Abstract
This cross-sectional study examined whether performance on the computerized Paired Associate Learning (PAL) task from the Cambridge Neuropsychological Test Automated Battery is associated with amyloid positivity as measured by Positron Emission Tomography, regional volume composites as measured by Magnetic Resonance Imaging, and cognitive impairment. Participants from the BIOCARD Study (N = 73, including 62 cognitively normal and 11 with mild cognitive impairment; M age = 70 years) completed the PAL task, a comprehensive clinical and neuropsychological assessment, and neuroimaging as part of their annual study visit. In linear regressions covarying age, sex, years of education and diagnosis, higher PAL error scores were associated with amyloid positivity but not with medial temporal or cortical volume composites. By comparison, standard neuropsychological measures of episodic memory and global cognition were unrelated to amyloid positivity, but better performance on the verbal episodic memory measures was associated with larger cortical volume composites. Participants with mild cognitive impairment demonstrated worse cognitive performance on all of the cognitive measures, including the PAL task. These findings suggest that this computerized visual paired associate learning task may be more sensitive to amyloid positivity than standard neuropsychological tests, and may therefore be a promising tool for detecting amyloid positivity in non-demented participants.
Keywords: cognition, paired associate learning, computerized tasks, amyloid positivity, Mild Cognitive Impairment
There is increasing evidence for subtle but significant changes in cognition during the preclinical phase of Alzheimer’s disease (AD), as measured by computerized neuropsychological tests (e.g., Buckley et al., 2017; De Jager et al., 2005; Polcher et al., 2017; Rentz et al., 2013; Stricker et al., 2020). However, less is known about whether computerized tasks are sensitive to biomarker changes during early disease phases. Consistent with the recognized need to identify brief, low-cost markers that are sensitive to both early cognitive changes and the presence of AD pathology, this study examined the relationship of a computerized test of visual paired associate learning to imaging biomarkers.
The Paired Associates Learning (PAL) task from the Cambridge Neuropsychological Test Automated Battery (CANTAB®; Cambridge Cognition, 2019) is a computerized task that assesses visual learning and memory (Barnett et al., 2016). Prior studies have demonstrated impaired CANTAB PAL performance among individuals with mild cognitive impairment (MCI) and mild AD dementia (e.g., Blackwell et al., 2004; de Jager et al., 2002; Egerházi et al., 2007; Fowler et al., 2002; Junkkila et al., 2012; Reijs et al., 2017), and that PAL performance, in combination with other measures, predicts progression to dementia with high accuracy (Blackwell et al., 2004; Fowler et al., 2002; Mitchell et al., 2009). However, prior studies evaluating PAL performance and biomarkers of AD pathology are more limited.
Most prior studies examining the relationship of PAL performance and AD biomarkers have used measures from cerebrospinal fluid (CSF). Collectively, these studies have demonstrated an association between PAL performance and CSF measures of beta-amyloid and tau (Reijs et al., 2017; Nathan et al., 2017; Salvadori et al., 2020; Soldan et al., 2016; but see Galluzzi et al., 2016). In contrast, only one prior study, to our knowledge, has evaluated the relationship between CANTAB PAL performance and amyloid burden as measured by Positron Emission Tomography (PET), and found no association among cognitively normal older adults (Konijnenberg et al., 2019). Studies that have evaluated the relationship of PAL performance to brain structure as measured by Magnetic Resonance Imaging (MRI) have shown an association with medial temporal lobe integrity among individuals with MCI (Meyer et al., 2013; Nathan et al., 2017), though null results have also been reported (Salvadori et al., 2020).
Expanding prior work, this study examined the cross-sectional association of PAL performance with 1) amyloid positivity as measured by PET imaging, 2) regional volume composites as measured by MRI, and 3) diagnosis in a sample of well-characterized participants without dementia (62 with normal cognition, 11 with MCI). As a secondary goal, we compared the results of the PAL analyses to standard paper and pencil neuropsychological measures to determine if the results were unique to the PAL task, or more widely observed for other neuropsychological tests. We hypothesized that performance on the PAL task would be more sensitive to AD biomarkers, relative to standard neuropsychological tests.
Methods
Study Design
This study reports cross-sectional data from the BIOCARD study, an ongoing, longitudinal study that was designed to identify variables among cognitively normal individuals associated with the subsequent development of symptoms of MCI or dementia (see Supplemental Materials 1 for additional study details). All data reported here were collected between 2015–2019. The present analyses included data from 73 participants without dementia (62 cognitively normal, 11 MCI) who completed the CANTAB PAL task within 16 months of their first amyloid PET scan (M days between PAL assessment and PET scan acquisition = 178, SD = 199). Of these, n = 2 were excluded from the MRI analyses because their MRI scan was collected prior to their PET scan, and more than 16 months before their PAL assessment. All participants signed informed consent forms approved by the JHU Institutional Review Board.
Clinical and Cognitive Assessments
Annual JHU visits include clinical and neuropsychological assessments, and annual consensus diagnostic reviews (see Supplemental Materials 1 and Albert et al., 2014). Briefly, the diagnostic criteria follow the recommendations incorporated in the National Institute on Aging and the Alzheimer’s Association working group reports for the diagnosis of MCI (Albert et al., 2011) and dementia due to AD (McKhann et al., 2011). This includes establishing a syndromic diagnosis (i.e., cognitively normal, MCI, impaired not MCI, dementia) based on: (1) clinical data pertaining to the medical, neurological, and psychiatric status of the individual; (2) reports of changes in cognition by the individual and by collateral sources, based on the Clinical Dementia Rating interview (Hughes et al., 1982; Morris, 1993); and (3) decline in cognitive performance, based on review of longitudinal testing.
In the CANTAB PAL task (https://www.cambridgecognition.com/cantab/; Cambridge Cognition, 2019), participants are instructed to remember the location of colorful abstract patterns presented within several possible locations on an iPad screen. In the data presented here, participants could complete up to 5 stages, which involve learning one, two, three, six or eight pattern-location pairings. For each trial, participants are first presented with gray boxes configured in a circle on a black background, indicating possible target locations. All boxes are “opened” in a randomized order, revealing either an empty box or a pattern to be remembered. After the final box is “opened,” the previously presented patterns are sequentially presented in the middle of the screen. Participants respond by tapping the location in which the pattern appeared. If all patterns are correctly recalled at any given stage, the task advances to the next successively difficult stage. If any of the patterns are incorrectly recalled, the trial is repeated, with up to ten attempts per stage at the 6- and 8-item stages. The outcome variable in the present analyses was the total number of errors across all stages, adjusted for the estimated number of errors a participant would have made on any problems not attempted due to a previous failure (i.e., ‘total errors adjusted’), referred to as PALTEA (max = 120).
The standard neuropsychological test scores examined for purposes of comparison included: (1) Verbal Paired Associates immediate recall (Wechsler Memory Scale - Revised (WMS-R); Wechsler, 1987), as it involves paired associate memory, like the PAL. (2) A verbal episodic memory composite score, as composite scores have been reported to have superior psychometric properties for studying early AD cognitive change compared to individual tests (Langbaum et al., 2014, 2015). This composite score (Soldan et al., 2019) was derived from confirmatory factor analysis, and calculated by summing weighted z-scores for WMS-R Logical Memory delayed recall, WMS-R Paired Associates immediate recall, and California Verbal Learning Test trial 1–5 recall (Delis et al., 1987). It therefore reflects a range of episodic memory processes from multiple neuropsychological tests, including delayed story recall, immediate paired associate recall, and repetition learning. (3) The Mini-Mental State Examination (MMSE; Folstein et al., 1975), as it is a commonly used measure of global cognition.
PiB PET image acquisition and processing
Dynamic 11C-labeled Pittsburgh compound B (PiB) PET scans were obtained on a GE Advance PET scanner in 3D mode, acquired over 70 minutes immediately following an intravenous bolus injection of 11C-PiB. The PiB PET scans were processed using a method described in detail previously (Bilgel et al., 2018; Walker et al., 2020). Briefly, the anatomical label image was transformed from MRI to PET space and the PET PiB distribution volume ratio (DVR) image was generated using cerebellar gray matter as the reference region. Mean cortical DVR (cDVR) was calculated by averaging DVR values across cortical regions, using parcellation maps generated by MRICloud (Walker et al., 2020). PiB positivity was defined as a mean cDVR threshold of 1.06 based on two-class Gaussian mixture modeling (Bilgel et al., 2016).
MRI acquisition and image processing
MRI scans were acquired on a 3T MR system (Philips Healthcare, Best, The Netherlands). The protocol included magnetization-prepared rapid gradient echo (MPRAGE) scans for structural brain imaging (TR=6.8ms, TE=3.1ms, shot interval 3000ms, flip angle=8°, FOV=240×256mm2, 170 slices with 1×1×1.2mm3 voxels, and scan duration=5min59s). Brain volumes were segmented and quantified using an automatic processing tool, MRICloud (Mori et al., 2016; www.MRICloud.org). A brief description of steps involved in MRICloud’s highly reproducible (Rezende et al., 2019) T1-weighted image processing is provided in Supplemental Materials 2. These analyses focused on two volumetric composites similar to those used in prior work in this cohort, consisting of regions previously shown to be ‘vulnerable’ to neuronal injury in AD: (1) the MTL volume composite included the hippocampus, entorhinal cortex, and amygdala (Pettigrew et al., 2017), and (2) the cortical volume composite included the inferior temporal gyrus, middle temporal gyrus, middle and superior temporal gyri poles, angular gyrus, superior parietal gyrus, precuneus, and posterior cingulate gyrus (Pettigrew et al., 2016). The composites were created as follows: for each region, volumes of the left and right hemispheres were first averaged. Left/right averages were then regressed on intracranial volume (ICV; i.e., total volume of brain tissues, ventricles, and sulci) to normalize for head size. The standardized residuals from these regression models were averaged to create the two composites described above.
Statistical analysis
Group differences in descriptive statistics were assessed by Wilcoxon rank sum tests for continuous variables and chi-square tests for binary variables.
Cross-sectional relationships between PAL performance and imaging biomarkers were examined with linear regression models, using separate models for amyloid positivity, the MTL volume composite, and the cortical volume composite. Comparable models were run for the three standard neuropsychological test scores. Model covariates included age (at cognitive assessment), sex, years of education, and diagnosis (normal vs. MCI). All continuous variables were standardized prior to model fitting and p < 0.05 was considered significant. Effect sizes were calculated using Hedges’ g, given the unequal group sizes.
A series of sensitivity analyses were also run. The first examined the impact of prior PAL task exposure (0, 1) on PALTEA performance. Additional models evaluated a continuous measure of cortical amyloid burden (i.e., cDVR, instead of PiB positivity), and the individual MTL regions. All analyses were run in R (version 4.0.3).
Results
On average, participants with MCI had higher PALTEA scores (i.e., more errors) and lower performance on the standard neuropsychological measures relative to those with normal cognition; they also had higher rates of PiB positivity and smaller MRI volume composites, though these biomarker measures did not differ between groups (Table 1).
Table 1.
Demographic and descriptive statistics, by diagnosis. Values reflect mean (SD) [range] unless otherwise indicated.
| All subjects | Cognitively normal | MCI | Effect size ^ | |
|---|---|---|---|---|
| N | 73 | 62 | 11 | |
| Age | 69.9 (8.1) | 69.3 (7.9) | 73.3 (8.8) | 0.49 |
| Female sex, N (%) | 50 (69%) | 42 (68%) | 8 (73%) | |
| White race, N (%) | 69 (94.52%) | 60 (96.77%) | 9 (81.82%) | |
| Years of education | 17.1 (2.4) | 17.1 (2.4) | 17.1 (2.4) | 0.00 |
| PALTEA scores | 21.7 (25.5) [0–120] | 17.3 (21. 4) [0–120] | 46.5 (32.7) [19–120] * | 1.24 |
| Verbal Paired Associates, immediate recall | 20.4 (2.9) [13–24] | 20.8 (2.8) [13–24] | 18.4 (2.7) [13–21] * | 0.85 |
| Verbal episodic memory composite score | 1.46 (1.54) [−2.08–4.74] | 1.78 (1.36) [−0.78–4.74] | −0.34 (1.19) [−2.08–1.48] * | 1.57 |
| MMSE | 29.0 (1.3) [24–30] | 29.4 (0.8) [27–30] | 27.1 (1.6) [24–29] * | 2.28 |
| PiB positive, N (%) | 24 (33%) | 20 (32%) | 4 (36%) | |
| Cortical DVR | 1.08 (0.14) | 1.08 (0.14) | 1.10 (0.17) | 0.14 |
| MTL volume composite # | −0.013 (0.79) | 0.031 (0.79) | −0.318 (0.71) | 0.45 |
| Cortical volume composite # | 0.050 (0.43) | 0.065 (0.45) | −0.047 (0.22) | 0.26 |
Significant group difference, p < 0.05, using Wilcoxon rank sum tests for continuous variables and chi-square tests for dichotomous variables.
n = 71
Hedges’ g
Abbreviations. DVR, distribution volume ratio. MCI, mild cognitive impairment. MMSE, Mini-Mental State Examination. MTL, medial temporal lobe. PALTEA, paired associate learning total errors adjusted. PET, Positron Emission Tomography.
PAL performance and imaging biomarkers
Higher PAL error scores were associated with amyloid positivity (effect size, Hedges’ g=0.60) (Table 2, Figure 1). The pattern of results was similar using cDVR (estimate=0.21, 95% CI (−0.03, 0.43), p=0.08). In contrast, PAL performance was unrelated to the MTL and cortical volume composites, and to the individual MTL regions (see Supplemental Materials 3). In all models, PAL error scores were higher among participants with MCI, but PAL performance was unrelated to age, sex, or years of education. All patterns of results were unchanged when an indicator for prior PAL task exposure was included as an additional model covariate; notably prior task exposure was not significantly related to PAL performance (p>0.09; data not shown). The patterns of results were similar when amyloid positivity and MRI measures were included as simultaneous model predictors (data not shown).
Table 2.
Association of CANTAB PAL performance and imaging biomarkers.
| PiB PET: amyloid positivity | MRI: MTL volume composite | MRI: Cortical volume composite | ||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | |
| Age | 0.020 (−0.195, 0.236) | 0.85 | 0.049 (−0.196, 0.293) | 0.69 | 0.074 (−0.162, 0.31) | 0.53 |
| Sex, female | −0.129 (−0.597, 0.339) | 0.58 | −0.143 (−0.667, 0.382) | 0.59 | −0.078 (−0.584, 0.428) | 0.76 |
| Education | −0.018 (−0.234, 0.199) | 0.87 | −0.041 (−0.278, 0.196) | 0.73 | −0.032 (−0.27, 0.205) | 0.79 |
| Diagnosis | 1.120 (0.521, 1.719) | < 0.001 | 1.052 (0.362, 1.741) | 0.003 | 1.071 (0.38, 1.761) | 0.003 |
| Imaging biomarker | 0.543 (0.083, 1.002) | 0.02 | −0.126 (−0.378, 0.126) | 0.32 | −0.080 (−0.316, 0.157) | 0.50 |
Note: In these models, PALTEA score was the dependent variable and independent variables are listed in the first column.
Abbreviations. CANTAB, Cambridge Neuropsychological Test Automated Battery. CI, confidence interval. MTL, medial temporal lobe. MRI, Magnetic Resonance Imaging. PAL, paired associate learning. PET, Positron Emission Tomography.
Figure 1.
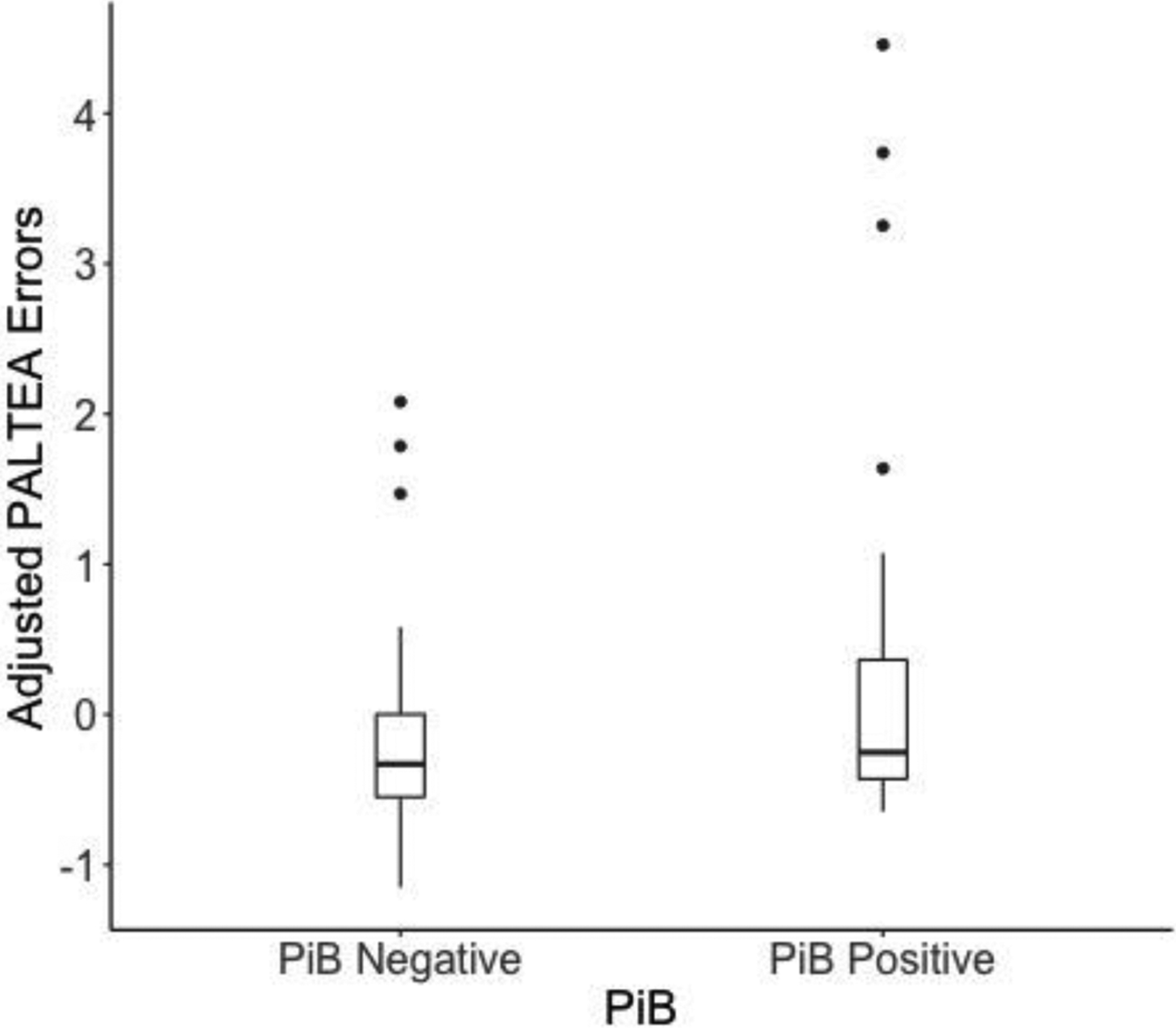
Association between PAL error scores and PiB positivity (adjusted for age, sex, years of education, and diagnosis).
Standard neuropsychological measures and imaging biomarkers
The three neuropsychological measures were unrelated to amyloid positivity (similarly, for cDVR, all p-values>0.33, data not shown), the MTL volume composite, and to the individual MTL regions. However, better performance on the verbal Paired Associates task and the verbal episodic memory composite score (but not MMSE) was associated with larger cortical volume composites (Table 3). In all models, neuropsychological performance was lower among participants with MCI and males, with trends for lower performance with fewer years of education.
Table 3.
Association of standard neuropsychological measures and imaging biomarkers.
| PiB PET: amyloid positivity | MRI: MTL volume composite | MRI: Cortical volume composite | ||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | |
| Verbal Paired Associates immediate recall | ||||||
| Age | −0.012 (−0.239, 0.214) | 0.91 | −0.050 (−0.279, 0.179) | 0.66 | 0.016 (−0.198, 0.231) | 0.88 |
| Sex, female | 0.762 (0.287, 1.236) | 0.002 | 0.755 (0.264, 1.246) | 0.003 | 0.832 (0.372, 1.292) | 0.001 |
| Education | 0.172 (−0.048, 0.393) | 0.12 | 0.148 (−0.074, 0.370) | 0.19 | 0.205 (−0.011, 0.420) | 0.06 |
| Diagnosis | −0.857 (−1.466, −0.249) | 0.006 | −1.097 (−1.742, −0.452) | 0.001 | −1.031 (−1.659, −0.404) | 0.002 |
| Imaging biomarker | −0.038 (−0.505, 0.428) | 0.87 | −0.091 (−0.327, 0.144) | 0.44 | 0.215 (0.000, 0.430) | 0.05 |
| Verbal episodic memory composite score | ||||||
| Age | −0.035 (−0.24, 0.171) | 0.74 | −0.038 (−0.251, 0.175) | 0.72 | −0.003 (−0.195, 0.190) | 0.98 |
| Sex, female | 0.661 (0.231, 1.092) | 0.003 | 0.714 (0.257, 1.170) | 0.003 | 0.718 (0.305, 1.132) | 0.001 |
| Education | 0.169 (−0.031, 0.369) | 0.10 | 0.174 (−0.033, 0.380) | 0.10 | 0.217 (0.023, 0.411) | 0.03 |
| Diagnosis | −1.396 (−1.948, −0.844) | < 0.001 | −1.517 (−2.117, −0.917) | < 0.001 | −1.476 (−2.039, −0.912) | < 0.001 |
| Imaging biomarker | −0.127 (−0.550, 0.297) | 0.55 | 0.046 (−0.173, 0.265) | 0.68 | 0.283 (0.090, 0.476) | 0.005 |
| MMSE | ||||||
| Age | −0.086 (−0.271, 0.099) | 0.36 | −0.103 (−0.293, 0.087) | 0.28 | −0.084 (−0.267, 0.099) | 0.37 |
| Sex, female | 0.472 (0.083, 0.861) | 0.02 | 0.484 (0.076, 0.893) | 0.02 | 0.512 (0.120, 0.905) | 0.01 |
| Education | 0.141 (−0.040, 0.322) | 0.12 | 0.128 (−0.056, 0.313) | 0.17 | 0.142 (−0.042, 0.327) | 0.13 |
| Diagnosis | −1.764 (−2.263, −1.266) | < 0.001 | −1.945 (−2.482, −1.409) | < 0.001 | −1.927 (−2.463, −1.392) | < 0.001 |
| Imaging biomarker | 0.012 (−0.370, 0.394) | 0.95 | −0.041 (−0.237, 0.155) | 0.68 | 0.036 (−0.147, 0.220) | 0.69 |
Note: In these models, the neuropsychological measure was the dependent variable and independent variables are listed in the first column.
Abbreviations. CI, confidence interval. MMSE, Mini-Mental State Examination. MTL, medial temporal lobe. MRI, Magnetic Resonance Imaging. PET, Positron Emission Tomography.
Discussion
In this cross-sectional study, PAL performance was associated with amyloid positivity. The association between PAL performance and amyloid positivity was moderate in magnitude (effect size=0.60). However, PAL scores were not associated with the MRI measures. In comparison, scores on a subset of standard neuropsychological assessments were associated with the cortical volume composite, but not amyloid positivity. Participants with MCI demonstrated worse performance on all of the cognitive measures, including the PAL task. Together, these results indicate that the PAL task may serve as a promising tool for detecting amyloid positivity in non-demented participants.
To our knowledge, only one prior study has examined the relationship of PAL performance to PET amyloid burden. Although Konijnenberg et al. (2019) found no association between PAL performance and amyloid levels, levels of amyloid positivity were lower (14% vs. 33% in this study), likely due to the fact that all participants were cognitively normal. Prior studies using amyloid measured in CSF have been mixed. Reijs et al. (2017) reported an association between higher PAL error scores and lower CSF amyloid-β42 in a large sample of participants with normal cognition, MCI, and dementia, and that this association did not differ by diagnosis. However, other studies have found no relationships with CSF amyloid biomarkers in cognitively normal (Konijnenberg et al., 2019) and MCI (Galluzi et al., 2016; Nathan et al., 2017; Salvadori et al., 2020) groups. Of note, studies of other computerized visual paired associate learning tasks have been similarly mixed (e.g., Lim et al., 2013; Racine et al., 2016). One possible reason for these discrepant findings may be the greater variability in PAL performance and amyloid levels afforded by studies including both cognitively normal and impaired participants (including the current study), compared to studies among a single diagnostic group. In support of this, the association between PAL performance and amyloid positivity was attenuated when the analyses were restricted to participants with normal cognition (p = 0.23), suggesting the range in variability may be important.
Prior studies in MCI participants have primarily reported associations between PAL performance and CSF levels of tau, including total tau, phosphorylated tau (p-tau), and the ratios of tau/beta-amyloid (Nathan et al., 2017; Reijs et al., 2017; see also Soldan et al., 2016), as well as worse PAL performance among participants with an ‘AD-like’ CSF profile (Salvadori et al., 2020). We therefore cannot rule out the possibility that the results reported here reflect the combined impact of amyloid and tau. Consistent with this, PiB PET amyloid burden is most strongly related to CSF p-tau/Aβ42 and tau/Aβ42 ratios, relative to the individual analytes alone (Fagan et al., 2011). While this interpretation is still in line with the view that the PAL task may be useful in the detection of PET amyloid positivity, additional studies with both amyloid and tau PET are needed.
It is noteworthy that all three standard neuropsychological measures were unrelated to amyloid positivity. However, better performance on the two verbal episodic memory measures was associated with larger cortical volume composites as well as diagnostic status. This may suggest that these tests are less sensitive than the PAL task to AD-specific biomarkers, but more sensitive to general neurodegeneration. We hypothesize that the sensitivity of the PAL to amyloid positivity may be due to the task’s multi-factorial nature. Studies examining the nature of errors on computerized visual paired associate learning tasks suggest that poor performance reflects impairments in both learning and aspects of executive function, such as strategy use (Baker et al., 2019; Harel et al., 2011; O’Donnell et al., 2011). Consistent with this hypothesis, amyloid burden has small but significant relationships with several cognitive domains (e.g., episodic memory, executive function, visuospatial) (Baker et al., 2017; Han et al., 2017; Hedden et al., 2013; Jansen et al., 2018; Pike et al., 2007).
PAL performance was unrelated to the MTL and cortical volume composites, which may be because the participants were largely cognitively normal. The few prior studies that have reported associations between PAL performance and structural MTL integrity have been conducted among individuals with MCI (Meyer et al., 2013; Nathan et al., 2017) who likely have more atrophy than the combined group of participants included here. Given that participants with MCI continued to demonstrate poorer PAL performance after accounting for both PET and MRI biomarkers, it may be that the early accumulation of AD pathology alters aspects of brain function that are important for PAL performance, in the absence of significant atrophy (Dickerson & Sperling, 2008; Pasquini et al., 2019). Consistent with this, a prior functional MRI study reported altered MTL activation (i.e., hyperactivation and hypoactivation) during PAL task performance among individuals with MCI (de Rover et al., 2011). While this provides an additional possible mechanism underlying poorer PAL performance among participants with MCI, in the absence of significant diagnostic group differences in the biomarker measures included here, future studies should examine this possibility by simultaneously measuring task-induced activations and AD biomarkers.
The PAL task has several features that make it a promising tool for clinical applications, compared to paper and pencil neuropsychological tests. PAL task administration can be standardized across individuals and time points, and performance scored immediately. The task also uses a large number of randomly selected, abstract and nonverbal stimuli, which may reduce the influence of other factors such as sex, education, and culture. In the present analyses, for example, PAL performance was associated with diagnosis and amyloid positivity, but not demographic characteristics such as sex and years of education, and PAL performance was not impacted by prior task exposure. In contrast, the standard neuropsychological measures (which were unrelated to amyloid positivity) were related to sex and years of education, and practice effects are well established (e.g., Calamia et al., 2012). The PAL task may therefore be useful as a brief, low-cost screening tool for determining whether to pursue more invasive or expensive biomarker procedures.
These findings should be interpreted within the context of the study’s limitations. Study participants were highly educated, primarily White, and have a strong family history of dementia due to AD. Additionally, the sample size was modest and some analyses (e.g., diagnostic group comparisons) may have been underpowered. These results therefore need replication in larger, more diverse cohorts.
Conclusions
Higher PAL error scores, but not standard measures of episodic memory and global cognition, were associated with amyloid positivity. These results suggest that CANTAB PAL performance may be more sensitive to amyloid positivity than standard neuropsychological tests. This task may therefore be a useful and inexpensive screening tool for clinical trials.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (grant numbers U19-AG033655, P50-AG005146), and in part by the Intramural Research Program of the National Institute on Aging, NIH. The BIOCARD Study consists of 7 Cores and two Projects with the following members: (1) the Administrative Core (Marilyn Albert, Rostislav Brichko); (2) the Clinical Core (Marilyn Albert, Anja Soldan, Corinne Pettigrew, Rebecca Gottesman, Greg Pontone, Leonie Farrington, Jules Gilles, Nicole Johnson, Maura Grega, Gay Rudow, Scott Rudow); (3) the Imaging Core (Michael Miller, Susumu Mori, Tilak Ratnanather, Andrea Faria, Anthony Kolasny, Kenichi Oishi, Laurent Younes); (4) the Biospecimen Core (Abhay Moghekar, Jacqueline Darrow, Alexandria Lewis); (5) the Informatics Core (Ann Ervin, Roberta Scherer, David Shade, Jennifer Jones, Hamadou Coulibaly, Kathy Moser); (6) the Biostatistics Core (Mei-Cheng Wang, Yuxin (Daisy) Zhu, Jiangxia Wang); (7) the Neuropathology Core (Juan Troncoso, Javier Redding, Karen Fisher); (8) Project 1 (Paul Worley, Jeremy Walston), and (9) Project 2 (Mei-Cheng Wang, Yifei Sun). The authors are grateful to the members of the BIOCARD Scientific Advisory Board who provide continued oversight and guidance regarding the conduct of the study including: Drs. David Holtzman, William Jagust, David Knopman, Walter Kukull, and Kevin Grimm, and Drs. John Hsiao and Laurie Ryan, who provide oversight on behalf of the National Institute on Aging. The authors thank the members of the BIOCARD Resource Allocation Committee who provide ongoing guidance regarding the use of the biospecimens collected as part of the study, including: Drs. Constantine Lyketsos, Carlos Pardo, Gerard Schellenberg, Leslie Shaw, Madhav Thambisetty, and John Trojanowski.
The authors acknowledge the contributions of the Geriatric Psychiatry Branch of the intramural program of NIMH who initiated the study (Principal investigator: Dr. Trey Sunderland). The authors are indebted to Dr. Karen Putnam, who provided documentation of the Geriatric Psychiatry Branch study procedures and the data files received from NIMH.
Funding:
This work was supported by grants from the National Institute on Aging (U19-AG033655, P30-AG066507).
Footnotes
Conflicts of Interest: CP, AS, RB, YZ, MCW, KK and MB report no disclosures. Michael I. Miller owns Anatomy Works, with Susumu Mori serving as its CEO. This arrangement is being managed by Johns Hopkins University in accordance with its conflict of interest policies. Marilyn Albert is an advisor to Eli Lily.
Ethics Approval: This study was approved by the Johns Hopkins University Institutional Review Board.
Consent to Participate: Written informed consent was obtained from all participants.
Availability of Data:
Anonymized data used in the analyses presented in this report are available on request from qualified investigators (biocard-se.org).
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, … Phelps CH (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M, Soldan A, Gottesman R, McKhann G, Sacktor N, Farrington L, … Selnes O (2014). Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Current Alzheimer Research, 11(8), 773–784. 10.2174/156720501108140910121920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JE, Lim YY, Pietrzak RH, Hassenstab J, Snyder PJ, Masters CL, & Maruff P (2017). Cognitive impairment and decline in cognitively normal older adults with high amyloid-β: A meta-analysis. Alzheimer’s & Dementia, 6, 108–121. 10.1016/j.dadm.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JE, Pietrzak RH, Laws SM, Ames D, Villemagne VL, Rowe CC, … Lim YY (2019). Visual paired associate learning deficits associated with elevated beta-amyloid in cognitively normal older adults. Neuropsychology, 33(7), 964–974. 10.1037/neu0000561 [DOI] [PubMed] [Google Scholar]
- Barnett JH, Blackwell AD, Sahakian BJ, & Robbins TW (2016). The Paired Associates Learning (PAL) Test: 30 Years of CANTAB Translational Neuroscience from Laboratory to Bedside in Dementia Research. Current Topics in Behavioral Neurosciences, 28, 449–474. 10.1007/7854_2015_5001 [DOI] [PubMed] [Google Scholar]
- Bilgel M, An Y, Helphrey J, Elkins W, Gomez G, Wong DF, … Resnick SM (2018). Effects of amyloid pathology and neurodegeneration on cognitive change in cognitively normal adults. Brain, 141(8), 2475–2485. 10.1093/brain/awy150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgel M, An Y, Zhou Y, Wong DF, Prince JL, Ferrucci L, & Resnick SM (2016). Individual estimates of age at detectable amyloid onset for risk factor assessment. Alzheimer’s & Dementia, 12(4), 373–379. 10.1016/j.jalz.2015.08.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, & Hodges JR (2004). Detecting dementia: novel neuropsychological markers of preclinical Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 17(1–2), 42–48. 10.1159/000074081 [DOI] [PubMed] [Google Scholar]
- Buckley RF, Sparks KP, Papp KV, Dekhtyar M, Martin C, Burnham S, Sperling RA, & Rentz DM (2017). Computerized cognitive testing for use in clinical trials: A comparison of the NIH Toolbox and Cogstate C3 batteries. The Journal of Prevention of Alzheimer’s Disease, 4(1), 3–11. 10.14283/jpad.2017.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamia M, Markon K, & Tranel D (2012). Scoring higher the second time around: Meta-analyses of practice effects in neuropsychological assessment. The Clinical Neuropsychologist, 26(4), 543–570. 10.1080/13854046.2012.680913 [DOI] [PubMed] [Google Scholar]
- Cambridge Cognition (2019). CANTAB® [Cognitive assessment software]. All rights reserved. www.cantab.com
- de Jager CA, Milwain E, & Budge M (2002). Early detection of isolated memory deficits in the elderly: the need for more sensitive neuropsychological tests. Psychological Medicine, 32(3), 483–491. 10.1017/s003329170200524x [DOI] [PubMed] [Google Scholar]
- de Jager C, Blackwell AD, Budge MM, & Sahakian BJ (2005). Predicting cognitive decline in healthy older adults. The American Journal of Geriatric Psychiatry, 13(8), 735–740. 10.1176/appi.ajgp.13.8.735 [DOI] [PubMed] [Google Scholar]
- de Rover M, Pironti VA, McCabe JA, Acosta-Cabronero J, Arana FS, Morein-Zamir S, … Sahakian BJ (2011). Hippocampal dysfunction in patients with mild cognitive impairment: a functional neuroimaging study of a visuospatial paired associates learning task. Neuropsychologia, 49(7), 2060–2070. 10.1016/j.neuropsychologia.2011.03.037 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA (1987). California Verbal Learning Test. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Dickerson BC, & Sperling RA (2008). Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: Insights from functional MRI studies. Neuropsychologia, 46(6), 1624–1635. 10.1016/j.neuropsychologia.2007.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerházi A, Berecz R, Bartók E, & Degrell I (2007). Automated Neuropsychological Test Battery (CANTAB) in mild cognitive impairment and in Alzheimer’s disease. Progress in Neuro-psychopharmacology & Biological Psychiatry, 31(3), 746–751. 10.1016/j.pnpbp.2007.01.011 [DOI] [PubMed] [Google Scholar]
- Fagan AM, Shaw LM, Xiong C, Vanderstichele H, Mintun MA, Trojanowski JQ, Coart E, Morris JC, & Holtzman DM (2011). Comparison of analytical platforms for cerebrospinal fluid measures of β-amyloid 1–42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Archives of neurology, 68(9), 1137–1144. 10.1001/archneurol.2011.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, & Louis WJ (2002). Paired associate performance in the early detection of DAT. Journal of the International Neuropsychological Society, 8(1), 58–71. [PubMed] [Google Scholar]
- Galluzzi S, Marizzoni M, Babiloni C, Albani D, Antelmi L, Bagnoli C, … PharmaCog Consortium (2016). Clinical and biomarker profiling of prodromal Alzheimer’s disease in workpackage 5 of the Innovative Medicines Initiative PharmaCog project: A ‘European ADNI study’. Journal of Internal Medicine, 279(6), 576–591. 10.1111/joim.12482 [DOI] [PubMed] [Google Scholar]
- Han SD, Nguyen CP, Stricker NH, & Nation DA (2017). Detectable Neuropsychological Differences in Early Preclinical Alzheimer’s Disease: A Meta-Analysis. Neuropsychology Review, 27(4), 305–325. 10.1007/s11065-017-9345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel BT, Darby D, Pietrzak RH, Ellis KA, Snyder PJ, & Maruff P (2011). Examining the nature of impairment in visual paired associate learning in amnestic mild cognitive impairment. Neuropsychology, 25(6), 752–762. 10.1037/a0024237 [DOI] [PubMed] [Google Scholar]
- Hedden T, Oh H, Younger AP, & Patel TA (2013). Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology, 80(14), 1341–1348. 10.1212/WNL.0b013e31828ab35d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, & Martin RL (1982). A new clinical scale for the staging of dementia. The British Journal of Psychiatry, 140, 566–572. 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Tijms BM, Fagan AM, Hansson O, Klunk WE, … Zetterberg H (2018). Association of Cerebral Amyloid-β Aggregation With Cognitive Functioning in Persons Without Dementia. JAMA Psychiatry, 75(1), 84–95. 10.1001/jamapsychiatry.2017.3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junkkila J, Oja S, Laine M, & Karrasch M (2012). Applicability of the CANTAB-PAL computerized memory test in identifying amnestic mild cognitive impairment and Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 34(2), 83–89. 10.1159/000342116 [DOI] [PubMed] [Google Scholar]
- Konijnenberg E, den Braber A, Ten Kate M, Tomassen J, Mulder SD, Yaqub M, … Visser PJ (2019). Association of amyloid pathology with memory performance and cognitive complaints in cognitively normal older adults: A monozygotic twin study. Neurobiology of Aging, 77, 58–65. 10.1016/j.neurobiolaging.2019.01.006 [DOI] [PubMed] [Google Scholar]
- Langbaum JB, Hendrix S, Ayutyanont N, Bennett DA, Shah RC, Barnes LL, Lopera F, Reiman EM, & Tariot PN (2015). Establishing composite cognitive endpoints for use in preclinical Alzheimer’s disease trials. The Journal of Prevention of Alzheimer’s Disease, 2(1), 2–3. 10.14283/jpad.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbaum JB, Hendrix SB, Ayutyanont N, Chen K, Fleisher AS, Shah RC, Barnes LL, Bennett DA, Tariot PN, & Reiman EM (2014). An empirically derived composite cognitive test score with improved power to track and evaluate treatments for preclinical Alzheimer’s disease. Alzheimer’s & Dementia, 10(6), 666–674. 10.1016/j.jalz.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Ellis KA, Ames D, Darby D, Harrington K, Martins RN, … AIBL Research Group (2013). Aβ amyloid, cognition, and APOE genotype in healthy older adults. Alzheimer’s & Dementia, 9(5), 538–545. 10.1016/j.jalz.2012.07.004 [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, … Phelps CH (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer P, Feldkamp H, Hoppstädter M, King AV, Frölich L, Wessa M, & Flor H (2013). Using voxel-based morphometry to examine the relationship between regional brain volumes and memory performance in amnestic mild cognitive impairment. Frontiers in Behavioral Neuroscience, 7, 89. 10.3389/fnbeh.2013.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Arnold R, Dawson K, Nestor PJ, & Hodges JR (2009). Outcome in subgroups of mild cognitive impairment (MCI) is highly predictable using a simple algorithm. Journal of Neurology, 256(9), 1500–1509. 10.1007/s00415-009-5152-0 [DOI] [PubMed] [Google Scholar]
- Mori S, Wu D, Ceritoglu C, Li Y, Kolasny A, Vaillant MA, … Miller MI (2016). MRICloud: Delivering high-throughput MRI neuroinformatics as cloud-based software as a service. Computing in Science & Engineering, 18, 21–35. 10.1109/mcse.2016.93 [DOI] [Google Scholar]
- Morris JC (1993). The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology, 43(11), 2412–2414. 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Nathan PJ, Lim YY, Abbott R, Galluzzi S, Marizzoni M, Babiloni C, … PharmaCog Consortium (2017). Association between CSF biomarkers, hippocampal volume and cognitive function in patients with amnestic mild cognitive impairment (MCI). Neurobiology of Aging, 53, 1–10. 10.1016/j.neurobiolaging.2017.01.013 [DOI] [PubMed] [Google Scholar]
- O’Donnell J, Pietrzak RH, Ellis KC, Snyder PJ, & Maruff P (2011). Understanding failure of visual paired associate learning in amnestic mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology, 33(10), 1069–1078. 10.1080/13803395.2011.596821 [DOI] [PubMed] [Google Scholar]
- Pasquini L, Rahmani F, Maleki-Balajoo S, La Joie R, Zarei M, Sorg C, Drzezga A, & Tahmasian M (2019). Medial temporal lobe disconnection and hyperexcitability across Alzheimer’s disease stages. Journal of Alzheimer’s Disease Reports, 3(1), 103–112. 10.3233/ADR-190121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew C, Soldan A, Sloane K, Cai Q, Wang J, Wang MC, … BIOCARD Research Team (2017). Progressive medial temporal lobe atrophy during preclinical Alzheimer’s disease. NeuroImage: Clinical, 16, 439–446. 10.1016/j.nicl.2017.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew C, Soldan A, Zhu Y, Wang MC, Moghekar A, Brown T, …BIOCARD Research Team (2016). Cortical thickness in relation to clinical symptom onset in preclinical AD. NeuroImage: Clinical, 12, 116–122. 10.1016/j.nicl.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, … Rowe CC (2007). Beta-amyloid imaging and memory in non-demented individuals: Evidence for preclinical Alzheimer’s disease. Brain, 130(Pt 11), 2837–2844. 10.1093/brain/awm238 [DOI] [PubMed] [Google Scholar]
- Polcher A, Frommann I, Koppara A, Wolfsgruber S, Jessen F, & Wagner M (2017). Face-name associative recognition deficits in subjective cognitive decline and mild cognitive impairment. Journal of Alzheimer’s Disease, 56(3), 1185–1196. 10.3233/JAD-160637 [DOI] [PubMed] [Google Scholar]
- Reijs B, Ramakers I, Köhler S, Teunissen CE, Koel-Simmelink M, Nathan PJ, … Visser PJ (2017). Memory correlates of Alzheimer’s disease cerebrospinal fluid markers: A longitudinal cohort study. Journal of Alzheimer’s Disease, 60(3), 1119–1128. 10.3233/JAD-160766 [DOI] [PubMed] [Google Scholar]
- Racine AM, Clark LR, Berman SE, Koscik RL, Mueller KD, Norton D, … Johnson SC (2016). Associations between performance on an abbreviated CogState battery, other measures of cognitive function, and biomarkers in people at risk for Alzheimer’s disease. Journal of Alzheimer’s Disease, 54(4), 1395–1408. 10.3233/JAD-160528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Parra Rodriguez MA, Amariglio R, Stern Y, Sperling R, & Ferris S (2013). Promising developments in neuropsychological approaches for the detection of preclinical Alzheimer’s disease: A selective review. Alzheimer’s Research & Therapy, 5(6), 58. 10.1186/alzrt222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende T, Campos BM, Hsu J, Li Y, Ceritoglu C, Kutten K, França Junior MC, Mori S, Miller MI, & Faria AV (2019). Test-retest reproducibility of a multi-atlas automated segmentation tool on multimodality brain MRI. Brain and behavior, 9(10), e01363. 10.1002/brb3.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadori N, Biscetti L, Eusebi P, Farotti L, Chipi E, Chiappiniello A, … Parnetti L (2020). Relationship of neuropsychological assessment with brain MRI measures and CSF biomarkers in patients with mild cognitive impairment. Current Neurobiology, 11(2), 48–56. [Google Scholar]
- Soldan A, Moghekar A, Walker KA, Pettigrew C, Hou X, Lu H, Miller MI, Alfini A, Albert M, Xu D, Xiao MF, Worley P, & BIOCARD Research Team (2019). Resting-state functional connectivity is associated with cerebrospinal fluid levels of the synaptic protein NPTX2 in non-demented older adults. Frontiers in Aging Neuroscience, 11, 132. 10.3389/fnagi.2019.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A, Pettigrew C, Moghekar A, Albert M, & BIOCARD Research Team (2016). Computerized cognitive tests are associated with biomarkers of Alzheimer’s disease in cognitively normal individuals 10 years prior. Journal of the International Neuropsychological Society, 22(10), 968–977. 10.1017/S1355617716000722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker NH, Lundt ES, Albertson SM, Machulda MM, Pudumjee SB, Kremers WK, Jack CR, Knopman DS, Petersen RC, & Mielke MM (2020). Diagnostic and prognostic accuracy of the Cogstate Brief Battery and Auditory Verbal Learning Test in preclinical Alzheimer’s disease and incident mild cognitive impairment: Implications for defining subtle objective cognitive impairment. Journal of Alzheimer’s Disease, 76(1), 261–274. 10.3233/JAD-200087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Gross AL, Moghekar AR, Soldan A, Pettigrew C, Hou X, … Walston J (2020). Association of peripheral inflammatory markers with connectivity in large-scale functional brain networks of non-demented older adults. Brain, Behavior, and Immunity, 87, 388–396. 10.1016/j.bbi.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1987). Wechsler Memory Scale - Revised Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data used in the analyses presented in this report are available on request from qualified investigators (biocard-se.org).


