Abstract
The pandemic of COVID-19 has caused >5 million deaths in the world. One of the leading causes of the severe form of COVID-19 is the production of massive amounts of proinflammatory cytokines. Epigenetic mechanisms, such as histone/DNA methylation, miRNA, and long noncoding RNA, are known to play important roles in the regulation of inflammation. In this study, we investigated if hospitalized COVID-19 patients exhibit alterations in epigenetic pathways in their PBMCs. We also compared gene expression profiles between healthy controls and COVID-19 patients. Despite individual variations, the expressions of many inflammation-related genes, such as arginase 1 and IL-1 receptor 2, were significantly upregulated in COVID-19 patients. We also found the expressions of coagulation-related genes Von Willebrand factor and protein S were altered in COVID-19 patients. The expression patterns of some genes, such as IL-1 receptor 2, correlated with their histone methylation marks. Pathway analysis indicated that most of those dysregulated genes were in the TGF-β, IL-1b, IL-6, and IL-17 pathways. A targeting pathway revealed that the majority of those altered genes were targets of dexamethasone, which is an approved drug for COVID-19 treatment. We also found that the expression of bone marrow kinase on chromosome X, a member of TEC family kinases, was increased in the PBMCs of COVID-19 patients. Interestingly, some inhibitors of TEC family kinases have been used to treat COVID-19. Overall, this study provides important information toward identifying potential biomarkers and therapeutic targets for COVID-19 disease.
Key Points
Expressions of many inflammation-related genes are altered in COVID-19 patients.
Some of these changes correlate with their histone methylation marks.
Introduction
The pandemic of COVID-19 disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in >250 million infected cases and >5 million deaths in the world. In the United States, >40 million people have been infected with the virus. Although most infected people are asymptomatic or with mild symptoms, ∼5–10% of the individuals develop a severe form of the disease with clinical features such as pneumonia, acute respiratory distress syndrome, cytokine storm, and multiorgan failure, which could lead to death (1). One of the major underlying causes of death in COVID-19 patients is the induction of cytokine storm, during which massive amounts of proinflammatory cytokines are produced, causing tissue damage and organ failure. The proinflammatory cytokines induced include IL-6, IL-8, and TNF-α (2, 3). It is believed that SARS-CoV-2 infection activates various immune cells, including macrophages, NK cells, T cells, and dendritic cells. As a result, multiple inflammatory pathways, such as the JAK/STAT signaling, TNF-α pathway, and TLR pathway, are stimulated, leading to the release of proinflammatory cytokines and cytokine storm (4, 5). Although drugs such as dexamethasone, a corticosteroid, have been shown to reduce mortality in patients with the severe form of COVID-19 (6, 7), treating COVID-19 patients with immunosuppressive drugs has a limited effect due to the inability to control the cytokine storm once initiated.
We and the others have shown that epigenetic mechanisms such as histone/DNA methylation, miRNA, and long noncoding RNA play important roles in the regulation of cytokine production and inflammation (8, 9). Dysregulation of miRNA in PBMCs of sepsis patients who also develop cytokine storm correlates with clinical manifestations and inflammation (10). In posttraumatic stress disorder patients, we have shown that the expression of proinflammatory cytokines is associated with altered histone/DNA modification and noncoding RNA expression (miRNA and long noncoding RNA) (11, 12). In animal models of inflammatory disease, we have shown that epigenetic changes in Th cells associate with disease development (9, 13). More importantly, treatment with anti-inflammatory agents such as Δ9-tetrahydrocannabinol and cannabidiol reduces the symptoms and partially reverses these epigenetic changes, highlighting the important role of epigenetic modification in immune response (9, 13, 14).
PBMCs, which include primarily T cells, B cells, and monocytes, are key players in the peripheral immune system that produce proinflammatory and anti-inflammatory cytokines and chemokines during an infection. Thus, genetic and epigenetic studies using these cells provide useful clues on the ongoing systemic inflammation. In the current study, we used next-generation sequencing–based approaches to examine genome-wide histone methylation and gene expression profiles in the PBMCs of COVID-19 patients. We focused on two histone marks, H3K4me3 and H3K27me3. These two marks are relatively well studied. The presence of H3K4me3 in the promoter region is associated with transcriptional activation, whereas H3K27me3 is associated with transcriptional repression (15, 16). In immune cells, these two marks are often present in the same genomic locations, allowing the cell to quickly turn the transcription on and off in response to the environmental stimuli (16). In our previous studies, we have found that these two histone marks correlate with the expressions of some immune regulatory genes in human PBMCs (17). In the current study, we also examined global gene expression in PBMCs using RNA sequencing (RNA-seq). Despite individual variations, the expressions of many genes were altered in COVID-19 patients. To assess the expression of genes of interest in a larger cohort, the RNA-seq data from a recent multiomics data set (18) were also analyzed, and the findings are reported. Among the upregulated genes in COVID-19 patients, the majority were associated with the regulation of inflammation.
Materials and Methods
Patient samples
Blood samples were provided by Richland Hospital of Prisma Health in Columbia, SC. The COVID-19 patients were those infected and admitted to the hospital, whereas the controls were healthy volunteer donors. All controls and patients were between 25 and 78 y old and gave written consent. The study was approved by the institutional review board of Prisma Health and the University of South Carolina. In this study, we included six healthy controls and six COVID-19 patients. The healthy controls included four Caucasians and two Asians, four males and two females, with an average age of 32.5 y. The COVID-19 patients included five males and one female with the average age of 63 y. The COVID-19 patients were African Americans. All COVID-19 patients had severe symptoms and were admitted to intensive care unit (ICU). The blood samples were taken between 7 and 14 d after they were admitted to the hospital (see Supplemental File 1 for patient information and WBC differential).
PBMC isolation
Peripheral blood samples were drawn into EDTA-coated tubes. Samples were processed immediately using Ficoll-Paque Plus (GE Healthcare) according to the provided protocol. The average amount of PBMC in controls was 1.7 × 106/ml, and in COVID-19 patients, it was 4.7 × 106/ml. Isolated PBMCs were resuspended in PBS. Total RNA, genomic DNA, and protein were isolated using the AllPrep DNA/RNA/Protein Kit (Qiagen, Germantown, MD).
Chromatin immunoprecipitation sequencing and RNA-seq
Chromatin immunoprecipitation sequencing (ChIP-seq) was performed as described previously (9). Briefly, histone and DNA were cross-linked by formaldehyde, and chromatins were fragmented by sonication using Bioruptor (Diagenode, Denville, NJ). The H3K4me3 and H3K27me3 Abs for ChIP were purchased from Abcam (Cambridge, MA). After the immunoprecipitated chromatins were reverse cross-linked and purified, sequencing libraries were prepared using the Illumina DNA sample preparation kit (Illumina, San Diego, CA). For RNA-seq, libraries were prepared with the NEBNext Ultra RNA Library Prep Kit (New England Biolabs, Ipswich, MA). Each sample had its unique index, and pooled samples were sequenced by Illumina NextSeq 550. In ChIP-seq, we tested five controls and five COVID-19 samples, and in RNA-seq, there were six samples in each group.
Real-time PCR and Western blotting
RNA was reversely transcribed into cDNA using random primers and SuperScript II reverse transcriptase (Invitrogen, Waltham, MA) according to the manufacturer’s instructions. The relative abundance of gene expression was determined by real-time PCR using 18S rRNA as the internal reference. For miRNA quantification, cDNA was prepared using the miScript II RT Kit, and PCR was performed using the miScript Primer Assay Kit (both from Qiagen). Snod96 was used as the internal reference for miRNA. The average amount in the control samples was set as 1. The error bars in quantitative PCR results were SEM. Proteins isolated by the Qiagen DNA/RNA/Protein Kit were used for Western blotting. Anti–bone marrow kinase on chromosome X (BMX) Ab was purchased from Abcam, and anti–β-actin and anti-GADPH Abs were from Cell Signaling Technology (Danvers, MA).
Data analysis of University of South Carolina samples
For ChIP-seq, sequencing reads were mapped to human genome build hg19 using Bowtie software (19). SICER was used for the peak calling (20, 21). The peaks in WIG file format were visualized in the IGB genome browser (https://www.bioviz.org). Differentially associated histone marks were analyzed by Partek package (https://www.partek.com). Fold change >2 and p values <0.05 were considered as significant. For RNA-seq data, reads were mapped with TopHat2 (22). Differentially expressed genes were determined by DESeq2 (23). An adjusted p value <0.05 was considered as significant. Functional enrichment analysis of significantly altered genes was performed using g:Profiler (biit.cs.ut.ee/gprofiler). Ingenuity Pathway Analysis (Qiagen) was used to identify the canonical pathways and top networks in which the significantly altered genes are involved.
Meta-analysis of RNA-seq data from a multiomic data set
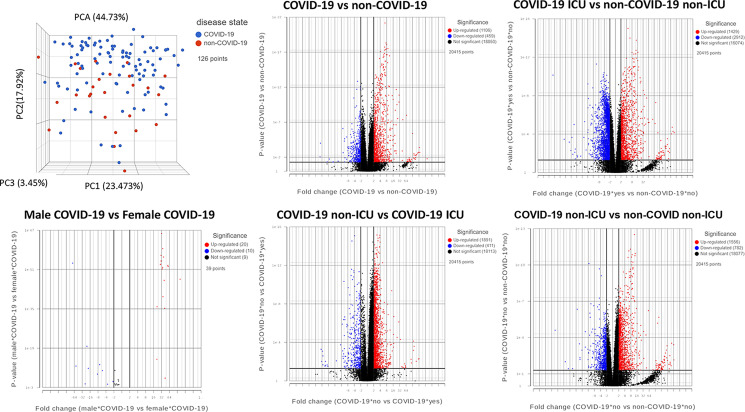
Human RNA-seq data were downloaded from the Gene Expression Omnibus (series GSE157103) (18) and analyzed via Partek. This included 126 plasma and leukocyte samples (100 COVID-19 patients and 26 non–COVID-19 patients) with differing degrees of disease severity. These data, along with lipidomic, proteomic, and metabolomic data, are publicly available and curated in a user-friendly database (https://covid-omics.app:8080/) (18). The raw data and all supporting information were uploaded into Partek, prealignment quality control was performed, bases were trimmed with default settings, STAR-2.7.8a aligned, and reads were quantified to the hg19 reference using the Partek E/M annotation model. Gene counts were filtered to exclude maximum features ≤1.0 and normalized with recommended settings (cpm add: 1E-4), resulting in 126 samples with 20,415 features. Principal component analysis (PCA), differential analysis using the Partek gene-specific analysis (GSA) algorithm, and descriptive statistics were performed. GSA results were filtered using the false discovery rate (FDR) ≤0.05 and are reported in gene lists and volcano plots.
Results
Genome-wide histone H3K4me3 and H3K27me3 methylation in PBMCs
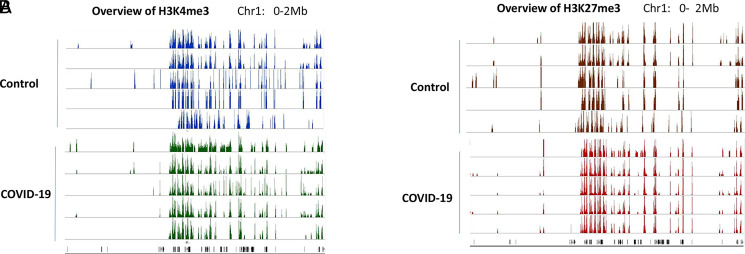
Because H3K4me3 and H3K27me3 are two well-studied histone marks that regulate gene expression, we first investigated whether the overall histone methylation status was altered in PBMCs from COVID-19 patients. To that end, ChIP-seq was performed in samples collected from five healthy controls and five COVID-19 patients. Interestingly, the histone methylation patterns in each individual were very similar. There was no overall difference in these two histone marks in PBMCs between controls and COVID-19 patients. In addition, H3K4me3 and H3K27me3 that seem to be associated with opposite functions in regulating gene expression coexisted in the same regions (Fig. 1). Such so-called “bivalent domains,” in which the DNA segments have both repressive and activating histone modifications, allow the genes to be turned on and off rapidly depending on environmental signals. This result was consistent with our previous ChIP-seq data from immune cells in inflammatory disease models (9).
FIGURE 1.
Histone methylation profile in PBMCs. H3K4me3 (A) and H3K27me3 (B) in PBMCs from five controls and five COVID-19 patients were examined by ChIP-seq. The methylation marks in the region of Chr:1 0–2 Mb were visualized in IGB genome browser and presented as examples of global histone methylation profile.
Histone marks in individual genes
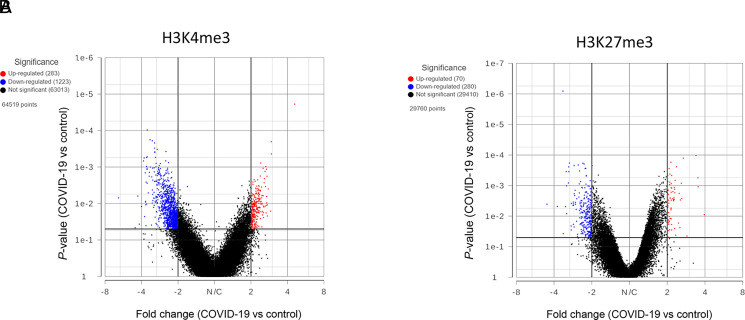
Although there was no significant difference in overall global histone methylation status in PBMCs between the controls and COVID-19 patients, the signal intensity in individual genes might differ, as shown by us previously in other models (9, 17). By comparing the levels of H3K4me3 and H3K27me3 within the 5 kb upstream and downstream of the transcription start site (TSS), we identified genes that have significant differences in histone mark intensity between the controls and COVID-19 patient samples (Fig. 2, Supplemental File 2). Overall, there were more genes with altered H3K4me3 near the TSS. However, those differences may or may not lead to altered gene expressions, because gene expression is regulated by multiple mechanisms.
FIGURE 2.
Genes with altered histone marks. H3K4me3 (A) and H3K27me3 (B) signals within 5 kb upstream and downstream of TSS in PBMCs of five controls and five COVID-19 patients were quantified by Partek software using normalized sequencing counts. Red dots represent the genes with significantly increased histone mark in the COVID-19 samples, whereas blue dots are genes with reduced mark.
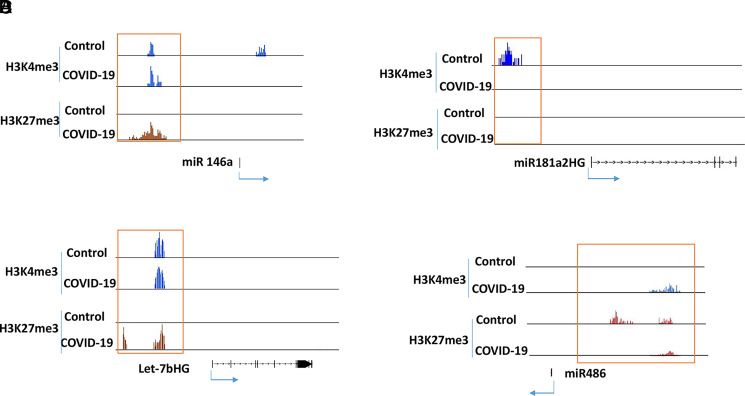
It has been reported that there are changes in the expression of some miRNAs in PBMCs of COVID-19 patients (24); therefore, we tested if these genes were associated with histone modifications. For example, it has been shown that miR-146a is consistently downregulated in COVID-19 patients, whereas miR181a-2 is only downregulated in severe COVID-19 cases (25, 26). In our ChIP-seq data, miR-146a had increased suppressive H3K27me3 in the patient samples, whereas the miR181a-2 host gene had a more active H3K4me3 mark in the controls (Fig. 3A, 3B). A recent study showed that let-7b targets the TLR4/NF-κB pathway and overexpressing let-7b suppresses the expression of IL-6, IL-8, and TNF-α and improves survival in a murine sepsis model (27). These proinflammatory cytokines are increased in patients with COVID-19 (28, 29), and the let-7b host gene in patient samples had a suppressive H3K27me3 mark (Fig. 3C), suggesting a decreased expression. In contrast, miR-486 was increased in the PBMCs of COVID-19 patients (25), and it was associated with H3K4me3 (Fig. 3D). These results suggested that the expression of some miRNAs correlated with these two histone marks.
FIGURE 3.
Histone methylation in selected miRNAs. ChIP-seq results from five controls and five COVID-19 patients were combined into two groups (control and COVID-19) for analysis. Histone marks in miR-146a (A), miR181a2HG (B), Let-7bHG (C) and miR-486 (D) were visualized in IGB genome browser.
Gene expression profile in PBMCs
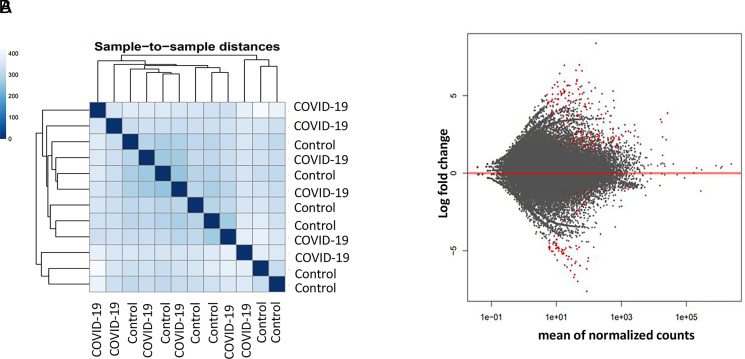
To determine how SARS-CoV-2 infection affects gene expression in PBMCs, RNA-seq was performed in samples from six controls and six COVID-19 patients. Based on the overall gene expression prolife, controls and patients did not form clusters as shown in sample-to-sample distances, suggesting the overall gene expression pattern did not differ drastically (Fig. 4A). Nonetheless, there were ∼160 genes that were significantly increased in COVID-19 patients, whereas ∼60 genes were downregulated (Fig. 4B, Supplemental File 2).
FIGURE 4.
Gene expression profile in PBMCs of control and COVID-19 patients. Gene expressions in six controls and six COVID-19 patients were determined by RNA-seq. The results were analyzed by DESeq2 software. (A) Sample-to-sample distance was determined by comparing overall gene expression profile. (B) Red dots are genes with significant difference in expression level between in the control and patient group. Adjusted p value <0.05 was considered significant.
Differentially expressed genes in PBMCs of COVID-19 patients
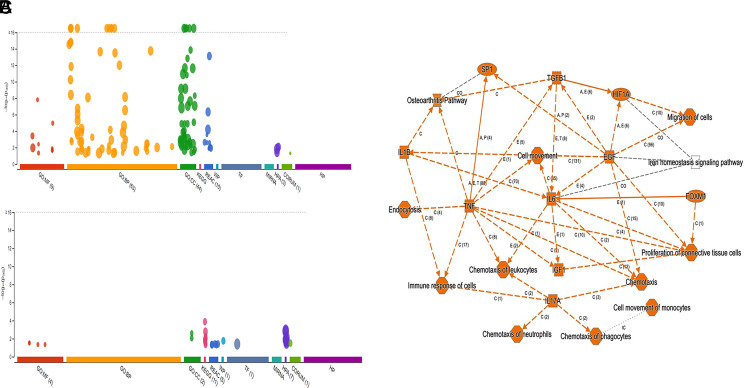
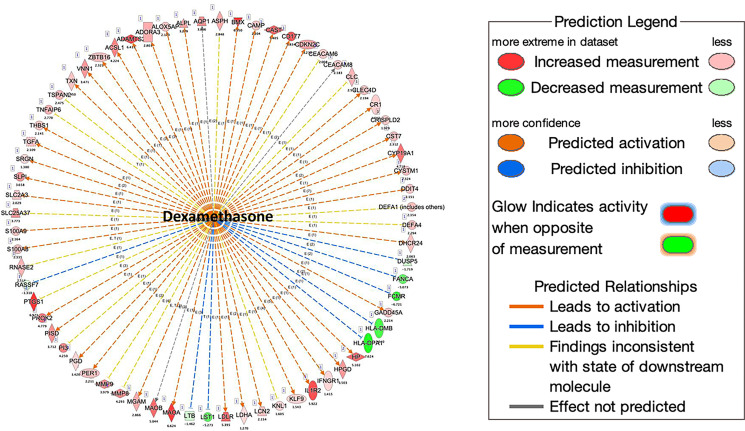
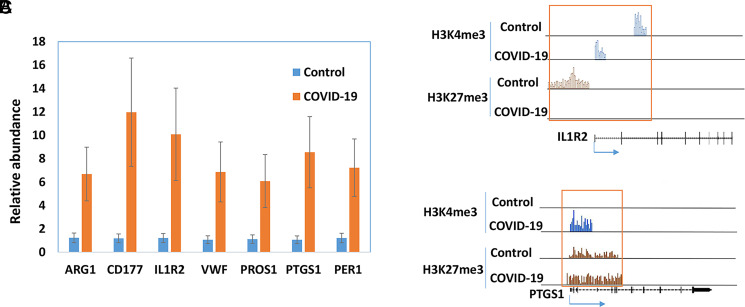
Pathway analysis by G-profiler revealed that the majority of the increased genes were enriched in Gene Ontology: Biological Processes, whereas none of the downregulated genes were in Gene Ontology: Biological Processes (Fig. 5A, 5B). Further analysis showed that most upregulated genes in COVID-19 patients were related to immune response, including the TGF-β, IL-1b, IL-6, and IL-17 pathway (Fig. 5C). We also used Ingenuity Pathway Analysis to examine whether these altered genes were enriched in some drug-targeting pathways. The dexamethasone-targeting pathway was the most enriched one (62 upregulated and 6 downregulated genes; see Supplemental File 2 for the list of the genes) (Fig. 6). Dexamethasone is an anti-inflammatory drug and has been used in hospitalized patients with COVID-19 (6, 7). Among those upregulated genes, some were well-known proinflammatory or anti-inflammatory genes. For example, arginase 1 (ARG1) is highly expressed in monocytes and has an anti-inflammatory function (30). CD177 is expressed in neutrophils and upregulated during inflammation (31). IL-1 receptor 2 (IL-1R2) is an anti-inflammatory gene but often overexpressed during inflammation (32). PG-endoperoxide synthase 1 is a target of nonsteroidal anti-inflammatory drugs (33). There were other interesting genes that were upregulated in COVID-19 patients. Period 1 (PER1) is a circadian gene that has been reported to be dysregulated during inflammatory processes (34). Von Willebrand factor (VWF), a blood-clotting protein, was upregulated in COVID-19 patients (35). Protein S (PROS1), a cofactor that regulates blood clotting, was also upregulated in patients (36). The expressions of these selected genes were further validated by real-time RT-PCR (Fig. 7A). We also examined H3K4me3 and H3K27me3 marks of these genes. Although the expression of these genes might be regulated by other mechanisms, the histone marks in some genes were consistent with their expression patterns (Fig. 7B, 7C).
FIGURE 5.
Functional enrichment analysis of significantly altered genes in COVID-19 patients. Enrichment analysis of upregulated genes (A) and downregulated genes (B) in PBMCs of a COVID-19 patient was performed using g:GOSt functional profiling in g:Profiler. Pathway enrichment of both upregulated and downregulated genes was performed using Qiagen Ingenuity Pathway Analysis (C).
FIGURE 6.
Downstream analysis of altered genes in COVID-19 patients. The downstream analysis was performed using Qiagen Ingenuity Pathway Analysis. More than one third of upregulated genes are downstream targets of dexamethasone.
FIGURE 7.
Expressions of selected genes in PBMCs of COVID-19 patients. The expressions of selected genes were quantified by real-time RT-PCR in PBMCs of healthy controls and patients with COVID-19 (n = 5). (A) The average amount in the controls was set as 1, and the error bars show SEM. The histone marks in IL-1R2 (B) and PG-endoperoxide synthase 1 (PTGS1) (C) were obtained from ChIP-seq data.
Increased expression of BMX kinase
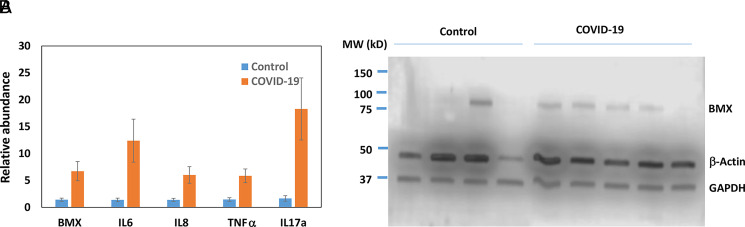
One of the most significantly increased genes identified by RNA-seq in the PBMCs of COVID-19 patients was BMX kinase (Supplemental File 2). BMX belongs to a group of nonreceptor tyrosine kinases called the TEC family. Interestingly, the BMX gene is located next to the ACE2 gene, which is the receptor for SARS-CoV-2. The expression of BMX was further validated by real-time PCR (Fig. 8A). It is known that BMX exerts its proinflammatory function, in part, by inducing the expression of IL-6 and IL-8 cytokines. Indeed, their expressions were increased in patient samples (Fig. 8A). TNF-α, another downstream of BMX, was also increased (Fig. 8A). IL-17 is a known proinflammatory cytokine that was also increased (Fig. 8A). Somehow these cytokines were not identified as significantly increased genes in RNA-seq data. However, real-time PCR results indicated that their expressions were increased in the patients (Fig. 8A). We further examined the expression of BMX at the protein level. In four control samples, only one had a high level of BMX. In contrast, four out of five patient samples had elevated protein levels of BMX (Fig. 8B).
FIGURE 8.
Expression of BMX in PBMCs. (A) The expression of BMX and its downstream genes in the control and COVID-19 samples (n = 5) was quantified by real-time RT-PCR. The average amount in the control was set as 1, and the error bars represent SEM. (B) The protein levels of BMX in PBMCs were determined by Western blotting.
Meta-analysis of COVID-19 and non–COVID-19 patients
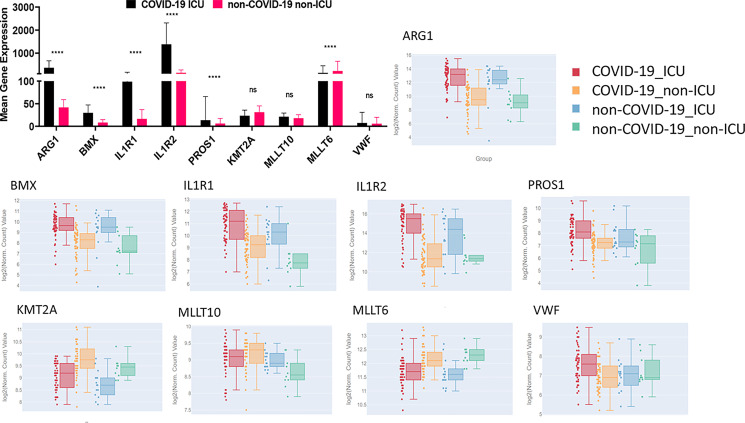
Due to the small sample size and demographic difference in this study, we investigated to determine whether those significantly altered genes were also identified in other studies. A recent multiomics cohort (18) was analyzed for RNA-seq gene expression changes between specific groups in human plasma and leukocyte samples. The PCA shows dimensional reduction between disease state of the 126 samples as COVID-19–positive (COVID-19) or COVID-19–negative (non–COVID-19). Differential gene expressions between specific comparisons are reported as volcano plots showing FDR significance ≤0.05 (Fig. 9). These genes are reported in Supplemental File 3. Sex differences (male and female) are also reported between 39 individuals positive for COVID-19. Expression of select genes in patients positive (COVID-19) or negative for COVID-19 (non–COVID-19) and among the COVID-19 positives who were admitted to the ICU or not (non-ICU) are depicted as reported in the database provided by the authors (https://covid-omics.app:8080/) (18). Furthermore, the expression of select genes of interest was plotted for the comparison between COVID-19 ICU versus non–COVID-19 non-ICU to resemble the current study. Interestingly, the majority of the changes seen, such as increased expression of ARG1, BMX, IL1R2, and PROS1, were significantly increased in COVID-19 ICU patients when compared with non–COVID-19 non-ICU (Fig. 10), which was similar to the data found in the current study (Figs. 7 and 8). However, the data set did not indicate significant alterations in VWF and histone modification enzymes KMT2A and MLLT10, whereas we found their expressions were altered in COVID-19 patients (Supplemental File 2).
FIGURE 9.
Meta-analysis of COVID-19 and non–COVID-19 patients. PCA of patient samples grouped by disease, COVID-19 (blue) and non–COVID-19 (red). Groups include COVID-19–positive (COVID-19), COVID-19–negative (non–COVID-19), males positive for COVID-19 (Male COVID-19), females positive for COVID-19 (Female COVID-19), COVID-19 patients admitted to the ICU (COVID-19 ICU), COVID-19 patients not admitted to the ICU (COVID-19 non-ICU), non–COVID-19 patients admitted to the ICU (non–COVID-19 ICU), and non–COVID-19 patients not admitted to the ICU (non–COVID-19 non-ICU). Volcano plots of differential gene expression analysis filtered by significance of FDR ≤0.05 for the comparisons shown, where upregulated genes are shown in red, downregulated genes in blue, and not significant genes in black.
FIGURE 10.
Expression of select genes in plasma and leukocytes of COVID-19 patients. Mean gene expression of specific genes of interest in COVID-19 ICU patients (black) and non–COVID-19 non-ICU patients (pink) (top left). Significance represents p values calculated by differential gene analysis (GSA). The Web site–based tool provided [Overmyer et al. (18)] was used to generate the figures representing gene expression trends across all the groups, where COVID-19 ICU is red, COVID-19 non-ICU is yellow, non–COVID-19 ICU is blue, and non–COVID-19 non-ICU is green. Error bars represent SEM.
Discussion
Excessive inflammation is the major cause of the severe form of COVID-19. During inflammation, immune cells are activated and gene expression is increased. Although gene expression is usually correlated with histone marks, the overall levels of H3K4me3 and H3K27me3 do not differ significantly. This result is consistent with our previous results from mouse models of inflammatory disease (9). The result is not unexpected because there are many histone modifications. The gene expression can be regulated by other histone marks. In addition to histone modification, DNA methylation also plays an important role in gene expression. Interestingly, in our RNA-seq data, KMT2A, MLLT6, and MLLT10 were found to be downregulated (Supplemental File 2). KMTA2A (histone-lysine N-methyltransferase 2A), also known as MLL1 (myeloid/lymphoid or mixed-lineage leukemia 1), is an enzyme that can cause H3K4me3 methylation. It can also lead to mono- and dimethylation of H3K4 (37). MLLT6, a PHD finger-containing protein, and MLLT10 (histone lysine methyltransferase DOT1L cofactor) are involved in chromosomal rearrangement resulting in various types of leukemia (38, 39). In some acute leukemia, MLLT6 and MLLT10 are fused with the KMT2A gene, leading to the transcription activity of certain genes in T cells (40, 41). However, deletion of KMT2A in mouse only causes decreased H3K4me3 in certain genes (42, 43). Besides KMT2A, there are at least six other family members (KMT2B–G) responsible for H3K4 methylation (43). Therefore, we do not expect that altered expression of KMT2A and MLLT10 in COVID-19 patients will lead to a significant change in the global H3K4me3 level. Nevertheless, genes that have altered histone marks should be further investigated. The histone marks in some genes are consistent with their expressions. In our previous study, miR-146a was found to be one of the most significantly downregulated miRNAs in PBMCs of sepsis patients (10). It is also one of the most downregulated miRNAs in COVID-19 patients (25). This suggests that the downregulation could be due to the increased H3K27me3 in its promoter region. We have demonstrated that miR-146a directly targets IL-6 (10), and IL-6 promotes monocyte proliferation, which is a hallmark for both sepsis and COVID-19. One limitation of this ChIP-seq study is that we only examined two histone methylation marks. Other histone modifications could also have important roles in regulating gene expression in COVID-19 patients. Another pitfall of this study is that these samples were collected at one time point and only reflected the level of histone mark at that time point. It is known that the alteration in histone modification status is a dynamic process. If the samples were collected from the same patient at different time points during the disease development, it would be more informative in terms of how histone modifications regulate gene expression in the PBMCs of COVID-19 patients during the course of the disease.
One of the most significantly upregulated genes in COVID-19 PBMCs is ARG1. It has been reported that the expression of ARG1 is increased in sepsis patients (44, 45). A recent study also shows that ARG1 is upregulated in COVID-19 patients (46). ARG1 is known to play an important role in immune response and is expressed by many types of immune cells, such as monocytes, macrophages, neutrophils, and myeloid-derived suppressor cells. During infection, l-arginine is converted to NO by inducible NO synthase in proinflammatory immune cells, such as M1 macrophages, in an effort to kill the pathogens. l-Arginine can also be converted to ornithine by ARG1 in M2 macrophages to suppress inflammation and repair tissue damage. It has been suggested that an increased level of ARG1 may lead to the depletion of l-arginine in the system, which, in turn, limits the production of NO and anti-infection activity. It has also been suggested that upregulation of ARG1 is associated with elevated viral load (47, 48). Another anti-inflammatory gene that is upregulated in the PBMCs of COVID-19 patients is IL-1R2. The IL-1–mediated signaling pathway is critical to immune response and is tightly regulated. IL-1R1 and IL-1R2 are two main receptors of IL-1. Although IL-1R1 is widely expressed, IL-1R2 is mainly expressed by monocytes, macrophages, and neutrophils. IL-1R1 is responsible for signal transduction and initiates inflammatory response after binding to IL-1. However, IL-1R2 is a decoy receptor, and the binding of IL-1 to IL-1R2 does not trigger signaling (32, 49). As a result, IL-1R2 competes with IL-1R1 for ligand binding and servers as a negative-feedback mechanism. It has been reported that plasma levels of IL-1R2 are increased in infectious diseases, including sepsis and acute respiratory distress syndrome, as well as in other inflammatory diseases, such as multiple sclerosis, rheumatoid arthritis, and inflammatory bowel disease (50–52). Therefore, ARG1 and IL-1R2 can serve as potential biomarkers and therapeutic targets for infectious diseases, including COVID-19.
Our RNA-seq results also show an increased expression of two coagulation-related genes, VWF and PROS1. VWF is a blood-clotting factor synthesized by endothelial cells and platelets. A low level of VWF is associated with bleeding, whereas a high level of VWF may lead to blood clotting (53). It has been reported that COVID-19 patients have high levels of VWF in their blood, which may contribute to blood clots in the lungs and other organs (54, 55). VWF synthesized by endothelial cells is stored in Weibel-Palade bodies and released when the vessel wall is damaged. It is believed that increased VWF in COVID-19 patients is due to virus-induced endothelial cell damage. The results from this study suggest that the increased expression of VWF may also contribute to an elevated VWF level. Interestingly, the expression of PROS1, a vitamin K–dependent anticoagulant, is also increased in COVID-19 patients. There is speculation that PROS1 may be depleted in patients with blood clots because blood clotting consumes soluble coagulation proteins, including PROS1 (56). However, it is unclear whether the level of PROS1 is indeed decreased in the blood of COVID-19 patients. If so, the increased RNA level of PROS1 could be a compensation mechanism. Nevertheless, the result suggests a dysregulation within the coagulation system in patients with COVID-19. Abnormal blood clotting is one of the main symptoms in patients with COVID-19.
BMX is a member of the TEC family, a group of nonreceptor tyrosine kinases including hepatocellular carcinoma (TEC), Bruton’s tyrosine kinase (BTK), and IL-2–inducible T cell kinase (57). This group of kinases is known to be critical in immune response (58). For example, BTK is required for BCR signaling. Mutations in the BTK gene can lead to an absence of B cells in peripheral blood (59). It has been shown that BMX is activated by TLR agonists and is associated with MyD88 and focal adhesion kinase in rheumatoid arthritis synovial fibroblasts (60). Downregulation of BMX inhibits LPS-induced IL-6 expression in synovial fibroblasts, whereas overexpression of BMX increases LPS-induced IL-6 (61, 62). BMX is also required for TNF-α– and IL-1β–induced expression of IL-8 (63). Although in our RNA-seq data, the RNA levels of IL-6 and IL-8 did not differ significantly, real-time PCR showed that their expressions were increased in PBMCs of COVID-19 patients. Interestingly, in recent clinical trials, administration of BTK inhibitors acalabrutinib and ibrutinib to the hospitalized COVID-19 patients resulted in improved oxygenation and decreased the disease severity (64, 65). Further studies indicate that the activity of BTK in macrophages of COVID-19 patients is increased, whereas the total protein level does not differ (64). Acalabrutinib and ibrutinib have been approved to treat chronic lymphocytic leukemia because they inhibit lymphocyte activation (66). It is assumed that reducing lymphocyte activity alleviates the hyperinflammation caused by the viruses. Acalabrutinib and ibrutinib are also potent inhibitors of other TEC family kinases, including BMX. Because the expression of BMX is increased in the PBMCs of COVID-19 patient, it is possible that the effects of these drugs may also be due to the inhibition of BMX.
Our patient sample data have limitations. The sample size is small, and the changes in gene expression could be due to individual difference, which is more pronounced in human studies. To assess our findings on a larger scale, we employed in silico analysis of the data generated by Overmyer et al. (18) using the provided online portal as well as analysis of the raw data. The results showed that when COVID-19 ICU versus non–COVID-19 non-ICU was compared, ARG1, BMX, IL1R1, IL1R2, PROS1, and MLLT6 were significantly altered, and these changes were similar to our patient findings. On the contrary, KMT2A, MLLT10, and VWF were not significantly changed in their studies. This discrepancy could be caused by small sample size and individual variations. Another reason for the discrepancy is that in their study, the COVID-19 patients were compared with other non–COVID-19 patients admitted to the hospital, whereas in our study, the controls were healthy individuals. Interestingly, the authors reported increased expression of platelet-associated proteins, including VWF, in COVID-19 samples compared with non–COVID-19 samples. VWF has been implicated in COVID-19–associated endotheliopathy (67). In addition, the expressions of some known inflammatory cytokines, such as IL-6, IL-8, and IL-17, were not significantly different in RNA-seq data from the current study. However, the differential expression could be detected by real-time PCR, which is more sensitive than RNA-seq. Despite these limitations, the results are consistent with the pathology of COVID-19 disease. Pathway analysis indicates that the altered genes are enriched in pathways known to be involved in COVID-19. Interestingly, more than one third of the upregulated genes were downstream targets of dexamethasone. Dexamethasone is a glucocorticoid that has been widely used to suppress inflammation and cytokine storm. Dexamethasone acts through the binding to the glucocorticoid receptor, which is ubiquitously expressed. The ligand-activated receptor works as a transcription factor to activate or suppress the expression of many genes (68). Glucocorticoids have been used to treat cytokine storm in SARS, Middle East respiratory syndrome, and, recently, COVID-19 (7).
Supplementary Material
This work was supported in part by National Institutes of Health Grants P01AT003961, R01AT006888, and R01MH094755, National Institute of Allergy and Infectious Diseases Grants R01AI123947 and R01AI129788, and National Institute of General Medical Sciences Grant P20GM103641 to M.N. and P.S.N.
The online version of this article contains supplemental material.
- ARG1
- arginase 1
- BMX
- bone marrow kinase on chromosome X
- BTK
- Bruton’s tyrosine kinase
- ChIP-seq
- chromatin immunoprecipitation sequencing
- FDR
- false discovery rate
- GSA
- gene-specific analysis
- IL-1R2
- IL-1 receptor 2
- PCA
- principal component analysis
- PROS1
- protein S
- RNA-seq
- RNA sequencing
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- TSS
- transcription start site
- VWF
- Von Willebrand factor
Disclosures
The authors have no financial conflicts of interest.
References
- 1. Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., et al. 2020. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 130: 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pedersen S. F., Ho Y. C.. 2020. SARS-CoV-2: a storm is raging. J. Clin. Invest. 130: 2202–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choudhary S., Sharma K., Silakari O.. 2021. The interplay between inflammatory pathways and COVID-19: a critical review on pathogenesis and therapeutic options. Microb. Pathog. 150: 104673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan L. Y., Komarasamy T. V., Rmt Balasubramaniam V.. 2021. Hyperinflammatory immune response and COVID-19: a double edged sword. Front. Immunol. 12: 742941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tomazini B. M., Maia I. S., Cavalcanti A. B., Berwanger O., Rosa R. G., Veiga V. C., Avezum A., Lopes R. D., Bueno F. R., Silva M. V. A. O., et al. COALITION COVID-19 Brazil III Investigators . 2020. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 324: 1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Horby P., Lim W. S., Emberson J. R., Mafham M., Bell J. L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., et al. RECOVERY Collaborative Group . 2021. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 384: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang X., Bam M., Becker W., Nagarkatti P. S., Nagarkatti M.. 2020. Long noncoding RNA AW112010 promotes the differentiation of inflammatory T cells by suppressing IL-10 expression through histone demethylation. J. Immunol. 205: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang X., Hegde V. L., Rao R., Zhang J., Nagarkatti P. S., Nagarkatti M.. 2014. Histone modifications are associated with Δ9-tetrahydrocannabinol-mediated alterations in antigen-specific T cell responses. J. Biol. Chem. 289: 18707–18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou J., Chaudhry H., Zhong Y., Ali M. M., Perkins L. A., Owens W. B., Morales J. E., McGuire F. R., Zumbrun E. E., Zhang J., et al. 2015. Dysregulation in microRNA expression in peripheral blood mononuclear cells of sepsis patients is associated with immunopathology. Cytokine 71: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bam M., Yang X., Zhou J., Ginsberg J. P., Leyden Q., Nagarkatti P. S., Nagarkatti M.. 2016. Evidence for epigenetic regulation of pro-inflammatory cytokines, interleukin-12 and interferon gamma, in peripheral blood mononuclear cells from PTSD patients. J. Neuroimmune Pharmacol. 11: 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bam M., Yang X., Zumbrun E. E., Zhong Y., Zhou J., Ginsberg J. P., Leyden Q., Zhang J., Nagarkatti P. S., Nagarkatti M.. 2016. Dysregulated immune system networks in war veterans with PTSD is an outcome of altered miRNA expression and DNA methylation. Sci. Rep. 6: 31209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang X., Bam M., Nagarkatti P. S., Nagarkatti M.. 2019. Cannabidiol regulates gene expression in encephalitogenic T cells using histone methylation and noncoding RNA during experimental autoimmune encephalomyelitis. Sci. Rep. 9: 15780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang X., Bam M., Nagarkatti P. S., Nagarkatti M.. 2016. RNA-seq analysis of δ9-tetrahydrocannabinol-treated T cells reveals altered gene expression profiles that regulate immune response and cell proliferation. J. Biol. Chem. 291: 15460–15472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernstein B. E., Mikkelsen T. S., Xie X., Kamal M., Huebert D. J., Cuff J., Fry B., Meissner A., Wernig M., Plath K., et al. 2006. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125: 315–326. [DOI] [PubMed] [Google Scholar]
- 16. Wei G., Wei L., Zhu J., Zang C., Hu-Li J., Yao Z., Cui K., Kanno Y., Roh T. Y., Watford W. T., et al. 2009. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30: 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bam M., Yang X., Busbee B. P., Aiello A. E., Uddin M., Ginsberg J. P., Galea S., Nagarkatti P. S., Nagarkatti M.. 2020. Increased H3K4me3 methylation and decreased miR-7113-5p expression lead to enhanced Wnt/β-catenin signaling in immune cells from PTSD patients leading to inflammatory phenotype. Mol. Med. 26: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Overmyer K. A., Shishkova E., Miller I. J., Balnis J., Bernstein M. N., Peters-Clarke T. M., Meyer J. G., Quan Q., Muehlbauer L. K., Trujillo E. A., et al. 2021. Large-scale multi-omic analysis of COVID-19 severity. Cell Syst. 12: 23–40.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Langmead B., Trapnell C., Pop M., Salzberg S. L.. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zang C., Schones D. E., Zeng C., Cui K., Zhao K., Peng W.. 2009. A clustering approach for identification of enriched domains from histone modification ChIP-Seq data. Bioinformatics 25: 1952–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blankenberg D., Von Kuster G., Coraor N., Ananda G., Lazarus R., Mangan M., Nekrutenko A., Taylor J.. 2010. Galaxy: a web-based genome analysis tool for experimentalists. Curr. Protoc. Mol. Biol. Chapter 19: Unit 19.10.11–19.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S. L.. 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Love M. I., Huber W., Anders S.. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donyavi T., Bokharaei-Salim F., Baghi H. B., Khanaliha K., Alaei Janat-Makan M., Karimi B., Sadri Nahand J., Mirzaei H., Khatami A., Garshasbi S., et al. 2021. Acute and post-acute phase of COVID-19: analyzing expression patterns of miRNA-29a-3p, 146a-3p, 155-5p, and let-7b-3p in PBMC. Int. Immunopharmacol. 97: 107641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang H., Gao Y., Li Z., Miao Y., Huang Z., Liu X., Xie L., Li H., Wen W., Zheng Y., Su W.. 2020. The noncoding and coding transcriptional landscape of the peripheral immune response in patients with COVID-19. Clin. Transl. Med. 10: e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang S., Amahong K., Sun X., Lian X., Liu J., Sun H., Lou Y., Zhu F., Qiu Y.. 2021. The miRNA: a small but powerful RNA for COVID-19. Brief. Bioinform. 22: 1137–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen B., Han J., Chen S., Xie R., Yang J., Zhou T., Zhang Q., Xia R.. 2021. MicroLet-7b regulates neutrophil function and dampens neutrophilic inflammation by suppressing the canonical TLR4/NF-κB pathway. Front. Immunol. 12: 653344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Del Valle D. M., Kim-Schulze S., Huang H. H., Beckmann N. D., Nirenberg S., Wang B., Lavin Y., Swartz T. H., Madduri D., Stock A., et al. 2020. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 26: 1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leisman D. E., Ronner L., Pinotti R., Taylor M. D., Sinha P., Calfee C. S., Hirayama A. V., Mastroiani F., Turtle C. J., Harhay M. O., et al. 2020. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 8: 1233–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Monticelli L. A., Buck M. D., Flamar A. L., Saenz S. A., Tait Wojno E. D., Yudanin N. A., Osborne L. C., Hepworth M. R., Tran S. V., Rodewald H. R., et al. 2016. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat. Immunol. 17: 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stroncek D. F., Caruccio L., Bettinotti M.. 2004. CD177: a member of the Ly-6 gene superfamily involved with neutrophil proliferation and polycythemia vera. J. Transl. Med. 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peters V. A., Joesting J. J., Freund G. G.. 2013. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav. Immun. 32: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harris S. G., Padilla J., Koumas L., Ray D., Phipps R. P.. 2002. Prostaglandins as modulators of immunity. Trends Immunol. 23: 144–150. [DOI] [PubMed] [Google Scholar]
- 34. Takahashi S., Yokota S., Hara R., Kobayashi T., Akiyama M., Moriya T., Shibata S.. 2001. Physical and inflammatory stressors elevate circadian clock gene mPer1 mRNA levels in the paraventricular nucleus of the mouse. Endocrinology 142: 4910–4917. [DOI] [PubMed] [Google Scholar]
- 35. Kawecki C., Lenting P. J., Denis C. V.. 2017. von Willebrand factor and inflammation. J. Thromb. Haemost. 15: 1285–1294. [DOI] [PubMed] [Google Scholar]
- 36. Rigby A. C., Grant M. A.. 2004. Protein S: a conduit between anticoagulation and inflammation. Crit. Care Med. 32(Suppl): S336–S341. [DOI] [PubMed] [Google Scholar]
- 37. Bochyńska A., Lüscher-Firzlaff J., Lüscher B.. 2018. Modes of interaction of KMT2 histone H3 lysine 4 methyltransferase/COMPASS complexes with chromatin. Cells 7: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaplin T., Bernard O., Beverloo H. B., Saha V., Hagemeijer A., Berger R., Young B. D.. 1995. The t(10;11) translocation in acute myeloid leukemia (M5) consistently fuses the leucine zipper motif of AF10 onto the HRX gene. Blood 86: 2073–2076. [PubMed] [Google Scholar]
- 39. Saha V., Chaplin T., Gregorini A., Ayton P., Young B. D.. 1995. The leukemia-associated-protein (LAP) domain, a cysteine-rich motif, is present in a wide range of proteins, including MLL, AF10, and MLLT6 proteins. Proc. Natl. Acad. Sci. USA 92: 9737–9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meyer C., Kowarz E., Hofmann J., Renneville A., Zuna J., Trka J., Ben Abdelali R., Macintyre E., De Braekeleer E., De Braekeleer M., et al. 2009. New insights to the MLL recombinome of acute leukemias. Leukemia 23: 1490–1499. [DOI] [PubMed] [Google Scholar]
- 41. Chen X., Wang F., Zhang Y., Wang M., Tian W., Teng W., Ma X., Guo L., Fang J., Zhang Y., et al. 2019. Panoramic view of common fusion genes in a large cohort of Chinese de novo acute myeloid leukemia patients. Leuk. Lymphoma 60: 1071–1078. [DOI] [PubMed] [Google Scholar]
- 42. Vallianatos C. N., Raines B., Porter R. S., Bonefas K. M., Wu M. C., Garay P. M., Collette K. M., Seo Y. A., Dou Y., Keegan C. E., et al. 2020. Mutually suppressive roles of KMT2A and KDM5C in behaviour, neuronal structure, and histone H3K4 methylation. [Published erratum appears in 2020 Commun. Biol. 3: 331.] Commun. Biol. 3: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Husmann D., Gozani O.. 2019. Histone lysine methyltransferases in biology and disease. Nat. Struct. Mol. Biol. 26: 880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Washburn M. L., Wang Z., Walton A. H., Goedegebuure S. P., Figueroa D. J., Van Horn S., Grossman J., Remlinger K., Madsen H., Brown J., et al. 2019. T cell- and monocyte-specific RNA-sequencing analysis in septic and nonseptic critically ill patients and in patients with cancer. J. Immunol. 203: 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Severino P., Silva E., Baggio-Zappia G. L., Brunialti M. K., Nucci L. A., Rigato O. Jr., da Silva I. D., Machado F. R., Salomao R.. 2014. Patterns of gene expression in peripheral blood mononuclear cells and outcomes from patients with sepsis secondary to community acquired pneumonia. PLoS One 9: e91886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Derakhshani A., Hemmat N., Asadzadeh Z., Ghaseminia M., Shadbad M. A., Jadideslam G., Silvestris N., Racanelli V., Baradaran B.. 2021. Arginase 1 (Arg1) as an up-regulated gene in COVID-19 patients: a promising marker in COVID-19 immunopathy. J. Clin. Med. 10: 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burrack K. S., Morrison T. E.. 2014. The role of myeloid cell activation and arginine metabolism in the pathogenesis of virus-induced diseases. Front. Immunol. 5: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang Z., Ming X. F.. 2014. Functions of arginase isoforms in macrophage inflammatory responses: impact on cardiovascular diseases and metabolic disorders. Front. Immunol. 5: 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Molgora M., Supino D., Mantovani A., Garlanda C.. 2018. Tuning inflammation and immunity by the negative regulators IL-1R2 and IL-1R8. Immunol. Rev. 281: 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Deuren M., van der Ven-Jongekrijg J., Vannier E., van Dalen R., Pesman G., Bartelink A. K., Dinarello C. A., van der Meer J. W.. 1997. The pattern of interleukin-1beta (IL-1beta) and its modulating agents IL-1 receptor antagonist and IL-1 soluble receptor type II in acute meningococcal infections. Blood 90: 1101–1108. [PubMed] [Google Scholar]
- 51. Kovach M. A., Stringer K. A., Bunting R., Wu X., San Mateo L., Newstead M. W., Paine R., Standiford T. J.. 2015. Microarray analysis identifies IL-1 receptor type 2 as a novel candidate biomarker in patients with acute respiratory distress syndrome. Respir. Res. 16: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dinarello C. A. 2011. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117: 3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Peyvandi F., Garagiola I., Baronciani L.. 2011. Role of von Willebrand factor in the haemostasis. Blood Transfus. 9(Suppl 2): s3–s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ward S. E., Curley G. F., Lavin M., Fogarty H., Karampini E., McEvoy N. L., Clarke J., Boylan M., Alalqam R., Worrall A. P., et al. Irish COVID-19 Vasculopathy Study (ICVS) Investigators . 2021. Von Willebrand factor propeptide in severe coronavirus disease 2019 (COVID-19): evidence of acute and sustained endothelial cell activation. Br. J. Haematol. 192: 714–719. [DOI] [PubMed] [Google Scholar]
- 55. Mancini I., Baronciani L., Artoni A., Colpani P., Biganzoli M., Cozzi G., Novembrino C., Boscolo Anzoletti M., De Zan V., Pagliari M. T., et al. 2021. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J. Thromb. Haemost. 19: 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Esmon C. T. 2013. Molecular circuits in thrombosis and inflammation. Thromb. Haemost. 109: 416–420. [DOI] [PubMed] [Google Scholar]
- 57. Schwartzberg P. L., Finkelstein L. D., Readinger J. A.. 2005. TEC-family kinases: regulators of T-helper-cell differentiation. Nat. Rev. Immunol. 5: 284–295. [DOI] [PubMed] [Google Scholar]
- 58. Horwood N. J., Urbaniak A. M., Danks L.. 2012. Tec family kinases in inflammation and disease. Int. Rev. Immunol. 31: 87–103. [DOI] [PubMed] [Google Scholar]
- 59. Middendorp S., Dingjan G. M., Maas A., Dahlenborg K., Hendriks R. W.. 2003. Function of Bruton’s tyrosine kinase during B cell development is partially independent of its catalytic activity. J. Immunol. 171: 5988–5996. [DOI] [PubMed] [Google Scholar]
- 60. Semaan N., Alsaleh G., Gottenberg J. E., Wachsmann D., Sibilia J.. 2008. Etk/BMX, a Btk family tyrosine kinase, and Mal contribute to the cross-talk between MyD88 and FAK pathways. J. Immunol. 180: 3485–3491. [DOI] [PubMed] [Google Scholar]
- 61. Palmer C. D., Mutch B. E., Page T. H., Horwood N. J., Foxwell B. M.. 2008. Bmx regulates LPS-induced IL-6 and VEGF production via mRNA stability in rheumatoid synovial fibroblasts. Biochem. Biophys. Res. Commun. 370: 599–602. [DOI] [PubMed] [Google Scholar]
- 62. Palmer C. D., Mutch B. E., Workman S., McDaid J. P., Horwood N. J., Foxwell B. M.. 2008. Bmx tyrosine kinase regulates TLR4-induced IL-6 production in human macrophages independently of p38 MAPK and NFkappB activity. Blood 111: 1781–1788. [DOI] [PubMed] [Google Scholar]
- 63. Gottar-Guillier M., Dodeller F., Huesken D., Iourgenko V., Mickanin C., Labow M., Gaveriaux S., Kinzel B., Mueller M., Alitalo K., et al. 2011. The tyrosine kinase BMX is an essential mediator of inflammatory arthritis in a kinase-independent manner. J. Immunol. 186: 6014–6023. [DOI] [PubMed] [Google Scholar]
- 64. Roschewski M., Lionakis M. S., Sharman J. P., Roswarski J., Goy A., Monticelli M. A., Roshon M., Wrzesinski S. H., Desai J. V., Zarakas M. A., et al. 2020. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 5: eabd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Treon S. P., Castillo J. J., Skarbnik A. P., Soumerai J. D., Ghobrial I. M., Guerrera M. L., Meid K., Yang G.. 2020. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. [Published erratum appears in 2021 Blood 137: 1561.] Blood 135: 1912–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Isaac K., Mato A. R.. 2020. Acalabrutinib and its therapeutic potential in the treatment of chronic lymphocytic leukemia: a short review on emerging data. Cancer Manag. Res. 12: 2079–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Goshua G., Pine A. B., Meizlish M. L., Chang C. H., Zhang H., Bahel P., Baluha A., Bar N., Bona R. D., Burns A. J., et al. 2020. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 7: e575–e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liberman A. C., Budziñski M. L., Sokn C., Gobbini R. P., Steininger A., Arzt E.. 2018. Regulatory and mechanistic actions of glucocorticoids on T and inflammatory cells. Front. Endocrinol. (Lausanne) 9: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.