Abstract
Elevated measures of matrix metalloproteinases (MMPs) are associated with acute myocardial infarction (MI), but it is not known how long these changes persist post-MI or if these measures differ between atherothrombotic versus non-atherothrombotic MI. MMPs-2, 3, and 9 were measured in 80 subjects with acute MI (atherothrombotic and non-atherothrombotic MI) or stable coronary artery disease (CAD). Measurements were made at, the time of acute MI, and > 3-month following acute MI (quiescent phase). Outcome measures were compared between groups and between time of acute MI and quiescent post-MI follow-up using Wilcoxon’s and repeated measures analysis of variance. Forty-nine subjects met the criteria for acute MI with clearly defined atherothrombotic (n = 22) and non-atherothrombotic (n = 12) subsets. Fifteen subjects met criteria for stable CAD. MMP-3 was higher in acute MI versus stable CAD subjects at the time of acute MI: (453 vs. 217 pg/mL, p = 0.010) but not at quiescent phase follow-up (p > 0.05). MMP-9 was higher in acute MI versus stable CAD subjects at the time of acute MI: (412 vs. 168 pg/mL, p = 0.002) but not at the quiescent phase follow-up (p > 0.05). MMP-9 was higher at the time of acute MI versus quiescent phase follow-up in acute MI (412 vs. 213 pg/mL, p = 0.001) and atherothrombotic MI specifically (458 vs. 212 pg/mL, p = 0.001). No difference in MMP-2, 3, or 9 was observed between atherothrombotic versus non-atherothrombotic MI subgroups. MMPs-3 and 9 are significantly elevated in acute MI verses stable CAD subjects at time of acute MI but not different at quiescent phase follow-up. MMP-9 is elevated at the time of acute MI and specifically in acute atherothrombotic MI at time of MI versus quiescent phase follow-up.
Keywords: Atherothrombosis, Acute myocardial infarction, Metalloproteinases, Extracellular matrix
Introduction
Myocardial infarction (MI) is a major cause of death and disability worldwide [1, 2]. An atherosclerotic plaque is the underlying substrate responsible for acute MI. The atherosclerotic plaque is a heterogeneous population of biological substances, collectively embedded by an extracellular matrix (ECM) [3-6]. The ECM is a complex network of collagen, glycoproteins, and enzymes, which undergo constant remodeling to provide structural and chemical support to the coronary vasculature [7]. The matrix metalloproteinases (MMPs) play a central role in ECM degradation by selectively hydrolyzing individual components of the ECM [7, 8]. As a part of their normal physiological function, MMPs maintain the vessel’s integrity by breaking down ECM while new matrix is being synthesized. They achieve this vascular remodeling by facilitating cell turnover, inflammatory signals, angiogenesis, and collagen degradation [9]. This upkeep is necessary to avoid weakening of the ECM from continuous mechanical stresses. However, these otherwise normally functioning mechanisms of MMPs also facilitate Glagov remodeling and degradation of the fibrous cap surrounding atherosclerotic plaque resulting in a plaque prone to rupture and ensuing atherothrombosis [6, 10-14].
The most commonly encountered perpetrator of an acute MI is the rupture of an atherosclerotic plaque, which in turn precipitates an uncontrolled thrombotic response resulting in an acute atherothrombotic MI [15-17]. Nonetheless, multiple non-atherothrombotic MI etiologies such as coronary vasospasm and demand ischemia, and non-MI causes of myocardial injury are now known to exist and necessitate treatment distinctive from atherothrombotic MI [17]. Multiple studies have reported that acute non-atherothrombotic MI is at least as common as thrombotic MI and is associated with greater mortality [18-22]. The Fourth Universal Definition of Myocardial Infarction (UDMI) presents an international consensus definition for etiologically distinct classes of MIs that can be simplified into two classes: atherothrombotic and non-atherothrombotic MI [17].
While MMPs are known to be elevated in the blood following an acute myocardial infarction (MI) [7, 23-25], it is not known how long these changes persist post-MI or if these measures differ between acute atherothrombotic and non-atherothrombotic MI. Therefore, we sought to study the progression of MMPs during and after an acute MI as compared to subjects with stable CAD undergoing a cardiac catheterization, and between atherothrombotic and non-atherothrombotic MI. We hypothesize that MMPs will differ between acute MI and stable CAD subjects at the time of acute MI but not after the acute disease has resolved (quiescent phase). Furthermore, we hypothesize that MMP levels will differ between acute atherothrombotic versus non-atherothrombotic MI.
Materials and methods
Study design and population
Following Institutional Review Board (IRB) approval, participants were recruited from University of Louisville Hospital and Jewish Hospital in Louisville, Kentucky between March 2012 and August 2013. Two groups of subjects were enrolled in the study: acute MI and stable CAD. Inclusion criteria for both groups required that each subject be greater than 18 years of age and undergo cardiac catheterization with coronary angiography within 48 h. Specific inclusion and exclusion criteria are detailed in supplemental material (Appendix Table 1). Subjects who received fibrinolytic therapy were excluded from this study. All subjects provided signed informed consent and study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Sample collection and time-points
Sample collection and processing were completed under an established standardized operating protocol, as described in our previous studies [26-28]. Blood samples were collected in ethylene-diamine-tetraacetic acid (EDTA) coated vacutainer tubes. Time between phlebotomy and processing was standardized at 45 min. Plasma derived from the EDTA tubes was aliquoted and stored in a – 81 °C freezer within 2 h of blood draw. Potential confounders from time of blood draw to storage was examined for association with all analytes and a regression analysis was performed.
Blood samples were collected at two distinct phases: acute and quiescent. Acute phase consisted of two time-points: T0 (immediately at start of cardiac catheterization) and T6 (6 h post T0). Quiescent phase was a follow-up during which the subject was free of any recent acute illness for a minimum of 3 months. Acute phase samples were collected from an arterial sheath after a 5–10 mL waste draw, whereas for the quiescent phase samples, virgin peripheral veins were phlebotomized with a 5–10 mL waste draw.
Coronary angiography and histological analysis
Angiograms were systematically evaluated in all subjects by the Angiographic Core Laboratory at the Johns Hopkins University (Baltimore, Maryland) by a technician and physician blinded to all other subject data. Coronary aspiration was left to the discretion of the treating interventional cardiologist. The standard of care at the enrollment sites for this study included thrombus aspiration in ST elevation myocardial infarction patients. If aspiration was attempted, the aspirate was immediately filtered and preserved in formalin for histological evaluation by a pathologist specializing in coronary thrombosis at CVPath, Incorporated (Gaithersburg, Maryland). The pathologist was blinded to all other subject data except the vessel from which the aspirate was obtained.
Biochemical analysis
Clinical laboratory data was tested at two independent CLIA approved laboratories at the University of Louisville Hospital (ULH) and KentuckyOne Health Jewish Hospital (JH). Troponin criteria for outcomes are detailed in supplemental material (Appendix Methods, Biochemical Data).
Sample testing via enzyme-linked immunosorbent assay (ELISA)
Quantification of MMPs 2, 3, and 9 was performed using the Affymetrix Human ProcartaPlex Multiplex Immunoassay MMP Panel II (Santa Clara, CA) kits and the Luminex MAGPIX instrument (Austin, Texas). The MMP concentrations were recorded in picograms per milliliter (pg/mL) units. Assay optimization and validation were performed in control subjects prior to testing study samples. MMP concentrations for all study samples were measured in triplicates, the three measures were averaged to obtain a single measurement value. A single operator, who is an expert in laboratory techniques, completed all the sample measurements. Sample testing is further detailed in supplemental material [Appendix Methods, Sample Testing via Enzyme-Linked Immunosorbent Assay (ELISA)].
Study group classification
Four subject groups were defined for this study a priori: stable CAD, acute MI, atherothrombotic MI, non-atherothrombotic MI (Appendix Table 2). Atherothrombotic MI and non-atherothrombotic MI subjects were a subset of the acute MI group. We developed novel conservative criteria, a priori, eliminating borderline cases from analysis in order to limit confounding factors from misclassification and to produce an ideal cohort for discovering new biology related to acute MI. Our criteria are a variation of criteria previously proposed by our group [26, 28-30]. Borderline MI subjects that did not identify with study group criteria were excluded from the analysis. We believe these criteria are more robust than any other published criteria for distinguishing between atherothrombotic MI and non-atherothrombotic MI.
Outcome measures
The primary outcome is the concentration of MMP-2, 3, and 9 at the acute phase, the post MI quiescent phase, and the absolute change from acute phase to follow-up in acute MI and stable CAD participants. A secondary analysis was conducted to evaluate differences in MMP-2, 3, and 9 between atherothrombotic MI and non-atherothrombotic MI.
Statistical analysis
Frequencies and percentages were reported for categorical variables, with Fisher’s exact test for comparison of the study population. Means and standard deviations were reported for continuous distributions. Shapiro–Wilk was used to determine the normal distribution. Normally distributed outcome measures were compared between groups using Student’s or Welch’s t tests if the heterogeneity of variances assumption was violated. Mann–Whitney U test was employed for non-normal distributions. Wilcoxon rank-sum test was used to compare differences between study groups at individual time-points for each analyte (MMP-2, 3, and 9).
The acute time-point was created by calculating the mean vector of the matrix using time-points T0 (immediately at start of cardiac catheterization) and T6 (6 h post T0).
Repeated measures analysis of variance (ANOVA) were employed to compare levels of each analyte (MMP-2, 3, and 9) over acute versus quiescent time-points for each individual study group and sub-group. Fixed effects of the model were: group, time-point: acute (T0, T6) versus quiescent (TF/U), and group × time-point. The random effects were the study subjects. The repeated models tested if the means of the fixed effects for each MMP differed by study groups or across time-points. It also measured whether the time-course differed by the study groups. Type III sum of squares F-tests P-values are reported for determining statistical significance. Further analysis of the sub-groups comparing the atherothrombotic MI and non-atherothrombotic MI at different time-points was also done. Random effects for study subject assumed a compound symmetry covariance structure.
Post-hoc power was calculated using means of the groups, size of the group, number of groups, detectable difference-calculated form the proportion of variance, and correlation across measurements, and the number of measurements per subject [31, 32].
All analyses and graphics were performed using R Studio version 3.5.1 (2018-07-02).
Results
Out of the 80 subjects enrolled in this study, 16 subjects did not meet analytical inclusion criteria or had missing data and were not included in the analysis (Appendix Fig. 1). Sixty-four subjects met criteria for study analysis, out of which 15 subjects met the phenotype criteria for stable CAD and 49 subjects for acute MI (Table 1). In the acute MI group, 22 subjects met criteria for atherothrombotic MI, and 12 for non-atherothrombotic MI, 15 borderline cases were not included in the analysis (Appendix Fig. 1).
Table 1.
Cohort characteristics
| Characteristic | Acute MI group (N = 49) | Stable CAD group (N = 15) |
P value |
|---|---|---|---|
| Age (mean ± SD) years | 58.59 ± 14.42 | 61.33 ± 8.86 | 0.49 |
| Males (%) | 65.3 | 53.3 | 0.59 |
| Caucasian race (%) | 77.6 | 93.3 | 0.32 |
| Current smoker (%) | 53.1 | 20.0 | 0.05 |
| Currently consumes alcohol (%) | 32.7 | 46.7 | 0.50 |
| History of dyslipidemia (%) | 46.9 | 86.7 | 0.047 |
| History of diabetes mellitus (%) | 10.2 | 40.0 | 0.022 |
| History of hypertension (%) | 61.2 | 93.3 | 0.042 |
| History of atherosclerosis (%) (MI, CAD, PCI, CABG) | 30.6 | 93.3 | < 0.001 |
| History of congestive heart failure (%) | 8.2 | 6.7 | 1.00 |
| HR at time of presentation (mean ± SD) | 84.90 ± 24.98 | 65.87 ± 9.59 | 0.006 |
| MAP at time of presentation (mean ± SD) | 102.77 ± 24.72 | 91.36 ± 14.29 | 0.10 |
| BMI at time of presentation (mean ± SD) | 27.54 ± 7.24 | 33 ± 7.08 | 0.013 |
| Baseline troponin (mean ± SD, range) mg/dL | 5.59 ± 10.83 | 0.01 ± 0.00 | 0.05 |
| Peak troponin (mean ± SD, range) | 33.53 ± 37.94 | 0.01 ± 0.00 | 0.002 |
| Glucose at baseline (mean ± SD, range) | 142.14 ± 56.46 | 131.60 ± 30.61 | 0.493 |
| Creatinine at baseline (mean ± SD, range) | 1.13 ± 0.79 | 0.92 ± 0.17 | 0.32 |
| ST elevation on EKG at baseline (%) | 63.3 | 0.0 | < 0.001 |
| At least one vessel with ≥ 50% coronary stenosis on enrollment angiogram (%) | 73.5 | 40.0 | 0.03 |
| Aspirin use at time of enrollment (%) | 34.7 | 100 | < 0.001 |
| Clopidogrel use at enrollment (%) | 10.2 | 66.7 | < 0.001 |
| Statin use at time of enrollment (%) | 32.7 | 80 | 0.003 |
| ACE-I/ARB use at time of enrollment (%) | 28.6 | 66.7 | 0.018 |
| Beta blocker use at time of enrollment (%) | 24.5 | 66.7 | 0.007 |
| Calcium channel blocker use at time of enrollment (%) | 14.3 | 53.3 | 0.006 |
| NSAIDs use at time of enrollment (%) | 38.8 | 0 | 0.011 |
Bold values indicate statistical significance (p < 0.05)
ACE-I Angiotensin converting enzyme inhibitor, ARB Angiotensin II receptor blockers, BMI Body Mass Index, CABG coronary artery bypass grafting, CAD coronary artery disease, DBP diastolic blood pressure, EKG electrocardiogram, HR heart rate, MAP mean arterial pressure, MI myocardial infarction, NSAIDs nonsteroidal anti-inflammatory drugs, PCI percutaneous coronary intervention, SBP systolic blood pressure, SD standard deviation
Cohort characteristics are presented in Table 1. Independent of variables that defined the individual study groups, current smoking and heart rate were higher, and history of diabetes, hypertension, and body mass index were lower in acute MI subjects as compared to stable CAD subjects (Table 1). Cohort characteristics of the MI sub-groups (atherothrombotic versus non-atherothrombotic MI) are detailed in Appendix Table 3.
Metalloproteinase-2
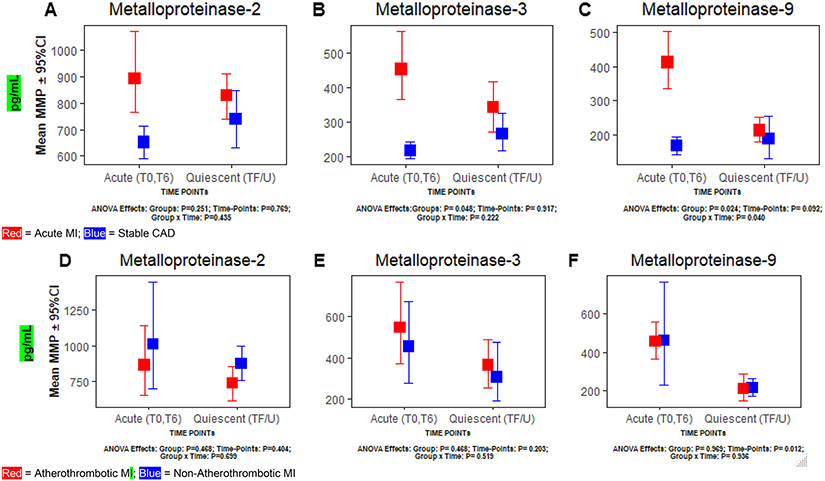
A significant difference in MMP-2 was not observed between acute MI versus stable CAD or atherothrombotic versus non-atherothrombotic MI at the acute or quiescent phase (TF/U) time-points (all p > 0.05) (Table 2). Significant differences were not observed for MMP-2 between acute versus quiescent phase (TF/U) in any of the study groups (acute MI, stable CAD, atherothrombotic, non-atherothrombotic MI) (all p > 0.05) (Table 3). Significant interaction between any study group and time-point was not observed in MMP-2 (p > 0.05) (Fig. 1a and d).
Table 2.
Comparison of metalloproteinases-2, 3, and 9 levels between Primary Study Groups and Study Sub-Groups at acute and quiescent (~ 3-month follow-up) time points
| Analyte | Time-point | Primary study groups analysis |
Study sub-group analysis |
||||
|---|---|---|---|---|---|---|---|
| Acute MI (n = 49) (Mean ± SD) pg/mL |
Stable CAD (n = 15) (Mean ± SD) pg/mL |
P value* | Atherothrombotic MI (n = 22) (Mean ± SD) pg/mL |
Non-Atherothrom- botic MI (n =12) (Mean ± SD) pg/mL |
P value* | ||
| MMP-2 | Acute | 893.25 ± 736.76 | 651.39 ± 172.86 | 0.078 | 862.55 ± 837.30 | 1011.39 ± 959.55 | 0.51 |
| Quiescent F/U | 828.25 ± 261.08 | 738.89 ± 209.84 | 0.33 | 736.98 ± 261.39 | 870.83 ± 200.51 | 0.28 | |
| MMP-3 | Acute | 453.03 ± 490.19 | 216.53 ± 67.40 | 0.010 | 546.28 ± 619.51 | 454.85 ± 489.97 | 0.25 |
| Quiescent F/U | 341.82 ± 226.45 | 265.96 ± 109.21 | 0.24 | 364.91 ± 239.88 | 306.40 ± 240.48 | 0.61 | |
| MMP-9 | Acute | 411.73 ± 419.60 | 168.34 ± 75.63 | 0.002 | 457.86 ± 323.39 | 460.87 ± 680.61 | 0.98 |
| Quiescent F/U | 213.35 ± 113.13 | 188.08 ± 121.35 | 0.48 | 211.73 ± 144.97 | 216.62 ± 74.92 | 0.53 | |
Bold values indicate statistical significance (p < 0.05)
Acute Phase Values at acute time-points T0 (at time of cardiac catheterization) and T6 (6 h post cardiac catheterization), CAD coronary artery disease, CI confidence interval, MI myocardial infarction, MMP matrix metalloproteinase, Primary Study Groups stable CAD versus acute MI, Sub-Groups atherothrombotic versus non-atherothrombotic MI, SD standard deviation, T0 at time of cardiac catheterization, T6 6 h post T0, Quiescent ~ 3 Month follow-up during a healthy state (without any illness)
P-values calculated via Wilcoxon rank-sum test for comparison between Primary Study Groups and for comparison between Study Sub-Groups
Table 3.
Metalloproteinases-2, 3, and 9 levels at time of acute event (acute MI or cardiac catheterization for stable CAD) versus quiescent phase follow-up (minimum of 3 months following acute event and free of acute illness) for Primary Groups and Sub-Groups
| Groups | Analyte | Acute phase (Mean ± SD) pg/mL |
Quiescent phase (Mean ± SD) pg/mL |
P value* |
|---|---|---|---|---|
| Stable CAD (n = 15) | MMP-2 | 651.39 ± 172.86 | 738.89 ± 209.84 | 0.25 |
| MMP-3 | 216.53 ± 67.40 | 265.96 ± 109.21 | 0.14 | |
| MMP-9 | 168.34 ± 75.63 | 188.08 ± 121.35 | 0.71 | |
| Acute MI (n = 49) | MMP-2 | 893.25 ± 736.76 | 828.25 ± 261.08 | 0.18 |
| MMP-3 | 453.03 ± 490.19 | 341.82 ± 226.45 | 0.40 | |
| MMP-9 | 411.73 ± 419.60 | 213.35 ± 113.13 | 0.001 | |
| Atherothrombotic MI (n = 22) | MMP-2 | 862.55 ± 837.30 | 736.98 ± 261.39 | 0.61 |
| MMP-3 | 546.28 ± 619.51 | 364.91 ± 239.88 | 0.30 | |
| MMP-9 | 457.86 ± 323.39 | 211.73 ± 144.97 | 0.001 | |
| Non-Atherothrombotic MI (n = 12) | MMP-2 | 1011.39 ± 959.55 | 870.83 ± 200.51 | 0.30 |
| MMP-3 | 454.85 ± 489.97 | 306.40 ± 240.48 | 0.54 | |
| MMP-9 | 460.87 ± 680.61 | 216.62 ± 74.92 | 0.16 |
Bold values indicate statistical significance (p < 0.05)
Acute Phase Values at acute time-points T0 (at time of cardiac catheterization) and T6 (6 h post cardiac catheterization), CAD coronary artery disease, CI confidence interval, MI myocardial infarction, MMP matrix metalloproteinase, Primary Study Groups stable CAD versus acute MI, Sub-Groups atherothrombotic versus non-atherothrombotic MI, SD standard deviation, T0 at time of cardiac catheterization, T6 6 h post T0, Quiescent Phase ~ 3 month follow-up during a healthy state (without any illness)
P values calculated via Wilcoxon signed-rank test for comparison of means between acute and quiescent time-points
Fig. 1.
Comparing changes in metalloproteinases-2, 3, and 9 between acute and quiescent time-points, a–c Primary analysis—acute myocardial infarction versus stable coronary artery disease; d–f Sub-Group analysis—atherothrombotic versus non-atherothrombotic myocardial infarction. CAD Coronary artery disease, CI confidence interval, MI myocardial infarction, SD standard deviation, T0 at time of cardiac catheterization, T6 6 hours post T0, Quiescent Phase (TF/U) ~ 3 month follow-up during a healthy state (without any illness). *P values calculated via repeated measures ANOVA
Metalloproteinase-3
At the acute phase, MMP-3 was significantly higher in acute MI subjects (453.03 ± 490.19) as compared to stable CAD subjects (216.53 ± 67.40, p = 0.010) (Table 2, Fig. 1b). MMP-3 was not significantly different at quiescent phase time-point (TF/U) between acute MI versus stable CAD groups (p > 0.05) (Table 2, Fig. 1b).
Significant differences were not observed for MMP-3 between acute versus quiescent phase in any of the study groups (acute MI, stable CAD, atherothrombotic, non-atherothrombotic MI) (all p > 0.05) (Table 3). Although mean MMP-3 for the acute phase was higher in acute MI versus stable CAD subjects (p = 0.048) (Fig. 1b), no significant difference was observed in atherothrombotic versus non-atherothrombotic MI and no significant interaction between study groups and time-point was observed for MMP-3 in any study group (p > 0.05) (Fig. 1b and e).
Metalloproteinase-9
At the acute phase, MMP-9 was significantly higher in acute MI subjects (411.73 ± 419.60) as compared to stable CAD subjects (168.34 ± 75.63, p = 0.002) (Table 2, Fig. 1c). No significant difference in MMP-9 was observed at the quiescent time-point between acute MI versus stable CAD groups or at any time between the atherothrombotic and non-atherothrombotic MI groups (p > 0.05) (Table 2, Fig. 1f).
MMP-9 was significantly higher during acute phase (411.73 ± 419.60) as compared to quiescent phase (213.35 ± 113.13, p = 0.001) in the acute MI group (Table 3, Fig. 1c). MMP-9 was also significantly higher at the acute phase (457.86 ± 323.39) when compared to the quiescent phase (211.73 ± 144.97, p = 0.001) in the atherothrombotic MI sub-group (Table 3, Fig. 1f). In contrast, no difference was observed between the acute and quiescent phase among the stable CAD and non-atherothrombotic MI groups (Table 3, Fig. 1). MMP-9 showed significant interaction between group and time-points in the acute MI (p = 0.040) but not for the stable CAD group (Fig. 1c) or the atherothrombotic versus non-atherothrombotic MI sub-groups (Fig. 1f).
The median time (in hours) from blood draw and collection to storage at – 81 °C in the acute MI subjects (1.68, IQR 1.50, 1.92) did not differ significantly from the stable CAD subjects (1.62, IQR 1.45, 1.75; P = 0.067). The time from collection to storage accounted for about 3.2% in the variability of the MMP-3 P = 0.017 (Appendix Table 5). In the regression analysis of the time of sample collection to storage at – 81 °C and the mean analytes levels, none of the analytes exhibited a significant relationship with the time from collection to storage. This remained after adjusting for the groups.
Post-hoc power calculation was performed for 64 subjects (49 acute MI and 15 stable CAD) with 3 distinct time-point measurements. For an expected moderate effect size of 0.50 SD pg/mL units, an alpha of 0.05 and assuming compound symmetry, repeated measures power was calculated at 80% for detecting a difference between acute MI and stable CAD (primary analysis) and 53% for detecting a difference between atherothrombotic and non-atherothrombotic MI subtypes (secondary analysis).
Discussion
In this prospective study of subjects with acute MI and stable CAD, there were three major findings with important clinical implications. First, MMP-3 and MMP-9 were significantly elevated in MI subjects, at the time of acute MI, but not at a quiescent phase follow-up, when compared to stable CAD subjects. Second, within acute MI subjects, MMP-9 is significantly higher at the time of acute MI, specifically atherothrombotic MI, as compared to a quiescent phase follow-up (~ 3 months post-acute MI). Third, a significant difference in MMP-2, MMP-3, or MMP-9 was not observed between atherothrombotic and non-atherothrombotic MI subjects.
MMP-2 is secreted by cardiomyocytes, endothelial cells, vascular smooth muscle cells, fibroblasts, and macrophages, and is involved in matrix degradation, angiogenesis, and inflammatory responses [25, 33, 34]. It has a high basal activity and is crucial during tissue turnover. MMP-3 is mainly secreted by cardiac fibroblasts and macrophages, and is actively involved in the breakdown of ECM components [25, 33-35]. MMP-3 also activates other MMPs and has a broad effect potential on ECM components [35]. MMP-9 is predominantly secreted by cardiomyocytes, endothelial cells, neutrophils, fibroblast, and macrophages, and shares a similar functionality of ECM degradation with that of MMP-2 [25, 33, 34]. MMP-9 expression increases considerably shortly after acute MI in the peripheral circulation and from samples collected from the great cardiac veins, this may suggest active plague rupture [36, 37].
The existing literature acknowledges a temporal association between MMPs and acute MI [25, 34, 38-40]. However, the progression of MMPs from the time of an acute MI through a state of recovery has not been reported previously. Our study fills this important knowledge gap by demonstrating that the elevation of MMP-3 and 9 at the time of acute MI resolves by a quiescent phase follow-up (approximately 3 months). There is growing evidence about the role of MMPs in facilitating the degeneration of the fibrous cap of an unstable plaque [7]. Inflammatory and smooth muscle cells that express MMPs, especially in vulnerable plaques, orchestrate the final outcome of acute MI [7]. MMPs undertake a vigorous degradation of the ECM and an increased pro-inflammatory signaling during the acute phase of an MI [9], which is to the hypothesized purpose of recycling necrolyzed cardiomyocytes and other tissue debris [34]. Kai et al. investigated MMP-2 and MMP-9 levels in subjects with acute coronary syndrome (ACS) as compared to stable angina and healthy volunteers and found that both MMP-2 and MMP-9 are significantly elevated during the first 2 days of an ACS episode as compared to stable angina or healthy volunteers [37]. Our finding concurs with the Kai et al. study (N = 67) with respect to the observation of higher MMP-9 during the acute phase of an MI. However, in contrast to the Kai et al. study’s results, our study did not find a significant difference in MMP-2 levels between acute MI and stable CAD groups. This observed phenomenon may be due to the higher constitutive activity of MMP-2 [34]. While MMP-2 exhibited a decreasing trend over time in our study, this descent was not statistically significant. On the other hand, MMPs-3 and 9 exhibited a statistically significant two-fold higher levels in the acute MI group as compared to the stable CAD group.
Our findings of increased MMP-3 levels following acute MI is consistent with findings by Cavusoglu et al. and Abd El-Aziz et al. [41, 42]. These studies noted both the diagnostic and prognostic implications of elevated MMP-3, while our study was of diagnostic importance. These studies also presented MMPs’ polymorphisms of diagnostic importance in subpopulations and outcomes following acute MI [41, 42]. The elevated levels of MMP-9 during the time of an acute MI in our study were significantly reduced to about half at the time of a quiescent phase follow-up (approximately 3 months post MI). However, this trend was not exclusive to one specific study sub-group (atherothrombotic or non-atherothrombotic MI). We found no evidence of significant differences between atherothrombotic and non-atherothrombotic subjects in any of the investigated analytes (MMP-2, MMP-3, or MMP-9).
Our findings of significantly higher levels of MMP-9 at the time of acute MI, specifically atherothrombotic MI, as compared to a quiescent phase follow-up (~ 3 months post-acute MI), may be secondary to inducible transcription of matrix metalloproteinase which may be an important determinant of plaque rupture resulting in cardiovascular events. This is the first study to demonstrate a difference in MMP activity between these distinct acute MI subtypes. Identification of relevant differences between acute MI subtypes, as demonstrated here with MMP-9, [16]; will allow for a greater understanding of the unique pathobiology of these MI subtypes. Greater understanding of acute MI subtype pathobiology is needed to develop diagnostics, prevention and treatment strategies specific to these etiologically distinct MI subtypes (precision medicine).
Limitations
Our study time-points in the acute phase did not extend beyond 6 h post-event. We also did not type for haplotypes or the functional variants of these MMPs. This limits our ability to compare our findings to other studies reporting elevations 72 h to 4 days post-MI and the variants of the analytes. Ours is the first study comparing acute MI and atherothrombotic and non-atherothrombotic MI subtypes, however, we were limited by small sample size. Although residual confounding can never be completed ruled out in human subject research we believe our stringently study designed, including rigorous objective criteria for identifying study phenotypes, and repeat measures to allow for intra-subject comparisons (acute versus quiescent phase) provides the most rigorous control of confounding possible for this disease process. We also acknowledge that MMP testing is not currently widely available in clinical practice but believe proving utility will drive testing innovation and availability. Medications used for the treatment of cardiovascular diseases such as angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers, beta-blockers, and statins have been previously described as indirect inhibitors of MMPs [43-49]. The stable CAD group in our study has reported a significantly higher use of these medications at the time of enrollment, which could have resulted in lower MMP concentrations in the stable CAD group.
Conclusions
MMPs-3 and 9 are elevated at the time of acute MI and significantly decrease by a quiescent phase follow-up (approximately 3 months). This pattern appears to be consistent among thrombotic and non-thrombotic MI but is most pronounced for atherothrombotic MI and MMP-9. MMP-2, 3, and 9 do not differentiate atherothrombotic from non-atherothrombotic MIs. The role of MMP’s in acute MI subtypes, atherothrombotic versus non-atherothrombotic, warrants further investigation.
Supplementary Material
Highlights.
Metalloproteinases are significantly elevated at the time of acute myocardial.
Infarction (AMI) and significantly decrease over a median follow up time of about 3 months.
At the time of atherothrombotic MI, the levels of the metalloproteinase-9 are elevated compared to a quiescent phase 3-month follow up.
Metalloproteinases do not differentiate atherothrombotic MI from non-atherothrombotic MI.
Acknowledgments
We would like to thank all of the subjects who generously consented to participate in this study. We also appreciate the University of Louisville Diabetes and Obesity Center, the University of Louisville and the KentuckyOne Jewish Hospitals, CVPath Institute, Inc., Gaithersburg, Maryland, and the Johns Hopkins Quantitative Angiographic Core Laboratory. This work was supported in part by a grant from the American Heart Association (11CRP7300003), the National Institute of General Medical Sciences (1P20 GM103492).
Footnotes
Conflict of interest All authors have no conflict of interest to disclose.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11239-019-02004-7) contains supplementary material, which is available to authorized users.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR,Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS,American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S (2019) Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139(10):e56–e528. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 2.Disease GBD, Injury I, Prevalence C (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1789–1858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett MR, Sinha S, Owens GK (2016) Vascular smooth muscle cells in atherosclerosis. Circ Res 118(4):692–702. 10.1161/CIRCRESAHA.115.306361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentzon JF, Otsuka F, Virmani R, Falk E (2014) Mechanisms of plaque formation and rupture. Circ Res 114(12):1852–1866. 10.1161/CIRCRESAHA.114.302721 [DOI] [PubMed] [Google Scholar]
- 5.Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352(16):1685–1695. 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Touchard A, Henry TD, Sangiorgi G, Spagnoli LG, Mauriello A, Conover C, Schwartz RS (2005) Extracellular proteases in atherosclerosis and restenosis. Arterioscler Thromb Vasc Biol 25(6):1119–1127. 10.1161/01.ATV.0000164311.48592.da [DOI] [PubMed] [Google Scholar]
- 7.Jones CB, Sane DC, Herrington DM (2003) Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res 59(4):812–823. 10.1016/s0008-6363(03)00516-9 [DOI] [PubMed] [Google Scholar]
- 8.Katsuda S, Kaji T (2003) Atherosclerosis and extracellular matrix. J Atheroscler Thromb 10(5):267–274. 10.5551/jat.10.267 [DOI] [PubMed] [Google Scholar]
- 9.Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15(12):786–801. 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celentano DC, Frishman WH (1997) Matrix metalloproteinases and coronary artery disease: a novel therapeutic target. J Clin Pharmacol 37(11):991–1000 [DOI] [PubMed] [Google Scholar]
- 11.Korshunov VA, Schwartz SM, Berk BC (2007) Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov’s phenomenon. Arterioscler Thromb Vasc Biol 27(8):1722–1728. 10.1161/ATVBAHA.106.129254 [DOI] [PubMed] [Google Scholar]
- 12.Libby P (2012) Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 32(9):2045–2051. 10.1161/ATVBAHA.108.179705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varnava AM, Mills PG, Davies MJ (2002) Relationship between coronary artery remodeling and plaque vulnerability. Circulation 105(8):939–943. 10.1161/hc0802.104327 [DOI] [PubMed] [Google Scholar]
- 14.Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and bio-chemistry. Circ Res 92(8):827–839. 10.1161/01.RES.0000070112.80711.3D [DOI] [PubMed] [Google Scholar]
- 15.Burke AP, Farb A, Malcom GT, Liang Y, Smialek JE, Virmani R (1999) Plaque rupture and sudden death related to exertion in men with coronary artery disease. JAMA 281(10):921–926 [DOI] [PubMed] [Google Scholar]
- 16.DeFilippis AP, Nasir K, Blaha MJ (2019) Myocardial infarction as a clinical end point in research. Circ Res 124(12):1701–1703. 10.1161/CIRCRESAHA.119.315101 [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial I (2018) Fourth universal definition of myocardial infarction. J Am Coll Cardiol 72(18):2231–2264. 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 18.Gore MO, Seliger SL, Defilippi CR, Nambi V, Christenson RH, Hashim IA, Hoogeveen RC, Ayers CR, Sun W, McGuire DK, Ballantyne CM, de Lemos JA (2014) Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol 63(14):1441–1448. 10.1016/j.jacc.2013.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Javed U, Aftab W, Ambrose JA, Wessel RJ, Mouanoutoua M, Huang G, Barua RS, Weilert M, Sy F, Thatai D (2009) Frequency of elevated troponin I and diagnosis of acute myocardial infarction. Am J Cardiol 104(1):9–13. 10.1016/j.amjcard.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 20.Wong P, Murray S, Ramsewak A, Robinson A, van Heyningen C, Rodrigues E (2007) Raised cardiac troponin T levels in patients without acute coronary syndrome. Postgrad Med J 83(977):200–205. 10.1136/pgmj.2006.049080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong P, Ramsewak A, Murray S, Robinson A, Robinson D, Rodrigues E (2007) Effects of comorbidity and hospital care on 6-month mortality in patients with elevated cardiac troponin T. Postgrad Med J 83(979):332–337. 10.1136/pgmj.2006.053082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong PS, Jones JD, Ashrafi R, Khanzada O, Wickramarachchi U,Keen TH, Robinson DR (2012) Early and late mortality in hospitalised patients with raised cardiac troponin T. Postgrad Med J 88(1042):437–442. 10.1136/postgradmedj-2011-130466 [DOI] [PubMed] [Google Scholar]
- 23.Hlatky MA, Ashley E, Quertermous T, Boothroyd DB, Ridker P, Southwick A, Myers RM, Iribarren C, Fortmann SP, Go AS, Atherosclerotic Disease VF, Genetic Epidemiology S (2007) Matrix metalloproteinase circulating levels, genetic polymorphisms, and susceptibility to acute myocardial infarction among patients with coronary artery disease. Am Heart J 154(6):1043–1051. 10.1016/j.ahj.2007.06.042 [DOI] [PubMed] [Google Scholar]
- 24.Liu P, Sun M, Sader S (2006) Matrix metalloproteinases in cardiovascular disease. Can J Cardiol 22(Suppl B):25B–30B. 10.1016/s0828-282x(06)70983-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phatharajaree W, Phrommintikul A, Chattipakorn N (2007) Matrix metalloproteinases and myocardial infarction. Can J Cardiol 23(9):727–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFilippis AP, Trainor PJ, Hill BG, Amraotkar AR, Rai SN, Hirsch GA, Rouchka EC, Bhatnagar A (2017) Identification of a plasma metabolomic signature of thrombotic myocardial infarction that is distinct from non-thrombotic myocardial infarction and stable coronary artery disease. PLoS ONE 12(4):e0175591. 10.1371/journal.pone.0175591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amraotkar AR, Song DD, Otero D, Trainor PJ, Ismail I, Kothari V,Singh A, Moore JB, Rai SN, DeFilippis AP (2017) Platelet count and mean platelet volume at the time of and after acute myocardial infarction. Clin Appl Thromb Hemost 23(8):1052–1059. 10.1177/1076029616683804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sultan A, Zheng Y, Trainor PJ, Siow Y, Amraotkar AR, Hill BG, DeFilippis AP (2017) Circulating prolidase activity in patients with myocardial infarction. Front Cardiovasc Med 4:50. 10.3389/fcvm.2017.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amraotkar AR, Ghafghazi S, Trainor PJ, Hargis CW, Irfan AB, Rai SN, Bhatnagar A, DeFilippis AP (2017) Presence of multiple coronary angiographic characteristics for the diagnosis of acute coronary thrombus. Cardiol J 24(1):25–34. 10.5603/CJ.a2017.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeFilippis AP, Chernyavskiy I, Amraotkar AR, Trainor PJ, Kothari S, Ismail I, Hargis CW, Korley FK, Leibundgut G, Tsimikas S, Rai SN, Bhatnagar A (2016) Circulating levels of plasminogen and oxidized phospholipids bound to plasminogen distinguish between atherothrombotic and non-atherothrombotic myocardial infarction. J Thromb Thrombolysis 42(1):61–76. 10.1007/s11239-015-1292-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller KE, Lavange LM, Ramey SL, Ramey CT (1992) Power calculations for general linear multivariate models including repeated measures applications. J Am Stat Assoc 87(420):1209–1226. 10.1080/01621459.1992.10476281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schafer T, Schwarz MA (2019) The meaningfulness of effect sizes in psychological research: differences between sub-disciplines and the impact of potential biases. Front Psychol 10:813. 10.3389/fpsyg.2019.00813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beilby JP, Chapman CM, Palmer LJ, McQuillan BM, Thompson PL, Hung J (2005) Stromelysin-1 (MMP-3) gene 5A/6A promoter polymorphism is associated with blood pressure in a community population. J Hypertens 23(3):537–542 [DOI] [PubMed] [Google Scholar]
- 34.DeLeon-Pennell KY, Meschiari CA, Jung M, Lindsey ML (2017) Matrix metalloproteinases in myocardial infarction and heart failure. Prog Mol Biol Transl Sci 147:75–100. 10.1016/bs.pmbts.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newby AC, Southgate KM, Davies M (1994) Extracellular matrix degrading metalloproteinases in the pathogenesis of arteriosclerosis. Basic Res Cardiol 89(Suppl 1):59–70. 10.1007/978-3-642-85660-0_6 [DOI] [PubMed] [Google Scholar]
- 36.Inokubo Y, Hanada H, Ishizaka H, Fukushi T, Kamada T, Okumura K (2001) Plasma levels of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 are increased in the coronary circulation in patients with acute coronary syndrome. Am Heart J 141(2):211–217. 10.1067/mhj.2001.112238 [DOI] [PubMed] [Google Scholar]
- 37.Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, Ueno T, Sugi K, Imaizumi T (1998) Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol 32(2):368–372 [DOI] [PubMed] [Google Scholar]
- 38.DeCoux A, Lindsey ML, Villarreal F, Garcia RA, Schulz R (2014) Myocardial matrix metalloproteinase-2: inside out and upside down. J Mol Cell Cardiol 77:64–72. 10.1016/j.yjmcc.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng B, Prasan A, Fung KC, Solanki V, Bruce D, Freedman SB, Brieger D (2005) Elevated circulating levels of matrix metalloproteinase-9 and −2 in patients with symptomatic coronary artery disease. Intern Med J 35(6):331–335. 10.1111/j.1445-5994.2005.00822.x [DOI] [PubMed] [Google Scholar]
- 40.Yabluchanskiy A, Li Y, Chilton RJ, Lindsey ML (2013) Matrix metalloproteinases: drug targets for myocardial infarction. Curr Drug Targets 14(3):276–286 [PMC free article] [PubMed] [Google Scholar]
- 41.Abd El-Aziz TA, Mohamed RH (2016) Matrix metalloproteinase 3 gene polymorphism and its level predict morbidity after acute myocardial infarction. Am J Clin Pathol 145(1):134–139. 10.1093/ajcp/aqv008 [DOI] [PubMed] [Google Scholar]
- 42.Cavusoglu E, Marmur JD, Kassotis JT, Yanamadala S, Chopra V, Eng C (2016) Usefulness of plasma matrix metalloproteinase-3 levels to predict myocardial infarction in men with and without acute coronary syndrome. Am J Cardiol 117(6):881–886. 10.1016/j.amjcard.2015.12.022 [DOI] [PubMed] [Google Scholar]
- 43.Luan Z, Chase AJ, Newby AC (2003) Statins inhibit secretion of metalloproteinases-1, −2, −3, and −9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol 23(5):769–775. 10.1161/01.ATV.0000068646.76823.AE [DOI] [PubMed] [Google Scholar]
- 44.Kuntze LB, Antonio RC, Izidoro-Toledo TC, Meschiari CA, Tanus-Santos JE, Gerlach RF (2014) Captopril and lisinopril only inhibit matrix metalloproteinase-2 (MMP-2) activity at millimolar concentrations. Basic Clin Pharmacol Toxicol 114(3):233–239 [DOI] [PubMed] [Google Scholar]
- 45.Jones K, Saxon L, Cunningham W, Adams P, Guideline Development G (2013) Secondary prevention for patients after a myocardial infarction: summary of updated NICE guidance. BMJ 347:f6544. 10.1136/bmj.f6544 [DOI] [PubMed] [Google Scholar]
- 46.Cimmino G, Ibanez B, Giannarelli C, Prat-Gonzalez S, Hutter R, Garcia M, Sanz J, Fuster V, Badimon JJ (2011) Carvedilol administration in acute myocardial infarction results in stronger inhibition of early markers of left ventricular remodeling than metoprolol. Int J Cardiol 153(3):256–261. 10.1016/j.ijcard.2010.08.018 [DOI] [PubMed] [Google Scholar]
- 47.Amraotkar AR, Hargis CW, Cambon AC, Rai SN, Keith MC, Ghafghazi S, Bolli R, DeFilippis AP (2015) Comparison of coli-form contamination in non-municipal waters consumed by the mennonite versus the non-mennonite rural populations. Environ Health Prev Med 20(5):338–346. 10.1007/s12199-015-0472-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto D, Takai S, Jin D, Inagaki S, Tanaka K, Miyazaki M (2007) Molecular mechanism of imidapril for cardiovascular protection via inhibition of MMP-9. J Mol Cell Cardiol 43(6):670–676. 10.1016/j.yjmcc.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 49.Pfeffer MA (1998) ACE inhibitors in acute myocardial infarction: patient selection and timing. Circulation 97(22):2192–2194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.