This cohort study uses Danish national registry data to assess the association of maternal autoimmune diseases before childbirth with risk of mental disorders among offspring up to early adulthood.
Key Points
Question
Is prenatal maternal autoimmune disease associated with an increased long-term risk of mental disorders in offspring?
Findings
In this cohort study of 2 254 234 singleton infants with up to 38 years of follow-up, prenatal maternal autoimmune diseases were associated with increased risks of a wide range of mental disorders in offspring. Maternal type 1 diabetes and rheumatoid arthritis during pregnancy were associated with offspring’s mental health up to early adulthood.
Meaning
The findings suggest that offspring of mothers with autoimmune diseases may benefit from long-term surveillance for mental disorders.
Abstract
Importance
Maternal immune activation during pregnancy is associated with increased risks of several mental disorders in offspring during childhood, but little is known about how maternal autoimmune diseases during pregnancy are associated with mental health in offspring during and after childhood.
Objective
To investigate the association between maternal autoimmune diseases before childbirth and risk of mental disorders among offspring up to early adulthood.
Design, Setting, and Participants
This population-based nationwide cohort study used data from Danish national registers on singletons born in Denmark from 1978 to 2015 with up to 38 years of follow-up. Data analyses were conducted from March 1, 2020, through September 30, 2021.
Exposures
Maternal autoimmune disease diagnosed before or during pregnancy according to the Danish National Patient Register.
Main Outcomes and Measures
The main outcome was mental disorders, defined by hospital diagnoses, in offspring. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% CIs for mental disorders.
Results
Of the 2 254 234 singleton infants included in the study (median age, 16.7 years [IQR, 10.5-21.7 years]; 51.28% male), 2.26% were born to mothers with autoimmune diseases before childbirth. Exposed participants had an increased risk of overall mental disorders compared with their unexposed counterparts (HR, 1.16; 95% CI, 1.13-1.19; incidence, 9.38 vs 7.91 per 1000 person-years). Increased risks of overall mental disorders in offspring were seen in different age groups for type 1 diabetes (1-5 years: HR, 1.35 [95% CI, 1.17-1.57]; 6-18 years: HR, 1.24 [95% CI, 1.15-1.33]; >18 years: HR, 1.19 [95% CI, 1.09-1.30]) and rheumatoid arthritis (1-5 years: HR, 1.42 [95% CI, 1.16-1.74]; 6-18 years: HR, 1.19 [95% CI, 1.05-1.36]; >18 years: HR, 1.28 [95% CI, 1.02-1.60]). Regarding specific mental disorders, increased risk after exposure to any maternal autoimmune disorder was observed for organic disorders (HR, 1.54; 95% CI, 1.21-1.94), schizophrenia (HR, 1.35; 95% CI, 1.21-1.51), obsessive-compulsive disorder (HR, 1.42; 95% CI, 1.24-1.63), mood disorders (HR, 1.12; 95% CI, 1.04-1.21), and a series of neurodevelopmental disorders (eg, childhood autism [HR, 1.21; 95% CI, 1.08-1.36] and attention-deficit/hyperactivity disorder [HR, 1.19; 95% CI, 1.12-1.26]).
Conclusions and Relevance
In this cohort study in Denmark, prenatal exposure to maternal autoimmune diseases was associated with increased risks of overall and type-specific mental disorders in offspring. Maternal type 1 diabetes and rheumatoid arthritis during pregnancy were associated with offspring’s mental health up to early adulthood. Individuals prenatally exposed to autoimmune disease may benefit from long-term surveillance for mental disorders.
Introduction
The prevalence of autoimmune diseases is higher than 5%, with these diseases having a high burden.1 Most autoimmune diseases are more common in females predominantly during the childbearing years.1,2 Animal studies suggest that maternal immune activation can induce alterations in brain morphologic features, abnormal neurotransmitter activity, and behavioral deficits in offspring3,4,5; these alterations support a role of intrauterine exposure to immunologic insults in the cause of mental disorders. Empirical evidence has suggested an association between certain maternal autoimmune diseases (eg, type 1 diabetes, rheumatoid arthritis) and specific mental disorders (eg, autism spectrum disorder, attention-deficit/hyperactivity disorder [ADHD]) in offspring.6,7,8,9,10,11,12,13,14,15 However, most previous population-based studies were restricted to certain maternal autoimmune diseases and mental disorders in offspring during childhood and had small sample sizes. Using a Danish registry-based cohort of more than 2 million live-born singletons with up to 38 years of follow-up, we investigated the associations between a wide range of maternal autoimmune diseases diagnosed before childbirth and the risks of the full spectrum of mental disorders in offspring during childhood and early adulthood.
Methods
This cohort study was approved by the Danish Data Protection Agency. By Danish law, no informed consent was required because this was a register-based study using anonymized data. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. Data analyses were performed from March 1, 2020, through September 30, 2021.
Study Population
We conducted a population-based cohort study using data from Danish national registers.16 A total of 2 272 473 live-born singletons delivered in Denmark from 1978 to 2015 were identified from the Danish Medical Birth Registry.17 We excluded 18 239 infants who died or emigrated from Denmark before 1 year of age. The final cohort consisted of 2 254 234 births with 38 916 359 person-years.
Assessment of Autoimmune Diseases
Offspring born to mothers diagnosed with autoimmune disease before childbirth were considered to have been prenatally exposed to maternal autoimmune disease. Information on autoimmune diseases was obtained from the Danish National Patient Register (NPR).18 The diagnostic systems used were the International Classification of Diseases, Eighth Revision (ICD-8) from 1969 to 1993 and the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) from 1994 onward. Using data from the NPR, information on 29 different autoimmune diseases in mothers of cohort members was retrieved (eTable 1 in the Supplement). The time of onset was defined as the first day of the first hospital contact for each autoimmune disease diagnosis. If a mother had a history of more than 1 autoimmune disease, the first date of the earliest diagnosed autoimmune disease was set as the onset time for any autoimmune disease. The first recorded date of diagnosis of autoimmune disease was used to assess whether the diagnosis was made before delivery.
Mental Disorders in Offspring
Information on mental disorders was obtained from the Psychiatric Central Research Register and the NPR.18,19 The specific diagnoses included in each group of disorders according to ICD-10 and ICD-8 codes are presented in eTable 2 in the Supplement.20,21 For each specific mental disorder, the date of onset was defined as the first day of the first hospital contact. Individuals with more than 1 disorder were included in the numerator for each specific disorder.
Potential Confounders
We included the following potential confounders: sex of the child (male or female), calendar period of birth (1978-1985, 1986-1994, 1995-2005, or 2006-2015), parental psychiatric history before childbirth (yes or no), and maternal characteristics, including parity (1, 2, or ≥3 births), maternal age at birth (<20, 20-24, 25-29, 30-34, or ≥35 years), highest attained educational level (≤9, 10-14, or ≥15 years), cohabitation with a partner (yes or no), residence (Copenhagen, city with ≥100 000 inhabitants, or other), and country of birth (Denmark, other). Missing data for each variable were coded separately.
Statistical Analysis
Follow-up for offspring started at the earliest possible age at onset of the disorder (for each disorder separately) (eTable 2 in the Supplement) until the date of the first diagnosis, death, emigration, or December 31, 2016, whichever came first. Cox proportional hazards regression was used to estimate hazard ratios (HRs) with 95% CIs to assess the associations of interest, with offspring’s age as the time scale. Offspring born to mothers without any autoimmune disease diagnosis before delivery were treated as the reference group. Based on the main hypothesis in this study, the association between overall maternal autoimmune diseases and any mental disorder in offspring was the primary outcome analyzed. The log-minus-log survival curves were parallel (eFigure in the Supplement); thus, it was assumed that the proportional hazards assumption was not violated. We also estimated whether the overall risk of mental disorders in offspring associated with specific maternal autoimmune diseases differed by offspring’s age (≤5, 6-18, and >18 years).
In sensitivity analyses, we evaluated the associations by comorbidity of different types of autoimmune diseases. We excluded preterm births to assess whether the risk pattern was modified by fetal maturity in utero. In addition, we restricted the analyses to individuals without congenital malformations of the nervous system and chromosomal abnormalities to consider their potential associations with mental disorders. Because of the change in ICD version in 1994 and the extension of the NPR with data from 3 departments (inpatient, outpatient, and emergency) in 1995, we performed subanalyses restricted to individuals born after 1997. To explore potential residential confounding from genetic factors and shared familial factors, we used paternal autoimmune diseases as the negative control exposure and evaluated associations between paternal autoimmune diseases diagnosed before childbirth and mental disorders in children. To elucidate the role of maternal medication use for autoimmune diseases or mental disorders before or during pregnancy, we obtained information on medications by Anatomical Therapeutic Chemical codes from the Danish National Prescription Registry (eTable 3 in the Supplement).22 We adjusted for these variables in the Cox proportional hazards regression model. Because the Danish National Prescription Registry was established in 1995, only participants born between 1998 and 2015 were included. Also, we applied multiple imputation to address missing values and propensity score methods to validate the robustness of the results. All analyses were conducted using SAS, version 9.4 (SAS Institute).
Results
Of the 2 254 234 singleton infants included (median age, 16.7 years [IQR, 10.5-21.7 years]; 1 155 982 [51.28%] male), 50 863 (2.26%) were born to mothers with autoimmune diseases diagnosed before or during pregnancy and 2 203 371 were born to mothers without autoimmune diseases (97.74%). Compared with mothers without autoimmune disease, mothers with autoimmune diseases were more likely to be older at the time of pregnancy, to have more than 1 child, to have higher educational level, and to live alone. Compared with unexposed offspring, offspring exposed to maternal autoimmune diseases were more likely to be born preterm (Table).
Table. Baseline Characteristics of the Offspring According to Prenatal Exposure to Maternal Autoimmune Diseases.
| Characteristics | Maternal autoimmune disease, No. (%) | |
|---|---|---|
| Exposed offspring (n = 50 863) | Unexposed offspring (n = 2 203 371) | |
| Sex | ||
| Female | 24 997 (49.15) | 1 072 255 (48.66) |
| Male | 25 863 (50.85) | 1 130 119 (51.29) |
| Missing | 3 (0.01) | 997 (0.05) |
| Calendar year of birth | ||
| 1978-1985 | 3187 (6.27) | 424 492 (19.27) |
| 1986-1994 | 6232 (12.25) | 538 798 (24.45) |
| 1995-2005 | 16 391 (32.23) | 679 775 (30.85) |
| 2006-2016 | 25 053 (49.26) | 560 306 (25.43) |
| Gestational age at birth, wk | ||
| <37 | 5161 (10.15) | 94 899 (4.31) |
| ≥37 | 44 991 (88.46) | 2 028 343 (92.06) |
| Missing | 711 (1.40) | 80 129 (3.64) |
| Maternal parity | ||
| 1 | 21 193 (41.67) | 986 635 (44.78) |
| 2 | 19 583 (38.50) | 818 434 (37.14) |
| ≥3 | 10 087 (19.83) | 398 302 (18.08) |
| Maternal age at childbirth, y | ||
| <20 | 332 (0.65) | 39 822 (1.81) |
| 20-34 | 38 590 (75.87) | 1 839 428 (83.48) |
| ≥35 | 11 941 (23.48) | 324 121 (14.71) |
| Maternal educational level at childbirth, y | ||
| 0-9 | 10 902 (21.43) | 595 282 (27.02) |
| 10-14 | 22 309 (43.86) | 951 005 (43.16) |
| ≥15 | 17 136 (33.69) | 613 715 (27.85) |
| Missing | 516 (1.01) | 43 369 (1.97) |
| Maternal cohabitation at childbirth | ||
| Yes | 27 003 (53.09) | 1 214 548 (55.12) |
| No | 23 851 (46.89) | 986 215 (44.76) |
| Missing | 9 (0.02) | 2608 (0.12) |
| Maternal residence at childbirth | ||
| Copenhagen | 6219 (12.23) | 244 865 (11.11) |
| City with ≥100 000 inhabitants | 7110 (13.98) | 280 727 (12.74) |
| Other | 37 534 (73.79) | 1 677 779 (76.15) |
| Maternal country of birth | ||
| Denmark | 46 124 (90.68) | 1 955 104 (88.73) |
| Other | 4712 (9.26) | 243 512 (11.05) |
| Missing | 27 (0.05) | 4755 (0.22) |
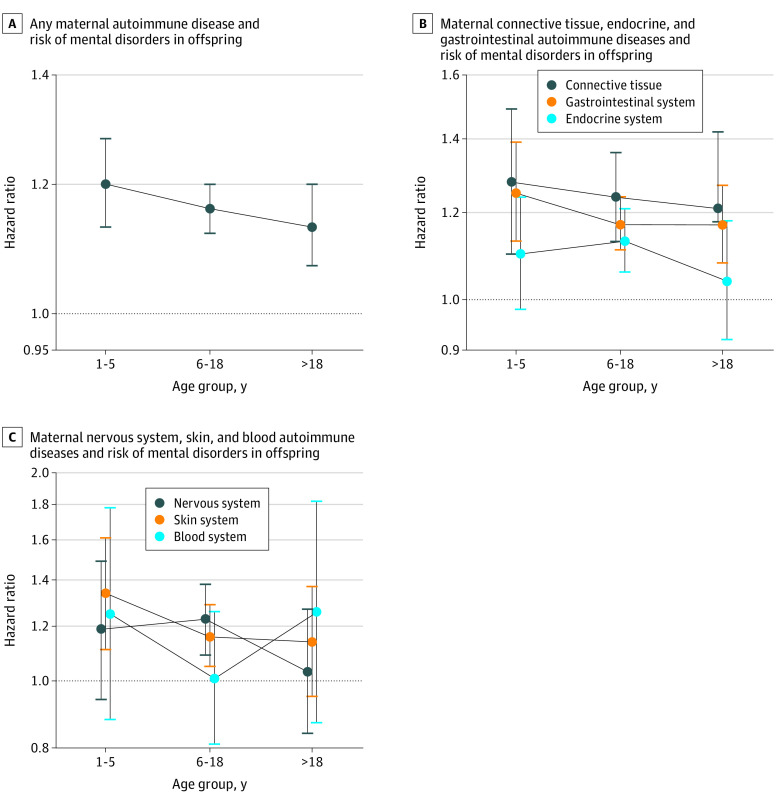
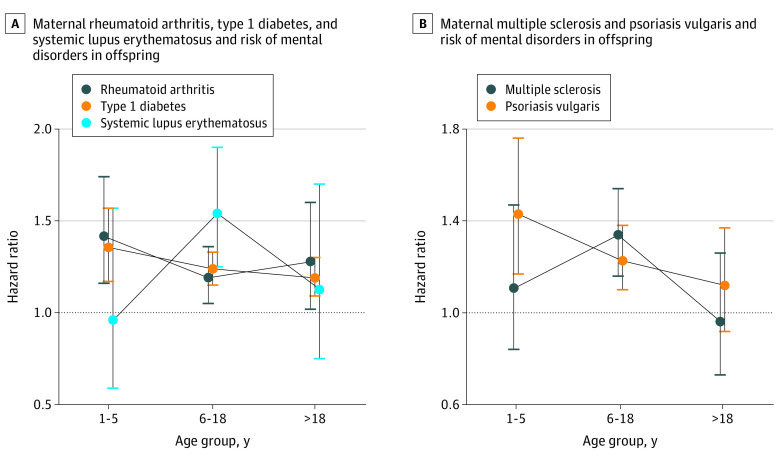
During the follow-up period of up to 38 years (median, 17 years [IQR, 8-25 years]), 5460 offspring of mothers with autoimmune diseases (10.73%) and 303 092 offspring of mothers without autoimmune diseases (13.76%) were diagnosed with a mental disorder; the incidence of any mental disorder was higher among exposed offspring (9.38 per 1000 person-years) compared with unexposed offspring (7.91 per 1000 person-years) (Figure 1). Overall, offspring exposed to any maternal autoimmune disease had a 16% higher risk of overall mental disorders than did unexposed offspring (HR, 1.16; 95% CI, 1.13-1.19). When we classified autoimmune disease according to organ system, increased risk of developing any mental disorder in children was observed for most system types of maternal autoimmune diseases (connective tissue system: HR, 1.24 [95% CI, 1.16-1.33]; endocrine system: HR, 1.19 [1.14-1.24]; gastrointestinal system: HR, 1.11 [95% CI, 1.05-1.17]; blood system: HR, 1.10 [95% CI, 0.93-1.30]; nervous system: HR, 1.17 [95% CI, 1.07-1.29]; skin system: HR, 1.19 [95% CI, 1.10-1.29]). Specifically, the highest overall risk for mental disorders in offspring was observed for primary biliary cirrhosis (HR, 3.07; 95% CI, 1.75-5.41) but with small numbers of events (12 of 61 births). The 5 most common maternal autoimmune diseases associated with the overall risk of mental disorders in offspring were type 1 diabetes (HR, 1.24; 95% CI, 1.18-1.30), rheumatoid arthritis (HR, 1.25; 95% CI, 1.14-1.38), systemic lupus erythematosus (HR, 1.34; 95% CI, 1.13-1.60), multiple sclerosis (HR, 1.21; 95% CI, 1.08-1.36), and psoriasis vulgaris (HR, 1.24; 95% CI,1.14-1.36). Those associations existed in each age group for any autoimmune disease (1-5 years: HR, 1.20 [95% CI, 1.13-1.28]; 6-18 years: HR, 1.16 [95% CI, 1.12-1.20]; >18 years: HR, 1.13 [95% CI, 1.07-1.20]), endocrine system (1-5 years: HR, 1.25 [95% CI, 1.13-1.39]; 6-18 years: HR, 1.17 [95% CI,1.11-1.24]; >18 years: HR, 1.17 [95% CI, 1.08-1.27]), and connective tissue autoimmune diseases (1-5 years: HR, 1.28 [95% CI, 1.10-1.49]; 6-18 years: HR, 1.24 [95% CI, 1.13-1.36]; 18 years: HR, 1.21 [95% CI, 1.02-1.42]), type 1 diabetes (1-5 years: HR, 1.35 [95% CI, 1.17-1.57]; 6-18 years: HR, 1.24 [95% CI, 1.15-1.33]; >18 years: HR, 1.19 [95% CI, 1.09-1.30]), and rheumatoid arthritis (1-5 years: HR, 1.42 [95% CI, 1.16-1.74]; 6-18 years: HR, 1.19 [95% CI, 1.05-1.36]; >18 years: HR, 1.28 [95% CI, 1.02-1.60]) but differed by age groups for systemic lupus erythematosus, multiple sclerosis, and psoriasis vulgaris (Figure 2 and Figure 3 and eTable 4 in the Supplement).
Figure 1. Associations Between Specific Maternal Autoimmune Diseases Diagnosed Before Childbirth and Risk of Overall Mental Disorders in Offspring.
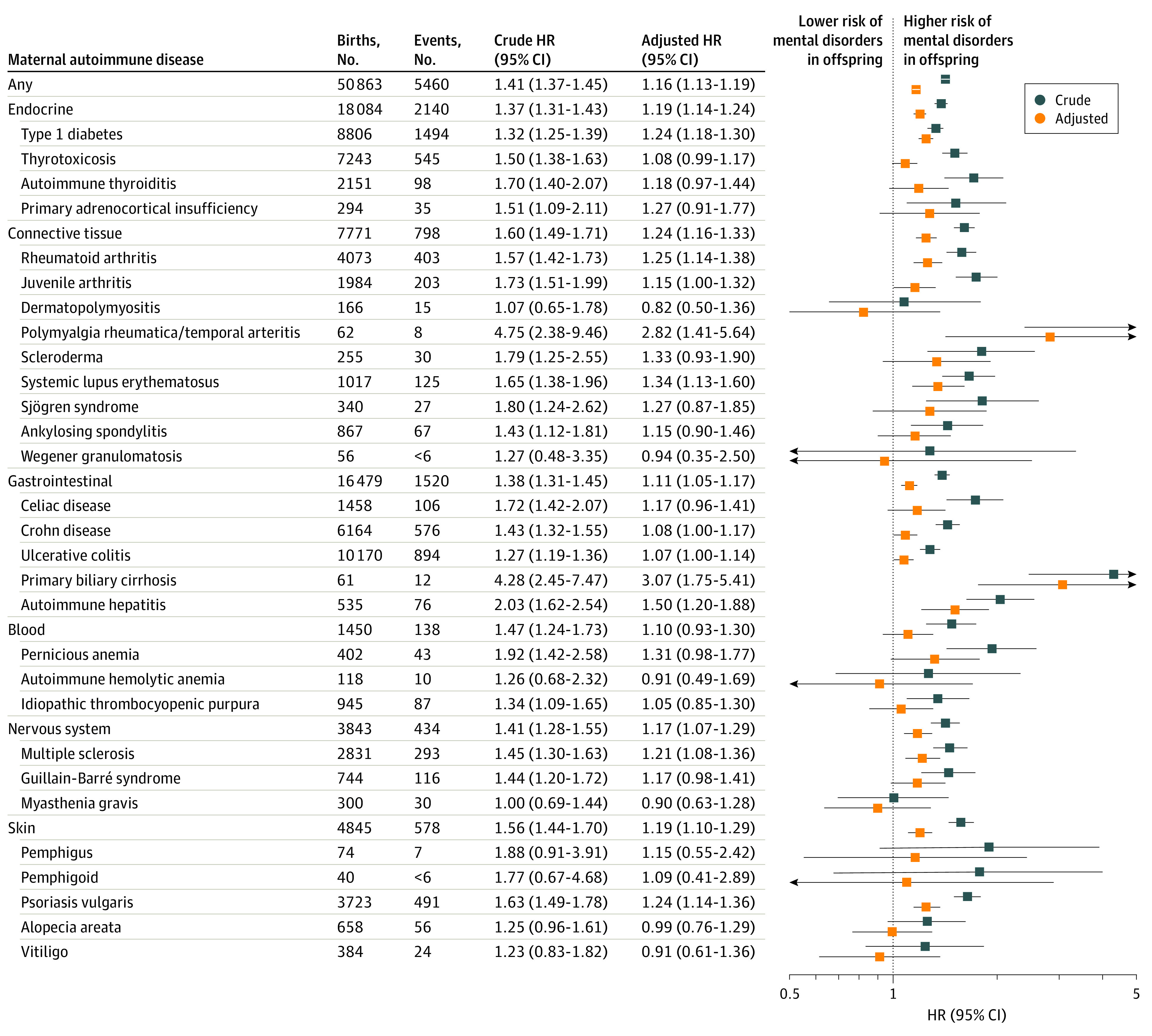
In the adjusted model, the associations were controlled for parental psychiatric history, maternal characteristics (parity, age at birth, highest educational level, cohabitation with a partner, residence, and birth country), and birth characteristics (child’s sex, calendar year of birth). Markers represent estimates, with horizontal lines indicating 95% CIs. HR indicates hazard ratio.
Figure 2. Associations Between Offspring’s Overall Mental Disorders and Maternal Organ System–Specific Autoimmune Diseases by Offspring’s Age.
Hazard ratios were adjusted for parental psychiatric history, maternal characteristics (parity, age at birth, highest educational level, cohabitation with a partner, residence, and birth country), and birth characteristics (child’s sex, calendar year of birth). Error bars indicate 95% CIs.
Figure 3. Associations Between Offspring’s Overall Mental Disorders With 5 Common Maternal Autoimmune Diseases by Offspring Age.
Hazard ratios were adjusted for parental psychiatric history, maternal characteristics (parity, age at birth, highest educational level, cohabitation with a partner, residence, and birth country), and birth characteristics (child’s sex, calendar year of birth). Error bars indicate 95% CIs.
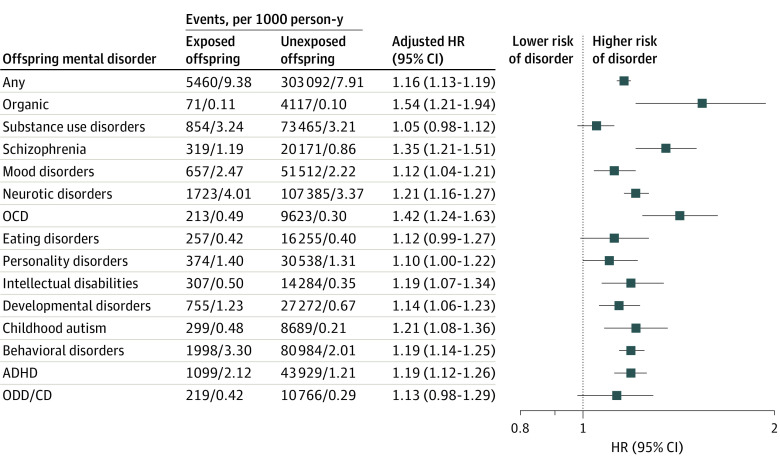
Figure 4 shows the HRs for all studied categories of mental disorders in offspring after prenatal exposure to any maternal autoimmune disease compared with their unexposed counterparts. Higher risk was observed for organic disorders (HR, 1.54; 95% CI, 1.21-1.94), obsessive-compulsive disorder (HR, 1.42; 95% CI, 1.24-1.63), schizophrenia (HR, 1.35; 95% CI, 1.21-1.51), and mood disorders (HR, 1.12; 95% CI, 1.04-1.21). Offspring with maternal autoimmune disease history had slightly increased risks for a wide range of neurodevelopmental disorders, for example, intellectual disability (HR, 1.19; 95% CI, 1.07-1.34), childhood autism (HR, 1.21; 95% CI, 1.08-1.36), and ADHD (HR, 1.19; 95% CI, 1.12-1.26). The number of comorbid maternal autoimmune diseases did not substantially modify the increased risk for overall or specific mental disorders in offspring (eTable 5 in the Supplement).
Figure 4. Association of Offspring’s Risk of Specific Mental Disorders With Prenatal Exposure to Any Autoimmune Disease.
Hazard ratios were adjusted for parental psychiatric history, maternal characteristics (parity, age at birth, highest educational level, cohabitation with a partner, residence, and birth country), and birth characteristics (adult’s sex, calendar year of birth). Markers represent estimates, with horizontal lines indicating 95% CIs. ADHD indicates attention-deficit/hyperactivity disorder; OCD, obsessive-compulsive disorder; ODD/CD, oppositional defiant disorder/conduct disorder.
In sensitivity analysis, effect estimates from analyses excluding preterm births were mostly consistent with those observed in the main analyses (eTables 6 and 7 in the Supplement). Results from separate analyses restricted to individuals without diagnoses of congenital malformations of the nervous system or chromosomal abnormalities or those who were born after 1997 were similar to those obtained in the primary analyses (eTables 8-11 in the Supplement). In addition, except for type 1 diabetes and psoriasis, most paternal autoimmune diseases diagnosed before the child was born were not significantly associated with the risk of overall mental disorders in offspring (eTable 12 in the Supplement). Increased risks were observed for organic disorders, schizophrenia, and obsessive-compulsive disorder in children with fathers with diagnoses of autoimmune diseases before the child was born (eTable 13 in the Supplement) but not for mood disorders or a wide range of neurodevelopmental disorders (eg, intellectual disability, childhood autism, and ADHD) compared with mothers who had autoimmune disorders (Figure 4). The results remained similar to those obtained in the primary analyses after adjusting for medication used before or during pregnancy (eTables 14 and 15 in the Supplement). The results from multiple imputation with 10 replications and propensity score methods yielded results similar to those of primary analyses (eTables 16-19 in the Supplement). In addition, we observed that children born to mothers with autoimmune diseases diagnosed before childbirth had an 11% increased risk of suicide, but the result was not statistically significant (HR, 1.11; 95% CI, 0.79-1.57) (eTable 20 in the Supplement).
Discussion
This nationwide population-based study involving births of 2 254 234 singleton infants provided estimates regarding the associations between prenatal exposure to various maternal autoimmune diseases and the full spectrum of mental disorders in offspring. Maternal autoimmune disease diagnosed before childbirth was associated with a 16% increased overall risk of mental disorders in offspring. Prenatal exposure to most common specific autoimmune diseases, such as type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and psoriasis, was associated with a significantly increased overall risk of mental disorders in offspring; the association of type 1 diabetes and rheumatoid arthritis with this risk lasted into early adulthood. In particular, children of mothers with autoimmune diseases diagnosed before delivery were more likely to develop a wide range of neurodevelopmental disorders (eg, intellectual disability, childhood autism, and ADHD), organic disorders, obsessive-compulsive disorder, schizophrenia, and mood disorders.
To our knowledge, this is the first study to analyze the association between a wide range of maternal autoimmune diseases diagnosed before childbirth and offspring’s full spectrum of mental disorders. The observed increased overall risk in offspring was apparent for most specific autoimmune diseases but varied (eg, primary biliary cirrhosis: HR, 3.07 [95% CI, 1.75-5.41] multiple sclerosis: HR, 1.21 [95% CI, 1.08-1.36]) perhaps because of the differences in pathogenicity or the degree of autoimmunity among diverse autoimmune diseases23 and statistical variance owing to small numbers of events (eg, 12 of 61 births with maternal primary biliary cirrhosis). Our findings corroborate the results of previous studies, suggesting an association of maternal autoimmune diseases with offspring’s mental disorders.7,9,10 However, direct comparisons between findings from earlier studies and our study are difficult because of differences in study design, case definition, and prevalence of autoimmune diseases in various regions. Of note, most of the previous investigations did not consider the temporal association between maternal autoimmune diseases and offspring’s mental disorders (ie, that maternal autoimmune diseases should be diagnosed before childbirth). Although some longitudinal studies have taken this into account,15,24,25 failure to limit the period of exposure before childbirth, together with lack of consideration for adverse birth outcomes (eg, preterm birth), are the main limitations of those earlier studies. Our study adds important epidemiological evidence to support the proposed maternal immune activation mechanism found in previous animal research.3,4,5
Most previous studies have focused on maternal autoimmune diseases and risks of selected specific mental disorders, and the results have been inconsistent.7,8,10,11,13,15,25,26,27,28 To our knowledge, no previous study has considered simultaneously a wide range of maternal autoimmune diseases and a large spectrum of mental disorders in offspring. In this study, we evaluated risks of the full spectrum of mental disorders in offspring born to mothers with autoimmune diseases. We found that prenatal exposure to maternal autoimmune diseases was associated with modestly elevated risks of neurodevelopmental disorders in childhood (eg, intellectual disabilities: HR, 1.19 [95% CI, 1.07-1.34]; childhood autism: HR, 1.21 [95% CI, 1.08-1.36]; ADHD: HR, 1.19 [95% CI, 1.12-1.26]). Previous reports have noted that maternal autoimmune diseases were more common among children with autism than among healthy control individuals, suggesting a possible association between autism and maternal autoimmune disease history.7,28 However, a nested case-control study that collected data on any autoimmune disease diagnosis in mothers in the 2 years before and after pregnancy found no such association.29 A limited number of studies focused on ADHD, another common neurodevelopmental disorder in childhood.9,14,30,31 Our study extended the finding of a recent hybrid study in which ADHD risk in offspring was the main outcome of interest and both original data from a cohort and meta-analyses of published studies were included.14 Results from a Danish study suggested that mothers with an autoimmune disease were more likely to have a child with poorer school performance than their unexposed counterparts,12 suggesting a potential association of maternal autoimmune activity with fetal brain development. To our knowledge, no previous study investigated the direct association between maternal autoimmune diseases and offspring’s risk of intellectual disability. However, direct comparisons and interpretations of these findings are hampered by different definitions of the exposure time window for maternal autoimmune diseases and limitations in study design, including small sample sizes and reliance on self-reported history of autoimmune diseases. Our study provides further evidence that maternal history of autoimmune disease before childbirth may be associated with increased risks of many other mental outcomes, such as organic disorders, mood disorders, and obsessive-compulsive disorder.
Several potential mechanisms have been proposed. Autoimmune diseases and mental disorders are heritable disorders and may share common genetic risk factors.32 Another alternative hypothesis could be that mothers who had disturbed autoimmunity before giving birth may transfer autoantibodies to the fetus during pregnancy.33 These transferred antibodies may activate the immune system in the child and alter normal brain development.34,35 Our finding that children born to mothers with autoimmune diseases diagnosed before childbirth had a higher risk of mental disorders would support the maternal transmission hypothesis. Previous studies have suggested that women with autoimmune disease have an increased risk of developing certain obstetric complications (eg, preterm births),36,37,38 which in turn may be associated with increased risk of mental disorders in the child.39,40,41 However, in analyses restricted to term births, the risk of mental disorders remained increased, suggesting that the associations cannot be explained entirely by these factors. Further studies are needed to gain a better understanding of the underlying mechanisms.
Strengths and Limitations
Our study has several strengths. First, the prospectively collected registry data including all live births in Denmark minimized the potential selection bias and recall bias. Second, a large sample size of more than 2 million births provided sufficient statistical power to perform detailed subgroup analyses that, to our knowledge, have not been done previously. Third, the availability of sociodemographic and clinical information enabled consideration of a wide range of important confounding factors.
This study also has limitations. First, we lacked information on the potential exacerbation of autoimmune disease activity during pregnancy. The effect of autoimmune disease activity during pregnancy on the fetal environment and the potential transfer of maternal antibodies to the fetus are relevant and should be investigated in further studies. Second, we cannot rule out detection bias in our study because mothers with chronic autoimmune disease may use health care more frequently and, consequently, may more readily seek care for their children. Third, the study period spanned almost 4 decades, and advances in medical care over time and changes in the diagnostic criteria may have influenced the exposures and outcomes. However, adjustment for calendar time in the analyses would partially alleviate the effects of temporal changes in medical care. Fourth, we were not able to investigate the risk of mental disorders in late adulthood because the maximum follow-up was 38 years; however, to our knowledge, this study had the longest follow-up period for investigating maternal autoimmune diseases and offspring mental disorders. Fifth, the absence of information from primary care and the delayed inclusion of outpatient records in the Psychiatric Central Research Register and the NPR might have resulted in underestimation of the associations. This concern was partially addressed in our sensitivity analyses restricted to individuals born after 1997, when outpatient records were also included in the NPR and the Psychiatric Central Register; that analysis revealed similar results.
Conclusions
In this large Danish population-based cohort study, we found that in utero exposure to a maternal autoimmune disease was associated with increased risks of overall and most type-specific mental disorders in offspring. These risks varied according to different subtypes of autoimmune diseases and specific mental disorders. Maternal type 1 diabetes and rheumatoid arthritis before childbirth were associated with overall mental disorders in offspring up to early adulthood. These findings suggest that a wide spectrum of mental disorders should be monitored in offspring of mothers with autoimmune diseases before or during pregnancy and especially for certain maternal autoimmune diseases (eg, type 1 diabetes and rheumatoid arthritis). Individuals prenatally exposed to autoimmune disease may benefit from long-term surveillance for mental disorders. If confirmed in future studies, our findings may have important implications for early screening and appropriate intervention for children at high risk of mental disorders.
eTable 1. Diagnostic Classification of Autoimmune Diseases Considered According to the ICD-10 and Equivalent ICD-8 Diagnoses
eTable 2. Diagnostic Classification of Mental Disorders Considered According to the ICD-10 and Equivalent ICD-8 Diagnoses
eTable 3. Anatomical Therapeutic Chemical (ATC) Codes for Autoimmune Disease and Mental Disorders Medications
eTable 4. Associations Between Specific Maternal Autoimmune Diseases and Risk of Any Mental Disorder in the Offspring by Offspring’s Age
eTable 5. Hazard Ratios of Any Mental Disorder Among the Offspring Stratified by the Numbers of Comorbid Maternal Autoimmune Diseases
eTable 6. Associations Between Specific Maternal Autoimmune Diseases Diagnosed Before Childbirth and Overall Mental Disorders Among Term-Born Individuals
eTable 7. Hazard Ratios of Specific Mental Disorders According to Prenatal Exposure to Any Maternal Autoimmune Disease Among Term-Born Individuals
eTable 8. Associations Between Specific Maternal Autoimmune Diseases Diagnosed Before Child Birth and Overall Mental Disorders Among Individuals Without Congenital Malformations of the Nervous System or Chromosomal Abnormalities
eTable 9. Hazard Ratios of Specific Mental Disorder According to Prenatal Exposure to Any Maternal Autoimmune Disease Among Individuals Without Congenital Malformations of the Nervous System and Chromosomal Abnormalities
eTable 10. Associations Between Specific Autoimmune Diseases of Mothers and Overall Mental Disorders in the Offspring Born During 1998-2007
eTable 11. Associations Between Specific Autoimmune Diseases of Mothers and Overall Mental Disorders in the Offspring Born During 2008-2015
eTable 12. Associations Between Specific Paternal Autoimmune Diseases Diagnosed Before Childbirth and Overall Mental Disorders in Offspring
eTable 13. Hazard Ratios of Specific Mental Disorder in Offspring According to Prenatal Exposure to Any Paternal Autoimmune Disease Diagnosed Before Childbirth
eTable 14. Associations Between Specific Maternal Autoimmune Diseases Diagnosed Before Childbirth and Overall Mental Disorders, Adjusted for Medications Used by Mothers Before Childbirth in Addition to the Variables in the Main Model
eTable 15. Hazard Ratios of Specific Mental Disorder According to Prenatal Exposure to Any Maternal Autoimmune Disease, Adjusted for Medications Used by Mothers Before Childbirth in Addition to the Variables in the Main Model
eTable 16. Associations Between Specific Maternal Autoimmune Diseases Diagnosed Before Childbirth and Overall Mental Disorders in Offspring, With Multiple Imputation for Missing Data
eTable 17. Hazard Ratios of Specific Mental Disorder According to Prenatal Exposure to Any Maternal Autoimmune Disease, With Multiple Imputation for Missing Data
eTable 18. Associations Between Specific Maternal Autoimmune Diseases Diagnosed Before Childbirth and Overall Mental Disorders in Offspring, Using Propensity Score Methods
eTable 19. Hazard Ratios of Specific Mental Disorder Among Individuals Born to Mothers With Any Autoimmune Disease, Using Propensity Score Methods
eTable 20. Association Between Overall Maternal Autoimmune Diseases Diagnosed Before Childbirth and Suicide in Offspring
eFigure. The Log-Minus-Log Survival Curve
References
- 1.Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB. Epidemiology of autoimmune diseases in Denmark. J Autoimmun. 2007;29(1):1-9. doi: 10.1016/j.jaut.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tincani A, Dall’Ara F, Lazzaroni MG, Reggia R, Andreoli L. Pregnancy in patients with autoimmune disease: a reality in 2016. Autoimmun Rev. 2016;15(10):975-977. doi: 10.1016/j.autrev.2016.07.017 [DOI] [PubMed] [Google Scholar]
- 3.Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23(1):116-123. doi: 10.1016/j.bbi.2008.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luchicchi A, Lecca S, Melis M, et al. Maternal immune activation disrupts dopamine system in the offspring. Int J Neuropsychopharmacol. 2016;19(7):pyw007. doi: 10.1093/ijnp/pyw007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrich E, Piontkewitz Y, Peretz A, Weiner I, Attali B. Maternal immune activation produces neonatal excitability defects in offspring hippocampal neurons from pregnant rats treated with poly I:C. Sci Rep. 2016;6(1):19106. doi: 10.1038/srep19106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman S, Nielsen J, Jølving LR, Nøhr EA, Nørgård BM. Long-term motor and cognitive function in the children of women with inflammatory bowel disease. J Crohns Colitis. 2020;14(12):1709-1716. doi: 10.1093/ecco-jcc/jjaa106 [DOI] [PubMed] [Google Scholar]
- 7.Keil A, Daniels JL, Forssen U, et al. Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology. 2010;21(6):805-808. doi: 10.1097/EDE.0b013e3181f26e3f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai PH, Yu KH, Chou IJ, et al. Risk of autism spectrum disorder in children born to mothers with systemic lupus erythematosus and rheumatoid arthritis in Taiwan. Joint Bone Spine. 2018;85(5):599-603. doi: 10.1016/j.jbspin.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Nielsen PR, Benros ME, Dalsgaard S. Associations between autoimmune diseases and attention-deficit/hyperactivity disorder: a nationwide study. J Am Acad Child Adolesc Psychiatry. 2017;56(3):234-240.e1. doi: 10.1016/j.jaac.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 10.Mataix-Cols D, Frans E, Pérez-Vigil A, et al. A total-population multigenerational family clustering study of autoimmune diseases in obsessive-compulsive disorder and Tourette’s/chronic tic disorders. Mol Psychiatry. 2018;23(7):1652-1658. doi: 10.1038/mp.2017.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atladóttir HO, Pedersen MG, Thorsen P, et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124(2):687-694. doi: 10.1542/peds.2008-2445 [DOI] [PubMed] [Google Scholar]
- 12.Mouridsen SE, Rich B, Isager T, Nedergaard NJ. Autoimmune diseases in parents of children with infantile autism: a case-control study. Dev Med Child Neurol. 2007;49(6):429-432. doi: 10.1111/j.1469-8749.2007.00429.x [DOI] [PubMed] [Google Scholar]
- 13.Rom AL, Wu CS, Olsen J, Jawaheer D, Hetland ML, Mørch LS. Parental rheumatoid arthritis and autism spectrum disorders in offspring: a Danish nationwide cohort study. J Am Acad Child Adolesc Psychiatry. 2018;57(1):28-32.e1. doi: 10.1016/j.jaac.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 14.Nielsen TC, Nassar N, Shand AW, et al. Association of maternal autoimmune disease with attention-deficit/hyperactivity disorder in children. JAMA Pediatr. 2021;175(3):e205487. doi: 10.1001/jamapediatrics.2020.5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalsgaard S, Waltoft BL, Leckman JF, Mortensen PB. Maternal history of autoimmune disease and later development of Tourette syndrome in offspring. J Am Acad Child Adolesc Psychiatry. 2015;54(6):495-501.e1. doi: 10.1016/j.jaac.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541-549. doi: 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 17.Bliddal M, Broe A, Pottegård A, Olsen J, Langhoff-Roos J. The Danish Medical Birth Register. Eur J Epidemiol. 2018;33(1):27-36. doi: 10.1007/s10654-018-0356-1 [DOI] [PubMed] [Google Scholar]
- 18.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7)(suppl):30-33. doi: 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 19.Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health. 2011;39(7)(suppl):54-57. doi: 10.1177/1403494810395825 [DOI] [PubMed] [Google Scholar]
- 20.Plana-Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827-1835. doi: 10.1016/S0140-6736(19)32316-5 [DOI] [PubMed] [Google Scholar]
- 21.Svahn MF, Hargreave M, Nielsen TS, et al. Mental disorders in childhood and young adulthood among children born to women with fertility problems. Hum Reprod. 2015;30(9):2129-2137. doi: 10.1093/humrep/dev172 [DOI] [PubMed] [Google Scholar]
- 22.Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7)(suppl):38-41. doi: 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 23.Richard-Miceli C, Criswell LA. Emerging patterns of genetic overlap across autoimmune disorders. Genome Med. 2012;4(1):6. doi: 10.1186/gm305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jølving LR, Nielsen J, Beck-Nielsen SS, et al. The association between maternal chronic inflammatory bowel disease and long-term health outcomes in children—a nationwide cohort study. Inflamm Bowel Dis. 2017;23(8):1440-1446. doi: 10.1097/MIB.0000000000001146 [DOI] [PubMed] [Google Scholar]
- 25.Vinet É, Pineau CA, Clarke AE, et al. Increased risk of autism spectrum disorders in children born to women with systemic lupus erythematosus: results from a large population-based cohort. Arthritis Rheumatol. 2015;67(12):3201-3208. doi: 10.1002/art.39320 [DOI] [PubMed] [Google Scholar]
- 26.Flannery KA, Liederman J. A test of the immunoreactive theory for the origin of neurodevelopmental disorders in the offspring of women with immune disorder. Cortex. 1994;30(4):635-646. doi: 10.1016/S0010-9452(13)80240-7 [DOI] [PubMed] [Google Scholar]
- 27.Wu S, Ding Y, Wu F, et al. Family history of autoimmune diseases is associated with an increased risk of autism in children: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2015;55:322-332. doi: 10.1016/j.neubiorev.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 28.Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, McDougle CJ. Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics. 2003;112(5):e420. doi: 10.1542/peds.112.5.e420 [DOI] [PubMed] [Google Scholar]
- 29.Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med. 2005;159(2):151-157. doi: 10.1001/archpedi.159.2.151 [DOI] [PubMed] [Google Scholar]
- 30.Chen MH, Su TP, Chen YS, et al. Comorbidity of allergic and autoimmune diseases among patients with ADHD. J Atten Disord. 2017;21(3):219-227. doi: 10.1177/1087054712474686 [DOI] [PubMed] [Google Scholar]
- 31.Butwicka A, Frisén L, Almqvist C, Zethelius B, Lichtenstein P. Risks of psychiatric disorders and suicide attempts in children and adolescents with type 1 diabetes: a population-based cohort study. Diabetes Care. 2015;38(3):453-459. doi: 10.2337/dc14-0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium . Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18(2):199-209. doi: 10.1038/nn.3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JY, Huerta PT, Zhang J, et al. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. 2009;15(1):91-96. doi: 10.1038/nm.1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Croen LA, Braunschweig D, Haapanen L, et al. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry. 2008;64(7):583-588. doi: 10.1016/j.biopsych.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howarth C, Gazis A, James D. Associations of type 1 diabetes mellitus, maternal vascular disease and complications of pregnancy. Diabet Med. 2007;24(11):1229-1234. doi: 10.1111/j.1464-5491.2007.02254.x [DOI] [PubMed] [Google Scholar]
- 37.Clowse ME. Lupus activity in pregnancy. Rheum Dis Clin North Am. 2007;33(2):237-252, v. doi: 10.1016/j.rdc.2007.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed SD, Vollan TA, Svec MA. Pregnancy outcomes in women with rheumatoid arthritis in Washington State. Matern Child Health J. 2006;10(4):361-366. doi: 10.1007/s10995-006-0073-3 [DOI] [PubMed] [Google Scholar]
- 39.Schlotz W, Phillips DIW. Fetal origins of mental health: evidence and mechanisms. Brain Behav Immun. 2009;23(7):905-916. doi: 10.1016/j.bbi.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 40.Larsson HJ, Eaton WW, Madsen KM, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol. 2005;161(10):916-925. doi: 10.1093/aje/kwi123 [DOI] [PubMed] [Google Scholar]
- 41.Linnet KM, Wisborg K, Agerbo E, Secher NJ, Thomsen PH, Henriksen TB. Gestational age, birth weight, and the risk of hyperkinetic disorder. Arch Dis Child. 2006;91(8):655-660. doi: 10.1136/adc.2005.088872 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Diagnostic Classification of Autoimmune Diseases Considered According to the ICD-10 and Equivalent ICD-8 Diagnoses
eTable 2. Diagnostic Classification of Mental Disorders Considered According to the ICD-10 and Equivalent ICD-8 Diagnoses
eTable 3. Anatomical Therapeutic Chemical (ATC) Codes for Autoimmune Disease and Mental Disorders Medications
eTable 4. Associations Between Specific Maternal Autoimmune Diseases and Risk of Any Mental Disorder in the Offspring by Offspring’s Age
eTable 5. Hazard Ratios of Any Mental Disorder Among the Offspring Stratified by the Numbers of Comorbid Maternal Autoimmune Diseases
eTable 6. Associations Between Specific Maternal Autoimmune Diseases Diagnosed Before Childbirth and Overall Mental Disorders Among Term-Born Individuals
eTable 7. Hazard Ratios of Specific Mental Disorders According to Prenatal Exposure to Any Maternal Autoimmune Disease Among Term-Born Individuals
eTable 8. Associations Between Specific Maternal Autoimmune Diseases Diagnosed Before Child Birth and Overall Mental Disorders Among Individuals Without Congenital Malformations of the Nervous System or Chromosomal Abnormalities
eTable 9. Hazard Ratios of Specific Mental Disorder According to Prenatal Exposure to Any Maternal Autoimmune Disease Among Individuals Without Congenital Malformations of the Nervous System and Chromosomal Abnormalities
eTable 10. Associations Between Specific Autoimmune Diseases of Mothers and Overall Mental Disorders in the Offspring Born During 1998-2007
eTable 11. Associations Between Specific Autoimmune Diseases of Mothers and Overall Mental Disorders in the Offspring Born During 2008-2015
eTable 12. Associations Between Specific Paternal Autoimmune Diseases Diagnosed Before Childbirth and Overall Mental Disorders in Offspring
eTable 13. Hazard Ratios of Specific Mental Disorder in Offspring According to Prenatal Exposure to Any Paternal Autoimmune Disease Diagnosed Before Childbirth
eTable 14. Associations Between Specific Maternal Autoimmune Diseases Diagnosed Before Childbirth and Overall Mental Disorders, Adjusted for Medications Used by Mothers Before Childbirth in Addition to the Variables in the Main Model
eTable 15. Hazard Ratios of Specific Mental Disorder According to Prenatal Exposure to Any Maternal Autoimmune Disease, Adjusted for Medications Used by Mothers Before Childbirth in Addition to the Variables in the Main Model
eTable 16. Associations Between Specific Maternal Autoimmune Diseases Diagnosed Before Childbirth and Overall Mental Disorders in Offspring, With Multiple Imputation for Missing Data
eTable 17. Hazard Ratios of Specific Mental Disorder According to Prenatal Exposure to Any Maternal Autoimmune Disease, With Multiple Imputation for Missing Data
eTable 18. Associations Between Specific Maternal Autoimmune Diseases Diagnosed Before Childbirth and Overall Mental Disorders in Offspring, Using Propensity Score Methods
eTable 19. Hazard Ratios of Specific Mental Disorder Among Individuals Born to Mothers With Any Autoimmune Disease, Using Propensity Score Methods
eTable 20. Association Between Overall Maternal Autoimmune Diseases Diagnosed Before Childbirth and Suicide in Offspring
eFigure. The Log-Minus-Log Survival Curve