraub et al., 2010). Making matters worse, the management of these conditions often poses serious clinical challenges. The best-validated therapeutic strategy for these problems is the dose reduction or discontinuation of pramipexole (Grall-Bronnec et al., 2018). However, the taper of these drugs often leads to a dopamine agonist withdrawal syndrome, characterized by anxiety, panic attacks, diaphoresis, fatigue, dysphoria, and depression, which cause significant distress and impair functioning and are often refractory to other dopamine replacement therapy agents (Nirenberg, 2013). Additionally, pramipexole discontinuation is not always successful in alleviating impulse-control disorders and can exacerbate PD motor symptoms (Nirenberg, 2013; Vilas et al., 2012). Unfortunately, no FDA-approved alternative treatments are currently available for these patients, underscoring the need for novel therapies to prevent iatrogenic impulse-control disorders or mitigate their severity. This background highlights the urgent need for novel, effective treatments for pramipexole-induced impulse-control disorders.
We previously developed a novel rat probability-discounting task that can specifically capture the reactivity to highly disadvantageous choices (Pes et al., 2017). Using this paradigm, we found that pramipexole caused a very mild increase in the discounting of probabilistic losses (Pes et al., 2017). These effects, however, were markedly magnified by concomitant treatment with the monoamine-depleting drug reserpine at low daily doses that did not intrinsically affect locomotor and operant behavior. In these conditions, the effects of pramipexole on presynaptic receptors were ablated by reserpine, suggesting that pramipexole alters probability discounting via activation of postsynaptic dopamine receptors (Pes et al., 2017). Notably, we found that the combination of reserpine and pramipexole elevated the expression of D3, but not D2, receptors in the nucleus accumbens (NAc) (Orrù et al., 2020); however, neither D2 nor D3 antagonists were able to reverse the increase in probability discounting in rats subjected to this treatment (Orrù et al., 2020).
We previously documented that the steroidogenesis inhibitor finasteride (N-(2-methyl-2-propyl)-3-oxo-4-aza-5α-androst-1-ene-17β carboxamide) markedly reduced the severity of gambling disorder in two PD patients (Bortolato et al., 2012). This drug is the prototypical inhibitor of the enzyme 5α-reductase (5αR), which catalyzes the saturation of the 4,5-double bond of the A ring of ketosteroids, such as testosterone, progesterone, and deoxycorticosterone. finasteride is approved to treat benign prostatic hyperplasia and male-pattern baldness (Paba et al., 2011). These therapeutic effects reflect the best-characterized mechanism of action of finasteride, namely the inhibition of the conversion of testosterone into dihydrotestosterone. We recently documented that finasteride reduces impulsivity (Godar et al., 2019a) and opioid-seeking behavior (Bossé et al., 2020). Furthermore, previous studies from our group documented that this drug elicits antidopaminergic effects, even though it does not bind to dopamine receptors (Bortolato et al., 2008; Devoto et al., 2012; Frau et al., 2016).
Building on these premises, the present study aimed to assess whether finasteride may oppose the enhancement in probability discounting and upregulation of accumbal D3 receptors caused by the combination of reserpine and pramipexole.
2. MATERIALS AND METHODS
2.1. Animals.
Three-month-old male Long-Evans rats (Charles River, Wilmington, MA, USA) were single-housed within rooms maintained at 22 ± 2 °C and 60% humidity, on an inverted 12/12 h light/dark cycle (lights on at 7:00 PM). Following acclimation to the housing facilities, animals were handled daily for 5 min, and underwent a food-restriction regimen, which kept them at 85–90% of their free-feeding weight throughout the study. Experimental procedures began on the eighth day of food restriction. Behavioral measurements were carried out and analyzed by trained experimenters in a blinded fashion. All experimental procedures were compliant with the NIH guidelines and approved by the IACUC of the University of Utah.
2.2. Drugs.
pramipexole (Accela Biochem, San Diego, CA, USA) was dissolved in saline (1 ml/kg) and administered 30 min prior to behavioral testing at the dose of 0.3 mg/kg/day (SC). reserpine (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in saline (1 ml/kg) and administered daily (1 mg/kg/day, SC) 22 h before behavioral testing. finasteride (Carbosynth Limited, Compton, UK) was suspended in a vehicle solution containing 5% DMSO, 5% Tween80, and 90% sterile saline (0.9% NaCl) and administered at 25–50 mg/kg/day, IP, one hour before testing. Doses were based on our previous studies (Pes et al., 2017; Bossé et al., 2020).
2.3. Probability discounting.
Studies were based on a modified version of the probability-discounting protocol described by Pes et al (2017), optimized to study reactivity to winning probabilities (WPs) ranging from advantageous to extremely disadvantageous. Animals (n=64) were tested daily between 10 AM and 4 PM, for 7 days/week, in operant chambers (31 x 21 x 24 cm; Med Associates, St. Albans, VT, USA), enclosed in sound-attenuating cabinets. Each chamber contained a central food receptacle from which food pellets were dispensed (45 mg; Bioserve, Frenchtown, NJ, USA), as well as one permanent lever (in the center) and two retractable levers (one on each side). Each chamber was also equipped with a fan, a house light and stimulus lights located above each lever. All behavioral data were recorded on a PC, using custom software (Med-PC IV, Med Associates). Training included five distinct phases:
Phase 1. Acclimation. Rats were first acclimated to the operant chambers and trained to retrieve food pellets from the dispenser in a single 30-min session, during which pellets were delivered at a variable rate averaging one/min.
Phase 2. Fixed-ratio (FR) reinforcement. Next, each animal was trained to press the center lever within a 30-s time allotment, using a FR1 schedule of reinforcement for 50 trials. During this training, the side levers remained retracted. The FR value was gradually increased to 5, while the time allotment for each lever press was reduced to 10 s. All animals completed center-lever training within 5 sessions.
Phase 3. Discrete trials. In this stage, rats were trained to perform a single side lever press following 5 center lever presses. A single press on a side lever within the allotted time (30 s to start and gradually reduced to 10 s) resulted in the delivery of one food pellet. Whenever the animal failed to press a lever within the allotted time, the lever retracted, the house light turned off without food delivery, and the trial was scored as an omission. Each side lever was presented 25 times per session, and the order of side lever presentations was randomized across the session. Trials were separated by a 5-s intertrial interval. Rats that completed 50 successful trials/session over two consecutive sessions proceeded to the next phase.
Phase 4. Lever discrimination training. Throughout this phase, animals were trained to associate each of the two side levers with the assignment of either 1 or 2 pellets. Sessions consisted of 4 blocks of 20 trials (4 forced-choice trials followed by 16 free-choice trials). The algorithm of each training session is shown in Fig. 1. Each trial began with the activation of the house light, and rats were required to engage in five center lever presses in order to proceed to the forced-choice or free-choice (as appropriate) portion of the trial. During the first 4 trials of each block (forced-choice), animals were presented with only one of the two levers, associated with either 1 or 2 food pellets (dispensed upon every lever press, to consolidate the association of each lever with its respective reward value). During the remaining 16 trials (free-choice), both side levers were presented to the rat (Fig. 2A). Once a side lever was pressed, both side levers retracted, the appropriate reward (or lack thereof) was dispensed, and lights were turned off to signal the end of the trial. After each trial, the house light was extinguished for 15 seconds before the beginning of a new trial. The two different reward sizes were associated with each lever in a counterbalanced order, which remained consistent throughout the whole study. Rats proceeded to the next phase after selecting the two-pellet lever on >85% of the trials for each block over two consecutive sessions. Animals that did not reach this criterion were omitted from the study. On average, the preference for the two-pellet lever reached full stability on day 15 of this phase and ranged between 83.7% and 91.2%.
Phase 5. Probability discounting task. In this phase, rats performed the same task as in Phase 4, but the two-pellet lever delivered its associated reward in a ‘probabilistic’ fashion. Probabilities were associated with WPs at 50%, 25%, 12.5% and 6.25% for each block and presented in a descending fashion (Fig. 2B). Conversely, the selection of the one-pellet lever (termed ‘certain’ from now on, to be distinguished from its ‘probabilistic’ alternative) always resulted in a single pellet reward after every press. Risk propensity was measured by a probabilistic choice index, defined as the ratio of free-choice probabilistic lever selections over the total number of free-choice trials for each WP block. Lever-press latency and number of trial omissions were also monitored; the latter parameter, however, was consistently <1/trial throughout the whole study, irrespective of treatments.
Figure 1.
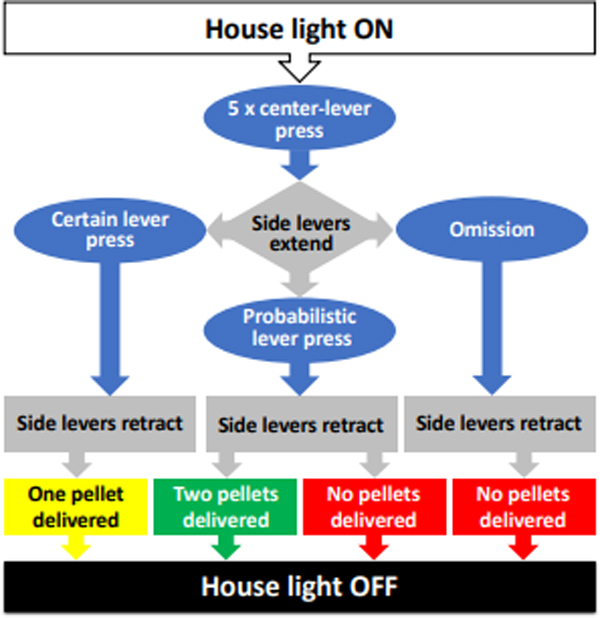
Schematic algorithm of a free-choice trial in the probability discounting task. Rats were presented with two alternative options, each associated with one lever: 1) a ‘certain’ option, consisting of a single pellet of food delivered after each lever press; and 2) a ‘probabilistic’ option, consisting of either no reward (a “loss”) or a two-pellet reward (a “win”), dispensed at variable degrees of winning probability (WP, defined as the likelihood that a lever press will dispense a two-pellet food reward).
Figure 2.
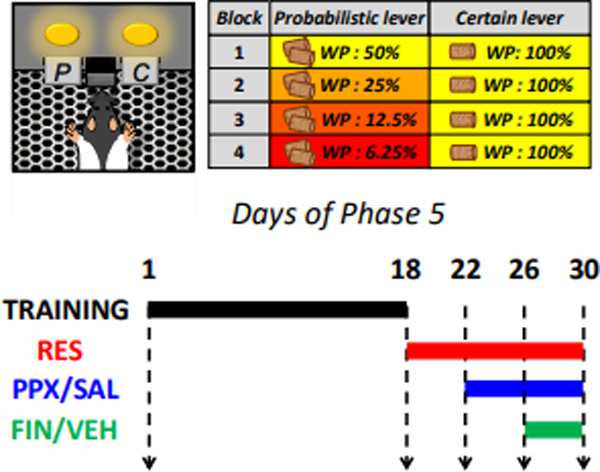
Experimental procedures related to the Phase 5 of operant testing (probability discounting task). A) Schematic representation of a free-choice trial in the probability discounting task. Rats were presented with a ‘certain’ lever (C, consisting of a single pellet of food delivered after each lever press; and a ‘probabilistic’ (P) lever, associated with the delivery of either no reward or a two-pellet reward (a “win”), dispensed at variable degrees of winning probability (WP, defined as the likelihood that a lever press will dispense a two-pellet food reward). B) Synoptic table of WPs associated with the probabilistic and certain levers throughout the four blocks of the probability-discounting session. The alternatives ranged from even (50% WP for two pellets vs 100% WP for one pellet) to extremely disadvantageous (6.25% for two pellets vs 100% for one pellet) conditions. The block sequence was presented in a descending fashion and counterbalanced across treatment groups. C) Timeline of the second experiment. Following 18 days of training, all rats were subjected to daily injections of reserpine (1 mg/kg/day, SC). On day 23, half of the rats were treated with pramipexole (pramipexole, 0.3 mg/kg/day, SC) or saline injection (SAL). Upon verification of stability of these effects, each group was further divided in two sub-groups with equivalent performances, which were assigned to rats were treated with finasteride (finasteride, 25/kg/day, IP) or vehicle (VEH) (from day 27 through day 30).
The analysis of rat behavior was complemented by analyses of the following parameters:
probability discrimination index, calculated as the within-session differences between the probabilistic choice at 50% and 6.25% WP;
win-stay ratio, calculated as the number of ‘wins’ in each block (excluding the last two blocks, in which rats experienced primarily losses) followed by the selection of the probabilistic lever / Total number of ‘wins’;
lose-switch ratio, calculated as the number of ‘losses’ in each block (excluding the first, in which no losses could occur) followed by the selection of the ‘certain’ lever / Total number of ‘losses’;
Throughout the probability discounting task, different drug treatments were initiated when rats reached a stable baseline of behavior. Stability was analyzed using a one-way ANOVA for repeated measures using WPs and testing days as within-subject factors. Stability was achieved when the analysis of the probabilistic choice index revealed that a main effect (P<0.05) for winning probabilities was not accompanied by significant effects for testing days.
In the first experiment (n=48), Phase 5 lasted 20 days. In the first 16 days, rats were trained on the probability discounting task. In the following 4 days, rats were subjected to finasteride treatment (25–50 mg/kg/day, IP). Rats were divided into three groups (n=16/group) to test the effects of different finasteride doses on probability discounting in comparison with its vehicle (n=12/group) for 4 days.
The second experiment (n=64) was aimed at studying the effects of the highest dose of finasteride that did not intrinsically impair probability discounting (25 mg/kg, IP) on the alterations of probability-discounting induced by the combination of reserpine and pramipexole. Our analysis was limited to this co-treatment, since our previous results (Pes et al., 2017) showed that the effects of pramipexole alone produced had very limited size (η2 <0.20); preliminary power analyses estimated that, under similar circumstances, any significant reduction of pramipexole-mediated effect could only detectable with extremely large groups (n>45 rats/group). Phase 5 lasted 30 days (Fig. 2C). The first 18 days were used for probability-discounting. In the following 12 days, rats were subjected to several pharmacological treatments. All animals were treated with reserpine (from day 19 onwards); on day 23, rats were subjected to into two groups (n=32) by simple randomization. Average probabilistic choices for each group (calculated using the mean values of all 5 WP blocks for each rat) were found to be equivalent by two one-sided tests (TOST; Schuirmann, 1987), with a lower and upper bound of 5% change considered to reflect significant differences (Ps< 0.001). Each group was randomly assigned to daily injections of either pramipexole or its vehicle. In conformity with our previous results (Pes et al., 2017), rats reached stability on day 26 (see below). From day 27 onward, we treated two groups with either finasteride (25 mg/kg, IP) or its vehicle. Treatment was continued for 4 days, when behavioral performance reached stability.
2.4. Western blot.
On the last day of the second experiment, 60 min after pramipexole and finasteride injection, rats were sacrificed, and their NAc and caudate-putamen (CPu) were harvested. For crude synaptosomal fractions, samples were weighed and homogenized on ice by using a glass-Teflon tissue grinder in homogenization buffer containing 10 mM Tris-HCl pH 7.4, 5 mM EDTA, 320 mM sucrose, protease and phosphatase inhibitor cocktail. Homogenates were centrifuged at 500 × g for 10 min at 4°C to precipitate nuclei; supernatant fraction was collected and centrifuged at 20,000 x g for 30 min at 4°C. The resulting pellet (P2) was solubilized in Cell Lysis Buffer (Cell Signaling, Frankfurt, Germany) supplemented with protease and phosphatase inhibitor cocktail. Small aliquots of the homogenate were used for protein determination by a modified Lowry protein assay method (DC protein assay, Bio-Rad Laboratories, Hercules, CA, USA).
Western blotting was performed as previously described (Godar et al., 2019b), with slight modifications. Equal amounts of proteins (30 μg) were separated on a 4–15% Criterion TGX Stain free Precast Gel (Bio-Rad Laboratories) by electrophoresis and transferred to a polyvinylidene difluoride membrane using the Trans-Blot Turbo Transfer system (Bio-Rad Laboratories). Membranes were probed for total protein content using the stain free capabilities of the Criterion Stain Free gels and the ChemiDoc Touch system (Bio-Rad Laboratories). Membranes were then blocked with 3% BSA (Sigma-Aldrich) in TRIS-buffered saline supplemented with 0.1% Tween 20 for 2 h at room temperature and then incubated overnight with primary antibodies at 4 °C. Primary antibodies used in this study include the following: anti-D2 dopamine receptor (ab130295, Abcam, Cambridge, UK); anti-D3 dopamine receptor (Abcam, ab142114); anti-β actin (Sigma-Aldrich). After washing, membranes were incubated with HRP-conjugated secondary antibodies. Antibody binding was detected using Clarity ECL substrate (Bio-Rad Laboratories) and proteins were analyzed by the ChemiDoc Touch system and the Image Lab software (Bio-Rad Laboratories). Samples from each treatment group were immunoblotted and analyzed together. To control for equal loading, blots incubated with antibodies against dopamine D3 and D2 receptors were stripped and re-probed using anti β-actin. Bands were quantified in arbitrary units and normalized using the software Image Lab (Bio-Rad Laboratories) using β-actin as loading controls.
2.5. Data analyses.
Normality and homoscedasticity were preliminarily verified using Kolmogorov-Smirnov and Bartlett’s tests. Data were analyzed with one, two- or three-way ANOVAs, followed by Tukey’s test for post-hoc comparisons. Significance threshold was set at 0.05.
3. RESULTS
3.1. Probability discounting.
In the first experiment, rats displayed significant differences across various WP blocks on day 4 of Phase 5 (P<0.01). Post-hoc analyses indicated significant reductions in probabilistic choice between all blocks (Ps<0.05) compared to the 50% WP block. Throughout Phase 5, the probability curve became progressively steeper and reached stability on day 16. Post-hoc analyses indicated significant differences in probabilistic choice between all blocks (Ps<0.001). The behavioral effects of the 50 mg/kg dose of finasteride could not be evaluated since this dose caused a significant increase in omissions (with most animals omitting > 50% of the choices; data not shown). In contrast, the analysis of the effects of finasteride (Fig. 3A) at the dose of 25 mg/kg showed that this regimen did not cause any significant increase in lever-press omissions. While a significant effect for the interaction of treatment and WP block [F(3,90)=3.93, P=0.01, η2: 0.12] was found, post-hoc comparisons revealed that finasteride did not alter the probabilistic choice at any WP block. The analysis of the probability differential index revealed that finasteride significantly reduced this parameter [F(1,30)=12.95, P=0.001] (Fig. 3B), pointing to a possible effect of finasteride on choice discrimination. Even so, finasteride failed to modify either the loss-switch [Fig. 3C; treatment x WP block interaction: F(3,90)=2.00, NS] or win-stay ratios [Fig. 3D; treatment x WP block interaction: F(1,30)=2.21, NS].
Figure 3.
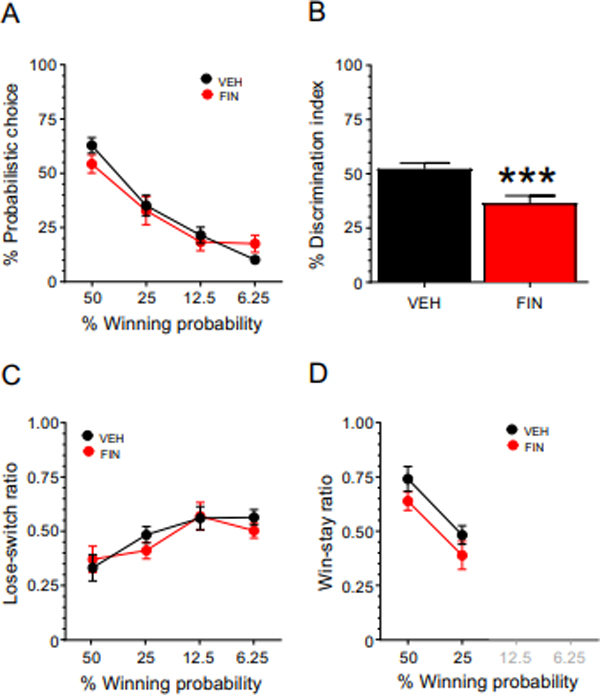
Effects of finasteride (finasteride, 25 mg/kg, IP) treatment on A) probabilistic decision making; B) differential index; C) lose-switch ratio; and D) win-stay ratio. Data are shown as mean ± SEM. Two-way repeated-measure ANOVA followed by Tukey’s test, VEH n = 12; FIN n = 16. ***, P = 0.001 for comparisons between finasteride- and vehicle (VEH)-treated animals. For further details, see text.
Building on these results, we next tested the effects of finasteride (25 mg/kg, IP) on the effects of pramipexole and reserpine (Fig. 4) using a different set of rats. The effects of finasteride were analyzed by a 3-way, repeated measure ANOVA design, and revealed that pramipexole significantly altered probability discounting [Fig. 4A: pramipexole x block interaction: F(3,180)=23.22, P<0.0000001; η2: 0.28] at the 25% (P<0.001), 12.5% (P<0.001), and 6.25% (P<0.0001) WP blocks. Conversely, while finasteride did not intrinsically affect probability discounting [finasteride x block interaction: F(3,180)=0.47, NS; η2: 0.01], it significantly opposed the effects of pramipexole in the 6.25% WP block [pramipexole x finasteride x block interaction: F(3,180)=8.63, P<0.0001; η2: 0.13; post-hoc for comparisons between pramipexole-vehicle and pramipexole-finasteride at 6.25% WP: P=0.02]. The analysis of the differential index confirmed that pramipexole markedly reduced this index [Fig. 4B; F(1,60)=34.63, η2: 0.37; P<0.00001]. However, while a significant interaction between finasteride and pramipexole was found [F(1,60)=12.85, η2: 0.18; P<0.001], post-hoc comparisons revealed only a marginal statistical trend for the comparison between vehicle-pramipexole and finasteride-pramipexole (P=0.10). The analysis of the lose-switch ratio showed that pramipexole significantly altered this index [Fig. 4C: pramipexole x block interaction: F(3,180)=6.54, P<0.001; η2: 0.10] at the 6.25% (P<0.01) WP block. finasteride did not intrinsically affect this index [finasteride x block interaction: F(3,180)=1.32, NS; η2: 0.02], but marginally opposed the effects of pramipexole in the 6.25% WP block [pramipexole x finasteride x block interaction: F(3,180)=3.64, P=0.01; η2: 0.06; post-hoc for comparisons between pramipexole-vehicle and pramipexole-finasteride at 6.25% WP: P=0.07]. Finally, finasteride, pramipexole, and their interaction failed to modify the win-stay ratio (Fig. 4D) [pramipexole x finasteride x block interaction: F(1,60)=2.30, NS].
Figure 4.
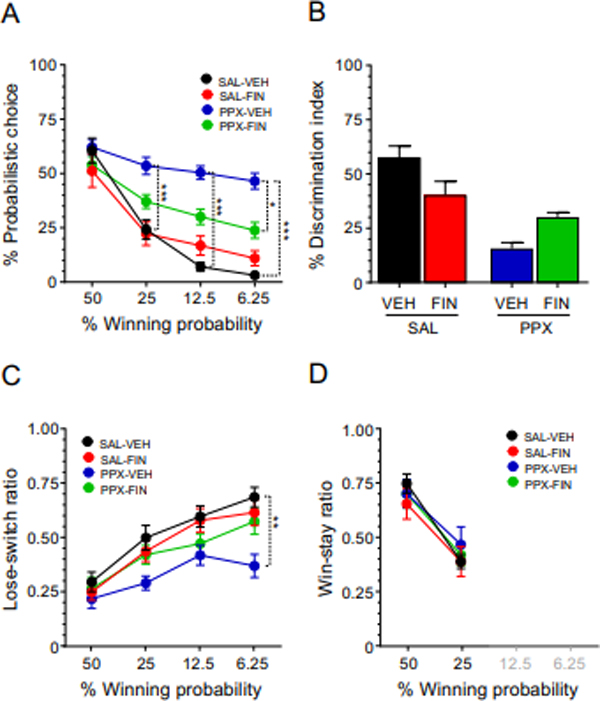
Effects of the combination of finasteride (finasteride, 25 mg/kg, SC) and pramipexole (pramipexole, 0.3 mg/kg/day, SC) on A) probabilistic decision making; B) differential index; C) lose-switch ratio; and D) win-stay ratio. Data are shown as mean ± SEM. All animals were also subjected to reserpine (RES, 1 mg/kg/day, SC) treatment. Three-way repeated measure ANOVA followed by Tukey’s test, n = 16/group. ***, P < 0.001. VEH, vehicle; SAL, saline. For further details, see text.
3.2. Western blotting.
Our previous results showed that animals treated with the combination of reserpine and pramipexole altered probability discounting in relation to disadvantageous options and a significant increase in D3 receptors selectively in the NAc (Orrù et al., 2020). Thus, we investigated whether the effect of finasteride on probability discounting was associated with modifications in D3 receptor expression in striatal areas. Western blot analysis of dopamine D3 receptors in synaptosomal fractions of NAc revealed that the combination of reserpine and pramipexole led to a selective upregulation of D3 receptor membrane expression in the NAc, as previously demonstrated (Fig.5A). Strikingly, finasteride countered this D3 receptor upregulation, reducing the membrane expression of these proteins in the NAc [F(2,19)= 4.48, P = 0.02] (Fig. 5A). Conversely, no effect of finasteride was observed on D2 receptor [F(2,19)= 0.11, NS] (Fig. 5B). In the CPu, no effects of finasteride were observed on the expression of either D3 [F(2,19)= 0.02, NS] (Fig. 5C) or D2 receptors [F(2,19)= 0.70, NS] (Fig. 5D).
Figure 5.
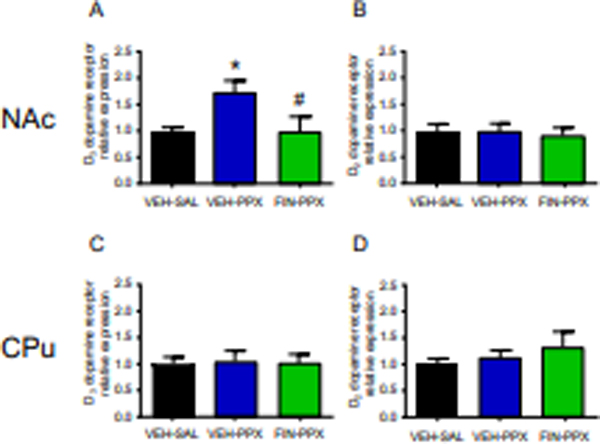
Effects of finasteride (finasteride, 25 mg/kg, SC) and pramipexole (pramipexole, 0.3 mg/kg/day, SC) on the levels of D3 and D2 dopamine receptor proteins in the Nucleus Accumbens (NAc; A, B) and caudate-putamen (CPu; C, D) of reserpine (RES)-treated rats. On the last day of the probability-discounting paradigm, immediately after completion of the behavioral task, animals were sacrificed, and their brain tissues (NAc and CPu) were harvested. Levels of dopamine D3 and D2 receptors in the NAc (A, B) and in the CPu (C, D) were analyzed by western blot. Data are shown as mean ± SEM and expressed as ratio relative to reserpinized rats treated with saline and vehicle. One-way ANOVA followed by Tukey’s test, n = 7–8/group. * P < 0.05 compared with reserpinized animals treated with saline and vehicle. #, P < 0.05 compared with reserpinized animals treated with pramipexole and vehicle.
4. DISCUSSION
The main result of this study is that the steroidogenic inhibitor finasteride effectively counters the probability-discounting alterations produced by pramipexole in rats pre-exposed to a mild reserpine treatment. This finding extends previous evidence showing that finasteride exerts anti-impulsive properties across a wide range of several behavioral paradigms, ranging from delay discounting (Godar et al., 2019a) to the wire-beam bridge (Godar et al., 2019a), a paradigm specifically designed by our group to measure risk-taking and venturesomeness (Bortolato et al., 2009; Festucci et al., 2021). Given that finasteride is approved for clinical use, these results collectively suggest that this drug may elicit therapeutic effects across various constructs of impulsivity and be a valuable treatment for multiple impulse-control disorders (both iatrogenic and idiopathic) or other disorders characterized by poor impulse control. From this perspective, it is worth mentioning that the results of this study resonate with our previous clinical observations on finasteride’s efficacy in reducing the severity of pathological gambling in PD patients treated with pramipexole and other dopamine-replacement drugs (Bortolato et al., 2012). Indeed, shallow probability discounting is a distinctive psychological feature of pathological gamblers (Madden et al., 2009; Miedl et al., 2012; Kyonka and Schutte, 2018). Notably, finasteride reduced the pathological gambling elicited by relatively low doses of pramipexole (1.4–2.1 mg/d); in line with these results, our protocol showed that this steroidogenic inhibitor worked on relatively low doses of pramipexole, which were sufficient to alter probability discounting without compromising the overall behavioral performance of the animals; indeed, in preliminary studies, we found that higher doses of pramipexole (≥ 2 mg/kg/day) dramatically reduced the engagement of rats in the task itself, irrespective of reserpine co-treatment.
As previously reviewed (Frau and Bortolato, 2019), the opportunity of repurposing finasteride as a potential therapy for iatrogenic complications caused by dopamine-replacement treatments in PD is particularly intriguing, also given our recent discovery that this drug and other 5αR inhibitors reduce the severity of levodopa-induced dyskinesias in rodent models (Frau et al., 2017a; Fanni et al., 2019) and counter the behavioral effects of dopaminergic agonists without causing extrapyramidal motor effects (Bortolato et al., 2008; Devoto et al., 2012; Frau et al., 2013; Frau et al., 2016). Given the high incidence of impulse-control disorders in PD patients with levodopa-induced dyskinesias (Biundo et al., 2017), these data point to the possibility that finasteride and similar drugs may reduce the severity of some adverse motor and behavioral outcomes caused by dopaminergic medications in PD. This avenue is quite appealing, given that available strategies for these iatrogenic problems are minimal. For example, preliminary pharmacoepidemiologic analyses suggest that pramipexole-associated gambling and impulse-control disorders are not susceptible to the therapeutic effects of the primary drugs used for idiopathic gambling (such as glutamatergic modulators and selective serotonin reuptake inhibitors) (Jeon and Bortolato, 2020). Furthermore, the paucity of available treatments for pramipexole-associated impulse-control disorders is further underscored by our recent finding that neither D2 nor D3 receptor antagonists had significantly ameliorative effects on the adverse effects of pramipexole in the same rat model used in this study (Orrù et al., 2020). Future studies are warranted to verify whether finasteride or other 5αR inhibitors may be viable strategies to reduce the adverse events of dopaminergic therapies in PD. Although the safety profile of finasteride has generally been regarded as satisfactory for many patients, any potential therapeutic development of this drug as a treatment for iatrogenic problems in PD should be pursued with extreme caution, given that recent evidence has shown that this drug increases the risk of depression and suicidality (Rahimi-Ardabili et al., 2006; Irwig, 2012; Traish et al., 2015; Nguyen et al., 2021, Ali et al., 2015). In some patients, these symptoms persist even after the discontinuation of finasteride therapy (Ganzer et al., 2015; Gray and Semla, 2019; Diviccaro et al., 2019). Related to this issue, we should note that the 50 mg/kg dose of finasteride led to a dramatic reduction in the rats’ engagement with lever pressing - an “amotivational response” that has been documented in response to depressogenic manipulations in rodents (Dieterich et al., 2019; 2021). This finding is in keeping with our previous observation of a generalized reduction of locomotor and exploratory activity, as well as depression-like reactions in finasteride-treated rats (Bortolato et al., 2008; Godar et al., 2019a). Given that PD patients have a relatively high incidence of depression (Cummings, 1992) and that mood disorders are highly associated with gambling and impulsive-compulsive behaviors in this clinical population (Santos-Garcia et al., 2021), these results advocate for extreme caution in the therapeutic use of finasteride in these patients. That said, in our own clinical experience, several male patients with PD are typically treated with finasteride other 5αR inhibitors for concomitant prostate problems; thus, the adverse outcome of these drugs may only refer to a subset of vulnerable subjects.
As previously shown (Pes et al., 2017; Orrù et al., 2020), the association of reserpine and pramipexole increased the tendency of rats to overvalue reinforcement with lower probabilistic odds (25%, 12.5%, and 6.25%). These data align with the indication that pramipexole exaggerates the affective response to rewards in humans (Ye et al., 2011). Like in our previous studies (Orrù et al., 2020), pramipexole decreased the discrimination index, suggesting that this drug reduces the ability to discriminate between different options associated with varying levels of advantage. In line with these findings, pramipexole has been shown to make rats more indifferent to alternative options, irrespective of temporal delays (Madden et al., 2010). Interestingly, while the 25 mg/kg dose of finasteride significantly opposed the effects of pramipexole on the 6.25% choice, this reversal was only partial, potentially reflecting the adverse impact of finasteride on the discrimination index.
In keeping with our previous findings (Orrù et al., 2020), the combination of reserpine and pramipexole led to a selective up-regulation of D3 receptor membrane expression in the NAc. Strikingly, finasteride countered this upregulation; given pramipexole is a potent D3 receptor agonist, the mechanism of finasteride is likely to be based on the downregulation of the main target of this drug. These findings are in accord with previous data from our group, showing that this steroidogenic inhibitor blocks some of the phenotypic effects of selective D3 receptor agonists (Frau et al., 2016). The idea that finasteride reversed the changes in probability discounting caused by pramipexole in RES-treated rats appears to suggest that D3 receptor activation may be the principal mechanism accounting for the adverse effects of this drug on impulsivity (Seeman, 2015). In support of this notion, the nucleus accumbens has one of the highest densities of D3 receptors in the brain (Murray et al., 1994). That said, our previous data do not support the direct involvement of D3 receptors in the effects of pramipexole, given that their antagonism did not reverse the effects of pramipexole on probability discounting (Orrù et al., 2020). Furthermore, our previous data (Pes et al., 2017) showed that presynaptic mechanisms are not likely implicated in the molecular processes whereby pramipexole affects probabilistic choice, likely suggesting that this dopaminergic agonist enhances impulsivity via activation of either post- or extrasynaptic D3 receptors.
Several limitations of the present study should be acknowledged. First, our results cannot explain the mechanisms whereby finasteride opposes the effects of pramipexole in probability discounting and reduces the membrane expression of D3 receptors. As mentioned above, finasteride inhibits 5αR, the enzyme catalyzing the rate-limiting step of the biosynthesis of many neurosteroids, including allopregnanolone (AP), a critical regulator of the stress response. Our previous work has shown that finasteride treatment leads to the accumulation of steroid precursors, such as pregnenolone and progesterone, and a reduction of the synthesis of AP (Frau et al., 2015; Frau et al., 2017b). Our behavioral studies suggest that both these mechanisms may contribute to the effects of finasteride. For example, previous work in our lab has shown that AP can partially counter some of the behavioral effects induced by sleep deprivation (Frau et al., 2017b). In line with this idea, we have shown that AP and finasteride have opposite effects in animal models of neuropsychiatric conditions, such as Tourette syndrome (Mosher et al., 2017). At the same time, our recent results on the effects of finasteride in opioid self-administration suggest that these therapeutic outcomes may reflect the elevation of steroid precursors, such as pregnenolone and dehydroepiandrosterone (DHEA) (Bossé et al., 2020). It is likely that some of the modifications of the steroid profile induced by finasteride may affect the expression of D3 receptors. For example, testosterone (whose metabolism is decreased by finasteride) has been shown to reduce D3 receptor transcript levels in the striatum of male rats (Purves-Tyson et al., 2014). Alternatively, DHEA and pregnanolone may reduce D3 receptor expression by binding to microtubule-associated proteins (Murakami et al., 2000; Laurine et al., 2003) and dysregulate cytoskeletal functions and intracellular trafficking of D3 receptors.
Another critical limitation of the present study is that all experiments were exclusively run using male rats. While this design was informed by the male preponderance of PD-associated gambling (Weintraub et al., 2006) and the exclusive clinical approval of finasteride for male patients, it should be noted that off-label use of this drug has been studied for several conditions, including hirsutism and female-pattern hair loss (Venturoli et al.,1999; Bayram et al., 2003; Won et al., 2018; Iamsumang et al., 2020; Hu et al., 2019). Thus, future studies will be needed to verify whether the same behavioral effects may be present in female rats. By the same token, while reserpine has been used as a pharmacological model of PD (Leão et al., 2015), the effects of finasteride should be verified in complementary models of gambling-like activity associated with lesional models of PD. For example, Rokosik and Napier (2012) studied the effects of pramipexole on probability discounting in a model of nigrostriatal lesions induced by bilateral injections of 6-hydroxydopamine in the dorsal striatum. Given the motoric impairments associated with this model, the authors had to use intracranial self-stimulation as the positive reinforcer. A possible limitation of this model is that the dose regimen of pramipexole needed to rescue motor functions (2 mg/kg) is significantly higher than those used in our study and thus non-specifically activate not just D3, but also D2 receptors. The other key problem with the use of lesional models of PD in operant protocols is the lack of available controls treated with vehicle, given the reliance of these paradigms on motor skills (for lever pressing etc.). That said, rat models of 6-hydroxydopamine bilateral lesion of the posterior ventral temporal area have been shown to lead to increased preference for pramipexole and the D3 receptor selective agonist PD128907 (Ouachikh et al., 2013). Testing our model in these rats (which do not exhibit major locomotor problems) would also help clarify whether the observed increase in incidence of impulsive compulsive disorders in PD may reflect the concomitant degenerative alteration of the mesolimbic system observed in some PD patients (Alberico et al., 2015). This idea would also be in line with research showing that impulsive-compulsive problems induced by dopaminergic agonists in PD display a decrease in baseline activity and reduced dopamine transporter binding - a well-established marker of dopaminergic fiber integrity - of the mesolimbic system (Cilia et al., 2010: Rao et al., 2010).
These limitations notwithstanding, the results of this study confirm that finasteride may serve as a novel strategy for impulse-control disorders and pathological gambling caused by dopaminergic agonists, such as pramipexole and ropinirole. It is worth noting that pathological gambling has recently been framed as a behavioral addiction (Mann et al., 2016); in line with this perspective, we and others showed that, in animal models, finasteride reduces the self-administration of several substances of abuse, including opioids (Bossé et al., 2020) and alcohol (Ford et al., 2008). These data raise the possibility that the antidopaminergic properties of finasteride may have universal effects across addictive disorders and may therefore be a valuable therapeutic strategy for the comorbidity of drug- and alcohol-dependent patients who have an increased risk of engaging in pathological gambling (Castellani and Rugle, 1995; Daghestani et al., 1996; Spunt et al., 1998; Petry, 2001). Thus, understanding the molecular underpinnings of the effects of finasteride in this and other behavioral models of impulsivity may pave the avenues for the development of novel treatments for both behavioral and substance addictions.
Acknowledgments:
We would like to thank Hunter Strathman, Eva Vigato, Jaipal Singh, and Gagandeep Singh for their technical assistance. Finally, we would like to remember and thank the late Stephen Fowler for his invaluable mentorship and precious intellectual contributions, which were critical to designing the rat model used in this study. On a personal level, he was a dear friend and an irreplaceable inspiration, and we deeply mourn his loss. This article is dedicated to his memory.
Support:
The present work was partially supported by the NIH grant R21DA049530 (to MB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures. The authors of the present manuscript do not declare any conflicts of interest.
REFERENCES
- Alberico SL, Cassell MD, & Narayanan NS (2015). The Vulnerable Ventral Tegmental Area in Parkinson’s Disease. Basal ganglia, 5(2–3), 51–55. 10.1016/j.baga.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AK, Heran BS, & Etminan M (2015). Persistent Sexual Dysfunction and Suicidal Ideation in Young Men Treated with Low-Dose finasteride: A Pharmacovigilance Study. Pharmacotherapy, 35(7), 687–695. 10.1002/phar.1612 [DOI] [PubMed] [Google Scholar]
- Bayram F, Müderris I, Güven M, Ozçelik B, & Keleştimur F (2003). Low-dose (2.5 mg/day) finasteride treatment in hirsutism. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology, 17(5), 419–422. 10.1080/09513590312331290328 [DOI] [PubMed] [Google Scholar]
- Biundo R, Weis L, Abbruzzese G, Calandra-Buonaura G, Cortelli P, Jori MC, Lopiano L, Marconi R, Matinella A, Morgante F, Nicoletti A, Tamburini T, Tinazzi M, Zappia M, Vorovenci RJ, & Antonini A (2017). Impulse control disorders in advanced Parkinson’s disease with dyskinesia: The ALTHEA study. Movement disorders : official journal of the Movement Disorder Society, 32(11), 1557–1565. 10.1002/mds.27181 [DOI] [PubMed] [Google Scholar]
- Bortolato M, Cannas A, Solla P, Bini V, Puligheddu M, & Marrosu F (2012). finasteride attenuates pathological gambling in patients with Parkinson disease. Journal of clinical psychopharmacology, 32(3), 424–425. 10.1097/JCP.0b013e3182549c2a [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orrù M, Bourov Y, Marrosu F, Mereu G, Devoto P, & Gessa GL (2008). Antipsychotic-like properties of 5-alpha-reductase inhibitors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 33(13), 3146–3156. 10.1038/npp.2008.39 [DOI] [PubMed] [Google Scholar]
- Bortolato M, Godar SC, Davarian S, Chen K, Shih JC (2009) Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase B-deficient mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 34(13), 2746–2757. 10.1038/npp.2009.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossé GD, Cadeddu R, Floris G, Farero RD, Vigato E, Lee SJ, Zhang T, Gaikwad NW, Keefe KA, Phillips PE, Bortolato M, & Peterson RT (2021). The 5α-reductase inhibitor finasteride reduces opioid self-administration in animal models of opioid use disorder. The Journal of clinical investigation, 131(10), e143990. 10.1172/JCI143990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani B, & Rugle L (1995). A comparison of pathological gamblers to alcoholics and cocaine misusers on impulsivity, sensation seeking, and craving. The International journal of the addictions, 30(3), 275–289. 10.3109/10826089509048726 [DOI] [PubMed] [Google Scholar]
- Cilia R, Ko JH, Cho SS, van Eimeren T, Marotta G, Pellecchia G, Pezzoli G, Antonini A, & Strafella AP (2010). Reduced dopamine transporter density in the ventral striatum of patients with Parkinson’s disease and pathological gambling. Neurobiology of disease, 39(1), 98–104. 10.1016/j.nbd.2010.03.013 [DOI] [PubMed] [Google Scholar]
- Cummings JL. Depression and Parkinson’s disease: a review. Am J Psychiatry 1992;149(4):443–454. doi: 10.1176/ajp.149.4.443 [DOI] [PubMed] [Google Scholar]
- Daghestani AN, Elenz E, & Crayton JW (1996). Pathological gambling in hospitalized substance abusing veterans. The Journal of clinical psychiatry, 57(8), 360–363. [PubMed] [Google Scholar]
- Devoto P, Frau R, Bini V, Pillolla G, Saba P, Flore G, Corona M, Marrosu F, & Bortolato M (2012). Inhibition of 5α-reductase in the nucleus accumbens counters sensorimotor gating deficits induced by dopaminergic activation. Psychoneuroendocrinology, 37(10), 1630–1645. 10.1016/j.psyneuen.2011.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich A, Liu T, & Samuels BA (2021). Chronic non-discriminatory social defeat stress reduces effort-related motivated behaviors in male and female mice. Translational psychiatry, 11(1), 125. 10.1038/s41398-021-01250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich A, Srivastava P, Sharif A, Stech K, Floeder J, Yohn SE, & Samuels BA (2019). Chronic corticosterone administration induces negative valence and impairs positive valence behaviors in mice. Translational psychiatry, 9(1), 337. 10.1038/s41398-019-0674-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diviccaro S, Melcangi RC, & Giatti S (2019). Post-finasteride syndrome: An emerging clinical problem. Neurobiology of stress, 12, 100209. 10.1016/j.ynstr.2019.100209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, & Ahlskog JE (2005). Pathological gambling caused by drugs used to treat Parkinson disease. Archives of neurology, 62(9), 1377–1381. 10.1001/archneur.62.9.noc50009 [DOI] [PubMed] [Google Scholar]
- Driver-Dunckley E, Samanta J, & Stacy M (2003). Pathological gambling associated with dopamine agonist therapy in Parkinson’s disease. Neurology, 61(3), 422–423. 10.1212/01.wnl.0000076478.45005.ec [DOI] [PubMed] [Google Scholar]
- Etminan M, Sodhi M, Samii A, Procyshyn RM, Guo M, & Carleton BC (2017). Risk of Gambling Disorder and Impulse Control Disorder With Aripiprazole, Pramipexole, and Ropinirole: A Pharmacoepidemiologic Study. Journal of clinical psychopharmacology, 37(1), 102–104. 10.1097/JCP.0000000000000634 [DOI] [PubMed] [Google Scholar]
- Fanni S, Scheggi S, Rossi F, Tronci E, Traccis F, Stancampiano R, De Montis MG, Devoto P, Gambarana C, Bortolato M, Frau R, & Carta M (2019). 5alpha-reductase inhibitors dampen L-DOPA-induced dyskinesia via normalization of dopamine D1-receptor signaling pathway and D1-D3 receptor interaction. Neurobiology of disease, 121, 120–130. 10.1016/j.nbd.2018.09.018 [DOI] [PubMed] [Google Scholar]
- Festucci F, Buccheri C, Parvopassu A, Oggiano M, Bortolato M, Laviola G, Curcio G, & Adriani W (2021). “Himalayan Bridge”: A New Unstable Suspended Bridge to Investigate Rodents’ Venturesome Behavior. Frontiers in behavioral neuroscience, 15, 637074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Yoneyama N, Strong MN, Fretwell A, Tanchuck M, & finasteriden DA (2008). Inhibition of 5alpha-reduced steroid biosynthesis impedes acquisition of ethanol drinking in male C57BL/6J mice. Alcoholism, clinical and experimental research, 32(8), 1408–1416. 10.1111/j.1530-0277.2008.00718.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau R, & Bortolato M (2019). Repurposing steroidogenesis inhibitors for the therapy of neuropsychiatric disorders: Promises and caveats. Neuropharmacology, 147, 55–65. 10.1016/j.neuropharm.2018.05.013 [DOI] [PubMed] [Google Scholar]
- Frau R, Abbiati F, Bini V, Casti A, Caruso D, Devoto P, & Bortolato M (2015). Targeting neurosteroid synthesis as a therapy for schizophrenia-related alterations induced by early psychosocial stress. Schizophrenia research, 168(3), 640–648. 10.1016/j.schres.2015.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau R, Bini V, Soggiu A, Scheggi S, Pardu A, Fanni S, Roncada P, Puligheddu M, Marrosu F, Caruso D, Devoto P, & Bortolato M (2017b). The Neurosteroidogenic Enzyme 5α-Reductase Mediates Psychotic-Like Complications of Sleep Deprivation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 42(11), 2196–2205. 10.1038/npp.2017.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau R, Mosher LJ, Bini V, Pillolla G, Pes R, Saba P, Fanni S, Devoto P, & Bortolato M (2016). The neurosteroidogenic enzyme 5α-reductase modulates the role of D1 dopamine receptors in rat sensorimotor gating. Psychoneuroendocrinology, 63, 59–67. 10.1016/j.psyneuen.2015.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau R, Pillolla G, Bini V, Tambaro S, Devoto P, & Bortolato M (2013). Inhibition of 5α-reductase attenuates behavioral effects of D1-, but not D2-like receptor agonists in C57BL/6 mice. Psychoneuroendocrinology, 38(4), 542–551. 10.1016/j.psyneuen.2012.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau R, Savoia P, Fanni S, Fiorentini C, Fidalgo C, Tronci E, Stancampiano R, Meloni M, Cannas A, Marrosu F, Bortolato M, Devoto P, Missale C, & Carta M (2017a). The 5-alpha reductase inhibitor finasteride reduces dyskinesia in a rat model of Parkinson’s disease. Experimental neurology, 291, 1–7. 10.1016/j.expneurol.2017.01.012 [DOI] [PubMed] [Google Scholar]
- Ganzer CA, Jacobs AR, & Iqbal F (2015). Persistent sexual, emotional, and cognitive impairment post-finasteride: a survey of men reporting symptoms. American journal of men’s health, 9(3), 222–228. 10.1177/1557988314538445 [DOI] [PubMed] [Google Scholar]
- Godar SC, Cadeddu R, Floris G, Mosher LJ, Mi Z, Jarmolowicz DP, Scheggi S, Walf AA, Koonce CJ, Frye CA, Muma NA, & Bortolato M (2019a). The Steroidogenesis Inhibitor finasteride Reduces the Response to Both Stressful and Rewarding Stimuli. Biomolecules, 9(11), 749. 10.3390/biom9110749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar SC, Mosher LJ, Scheggi S, Devoto P, Moench KM, Strathman HJ, Jones CM, Frau R, Melis M, Gambarana C, Wilkinson B, DeMontis MG, Fowler SC, Coba MP, Wellman CL, Shih JC, & Bortolato M (2019b). Gene-environment interactions in antisocial behavior are mediated by early-life 5-HT2A receptor activation. Neuropharmacology, 159, 107513. 10.1016/j.neuropharm.2019.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grall-Bronnec M, Victorri-Vigneau C, Donnio Y, Leboucher J, Rousselet M, Thiabaud E, Zreika N, Derkinderen P, & Challet-Bouju G (2018). Dopamine Agonists and Impulse Control Disorders: A Complex Association. Drug safety, 41(1), 19–75. 10.1007/s40264-017-0590-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SL, & Semla TP (2019). Post-finasteride syndrome. BMJ (Clinical research ed.), 366, l5047. 10.1136/bmj.l5047 [DOI] [PubMed] [Google Scholar]
- Hu AC, Chapman LW, & Mesinkovska NA (2019). The efficacy and use of finasteride in women: a systematic review. International journal of dermatology, 58(7), 759–776. 10.1111/ijd.14370 [DOI] [PubMed] [Google Scholar]
- Iamsumang W, Leerunyakul K, & Suchonwanit P (2020). finasteride and Its Potential for the Treatment of Female Pattern Hair Loss: Evidence to Date. Drug design, development and therapy, 14, 951–959. 10.2147/DDDT.S240615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwig MS (2012). Depressive symptoms and suicidal thoughts among former users of finasteride with persistent sexual side effects. The Journal of clinical psychiatry, 73(9), 1220–1223. 10.4088/JCP.12m07887 [DOI] [PubMed] [Google Scholar]
- Jeon N, Bortolato M. What drugs modify the risk of iatrogenic impulse-control disorders in Parkinson’s disease? A preliminary pharmacoepidemiologic study. PLoS One. 2020;15(1):e0227128. 10.1371/journal.pone.0227128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyonka EGE, Schutte NS. Probability discounting and gambling: a meta-analysis. Addiction 2018;113(12):2173–2181. 10.1111/add.14397 [DOI] [PubMed] [Google Scholar]
- Laurine E, Lafitte D, Grégoire C, Sérée E, Loret E, Douillard S, Michel B, Briand C, & Verdier JM (2003). Specific binding of dehydroepiandrosterone to the N terminus of the microtubule-associated protein MAP2. The Journal of biological chemistry, 278(32), 29979–29986. [DOI] [PubMed] [Google Scholar]
- Leão AH, Sarmento-Silva AJ, Santos JR, Ribeiro AM, & Silva RH (2015). Molecular, Neurochemical, and Behavioral Hallmarks of Reserpine as a Model for Parkinson’s Disease: New Perspectives to a Long-Standing Model. Brain pathology (Zurich, Switzerland), 25(4), 377–390. 10.1111/bpa.12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman A, Ranhosky A, & Korts D (1997). Clinical evaluation of pramipexole in advanced Parkinson’s disease: results of a double-blind, placebo-controlled, parallel-group study. Neurology, 49(1), 162–168. 10.1212/wnl.49.1.162 [DOI] [PubMed] [Google Scholar]
- Madden GJ, Johnson PS, Brewer AT, Pinkston JW, & Fowler SC (2010). Effects of pramipexole on impulsive choice in male wistar rats. Experimental and clinical psychopharmacology, 18(3), 267–276. 10.1037/a0019244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, & Johnson PS (2009). Pathological gamblers discount probabilistic rewards less steeply than matched controls. Experimental and clinical psychopharmacology, 17(5), 283–290. 10.1037/a0016806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Fauth-Bühler M, Higuchi S, Potenza MN, & Saunders JB (2016). Pathological gambling: a behavioral addiction. World psychiatry : official journal of the World Psychiatric Association (WPA), 15(3), 297–298. 10.1002/wps.20373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedl SF, Peters J, & Büchel C (2012). Altered neural reward representations in pathological gamblers revealed by delay and probability discounting. Archives of general psychiatry, 69(2), 177–186. 10.1001/archgenpsychiatry.2011.1552 [DOI] [PubMed] [Google Scholar]
- Mierau J, Schneider FJ, Ensinger HA, Chio CL, Lajiness ME, & Huff RM (1995). Pramipexole binding and activation of cloned and expressed dopamine D2, D3 and D4 receptors. European journal of pharmacology, 290(1), 29–36. 10.1016/0922-4106(95)90013-6 [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Nicolas A, Denesle R, & Gomez-Mancilla B (1999). Restless legs syndrome improved by pramipexole: a double-blind randomized trial. Neurology, 52(5), 938–943. 10.1212/wnl.52.5.938 [DOI] [PubMed] [Google Scholar]
- Moore TJ, Glenmullen J, & Mattison DR (2014). Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA internal medicine, 174(12), 1930–1933. 10.1001/jamainternmed.2014.5262 [DOI] [PubMed] [Google Scholar]
- Mosher LJ, Godar SC, Nelson M, Fowler SC, Pinna G, & Bortolato M (2017). Allopregnanolone mediates the exacerbation of Tourette-like responses by acute stress in mouse models. Scientific reports, 7(1), 3348. 10.1038/s41598-017-03649-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Fellous A, Baulieu EE, & Robel P (2000). Pregnenolone binds to microtubule-associated protein 2 and stimulates microtubule assembly. Proceedings of the National Academy of Sciences of the United States of America, 97(7), 3579–3584. 10.1073/pnas.97.7.3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AM, Ryoo HL, Gurevich E, & Joyce JN (1994). Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proceedings of the National Academy of Sciences of the United States of America, 91(23), 11271–11275. 10.1073/pnas.91.23.11271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DD, Marchese M, Cone EB, Paciotti M, Basaria S, Bhojani N, & Trinh QD (2021). Investigation of Suicidality and Psychological Adverse Events in Patients Treated With finasteride. JAMA dermatology, 157(1), 35–42. 10.1001/jamadermatol.2020.3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ (2013). Dopamine agonist withdrawal syndrome: implications for patient care. Drugs & aging, 30(8), 587–592. 10.1007/s40266-013-0090-z [DOI] [PubMed] [Google Scholar]
- Orrù M, Strathman HJ, Floris G, Scheggi S, Levant B, & Bortolato M (2020). The adverse effects of pramipexole on probability discounting are not reversed by acute D2 or D3 receptor antagonism. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology, 32, 104–119. 10.1016/j.euroneuro.2020.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouachikh O, Dieb W, Durif F, & Hafidi A (2013). Differential behavioral reinforcement effects of dopamine receptor agonists in the rat with bilateral lesion of the posterior ventral tegmental area. Behavioural brain research, 252, 24–31. 10.1016/j.bbr.2013.05.042 [DOI] [PubMed] [Google Scholar]
- Paba S, Frau R, Godar SC, Devoto P, Marrosu F, & Bortolato M (2011). Steroid 5α-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders. Current pharmaceutical design, 17(2), 151–167. 10.2174/138161211795049589 [DOI] [PubMed] [Google Scholar]
- Pes R, Godar SC, Fox AT, Burgeno LM, Strathman HJ, Jarmolowicz DP, Devoto P, Levant B, Phillips PE, Fowler SC, & Bortolato M (2017). Pramipexole enhances disadvantageous decision-making: Lack of relation to changes in phasic dopamine release. Neuropharmacology, 114, 77–87. 10.1016/j.neuropharm.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM (2001). Substance abuse, pathological gambling, and impulsiveness. Drug and alcohol dependence, 63(1), 29–38. 10.1016/s0376-8716(00)00188-5 [DOI] [PubMed] [Google Scholar]
- Purves-Tyson TD, Owens SJ, Double KL, Desai R, Handelsman DJ, Weickert CS. Testosterone induces molecular changes in dopamine signaling pathway molecules in the adolescent male rat nigrostriatal pathway. PLoS One 2014;9(3):e91151. Published 2014 Mar 11. 10.1371/journal.pone.0091151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi-Ardabili B, Pourandarjani R, Habibollahi P, & Mualeki A (2006). finasteride induced depression: a prospective study. BMC clinical pharmacology, 6, 7. 10.1186/1472-6904-6-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Mamikonyan E, Detre JA, Siderowf AD, Stern MB, Potenza MN, & Weintraub D (2010). Decreased ventral striatal activity with impulse control disorders in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society, 25(11), 1660–1669. 10.1002/mds.23147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokosik SL, & Napier TC (2012). Pramipexole-induced increased probabilistic discounting: comparison between a rodent model of Parkinson’s disease and controls. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 37(6), 1397–1408. 10.1038/npp.2011.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-García D, de Deus Fonticoba T, Cores Bartolomé C, Suárez Castro E, Jesús S, Mir P, Pascual-Sedano B, Pagonabarraga J, Kulisevsky J, HernándezVara J, Planellas LL, Cabo-López I, Seijo-Martínez M, Legarda I, Carrillo Padilla F, Caballol N, Cubo E, Nogueira V, Alonso Losada MG, López Ariztegui N, et al. COPPADIS Study Group (2021). Depression is Associated with Impulse-compulsive Behaviors in Parkinson’s disease. Journal of affective disorders, 280(Pt B), 77–89. 10.1016/j.jad.2020.11.075 [DOI] [PubMed] [Google Scholar]
- Scheggi S, Guzzi F, Braccagni G, De Montis MG, Parenti M, & Gambarana C (2020). Targeting PPARα in the rat valproic acid model of autism: focus on social motivational impairment and sex-related differences. Molecular autism, 11(1), 62. 10.1186/s13229-020-00358-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuirmann DJ (1987). A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. Journal of pharmacokinetics and biopharmaceutics, 15(6), 657–680. 10.1007/BF01068419 [DOI] [PubMed] [Google Scholar]
- Seeman P (2015). Parkinson’s disease treatment may cause impulse-control disorder via dopamine D3 receptors. Synapse (New York, N.Y.), 69(4), 183–189. 10.1002/syn.21805 [DOI] [PubMed] [Google Scholar]
- Spunt B, Dupont I, Lesieur H, Liberty HJ, & Hunt D (1998). Pathological gambling and substance misuse: a review of the literature. Substance use & misuse, 33(13), 2535–2560. 10.3109/10826089809059340 [DOI] [PubMed] [Google Scholar]
- Traish AM, Melcangi RC, Bortolato M, Garcia-Segura LM, & Zitzmann M (2015). Adverse effects of 5α-reductase inhibitors: What do we know, don’t know, and need to know?. Reviews in endocrine & metabolic disorders, 16(3), 177–198. 10.1007/s11154-015-9319-y [DOI] [PubMed] [Google Scholar]
- Venturoli S, Marescalchi O, Colombo FM, Macrelli S, Ravaioli B, Bagnoli A, Paradisi R, & Flamigni C (1999). A prospective randomized trial comparing low dose flutamide, finasteride, ketoconazole, and cyproterone acetate-estrogen regimens in the treatment of hirsutism. The Journal of clinical endocrinology and metabolism, 84(4), 1304–1310. 10.1210/jcem.84.4.5591 [DOI] [PubMed] [Google Scholar]
- Vilas D, Pont-Sunyer C, & Tolosa E (2012). Impulse control disorders in Parkinson’s disease. Parkinsonism & related disorders, 18 Suppl 1, S80–S84. 10.1016/S1353-8020(11)70026-8 [DOI] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, & Lang AE (2010). Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Archives of neurology, 67(5), 589–595. 10.1001/archneurol.2010.65 [DOI] [PubMed] [Google Scholar]
- Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, Moberg PJ, & Stern MB (2006). Association of dopamine agonist use with impulse control disorders in Parkinson disease. Archives of neurology, 63(7), 969–973. 10.1001/archneur.63.7.969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won YY, Lew BL, & Sim WY (2018). Clinical efficacy of oral administration of finasteride at a dose of 2.5 mg/day in women with female pattern hair loss. Dermatologic therapy, 31(2), e12588. 10.1111/dth.12588 [DOI] [PubMed] [Google Scholar]
- Ye Z, Hammer A, Camara E, & Münte TF (2011). Pramipexole modulates the neural network of reward anticipation. Human brain mapping, 32(5), 800–811. 10.1002/hbm.21067 [DOI] [PMC free article] [PubMed] [Google Scholar]


