Abstract
Purpose
Adults with autism spectrum disorder (ASD) consistently report worse functional health and well-being, compared to neurotypical (NT) peers. In a series of studies, we aimed to elucidated the effects of sex, age, and their interaction on health-related quality of life (HRQoL) and evaluated the effectiveness of mindfulness-based stress reduction (MBSR) for improving health-, disability-, and autism-related QoL, with possible sex and age outcome moderators, in adults with ASD.
Methods
Study 1 used the 36-Item Short Form Survey to compare mental and physical HRQoL composite scores in adults with ASD (n = 67) and matched NT adults (n = 66). Study 2 was a randomized pilot evaluation of the effect of MBSR, compared to an active control intervention with social support and relaxation education (support/education; n = 56), on the World Health Organization QoL BREF, Disability, and Autism-Specific scales in adults with ASD.
Results
In Study 1, we replicated findings that mental HRQoL is worse in both men and women with ASD, compared to NT counterparts, but physical HRQoL is only worse in women with ASD. We present novel findings that older age is associated with better mental HRQoL in women with ASD only. In Study 2, MBSR improved disability-related QoL in adults with ASD over and above the support/education intervention, but both interventions improved mental HRQoL. Lastly, both interventions were more effective for HRQoL improvements in women with ASD.
Conclusion
Findings encourage precision medicine approaches tailored to age and sex groups for best HRQoL outcomes in adults with ASD.
ClinicalTrials.gov Identifier:
Keywords: Autism spectrum disorder, Quality of life, Mental health, Aging, Sex differences, Mindfulness
Introduction
The first population-based estimates of adults living with autism spectrum disorder (ASD) recently projected nearly six million in the United States [1]. While research on adults with ASD lags far behind that dedicated to children, one consistent finding is reduced quality of life (QoL), compared to neurotypical (NT) counterparts [2–6]. QoL is defined as ‘[an] individual’s perceptions of their position in life in the context of the culture and value systems in which they live, and in relation to their goals, expectations, standards, and concerns’ [7]. This is a subjective assessment of how the individual relates to the world in their current setting [4]. Operational definitions of QoL that are particularly affected in adults with ASD include what people are able to do and how they feel which, with attributions to health, are measures of HRQoL [8]. Considering the pervasiveness of reduced subjective well-being and functioning in adults with ASD, compared to their NT peers, it’s important to understand the factors which influence HRQoL and effective interventions in this population.
Influence of age on QoL in adults with ASD
Limited information exists on the impact of QoL as adults with ASD age, and using research on youth to promote untested projections in adults with ASD negates influencers specific to aging [9]. When examined across the lifespan (ages 5 through 65), individuals with ASD reported lower HRQoL and social QoL across all ages, compared to a NT sample [10]. However, among studies that have examined age as a moderating factor, there are disparate findings. Moss et al. [11] found that older age was associated with poorer physical health, but there was no NT comparison group. It is well known that physical HRQoL declines with age in the general population [12]. Mason et al. [4] found the same trend of older age being associated with poorer mental HRQoL. Although they also did not have a NT comparison group, mental HRQoL, unlike physical HRQoL, does not decline with age in the general population [12]. Therefore, mental HRQoL may represent an age-related vulnerability for adults with ASD. This same group later published that HRQoL and social QoL scores in adults with ASD over 55 years of age are on average one standard deviation below normative scores for the United Kingdom [13]. Conversely, while van Heijst and Geurts [6] confirmed reduced HRQoL in a sample of older adults with ASD, their general QoL meta-analysis found no effect of age. Importantly, they did not examine sex-by-age interactions, which may explain variability in findings, as elaborated on below. Since 50,000 teens with ASD in the US become adults every year [14], it is imperative to reconcile how aging will affect their QoL outcomes.
Influence of sex on QoL in adults with ASD
Similar to the problems with extrapolating childhood research to adults, generalizing results obtained from male-only or male-biased samples can misrepresent the needs of females [15]. Currently, there is an estimated 3:1, male:female prevalence ratio in ASD [16]. However, previous estimates had even higher ratios [17], which has resulted in ASD research being disproportionately based on males. Only a few studies have investigated QoL sex differences specific to adults with ASD. Women with ASD report reduced physical HRQoL compared to men with ASD, while men with ASD report reduced social QoL compared to women with ASD [4]. Others have found women with ASD report reduced mental HRQoL and social QoL, compared to men with ASD [18]. In a study across the lifespan, Graham Holmes et al. [10] reported reduced physical HRQoL in females. Further analysis of age groups revealed that this was driven by adult self-rating, parent ratings of children and adolescent self-ratings did not show sex differences [10]. This suggests disparities between females with ASD and males with ASD may increase with age, but this hypothesis needs to be systematically evaluated. Understanding how potential complex sex by age interactions best predict QoL in adults with ASD is necessary to provide optimal interventions.
Interventions for improving QoL in adults with ASD
QoL is an ideal modifiable intervention target to improve a wide range of important outcomes in adults with ASD. For example, an adult longitudinal study found higher social QoL was associated with better adult outcomes such as employment, living independently, and close relationships [11]. Moreover, pinpointing the modifiable underpinnings of QoL in adults with ASD may help identify effective interventions. Khanna et al. [19] found problem-focused (a.k.a. adaptive) coping strategies and social support as two of the top factors for improving HRQoL in adults with ASD. Further, a systematic review found increasing social participation is one route by which adults with ASD can improve QoL [20]. Lastly, Smith et al. [21] outlined a complex interplay between anxiety and mental HRQoL and social QoL in adults with ASD suggesting addressing anxiety symptoms may contribute significantly to improved QoL. Mindfulness-based stress reduction (MBSR) is a structured training program that aims to provide adaptive coping, focused attention, and cognitive restructurings skills to distressed populations [22]. The program is taught in a group context which imbeds social support. Emerging research suggests MBSR is effective in reducing anxiety and/or depressive symptoms in adults with ASD [23–27]. Combined, this suggests MBSR may be an ideal intervention for improving HRQoL in adults with ASD.
Mindfulness-based stress reduction and QoL
MBSR has been shown to improve QoL across a diverse demographic, which was most notably demonstrated in a meta-analysis of 31 randomized controlled trials with 1942 participants [28]. Mindfulness-based interventions have also been shown to improve QoL in parents of children with ASD [29, 30]. However, MBSR’s impact on QoL specifically in adults with ASD has not yet been examined in a controlled study. In an uncontrolled design, Kiep et al. [23] found a modified MBSR improved general psychosomatic well-being on the Symptom Checklist-90 in adults with ASD. More recently, a meta-analysis was performed on mindfulness intervention studies for both children and adults with ASD and caregivers where the authors averaged all outcome measure to represent “subjective well-being” [31]. Findings were overwhelmingly positive, but a need for more controlled research was noted [31].
The present study
The present study aimed to identify the effects of sex and age, and possible interactions, on HRQoL in a cross-sectional sample of adults with and without ASD (Study 1). Then, we evaluated the effectiveness of MBSR for improving HRQoL in adults with ASD (Study 2). In Study 1, we hypothesized HRQoL would be reduced in adults with ASD, compared to NT adults, with a sex-by-diagnosis interaction, such that women with ASD would have the lowest HRQoL of all groups, especially in the physical health domain. We also hypothesized a sex-by-age-by-diagnosis interaction, such that increasing age would be associated with worse HRQoL specific to women with ASD. In Study 2, we hypothesized an MBSR intervention would lead to HRQoL improvements in adults with ASD over and above a control intervention. Further, we ran exploratory analyses to determine if sex or age impacted the intervention-related HRQoL benefits. This combined study approach was taken in order to inform precision medicine needs (Study 1) and effective interventions (Study 2) for adults with ASD across the lifespan.
Methods
Study 1
Participants and HRQoL measure
Data in this cross-sectional study were derived from a larger study investigating sex differences in aging in ASD (n = 195). This study began in 2015 and HRQoL measures were added in 2017. We present HRQoL data from 67 participants with ASD and 66 NT participants ages 18–71. This sample has been previously described [32, 33]. In brief, recruitment efforts for ASD participants were conducted primarily using the Southwest Autism Research and Resource Center (SARRC) database, where clients and research participants voluntarily enroll to be contacted for future studies. Other efforts included presentations at community groups and flyers. For NT participants, recruitment occurred via snowballing and community fliers. Snowball sampling is a nonprobability sampling technique where existing study subjects recruit future subjects from among their acquaintances. For participants with ASD, inclusion criteria included a diagnosis confirmation by an experienced, research reliable psychometrist who administered the Autism Diagnostic Observation Schedule-2, module 4 (ADOS-2; [34]) as well as a brief psychiatric history interview. Using the DSM-5 checklist [35], a psychologist with 25 years of ASD diagnostics experience reviewed assessment results and confirmed diagnosis criteria were met. For NT participants, criteria for inclusion were as follows: (1) no suspected/confirmed diagnosis of ASD, (2) Social Responsiveness Scale-2 Adult Self-report (SRS-2; [36]) T-scores ≤ 66, (3) no first-degree relative with an ASD diagnosis. For all participants, the following were inclusion criteria: (1) Kaufman Brief Intelligence Test—2nd Edition (KBIT-2; [37]) full-scale IQ score > 70; Mini Mental State Exam (MMSE; [38]) score ≥ 26; no self-reported history of neurological illness (exception: single-episode childhood seizure), head injury with loss of consciousness, or known genetic disorders; and no current use of seizure medications. With the exception of schizophrenia, other psychiatric conditions were non-exclusionary given the prevalence of co-morbid conditions in ASD [39]. Detailed self-report of psychiatric and other health-related problems were recorded and are reported by group in Supp. Table 1.
Once participants were enrolled in the study, they completed the paper–pencil 36-Item Short Form Health Survey (SF-36), without modifications, as a HRQoL measure [40]. HRQoL items, such as SF-36, represent multiple operational indicators of health, including behavioral function and dysfunction, distress and well-being, objective reports and subjective ratings, and both favorable and unfavorable self-evaluations of general health status [8]. This study met ethical standards for research as described by Arizona State University and the Declaration of Helsinki 2000 revision. All participants provided written consent approved by the Institutional Review Board.
Statistical analysis
Independent samples t-tests or chi-squared tests were conducted to ensure ASD and NT groups were similar in age, sex distribution, and IQ. The SF-36 yields norm-based scores based on eight generic functioning and well-being domains that are aggregated into physical and mental component summary scores with reliability of 0.95 and 0.93, respectively [8]. For both metrics, higher scores represent greater HRQoL, with mean = 50, SD = 10, in the general US population. A multiple regression analysis was performed on each composite score separately with the following factors: diagnosis, age, sex, diagnosis by age, diagnosis by sex, and diagnosis by sex by age. Age was treated as a continuous variable and all possible interactions were tested simultaneously in one model for an overall F statistic. After Bonferroni correction, alpha was set at 0.025 for the overall F statistic of each of the two regression models. In the presence of an overall F that survived correction, factor significance was set at alpha = 0.05. With our sample size, we had > 99% power to detect diagnosis effects on HRQoL based on Graham Holmes et al. [10] effect sizes, and 70% power to detect sex-by-diagnosis interactions based on Kamio et al. [18] effect sizes.
Study 2
Participants and QoL measure
For a Consolidated Standards of Reporting Trials (CONSORT) Checklist of information to include when reporting a randomized trial see Supp. Table 2. This longitudinal pilot study recruited via the same methods in Study 1 and enrolled 70 adults across two cohorts who self-reported an ASD diagnosis. Inclusion criteria were comprised of meeting ASD criteria on the Autism Diagnostic Observation Schedule-2 [34], administered by an experienced research-reliable administrator at SARRC, and an IQ score > 70 as measured by the KBIT-2. Exclusion criteria were comprised of history of traumatic brain injury, substance abuse, and active suicide ideation. Due to our goal of recruiting a representative group of intellectually-able adults with ASD, we did not exclude adults on the basis of sex, age, or general comorbidities (see Supp. Table 3 for detailed self-report of psychiatric and other health-related problems).
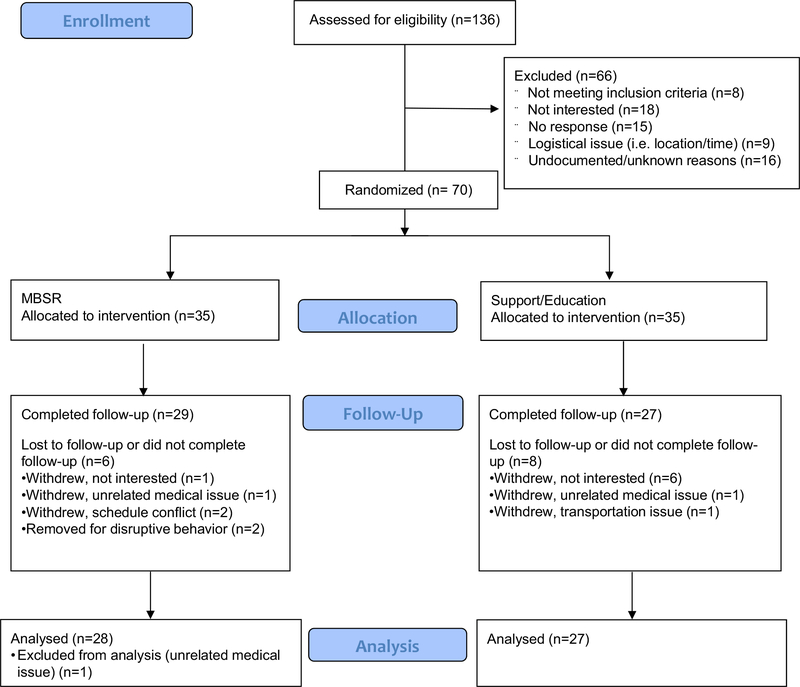
Participants were randomly assigned (via random number generator) to a parallel, eight-week MBSR group or a social support/relaxation education (support/education) control group with a 1-to-1 allocation ratio (See CONSORT Flow Diagram, Fig. 1. The PI generated the random allocation sequence, enrolled participants, and assigned participants to interventions. Those assessing outcomes were blinded after assignment to interventions, and every attempt was made for participants to be blinded by referring to both groups as “stress reduction” groups. Fourteen participants were lost to follow-up and one participant was excluded from the analysis because of a family crisis that severely affected their mental health and was unrelated to the study. The remaining 55 participants (MBSR group, n = 28; support/education group, n = 27) had pre and post-intervention QoL data. This sample size was determined as adequate for assessing HRQoL based on an effect size of d = 0.60 for MBSR effects on general psychosomatic well-being in adults with ASD [23], which requires a total sample size of 47 for 80% power.
Fig. 1.
Consolidated standards of reporting trials (CONSORT) flow diagram
Self-reported QoL was assessed with the World Health Organization (WHO) QoL Brief Version (BREF; [41]), Disability Assessment Schedule (DAS) [42], and autism-specific (AS QoL) items [43] during MRI visits within four weeks before and after the intervention (2018–2019; MRI data presented elsewhere, [24, 25]). Additionally, an informant report WHODAS was collected [44]. The WHO QoL assessment was chosen over the SF-36 because of the recent addition of AS QoL items [43]. Two participants were missing WHOBREF data, four and 14 participants were missing WHODAS self-report and proxy-report data, respectively; two were missing AS QoL data. Participant demographics can be found in Table 4. QoL measures were secondary outcomes. Primary outcomes were depression and anxiety measures, which have been previously published [24, 25]. This study was conducted in compliance with Arizona State University’s ethical standards for research and the Declaration of Helsinki 2000 revision. All participants provided written consent approved by the Institutional Review Board. Data were monitored via an Independent Monitor who is a Neurologist specializing in ASD. Full trial protocol can be accessed at ClinicalTrials.gov Identifier: NCT04017793.
Table 4.
Study 2: intervention group demographics
| MBSR (n = 28) | Support/Education (n = 27) | Statistics | |
|---|---|---|---|
| Age (years) | Mean = 30.32 ± 11.74 Range = 18–64 |
Mean = 32.56 ± 14.83 Range = 18–72 |
t(53) = 0.621, p = 0.537 |
| Sex (males/females) | 16/12 | 18/9 | X2(1) = 0.528, p = 0.467 |
| IQ (KBIT-2 composite) | Mean = 101.56 ± 16.33 Range = 70–131 |
Mean = 106.69 ± 16.11 Range = 70–139 |
t(53) = 1.174, p = 0.246 |
| Autism severity (ADOS-2 total) | Mean = 11.86 ±4.09 Range = 8–20 |
Mean = 10.81 ± 3.52 Range = 5–17 |
t(53) = − 1.012, p = 0.316 |
| Education (years) | Mean = 14.25 ± 2.21 Range = 12–19 |
Mean = 15.42 ± 2.86 Range = 12–20 |
t(46) = 1.582, p = 0.121 |
Intervention groups
The intervention groups were conducted at Arizona State University during early evening weekday hours (~ 5:30–7:30 pm Tuesday or Wednesday) in 2018 (Cohort 1) and 2019 (Cohort 2). The MBSR intervention was minorly adapted from Dr. Jon Kabat-Zinn’s original protocol and included adaptations similar to Spek et al. [27]: (i) class duration was reduced from 2.5 to 2 h, (ii) there was no required full day retreat, (iii) metaphorical references were removed from the instructions. Novel to MBSR studies in ASD, the 2-h weekly classes were co-led by a certified MBSR instructor and an ASD clinician for an 8-week period, as opposed to solely an MBSR instructor. Participants received daily email reminders to practice techniques taught in class for ~ 45 min. The overarching theme of practices involved continuous redirection of attention toward present moment experience of bodily sensations, sounds, emotions, and thoughts. Participants were encouraged to adopt a nonjudgmental and nonreactive attitude toward their experience as they were introduced to and practiced various exercises including body scanning, mindful walking, eating, and talking, awareness of the breath, and loving compassion meditation. For Cohort 2, an optional half-day retreat was offered and two participants attended.
The social support and relaxation education group (support/education group) was led by team members (authors BBB and BP) for weekly 2-h sessions over an 8-week span. Instructors provided basic education on relaxation techniques provided by the National Center for Complementary and Integrative Health (https://nccih.nih.gov/health/stress/relaxation.htm; Braden et al. [45], similar to Jain et al. [46] and Hughes et al. [47]) and facilitated group sharing/discussion, offering an opportunity to share personal experiences with stress and coping strategies. Participants were encouraged to pay attention to stressors throughout the week, practice relaxation techniques regularly at home, and to share additional resources with the group. For Cohort 2, an optional half-day social opportunity (board games was offered for the support/education group to mirror the MBSR retreat, one participant attended. Daily email reminders were sent to request that participants track stressors and techniques they utilized. An exit survey indicated most participants from both groups used techniques learned in class on an “every other day” basis. Importantly, we employed multiple strategies to ensure the integrity of findings in this pilot study where investigators had both intervention and analysis responsibilities. First, data was collected by lab members blinded to treatment conditions and hypotheses. Second, all analyses were performed blinded to group assignment. Third, blinded coders found equivalent fidelity ratings for each intervention on social support, and instructor’s believability, enthusiasm, and competence. Finally, pilot data were reanalyzed by a blinded scientist and yielded the same results/interpretation.
Statistical analysis
Independent samples t-tests or chi-squared tests were conducted to ensure MBSR and Support/Education groups were similar in age, sex distribution, IQ, and ASD severity. The WHOBREF and WHODAS scores were standardized based on Skevington et al. [41] and Andrews et al. [42]. The WHOBREF measures four domains: physical, psychological, social, and environment. The physical and psychological domains were selected from the BREF for best comparison to the SF-36 physical and mental composite scores. Across studies, WHOBREF and SF-36 generally show very high correlation and this was most robustly demonstrated in a large (n = 4628) investigation of diverse health conditions across 38 sites in the UK [48]. Specifically, the SF-36 physical composite score had the highest correlation with the physical BREF domain (r = 0.79) and the SF-36 mental composite score had the highest correlation with the psychological BREF domain (r = 0.69, [48]. The self and informant DAS each generated a total score, and the AS generated a total score. Reliability for the physical and psychological domains were 0.81 and 0.82, respectively, and were scored so that higher values represent better HRQoL [41]. Reliability for DAS total scores was high in previous studies and with these metrics lower values represent better disability-related QoL [42]. A repeated-measures ANOVA was performed with time (pre vs. post) as the within-subjects variable and each between-subjects variable separately. For BREF physical and BREF psychological, alpha was set at 0.025 after Bonferroni correction. With our sample size, we had > 99% power to detect MBSR-related improvements on HRQoL based on Kiep et al. [23] effect sizes.
For self DAS total, informant DAS total, and AS QoL total, analyses were exploratory given no prior study has investigated the effect of MBSR for adults with ASD on these variables. Exploratory analyses were also run to determine if sex or age impacted the intervention-related QoL improvements. For sex, two-way repeated-measures ANOVAs were performed with sex as the between-subjects variable and time as the within-subjects variable. We also ran two-way repeated-measures ANOVAs with sex and intervention group as between-subjects variables and time (pre vs. post) as the within-subjects variable. For age, we ran Pearson r correlations between age and change scores of variables of interest. Because of the exploratory nature, no correction for multiple comparison was employed, and alpha was set at 0.05 for two-tailed tests. We used an intent to treat approach for all analyses, and included all participants for whom post-intervention data were available. All but one participant in the final analyses attended ≥ 4 classes.
Results
Study 1
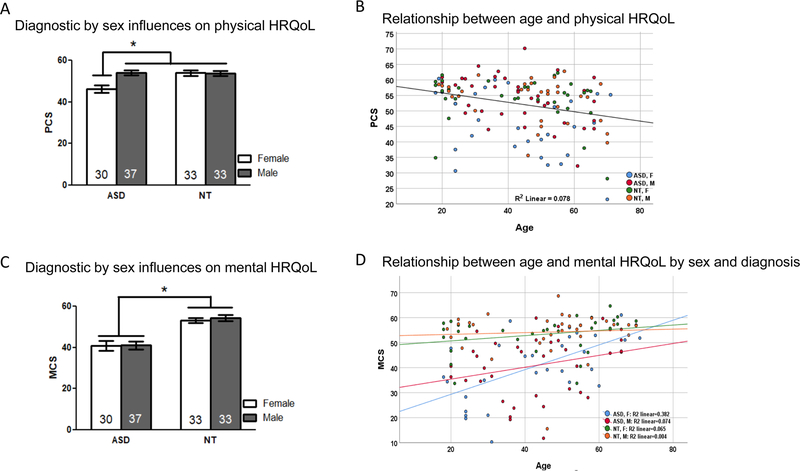
There were no significant differences between ASD and NT participants on age, IQ, or sex distribution (Table 1). Participants’ ages ranged from 18 to 71 years and averaged at 44 years of age. Descriptive statistics for SF-36 physical (PCS) and mental (MCS) composite scores can be found in Table 2. The multiple regression for the PCS was significant [F(7,126) = 5.716, p < 0.001; Adjusted R2 = 0.208] and found a significant diagnosis-by-sex interaction, such that physical HRQoL was lower in women with ASD only, as compared to men with ASD and NT men and women (Fig. 2a, Table 3). There was also a main effect of age, such that PCS was lower for older participants (Fig. 2b, Table 3). The multiple regression for MCS was significant [F(7,126) = 12.769, p < 0.001; Adjusted R2 = 0.395] and found a significant main effect of diagnosis, such that MCS was lower in adults with ASD compared to NT adults (Fig. 2c, Table 3). There was also a significant diagnosis-by-sex-by-age interaction such that increasing age was significantly associated with greater mental HRQoL in women with ASD [r(28) = 0.62, p < 0.001], but no other group (all p > 0.10; Fig. 2d, Table 3).
Table 1.
Study 1: diagnostic group demographics
| NT (n = 66) | ASD (n = 67) | Statistic | |
|---|---|---|---|
| Age (years) | Mean = 44.62 ± 16.681 Range = 18–70 |
Mean = 43.48 ± 15.057 Range = 18–71 |
t(131) = .415, p = 0.679 |
| Sex (males/females) | 33/33 | 37/30 | X2(1) = .364, p = 0.546 |
| IQ (KBIT-2 composite) | Mean = 108.12 ± 12.169 Range = 77–141 |
Mean = 107.07 ± 13.750 Range = 70–139 |
t(124) = .458, p = 0.648 |
| Education (years) | Mean = 16.39 ± 2.16 Range = 12–21 |
Mean = 15.78 ± 2.58 Range = 12–20 |
t(130) = − 1.49, p = 0.140 |
Table 2.
Study 1: SF-36 composite score descriptive statistics
| ASD (n = 67) |
NT (n = 66) |
|||
|---|---|---|---|---|
| Male (n = 37) Mean (SD) Range |
Female (n = 30) Mean (SD) Range |
Male (n = 33) Mean (SD) Range |
Female (n = 33) Mean (SD) Range |
|
| Physical | 53.99 (7.69) | 46.29 (10.20) | 53.63 (6.81) | 53.99 (7.50) |
| 32.23–70.19 | 21.46–60.42 | 35.63–62.79 | 28.14–62.24 | |
| Mental | 41.02 (12.29) | 40.87 (13.12) | 54.23 (8.78) | 53.11 (7.37) |
| 11.76–59.84 | 10.28–61.06 | 15.55–68.69 | 33.70–64.68 | |
Fig. 2.
A Mean (± SE) SF-36 physical composite scores (PCS) in ASD and NT groups separated by sex. Women with ASD had reduced physical health-related quality of life (HRQoL) compared to other groups. B The relationship between age and physical HRQoL across all participants shows worse physical HRQoL is associated with older age. C Mean (± SE) SF-36 mental composite scores (MCS) in ASD and NT groups separated by sex. Adults with ASD had reduced mental (HRQoL) with no sex differences. D The relationship between age and mental HRQoL in ASD and NT groups separated by sex. Only women with ASD show a significant positive association between age and mental HRQoL
Table 3.
Study 1: multiple regression for SF-36 physical and mental composite scores
| Beta coefficients | t Statistic | p Value | ||
|---|---|---|---|---|
| Physical | Diagnosis | .288 | .945 | .347 |
| Age | − .216 | − 2.023 | .045* | |
| Sex | − .002 | − .018 | .986 | |
| IQ | .041 | .490 | .625 | |
| Diagnosis × Age | − .320 | − 1.022 | .309 | |
| Diagnosis × Sex | − .617 | − 2.005 | .047* | |
| Diagnosis × Sex × Age | .223 | .732 | .466 | |
| Mental | Diagnosis | − .843 | − 3.171 | .002* |
| Age | .102 | 1.092 | .277 | |
| Sex | − .035 | − .366 | .715 | |
| IQ | − .030 | − .419 | .676 | |
| Diagnosis × Age | .337 | 1.234 | .220 | |
| Diagnosis × Sex | − .512 | − 1.906 | .059 | |
| Diagnosis × Sex × Age | .555 | 2.087 | .039* |
Significance = p<0.05
Study 2
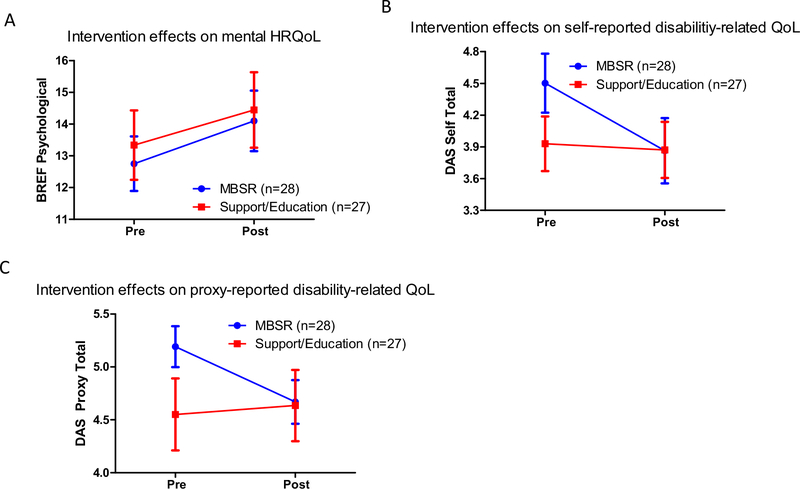
There were no significant baseline differences between MBSR and support/education participants on age, IQ, sex distribution, or ASD severity (Table 4). Descriptive statistics for BREF physical and psychological scores, DAS self and proxy total scores, and AS QoL total scores can be found in Table 5. Repeated measures ANOVA for BREF physical domain scores found no intervention effects; however, the psychological domain demonstrated a main effect of time, indicating improvements in both groups (F(1, 53) = 6.83, p = 0.01, ηp2 = 0.11; Fig. 3a). Exploratory DAS self and proxy total scores both showed group-by-time interactions [self: F(1, 52) = 6.90, p = 0.01, ηp2 = 0.12; proxy: F(1, 29) = 4.0, p = 0.05, ηp2 = 0.12], such that the MBSR group showed less difficulty in disability-related QoL after the intervention, but not the support/education group (Fig. 3b, c). There was also a main effect of time for DAS self (F(1, 52) = 6.90, p = 0.003, ηp2 = 0.16) but not DAS proxy; no main effects or interactions were detected for AS QoL total scores.
Table 5.
Study 2: WHO composite score descriptive statistics
| MBSR (n = 28) |
Support/Education (n = 27) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre |
Post |
Pre |
Post |
|||||
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| BREF physical | 15.126 (4.157) | 6.317–21.941 | 16.042 (5.008) | 4.084–24.174 | 15.782 (5.479) | 4.084–26.406 | 15.783 (6.061) | 6.316–26.406 |
| BREF psychological | 12.754 (4.557) | 4.802–21.728 | 14.103 (5.046) | 3.500–21.729 | 13.338 (5.691) | 4.802–24.334 | 14.447 (6.184) | 2.197–26.938 |
| DAS-self total | 4.502 (1.475) | 2.057–7.151 | 3.863 (1.632) | 1.679–7.717 | 3.929 (1.340) | 1.679–6.396 | 3.871 (1.378) | 1.679–6.208 |
| DAS-proxy total | 5.192 (1.026) | 3.566–7.340 | 4.669 (1.092) | 3.000–6.396 | 4.551 (1.772) | 1.679–8.283 | 4.635 (1.750) | 2.057–9.226 |
| AS total | 3.402 (.661) | 1.750–4.500 | 3.567 (.710) | 2.000–4.750 | 3.500 (.637) | 2.375–4.870 | 3.560 (.741) | 2.125–4.875 |
Fig. 3.
Pre- and post-intervention mean (± SE) WHOQOL scores in mindfulness-based stress reduction (MBSR) and support/education groups on A the BREF psychological domain, B self-report disability assessment schedule (DAS), and C proxy-report DAS. Both groups improved in mental health-related quality of life (HRQoL), but only the MBSR group improved in disability-related QoL
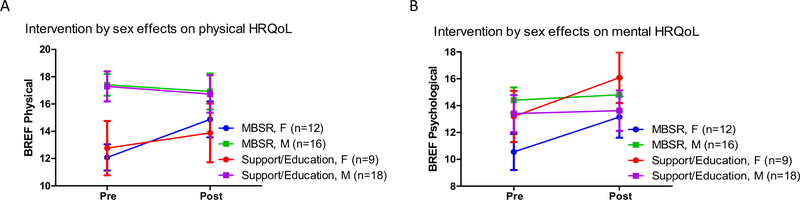
Exploratory analyses to determine if sex or age impacted the intervention-related QoL improvements identified significant sex-by-time interactions for both BREF physical (F(1, 52) = 6.02, p = 0.02, ηp2 = 0.10) and psychological (F(1, 52) = 6.44, p = 0.01, ηp2 = 0.11) scores, such that women improved more than men on both domains (Fig. 4, Supp. Table 4). This pattern did not interact with intervention group, and was similar for MBSR and support/education participants. There were no sex-by-time or sex-by-group-by time interactions for DAS or AS scores. Age did not correlate with change scores on any variables of interest.
Fig. 4.
Pre- and post-intervention mean (± SE) WHOQOL BREF scores in mindfulness-based stress reduction (MBSR) and support/education groups separated by sex on A the physical and B psychological domain. Women with ASD in both intervention groups improved in physical and mental HRQoL, but men did not significantly change
Discussion
The present studies aimed to (1) expand existing literature on sex and age, and possible interactive effects on physical and mental HRQoL in a cross-sectional sample of adults with and without ASD (Study 1), and (2) evaluated the effectiveness of MBSR for improving health-, disability-, and autism-related QoL in adults with ASD, and potential influences of sex and/or age (Study 2).
Study 1
As hypothesized HRQoL was worse in adults with ASD, but some effects depended on sex. Mental HRQoL was worse in both men and women with ASD, compared to their NT counterparts; however, physical HRQoL was only reduced in women with ASD. Worse mental HRQoL in adults with ASD, compared to NT adults, is consistent with other studies [3, 4, 10, 13], however, some have found this to be worse in women with ASD compared to men with ASD [18]. Our finding of worse physical HRQoL being specific to women with ASD is consistent with findings from Mason et al. [4] and Graham Holmes et al. [10]. For relationships between HRQoL and age, we replicated findings for physical HRQoL, but our hypothesis that increasing age would be associated with worse HRQoL specific to women with ASD was not supported. In line with Moss et al. [11] and research in the general population, we found increasing age was associated with worse physical HRQoL across all groups. Specifically, adults 45 years and over in the general population report about a 0.1–0.2 standard deviation decline per year [12], which seems to be consistent with adults with ASD. Our findings of similar age-related differences between NT and autistic adults for physical HRQoL is also consistent with the meta-analysis by van Heijst and Geurts [6].
For mental HRQoL, Mason et al. [4] previously found older age as being associated with poorer mental HRQoL in adults with ASD. Since this was not found in the van Heijst and Geurts [6] meta-analysis, a discrepant factor could be sex. Indeed, Graham Holmes et al. [10] reported reduced physical HRQoL in females, but further analysis of age groups revealed that this was driven by adult self-ratings; parent ratings of children and adolescent self-ratings did not show sex differences [10]. This suggests disparities between females with ASD and males with ASD may increase with age, and is why we hypothesized increasing age would be associated with worse HRQoL specific to women with ASD. For mental HRQoL, we did observe a sex by diagnosis by age interaction, but not as expected. Women with ASD were the only group to show a significant relationship between increasing age and better mental HRQoL. Upon further examination of the data in Mason et al. [4], their oldest group (61+) was no different from the youngest adults in mental HRQoL, but rather it was the middle-age groups that had worse mental HRQoL. Furthermore, their age-by-sex MANOVA interaction approached significance (p = 0.09), which could be driven by betther mental HRQoL in older women with ASD. Indeed Uljarević et al. [49] recently found older adults with ASD struggled less with mood disorder symptoms than middle-age adults with ASD, which is the same age pattern we recently identified for women with ASD and social cognitive abilities [24, 25]. Taken together, older women with ASD may have mental health resiliency.
Study 1 limitations
While our study provides novel insights into the complex interactive effects of age and sex on HRQoL in adults with ASD, there are some limitations that must be noted. First, we were slightly underpowered for interaction effects (i.e. 70% power for diagnosis-by-sex interaction), and were unable to compute power for three-way diagnosis-by-sex-by-age interactions, as results from a similar analysis has not been published to our knowledge. In spite of this, we detected a significant three-way interaction for mental HRQoL, but it is possible a subtler interaction is present for physical HRQoL that we were not powered to detect. It is also possible the three-way interaction for mental HRQoL is spurious, and thus, requires replication. Validity of findings are enhanced by our replication of main effects of ASD diagnosis on mental HRQoL and sex-by-diagnosis interactions for physical HRQoL. Second, inferring age relationships from a cross-sectional sample is always confounded by possible cohort effects. Our observed age effects warrant confirmation in a longitudinal study. Third, although the ASD and NT groups were very similar for overall health problems, they were not matched for psychiatric co-morbid conditions (Supp. Table 1). As recently put forth by Oakley et al. (50), worse HRQoL in adults with ASD may be better accounted for by depression and anxiety rather than autism symptoms. Future work should aim to determine if adults with ASD have worse HRQoL, over and above what can be accounted for by co-morbidities, compared to NT adults. Fourth, we did not include adults with ASD and comorbid intellectually disability (IQ < 70), which limits generalizability to the full ASD community. Last, but certainly not least, the ASD community has been very clear about the need for QoL measures specific to those with ASD [11, 51]. This need was met by McConachie et al. [43] after the inception of Study 1, therefore was not included. However, for Study 2, we specifically chose the WHO QoL measures in order to add their autism-specific questions to our battery. Future work will explore age and sex effects on the autism-specific QoL measures.
Study 1 conclusions
We replicated previous findings that mental HRQoL is worse in both men and women with ASD, compared to their NT counterparts, but that physical HRQoL is only reduced in women with ASD. Furthermore, we present novel findings that older age is associated with better mental HRQoL in women with ASD, but not men with ASD or either NT sex. Findings highlight the need to individualize interventions and services and to use age- and sex-based norms for comparing HRQoL outcomes in adults with ASD.
Study 2
For Study 2, our hypothesis was partially supported; MBSR led to disability-related QoL improvements in adults with ASD over and above a support/education control intervention. This pilot study found both interventions led to mental HRQoL improvements and neither intervention contributed to physical health or autism-specific QoL changes. But, our exploratory investigations revealed that sex impacted HRQoL benefits, such that only women in both intervention groups improved on physical HRQoL and women improved more than men on mental HRQoL.
We chose the WHO QoL battery for Study 2 in order to capitalize on the development of autism-specific QoL questions, in addition to the WHO disability module. We observed improvements on both self and proxy disability-related QoL that were specific to the MBSR intervention. This is not surprising given MBSR’s specific focus on providing adaptive coping skills [22], which is not communicated in NCCIH’s relaxation techniques handout. Khanna et al. [19] found coping to be a top factor for improving HRQoL in adults with ASD. Specific items on the WHO DAS may underly constructs that are particularly amendable to MBSR training (e.g. “being emotionally affected by health problems”, “concentrating on doing something for ten minutes”, “dealing with people you do not know”, “maintaining a friendship”, etcetera) and are challenging areas for many adults with ASD. MBSR’s focus on increasing emotional awareness and the adoption of attitudes of acceptance, nonjudgment, and nonreactivity toward present moment experience may be relevant to the disability-related improvements in QoL we observed. Such training may affect disability-related QoL by enhancing cognitive/affective functioning which has broad downstream effects pertaining to attention, interpersonal relationships, and one’s sense of wellbeing [52–54]. Although Nyklíček and Kuijpers [55] found the broadly defined ‘mindfulness’ construct mediates MBSR-elicited improvements in QoL, it is still unclear what facets of ‘mindfulness’ are responsible for disability-related QoL in adults with ASD. More psychological mechanistic research is warranted to shed light on this question is warranted.
While we are the first to identify an association between MBSR and improved disability-related QoL in adults with ASD, others have shown MBSR or other mindfulness programs improve WHO disability scores in various populations with depression [56, 57]. Additionally, mindfulness characteristics are associated with WHO disability scores in fibromyalgia patients and veterans with PTSD [58, 59]. In adults with ASD, Schmidt et al. [5] found aspects of the WHO disability module predicted overall life satisfaction, suggesting that MBSR may lead to overall life satisfaction improvements for adults with ASD over and above a social support/relaxation education control intervention. In the present study, neither group experienced improvements in autism-specific QoL. This is likely because neither the MBSR nor relaxation education were developed to address difficulties specific to being an adult on the spectrum.
Both MBSR and the support/education control intervention led to mental HRQoL improvements. We specifically designed the support/education intervention to control for the social support provided by MBSR, which our data suggests is sufficient to produce mental HRQoL improvements. This is not surprising given the other top factor Khanna et al. [19] identified for improving HRQoL in adults with ASD was social support. Additionally, a systematic review found increasing social participation is one route by which adults with ASD can improve QoL [20]. Our findings of MBSR improving mental HRQoL adds to the emerging research suggesting MBSR is effective in reducing anxiety and/or depressive symptoms in adults with ASD [23–27]. Moreover, our novel findings indicate the same mental HRQoL benefits can be gained from a social support group with freely available relaxation education. This is encouraging for those in the autistic community who may have financial or other barriers to accessing an MBSR group.
Our exploratory investigations revealed that sex impacted HRQoL benefits, such that only women in both intervention groups improved on physical HRQoL, and women improved more on mental HRQoL. In line with results from Study 1 and others [4, 10], women in both groups had worse physical HRQoL at pre-intervention. However, for mental HRQoL, men and women did not differ at pre-intervention, yet a sexby-time interaction was detected. This suggest women with ASD may respond better to interventions providing social support and stress reduction education for mental health outcomes, regardless of whether the education is mindfulness or relaxation. Since women with ASD report worse social QoL than men [10, 18], increased social support may have a greater residual impact on their mental HRQoL. Our other exploratory analysis did not find any associations between age and intervention outcomes. Taken together, social support through MBSR or a relaxation education intervention may be particularly effective for improving HRQoL in women with ASD across the adult lifespan.
Study 2 limitations
A strength of the present study was the use of an active control group to assess the specific benefits of mindfulness, while controlling for social support and stress reduction education and reducing bias [60]. However, this is a relatively small pilot study that needs to be replicated in large efficacy trials. Inclusion of a third, waitlist control group could be informative for future studies. In the present study, participants in the support/education group shared mindfulness resources with one another, such as mobile meditation apps, which may have bolstered the mental HRQoL improvements observed in that group. Future work will be more proactive to mitigate mindfulness contamination, and query exposure to mindfulness information or apps at post-intervention to be a control variable in analyses. Disability-related QoL improvements and interactive effects of sex on intervention-related improvements were exploratory analyses and uncorrected for multiple comparisons. These findings need to be validated in future studies. Similar to Study 1, our exclusion criteria of IQ scores < 70 prevented us from assessing the range of intellectual abilities that may benefit from these interventions. For example, MBSR might not be feasible for those with severe language impairments, limiting the generalizability to the larger population of adults with ASD. Lastly, health-and disability-related QoL improvements need to be interpreted in the absence of autism-specific QoL improvements. QoL measures that were not specifically developed for the ASD population (e.g. WHO BREF and DAS) may not effectively capture subjective well-being for autistic adults [43, 51].
Study 2 conclusions
This pilot study is the first to identify MBSR-associated benefits to disability-related QoL in adults with ASD over and above a support/education control intervention. Further, we contribute to the emerging research suggesting MBSR is effective in improving mental health in adults with ASD, and present novel findings that the same mental health benefits can be gained from a social support group with freely available relaxation education. The latter expands mental health options for the autistic community who may have financial or other barriers to accessing an MBSR group.
Overall conclusions
In a phased approach, this series of studies elucidated the effects of sex, age, and their interaction on HRQoL (Study 1) and evaluated the effectiveness of MBSR for improving health-, disability-, and autism-related QoL, with possible sex and age outcome moderators, in adults with ASD (Study 2). The most salient, collective findings are that: (1) men and women with ASD do not experience equivalent HRQoL reductions, and difficulties may be dynamic across the adult lifespan, (2) psychosocial interventions broadly may only be effective for improving HRQoL in women with ASD, and (3) MBSR may have specific efficacy for improving disability-related QoL in men and women with ASD across the adult lifespan. Ideally, this knowledge can be expanded and incorporated into precision medicine strategies for improving QoL in adults with ASD across the lifespan.
Supplementary Material
Funding
This work was supported by the National Institute of Health [K01MH116098; F31AT010976; F31MH122107]; Department of Defense [AR140105]; and Arizona Biomedical Research Commission [ADHS16-162413]. We would like to acknowledge Drs. Leslie Baxter and Christopher Smith for their contributions establishing our aging with ASD study.
Footnotes
Conflict of interest The authors have no conflicts to disclose.
Declarations
Ethical approval These studies were approved by Arizona State University’s Institutional Review Board.
Informed consent All participants provided written consent approved by the Institutional Review Board. All authors have read and approved the submission.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11136-021-03013-x.
Data availability
Data are made available upon request.
References
- 1.Dietz PM, Rose CE, McArthur D, & Maenner M (2020). National and state estimates of adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 10.1007/s10803-020-04494-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop-Fitzpatrick L, Mazefsky CA, & Eack SM (2018). The combined impact of social support and perceived stress on quality of life in adults with autism spectrum disorder and without intellectual disability. Autism, 22(6), 703–711. 10.1177/1362361317703090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin LY (2014). Quality of life of Taiwanese adults with autism spectrum disorder. PLoS ONE, 9(10), 21–26. 10.1371/journal.pone.0109567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mason D, McConachie H, Garland D, Petrou A, Rodgers J, & Parr JR (2018). Predictors of quality of life for autistic adults. Autism Research, 11(8), 1138–1147. 10.1002/aur.1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt L, Kirchner J, Strunz S, Broźus J, Ritter K, Roepke S, & Dziobek I (2015). Psychosocial functioning and life satisfaction in adults with autism spectrum disorder without intellectual impairment. Journal of Clinical Psychology, 71(12), 1259–1268. 10.1002/jclp.22225 [DOI] [PubMed] [Google Scholar]
- 6.Van Heijst BFC, & Geurts HM (2015). Quality of life in autism across the lifespan: A meta-analysis. Autism, 19(2), 158–167. 10.1177/1362361313517053 [DOI] [PubMed] [Google Scholar]
- 7.Orley J (1996). WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment: field trial version, December. World Health Organization, pp. 1–16. Retrieved from http://apps.who.int/iris/bitstream/handle/10665/63529/WHO-QOL-BREF.pdf?sequence=1&isAllowed=y
- 8.Ware JE (2000). SF-36 Health Survey update. Spine. 10.1097/00007632-200012150-00008 [DOI] [PubMed] [Google Scholar]
- 9.Brugha TS, Spiers N, Bankart J, Cooper SA, McManus S, Scott FJ, Smith J, & Tyrer F (2016). Epidemiology of autism in adults across age groups and ability levels. British Journal of Psychiatry, 209(6), 498–503. 10.1192/bjp.bp.115.174649 [DOI] [PubMed] [Google Scholar]
- 10.Graham Holmes L., Zampella CJ, Clements C, McCleery JP, Maddox BB, Parish-Morris J, Udhnani MD, Schultz RT, & Miller JS (2020). A lifespan approach to patientreported outcomes and quality of life for people on the autism spectrum. Autism Research. 10.1002/aur.2275 [DOI] [PubMed] [Google Scholar]
- 11.Moss P, Mandy W, & Howlin P (2017). Child and adult factors related to quality of life in adults with autism. Journal of Autism and Developmental Disorders, 47(6), 1830–1837. 10.1007/s10803-017-3105-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware JE, & Kosinski M (2001). SF-36 Physical & Mental Health Summary Scales: A manual for users of version 1 (2nd ed.). Quality Metric. [Google Scholar]
- 13.Mason D, Mackintosh J, McConachie H, Rodgers J, Finch T, & Parr JR (2019). Quality of life for older autistic people: The impact of mental health difficulties. Research in Autism Spectrum Disorders, 63(December 2018), 13–22. 10.1016/j.rasd.2019.02.007 [DOI] [Google Scholar]
- 14.Shattuck PT, Narendorf SC, Cooper B, Sterzing PR, Wagner M, & Taylor JL (2012). Postsecondary education and employment among youth with an autism spectrum disorder. Pediatrics, 129(6), 1042–1049. 10.1542/peds.2011-2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SK (2018). Sex as an important biological variable in biomedical research. BMB Reports, 51(4), 167–173. 10.5483/BMBRep.2018.51.4.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loomes R, Hull L, & Mandy WPL (2017). What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry. 10.1016/j.jaac.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 17.Icasiano F, Hewson PH, Machet P, Cooper C, & Marshall A (2004). Childhood autism spectrum disorder in the Barwon region: A community based study. Journal of Paediatrics and Child Health, 40(12), 696–701. 10.1111/j.1440-1754.2004.00513.x [DOI] [PubMed] [Google Scholar]
- 18.Kamio Y, Inada N, & Koyama T (2013). A nationwide survey on quality of life and associated factors of adults with high-functioning autism spectrum disorders. Autism, 17(1), 15–26. 10.1177/1362361312436848 [DOI] [PubMed] [Google Scholar]
- 19.Khanna R, Jariwala-Parikh K, West-Strum D, & Mahabaleshwarkar R (2014). Health-related quality of life and its determinants among adults with autism. Research in Autism Spectrum Disorders, 8(3), 157–167. 10.1016/j.rasd.2013.11.003 [DOI] [Google Scholar]
- 20.Tobin MC, Drager KDR, & Richardson LF (2014). A systematic review of social participation for adults with autism spectrum disorders: Support, social functioning, and quality of life. Research in Autism Spectrum Disorders, 8(3), 214–229. 10.1016/j.rasd.2013.12.002 [DOI] [Google Scholar]
- 21.Smith IC, Ollendick TH, & White SW (2019). Anxiety moderates the influence of ASD severity on quality of life in adults with ASD. Research in Autism Spectrum Disorders, 62(June 2018), 39–47. 10.1016/j.rasd.2019.03.001 [DOI] [Google Scholar]
- 22.Kabat-Zinn J (2003). Mindfulness-based interventions in context: Past, present, and future. Clinical Psychology: Science and Practice. 10.1093/clipsy/bpg016 [DOI] [Google Scholar]
- 23.Kiep M, Spek AA, & Hoeben L (2015). Mindfulness-based therapy in adults with an autism spectrum disorder: Do treatment effects last? Mindfulness, 6(3), 637–644. 10.1007/s12671-014-0299-x [DOI] [Google Scholar]
- 24.Pagni BA, Walsh MJM, Foldes E, Sebren A, Dixon MV, Guerithault N, & Braden BB (2020). The neural correlates of mindfulness-induced depression reduction in adults with autism spectrum disorder: A pilot study. Journal of Neuroscience Research, 98(6), 1150–1161. 10.1002/jnr.24600 [DOI] [PubMed] [Google Scholar]
- 25.Pagni BA, Walsh MJM, Rogers C, & Braden BB (2020). Social cognition in autism spectrum disorder across the adult lifespan: Influence of age and sex on reading the mind in the eyes task in a cross-sectional sample. Frontiers in Integrative Neuroscience, 14(September), 1–8. 10.3389/fnint.2020.571408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sizoo BB, & Kuiper E (2017). Cognitive behavioural therapy and mindfulness based stress reduction may be equally effective in reducing anxiety and depression in adults with autism spectrum disorders. Research in Developmental Disabilities, 64, 47–55. 10.1016/j.ridd.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 27.Spek AA, Van Ham N. C., & Nyklíček I (2013). Research in developmental disabilities mindfulness-based therapy in adults with an autism spectrum disorder: A randomized controlled trial. Research in Developmental Disabilities, 34(1), 246–253. 10.1016/j.ridd.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 28.Vibe M, Bjørndal A, Tipton E, Hammerstrøm K, & Kowalski K (2012). Mindfulness based stress reduction (MBSR) for improving health, quality of life, and social functioning in adults. Campbell Systematic Reviews, 8(1), 1–127. 10.4073/csr.2012.3 [DOI] [Google Scholar]
- 29.Hwang YS, Kearney P, Klieve H, Lang W, & Roberts J (2015). Cultivating mind: Mindfulness interventions for children with autism spectrum disorder and problem behaviours, and their mothers. Journal of Child and Family Studies, 24(10), 3093–3106. 10.1007/s10826-015-0114-x [DOI] [Google Scholar]
- 30.Rayan A, & Ahmad M (2016). Effectiveness of mindfulness-based interventions on quality of life and positive reappraisal coping among parents of children with autism spectrum disorder. Research in Developmental Disabilities, 55, 185–196. 10.1016/j.ridd.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 31.Hartley M, Dorstyn D, & Due C (2019). Mindfulness for children and adults with autism spectrum disorder and their caregivers: A meta-analysis. Journal of Autism and Developmental Disorders, 49(10), 4306–4319. 10.1007/s10803-019-04145-3 [DOI] [PubMed] [Google Scholar]
- 32.Baxter LC, Nespodzany A, Walsh MJM, Wood E, Smith CJ, & Braden BB (2019). The influence of age and ASD on verbal fluency networks. Research in Autism Spectrum Disorders, 63(March), 52–62. 10.1016/j.rasd.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh MJM, Baxter LC, Smith CJ, & Braden BB (2019). Age group differences in executive network functional connectivity and relationships with social behavior in men with autism spectrum disorder. Research in Autism Spectrum Disorders, 63(December 2017), 63–77. 10.1016/j.rasd.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism Diagnostic Observation Schedule–(ADOS-2) (2nd ed.). Los Angeles, CA: Western Psychological Corporation. [Google Scholar]
- 35.American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association. [Google Scholar]
- 36.Constantino JN (2012). Social Responsiveness Scale (2nd ed.). Western Psychological Services. [Google Scholar]
- 37.Kaufman AS, & Kaufman NL (2004). KBIT-2: Kaufman brief intelligence test (2nd ed.). Pearson Education, Inc. [Google Scholar]
- 38.Folstein MF, Folstein SE, & McHugh PR (1975). “Minimental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 39.Lever AG, & Geurts HM (2016). Psychiatric co-occurring symptoms and disorders in young, middle-aged, and older adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 10.1007/s10803-016-2722-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ware John E., & Sherbourne CD (1992). The MOS 36-item short-form health survey (Sf-36): I. conceptual framework and item selection. Medical Care. 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 41.Skevington SM, Lotfy M, & O’Connell KA (2004). The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial a Report from the WHOQOL Group. Quality of Life Research. 10.1023/B:QURE.0000018486.91360.00 [DOI] [PubMed] [Google Scholar]
- 42.Andrews G, Kemp A, Sunderland M, von Korff M., & Ustun TB (2009). Normative data for the 12 item WHO disability assessment schedule 2.0. PLoS ONE, 4(12), 1–6. 10.1371/journal.pone.0008343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McConachie H, Mason D, Parr JR, Garland D, Wilson C, & Rodgers J (2018). Enhancing the validity of a quality of life measure for autistic people. Journal of Autism and Developmental Disorders, 48(5), 1596–1611. 10.1007/s10803-017-3402-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Üstün TB (2010). Measuring health and disability: Manual for WHO Disability Assessment Schedule WHODAS 2.0. World Health Organization. [Google Scholar]
- 45.Braden BB, Pipe TB, Smith R, Glaspy TK, Deatherage BR, & Baxter LC (2016). Brain and behavior changes associated with an abbreviated 4-week mindfulness-based stress reduction course in back pain patients. Brain and Behavior, 6(3), 1–13. 10.1002/brb3.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain S, Shapiro SL, Swanick S, Roesch SC, Mills PJ, Bell I, & Schwartz GER (2007). A randomized controlled trial of mindfulness meditation versus relaxation training: Effects on distress, positive states of mind, rumination, and distraction. Annals of Behavioral Medicine. 10.1207/s15324796abm3301_2 [DOI] [PubMed] [Google Scholar]
- 47.Hughes JW, Fresco DM, Myerscough R, Van Dulmen MHM, Carlson LE, & Josephson R (2013). Randomized controlled trial of mindfulness-based stress reduction for prehypertension. Psychosomatic Medicine, 75(8), 721–728. 10.1097/PSY.0b013e3182a3e4e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skevington SM, & McCrate FM (2012). Expecting a good quality of life in health: assessing people with diversediseases and conditions using the WHOQOL-BREF. Health Expectations, 15, 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uljarević M, Hedley D, Rose-Foley K, Magiati I, Cai RY, Dissanayake C, Richdale A, & Trollor J (2019). Anxiety and depression from adolescence to old age in autism spectrum disorder. Journal of Autism and Developmental Disorders, 50(9), 3155–3165. 10.1007/s10803-019-04084-z [DOI] [PubMed] [Google Scholar]
- 50.Oakley BFM, Tillmann J, Ahmad J, Crawley D, San José Cáceres A., Holt R, & Loth E, (2021). How docore autism traits and associated symptoms relate to quality of life? Findings from the Longitudinal EuropeanAutism Project. Autism, 25(2), 389–404. 10.1177/1362361320959959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kapp SK (2018). Social support, well-being, and quality of life among individuals on the autism spectrum. Pediatrics, 141(April 2018), S362–S368. 10.1542/peds.2016-4300N [DOI] [PubMed] [Google Scholar]
- 52.Chiesa A, & Serretti A (2010). A systematic review of neurobiological and clinical features of mindfulness meditations. Psychological Medicine, 40(8), 1239–1252. 10.1017/S0033291709991747 [DOI] [PubMed] [Google Scholar]
- 53.Lao SA, Kissane D, & Meadows G (2016). Cognitive effects of MBSR/MBCT: A systematic review of neuropsychological outcomes. Consciousness and Cognition. 10.1016/j.concog.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 54.Mallya S, & Fiocco AJ (2016). Effects of mindfulness training on cognition and well-being in healthy older adults. Mindfulness, 7(2), 453–465. 10.1007/s12671-015-0468-6 [DOI] [Google Scholar]
- 55.Nyklíček I, & Kuijpers KF (2008). Effects of mindfulness-based stress reduction intervention on psychological wellbeing and quality of life: Is increased mindfulness indeed the mechanism? Annals of Behavioral Medicine, 35(3), 331–340. 10.1007/s12160-008-9030-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burnett-Zeigler I, Hong S, Waldron EM, Maletich C, Yang A, & Moskowitz J (2019). A mindfulness-based intervention for low-income African American women with depressive symptoms delivered by an experienced instructor versus a novice instructor. Journal of Alternative and Complementary Medicine, 25(7), 699–708. 10.1089/acm.2018.0393 [DOI] [PubMed] [Google Scholar]
- 57.Kladnitski N, Smith J, Uppal S, James MA, Allen AR, Andrews G, & Newby JM (2020). Transdiagnosticinternet-delivered CBT and mindfulness-based treatment for depression and anxiety: a randomised controlled trial.Internet Interv., 20, 10.1016/j.invent.2020.100310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brooks JM, Muller V, Sánchez J, et al. (2019). Mindfulness as aprotective factor against depressive symptoms in people with-fibromyalgia. Journal of Mental Health, 22, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyer EC, Frankfurt SB, Kimbrel NA, DeBeer BB, Gulliver SB, & Morrisette SB (2018). Theinfluence of mindfulness, self-compassion, psychological flexibility, and posttraumatic stress disorder on disabilityand quality of life over time in war veterans. Journal of Clinical Psychology, 74(7), 1272–1280. 10.1002/jclp.22596 [DOI] [PubMed] [Google Scholar]
- 60.Vibe M, Bjørndal A, Fattah S, Dyrdal GM, Halland E, & Tanner-Smith EE (2017). Mindfulness-based stress reduction (MBSR) for improving health, quality of life and social functioning in adults: A systematic review and meta-analysis. Campbell Systematic Reviews. 10.4073/csr.2017.11 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are made available upon request.