Abstract
Purpose:
This phase Ib trial was designed to evaluate the safety and early efficacy signal of the combination of imatinib and binimetinib in patients with imatinib-resistant advanced gastrointestinal stromal tumors (GISTs).
Patients and Methods:
This trial used a standard 3 + 3 design to determine the recommended phase II dose (RP2D). Additional patients were enrolled on an expansion cohort at the RP2D enriching for succinate dehydrogenase (SDH)-deficient GISTs to explore potential efficacy.
Results:
The trial enrolled nine patients in the dose-escalation cohort and 14 in the dose-expansion cohort including six with SDH-deficient GISTs. Imatinib 400 mg daily with binimetinib 45 mg twice daily was established as the RP2D. Dose-limiting toxicity (DLT) was asymptomatic grade 4 creatinine phosphokinase (CPK) elevation. The most common non-DLT grade 3/4 toxicity was asymptomatic CPK elevation (69.6%). Other common ≥grade 2 toxicities included peripheral edema (17.4%), acneiform rash (21.7%), anemia (30.4%), hypophosphatemia (39.1%), and aspartate aminotransferase (AST) increase (17.4%). Two serious adverse events occurred (grade 2 dropped head syndrome and grade 3 central retinal vein occlusion). No unexpected toxicities were observed. Limited clinical activity was observed in KIT-mutant GIST. For SDH-deficient GISTs, one of five had confirmed RECIST1.1 partial response (PR). The median progression-free survival (mPFS) in patients with SDH-deficient GIST was 45.1 months [95% confidence interval (CI), 15.8–not estimable (NE)]; the median overall survival (mOS) was not reached (95% CI, 31.6 months–NE). One patient with a refractory metastatic SDH-deficient GIST had an exceptional pathologic response and durable clinical benefit.
Conclusions:
The combination of imatinib and binimetinib is safe with manageable toxicity and has encouraging activity in SDH-deficient but not imatinib-refractory KIT/PDGFRA-mutant GISTs. The observed clinical benefits provide a motivation for a larger trial of the combination strategy in SDH-deficient GISTs.
Translational Relevance.
Combined targeting of the lineage-specific master transcription factor, ETV1, and signaling factor, KIT, by the combination of KIT and MEK inhibitors, is synergistic in preclinical gastrointestinal tumor (GIST) models. This phase Ib clinical trial was designed to evaluate the safety, determine the recommended phase II dose (RP2D), and early efficacy signal of the combination of imatinib and binimetinib in patients with refractory advanced KIT/PDGFRA-mutant and KIT/PDGFRA wild-type GISTs including SDH-deficient GISTs. This study showed that the imatinib/binimetinib combination was safe with manageable side effects and determined the RP2D. We observed durable and excellent pathologic responses in a small cohort of patients with SDH-deficient GISTs in the phase Ib expansion at RP2D. The promising clinical activity of the combination treatment in SDH-deficient GISTs observed here is worthy of further clinical investigation. A phase II study of the imatinib and binimetinib combination in patients with treatment-naïve advanced GISTs has met its prespecified primary endpoint based on the best objective response rate by RECIST1.1 and is reported separately.
Introduction
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal (GI) tract, occurring primarily in the stomach and small intestine and less frequently in the large intestine, rectum, and esophagus. The majority of GISTs are characterized by activating mutations in receptor tyrosine kinases (RTKs), KIT or PDGFRA. Approximately 15% of GISTs lack mutations in KIT/PDGFRA and are designated as KIT/PDGFRA wild-type (WT) GISTs. The majority of WT GISTs harbor genetic or epigenetic alterations that specifically inactivate the succinate dehydrogenase (SDH) pathway (1–4). SDH-deficient GISTs occur primarily in the pediatric and young adult population and in patients with the heritable Carney–Stratakis syndrome or the nonhereditary Carney triad. Unlike KIT/PDGFRA-mutant GISTs, SDH-deficient GISTs confer primary resistance to imatinib, the first-line standard-of-care (SOC) systemic therapy in advanced GISTs. They are also relatively insensitive to other tyrosine kinase inhibitors (TKIs; refs. 1, 4), and currently, there are no SOC therapeutic options for advanced SDH-deficient GISTs.
SDH is a component of both the Krebs cycle and the electron transport chain, and SDH deficiency leads to succinate accumulation, metabolic defects and a global genomic hypermethylation phenotype in SDH-deficient GISTs (4–6). Although SDH-deficient GISTs lack KIT/PDGFRA or other druggable oncogenic driver mutations, they share with other GISTs a common precursor, the interstitial cells of Cajal (ICC) and their shared lineage-specific dependency on KIT signaling for oncogenesis (7). ETV1, an ETS family transcription factor, has been uncovered as a lineage-specific survival factor in GISTs and its precursor ICCs (8). ETV1 is required for the growth and survival of both imatinib-sensitive and imatinib-resistant GISTs in vitro and GIST initiation and maintenance in vivo. ETV1 and mutant KIT/PDGFRA form a positive-feedback circuit in GIST pathogenesis, where active MAPK signaling downstream of active KIT/PDGFRA signaling stabilizes ETV1, and stabilized ETV1 enhances KIT expression (8–10). The fact that Etv1 is required for normal ICC lineage-specification and development and GIST tumor initiation in mouse models suggests that all GISTs regardless of genetic alterations, including the SDH-deficient GISTs, depend on ETV1 for growth and survival. Consistently, all human GISTs, including KIT/PDGFRA-WT GISTs, express high levels of ETV1 (11), indicating that ETV1 may be a novel therapeutic target for all GISTs. Preclinical studies showed that the combination of an MEK inhibitor (e.g., binimetinib) with a KIT inhibitor (e.g., imatinib) is synergistic and can durably inhibit the ETV1 protein level by simultaneously blocking the downstream MEK/ERK signaling and the feedback reactivation of the upstream KIT/PDGFRA signaling in GISTs (8, 9, 12). Furthermore, the Pea3 family ETS factors (ETV1/4/5) have been shown to directly regulate the homeostasis of the MAPK signaling pathways in multiple cancer types, including GISTs; and they are the critical mediators of early adaptive responses to MAPK pathway targeted therapies and subsequent development of therapeutic resistance (12–14).
Based on these preclinical studies, we initiated a phase Ib/II clinical trial to evaluate the safety, tolerability, and efficacy of targeting ETV1, using the combination of imatinib and an MEK inhibitor, binimetinib, in patients with advanced GISTs (NCT01991379). This is also a signal finding study to see whether targeting ETV1 protein stability can be an effective strategy for both the KIT/PDGFRA-mutant and the KIT/PDGFRA-WT GISTs including SDH-deficient GISTs. Initial results of the phase Ib portion of the study have shown that this combination is safe with manageable side effects (15). We have since accrued a small cohort of patients with SDH-deficient GISTs in the phase Ib expansion. Here, we report the safety and tolerability of the combination therapy and efficacy signal in SDH-deficient GISTs from the phase Ib study. The phase II portion of the trial is reported separately (16).
Patients and Methods
Patients
Adult patients (age ≥18) who had histologically confirmed advanced GISTs, an Eastern Cooperative Oncology Group (ECOG) performance score of 0 to 1, who had progressed on imatinib, and had adequate end-organ function were eligible to consent and participate. Additional key inclusion criteria were patients with measurable lesion(s) by RECIST1.1 and were able to take oral medications and sign informed consents. Key exclusion criteria included severe and/or uncontrolled medical diseases, active brain metastasis, history of retinal degenerative disease or central serious retinopathy or retinal vein occlusion (RVO), or neuromuscular disorders associated with elevated creatinine phosphokinase (CPK; e.g., inflammatory myopathies, muscular dystrophy, spinal muscular atrophy). Complete inclusion and exclusion criteria are available in the study protocol. The phase Ib study (dose-escalation and dose-expansion cohorts) was initially designed to accrue up to 18 patients. After observing an exceptional response in a patient with SDH-deficient GIST, the phase Ib expansion cohort was amended to accrue an additional five patients with SDH-deficient GISTs.
Trial oversight
The study was performed in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. The protocol, protocol amendments, and informed-consent documents were approved by the institutional review board (IRB) at Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY). All participants provided written informed consent. All biopsies and molecular testing were performed in accordance with the IRB-approved protocol.
Study design, treatment, and endpoints
This was a single-center (MSKCC), single-arm phase Ib study to evaluate the safety and tolerability and define the recommended phase II dose (RP2D) and early efficacy signal of imatinib and binimetinib combination in patients with advanced, imatinib-resistant GISTs. It consisted of two cohorts: (i) a dose-escalation cohort (n = 9) and (ii) a dose-expansion cohort at RP2D (n = 14) enriched for SDH-deficient GISTs (n = 6).
Dose-escalation was performed in standard 3 + 3 design (17). The standard dose of single-agent imatinib is 400 mg once daily. The single agent RP2D of binimetinib in phase II and III studies is 45 mg twice daily (18). Thus, the dose-escalation portion consists of three dose levels (−1, 1, 2) with the starting dose (level 1) of the combination at imatinib 400 mg once daily and binimetinib 30 mg twice daily; once the RP2D was determined, the phase Ib expanded at the RP2D to accrue a total of 24 patients, with a focus on SDH-deficient GISTs.
In the dose-escalation phase Ib portion, all eligible patients received imatinib 400 mg daily and binimetinib at the standard 3 + 3 escalation doses (see Protocol for dose-escalation table). In the phase Ib expansion cohort, all eligible patients received the RP2D (15), continuously on every 28-day cycle.
Disease assessments with CT or MRI were performed at baseline and then every 8 weeks for the initial 32 weeks and every 12 weeks until surgery, disease progression, death, or withdrawal. Adverse events (AEs) were graded by the investigator according to the Common Terminology Criteria for Adverse Events (version 4.03) until 28 days after discontinuation of study treatment.
The primary endpoint of phase Ib was to evaluate the safety and tolerability of the combination of imatinib and binimetinib, determine the maximum tolerated dose (MTD) and to define the RP2D. The secondary endpoints were to evaluate the objective response rate [ORR; complete response (CR) + partial response (PR)] by both RECIST1.1 and Choi criteria (19), progression-free survival (PFS), overall survival (OS), the clinical benefit rate [CBR; CR + PR + stable disease (SD)], and correlative studies by genomics and transcriptome with available archival tissue.
Statistical analysis
The primary endpoint of the phase Ib study was to determine the MTD of binimetinib in combination with imatinib in patients with advanced GISTs. The phase Ib pursued a standard 3 + 3 format, based on toxicities encountered during the first cycle of therapy. The phase Ib portion of the study would have a minimum sample size of four patients and a maximum of 18.
The dose-escalation proceeded within each cohort according to the dose-escalation schema and DLT evaluation in the study protocol. The first three patients enrolled at dose level 1. If dose level 1 was not found to be tolerable, then the next cohort would be enrolled at dose level -1. If dose level -1 was not found to be tolerable, then the study may be terminated based on discussions with the sponsor-investigator and the combination may be deemed intolerable. If zero of three patients or one of six patients experienced a DLT on dose level 2, this would be the RP2D.
The secondary endpoints of the phase Ib portion included ORR defined by RECIST 1.1 and by Choi criteria, PFS, and OS. ORR would be estimated as the proportion of patients who had CR or PR for each criterion. Patients who had not experienced the event of interest by the end of the study would be censored at the time of the last follow-up. PFS and OS were estimated using the Kaplan–Meier method.
All patients who received at least one dose of the combination of imatinib and binimetinib were included in the safety and toxicity analysis. All data reflect an interim data-cut on May 1, 2021 from patients enrolled between November 18, 2013 and May 1, 2021 (Fig. 1).
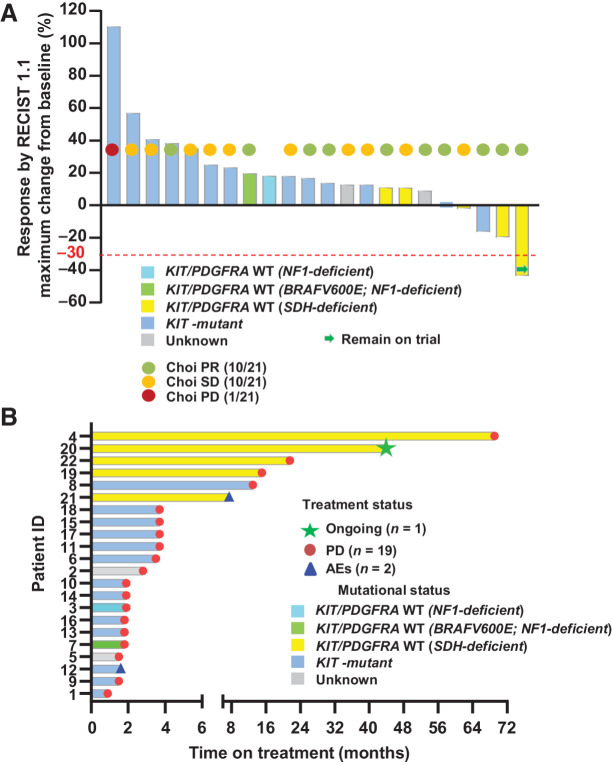
Figure 1.
Response rates (RECIST1.1, Choi) and duration of treatment. A, Response rates by RECIST1.1 and Choi criteria. Best objective responses by RECIST1.1 (n = 22), Choi responses (n = 21) around 8 weeks (end of cycle 2, first posttreatment scan) on combination imatinib and binimetinib treatment. The best RECIST1.1 responses are shown as percentage of change from baseline for patients who received the combination of imatinib and binimetinib and with at least one postbaseline scan. The known associated primary driver mutations in KIT, SDH complex, NF1, and BRAF are shown. The best ORR for all phase Ib patients was 4.5% (1/22 confirmed PR), two-sided 95% CI, 0.1 to 22.8. The best ORR for patients with SDH-deficient GIST was 20.0% (1/5 confirmed PR), two-sided 95% CI, 0.5 to 71.6. Choi response rate was 47.6% (95% CI, 25.7–70.2) and 60.0% (95% CI, 14.7–94.7) for all phase Ib patients and patients with SDH-deficient GISTs, respectively. B, Duration of treatment. AE, adverse events; PD, progression of disease; PR, partial response; SD, stable disease.
The study is registered at http://www.clinicaltrials.gov, under the identifier NCT01991379.
Genomic and transcriptomic studies
Sample preparation and quality control
RNA was extracted from tumor and normal tissues using a modification of the protocol for the DNA/RNA AllPrep kit (Qiagen). DNA from fresh-frozen tissues was extracted from tumor and normal tissue specimens using the DNeasy Blood and Tissue kit (Qiagen). DNA from formalin-fixed, paraffin-embedded (FFPE) samples was isolated using the QIAamp DNA FFPE Tissue kit (Qiagen). DNA from each specimen was initially quantified using the NanoDrop UV spectrophotometer and was further quantified with the Bioanalyzer assay (Agilent Technologies).
MSK-IMPACT
The MSK-IMPACT assay is a hybridization capture, next-generation sequencing (NGS) platform amenable to DNA from both fresh-frozen and FFPE samples for targeted sequencing as described previously (20, 21). The library construction and sequencing were performed by the MSKCC Integrated Genomics Operation Facility, Marie-Josée and Henry R. Kravis Center for Molecular Oncology. Alignment and single-nucleotide variant (SNV) and insertions and deletions (indel) calling were performed as described previously (20, 21). Copy-number analysis was performed as previously described (20, 21).
Whole-exome sequencing and somatic mutation analysis
Paired-end whole-exome sequencing (WES) was performed on tumor and matched normal samples by the MSKCC Integrated Genomics Operation Facility, Marie-Josée and Henry R. Kravis Center for Molecular Oncology. Exome capture was performed using the Agilent Exon 51MB hg19 v3, and the captured DNA was sequenced using the Illumina HiSeq platform. Somatic mutation detection was performed by the MSKCC Bioinformatics Core using standard algorithms. In brief, the raw sequencing reads were aligned to the reference human genome (hg19, build37) using the Burrows–Wheeler Aligner (doi: 10.1093/bioinformatics/btp324), duplicate reads were removed, realignment around indels was performed, and quality scores were recalibrated using the GATK analysis toolkit (doi:10.1101/gr.107524.110). Single base substitution calls were generated using MuTect (doi:10.1038/nbt.2514), and small somatic indels were identified using the haplotect and haplotypecaller algorithms. Variants were subsequently annotated using the Variant Effect Predictor. To reduce the number of false positives, only mutations detected at 5% allele frequency and those identified in the MSK-IMPACT gene panel were considered for this analysis. Variant calls with tumor depth less than 20, less than three supporting reads, with more than one matching read in the normal sample, and those located in sequenced regions with low mappability as defined by ENCODE (doi:10.1038/nature11247) and the RepeatMasker (doi:10.1002/0471250953.bi0410s25) were disregarded. To remove common variants, all calls were filtered against ExAC (doi:10.1038/nature19057); a variant was considered common if its minor allele frequency was above 0.0004. To further reduce sequencing and mapping artifacts, all variants were filtered against a panel of normal and FFPE samples available in-house. All remaining indels were visually reviewed using the Integrated Genomic Viewer (doi:10.1038/nbt.1754).
WES copy-number analysis
Allele-specific copy-number detection was performed using the FACETS algorithm (doi:10.1093/nar/gkw520). For each tumor-normal pair FACETS was run twice, once using a low sensitivity setting (Cval 300) to get estimates of purity and ploidy, and a second time, using these estimates and a higher sensitivity setting (Cval 100) to get finer granularity on the segmentation and copy-number calls.
RNA transcript studies
For cells from tissue culture (GIST48), RNA was isolated using the E.Z.N.A. Total RNA kit (Omega). RNA was extracted from frozen tumor and FFPE tumor samples using a modification of the protocol for the DNA/RNA AllPrep kit (Qiagen). For quantitative RT-PCR, RNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription kit (ABI), and PCR was run using Power SYBR Master Mix (ABI) on a Realplex machine (Eppendorf). Expression was normalized to the transcript amount of the ribosomal protein RPL27. The primer pairs used are listed in Supplementary Table S1. The gene expression data of ETV1 and KIT from archived tumor samples were profiled using the GeneChip Human Genome U133A 2.0 Array as previously described (22). Raw data were imported into Partek, quartile normalized, and log2 transformed.
Data availability
All datasets generated during and/or analyzed during the current study, including patient-level clinical data as well as all sequencing data are publicly available upon request from the corresponding author.
Results
Patients
From November 18, 2013 to May 1, 2021, a total of 24 patients were consented and enrolled in the phase Ib study; one enrolled patient had rapid clinical deterioration and discontinued the trial without receiving any study drug and therefore was removed from all analysis (Supplementary Fig. S1). Thus, nine patients were enrolled and treated in the dose-escalation cohort and 14 patients in the dose-expansion cohort, among whom six patients had SDH-deficient GISTs. All evaluable patients received at least 2 days of treatment with the combination of imatinib (400 mg daily) and binimetinib [30 mg twice daily (n = 3, dose level -1) or 45 mg twice daily (n = 20)]. The phase Ib dose-escalation and dose-expansion cohorts included 23 patients evaluable for safety and toxicity and 21 to 23 patients evaluable for various efficacy endpoints (Supplementary Fig. S1). The median age of the analytic cohort is 54.9 years (range, 30.2–74.6 years), 39.1% female, 82.6% patients with ECOG 0 (Table 1). The median number of prior therapies was three (range, 1–6), and 17 of 23 patients had at least three prior therapies, including imatinib, sunitinib, regorafenib, clinical trial drugs, among others. The molecular features of the GISTs included 13 patients with KIT-mutant refractory GIST and 10 had various detectable secondary resistant mutations in KIT exons 13, 14, 17, 18; six patients had SDH-deficient GISTs confirmed by MSK-IMPACT genetic assays and/or IHC analysis of SDH complex; one patient had an NF1-deficient GIST, and one had BRAFV600E-mutant and concurrent NF1-deficient GIST; two patients had no mutational data due to insufficient biopsy material (Table 1; Supplementary Table S2).
Table 1.
Patient characteristics.
| Characteristics | Number of patients (%; N = 23) |
|---|---|
| Median age, y (range) | 54.9 (30.2–74.6) |
| Sex | |
| Female | 9 (39.1) |
| Male | 14 (60.9) |
| ECOG status | |
| 0 | 19 (82.6) |
| 1 | 4 (4.3) |
| Number of prior therapies: | Median: 3 (range, 1–6) |
| 1–2 | 6 (26.1) |
| ≥3 | 17 (73.9) |
| Prior therapies: | |
| Imatinib | 23 (100) |
| Sunitinib | 19 (82.6) |
| Regorafenib | 11 (47.8) |
| Sorafenib | 7 (30.4) |
| Pazopanib | 4 (17.4) |
| Vemurafenib | 1 (4.3) |
| Dasatinib/ipilimumab trial | 2 (11.1) |
| Linsitinib trial | 1 (4.3) |
| BIIB021 trial | 1 (4.3) |
| Molecular characteristics: | |
| KIT-mutant (KIT exons 9, 11, 13, 14, 17; 10 patients with known imatinib secondary resistant mutations) | 13 (56.5) |
| NF1-deficient; KIT/PDGFRA-WT | 1 (4.3) |
| BRAF V600E; NF1-deficient; KIT/PDGFRA-WT | 1 (4.3) |
| SDH-deficient; KIT/PDGFRA-WT | 6 (26.1) |
| Unknown | 2 (8.7) |
Safety and treatment-associated toxicity
Overall, combination imatinib (400 mg daily) and binimetinib (45 mg twice daily) treatment is safe and has manageable toxicities with no unexpected toxicities observed. The most commonly observed toxicity of any grade and investigator attributed as possibly, probably, or definitely associated with either imatinib and/or binimetinib, included asymptomatic CPK elevation (100%), peripheral (60.9%), facial (43.5%) and periorbital (34.8%) edema, acneiform (65.2%) and maculopapular (65.2%) rash, diarrhea (52.2%), nausea (52.2%), vomiting (43.5%), anemia (82.6%), white blood cell decrease (82.6%), platelet count decrease (65.2%), hypophosphatemia (56.5%), hypomagnesemia (47.8%), hypocalcemia (56.5%), and alanine aminotransferase (ALT; 52.2%) and AST (95.7%) increase. Most common ≥ grade 2 toxicities included asymptomatic CPK elevation (73.9%), peripheral edema (17.4%), acneiform rash (21.7%), anemia (30.4%), hypophosphatemia (39.1%), and AST increase (17.4%). Most common grade 3/4 toxicity included asymptomatic CPK elevation (69.6%), lymphocyte decrease (8.7%), AST increase (8.7%; Table 2).
Table 2.
AEs at least possibly related to treatment (includes all grade 3 and 4 toxicities, and any grade ≥10% toxicities).
| AE | Any grade (≥10%) or grade 3/4; or SAE N = 23 (%) | All ≥ grade 2 N = 23 (%) | All grade 3 N = 23 (%) | All grade 4 N = 23 (%) |
|---|---|---|---|---|
| Fatigue | 10 (43.5) | 3 (13.0) | ||
| CPK elevation | 23 (100) | 17 (73.9) | 10 (43.5) | 6 (26.1) |
| Edema/fluid retention | ||||
| Peripheral (limbs) | 14 (60.9) | 4 (17.4) | ||
| Facial | 10 (43.5) | 1 (4.3) | ||
| Periorbital | 8 (34.8) | 3 (13.0) | ||
| Weight gain/trunk | 5 (21.7) | 1 (4.3) | ||
| Skin-related | ||||
| Rash (acneiform) | 15 (65.2) | 5 (21.7) | ||
| Rash (maculopapular) | 15 (65.2) | 3 (13.0) | ||
| Palmar-plantar | 2 (8.7) | |||
| Erythrodysesthesia | 7 (30.4) | 1 (4.3) | ||
| Dry skin | 5 (21.7) | |||
| Pruritis | 3 (13.0) | 2 (8.7) | ||
| Skin infection | 1 (4.3) | |||
| Skin hypopigmentation | ||||
| Gastrointestinal-related | ||||
| Diarrhea | 12 (52.2) | 2 (8.7) | ||
| Nausea | 12 (52.2) | 1 (4.3) | ||
| Vomiting | 10 (43.5) | 2 (8.7) | ||
| Mucositis, oral | 7 (30.4) | |||
| Dyspepsia | 6 (26.1) | 1 (4.3) | ||
| Anorexia | 3 (13.0) | 1 (4.3) | ||
| Gastroparesis | 1 (4.3) | |||
| Dry mouth | 3 (13.0) | |||
| Cardiac/pulmonary-related | ||||
| Dyspnea | 3 (13.0) | |||
| Hypertension | 4 (17.4) | 2 (8.7) | ||
| Pleural effusion | 1 (4.3) | |||
| Musculoskeletal-related | ||||
| Myalgia | 3 (13.0) | |||
| Dropped head syndrome | 1 (4.3) | 1 (4.3) | ||
| Eye/ocular-related | ||||
| Blurred vision | 3 (13.0) | |||
| Retinopathy (subretinal fluid) | 3 (13.0) | |||
| Retinal vascular disorder (retinal vein occlusion) | 1 (4.3) | 1 (4.3) | 1 (4.3) | |
| Hematologic-related | ||||
| Anemia | 19 (82.6) | 7 (30.4) | 1 (4.3) | |
| White blood cell decreased | 7 (30.4) | 1 (4.3) | ||
| Lymphocyte count decreased | 6 (26.1) | 3 (13.0) | 2 (8.7) | |
| Neutrophil count decreased | 4 (17.4) | 3 (13.0) | ||
| Platelet count decreased | 15 (65.2) | 1 (4.3) | ||
| Renal/electrolytes-related | ||||
| Hypophosphatemia | 13 (56.5) | 9 (39.1) | 1 (4.3) | |
| Hypomagnesemia | 11 (47.8) | 1 (4.3) | ||
| Hypocalcemia | 13 (56.5) | 1 (4.3) | 1 (4.3) | |
| Hypokalemia | 4 (17.4) | 1 (4.3) | 1 (4.3) | |
| Hyperkalemia | 4 (17.4) | 1 (4.3) | ||
| Hypernatremia | 6 (26.1) | |||
| Creatinine increased | 5 (21.7) | 1 (4.3) | ||
| Liver-related | ||||
| ALT increased | 12 (52.2) | 1 (4.3) | ||
| AST increased | 22 (95.7) | 4 (17.4) | 2 (8.7) | |
| Alk phos increased | 8 (34.8) | 1 (4.3) | ||
| Blood bilirubin increased | 3 (13.0) | 1 (4.3) | ||
Note: Items in bold indicate SAEs requiring discontinuation of therapy.
Abbreviations: Alk phos, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Two serious AEs (SAEs) occurred that were considered related to the study drug. One patient developed a grade 2 dropped head syndrome requiring discontinuation of therapy with full resolution of symptoms. One patient developed a grade 3 central retinal vein occlusion (CRVO) requiring discontinuation of study drug and intravitreal bevacizumab injection of the affected eye with subsequent full recovery of vision. The dropped head syndrome and CRVO are class effects of MEK inhibitors and are typically reversable with complete resolution of symptoms through dose reduction or discontinuation of drugs and appropriate medical management (23–25). The MEK inhibitor-associated CRVO has been observed to associate with hyperhomocysteinemia and MTHFR variants (23). There was no clinically significant grade 4 or grade 5 AEs at least possibly associated with study medications in this trial.
Efficacy in all phase Ib patients
At data cut-off, one patient treated at the RP2D of 22 (1/22) evaluable patients across all doses in phase Ib had confirmed RECIST1.1 PR. The best ORR was 4.5% [two-sided 95% confidence interval (CI), 0.1–22.8); 10 of 21 (47.6%, two-sided 95% CI, 25.7–70.2) evaluable patients had a Choi PR approximately 8 weeks (Fig. 1A). One patient with an SDH-deficient GIST who had RECIST PR remains on trial (treatment duration: 43.3 months at data cut-off); 19 patients progressed (range, 1.3–69.0 months); two patients discontinued trial because of treatment-associated toxicity (AE; Fig. 1B).
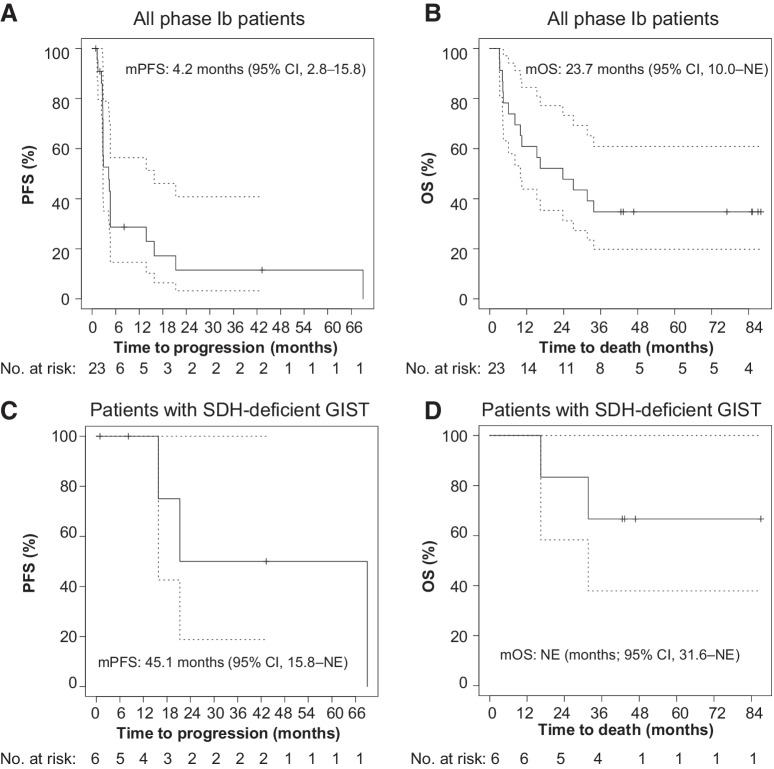
For all phase Ib patients including both dose-escalation and dose-expansion cohorts, the median PFS (mPFS) by RECIST1.1 was 4.2 months (95% CI, 2.8–15.8); 28.7% (95% CI, 14.6–56.4) and 11.5% (95% CI, 3.2–40.8) patients remained progression-free at 12 and 24 months, respectively (Fig. 2A). The median OS (mOS) was 23.7 months [95% CI, 10.0–not estimable (NE)]; 60.9% (95% CI, 43.9–84.5), and 47.8% (95% CI, 31.2–73.3) patients were alive at 12 and 24 months, respectively (Fig. 2B).
Figure 2.
Kaplan–Meier estimates of PFS and OS. PFS by RECIST1.1 (A) and OS (B) of all phase Ib patients. PFS (C) and OS (D) of patients with SDH-deficient GIST. mPFS is based on a Kaplan–Meier estimate of PFS, per investigator assessment. The mPFS was 4.2 months (95% CI, 2.8–15.8 months) and 45.1 months (95% CI, 15.8 months–NE) for all phase Ib patients and patients with SDH-deficient GISTs, respectively. The mOS was 23.7 months (95% CI, 10.0 months–NE), and not estimable (95% CI, 31.6 months–NE) for all phase Ib patients and patients with SDH-deficient GISTs, respectively.
Efficacy in patients with SDH-deficient GISTs
For the patients with SDH-deficient GISTs in the expansion cohort, one of five evaluable patients had confirmed RECIST1.1 PR with the best ORR of 20.0% (two-sided 95% CI, 0.5–71.6); three of five evaluable patients had Choi PR with the best ORR of 60.0% (two-sided 95% CI, 14.7–94.7). The CBR (RECIST1.1 PR + CR + SD) for the patients with SDH-deficient GISTs was 100% (95% CI, 39.8–100), 75% (95% CI, 19.4–99.4), and 50% (95% CI, 6.8–93.2), at 12 months, 18 months, and 24 months, respectively (Fig. 1A and B).
For the patients with SDH-deficient GISTs, the mPFS was 45.1 months (95% CI, 15.8–NE); 75.0% (95% CI, 42.6–100) and 50.0% (95% CI, 18.8–100) of patients remained progression-free at 18 and 24 months, respectively (Fig. 2C). The mOS for patients with SDH-deficient GISTs was not estimable (95% CI, 31.6 months–NE); 83.3% (95% CI, 58.3–100.0) and 66.7% (95% CI, 37.9–100) patients were alive at 18 and 36 months, respectively (Fig. 2D).
At data cut-off, the one patient with RECIST1.1 PR has continued response and remains on trial after 43.3 months. One patient with SD at 8.2 months developed CRVO and discontinued trial. Three patients discontinued trial due to disease progression. One patient had a prolonged disease stabilization for 69.0 months until disease progression and had an interim tumor biopsy at 22 months of combination treatment that allowed for pathologic response and molecular analysis of paired tumors (see below).
Exceptional pathologic and durable response in a patient with SDH-deficient GIST
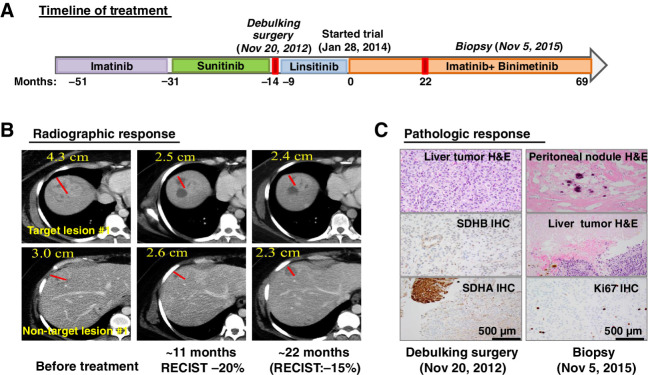
This is a 32-year-old female who was initially diagnosed with an SDH-deficient, KIT/PDGFRA-WT GIST localized in the stomach at age 19, in 2003. Initial therapy included subtotal gastrectomy with Roux-en-Y reconstruction, followed by 1 year of adjuvant imatinib therapy in a phase II clinical trial. Recurrent and metastatic disease in the gastrohepatic lymph nodes and liver, respectively, was confirmed by biopsy 6 years after the initial diagnosis. The patient was subsequently treated with imatinib in the randomized nilotinib versus imatinib phase III trial (ENESTg1; ref. 26) until clear progression of disease within 20 months of treatment. The patient then received sunitinib with slow but clear progression of disease for the following 1 year and 5 months. She then underwent debulking surgery in November 2012, followed by RECIST disease progression on linsitinib, an IGF-1R small-molecule inhibitor, in a clinical trial within 9 months (Fig. 3A).
Figure 3.
An example of durable treatment response in a patient with an SDH-deficient GIST. A, Treatment timeline and duration of various treatments the patient received for a metastatic SDH-deficient GIST. B, Representative CT images of the patient's metastatic liver lesions before, approximately 11 months and approximately 22 months after receiving the imatinib plus binimetinib combination therapy. One-dimension measurement in centimeters was provided for RECIST1.1 calculation at different time points. C, Treatment response by histopathologic studies. Representative images of histology and IHC stains for SDHB and SDHA, demonstrating dual SDH-deficiency, in the pretreatment tumor samples (debulking surgery; November 20, 2012) and the histology and the proliferation index marker, Ki67 IHC. The histology from the pretrial treatment liver lesions demonstrated more than 95% viable tumor and less than 5% treatment-associated necrosis. The histology from the on-treatment tumor samples (biopsy; November 5, 2015) showed 100% pathologic response with treatment-associated fibrosis, hyalinization, and dystrophic calcification in a resected metastatic peritoneal nodule and 70% pathologic response with treatment-associated necrosis in the metastatic liver lesion and less than 10% Ki67 IHC in the residual viable tumor. H&E, hematoxylin and eosin.
The patient then enrolled in the phase Ib trial of combination treatment of imatinib and binimetinib (NCT01991379). The patient received the RP2D of the combination, imatinib at 400 mg daily and binimetinib at 45 mg twice daily and tolerated the combination therapy well with manageable mild side effects. The patient had slow but steady disease regression in multiple liver lesions. By the fifth follow-up CT scan, after approximately 11 months of combination treatment, she showed approximately 20% reduction in tumor burden by RECIST, and approximately 38% reduction in CT density [Hounsfield unit (HU)], qualifying for a partial response by the Choi criteria (Fig. 3B; ref. 19). Her tumor then remained relatively stable with approximately 15% tumor burden reduction by RECIST by 22 months of treatment. At this point, one of the target lesions appeared to have slightly increased in density and size, whereas some of the nontarget lesions continued to respond with a decrease in size and CT density (HU) by imaging studies (Fig. 3B). Following 22 months of the combination treatment, the patient subsequently underwent laparotomy, lysis of adhesions, removal of a peritoneal nodule, and multiple Tru-Cut biopsies of liver lesions. The pathology revealed 100% fibrosis of a peritoneal metastatic nodule (a nontarget lesion) and about 70% treatment-associated necrosis of the liver metastasis (one of the target lesions), with the residual viable component having a proliferation index of less than 10%. These pathologic responses have been confirmed independently by two expert pathologists at our institution. This dramatic pathologic response is in stark contrast to the >95% tumor viability noted microscopically after sunitinib therapy (Fig. 3C). The patient continued the combination of imatinib and binimetinib clinical trial with stable disease for 69.0 months until disease progression.
RNA transcript analysis of SDH-deficient GIST tumors and pretreatment tumor specimen
To evaluate whether ETV1, the intended target of the imatinib/binimetinib combination, is present in SDH-deficient GISTs, we analyzed the ETV1 and KIT expression levels in our archived GIST tumors characterized by KIT or PDGFRA activating mutations, or KIT/PDGFRA-WT SDH-deficiency. We found that both the KIT/PDGFRA-mutant and the SDH-deficient GISTs expressed high levels of KIT and ETV1 (Fig. 4A). We then analyzed the pretreatment FFPE tumor sample of the patient above with an SDH-deficient GIST along with positive and negative FFPE sample controls and found that ETV1 is highly expressed in the SHD-deficient GIST of the index case (Fig. 4B).
Figure 4.
Molecular analyses of archived GISTs and the SDH-deficient GIST patient's pre- (debulking surgery; November 20, 2012) and on-treatment (biopsy; November 5, 2015) tumor samples. A, Normalized RNA expression levels of KIT and ETV1 from U133A microarray analyses in MSKCC archived GIST samples harboring KIT (n = 29) or PDGFRA (n = 4) activating mutations, or KIT/PDGFRA WT and SDH-deficient GIST (n = 15). Each dot represents one tumor sample. Error bars: 95% CI. B, Relative RNA expression level of KIT and ETV1 compared with RPL27 (a housekeeping gene) by qRT-PCR in the patient's FFPE pretreatment tumor samples, the ETV1-expressing GIST48 human cell line, and the ETV1-negative tumor samples control. n = 3, technical replicates; error bars: mean ± S.D. Genetic alterations identified by MSK-IMPACT assays (C) and by WES (D) of the pretreatment and on-treatment FFPE tumor samples from the patient with SDH-deficient GIST shown in Fig. 4. The MSK-IMPACT assay included the mean and minimum coverage of the targeted exons and the allele frequency detected for each permutation (C). *, the mutations detected by MSK-IMPACT. SDHA nonsense mutation was not detected by WES due to lack of coverage (D). But KDR missense mutation was identified in both the WES and IMPACT.
Genetic analysis of pretreatment and on-treatment tumor specimens
To understand the molecular mechanisms of the exceptional pathologic response, we first performed MSK-IMPACT, a clinically validated targeted NGS assay encompassing 410 cancer-associated genes (20, 21) and identified SDHA exon 2 p.R31* nonsense mutation in both the pretreatment and on-treatment tumor samples of the index case. We found an additional KDR p. V1334E missense mutation in the patient's on-treatment resected tumor samples (Fig. 4C). The KDR p.V1334E mutation resides in the last 22 amino acids from the C-terminus outside of the tyrosine kinase domain and is a variant of unknown significance (VUS). No additional mutations or copy-number variations were found. These data confirm the SDH-deficiency by IHC (Fig. 3C) and the lack of KIT/PDGFRA mutations.
Reasoning that the breadth of MSK-IMPACT is limited and excludes genes with less established roles in cancer, but which may still modulate response to therapy, we also performed WES of the FFPE pretreatment and on-treatment patient tumor samples to explore genetic perturbations comprehensively. Consistently, WES identified the presence of the same missense mutation (VUS) in KDR with 50% allelic frequency. WES also identified a number of other genetic alterations shared between the pre- and on-treatment tumor samples, including mutations in ITGB6, STAM, OR13C3, and several genetic alterations private to one of the pre- or on-treatment tumor samples (Fig. 4D; Supplementary Table S3). None of these genetic alterations has known functional significance. Overall, the genetic data are consistent with prior knowledge that SDH-deficient GISTs have very few genetic alterations, and SDHA/B/C/D-loss is the primary oncogenic event in the ICC context for GIST pathogenesis. These data indicate that the pathologic response is likely a result of targeting the ICC/GIST lineage-specific survival factor, ETV1, in the SDH-deficient GISTs.
Discussion
Lineage-specific oncogenic transcription factors [e.g., androgen receptor (AR), estrogen receptor, ETV1] can be excellent therapeutic targets because the toxicity is expected to be limited to the specific cell-type expressing the transcription factor. However, apart from nuclear receptors (e.g., AR), oncogenic transcription factors have been challenging to target. In GISTs, we have identified a novel therapeutic strategy to target the lineage-specific oncogenic transcription factor, ETV1, by targeting its protein stability, which depends on active MAPK signaling. Based on its critical role in ICC development and GIST pathogenesis, targeting ETV1 has the potential to bypass imatinib-resistance mechanisms that act at the level of KIT signaling. Previous studies suggest that ICC stem cells/GIST progenitors have low KIT expression and hence are intrinsically resistant to imatinib (27). Yet ICCs and GIST progenitors still depend on ETV1 for survival. These characteristics might account for part of the mechanisms of both primary and secondary resistance. Targeting ETV1 may offer a new way to address the intrinsically imatinib-resistant GIST stem cell/progenitor population, and therefore, may broadly benefit patients with GISTs, whether they have KIT/PDGFRA-mutant or SDH-deficient GISTs. Preclinical studies showed that targeting of ETV1 and KIT by the combination of binimetinib and imatinib was synergistic and could durably inhibit the ETV1 protein levels and decrease adaptive resistance compared to single-agent imatinib or binimetinib (8, 9, 12), which motivated this clinical investigation.
This phase Ib study showed that the combination of imatinib plus binimetinib at the RP2D is safe. Although no unexpected toxicity was seen, we observed two SAEs related to MEK inhibitor-associated class effects, including CRVO and dropped head syndrome. The most bothersome side effects for our patients were binimetinib-associated acneiform rash and binimetinib/imatinib-associated periorbital and peripheral edema. These were managed with prophylactic antibiotics, topical steroids, and ancillary support without the need for dose modifications. Overall, the combination therapy is reasonably tolerated with manageable toxicity.
In this phase Ib study, in patients who have imatinib-resistant KIT-mutant GISTs, the clinical activity of the imatinib and binimetinib combination therapy is limited due to the lack of an effective KIT inhibitor to suppress the binimetinib-induced feedback reactivation of the KIT/PDGFRA/MAPK signaling through the imatinib-resistant KIT-mutant variant alleles. In contrast, we observed promising clinical activity in a cohort of five patients with molecularly and histologically confirmed SDH-deficient GISTs. Although SDH-deficient GISTs can have indolent behavior initially, they typically exhibit more aggressive behavior when TKI treatment is indicated or upon progression of TKIs (4). Historically, RECIST responses are rare but can be observed upon multitargeted TKI treatments, e.g., sunitinib and regorafenib. The observed ORR ranges from 0% to 33% for a variety of TKIs, including imatinib [2.0% (1/49 patients)–8.3% (1/12 patients)] (4, 28), sunitinib [0% (0/7 patients)–14.2% (1/7 patients)–18.4% (7/38 patients)] (4, 29, 30), regorafenib [33% (2/6 patients)] (31), and no responses were observed for vandetanib and linsitinib (32, 33). For a subset of 12 patients with molecularly defined SDH-deficient GISTs, the mPFS was 9 months (95% CI, 3–58) in first-line imatinib therapy (400–800 mg/day; ref. 28). In KIT/PDGFRA-WT pediatric GISTs with the majority of patients with SDH-deficient GISTs, sunitinib treatment showed a mean duration of disease stabilization around 15 months (range, 7–21 + months) in a prospective study (30) and an mPFS of 15 months (range, 1–73 months) in a retrospective series (29). In imatinib-resistant or refractory SDH-deficient GISTs, the mPFS was 10 months with regorafenib treatment (31). Two recent trials specifically evaluating SDH-deficient GISTs with vandetanib and linsitinib showed an mPFS of 5.1 months and 10 months (95% CI, 7–25), respectively (32, 33). Similarly, we have only seen limited RECIST responses, one of five patients (20%) had RECIST PR with most patients demonstrating RECIST SD as best response. Nevertheless, it is encouraging to see that the combination of imatinib plus binimetinib demonstrated an mPFS of 45.1 months (95% CI, 15.8–NE), with a CBR of 75% at 18 months in patients with SDH-deficient GISTs in this phase Ib study.
We had the opportunity to examine the antitumor response to multiple TKIs in a single patient and sequential pre- and on-treatment tumor biopsy samples for molecular analysis. The patient with a metastatic SDH-deficient GIST has previously progressed on multiple systemic therapies, including imatinib, sunitinib, and linsitinib, with each therapy lasting less than 2 years of treatment. The patient, with documented disease progression, enrolled in this phase Ib trial with imatinib and binimetinib combination therapy, which was designed to target the ICC/GIST lineage-specific survival transcription factor, ETV1. In this study, the patient showed a modest radiographic response (RECIST approzimately −15% to −20%) but had a dramatic (70%–100%) pathologic response with durable clinical benefit. It is well known that SDH-deficient GISTs can have an indolent course. As shown in this patient, she had been treated with imatinib for 20 months with slow but clear disease progression. As her disease progressed, the duration of subsequent treatment became shorter, with sunitinib treatment for 17 months and linsitinib treatment for 9 months. Despite the relative slow pace of disease progression, pathology from the debulking surgery immediately after sunitinib treatment revealed more than 95% viable tumor and little treatment response, corroborating the lack of clinical benefit of sunitinib treatment. In contrast, this patient had derived clinical benefit from the imatinib and binimetinib combination treatment for 69 months with manageable side effects. Compared with the natural history of her disease in response to prior therapies, these observations highlight the durable response and significant clinical benefit achieved with this combination therapeutic strategy.
One apparent discrepancy of responses is between the modest radiographic response by RECIST (−15% to −20%) and the near-complete pathologic response (70%–100% treatment-associated necrosis and fibrosis) of biopsied metastatic tumors. These clinical efficacy differences have been previously observed and underlie the critical need to develop better modalities than CT/MRI-based RECIST to assess treatment responses in GISTs, especially SDH-deficient GISTs, which can be very hard to qualify radiographically. It is conceivable that when GISTs undergo fibrosis/hyalinization and/or treatment-associated necrosis, the residual “tumor” size cannot be used as an accurate assessment of the treatment response. Choi criteria that factor in both size reduction and changes in tumor density has been proposed as an alternative measure to assess treatment efficacy in GISTs (19), and is part of the secondary endpoint of the phase Ib clinical trial. Nevertheless, pathologic response remains the gold standard of treatment response. In comparison, the responses determined by the current imaging modalities can often be an underestimation of the true treatment response.
The clinical benefit and pathologic responses observed in this phase Ib trial in the SDH-deficient patients suggest that targeting ETV1 in the ICC/GIST lineage is clinically meaningful. The genetic and RNA characterization of the patient tumor specimens presented in Fig. 4 confirms the presence of high expression levels of ETV1 and KIT. Preclinical studies using in vitro and in vivo models have demonstrated the specificity of targeting the MAPK pathway and ETV1 protein stability with the combination of imatinib and binimetinib in GIST (9). The lack of KIT/PDGFRA or other MAPK pathway druggable genetic perturbations (e.g., BRAFV600E) further corroborates the concept that the treatment effect is due to on-target effect of ETV1. These studies highlight the clinical usefulness of discovering and targeting the lineage-specific dependence on ETV1 in GISTs. They also posit that the KIT/MEK inhibitor combination may be a novel and effective therapeutic strategy in SDH-deficient GISTs and possibly other GISTs with different genetic permutations but similar lineage-dependence and worth exploring further in future clinical trials with larger patient cohorts and more definitive efficacy endpoints.
Supplementary Material
Acknowledgments
We thank the patients and their families for participating in the study. The study was funded by Array BioPharma/Pfizer and supported by grants from the NIH/NCI (Cancer Center Support Grant P30CA008748 to P. Chi, L.-X. Qin, C.R. Antonescu, Y. Chen, W.D. Tap, S. Singer, A.M. Crago, S.P. D'Angelo, M.M. Gounder; Sarcoma SPORE P50CA217694 to P. Chi, L.-X. Qin, C.R. Antonescu, W.D. Tap, S. Singer, A.M. Crago, S.P. D'Angelo; P50CA140146 to C.R. Antonescu), Orphan Products Grants Program/FDA (R01FD005731 to P. Chi, L.-X. Qin, M.F. Berger, C.R. Antonescu, W.D. Tap; R01FD005105 to M.M. Gounder), Cycle for Survival and Geoffrey Beene Research Fund to P. Chi, Shuman Fund to P. Chi, C.R. Antonescu, W.D. Tap; GIST Cancer Research Fund to P. Chi, C.R. Antonescu; and GIST Cancer Awareness Fund to P. Chi; Grail to M.F. Berger.
This article is featured in Highlights of This Issue, p. 1475
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
P. Chi reports grants from Pfizer/Array BioPharma during the conduct of the study; grants and personal fees from Deciphera, personal fees from Exelixis, Zai Lab, and Novartis; and grants from Ningbo NewBay outside the submitted work. C.M. Kelly reports other support from Amgen, Merck, Servier, Kartos Therapeutics, Exicure, and Xencor and personal fees from Chemocentryx, Kartos Therapeutics and Immunicum outside the submitted work. S.P. D'Angelo reports other support from EMD Serono, Amgen, Nektar, Immune Design, GlaxoSmithKline, Incyte, Merck, Adaptimmune, Immunocore, Pfgizer (Alliance), Servier, Rain Therapeutics, Bristol Meyers Squibb, and Deciphera during the conduct of the study; and other support from EMD Serono, Amgen, Nektar, Immune Design, GlaxoSmithKline, Incyte, Merck, Adaptimmune, Immunocore, Pfizer (Alliance), Servier, Rain Therapeutics, Bristol Meyers Squibb, and Deciphera outside the submitted work. M.M. Gounder reports personal fees from Ayala, Bayer HealthCare, Boehringer Ingelheim, Daiichi Sankyo, Epizyme, Karyopharm, Rain Therapeutics, Springworks Therapeutics, Tracon, TYME Technologies, Guidepoint, GLG, Third Bridge, Flatiron Health, Medscape, More Health, Physicians Education Resource, touchIME, and Wolters Kluwer outside the submitted work. S. Movva reports grants from P30, P50, and FDA R01 during the conduct of the study and grants from Trillium, Hutchinson Medipharma, and Ascentage Pharma outside the submitted work. B.A. Nacev reports grants from the NIH during the conduct of the study and grants from the Connective Tissue Oncology Society, NIH outside the submitted work. A.M. Crago reports grants from NIH/NCI SPORE, NIH Cancer Center during the conduct of the study and personal fees and other support from Springworks Therapeutics outside the submitted work. M.F. Berger reports personal fees from Eli Lilly and Company and PetDx outside the submitted work. Y. Chen reports personal fees and other support from Oric Pharmaceuticals outside the submitted work. W.D. Tap reports other support from Array BioPharma and Novartis during the conduct of the study; personal fees from Eli Lilly and Company, EMD Serono, Mundipharma, C4 Therapeutics, Daiichi Sankyo, Blueprint, Agios Pharmaceuticals, NanoCarrier, Deciphera, Adcendo, Ayala, Kowa, Servier, Bayer HealthCare, Epizyme, Cogent, Medpacto, Foghorn, and Amgen outside the submitted work; in addition, W.D. Tap has a patent for Companion diagnostic for CDK4 inhibitors - 14/854,329 pending to MSK/SKI and a patent for Enigma and CDH18 as companion diagnostics for CDK4 inhibition - SKI2016-021-03 pending to MSK/SKI. W.D. Tap also reports relationships with Certis Oncology Solutions (Scientific Advisory Board), Atropos Therapeutics (stock ownership, cofounder), and Innova Therapeutics (stock ownership, Scientific Advisory Board). No disclosures were reported by the other authors.
Authors' Contributions
P. Chi: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing. L.-X. Qin: Data curation, formal analysis, investigation, methodology, writing–review and editing. N. Camacho: Formal analysis, writing–review and editing. C.M. Kelly: Investigation, writing–review and editing. S.P. D'Angelo: Investigation, writing–review and editing. M.A. Dickson: Investigation, writing–review and editing. M.M. Gounder: Investigation, writing–review and editing. M.L. Keohan: Investigation, writing–review and editing. S. Movva: Investigation, writing–review and editing. B.A. Nacev: Investigation, writing–review and editing. E. Rosenbaum: Investigation, writing–review and editing. K.A. Thornton: Investigation, writing–review and editing. A.M. Crago: Investigation, writing–review and editing. J.H. Francis: Investigation, writing–review and editing. M. Martindale: Data curation. H.T. Phelan: Data curation, investigation. M.D. Biniakewitz: Data curation, investigation. C.J. Lee: Data curation, investigation. S. Singer: Investigation, writing–review and editing. S. Hwang: Investigation, writing–review and editing. M.F. Berger: Formal analysis, investigation, methodology, writing–review and editing. Y. Chen: Conceptualization, formal analysis, investigation, writing–review and editing. C.R. Antonescu: Data curation, investigation, writing–review and editing. W.D. Tap: Conceptualization, supervision, investigation, writing–review and editing.
References
- 1. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer 2011;11:865–78. [DOI] [PubMed] [Google Scholar]
- 2. Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708–10. [DOI] [PubMed] [Google Scholar]
- 3. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577–80. [DOI] [PubMed] [Google Scholar]
- 4. Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, George S, et al. Molecular subtypes of KIT/PDGFRA wild-type gastrointestinal stromal tumors: a report from the national institutes of health gastrointestinal stromal tumor clinic. JAMA Oncol 2016;2:922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Killian JK, Kim SY, Miettinen M, Smith C, Merino M, Tsokos M, et al. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov 2013;3:648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flavahan WA, Drier Y, Johnstone SE, Hemming ML, Tarjan DR, Hegazi E, et al. Altered chromosomal topology drives oncogenic programs in SDH-deficient GISTs. Nature 2019;575:229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Janeway KA, Liegl B, Harlow A, Le C, Perez-Atayde A, Kozakewich H, et al. Pediatric KIT wild-type and platelet-derived growth factor receptor alpha-wild-type gastrointestinal stromal tumors share KIT activation but not mechanisms of genetic progression with adult gastrointestinal stromal tumors. Cancer Res 2007;67:9084–8. [DOI] [PubMed] [Google Scholar]
- 8. Chi P, Chen Y, Zhang L, Guo X, Wongvipat J, Shamu T, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature 2010;467:849–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ran L, Sirota I, Cao Z, Murphy D, Chen Y, Shukla S, et al. Combined inhibition of MAP kinase and KIT signaling synergistically destabilizes ETV1 and suppresses GIST tumor growth. Cancer Discov 2015;5:304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayashi Y, Bardsley MR, Toyomasu Y, Milosavljevic S, Gajdos GB, Choi KM, et al. Platelet-derived growth factor receptor-alpha regulates proliferation of gastrointestinal stromal tumor cells with mutations in KIT by stabilizing ETV1. Gastroenterology 2015;149:420–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ostrowski J, Polkowski M, Paziewska A, Skrzypczak M, Goryca K, Rubel T, et al. Functional features of gene expression profiles differentiating gastrointestinal stromal tumours according to KIT mutations and expression. BMC Cancer 2009;9:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie Y, Cao Z, Wong EW, Guan Y, Ma W, Zhang JQ, et al. COP1/DET1/ETS axis regulates ERK transcriptome and sensitivity to MAPK inhibitors. J ClinInvest 2018;128:1442–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang B, Krall EB, Aguirre AJ, Kim M, Widlund HR, Doshi MB, et al. ATXN1L, CIC, and ETS transcription factors modulate sensitivity to MAPK pathway inhibition. Cell Rep 2017;18:1543–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lito P, Rosen N, Solit DB. Tumor adaptation and resistance to RAF inhibitors. Nat Med 2013;19:1401–9. [DOI] [PubMed] [Google Scholar]
- 15. Chi P, Qin L, D'Angelo SP, Dickson MA, Gounder MM, Keohan ML, et al. A phase Ib/II study of MEK162 (binimetinib [BINI]) in combination with imatinib in patients with advanced gastrointestinal stromal tumor (GIST). J Clin Oncol 33:15s, 2015(suppl; abstr 10507). [Google Scholar]
- 16. Chi P, Qin LX, Nguyen B, Kelly CM, D'Angelo SP, Dickson MA, et al. Phase II trial of imatinib plus binimetinib in patients with treatment-naive advanced gastrointestinal stromal tumor. J Clin Oncol 2022 Jan 18:JCO2102029. doi: 10.1200/JCO.21.02029. Online ahead of print. Available from: https://ascopubs.org/doi/abs/10.1200/JCO.21.02029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ji Y, Li Y, Nebiyou Bekele B. Dose-finding in phase I clinical trials based on toxicity probability intervals. Clin Trials 2007;4:235–44. [DOI] [PubMed] [Google Scholar]
- 18. Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CM, Queirolo P, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 2013;14:249–56. [DOI] [PubMed] [Google Scholar]
- 19. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007;25:1753–9. [DOI] [PubMed] [Google Scholar]
- 20. Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 2015;17:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Won HH, Scott SN, Brannon AR, Shah RH, Berger MF. Detecting somatic genetic alterations in tumor specimens by exon capture and massively parallel sequencing. J Vis Exp 2013;80:e50710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Antonescu CR, Viale A, Sarran L, Tschernyavsky SJ, Gonen M, Segal NH, et al. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res 2004;10:3282–90. [DOI] [PubMed] [Google Scholar]
- 23. Francis JH, Diamond EL, Chi P, Jaben K, Hyman DM, Abramson DH. MEK inhibitor-associated central retinal vein occlusion associated with hyperhomocysteinemia and MTHFR variants. Ocul Oncol Pathol 2020;6:159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen X, Schwartz GK, DeAngelis LM, Kaley T, Carvajal RD. Dropped head syndrome: report of three cases during treatment with a MEK inhibitor. Neurology 2012;79:1929–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berger M, Amini-Adlé M, Maucort-Boulch D, Robinson P, Robinson P, Thomas L, et al. Left ventricular ejection fraction decrease related to BRAF and/or MEK inhibitors in metastatic melanoma patients: a retrospective analysis. Cancer Med 2020;9:2611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blay JY, Shen L, Kang YK, Rutkowski P, Qin S, Nosov D, et al. Nilotinib versus imatinib as first-line therapy for patients with unresectable or metastatic gastrointestinal stromal tumours (ENESTg1): a randomised phase 3 trial. Lancet Oncol 2015;16:550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bardsley MR, Horvath VJ, Asuzu DT, Lorincz A, Redelman D, Hayashi Y, et al. Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors. Gastroenterology 2010;139:942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heinrich MC, Rankin C, Blanke CD, Demetri GD, Borden EC, Ryan CW, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: analysis of phase 3 SWOG intergroup trial S0033. JAMA Oncol 2017;3:944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rutkowski P, Magnan H, Chou AJ, Benson C. Treatment of gastrointestinal stromal tumours in paediatric and young adult patients with sunitinib: a multicentre case series. BMC Cancer 2017;17:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Janeway KA, Albritton KH, Van Den Abbeele AD, D'Amato GZ, Pedrazzoli P, Siena S, et al. Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Pediatr Blood Cancer 2009;52:767–71. [DOI] [PubMed] [Google Scholar]
- 31. Ben-Ami E, Barysauskas CM, von Mehren M, Heinrich MC, Corless CL, Butrynski JE, et al. Long-term follow-up results of the multicenter phase II trial of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of standard tyrosine kinase inhibitor therapy. Ann Oncol 2016;27:1794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. von Mehren M, George S, Heinrich MC, Schuetze SM, Yap JT, Yu JQ, et al. Linsitinib (OSI-906) for the treatment of adult and pediatric wild-type gastrointestinal stromal tumors, a SARC phase II study. Clin Cancer Res 2020;26:1837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glod J, Arnaldez FI, Wiener L, Spencer M, Killian JK, Meltzer P, et al. A phase II trial of vandetanib in children and adults with succinate dehydrogenase-deficient gastrointestinal stromal tumor. Clin Cancer Res 2019;25:6302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated during and/or analyzed during the current study, including patient-level clinical data as well as all sequencing data are publicly available upon request from the corresponding author.