Abstract
Laryngopharyngeal reflux (LPR) is a syndrome caused by reflux of gastric contents into the pharynx or larynx which leads to symptoms of throat clearing, hoarseness, pain, globus sensation, cough, excess mucus production in the throat, and dysphonia. LPR is a challenging condition as there is currently no gold standard for diagnosis or treatment and thus presents a burden to the healthcare system. Strategies for treatment of LPR are numerous. Medical therapies include proton pump inhibitors, which are first line, H2 receptor antagonists, alginates, and baclofen. Other non-invasive treatment options include lifestyle therapy and the external upper esophageal sphincter compression device. Endoscopic and surgical options include anti-reflux surgery, magnetic sphincter augmentation, and transoral incisionless fundoplication. Functional laryngeal disorders and laryngeal hypersensitivity can present as LPR symptoms with or without GERD. Though there are minimal studies in this area, neuromodulators and behavioral interventions are potential treatment options. Given the complexity of these patients and numerous available treatment options, we propose a treatment algorithm to help clinicians diagnose and triage patients into an appropriate therapy.
Keywords: Laryngopharyngeal reflux, Extraesophageal reflux, Gastroesophageal reflux, Treatment
Introduction
Laryngopharyngeal reflux (LPR) is a syndrome where reflux of gastric contents causes laryngeal symptoms including throat clearing, hoarseness, pain, globus sensation, cough, excess mucus in the throat, and dysphonia.1–3 The incidence and prevalence of LPR are challenging to ascertain due to the lack of a gold standard diagnostic test; however, in a study of patients presenting with laryngeal and voice disorders, 50% were diagnosed with LPR based on 24-hour double-probe pH monitoring.4 Further, gastroesophageal reflux disease (GERD) is the most common gastrointestinal disease in North America with the last consensus from 2004-2011 indicating that symptoms of GERD affected between 18.1%-27.8% of individuals in the US and between 2.5%-33.1% of individuals worldwide.5 LPR presents both a diagnostic and treatment dilemma, as current diagnostic testing typically lacks in either sensitivity or specificity and our knowledge about the best treatment option remains unclear. Ambiguity exists in symptoms as well; extra-esophageal manifestation can occur independently from common GERD symptoms.6 Consequently, patients end up seeing on average 10 specialists and undergoing 6 tests in the initial year of evaluation, often without diagnostic clarity or improvement. 7–10 Current clinical approaches are estimated to cost $5,438/patient, equating to over $50 billion in annual health care costs. 7
There are several theories as to the pathophysiology behind LPR and why some individuals may be responsive to PPIs. The reflux theory explains that LPR develops from microaspiration of acid, bile acids, and pepsin that can directly injure the larynx and cause symptoms.6,11,12 This has been supported by the finding of elevated salivary pepsin and bile acid levels in patients with LPR, diagnosed via high-resolution manometry and combined multichannel intraluminal impedance and 24h pH monitoring.13 It has been postulated that even minor pepsin reflux reaching the larynx can be damaging, as the microenvironment at the level of the larynx is less protective against insults14 and this may explain why some patients do not respond to PPIs alone.15 Alternatively the reflex theory postulates that reflux of acidic gastric contents in the distal esophagus stimulates vagally induced laryngeal symptoms.16
Heterogeneous clinical presentations and pathophysiologic mechanisms driving laryngeal symptoms leads to differences observed in both incidence of and treatment outcomes for LPR. In this review we aim to outline current LPR treatment in an algorithmic approach and attempt to identify how clinicians can identify patients that may be more responsive to certain therapies.
Diagnosis of LPR
Unfortunately there is no diagnostic gold standard when assessing LPR.11 Therefore, optimal evaluation likely involves consultation with both an otolaryngologist and gastroenterologist in addition to certain targeted tests. Flexible laryngoscopy is an important initial test to exclude other laryngeal pathology.11 In addition, careful examination for rhinorrhea, nasal purulence or prominent lymphoid tissue at the posterior pharyngeal wall should be done to exclude other diagnoses such as allergic rhinitis or sinusitis. Ambulatory reflux monitoring plays an important diagnostic role to measure esophageal reflux burden.17 Recent guidelines recommend ambulatory reflux monitoring off acid suppression in the evaluation of LPR symptoms and recommend testing patients prior to starting empiric pharmacotherapy in patients with LPR symptoms in the absence of heartburn or regurgitation.17 This is guided by the fact that up to 50-60% of patients with isolated laryngeal symptoms will not have gastroesophageal reflux pathology and will not respond to anti-reflux therapies.11,17 On the other hand, a small proportion of patients with isolated laryngeal symptoms may have true LPR, commonly referred to as “silent reflux”.11,17 At present, data supports that the best outcomes are seen in patients with elevated esophageal acid burden, disrupted anti-reflux barrier, and concomitant presence of esophageal reflux symptoms.17,18 However, even in patients with pathologic reflux, laryngeal symptoms may not resolve with typical anti-reflux therapies.17 Ultimately, diagnosis of LPR remains a challenge and is an area that requires further investigation.
Dietary and Lifestyle Therapy
A plant based diet is hypothesized as enhancing laryngopharyngeal mucosal recovery due to altered microbiota of the hypopharyngeal-esophageal reflux events as measured on the hypopharyngeal-esophageal multichannel intraluminal impedance-pH monitoring.19 In a retrospective chart review of 85 patients with LPR treated with PPI and standard reflux precautions (avoidance of coffee, tea, chocolate, soda, greasy, fried, fatty, and spicy foods, and alcohol) and 99 patients with LPR treated with alkaline water and a 90% plant based Mediterranean diet and standard reflux precautions, researchers found that there was no difference in the proportion reaching a 6-point reduction in their RSI; however, when comparing the mean percent reduction in the RSI, the data significantly favored the plant-based diet approach.20
In another retrospective chart review of 65 patients with LPR, all patients were treated with pantoprazole 20mg twice daily and all were counseled on lifestyle changes as well as an alkaline, protein, low fat, low-acid diet.21 Patients were divided into 2 groups based on adherence to dietary and lifestyle recommendations and the researchers found that RSI and RFS significantly improved in both groups; however, the improvement in RSI was significantly higher in the patients who adhered to the dietary and lifestyle recommendations when compared to those who did not.21 With regards to the recommended lifestyle habits, these included stress control, tobacco cessation, small meals, hot lunch instead of a hot dinner, eating slowly, avoiding talking while eating, avoiding tight clothing, and avoiding certain drugs (non-steroidal anti-inflammatory drugs, corticosteroids, aspirin, theophylline, progesterone, iron supplementation, calcium channel blockers, nitroderivatives, and anticholinergic medications).21 The dietary changes were focused on avoiding fatty animal products (including meats/chicken/fish and dairy), fried foods, refined carbohydrates, nuts, spicy foods, shallots, onion, garlic, tomato, aspartame, rhubarb, blueberries, refined sugar, alcohol, coffee, tea, and citrus juices.21 Further supporting this diet is the knowledge that patients who consume high-fat, low-protein, high-sugar, and high-acid foods tend to have higher numbers of proximal reflux episodes with multichannel intraluminal impedance-pH monitoring.22
In a small study of 12 male and 8 female patients with PPI resistant LPR, it was found that on a strict, low-acid diet (all foods pH≥5), there was a statistically significant improvement in both the mean pre-diet versus post-diet RSI and RFS scores.23 Along this same line, another group of researchers conducted a retrospective study of patients completing an LPR induction program versus patients prescribed only anti-reflux medications and behavioral modifications.24 The LPR induction program included a 2-week induction diet (low fat foods, all with a pH≥5) followed by a similar but less strict diet, high dose anti-reflux medication, at least 16 oz alkaline water, and behavioral modifications (weight loss, smoking cessation, alcohol avoidance, minimizing tight clothing, avoiding eating 3 hours before lying down, taking PPI 30-60 minutes before meals).24 The control group had a high dose PPI (with or without H2 blocker) and behavioral modifications.24 Researchers found that patients undergoing the induction program (average of 32-day first follow-up) had significant improvements in their RSI scores whereas those in the control group (average of 62-day first follow-up) did not.24
With regards to obesity, in a retrospective review of 285 patients with clinical LPR (determined by history, RFS, and RSI) who underwent pH-probe studies and found that abnormal esophageal reflux events correlated significantly with increasing BMI, whereas abnormal pharyngeal reflux events did not correlate with increasing BMI.25
Overall, certain reflux-centric lifestyle adjustments (Table 1) as well as favoring lower fat, lower acid, plant-based foods seems reasonable to propose to patients hoping to reduce their symptomatic burden from LPR. In general it seems worthwhile to combine both medical treatment and lifestyle/dietary changes to maximize symptomatic improvement.
Table 1:
Therapeutic options for patients presenting with LPR symptoms
| Therapy | Dose/Frequency | Mechanism | Special Considerations |
|---|---|---|---|
| PPI | High-dose PPI for 8 weeks | Block secretion of gastric acid by binding irreversibly to the hydrogen-potassium ATPase pump on gastric parietal cells. | Patients with predominately nonacid reflux events may be non-responders |
| H2 Receptor Antagonists | Variable dosing regimens | Block secretion of gastric acid by reversibly binding to histamine H2 receptors on gastric parietal cells. | May be an option in patients for breakthrough symptoms on a PPI, but are unlikely to be effective in individuals with frequent symptoms |
| Alginates | GA, 10mL to 20mL after meals and/or at bedtime | Mucosal barrier near the gastroesophageal junction, prevention of gastric reflux by forming a mechanical raft above gastric content, and inhibition of pepsin and bile salts. | May be helpful in patients with nonacid or mixed acid reflux who are PPI non-responders and those prone to post-prandial acid pocket |
| Baclofen | Variable dosing regimens used in studies | Gamma-aminobutyric acid receptor type-B agonist that inhibits lower esophageal sphincter relaxation | Modest efficacy in LPR and does carry important side effect profile |
| Dietary and Lifestyle Therapy | Plant-based, low fat, low acid diet with standard reflux precautions | Acid suppression | Relatively effective and should be included when discussing symptom management |
| External UES Compression Device | 20-30 mmHg of cricoid pressure, nightly while sleeping | Increases the upper esophageal sphincter intraluminal pressure to prevent pharyngeal reflux events | Newer therapy with potential efficacy in LPR |
| Endoscopic and Surgical Interventions | Laparoscopic fundoplication, Magnetic sphincter augmentation, Transoral incisionless fundoplication | Re-creation of a gastro-esophageal flap valve to restore the anti-reflux barrier | Should only be considered if symptoms are refractory to medical therapy and the patient has objective findings of GERD |
| Pharmacologic Neuromodulators* | Variable dosing/frequency. Gabapentin, Pregabalin, Tricyclic Antidepressants, Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine Reuptake Inhibitors, and P2X3 antagonists. | Focused on laryngeal hypersensitivity and functional laryngeal disorders | Mainly studied in chronic cough, reflux hypersensitivity, and functional heartburn. |
| Speech Therapy* | Variable interventions | Focused on laryngeal hypersensitivity and functional laryngeal disorders | Mainly studied in chronic cough, requires experienced/trained practitioners |
| Behavioral Therapy* | Hypnotherapy and Cognitive Behavioral Therapy | Focused on laryngeal hypersensitivity and functional laryngeal disorders | Less robust evidence in laryngeal/esophageal disorders and requires experienced/trained practitioners |
Consider for patients who have laryngeal hypersensitivity or whom are not responding to therapy, as laryngeal hypersensitivity may be present in patients with LPR/GERD.
Pharmacotherapy
Proton Pump Inhibitors
Historically, management of patients with suspected LPR typically began with an empiric 8 to 12 week double-dose proton pump inhibitor (PPI) trial.6 Current guidelines recommend high dose PPI therapy given its high probability (91 to 99%) of restricting physiologic esophageal acid exposure.11,26 In a recent double-blind, placebo controlled trial in patients with persistent throat symptoms, researchers investigated the effectiveness of Lansoprazole 30mg BID for 16 weeks versus placebo and found that in 220 individuals who completed the study, mean RSI scores were not significantly different between groups, and these results held after 12 months of treatment.27 Similarly in the TOPPITS randomized controlled clinical trial, patients with persistent throat symptoms were also randomized to the aforementioned groups and researchers found that in 267 patients completing the primary outcomes, there was no significant difference between RSI scores at 16 weeks or 12 months of follow-up.28 Notably neither of these studies evaluated patients for objective findings of GERD. Several reviews and meta-analyses assessing the efficacy of PPIs in patients with suspected LPR have reported mixed results.29–33 In fact, in a recent systematic review, researchers evaluated published systematic reviews and meta-analyses that evaluated PPIs in patients with persistent throat symptoms and found 10 systematic reviews that were all at high risk of bias, except for one.34 The inconsistency in symptomatic response in these studies likely reflects the variability in study design with respect to dosing, frequency, and PPI choices, as well as the mixed response patients have to PPIs depending on their underlying disease pathology, which further complicates the ability to apply these results to patients.
There are two overarching explanations for the suboptimal response to PPI therapy in patients with suspected LPR. First, extra-esophageal symptoms are nonspecific for LPR and most studies examining LPR likely include a diluted and heterogeneous patient population, some of whom may not actually have LPR and instead have a range of other diagnoses including laryngeal hypersensitivity or functional laryngeal disorders. Consequently, recent studies have attempted to delineate which patients are more likely to respond to PPIs. A recent multicenter study of 302 adults with chronic laryngeal symptoms identified distinct phenotypes of patients, which included LPR/GERD with a hiatal hernia, LPR with mild GERD, no LPR or GERD, reflex cough, and mixed/possible obstructive EGJ.35 Individuals with LPR/GERD with hiatal hernia would likely be most responsive to PPI followed by LPR and LPR with mild GERD and reflux cough, thus separating patients into distinct phenotypic categories may help to inform which treatments are effective for which patients.35 Second, it is possible that esophago-pharyngeal refluxate is not entirely acidic, and prior studies demonstrate that acid reducing agents are likely ineffective in patients with nonacid reflux events.15,36,37 For these reasons, current societal guidelines recommend ambulatory reflux monitoring in patients with isolated extra-esophageal symptoms as opposed to empiric trials of PPI therapy in order to facilitate early identification of LPR and improve efficacy of PPI therapy (Table 1).
H2 Receptor Antagonists
H2 Receptor Antagonists are another class of drug that has been studied in LPR, but as is the case with GERD, is considered more of a second line therapy due to their short duration of action and lower potency of acid suppression compared to PPI.1 In one study of patients presenting with symptoms of chronic laryngitis, researchers treated patients with an escalating regimen of lifestyle changes, Famotidine 20mg nightly, followed by Omeprazole 20mg nightly.38 They found that symptoms resolved in 51% (93/182) on standard antireflux precautions, 54% (48/89) of those who failed standard reflux precautions responded to Famotidine 20mg nightly, and in the remaining 41 participants 34 (83%) had improvement in their symptoms with Omeprazole 20mg nightly.38 H2 Receptor Antagonists may be an option in patients for breakthrough symptoms on a PPI, but are unlikely to be effective in individuals with frequent symptoms given the aforementioned shortcomings.
Alginates
Alginates are an oral pharmacologic therapy that creates a barrier at the esophago-gastric junction and a mechanical raft above the gastric contents to prevent gastro-esophageal reflux events, whether acidic or nonacidic. In addition, Alginates inhibit pepsin and bile salts. Alginates are often used as an adjunct to PPI therapy in patients with GERD that have not symptomatically improved with acid suppresion.11 Placebo controlled trials demonstrate significant symptom improvement with alginates in patients with typical GERD symptoms.39,40
Alginates also improve symptoms in LPR patients. In one randomized controlled trial of patients with Reflex Symptom Index (RSI) scores of >10 and Reflux Finding Scores (RFS) scores of >5, the group that received Gaviscon Advanced had greater symptom improvement at 2, 4, and 6 months post-treatment as well as laryngoscopic improvement per the RFS at 6 months compared to the group that received no treatment.41 Limitations of this study include lack of a placebo and lack of PPI trial prior to treatment. On the other hand, a double-blind placebo-controlled study in patients with RSI>10 and RFS >5 comparing 8 weeks of Alginos Oral Suspension with a placebo, identified significant reduction of the total RSI, RFS, and total number of reflux events on multichannel intraluminal impedance pH monitoring in both arms, suggesting that liquid alginate is not superior to placebo.42 One of the notable components of this study was that all patients were counseled on lifestyle modification, which could have contributed to the similar findings in both groups. In a prospective study of patients with LPR and RSI >10, researchers found that Gaviscon Advance alone is effective in treating LPR and co-prescription with a high dose PPI did not offer an additional symptom improvement.43 Of note is that patients were not randomized and if patients were on a PPI prior to the trial initiation, this was optimized to high-dose twice daily dosing. Despite the mixed data, alginates are well tolerated and postulated to have a role in LPR, particularly in patients with nonacid or mixed reflux whose symptoms do not improve with an initial PPI trial.1 When used for LPR, the typical dose is Gaviscon Advanced with recommended doses ranging from 10 to 20mL after meals and/or at bedtime (Table 1). 41,43 42
Baclofen
Baclofen is a gamma-aminobutyric acid receptor type-B agonist that inhibits transient lower esophageal sphincter relaxations and potentially prevents reflux events, both acidic and non-acidic.44 Most extensively studied in GERD, a meta-analysis of 9 RCTs identified a significant reduction in the incidence of gastroesophageal reflux, the length of the reflux episodes, and the occurrence of transient lower esophageal sphincter relaxation when comparing patients on baclofen versus placebo.45
While baclofen studies are primarily targeting cough, there are potential LPR applications. In a study of 16 patients with refractory GERD induced chronic cough whom had failed omeprazole 20mg twice daily plus domperidone, baclofen improved cough in 56.3% and responders had a significantly lower number of acid reflux events when compared to non-responders.46 Notably 1 participant withdrew due to side effects (persistent nausea and diarrhea) and 3 participants withdrew because of deterioration or no improvement in their cough.46 In another study of 32 subjects with LPR symptoms despite PPI therapy, 53% met symptom response (>50% improvement in RSI from baseline) with lansoprazole 15mg twice daily and baclofen 10mg three times daily for 3 months and lifestyle counseling.44 The efficacy of baclofen in LPR is unclear given limited and low quality data. Also, given its challenging side effect profile, it is not routinely recommended as a first-line or adjunctive therapy in LPR (Table 1).
External Upper Esophageal Sphincter Compression Device
One of the newer and novel therapies being tested for LPR is the External Upper Esophageal Sphincter (UES) Compression Device (Figure 1). The idea behind this device came after researchers found that in individuals with typical reflux symptoms plus supra-esophageal symptoms (chronic cough, burning throat, or hoarseness) had impaired esophageal and UES response to simulated reflux events and thus these individuals could be at a greater risk of esophagopharyngeal reflux.47
Figure 1:

Image of the External Upper Esophageal Sphincter Compression Device
Based on this initial research, the utility of an External UES Compression Device was proposed and it was found that using 20-30 mmHg of cricoid pressure lead to a significant increase in the UES intraluminal pressure and thus prevented pharyngeal reflux events.48 The initial study conducted with this device enrolled 95 patients with esophagopharyngeal reflux with extra-esophageal symptoms and RSI > 13 whom were instructed to wear the External UES Compression Device at night.49 They found that RSI scores at 2 and 4-week follow-ups were significantly improved.49 Based on this research, the Reflux Band (Somna Therapeutics, Germantown, WI) was approved by the FDA.49 Another group tested the External UES Compression Device in 15 participants with an RSI ≥13 whom were trialed on 14 consecutive nights of therapy.50 They found that 29% were complete responders (>50% reduction in RSI and post-intervention RSI<13), 58% were partial responders (reduction from baseline RSI but not meeting complete responder criteria), and 14% were non-responders.50 Compared to baseline, mean RSI was significantly improved post-intervention and there was a statistically significant reduction in salivary pepsin in the group of complete responders.50 In a two-phase prospective clinical trial in adults with at least 8 weeks of laryngeal symptoms, participants were given double dose PPI for 4 weeks followed by the addition of the external UES compression device for 4 weeks, and researchers found that 55% of the 31 participants achieved either an RSI score ≤ 13 and/or a > 50% reduction in RSI.51 When comparing non-responders to responders, it was found that responders tended to have lower BMI (24.2 kg/m2 vs. 28.6, p=0.02), significantly higher salivary pepsin at baseline (145.0 ng/mL vs. 34.6 ng/mL, p=0,01) and tended to have a lower separation between the LES and crural diaphragm on manometry.51 Therefore, the authors suggest that individuals with a hiatal hernia, central obesity, and cough with mechanical reflux, may be less likely to respond to the external UES compression device with PPI therapy.51 Adverse events from this device were minimal.51 When contacted for the 3-month follow-up, 14 (67%) were still using the external UES compression device and seven had discontinued for intolerance (4, 57%), poor symptom control (2, 29%), and rash (1, 14%).51 Given this data it seems reasonable to consider the External UES Compression Device to be used at nighttime for patients with established GERD whom are also suffering from LPR whose symptoms are not fully controlled on a PPI (Table 1).51 Given the limited data and lack of randomized controlled trials, more information is needed to determine the duration of therapy and whether concomitant therapy, namely with a PPI, is warranted.
Endoscopic and Surgical Interventions
While the role of anti-reflux surgery is well established for PPI refractory GERD, its role is less clear in LPR and experts warn that a cautious approach is warranted when recommending surgical therapy for LPR.11 The most recent American Gastrointestinal Association (AGA) guidelines outline that surgical therapy can be considered in patients with extraesophageal symptoms whom are refractory to medical therapy and have objective findings of GERD.11 In addition it is noted that patients with mechanical defects (hiatal hernia) may also benefit.11
In a study of 27 patients with PPI refractory extra-esophageal symptoms (twice daily PPI for at least 12 weeks with a <50% improvement in symptoms) whom had objective findings of GERD and underwent a standard Nissen laparoscopic fundoplication for treatment, researchers found that 59% had at least partial improvement in their main symptom following fundoplication.52 Characteristics that helped predict which patients would symptomatically improve included individuals who also had heartburn with or without regurgitation and a distal esophageal acid exposure time (pH less than 4.0) of greater than 12%.52 Symptom improvement was assessed by asking patients to indicate their percentage improvement of their primary symptom and responders were defined has a greater than 50% improvement from baseline.52
In a review of 27 observational studies, researchers found that the effectiveness of antireflux surgery for LPR was anywhere from 10 to 93%.53 In another, more recent, review, 34 studies of patients with LPR who had fundoplication (29 studies laparoscopic, 1 study endoluminal/transoral, 3 studies with other approaches) were examined and researchers found that due to the extreme variability with regards to their diagnostic method, exclusion criteria, and outcomes used to determine efficacy of surgery, that the review was inconclusive with regards to whether fundoplication is effective for LPR.54
Magnetic sphincter augmentation (MSA) has shown success in patients with typical GERD, thus researchers retrospectively investigated the effects of MSA on patients with objective evidence of GERD on ambulatory pH monitoring and LPR symptoms and found that mean RSI scores significantly improved from 20.9 to 8.1 (p<0.01) after MSA.55
Another potential option for patients is endoscopic therapy with transoral incisionless fundoplication (TIF). In a study of 34 patients with GERD and/or LPR who underwent TIF, researchers found that TIF resulted in symptomatic improvement with mean RSI scores reduced from 19.2 pre-TIF on PPI to 6.1 post-TIF (p<0.001).56
Overall, endoscopic and surgical interventions (Table 1) should be considered as a last line treatment for patients with LPR as the outcomes are quite variable. In addition, only select patients, as outlined above, should be considered for surgery or endoscopic intervention. Further investigation regarding endoscopic and surgical therapies for LPR are warranted.
Treatment Approach for Functional Laryngeal Disorder and Laryngeal Hypersensitivity
Functional laryngeal disorders can present with a variety of symptoms, many of which can be challenging to distinguish from LPR. Diagnosis in this subset of patients is made when an individual demonstrates no reflux on pH testing and in whom other causes of laryngeal dysfunction have been excluded.57 To qualify, patients should have the following criteria for at least 3 months with symptom onset at least 6 months prior to diagnosis: 1) Laryngeal symptoms (cough, throat clearing, sore/burning throat) at least several times per week, 2) No evidence that GERD is the etiology, and 3) They should be free of sinus, endocrine, pulmonary, or laryngeal disorders other than just irritation.57 Functional laryngeal disorders are thought to be due in part to laryngeal hypersensitivity, which is hypothesized to occur after an insult, such as an upper respiratory tract infection or aspiration, or in association with another comorbidity, such as asthma or chronic rhinosinusitis, which then causes sensitization of the larynx and an exaggerated response to triggers.58 This hypersensitivity may explain why some individuals who are presumed to have LPR, do not respond to PPI treatment.59 This patient population can be hard to distinguish from LPR patients clinically, and likely represents a major confounder in accurate diagnosis and effective treatment of LPR. Further, laryngeal hypersensitivity can coexist with LPR, just as esophageal hypersensitivity can coexist with esophageal motility disorders and GERD, which can ultimately influence how these patients are managed. It is plausible that in patients who do not respond to PPIs, there are likely other factors, such as hypervigilance, elevated anxiety levels, allodynia, and hyperalgesia that are contributing to their symptoms.60 Given this information, clinicians should have a high index of suspicion of laryngeal hypersensitivity when treating patients with laryngeal symptoms whom are non-responders to typical treatments. In addition, validated laryngeal hypersensitivity scoring systems, such as the EHAS score for esophageal hypersensitivity, are needed.
Pharmacologic Neuromodulation
Neuromodulators are one of the main treatments used in patients with functional and laryngeal disorders due to hypersensitivity, and have been most studied with chronic cough.6 While these are typically regarded as different diagnoses, some treatment strategies may be extrapolated from experience with chronic cough and applied to treatment of laryngeal hypersensitivity and LPR patients more broadly.
Gabapentin (in doses of up to 1800 mg/d) has been shown in a randomized, double-blind, placebo-controlled trial in 62 patients to significantly improve patient’s scores on the Leicester Cough Questionnaire (LCQ) when compared to placebo.61 Current CHEST guidelines recommend considering a trial of Gabapentin in individuals with chronic unexplained cough.62 In another randomized placebo controlled trial of 40 patients with refractory chronic cough that compared speech therapy with and without the addition of Pregabalin, researchers found that patient’s scores on the LCQ improved with both interventions; however, in the speech therapy with Pregabalin group, there was a significantly greater improvement.62 In another study focused on patients presenting with chronic cough, globus sensation, odynophonia, and or odynophagia, researchers retrospectively reviewed 12 consecutive patients prescribed Pregabalin and found that out of the 10 who tolerated the medication, symptom severity rating improved from 3.9 pre-treatment to 1.2 post-treatment.63 Amitriptyline has also demonstrated effectiveness with a decrease in the cough severity and frequency in a randomized controlled study of 28 participants with cough due to post-viral vagal neuropathy when compared to codeine/guaifenesin.64 Neuromodulators, such as tricyclic antidepressants, serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors are also commonly used in patients with reflux hypersensitivity or functional heartburn.65
Other therapies have aimed to target neuronal hyperresponsiveness of the cough reflex, such as P2X3 receptors located in airway vagal afferent nerves, which are suspected to add to sensory neuron hypersensitization.66 In a double-blind, placebo-controlled, two-period, crossover study of 34 individuals, researchers found that cough frequency was significantly reduced in the treatment group (AF-219, an oral P2X3 antagonist) when compared to placebo.66 More recently, a P2X3 receptor antagonist (Gefapixant) was studied in a 12-week, phase 2b, randomized, double-blind placebo controlled study in patients with refractory cough.67 253 patients were randomly assigned to placebo or differing doses of Gefapixant, and researchers found that with a dose of 50mg twice daily there was a significant decrease in objective cough frequency, as measured through a 24-hour sound recording, when compared to placebo.67
Speech Therapy
In addition to medications for chronic cough, speech therapy has also been shown to be effective and is recommended by the current CHEST guidelines.62 In a study of 20 patients with cough and paradoxical vocal fold movement disorder who underwent treatment with a PPI for 6 months and 3-5 sessions of respiratory retaining therapy, a form of speech and breathing therapy that targets the abdominal and oropharyngeal musculature, researchers found that 100% had improvement in their cough and 85% had improvement in RSI.68 In a single blind, randomized, placebo controlled trial in 87 patients with chronic cough who underwent a speech pathology intervention or placebo intervention (education of healthy lifestyle such as relaxation, stress management, exercise, and diet), researchers found that the treatment group had significant improvements in their symptoms (cough, breathing, voice, upper airway, and limitations in performing everyday activity).69 The placebo group also had statistically significant improvements in breathing, cough, and limitations in performing everyday activities; however, the amount of improvement was significantly less than seen in the intervention cohort.69 Neuromodulators and speech therapy have both shown promise in patients with chronic cough and may potentially improve other laryngeal hypersensitivity symptoms; however, additional research into this area is warranted.
Behavioral Therapy
Hypnotherapy is another targeted treatment strategy in patients suffering from bothersome laryngeal symptoms. In a small case series of 10 patients presenting with globus sensation, researchers found that after a standardized, semi-structure, 7-session relaxation protocol (included modifying breathing and relaxing muscles) and 5-sessions of esophagus-directed hypnotically assisted relaxation, there was a statistically significant decrease in overall symptom severity.70 In another study examining 9 patients with functional heartburn who underwent 7 weekly sessions of a protocol targeting esophageal-directed hypnotherapy, researchers found statistically significant improvements in patient’s heartburn symptoms, visceral anxiety, and quality of life.71 In a recent review, the authors concluded that hypnotherapy for patients presenting with dysphagia, globus, functional chest pain/non-cardiac chest pain, dyspepsia, and functional heartburn was warranted based on current evidence.72
Cognitive Behavioral Therapy (CBT) has been well studied in patients with functional bowel disorders; however, the data for patients with esophageal complaints is less robust.65 In CBT, a health psychologist will work with the patient on skills such as stress management, cognitive restructuring, coping strategies, problem solving, and controlling anxiety.65
Though data for esophageal disorders is less robust than for other functional gastrointestinal disorders, behavioral therapies are safe and effective options to trial for patients suffering from functional esophageal symptoms,65,72–74 and this data can likely be extrapolated for use in patients with laryngeal hypersensitivity and functional laryngeal disorders. However, additional research in the area of behavioral therapies in the treatment of laryngeal hypersensitivity and functional laryngeal disorders is warranted.
Conclusion
LPR presents as a challenge both to the treating clinician and patients (Figure 2). In addition, the exact mechanism of disease pathology leading to LPR is unknown and likely due to a multitude of factors, which further complicates the picture. A variety of therapeutic options from lifestyle/dietary modifications, to medications, to the External UES Compression Device, and potentially even surgery exist for patients with LPR (Table 1). Though an empiric PPI trial is a simple first line therapeutic approach in individuals presenting with symptoms of LPR, this is no longer recommended as many patients fail PPIs and thus additional research is warranted to help define patient characteristics and diagnostic testing strategies that could potentially help to personalize the treatment algorithm. Based on LPR research, we propose the following treatment algorithm (Figure 3) that helps clinicians to systematically diagnose and triage patients to variable treatment options. Ultimately with improvement in our understanding of LPR, as well as our ability to diagnose and categorize patients into variable groups based on diagnostic testing, we can hopefully develop a more streamlined approach to manage these complex patients.
Figure 2:
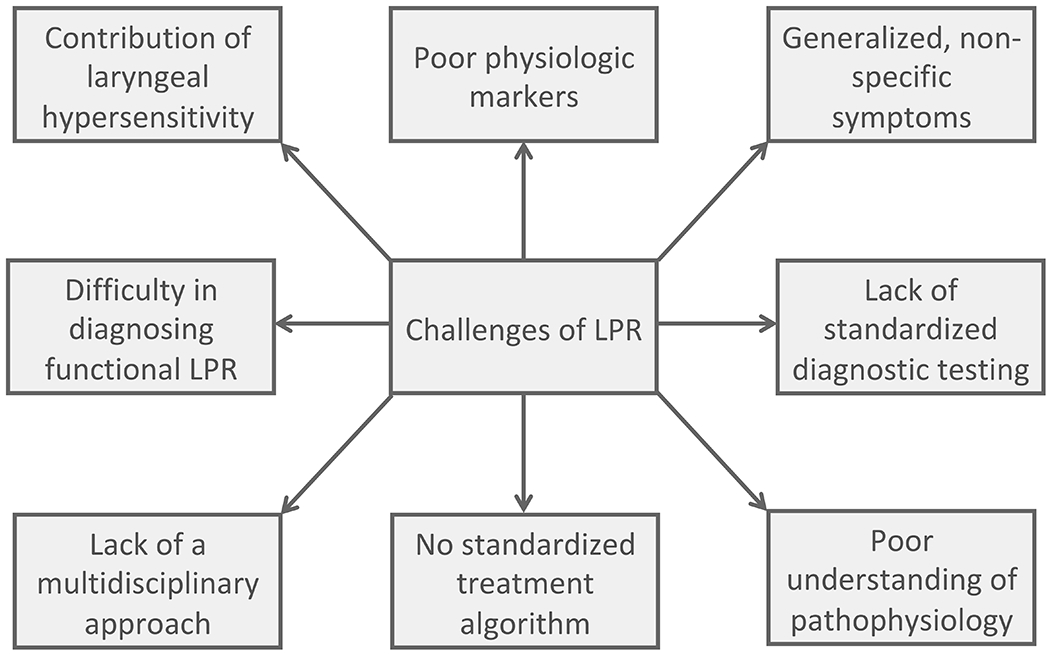
Conceptual diagram outlining both the diagnostic and therapeutic challenges of LPR.
Figure 3:
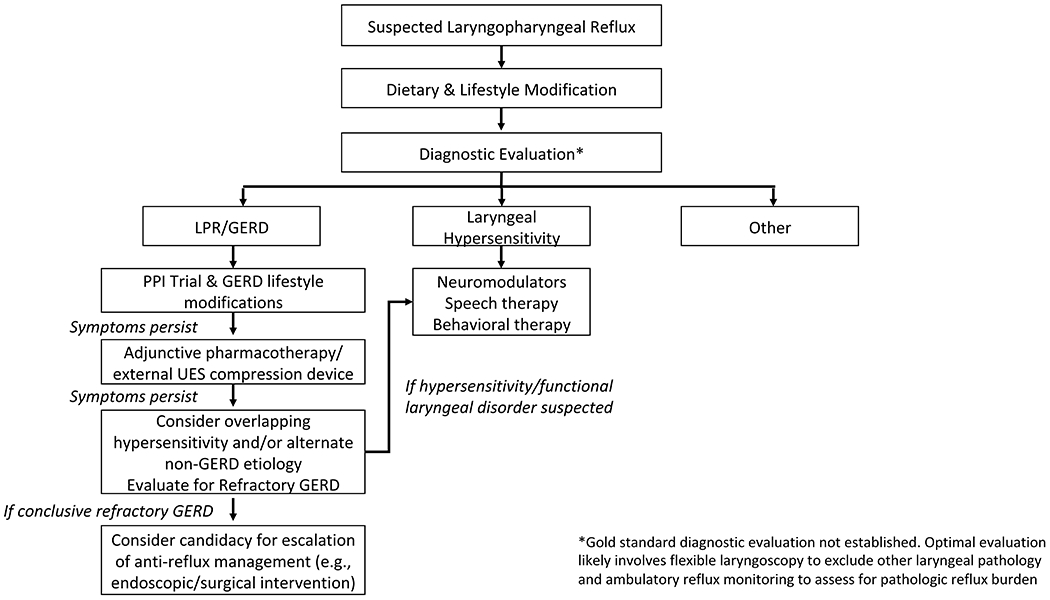
Proposed diagnostic algorithm for patients presenting with suspected LPR. Given the lack of a gold standard for diagnosis of LPR, we recommend flexible laryngoscopy and ambulatory reflux monitoring to assess the reflux burden. Pending the results, providers can treat patients with a sequential strategy for patients with LPR and GERD or Laryngeal hypersensitivity. For the LPR/GERD category, providers should initially start with a PPI trial with GERD lifestyle modifications, and if symptoms persist, move down the treatment algorithm trialing different therapies as outlined in the diagram.
Funding Support:
RY is supported by NIH K23 DK125266 (PI: Yadlapati).
Disclosures
RY: Consultant: Medtronic (Institutional), Ironwood Pharmaceuticals (Institutional), Phathom Pharmaceuticals; Research support: Ironwood Pharmaceuticals; Advisory Board with Stock Options: RJS Mediagnostix
AJK, EHW, PAW, THT: No relevant disclosures
References
- 1.Lechien JR, Akst LM, Hamdan AL, et al. Evaluation and Management of Laryngopharyngeal Reflux Disease: State of the Art Review. Otolaryngol Head Neck Surg. 2019;160(5):762–782. [DOI] [PubMed] [Google Scholar]
- 2.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus G. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101(8):1900–1920; quiz 1943. [DOI] [PubMed] [Google Scholar]
- 3.Olson NR. Laryngopharyngeal manifestations of gastroesophageal reflux disease. Otolaryngol Clin North Am. 1991;24(5):1201–1213. [PubMed] [Google Scholar]
- 4.Koufman JA, Amin MR, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol Head Neck Surg. 2000;123(4):385–388. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63(6):871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett CM, Patel D, Vaezi MF. Laryngopharyngeal Reflux and Atypical Gastroesophageal Reflux Disease. Gastrointest Endosc Clin N Am. 2020;30(2):361–376. [DOI] [PubMed] [Google Scholar]
- 7.Francis DO, Rymer JA, Slaughter JC, et al. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol. 2013;108(6):905–911. [DOI] [PubMed] [Google Scholar]
- 8.Lenderking WR, Hillson E, Crawley JA, Moore D, Berzon R, Pashos CL. The clinical characteristics and impact of laryngopharyngeal reflux disease on health-related quality of life. Value Health. 2003;6(5):560–565. [DOI] [PubMed] [Google Scholar]
- 9.Carrau RL, Khidr A, Crawley JA, Hillson EM, Davis JK, Pashos CL. The impact of laryngopharyngeal reflux on patient-reported quality of life. Laryngoscope. 2004;114(4):670–674. [DOI] [PubMed] [Google Scholar]
- 10.Carrau RL, Khidr A, Gold KF, et al. Validation of a quality-of-life instrument for laryngopharyngeal reflux. Arch Otolaryngol Head Neck Surg. 2005;131(4):315–320. [DOI] [PubMed] [Google Scholar]
- 11.Vaezi MF, Katzka D, Zerbib F. Extraesophageal Symptoms and Diseases Attributed to GERD: Where is the Pendulum Swinging Now? Clin Gastroenterol Hepatol. 2018;16(7):1018–1029. [DOI] [PubMed] [Google Scholar]
- 12.Cherry J, Margulies SI. Contact ulcer of the larynx. Laryngoscope. 1968;78(11):1937–1940. [DOI] [PubMed] [Google Scholar]
- 13.Sereg-Bahar M, Jerin A, Jansa R, Stabuc B, Hocevar-Boltezar I. Pepsin and bile acids in saliva in patients with laryngopharyngeal reflux - a prospective comparative study. Clin Otolaryngol. 2015;40(3):234–239. [DOI] [PubMed] [Google Scholar]
- 14.Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101(4 Pt 2 Suppl 53):1–78. [DOI] [PubMed] [Google Scholar]
- 15.Sharma N, Agrawal A, Freeman J, Vela MF, Castell D. An analysis of persistent symptoms in acid-suppressed patients undergoing impedance-pH monitoring. Clin Gastroenterol Hepatol. 2008;6(5):521–524. [DOI] [PubMed] [Google Scholar]
- 16.Wright RA, Miller SA, Corsello BF. Acid-induced esophagobronchial-cardiac reflexes in humans. Gastroenterology. 1990;99(1):71–73. [DOI] [PubMed] [Google Scholar]
- 17.Gyawali CP, Carlson DA, Chen JW, Patel A, Wong RJ, Yadlapati RH. ACG Clinical Guidelines: Clinical Use of Esophageal Physiologic Testing. Am J Gastroenterol. 2020;115(9):1412–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zerbib F, Bredenoord AJ, Fass R, et al. ESNM/ANMS consensus paper: Diagnosis and management of refractory gastro-esophageal reflux disease. Neurogastroenterol Motil. 2021;33(4):e14075. [DOI] [PubMed] [Google Scholar]
- 19.Lechien JR, De Vos N, Everard A, Saussez S. Laryngopharyngeal reflux: The microbiota theory. Med Hypotheses. 2021;146:110460. [DOI] [PubMed] [Google Scholar]
- 20.Zalvan CH, Hu S, Greenberg B, Geliebter J. A Comparison of Alkaline Water and Mediterranean Diet vs Proton Pump Inhibition for Treatment of Laryngopharyngeal Reflux. JAMA Otolaryngol Head Neck Surg. 2017;143(10):1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechien JR, Huet K, Khalife M, et al. Alkaline, protein, low-fat and low-acid diet in laryngopharyngeal reflux disease: Our experience on 65 patients. Clin Otolaryngol. 2019;44(3):379–384. [DOI] [PubMed] [Google Scholar]
- 22.Lechien JR, Bobin F, Muls V, et al. Patients with acid, high-fat and low-protein diet have higher laryngopharyngeal reflux episodes at the impedance-pH monitoring. Eur Arch Otorhinolaryngol. 2020;277(2):511–520. [DOI] [PubMed] [Google Scholar]
- 23.Koufman JA. Low-acid diet for recalcitrant laryngopharyngeal reflux: therapeutic benefits and their implications. Ann Otol Rhinol Laryngol. 2011;120(5):281–287. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Dehom S, Sanders S, Murry T, Krishna P, Crawley BK. Treating laryngopharyngeal reflux: Evaluation of an anti-reflux program with comparison to medications. Am J Otolaryngol. 2018;39(1):50–55. [DOI] [PubMed] [Google Scholar]
- 25.Halum SL, Postma GN, Johnston C, Belafsky PC, Koufman JA. Patients with isolated laryngopharyngeal reflux are not obese. Laryngoscope. 2005;115(6):1042–1045. [DOI] [PubMed] [Google Scholar]
- 26.Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol. 2005;100(2):283–289. [DOI] [PubMed] [Google Scholar]
- 27.O’Hara J, Stocken DD, Watson GC, et al. Use of proton pump inhibitors to treat persistent throat symptoms: multicentre, double blind, randomised, placebo controlled trial. BMJ. 2021;372:m4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson JA, Stocken DD, Watson GC, et al. Lansoprazole for persistent throat symptoms in secondary care: the TOPPITS RCT. Health Technol Assess. 2021;25(3):1–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karkos PD, Wilson JA. Empiric treatment of laryngopharyngeal reflux with proton pump inhibitors: a systematic review. Laryngoscope. 2006;116(1):144–148. [DOI] [PubMed] [Google Scholar]
- 30.Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2006;101(11):2646–2654. [DOI] [PubMed] [Google Scholar]
- 31.Guo H, Ma H, Wang J. Proton Pump Inhibitor Therapy for the Treatment of Laryngopharyngeal Reflux: A Meta-Analysis of Randomized Controlled Trials. J Clin Gastroenterol. 2016;50(4):295–300. [DOI] [PubMed] [Google Scholar]
- 32.Spantideas N, Drosou E, Bougea A, AlAbdulwahed R. Proton Pump Inhibitors for the Treatment of Laryngopharyngeal Reflux. A Systematic Review. J Voice. 2020;34(6):918–929. [DOI] [PubMed] [Google Scholar]
- 33.Lechien JR, Saussez S, Schindler A, et al. Clinical outcomes of laryngopharyngeal reflux treatment: A systematic review and meta-analysis. Laryngoscope. 2019;129(5):1174–1187. [DOI] [PubMed] [Google Scholar]
- 34.Cosway B, Wilson JA, O’Hara J. The acid test: Proton pump inhibitors in persistent throat symptoms: A systematic review of systematic reviews. Clin Otolaryngol. 2021. [DOI] [PubMed] [Google Scholar]
- 35.Yadlapati R, Kaizer AM, Sikavi DR, et al. Distinct Clinical Physiologic Phenotypes of Patients With Laryngeal Symptoms Referred for Reflux Evaluation. Clin Gastroenterol Hepatol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tutuian R, Vela MF, Hill EG, Mainie I, Agrawal A, Castell DO. Characteristics of symptomatic reflux episodes on Acid suppressive therapy. Am J Gastroenterol. 2008;103(5):1090–1096. [DOI] [PubMed] [Google Scholar]
- 37.Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut. 2006;55(10):1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanson DG, Kamel PL, Kahrilas PJ. Outcomes of antireflux therapy for the treatment of chronic laryngitis. Ann Otol Rhinol Laryngol. 1995;104(7):550–555. [DOI] [PubMed] [Google Scholar]
- 39.Reimer C, Lodrup AB, Smith G, Wilkinson J, Bytzer P. Randomised clinical trial: alginate (Gaviscon Advance) vs. placebo as add-on therapy in reflux patients with inadequate response to a once daily proton pump inhibitor. Aliment Pharmacol Ther. 2016;43(8):899–909. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson J, Wade A, Thomas SJ, Jenner B, Hodgkinson V, Coyle C. Randomized clinical trial: a double-blind, placebo-controlled study to assess the clinical efficacy and safety of alginate-antacid (Gaviscon Double Action) chewable tablets in patients with gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol. 2019;31(1):86–93. [DOI] [PubMed] [Google Scholar]
- 41.McGlashan JA, Johnstone LM, Sykes J, Strugala V, Dettmar PW. The value of a liquid alginate suspension (Gaviscon Advance) in the management of laryngopharyngeal reflux. Eur Arch Otorhinolaryngol. 2009;266(2):243–251. [DOI] [PubMed] [Google Scholar]
- 42.Tseng WH, Tseng PH, Wu JF, et al. Double-blind, placebo-controlled study with alginate suspension for laryngopharyngeal reflux disease. Laryngoscope. 2018;128(10):2252–2260. [DOI] [PubMed] [Google Scholar]
- 43.Wilkie MD, Fraser HM, Raja H. Gaviscon(R) Advance alone versus co-prescription of Gaviscon(R) Advance and proton pump inhibitors in the treatment of laryngopharyngeal reflux. Eur Arch Otorhinolaryngol. 2018;275(10):2515–2521. [DOI] [PubMed] [Google Scholar]
- 44.Lee YC, Jung AR, Kwon OE, Kang JW, Huh JH, Eun YG. The effect of baclofen combined with a proton pump inhibitor in patients with refractory laryngopharyngeal reflux: A prospective, open-label study in thirty-two patients. Clin Otolaryngol. 2019;44(3):431–434. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Shi S, Chen F, Lin J. The effects of baclofen for the treatment of gastroesophageal reflux disease: a meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2014;2014:307805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu XH, Yang ZM, Chen Q, et al. Therapeutic efficacy of baclofen in refractory gastroesophageal reflux-induced chronic cough. World J Gastroenterol. 2013;19(27):4386–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babaei A, Venu M, Naini SR, et al. Impaired upper esophageal sphincter reflexes in patients with supraesophageal reflux disease. Gastroenterology. 2015;149(6):1381–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaker R, Babaei A, Naini SR. Prevention of esophagopharyngeal reflux by augmenting the upper esophageal sphincter pressure barrier. Laryngoscope. 2014;124(10):2268–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slivers SL VM, Nimish B, et al. Prospective study of upper esophageal sphincter assist device for treating extraesophageal reflux. Otolaryngol Open J. 2016;2(1):8. [Google Scholar]
- 50.Yadlapati R, Craft J, Adkins CJ, Pandolfino JE. The Upper Esophageal Sphincter Assist Device Is Associated With Symptom Response in Reflux-Associated Laryngeal Symptoms. Clin Gastroenterol Hepatol. 2018;16(10):1670–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yadlapati R, Pandolfino JE, Greytak M, et al. Upper Esophageal Sphincter Compression Device as an Adjunct to Proton Pump Inhibition for Laryngopharyngeal Reflux. Dig Dis Sci. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Francis DO, Goutte M, Slaughter JC, et al. Traditional reflux parameters and not impedance monitoring predict outcome after fundoplication in extraesophageal reflux. Laryngoscope. 2011;121(9):1902–1909. [DOI] [PubMed] [Google Scholar]
- 53.Sidwa F, Moore AL, Alligood E, Fisichella PM. Surgical Treatment of Extraesophageal Manifestations of Gastroesophageal Reflux Disease. World J Surg. 2017;41(10):2566–2571. [DOI] [PubMed] [Google Scholar]
- 54.Lechien JR, Dapri G, Dequanter D, et al. Surgical Treatment for Laryngopharyngeal Reflux Disease: A Systematic Review. JAMA Otolaryngol Head Neck Surg. 2019;145(7):655–666. [DOI] [PubMed] [Google Scholar]
- 55.Ward MA, Ebrahim A, Kopita J, et al. Magnetic sphincter augmentation is an effective treatment for atypical symptoms caused by gastroesophageal reflux disease. Surg Endosc. 2020;34(11):4909–4915. [DOI] [PubMed] [Google Scholar]
- 56.Trad KS, Turgeon DG, Deljkich E. Long-term outcomes after transoral incisionless fundoplication in patients with GERD and LPR symptoms. Surg Endosc. 2012;26(3):650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel DA, Blanco M, Vaezi MF. Laryngopharyngeal Reflux and Functional Laryngeal Disorder: Perspective and Common Practice of the General Gastroenterologist. Gastroenterol Hepatol (N Y). 2018;14(9):512–520. [PMC free article] [PubMed] [Google Scholar]
- 58.Hull JH, Backer V, Gibson PG, Fowler SJ. Laryngeal Dysfunction: Assessment and Management for the Clinician. Am J Respir Crit Care Med. 2016;194(9):1062–1072. [DOI] [PubMed] [Google Scholar]
- 59.Roman S, Keefer L, Imam H, et al. Majority of symptoms in esophageal reflux PPI non-responders are not related to reflux. Neurogastroenterol Motil. 2015;27(11):1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kahrilas PJ, Keefer L, Pandolfino JE. Patients with refractory reflux symptoms: What do they have and how should they be managed? Neurogastroenterol Motil. 2015;27(9):1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583–1589. [DOI] [PubMed] [Google Scholar]
- 62.Gibson P, Wang G, McGarvey L, et al. Treatment of Unexplained Chronic Cough: CHEST Guideline and Expert Panel Report. Chest. 2016;149(1):27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halum SL, Sycamore DL, McRae BR. A new treatment option for laryngeal sensory neuropathy. Laryngoscope. 2009;119(9):1844–1847. [DOI] [PubMed] [Google Scholar]
- 64.Jeyakumar A, Brickman TM, Haben M. Effectiveness of amitriptyline versus cough suppressants in the treatment of chronic cough resulting from postviral vagal neuropathy. Laryngoscope. 2006;116(12):2108–2112. [DOI] [PubMed] [Google Scholar]
- 65.Riehl ME, Chen JW. The Proton Pump Inhibitor Nonresponder: a Behavioral Approach to Improvement and Wellness. Curr Gastroenterol Rep. 2018;20(7):34. [DOI] [PubMed] [Google Scholar]
- 66.Abdulqawi R, Dockry R, Holt K, et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2015;385(9974):1198–1205. [DOI] [PubMed] [Google Scholar]
- 67.Smith JA, Kitt MM, Morice AH, et al. Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir Med. 2020;8(8):775–785. [DOI] [PubMed] [Google Scholar]
- 68.Murry T, Tabaee A, Owczarzak V, Aviv JE. Respiratory retraining therapy and management of laryngopharyngeal reflux in the treatment of patients with cough and paradoxical vocal fold movement disorder. Ann Otol Rhinol Laryngol. 2006;115(10):754–758. [DOI] [PubMed] [Google Scholar]
- 69.Vertigan AE, Theodoros DG, Gibson PG, Winkworth AL. Efficacy of speech pathology management for chronic cough: a randomised placebo controlled trial of treatment efficacy. Thorax. 2006;61(12):1065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kiebles JL, Kwiatek MA, Pandolfino JE, Kahrilas PJ, Keefer L. Do patients with globus sensation respond to hypnotically assisted relaxation therapy? A case series report. Dis Esophagus. 2010;23(7):545–553. [DOI] [PubMed] [Google Scholar]
- 71.Riehl ME, Pandolfino JE, Palsson OS, Keefer L. Feasibility and acceptability of esophageal-directed hypnotherapy for functional heartburn. Dis Esophagus. 2016;29(5):490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riehl ME, Keefer L. Hypnotherapy for Esophageal Disorders. Am J Clin Hypn. 2015;58(1):22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vasant DH, Whorwell PJ. Gut-focused hypnotherapy for Functional Gastrointestinal Disorders: Evidence-base, practical aspects, and the Manchester Protocol. Neurogastroenterol Motil. 2019;31(8):e13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Riehl ME, Kinsinger S, Kahrilas PJ, Pandolfino JE, Keefer L. Role of a health psychologist in the management of functional esophageal complaints. Dis Esophagus. 2015;28(5):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]


