Abstract
In order to evaluate the arrhythmogenic potency of macrolide antibiotics in a quantitative manner, we analyzed the influence of clarithromycin (CAM), roxithromycin (RXM), and azithromycin (AZM) on Q-T intervals from pharmacokinetic and pharmacodynamic points of view and in comparison with the potency of erythromycin (EM) previously reported by us for rats. Male Sprague-Dawley rats were anesthetized, and CAM (6.6, 21.6, and 43.2 mg/kg of body weight/h), RXM (20 and 40 mg/kg/h), and AZM (40 and 100 mg/kg/h) were intravenously injected for 90 min to obtain the time courses of drug concentrations in plasma and the changes in the Q-T intervals during and after the drug injections. Distinct Q-T interval prolongation of up to 10 ms was observed with CAM at its clinical concentrations. RXM and AZM evoked Q-T interval prolongation at concentrations higher than their clinical ranges. The potencies for Q-T interval prolongation, assessed as the slope of the concentration-response relationship, were 6.09, 0.536, and 0.989 ms · ml/μg for CAM, RXM, and AZM, respectively. There was hysteresis between the change in the Q-T intervals and the time course of the plasma concentration of each drug. The rank order of clinical arrhythmogenicity was estimated to be EM > CAM > RXM > AZM, as assessed from the present results and our previous report for EM. In conclusion, RXM and AZM were estimated to be less potent at provoking arrhythmia than EM and CAM. These results should be useful for making a safer choice of an appropriate agent for patients with electrocardiographic risk factors.
Macrolide antibiotics have been widely used for the treatment of infections caused by gram-positive organisms. Many macrolide antibiotics inhibit the metabolic activity of cytochrome P450 3A4 in the liver and intestine in an irreversible manner (8, 22). With concomitant administration of macrolide antibiotics and potentially arrhythmogenic agents, such as terfenadine or astemizole, fatal ventricular arrhythmias, including torsades de pointes (TdP), were reported to occur as a result of an increase in the concentrations of the unchanged drugs in plasma (3, 12). Besides the metabolic inhibition, erythromycin (EM) and clarithromycin (CAM) themselves possess arrhythmogenic activities and induce Q-T interval prolongation, resulting in ventricular arrhythmia, such as TdP (14, 15, 17, 23). In isolated heart preparations from guinea pigs and dogs, EM was also shown to prolong the Q-T interval and action potential duration (1, 5). We have proved by a pharmacokinetic-pharmacodynamic analysis with rats that EM at its clinical range induces Q-T interval prolongation in a concentration-dependent manner (9). Taking these facts into consideration, extra care should be taken for erythromycin-treated patients with cardiographic risk factors.
For making the choice of macrolide antibiotics, it is important to quantitatively estimate and compare the arrhythmogenic potencies of macrolide antibiotics. However, few studies have been conducted to quantitatively evaluate the arrhythmogenicities of CAM, roxithromycin (RXM), or azithromycin (AZM) or to compare their arrhythmogenic risks with those of EM.
In the present study, we aimed to evaluate the arrhythmogenic risks of CAM, RXM, and AZM from pharmacokinetic and pharmacodynamic points of view and in comparison with the potency of EM using rat electrocardiograms (ECG). Data for EM are from our previous study (9), which was conducted with the same methods and the same model.
MATERIALS AND METHODS
Chemicals.
CAM, RXM, and AZM were kind gifts from Taisho Pharmaceuticals Co., Ltd. (Tokyo, Japan), Hoechst Marion Roussel Co., Ltd. (Tokyo, Japan), and Pfizer Pharmaceuticals Inc. (Tokyo, Japan), respectively. All other chemicals used were of reagent grade and were commercially available.
Pharmacodynamic experiments.
Male Sprague-Dawley rats weighing 250 to 350 g were purchased from Nihon Ikagaku Zairyou Co., Ltd. (Tokyo, Japan), and anesthetized with an intraperitoneal injection of urethane and α-chloralose (1.2 and 30 mg/kg of body weight, respectively). The precordial and limb hair was removed with hair-removing cream (Kanebo, Tokyo, Japan). With the animals restrained in a supine position, the trachea, right jugular vein, and right carotid artery were cannulated with polyethylene tubing. The body temperature was maintained at 37.5 ± 0.5°C throughout the experiments by a hot water-circulating heat pad placed beneath the animals. The ECG from the bipolar limb lead (lead II) was recorded and analyzed by the method of Ohtani et al. (18). The Q-T intervals were derived from the average shape of the ECG recording over 10 s. The Q-T intervals were not corrected for heart rate, since the Q-T intervals in rats have been reported not to be affected by heart rate, contrary to the situation for rabbits, guinea pigs, or humans (10, 19).
After stabilization of the ECG and body temperature, a physiological salt solution (135 mM NaCl, 11.9 mM NaHCO3, 5.4 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2) was infused into the jugular vein at rates of 0.80 to 2.08 ml/h for 90 min by an infusion pump (model 22; Harvard Apparatus, Cambridge, Mass.). CAM (6.6, 21.6, or 43.2 mg/kg/h), RXM (20 or 40 mg/kg/h), or AZM (40 or 100 mg/kg/h) was then infused for 90 min in the same manner. Each drug was dissolved in physiological salt solution by use of a stoichiometrically equivalent amount of phosphoric acid neutralized with 1 N NaOH. The doses were selected by the following method. As the clinical range of CAM evoked Q-T interval prolongation, we attempted to investigate the saturable concentration-response relationship as shown for EM and increased the dose to 43.2 mg/kg/h. For RXM or AZM, since significant Q-T interval prolongation was not observed with the clinical range, we increased the dose to evoke significant Q-T interval prolongation up to 10 ms; this increase might enable a pharmacodynamic comparison.
The Q-T intervals were measured at −10, 0, 1, 2, 3, 4, 5, 6, 7, 10, 15, 20, 30, 45, 60, 75, 91, 92, 93, 94, 95, 96, 97, 100, 105, 110, 120, 135, 150, 165, and 180 min after the start of infusion.
Pharmacokinetic experiments.
Pharmacokinetic experiments were performed with animals other than those used in the pharmacodynamic experiments to avoid possible effects of blood sampling on pharmacodynamic parameters. All conditions were identical to those used in the pharmacodynamic experiments mentioned above, with the exception of blood sampling from the carotid artery at 2, 5, 15, 45, 90, 92, 95, 105, 135, and 180 min after drug administration. Blood samples (200 to 400 μl) were centrifuged at 1,500 × g for 10 min to collect plasma. The concentrations of CAM, RXM, and AZM in plasma were determined by a high-performance liquid chromatographic procedure reported previously by Taninaka et al. (26).
Model analysis.
Pharmacokinetic parameters, i.e., V1, k21, α, and β, were calculated by simultaneous fitting of all the time profiles of plasma concentration to a conventional two-compartment open model with a first-order elimination process using a nonlinear least-squares regression program, MULTI (29), where V1 is the volume of distribution of the central compartment, k21 is the rate constant for transfer from the peripheral compartment to the central one, and α and β represent the exponential rate constants. Statistical comparisons for the discrimination of the pharmacokinetic model were carried out based on F statistics by the method described by Endrenyi and Patel (7).
Parameters such as the rate constant for elimination from the central compartment (k10), half-life of the elimination phase [t1/2(β)], volume of distribution at steady state (Vss), and total plasma clearance (CL) were calculated from the above parameters.
The effect compartment model introduced by Sheiner et al. (24) was applied for the analysis of Q-T interval prolongation. The pharmacological effect (E), the increase in the Q-T intervals in this study, was assumed to be related to the drug concentration in the effect compartment (Ce) by equation 1 or 2, depending upon the nature of the observed relationship:
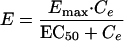 |
1 |
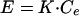 |
2 |
In these equations, Emax, EC50, and K denote the maximum effect, the concentration at which the half-maximal effect was evoked, and the slope of the concentration-response relationship (i.e., potency), respectively. Ce was calculated with a conventional two-compartment model with a zero-order infusion as follows (11):
with t ≤ 90,
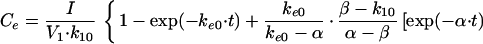 |
3 |
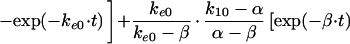 |
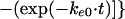 |
with t > 90,
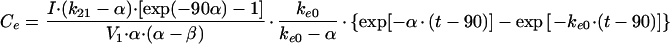 |
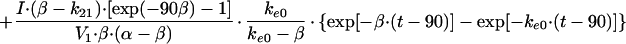 |
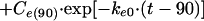 |
4 |
In these equations, ke0, I, and Ce(90) indicate the rate constant for elimination from the effect compartment, the rate of infusion of the drug, and the Ce at the end of the infusion (90 min), respectively.
Pharmacodynamic parameters, Emax, EC50, ke0, and K, were derived by simultaneous fitting of the ECG effects (E) at all infusion rates to equation 1 or 2 and equation 3 or 4 with naive averaging of data using nonlinear least-squares regression analysis (29).
Pharmacokinetic and pharmacodynamic data for EM.
All the pharmacokinetic and pharmacodynamic data for EM used for comparison in this study are from our previous study (9), which was conducted with the same experimental methods and the same model analysis.
RESULTS
Effects of macrolides on rat ECG.
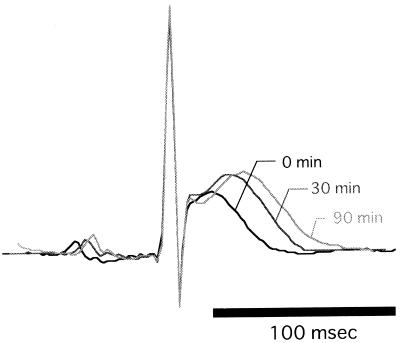
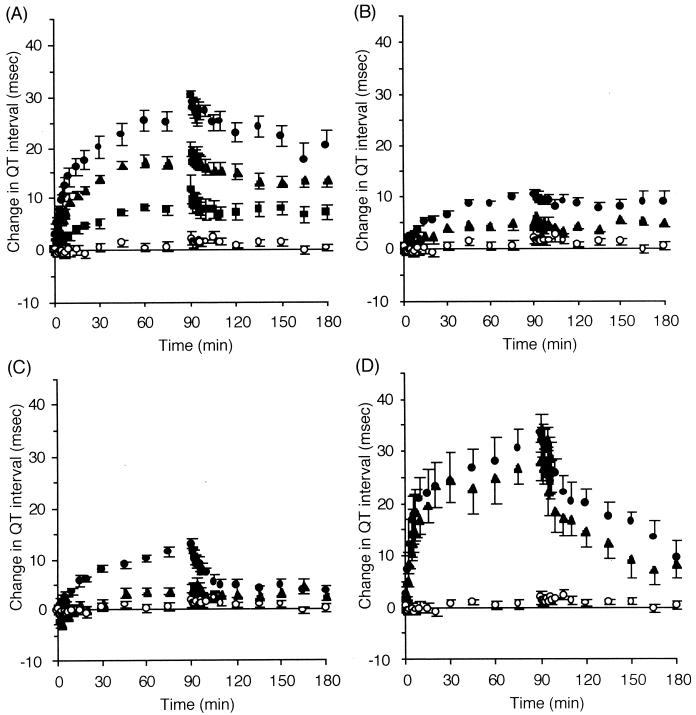
All the macrolides investigated induced Q-T interval prolongation in an infusion rate-dependent manner after the onset of infusion. Figure 1 shows a typical change in ECG shapes induced by CAM at an infusion rate of 21.6 mg/kg/h. The time courses of Q-T interval prolongation during and after the constant intravenous infusion of each drug are presented in Fig. 2. Macrolide-induced Q-T interval prolongation resumed after the cessation of infusion but with a lag time. The saturated nature of Q-T interval prolongation relative to the infusion rate was shown for CAM, while RXM and AZM did not show such a characteristic under the current experimental conditions. No macrolide affected the P-R interval or the QRS interval (data not shown).
FIG. 1.
Typical change in ECG shapes evoked by a constant intravenous infusion of CAM (21.6 mg/kg/h). The ECG shapes before and 30 and 90 min after the beginning of CAM infusion are shown in a superimposed manner.
FIG. 2.
Changes in Q-T intervals before and after intravenous infusion of CAM (■, 6.6 mg/kg/h [n = 5]; ▴, 21.6 mg/kg/h [n = 6]; ●, 43.2 mg/kg/h [n = 6]) (A), RXM (▴, 20 mg/kg/h [n = 5]; ●, 40 mg/kg/h [n = 5]) (B), and AZM (▴, 40 mg/kg/h [n = 5]; ●, 100 mg/kg/h [n = 5]) (C) compared with data previously reported by us (9) for EM (○, vehicle [n = 5]; ▴, 4.0 mg/kg/h [n = 5]; ●, 8.0 mg/kg/h [n = 4]) (D). Data are reported as mean and standard error of the mean.
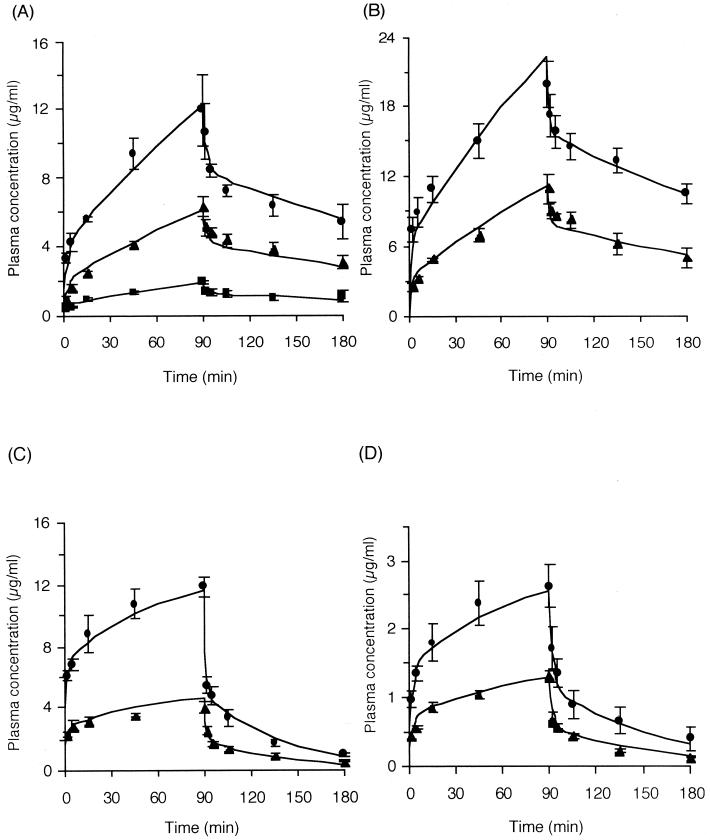
Pharmacokinetics of macrolides.
Figure 3 represents the time courses of plasma drug concentrations during and after the infusion of drugs. The two-compartment open model could successfully explain the pharmacokinetics of CAM, RXM, and AZM, and nonlinear kinetics were not observed for any macrolide examined. Table 1 shows the pharmacokinetic parameters of each macrolide.
FIG. 3.
Time courses of the concentrations in plasma of CAM (■, 6.6 mg/kg/h [n = 3]; ▴, 21.6 mg/kg/h [n = 3]; ●, 43.2 mg/kg/h [n = 3]) (A), RXM (▴, 20 mg/kg/h [n = 4]; ●, 40 mg/kg/h [n = 3]) (B), and AZM (▴, 40 mg/kg/h [n = 3]; ●, 100 mg/kg/h [n = 3]) (C) compared with data previously reported by us (9) for EM (▴, 4.0 mg/kg/h [n = 4]; ●, 8.0 mg/kg/h [n = 4]) (D). Data are reported as mean and standard error of the mean.
TABLE 1.
Pharmacokinetic parameters for CAM, RXM, and AZM, along with those for EM previously reported by us (9)
| Drug | α (min−1)a | β (min−1)a | V1 (ml/kg)a | kel (min−1)a | t1/2(β) (min) | Vss (liters/kg) | CL (ml/min/kg) |
|---|---|---|---|---|---|---|---|
| CAM | 0.421 ± 0.087 | 0.00467 ± 0.00097 | 418 ± 61 | 0.0606 ± 0.0134 | 149 | 4.70 | 25.3 |
| RXM | 0.872 ± 0.323 | 0.00457 ± 0.00106 | 111 ± 35 | 0.113 ± 0.043 | 152 | 2.41 | 12.5 |
| AZM | 0.846 ± 0.191 | 0.0188 ± 0.0020 | 285 ± 56 | 0.461 ± 0.094 | 36.8 | 3.34 | 131 |
| EM | 0.476 ± 0.079 | 0.0141 ± 0.0021 | 192 ± 24 | 0.229 ± 0.031 | 49.1 | 1.71 | 44.4 |
Parameters were estimated by a nonlinear least-squares method. Values are reported as estimates and standard deviations.
Pharmacokinetic-pharmacodynamic analysis of Q-T interval prolongation induced by macrolides.
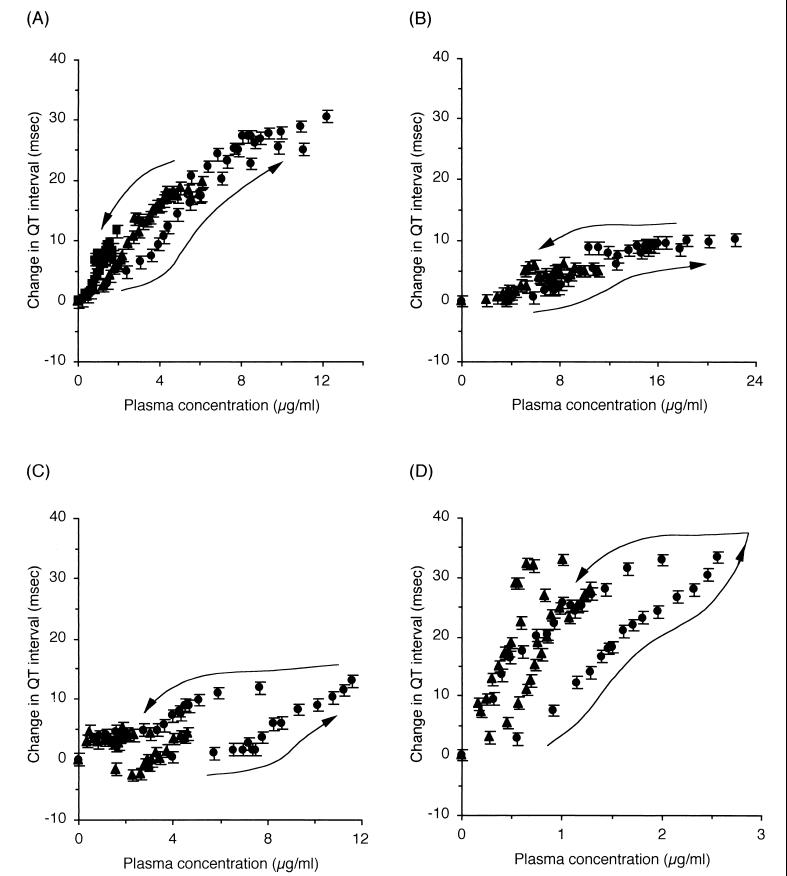
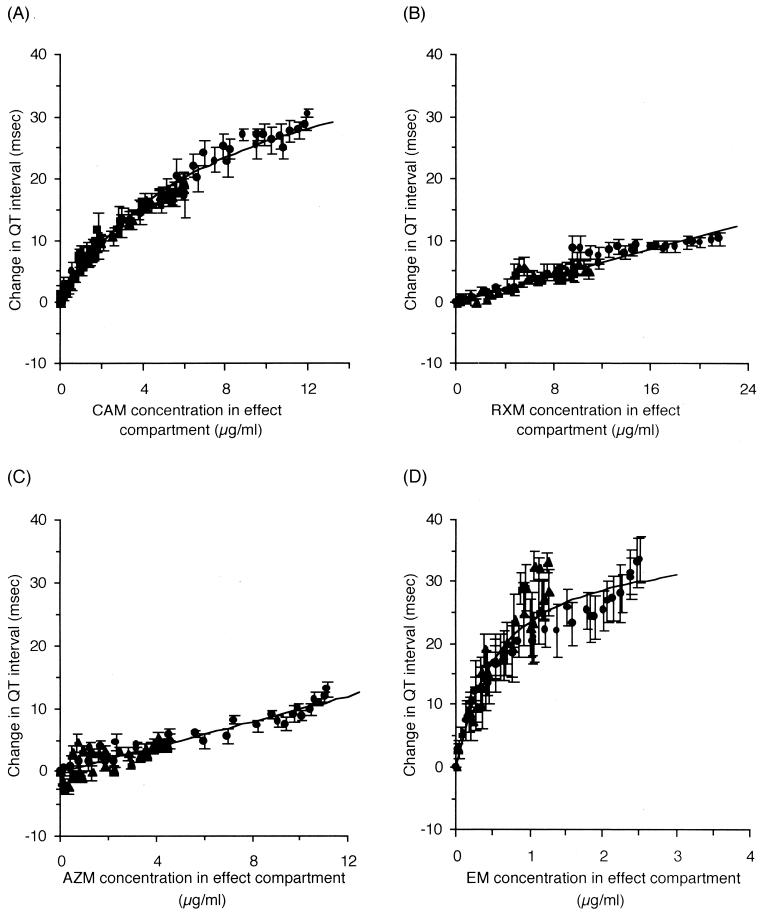
The effect compartment model introduced by Sheiner et al. (24) was applied to explain the counterclockwise hysteresis observed between plasma drug concentrations and Q-T interval prolongation (Fig. 4). For CAM, the Emax model described by equation 1 was applied to relate the drug concentration to the extent of Q-T interval prolongation, since saturation of the Q-T interval prolongation was observed. On the other hand, the linear model described by equation 2 was applied for RXM and AZM. Incorporating the time profiles of the drug concentrations estimated from the pharmacokinetic parameters (Table 1) as input functions, the time courses of Q-T interval prolongation at all the infusion rates were simultaneously fitted to equation 1 or 2 and to equation 3 or 4 to estimate the pharmacodynamic parameters. Figure 5 shows the relationship between the drug concentrations in the effect compartment and the extent of Q-T interval prolongation, together with the relationship for EM previously reported by us (9).
FIG. 4.
Relationships between drug concentrations in plasma and changes in Q-T interval (n = 5 or 6) for CAM (A), RXM (B), AZM (C), and EM (9) (D). Each arrow indicates the ECG recording time. Data are reported as mean and standard error of the mean. Symbols are the same as for Fig. 2 and 3.
FIG. 5.
Relationships between drug concentrations in the effect compartment and changes in Q-T interval (n = 5 or 6). The lines calculated from equations 1 or 2 are superimposed. (A) CAM. (B) RXM. (C) AZM. (D) EM. (9). Data are reported as mean and standard error of the mean. Symbols are the same as for Fig. 2 and 3.
DISCUSSION
Among macrolide antibiotics, EM, CAM, and spiramycin have been reported to induce Q-T interval prolongation (14, 15, 17, 23, 25). Moreover, EM was found to prolong the action potential duration in an electrophysiological study with an isolated guinea pig heart preparation (5). On the other hand, neither RXM nor AZM has been reported to be arrhythmogenic or implicated as having electrocardiographic activity. To make safer choices of macrolide antibiotics for patients with cardiac risk factors, the differences in the arrhythmogenic potencies of the drugs should be carefully taken into consideration. This study is the first to quantitatively compare the arrhythmogenic risks of macrolide antibiotics under the same conditions.
To relate the extent of Q-T interval prolongation to the drug concentration, the Emax model successfully explained the relationship for CAM as well as for EM (9). On the contrary, a linear model was used for RXM and AZM, since saturation of the Q-T interval prolongation was not observed with these agents. A plausible explanation for this finding is that these agents have a weak potency for Q-T interval prolongation at the concentration ranges used in this study. Although a concentration far exceeding the clinical range might provide a saturable relationship for RXM and AZM, higher infusion rates were experimentally nonfeasible due to the limited solubility of these drugs, since the rate of infusion of each drug was determined not by the flow rate of the solution but by the concentration of the drug. As the pharmacokinetics of all the agents showed linear properties with the current infusion rates (Fig. 3), the pharmacokinetic parameters based on the two-compartment model (Table 1) should be suitable as input functions for subsequent pharmacodynamic analyses.
The arrhythmogenic potency of a drug could be defined as the steepness of the concentration-response relationship, i.e., K in the linear model and Emax/EC50 in the Emax model, described by equations 2 and 1, respectively. The calculated potencies for Q-T interval prolongation were 6.09, 0.536, and 0.989 msec · ml/μg for CAM, RXM, and AZM, respectively (Table 2). In comparison with the potency of EM (63.5 msec · ml/μg), which we previously reported using the same experimental conditions (9), the extent of arrhythmogenicity of the four macrolides in rats was ranked as EM > CAM > AZM > RXM (Table 2).
TABLE 2.
Pharmacodynamic parameters for CAM, RXM, and AZM, along with those for EM previously reported by us (9)
| Drug | Emax (msec) | EC50 (μg/ml) | ke0 (min−1) | K or Emax/EC50 (ms · ml/μg) |
|---|---|---|---|---|
| CAM | 45.7 ± 1.8 | 7.51 ± 0.53 | 0.244 ± 0.022 | 6.09 |
| RXM | 0.220 ± 0.045 | 0.536 ± 0.012 | ||
| AZM | 0.0644 ± 0.0095 | 0.989 ± 0.034 | ||
| EM | 36.9 ± 2.0 | 0.581 ± 0.089 | 0.158 ± 0.023 | 63.5 |
Values are reported as estimates and standard deviations.
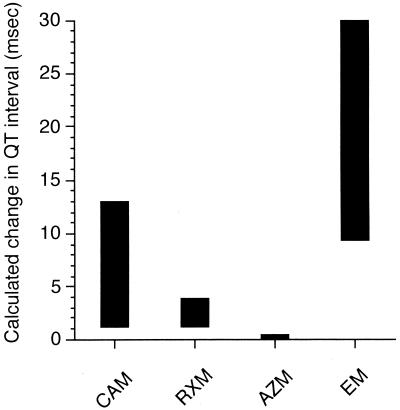
However, the clinical levels achieved in humans after the administration of regular doses must be considered. The therapeutic area under the curve, instead of the therapeutic plasma drug concentration, should be used as an index of the antimicrobial efficacy of macrolides. On the other hand, Q-T interval prolongation was indicated to be concentration dependent. We used the clinical plasma drug concentrations, which were attained after ordinary oral doses, instead of the therapeutic area under the curve. We applied these clinical concentrations to the pharmacodynamic model described by equation 1 or 2 to derive the estimated ranges for Q-T interval prolongation shown in Fig. 6. As the clinical concentrations of EM, CAM, RXM, and AZM in plasma were reported to be 0.2 to 2.0 (9), 0.2 to 3.0, 2.0 to 7.5, and 0.04 to 0.4 μg/ml, the estimated ranges for Q-T interval prolongation were 9.4 to 29.9, 1.2 to 13.0, 1.1 to 3.8, and 0.04 to 0.4 ms, respectively. These results suggested that the arrhythmogenic risk of the four macrolides should be ranked as EM > CAM > RXM > AZM.
FIG. 6.
Estimated ranges for Q-T interval prolongation evoked in rats by macrolides at clinical plasma drug concentration ranges.
The above rank order is consistent with previous findings. Honig et al. reported that after oral administration of a regular dose of macrolides to healthy volunteers, EM evoked 15 ms of Q-T interval prolongation on average, while CAM and AZM did not elicit obvious Q-T interval prolongation (13). Other studies reported the maximum effect of CAM for corrected Q-T interval prolongation in healthy volunteers as being 7 to 11 ms (4, 28). Clinical cases of TdP have been much more frequently associated with the use of EM and reached 49 cases in the MedWatch database of the Food and Drug Administration (6), while only a few such cases have been reported for CAM and no cases have been reported for RXM or AZM, to the best of our knowledge. Thus, our findings are also consistent with the difference in the incidence of TdP, although relative use of each macrolide may also affect the above difference in arrhythmogenicity.
The effect compartment was assumed to relate Q-T interval prolongation to plasma drug concentration because of the existence of a lag time, i.e., counterclockwise hysteresis, between the effect and the plasma drug concentration (Fig. 4). A possible explanation for this hysteresis is the delayed distribution into the effect site. Drug-induced Q-T interval prolongation is generally attributed to the inhibition of potassium channels on ventricular myocytes (31). Assuming that macrolide-induced Q-T interval prolongation is attributed to the inhibition of the potassium channels from the cytosolic side of the membrane, the rate-limiting permeability of the drugs may cause the delayed distribution. This assumption appears feasible, because it has been suggested that the immunosuppressants FK506 and rapamycin induce Q-T interval prolongation through FK506 binding protein-mediated potassium current inhibition (27).
Another explanation for the hysteresis is the generation of metabolites possessing Q-T interval-prolonging activity. Although several metabolites for macrolides, some of which are common to rats and humans, have been reported (21, 30), there are possibly several interspecies differences in the formation of metabolites. However, these metabolites are not detectable by electrochemical detection. In any case, unfortunately, we were unable to obtain them and could not conduct any investigation for metabolites. However, unchanged EM was reported to prolong action potential duration in vitro (1, 5). Moreover, we also found that the inhibitory action of unchanged EM on cardiac potassium current in isolated rat ventricular myocytes became stable 5 min after the onset of perfusion (unpublished observations). Taken together, these data indicate that the generation of metabolites may be less likely to have provided a significant contribution to the observed hysteresis.
We developed a rat model in which arrhythmogenicities of drugs can be successfully analyzed (18), since clinical studies of life-threatening adverse reactions, such as arrhythmia, may not be ethically feasible with humans. Potassium channels are believed to play an important role in the repolarization process of cardiac myocytes, and several differences between rats and humans have been found (2). However, the significance of the results of the present study with rats may not be dismissed because the pharmacokinetic-pharmacodynamic evaluations of Q-T interval prolongation based on rat ECG data have been in good accordance with the clinical findings for humans (9, 18, 20); however, the results should be carefully interpreted since there may be differences between rats and humans. Therefore, the present findings provide clinically significant information for making safer choices of macrolide antibiotics with regard to cardiographic adverse effects.
In conclusion, the clinical arrhythmogenic risks of the four macrolides examined were ranked as EM > CAM > RXM > AZM. This ranking was determined on the basis of Q-T interval prolongation from the pharmacokinetic and pharmacodynamic points of view, since the Q-T interval is widely agreed to be a valuable index of arrhythmogenicity in clinical settings (16).
REFERENCES
- 1.Antzelevitch C, Sun A-Q, Zhang Z-Q, Yan G-X. Cellular and ionic mechanisms underlying erythromycin-induced long QT intervals and torsade de pointes. J Am Coll Cardiol. 1996;28:1836–1848. doi: 10.1016/S0735-1097(96)00377-4. [DOI] [PubMed] [Google Scholar]
- 2.Barry D M, Nerbonne J M. Myocardial potassium channels: electrophysiological and molecular diversity. Annu Rev Physiol. 1996;58:363–394. doi: 10.1146/annurev.ph.58.030196.002051. [DOI] [PubMed] [Google Scholar]
- 3.Biglin K E, Faraon M S, Condtance T D, Lieh-Lai M. Drug-induced torsades de pointes: a possible interaction of terfenadine and erythromycin. Ann Pharmacother. 1994;28:282. doi: 10.1177/106002809402800226. [DOI] [PubMed] [Google Scholar]
- 4.Carr R A, Edmonds A, Shi H, Locke C S, Gustavson L E, Craft J C, Harris S I, Palmer R. Steady-state pharmacokinetics and electrocardiographic pharmacodynamics of clarithromycin and loratadine after individual or concomitant administration. Antimicrob Agents Chemother. 1998;42:1176–1180. doi: 10.1128/aac.42.5.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daleau P, Lessard E, Groleau M-F, Turgeon J. Erythromycin blocks the rapid component of the delayed rectifier potassium current and lengthens repolarization of guinea pig ventricular myocytes. Circulation. 1995;91:3010–3016. doi: 10.1161/01.cir.91.12.3010. [DOI] [PubMed] [Google Scholar]
- 6.Drici M-D, Knollmann B C, Wang W-X, Woosley R L. Cardiac actions of erythromycin. JAMA. 1998;280:1774–1776. doi: 10.1001/jama.280.20.1774. [DOI] [PubMed] [Google Scholar]
- 7.Endrenyi L, Patel M. Evaluation of two assumptions: single straight line, and single normal distribution. Trends Pharmacol Sci. 1991;12:293–296. doi: 10.1016/0165-6147(91)90580-l. [DOI] [PubMed] [Google Scholar]
- 8.Gorski J C, Jones D R, Haehner-Daniels B D, Hamman M A, O'Mara E M, Hall S D. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther. 1998;64:133–143. doi: 10.1016/S0009-9236(98)90146-1. [DOI] [PubMed] [Google Scholar]
- 9.Hanada E, Ohtani H, Kotaki H, Sawada Y, Sato H, Iga T. Pharmacodynamic analysis of the electrocardiographic interaction between disopyramide and erythromycin in rats. J Pharm Sci. 1999;88:234–240. doi: 10.1021/js980256r. [DOI] [PubMed] [Google Scholar]
- 10.Hayes E, Pugsley M K, Penz W P, Adaikan G, Walker M J A. Relationship between QaT and RR intervals in rats, guinea pigs, rabbits and primates. J Pharmacol Toxicol Methods. 1994;32:201–207. doi: 10.1016/1056-8719(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 11.Holford H G N, Sheiner L B. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet. 1981;6:429–453. doi: 10.2165/00003088-198106060-00002. [DOI] [PubMed] [Google Scholar]
- 12.Hong P K, Woosley R L, Zamani K, Conner D P, Cantilena L R., Jr Changes in the pharmacokinetics and electrocardiographic pharmacodynamics of terfenadine with concomitant administration of erythromycin. Clin Pharmacol Ther. 1992;52:231–238. doi: 10.1038/clpt.1992.135. [DOI] [PubMed] [Google Scholar]
- 13.Honig P K, Wortham D C, Zamani K, Cantilena L R. Comparison of the effect of the macrolide antibiotics erythromycin, clarithromycin and azithromycin on terfenadine steady-state pharmacokinetics and electrocardiographic parameters. Drug Investig. 1998;7:542–546. [Google Scholar]
- 14.Katapadi K, Kostandy G, Katapadi M, Hussain K M A, Schifter D. A review of erythromycin-induced malignant tachyarrhythmia—torsade de pointes: a case report. Angiology. 1997;48:821–826. doi: 10.1177/000331979704800909. [DOI] [PubMed] [Google Scholar]
- 15.Lee K L, Jim M-H, Tang S C, Tai Y-T. QT prolongation and torsades de pointes associated with clarithromycin. Am J Med. 1998;104:395–396. doi: 10.1016/s0002-9343(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 16.Lipicky R J. A viewpoint on drugs that prolong the QTc interval. Am J Cardiol. 1993;72:53B–54B. doi: 10.1016/0002-9149(93)90042-b. [DOI] [PubMed] [Google Scholar]
- 17.McComb J M, Campbell N P S, Cleland J. Recurrent ventricular tachycardia associated with QT prolongation after mitral valve replacement and its association with intravenous administration of erythromycin. Am J Cardiol. 1984;54:922–923. doi: 10.1016/s0002-9149(84)80237-4. [DOI] [PubMed] [Google Scholar]
- 18.Ohtani H, Hanada E, Yamamoto K, Sawada Y, Iga T. Pharmacokinetic-pharmacodynamic analysis of the electrocardiographic effects of terfenadine and quinidine in rats. Biol Pharm Bull. 1996;19:1189–1196. doi: 10.1248/bpb.19.1189. [DOI] [PubMed] [Google Scholar]
- 19.Ohtani H, Kotaki K, Sawada Y, Iga T. A comparative pharmacokinetic-pharmacodynamic study of the electrocardiographic effects of epinastine and terfenadine in rats. J Pharm Pharmacol. 1997;49:458–462. doi: 10.1111/j.2042-7158.1997.tb06824.x. [DOI] [PubMed] [Google Scholar]
- 20.Ohtani H, Sato H, Iga T, Kotaki H, Sawada Y, Iga T. Pharmacokinetic-pharmacodynamic analysis of the arrhythmogenic potency of a novel antiallergic agent, ebastine, in rats. Biopharm Drug Dispos. 1999;20:101–106. doi: 10.1002/(sici)1099-081x(199903)20:2<101::aid-bdd160>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Periti P, Mazzei T, Mini E, Novelli A. Clinical pharmacokinetic properties of the macrolide antibiotics. Effect of age and various pathophysiological states (part I) Clin Pharmacokinet. 1989;16:193–214. doi: 10.2165/00003088-198916040-00001. [DOI] [PubMed] [Google Scholar]
- 22.Periti P, Mazzei T, Mini E, Novelli A. Pharmacokinetic drug interactions of macrolides. Clin Pharmacokinet. 1992;23:106–131. doi: 10.2165/00003088-199223020-00004. [DOI] [PubMed] [Google Scholar]
- 23.Schoenenberger R A, Haefeli W E, Weiss P, Ritz R F. Association of intravenous erythromycin and potentially fatal ventricular tachycardia with Q-T prolongation (torsades de pointes) Br Med J. 1990;300:1375–1376. doi: 10.1136/bmj.300.6736.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheiner L B, Stanski D R, Vozeh S, Miller R D, Ham J. Simultaneous modelling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther. 1979;25:358–371. doi: 10.1002/cpt1979253358. [DOI] [PubMed] [Google Scholar]
- 25.Stramba-Madiale M, Guffanti S, Porta N, Frediani M, Beria G, Colnaghi C. QT interval prolongation and cardiac arrest during antibiotic therapy with spiramycin in a newborn infant. Am Heart J. 1993;126:740–742. doi: 10.1016/0002-8703(93)90438-f. [DOI] [PubMed] [Google Scholar]
- 26.Taninaka C, Ohtani H, Hanada E, Kotaki H, Sato H, Iga T. Determination of erythromycin, clarithromycin, roxithromycin and azithromycin in plasma by high-performance liquid chromatography with amperometric detection. J Chromatogr B. 2000;738:405–411. doi: 10.1016/s0378-4347(99)00512-5. [DOI] [PubMed] [Google Scholar]
- 27.Terashima A, Nakai M, Hashimoto T, Kawamata T, Taniguchi T, Yasuda M, Maeda K, Tanaka C. Single-channel activity of Ca2+-dependent K+ channel is modulated by FK506 and rapamycin. Brain Res. 1998;786:255–258. doi: 10.1016/s0006-8993(97)01435-2. [DOI] [PubMed] [Google Scholar]
- 28.Van Haarst A D, van't Klooster G A E, van Gerven J M A, Schoemaker R C, van Oene J C, Burggraaf J, Coene M-C, Cohen A F. The influence of cisapride and clarithromycin on QT intervals in healthy volunteers. Clin Pharmacol Ther. 1998;64:542–546. doi: 10.1016/S0009-9236(98)90137-0. [DOI] [PubMed] [Google Scholar]
- 29.Yamaoka K, Tanigawara Y, Nakagawa T, Uno T. A pharmacokinetic analysis program (MULTI) for microcomputer. J Pharmacobiodyn. 1981;4:879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki H, Hiroki S, Urano T, Inoue K, Shimada T. Effects of roxithromycin, erythromycin and troleandomycin on their N-demethylation by rat and human cytochrome P450 enzymes. Zenobiotica. 1996;26:1143–1153. doi: 10.3109/00498259609050259. [DOI] [PubMed] [Google Scholar]
- 31.Zehender M, Hohnloser S, Just H. QT-interval prolonging drugs: mechanisms and clinical relevance of their arrhythmogenic hazards. Cardiovasc Drugs Ther. 1991;5:515–530. doi: 10.1007/BF03029779. [DOI] [PubMed] [Google Scholar]