Abstract
On May 28, 2021, the U.S. Food and Drug Administration (FDA) granted accelerated approval to sotorasib (Lumakras™, Amgen) for the treatment of adults with advanced non-small cell lung cancer (NSCLC) with a Kirsten rat sarcoma proto-oncogene (KRAS) G12C mutation who have received at least one prior systemic therapy. The approval was based on CodeBreaK 100 (Study 20170543), a dose escalation and dose expansion trial in patients with an advanced, KRAS G12C-mutated, solid tumor. The overall response rate (ORR) observed in patients with KRAS G12C-mutated NSCLC treated with sotorasib (n = 124) was 36% (95% CI 28, 45). The median duration of response (DOR) was 10.0 months (95% CI 6.9, not estimable). The most common adverse reactions (≥20%) were diarrhea, musculoskeletal pain, nausea, fatigue, hepatotoxicity, and cough. This is the first approval of a targeted therapy for KRAS G12C-mutated NSCLC. Due to pharmacokinetic data and ORRs of patient cohorts who took sotorasib at lower doses in the dose escalation portion of CodeBreaK 100, a dose comparison study is being conducted as a post-marketing requirement (PMR).
Introduction
Locally advanced or metastatic NSCLC with KRAS G12C mutation is a genetically distinct form of lung cancer that is not curable with available therapy and comprises approximately 14% of advanced NSCLC cases. The KRAS G12C mutation leads to persistent downstream signaling to pathways promoting cell growth and division including the RAF-MEK-ERK pathway. KRAS has been described historically as an “undruggable target,” with multiple failed therapeutic efforts (1). Previous challenges to successful targeting of mutant KRAS included the risk of significant toxicity from off-target wildtype KRAS inhibition, the high affinity of KRAS for guanosine-5’-triphosphate (GTP) which thereby activates the signaling protein, and the perceived lack of suitable drug binding pockets (2, 3).
Unlike patients with NSCLC harboring other oncogenic driver mutations (e.g., epidermal growth factor receptor [EGFR] and anaplastic lymphoma kinase [ALK]), who are generally never-smokers, patients with KRAS-mutated NSCLC frequently have a smoking history (1, 4). KRAS-mutated NSCLC is often associated with high programmed death-ligand1 (PD-L1) expression, high tumor mutational burden, and responsiveness to first line immunotherapy. This last feature is distinct from other subtypes of lung cancer harboring a targetable mutation. (5, 6).
Prior to the approval of sotorasib, patients with KRAS G12C-mutated NSCLC were treated with therapies approved for NSCLC without a targetable mutation. First-line standard therapies include immunotherapy alone or in combination with platinum-based chemotherapy. The addition of immunotherapy to lung cancer treatment regimens has improved the median overall survival of patients with KRAS G12C-mutated NSCLC from 10–20 months with chemotherapy alone to 21–28 months (7, 8). However, the vast majority of KRAS G12C-mutated NSCLC patients progress on first line chemo-immunotherapy. Second-line treatment options are docetaxel monotherapy or in combination with ramucirumab, both intravenous (IV) therapies with limited efficacy (9).
Sotorasib is the first FDA-approved drug for advanced, KRAS G12C-mutated NSCLC. Herein, we provide a summary of FDA’s review of the marketing application that led to the approval of sotorasib for patients with advanced NSCLC that harbors a KRAS G12C mutation and progressed after at least one line of therapy.
Regulatory History
Sotorasib was granted Orphan Drug Designation for the treatment of KRAS G12C-mutated NSCLC on May 1, 2019 and received Breakthrough Therapy Designation on December 7, 2020. The NDA was submitted on December 16, 2020, and used the Real-Time Oncology Review pilot program. The application voluntarily utilized the Assessment Aid to facilitate FDA’s review (10).
Mechanism of Action
KRAS is an oncogene that encodes the small guanosine 5’-triphosphate hydrolase (GTPase) signal-transduction protein KRAS (11). It is a member of the RAS family of proteins, which function as molecular switches by alternating between inactive guanosine 5’-diphosphate (GDP)-bound and active GTP-bound states. Binding of GTP to RAS activates downstream signaling through multiple pathways including RAF/MEK/ERK pathway (12). Intrinsic GTPase and GDP–GTP exchange rates can vary among the different RAS mutants. The KRAS G12C activating mutation reduces GAP-mediated GTP hydrolysis resulting in accumulation of active GTP-bound KRAS (13, 14). KRAS-G12C exhibits near-wild-type intrinsic GTPase activity, thus enabling the development of covalent inhibitors that bind to the GDP-bound state of KRAS-G12C (15).
Previously unexploited utilization of a surface groove in KRAS G12C led to the development of sotorasib (1). Sotorasib is an oral small molecule inhibitor of the RAS GTPase family that binds irreversibly to the P2 pocket in the inactive GDP-bound form of KRAS. Sotorasib forms an irreversible, covalent bond with the unique cysteine of KRAS G12C, locking the protein in an inactive state (1). Sotorasib thereby blocks KRAS signaling, inhibits cell growth in vitro, tumor growth in vivo, and promotes apoptosis only in KRAS G12C tumor cell lines (16).
Clinical Pharmacology
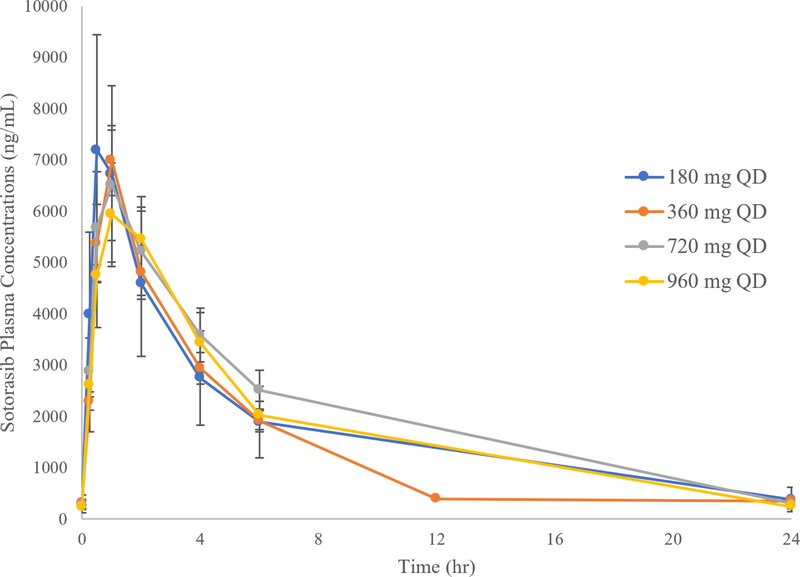
FDA reviewed subject-level data collected in CodeBreaK 100 (Study 20170543) and healthy volunteer studies to characterize the pharmacokinetics, pharmacodynamics, food effect, and drug–drug interactions of sotorasib. Systemic exposures (i.e., AUC0–24h and Cmax) of sotorasib after daily administration of doses ranging from 180 mg to 960 mg were similar (Table 1 and Figure 1). In addition, overall response rates (ORRs) ranging from 25–50% were observed in patients with NSCLC who were treated with lower doses of sotorasib in the dose escalation portion of the trial (n ranging from 3–16 per dose level) (Table 2).
Table 1.
Sotorasib Multiple-Dose PK Parameters on Day 8 Following Oral Administration of Various Dose Levels of Sotorasib QD under Fasted Conditions in Patients with KRAS G12C-Mutated Advanced Solid Tumors
| Daily Dose (mg) | N | tmax (hr) | Cmax (μg/mL) | AUC0–24h (hr*μg/mL) | t1/2,z (hr) |
|---|---|---|---|---|---|
| 180 | 6 | 0.73 (0.50–1.2) | 6.44 (7.63, 67%) | 31.7 (40.8, 89%) | 5.13 (1.99) |
| 360 | 24 | 1.0 (0.50–4.0) | 6.31 (7.33, 43%) | 38.9 (43.7, 49%) | 5.53 (1.84) |
| 720 | 11 | 1.1 (0.53–4.0) | 5.45 (6.76, 50%) | 42.1 (48.5, 49%) | 4.75 (1.16) |
| 960 | 24 | 1.1 (0.22–6.5) | 5.39 (6.82, 65%) | 32.4 (42.3, 75%) | 5.07 (1.08) |
Data are presented as geometric mean (arithmetic mean, CV%) for all PK parameters except for tmax and t1/2,z, which is presented as median (range) and mean (SD).
Figure 1. Mean Sotorasib Multiple-Dose (Day 8) Plasma Concentration-Time Profiles Following Oral Administration of Sotorasib QD under Fasted Conditions in Patients with KRAS G12C-Mutated Advanced Solid Tumors.
Plasma was collected over time from patients with KRAS G12C mutated advanced solid tumor who were enrolled in CodeBreaK 100 and receiving sotorasib at 180mg, 360mg, 720mg, or 960mg. Plasma concentrations of sotorasib were measured over time. Data are represented as Mean and Standard Error. Source U.S. Food and Drug Administration. NDA Multi-disciplinary Review and Evaluation (NDA 214665) and Approval Package: LUMAKRAS (sotorasib) (ref 19).
Table 2.
Overall Response Rates (ORR) Amongst Patients with KRAS G12C-Mutated NSCLC Treated with Sotorasib in Dose Escalation Cohorts in Study CodeBreaK 100
| Daily Dose (mg) | N | ORR N (%) (95% CI) |
|---|---|---|
| 180 | 3 | 1 (33) (0.8, 91) |
| 360 | 16 | 4 (25) (7, 52) |
| 720 | 6 | 3 (50) (12, 88) |
| 960 | 34 | 16 (47) (30, 65) |
CI, confidence interval; ORR, overall response rate.
Source: U.S. Food and Drug Administration. NDA Multi-disciplinary Review and Evaluation (NDA 214665) and Approval Package: LUMAKRAS (sotorasib) (ref 19).
Sotorasib is primarily metabolized by CYP3A4 and is mainly eliminated via the fecal route with minor renal excretion. Sotorasib may be taken with or without food. No clinically significant differences in the exposure of sotorasib were identified based on age (28 to 86 years), sex, body weight (37 to 158 kg), mild and moderate renal impairment or mild hepatic impairment. Sotorasib USPI recommends specific dose modification strategies for concomitant use of sotorasib with CYP3A inducers and substrates, gastric-acid-reducing agents, and P-glycoprotein (P-gp) substrates with a narrow therapeutic window.
Clinical Trials
The approval of sotorasib was based upon the results of CodeBreaK 100, a global, multicenter, multicohort, dose escalation and dose expansion trial that enrolled 427 patients with advanced KRAS G12C-mutated solid tumors, including 250 patients with NSCLC, as of September 1, 2020. All enrolled patients received at least one prior systemic therapy for their malignancy except in the treatment naïve NSCLC cohort. Oral doses of 180mg, 360mg, 720mg, and 960mg daily were studied in the dose escalation portion of the trial. Patients in the dose expansion portion of the study received sotorasib 960mg daily in 21-day cycles.
The primary efficacy endpoint of CodeBreaK 100 to support marketing approval was the ORR as determined by blinded independent central review (BICR); duration of response (DOR) was a key secondary efficacy endpoint.
Efficacy Results
The primary efficacy analysis population included 124 patients with KRAS G12C-mutated NSCLC who had at least one measurable lesion at baseline by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 and received sotorasib 960mg daily. Patients were primarily current or former smokers (93%), and most had received an immune checkpoint inhibitor (91%) or platinum-based chemotherapy (90%) previously. A total of 81% of patients had progressive disease after both platinum-based chemotherapy and an immune-checkpoint inhibitor. The majority of the patients in the primary efficacy population were White (82%) with African American and Asian patients represented at 2% and 15% respectively.
The ORR of the primary efficacy population as determined by BICR was 36% (95% CI: 28, 45). The median duration of response (DOR) was 10.0 months (range 1.3+, 11.1) with 58% of responders experiencing a DOR ≥6 months (Table 3). These results are based upon updated data provided to FDA at the time of new drug application (NDA) submission.
Table 3.
Summary of Overall Response and Duration of Responses per Blinded Independent Central Review in CodeBreaK 100
| Efficacy parameter | Primary Efficacy Population N = 124 |
Post-chemotherapy and Immunotherapy Population N = 100 |
|---|---|---|
|
| ||
|
Dose expansion portion (N = 124)
| ||
| Overall response rate, n (%) | 45 (36) | 31 (31) |
| Clopper-Pearson 95% CI | (28, 45) | (22, 41) |
| Complete Response | 3 (2.5) | 1 (1.0) |
| Duration of Response (DOR) | ||
| Median, months (95% CI) | 10.0 (6.9, NE) | 10 (6.9, NE) |
| Range, months | (1.3+, 11.1) | (1.3+, 11.1) |
| N (%) w/DOR ≥ 6 months | 26 (58) | 18 (58) |
CI: confidence interval; NE: not estimable
Source: LUMAKRAS (sotorasib) package insert (ref 16)
Safety Results
Nonclinical Toxicology
Toxicological assessment of sotorasib was performed in rats and Beagle dogs. In the 3-month rat toxicology study, renal toxicity was most notable, and characterized by renal tubular degeneration/necrosis and increased kidney weight with biomarkers of renal tubular injury. Partial recovery was observed after two months. Increases in cysteine S-conjugate β-lyase pathway metabolism in the rat kidney compared to human (17) may make rats more susceptible to renal toxicity due to local formation of a putative sulfur-containing metabolite (18). Renal toxicity was not identified in the 3-month dog toxicology study, although sotorasib induced findings in the liver, pituitary gland, and thyroid gland in dogs that may be due to an adaptive response to hepatocellular enzyme induction and secondary hypothyroidism (16, 19, 20).
Safety in the Intended Population
Safety analyses were conducted in all 204 patients with NSCLC enrolled in Study 20170543 who received at least one dose of sotorasib 960 mg daily in the fed or fasted state. Among patients in this primary safety population, 39% were exposed for at least 6 months and 3% were exposed for at least one year. The most common (>10%) adverse events (AEs) were diarrhea (42%), musculoskeletal pain (35%), hepatotoxicity (25%), nausea (26%), fatigue (26%), cough (20%), vomiting (17%), constipation (16%), dyspnea (16%), abdominal pain (15%), edema (15%), decreased appetite (13%), pneumonia (12%), and rash (12%). Serious AEs occurred in 50% of patients treated with sotorasib, the most common of which were pneumonia (8%), hepatotoxicity (3.4%), and diarrhea (2%). Permanent discontinuation of sotorasib due to an AE occurred in 9% of patients; the most common reason for discontinuation was hepatotoxicity (4.9%).
Interstitial lung disease/pneumonitis and hepatotoxicity were identified as potentially significant safety issues. Interstitial lung disease/pneumonitis occurred in three patients in the primary safety population (1.5%), with one fatal case. All three patients had received prior treatment with an immune checkpoint inhibitor; however, the contribution of sotorasib could not be ruled out.
In FDA’s safety review, hepatotoxicity was defined as a group including preferred terms alanine aminotransferase (ALT) increased, aspartate aminotransferase (AST) increased, blood bilirubin increased, drug induced liver injury, hepatitis, hepatotoxicity, liver function test increased, and transaminases increased. While hepatotoxicity was one of the most commonly observed adverse events, only 12% of hepatotoxicity events were severe (Grade 3 or 4), and there were no fatal events. Of the 50 patients in the primary safety population who experienced an event of hepatotoxicity, 46% (n=23) required either a dose reduction or interruption, which resulted in resolution in 91% of the cases. In addition, 5% of the 357 patients treated with sotorasib 960 mg daily with an event of hepatoxicity were treated with systemic corticosteroids as part of its management. In all cases, steroids were administered in combination with dose modifications so the degree to which steroids contributed to hepatotoxicity mitigation is unclear.
While preclinical studies with sotorasib were concerning for a potential nephrotoxicity signal, renal toxicity events in the primary safety population were rare, mild to moderate, and did not require dose reduction or discontinuation.
The FDA review team considers the safety profile of sotorasib to be acceptable for the intended population in the context of a life-threatening disease. Information in the Warnings and Precautions and Dosage and Administration sections of the product labeling provides guidance to clinicians on monitoring and managing toxicities associated with sotorasib (16).
Regulatory Insights
The approval of sotorasib provides a new treatment option for patients with KRAS G12C-mutated NSCLC for whom a targeted therapy has been elusive for decades (Table 4). While the use of immunotherapies has improved overall survival for patients with KRAS G12C-mutated NSCLC, later lines of therapies have resulted in minimal benefit (7, 8, 21). To confirm the clinical benefit of sotorasib in patients with KRAS G12C-mutated advanced NSCLC with progression after at least one prior therapy, Amgen is conducting CodeBreaK 200 (Study 20190009) which compares the efficacy of sotorasib as a single agent to docetaxel, in the same refractory population as CodeBreaK 100, with progression free survival (PFS) as a primary endpoint. Although the clinical benefit of therapeutics granted accelerated approval for an oncologic indication is often confirmed in trials evaluating an earlier line of therapy, there may be inherent challenges in combining tyrosine kinase inhibitors with standard first-line therapies for NSCLC (immunotherapy with or without platinum-based chemotherapy) due to the additive toxicity of the regimens. Furthermore, the addition of immunotherapy to platinum-based chemotherapy for the treatment of patients with NSCLC without targetable oncologic driver mutations has resulted in a markedly improved overall survival over platinum-based chemotherapy alone, offering patients with KRAS G12C-mutated NSCLC an effective first-line therapy.
Table 4:
FDA Benefit-Risk Analysis
| Dimension | Evidence and Uncertainties | Conclusions and Reasons |
|---|---|---|
| Analysis of Condition | • 14% of metastatic NSCLC is KRAS G12C-mutated • Therapies after first-line immunotherapy, which offers survival benefit, are limited in number and benefit. |
Metastatic KRAS G12C mutated NSCLC is a life-threatening condition with poor survival. |
| Current Treatment Options | • First line therapies for patients with metastatic NSCLC without a targetable mutation include platinum-based chemotherapy with or without an immune checkpoint inhibitor. Second line therapies include immune checkpoint inhibitors and pemetrexed if not already given and docetaxel with or without ramucirumab. • |
Prior to the approval of sotorasib, there were no FDA-approved targeted therapies for patients with KRAS G12C-mutated NSCLC. |
| Benefit | • The ORR in the primary efficacy population of patients with KRAS G12C-mutated NSCLC (n=124) enrolled in CodeBreaK 100 was 36% (95% CI: 28, 45). The median DOR was 10.0 months (range 1.3+, 11.1); 58% of patients had a DOR ≥6 months. • ORRs ranging from 25–50% were observed in NSCLC patients who received lower doses of sotorasib (180–960 mg QD). |
A meaningful improvement in durable ORR compared to FDA-approved therapies for the second- line treatment of patients with advanced KRAS G12C-mutated NSCLC was observed with sotorasib. A PMR was issued for a dose optimization study. |
| Risk and Risk Management | • The most common (≥20%) TEAEs observed in patients with NSCLC enrolled in CodeBreaK 100 were diarrhea, musculoskeletal pain, nausea, fatigue, hepatotoxicity, and cough. • SAEs occurring ≥ 2% of patients were pneumonia, hepatotoxicity, and diarrhea. |
Information in the Warnings and Precautions and Dosage and Administration sections of product labeling address these toxicities adequately. |
CI, confidence interval; DOR, duration of response; FDA, Food and Drug Administration; KRAS, Kirsten rat sarcoma; NSCLC, non-small cell lung cancer; ORR, overall response rate; PD-L1, programmed death-ligand 1; PMR, post-marketing requirement; SAE, serious adverse event; TEAE, treatment emergent adverse event
Source: U.S. FDA NDA Multi-disciplinary Review and Evaluation (NDA 214665) and Approval Package (ref. 19).
As a part of the accelerated approval, a PMR was issued for a dose optimization study to investigate the safety and efficacy of a lower sotorasib daily dose as compared to the 960 mg daily dose. Sotorasib exhibited similar systemic exposures with multiple daily dosing across all dose levels (180 mg to 960 mg) administered in Study 20170543. Tumor responses were observed at lower sotorasib doses; however, the small numbers of patients enrolled at the lower dose levels resulted in wide and overlapping 95% confidence intervals.
During the NDA review period, agreement on the design of a PMR study to evaluate the anti-tumor activity and safety of sotorasib administered at 240 mg daily (two 120 mg tablets) compared to the approved dose (960 mg; eight 120 mg tablets) was reached. If a lower dose of sotorasib is found to be equally effective, patients may benefit from a reduced daily pill burden from the eight pills a day required for 960 mg of sotorasib. FDA is developing Project Optimus to investigate strategies to improve dose optimization of oncology therapies (22).
To address the limited numbers of African American patients in CodeBreaK 100, a PMC was issued for the submission of results of clinical trials enrolling a sufficient number of African American patients with KRAS G12C-mutated NSCLC to further characterize the safety and efficacy of sotorasib in this patient population (23). Of note, a total of 15% of the study population was Asian, which the FDA considers to be sufficiently representative of the overall population of Asian patients with KRAS G12C-mutated NSCLC. In Asian countries, the KRAS G12C mutation has been reported as having a low incidence (approximately 4% in an analysis of 11,951 tumor samples collected in China) (24).
As a part of the Agency-wide effort to assess the impact of the COVID-19 pandemic on clinical trials, FDA evaluated the protocol changes and protocol deviations that occurred in CodeBreaK 100 due to the pandemic. Notable protocol modifications included permitting alternative sites for imaging and laboratory assessments, telehealth assessments for adverse events, and direct shipment of sotorasib to patients’ homes. In the primary efficacy population, 13 patients (10%) had ≥ 1 important protocol deviation related to the COVID-19 pandemic, including missing data (excluding missing data for incorrect treatment, dose, or other treatment compliance) (10 patients, 8%), received an incorrect, incomplete, or partial dose of sotorasib (3 patients, 2.4%), and off-schedule procedures (1 patient, 0.8%). These study adaptations did not affect overall efficacy endpoints or interpretation of study results.
This application was reviewed under FDA’s Project Orbis, in collaboration with several international Orbis partners. Australia’s Therapeutic Goods Administration (TGA), Brazil’s Health Regulatory Agency, Health Canada, and the United Kingdom’s Medicines and Healthcare Products Regulatory Agency (MHRA).
Conclusion
Treatment with sotorasib results in a response rate that represents a clinically meaningful improvement over available therapies for second- and later-line treatment. This is a landmark approval for a population with a significant unmet medical need for which targeted therapies have remained elusive for decades. Mutant KRAS has been difficult to target due to its structure (25) and the results of CodeBreaK 100 are notable. Although the approved dose of sotorasib 960 mg daily may not be fully optimized, the overall risk-benefit profile of this novel treatment is positive at the currently approved dose. Future directions for improvement on the treatment options for patients with KRAS G12C-mutated NSCLC include the study of combination therapies as first-line treatment options for patients with metastatic disease, assessment of the efficacy of KRAS G12C inhibitors in the CNS, and the development of therapies and regimens targeting resistance mutations that may develop after treatment with sotorasib. The landmark development and approval of sotorasib is a significant advance for patients with KRAS G12C-mutated NSCLC, providing a new and long-awaited treatment option for a refractory population of lung cancer patients.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors report no financial interests or relationships with the commercial sponsors of any products discussed in this report.
Note: This is U.S. Government work. There are no restrictions on its use.
References
- 1.Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019; 575 (7781):217–223. [DOI] [PubMed] [Google Scholar]
- 2.Ostrem JM, Shokat KM. Direct small-molecule inhibitors of KRAS: from structural insights to mechanism-based design. Nat Rev Drug Discov. 2016. Nov;15(11):771–785. [DOI] [PubMed] [Google Scholar]
- 3.Nagasaka M, Li Y, Sukari A, Ou SI, Al-Hallak MN, Azmi AS. KRAS G12C Game of Thrones, which direct KRAS inhibitor will claim the iron throne? Cancer Treat Rev. 2020. Mar;84:101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan G, Zhang K, Ding J, Li J. Prognostic value of EGFR and KRAS in circulating tumor DNA in patients with advanced non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(20):33922–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karatrasoglou EA, Chatziandreou I, Sakellariou S, Stamopoulos K, Kavantzas N, Lazaris AC, et al. Association between PD-L1 expression and driver gene mutations in non-small cell lung cancer patients: correlation with clinical data. Virchows Arch. 2020. Aug;477(2):207–217. [DOI] [PubMed] [Google Scholar]
- 6.Burns TF, Borghaei H, Ramalingam SS, Mok TS, Peters S. Targeting KRAS-Mutant Non-Small-Cell Lung Cancer: One Mutation at a Time, With a Focus on KRAS G12C Mutations. J Clin Oncol. 2020. Dec 10;38(35):4208–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbst RS, Lopes G, Kowalski DM, Kasahara K, Wu Y, De Castro G Jr., et al. Association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in Keynote-042. Annals of Oncology. 2019; 30(11): X163–X164. [Google Scholar]
- 8.Gadgeel S, Rodriguez-Abreu D, Felip E, Esteban E, Speranza G, Reck M, et al. KRAS mutational status and efficacy in KEYNOTE-189: pembrolizumab (pembro) plus chemotherapy (chemo) vs placebo plus chemo as first-line therapy for metastatic non-squamous NSCLC. ESMO Immuno-Oncology Congress; 2019. [Google Scholar]
- 9.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small cell lung cancer after disease progression on platinum-based chemotherapy (REVEL): a multicenter, double-blind, randomized phase 3 trial. The Lancet. 2014; 384(9944): 665–673. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration. Assessment Aid. Available at: https://www.fda.gov/about-fda/oncology-center-excellence/assessment-aid
- 11.Chang EH, Gonda MA, Ellis RW, Scolnick EM, Lowy DR. Human genome contains four genes homologous to transforming genes of Harvey and Kirsten murine sarcoma viruses. Proc Natl Sci U S A. 1982; 79(16):4848–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cully M, Downward J. SnapShot: Ras Signaling. Cell. 2008; 133(7):1292–1292.e1. [DOI] [PubMed] [Google Scholar]
- 13.Scheffzek K, Ahmadian MR, Kabsch W, Wiesmuller L, Lautwein A, Schmitz F, et al. The Ras–RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997; 277(5324):333–338. [DOI] [PubMed] [Google Scholar]
- 14.Simanshu DK, Nissley DV, McCormick F. RAS proteins and their regulators in human disease. Cell. 2017; 170(1):17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore AR, Rosenberg DC, McCormick F et al. RAS-targeted therapies: Is the undruggable drugged? Nat Rev Drug Discov 19, 533–552 (2020). Available at: 10.1038/s41573-020-0068-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Food and Drug Administration, Drugs@FDA [database on the internet]. Sotorasib USPI. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214665s000lbl.pdf
- 17.Iyer RA, Anders MW. Cysteine conjugate beta-lyase-dependent biotransformation of the cysteine S-conjugates of the sevoflurane degradation product compound A in human, nonhuman primate, and rat kidney cytosol and mitochondria. Anesthesiology. 1996; 85(6):1454–1461. [DOI] [PubMed] [Google Scholar]
- 18.Werner JA, Davies R, Wahlstrom J, Dahal UP, Jiang M, Stauber J, et al. Mercapturate pathway metabolites of sotorasib, a covalent inhibitor of KRASG12C, are associated with renal toxicity in the Sprague Dawley rat. Toxicol Appl Pharmacol. 2021. Jul 15;423:115578. doi: 10.1016/j.taap.2021.115578. Epub 2021 May 15. PMID: 34004237 [DOI] [PubMed] [Google Scholar]
- 19.U.S. Food and Drug Administration. NDA Multi-disciplinary Review and Evaluation (NDA 214665) and Approval Package: LUMAKRAS (sotorasib). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214665Orig1s000TOC.cfm
- 20.Ishida K, Werner JA, Davies R, Fan F, Thomas B, Wahlstrom J, et al. Nonclinical Safety Profile of Sotorasib, a KRASG12C-Specific Covalent Inhibitor for the Treatment of KRAS p.G12C-Mutated Cancer. Int J Toxicol. 2021. Jun 17:10915818211022965. doi: 10.1177/10915818211022965. Online ahead of print. PMID: 34137282 [DOI] [PubMed] [Google Scholar]
- 21.Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000. May;18(10):2095–103. [DOI] [PubMed] [Google Scholar]
- 22.Shah M, Rahman A, Theoret MR, Pazdur R. The conundrum of oncology drug dosing: more is less and less is more. NEJM (in press). (identified) [DOI] [PubMed] [Google Scholar]
- 23.Nassar AH, Adib E, Kwiatkowski DJ. Distribution of KRAS G12C Somatic Mutations across Race, Sex, and Cancer Type. N Engl J Med. 2021; 384:185–187. [DOI] [PubMed] [Google Scholar]
- 24.Loong HH, Du N, Cheng C, Lin H, Guo J, Lin G, et al. KRAS G12C mutations in Asia: a landscape analysis of 11,951 Chinese tumor samples. Transl Lung Cancer Res. 2020; 9(5): 1759–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Lancet Oncology. Undruggable KRAS-time to rebrand? Lancet Oncol. 2021. Mar;22(3):289. doi: 10.1016/S1470-2045(21)00091-7. PMID: 33662279. [DOI] [PubMed] [Google Scholar]