Abstract
Study Objectives
Insomnia Disorder diagnoses require persistent sleep complaints despite “adequate sleep opportunity.” Significant Perinatal Sleep Disruption makes this diagnosis challenging. This longitudinal study distinguished between Insomnia Disorder and Perinatal Sleep Disruption and their sleep and mental health correlates.
Methods
One hundred sixty-three nulliparous females (age M ± SD = 33.35 ± 3.42) participating in a randomized controlled trial repeated the Insomnia Disorder module of the Duke Structured Interview for Sleep Disorders and Patient-Reported Outcome Measurement Information System measures for sleep and mental health at 30- and 35-weeks’ gestation, and 1.5, 3, 6, 12, and 24 months postpartum (944 interviews, 1009 questionnaires completed). We compared clinical features when Diagnostic and Statistical Manual of Mental Disorders (DSM-5) Insomnia Disorder criteria (without the Duration criterion) were: (1) met (Insomnia Disorder), (2) not met only because of the sleep opportunity criteria (Perinatal Sleep Disruption), and (3) not met due to other criteria (Low Complaint).
Results
Proportions of Insomnia Disorder were 16.0% and 19.8% during early and late third trimester, and ranged 5.3%–11.7% postpartum. If the sleep opportunity criteria were not considered, rates of Insomnia would be 2–4 times higher (21.4%–40.4%) across time-points. Mixed-effects models adjusting for covariates showed that compared to Low Complaint, both Insomnia Disorder and Perinatal Sleep Disruption scored significantly higher on insomnia and sleep disturbance scales, sleep effort, and sleep-related impairments (p values < .01), but depression and anxiety were comparable (p values > .12).
Conclusion
Assessing sleep complaints without considering sleep opportunities can result in over-diagnosis of Insomnia Disorder in the perinatal periods. Insomnia Disorder and Perinatal Sleep Disruption were both associated with adverse sleep and mood outcomes, and need to be carefully differentiated and appropriately addressed.
Clinical Trial Registration: The SEED Project (Sleep, Eat, Emotions, and Development): A randomized controlled pilot study of a perinatal sleep intervention on sleep and wellbeing in mothers and infants. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371634, Australian New Zealand Clinical Trials Registry: ACTRN12616001462471.
Keywords: Insomnia Disorder, sleep disruption, perinatal, pregnancy, postpartum, assessment, diagnosis, anxiety, depression
Statement of Significance.
This longitudinal study used structured clinical interview, the gold standard method for establishing diagnostic status, to distinguish between Insomnia Disorder and Perinatal Sleep Disruption from pregnancy all the way to 2 years postpartum. Findings highlight the importance of differentiating Insomnia Disorder from Perinatal Sleep Disruption. Establishing whether sleep complaints occur despite “adequate sleep opportunity” in both duration and quality was essential to prevent over-diagnosing Insomnia Disorder. Nevertheless, both Insomnia Disorder and Perinatal Sleep Disruption were associated with adverse sleep and mood outcomes, and both need to be addressed using appropriate intervention during the perinatal periods.
Introduction
Approximately 50%–73% of gestational parents report changes to their sleep-wake patterns during pregnancy and postpartum periods [1–4]. While estimated rates for self-report symptoms of Insomnia Disorder during the perinatal periods are high (17%–62%) [1, 2, 5, 6], not all women with sleep dissatisfaction meet the criteria for Insomnia Disorder. The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) characterizes Insomnia Disorder as difficulties initiating sleep, maintaining sleep, and/or early morning awakenings that cause clinically significant distress or daytime impairment, and occur despite adequate opportunity for sleep [7]. This “opportunity for sleep” needs to be adequate in both duration (i.e. sufficient time) and quality (i.e. conducive to sleep, and lack external disruption). This makes it difficult to effectively assess sleep complaints during the perinatal periods.
Insomnia disorder and sleep disruption in the perinatal periods
An important feature to consider when assessing perinatal sleep difficulties is sleep opportunity. As sleep quality begins to deteriorate in the second and third trimesters, 42%–75% of gestational parents attribute poor sleep quality to fetal movements, heartburn, back, neck, or joint pain, restless legs syndrome, frequent nighttime urination, breathing difficulties, and vivid dreams [8, 9]. During the postpartum period, infant sleep patterns and feeding schedules become a source of multiple nighttime awakenings, posing a major challenge to parental sleep [10–12]. These factors occur largely outside of one’s voluntary control and can create conditions that are not sleep-conducive and cause significant disruptions to sleep opportunities.
Making a diagnosis of Insomnia Disorder can be challenging as presentations of Perinatal Sleep Disruption and Insomnia Disorder can resemble one another. Common features of both Perinatal Sleep Disruption and Insomnia Disorder can include multiple nighttime awakenings, poor sleep quality, daytime fatigue, and irritability [8, 13]. However, unlike individuals experiencing Perinatal Sleep Disruption, individuals with Insomnia Disorder report persistent sleep difficulties even when presented with an undisrupted sleep opportunity (i.e. not needing to attend to baby at night). Individuals with Insomnia Disorder also report sleep difficulties when their internal/external environment is conducive to sleep (i.e. when free of pain and discomfort). Thus, considering “adequate sleep opportunity” is essential when differentiating between Perinatal Sleep Disruption and Insomnia Disorder, but is not clear in the existing literature.
Lack of distinction between Insomnia Disorder and Perinatal Sleep Disruption in the literature
Current research has not systematically distinguished Insomnia Disorder and sleep disruption for the perinatal periods. First, definitions for Insomnia Disorder remain broad (e.g. some refer to Insomnia as “the inability to remain asleep during the night” [14]) and rarely refer to “sleep opportunity” [15, 16]. The terms “Insomnia” and “sleep disturbance/disruption” are also interchangeably used to describe poor sleep duration and sleep quality in the perinatal periods [11, 17, 18]. For example, clinical documentations describe “reasons for Insomnia” as frequent bathroom visits, difficulty finding a comfortable position in bed, and restless legs, however, these experiences are typically more reflective of sleep disruption or other sleep disorders [19, 20]. Prevalence rates for Insomnia Disorder symptoms are also reported by citing studies that measured sleep disturbances more broadly (e.g. night awakenings), rather than Insomnia-specific symptoms [18]. Together, this has resulted in Insomnia Disorder symptoms being poorly characterized during the perinatal periods.
A recent meta-analysis identified 24 studies on Insomnia Disorder during the perinatal periods that utilized self-report questionnaires [15], and to the best of our knowledge, there has not been a longitudinal study that used gold standard structured clinical interview to establish the occurrence of Insomnia Disorder during pregnancy or postpartum. Using the Bergen Insomnia Scale (BIS), Sivertsen et al. estimated Insomnia Disorder prevalence at 32 weeks pregnancy and 8 weeks postpartum to be 60% [1]. However self-report scales such as these have the potential to overestimate Insomnia Disorder rates as they have not been validated against clinical interviews during perinatal periods [15], nor do they consider the context in which sleep complaints occur (i.e. physical discomfort, going to the bathroom, attending to baby). Because of these limitations, the heterogeneous nature of sleep disturbance during the perinatal periods have been overlooked, hindering effective assessment and potentially also management of different types of perinatal sleep difficulties. For example, sleep restriction, a key component of Cognitive Behavioral Therapy for Insomnia (CBT-I) [21], is likely, not appropriate for individuals with inadequate sleep opportunities; instead, they could benefit from appropriately timed daytime naps for managing sleepiness [22]. In contrast, daytime napping may perpetuate poor nighttime in the context of Insomnia Disorder.
Nevertheless, the existing literature consistently showed that Perinatal Sleep Disruption and Insomnia Disorder are associated with symptoms of depression and anxiety, as well as poorer parent-infant bonding [5, 6, 23–30]. Poor sleep quality during the perinatal periods is also associated with lower quality of life, daytime sleepiness, and fatigue [31–33]. Therefore, both Perinatal Sleep Disruption and Insomnia Disorder are highly relevant to parent-infant wellbeing, yet no study has distinguished and compared sleep and mental health correlates for these common sleep complaints.
Current study
This longitudinal study used structured clinical interviews to identify Insomnia Disorder, Perinatal Sleep Disruption, and Low Complaints in a community sample of nulliparous females who participated in a longitudinal randomized controlled trial. Definitions of these three diagnostic statuses are shown in Table 1, and detailed procedures of categorization are described in the Methods section. Briefly, Low Complaints captures interview outcomes when the frequency or distress/impairments associated with the sleep complaints were insufficient to meet Insomnia Disorder criteria. Both Insomnia Disorder and Perinatal Sleep Disruption meet frequency and distress/impairments for Insomnia Disorder but differ on the response to Criteria E regarding adequate sleep opportunities. Sleep complaints persist despite suitable sleep opportunity for Insomnia Disorder, but not for Perinatal Sleep Disruption. Thus, Perinatal Sleep Disruption captures responses when significant sleep complaints were mainly caused by external factors or physical discomfort. Interviews were repeated at seven time points, from the third trimester of pregnancy to 2 years postpartum.
Table 1.
Criteria for Insomnia Disorder, Perinatal Sleep Disruption, and Low Complaint statuses
| DSM-5 Insomnia criteria | Insomnia Disorder | Perinatal Sleep Disruption | Low Complaints |
|---|---|---|---|
| A. Dissatisfaction with sleep quantity or quality, associated with difficulties initiating sleep, maintaining sleep, or early morning awakenings. | Yes | Yes | Yes/No |
| B. Clinically significant distress or impairment in important areas of functioning.* | Yes | Yes | No/Yes (when no to C) |
| C. The sleep difficulty occurs at least 3 nights per week.* | Yes | Yes | No/Yes (when no to B) |
| D. The sleep difficulty is present for at least 3 months.† | |||
| E. The sleep difficulty occurs despite adequate opportunity for sleep. | Yes | No | |
| F–H. Symptoms not better explained by another sleep disorder, substance, or medical/mental health condition. | Yes | Yes | |
| Common examples: | “I toss and turn and it takes ages to fall asleep, even when my baby is sleeping” | “I’m getting bigger, get up every couple of hours to use toilet…Just wish I could get comfortable…” | “My sleep is not as good, but given the circumstance I think I’m sleeping okay” |
*Both B and C are met (Yes) for Insomnia Disorder and Perinatal Sleep Disruption; B and C are not simultaneously met for Low Complaints.
†Duration criteria was not used in this study to characterize current Insomnia Disorder status. See measures for details.
The aims of this study are:
To distinguish between Insomnia Disorder and Perinatal Sleep Disruption while exploring the impact of considering (or not considering) sleep opportunity on diagnostic status. We evaluated descriptive statistics to describe the proportion of participants meeting each diagnostic status from pregnancy to 2 years postpartum. The rates of Insomnia Disorder were compared if the DSM-5 sleep opportunity criteria were and were not considered.
To compare self-reported insomnia symptom severity, sleep disturbance, sleep-related impairment, sleep effort, dysfunctional beliefs/attitudes about sleep, depression, and anxiety amongst Insomnia Disorder, Perinatal Sleep Disruption, and Low Sleep Complaints diagnostic outcomes. We hypothesized that after adjusting for covariates and compared to Low Complaints, the Insomnia Disorder and Perinatal Sleep Disruption status would be associated with worse sleep and mental health outcomes; differences between Insomnia Disorder and Perinatal Sleep Disruption status are exploratory.
Methods
This is a secondary analysis of data from a randomized controlled trial comparing a behavioral sleep intervention with a “healthy diet” intervention [22] (Australian New Zealand Clinical Trials Registry: ACTRN12616001462471). Detailed methods are published in the study protocol [34]. The trial was advertised as “Sleep, Eat, Emotions, and Development (SEED)” during recruitment to promote the face validity of a “wellbeing” project so that the sample was not biased toward those with preexisting sleep concerns.
Participants
Participants were recruited in their third trimester from Childbirth Education classes at the Royal Women’s Hospital, a centrally located public hospital specialized in women’s health in Victoria, Australia. Inclusion criteria: (1) nulliparas; (2) singleton pregnancy; (3) over 18 years old; (4) English literacy; (5) had regular access to email and the internet. Exclusion criteria included: (1) current or planned shift work during participation in the study; (2) reporting severe and current medical or psychiatric conditions (e.g. lifetime Bipolar or Psychotic Disorder) or physiologically based sleep disorders (e.g. Periodic Limb Movement Disorder, Restless Leg Syndrome, Circadian Rhythm Sleep-Wake Disorders; Insomnia Disorder was not excluded); (3) on medication that affects sleep. Eligibility was determined through a telephone screening that included the Duke Structured Interview for Sleep Disorders (DSISD) [35] and the M.I.N.I. International Neuropsychiatric Interview 7.0 (MINI) [36], a diagnostic interview used for DSM-5 psychiatric disorders.
Procedures
After informed consent and telephone screening, eligible participants were randomized 1:1 to a “Healthy Sleep” (therapist-assisted CBT) or “Healthy Diet” (control) intervention. All participants received a 1-h telephone session and automated multimedia emails from the third trimester until 6 months postpartum. See published protocol [34] for details on the interventions. Assessments were conducted via telephone and online surveys at 28–30 (T1) and 35–36 (T2) weeks’ gestation, and 1.5 (T3), 3 (T4), 6 (T5), 12 (T6), and 24 (T7) months postpartum. Research assistants conducting post-baseline telephone interviews were blinded to treatment conditions. Participants received gift vouchers of $50, $100, $50, and $20 AUD at T3, T5, T6, and T7, respectively.
Measures
Structured interview.
Insomnia Disorder symptoms and diagnosis were assessed through the Insomnia Disorder module of the DSISD, a semi-structured interview for the DSM-5 diagnostic criteria for Insomnia Disorder (see Table 1), which has reported good reliability and validity [35]. The 3-month duration criteria for DSM-5 were not used in this study because: (1) sleep undergoes rapid changes during the perinatal transition, and (2) we were interested in capturing changes between assessments, some of which were completed less than 3 months apart.
To reduce participant burden, we asked a screening question “Have you been satisfied with your sleep?”. If a participant was satisfied with sleep, we discontinued the interview and classified the outcome as Low Complaints. For all other responses (e.g. not satisfied, uncertain), we administered the full module. The interviewer documented the frequency, duration, and distress/impairment associated with sleep concerns (e.g. nighttime awakenings) regardless of the causes. If an interview did not meet Criteria A, B, C, F–H simultaneously, it was categorized as Low Complaints.
Interviews that met Criteria A, B, C, F–H were further categorized as Insomnia Disorder (meet Criteria E on sleep opportunity) and Perinatal Sleep Disruption (did not meet Criteria E). To differentiate these, we asked participants “Do you have this sleep difficulty even when you have the time to sleep, and your sleep environment is comfortable and safe?” to establish whether these sleep concerns persist when they have adequate sleep opportunity (Criteria E in Table 1). In doing so, we explored common factors that may disrupt sleep during the perinatal periods (e.g. physical discomfort, pain, nighttime infant care), as well as other disrupting factors that may be applicable to the individual. The interviewer also assessed whether the sleep concerns were better accounted for by another sleep disorder, substance, or medical/mental health condition (Criteria F–H).
Table 1 describes the three diagnostic statuses against each of the DSM-5 Insomnia Disorder criterion.
Self-report questionnaires.
The Patient-Reported Outcome Measurement Information System Sleep Disturbance (PROMIS-SD) [38] and Sleep-Related Impairment (PROMIS-SRI) are 8-item instruments that measure sleep disturbance and sleep-related impairment. PROMIS instruments use a T-Score metric with a mean of 50 and SD of 10 (e.g. a score of 60 is one SD higher than the population mean). PROMIS instruments are commonly used to assess parental wellbeing and have been validated in the perinatal periods [39–42].
The Insomnia Severity Index (ISI) is a 7-item self-report measure of Insomnia Disorder symptom severity [43]. Scores range from 0 to 28, with 8–14 indicating sub-threshold Insomnia Disorder, 15–21 moderate clinical Insomnia Disorder, and 22–28 severe clinical Insomnia Disorder [44].
The following measures were used to assess Insomnia-related characteristics: Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS) [45], a 16-item measure where higher scores indicate greater endorsement of unhelpful sleep-related beliefs and attitudes; the Glasgow Sleep Effort Scale (GSES) [46] is a 7-item measure where higher scores indicate greater perceived sleep effort; the Ford Insomnia Response to Stress Test (FIRST) [47] is a 9-item measure with higher scores indicating greater vulnerability to Insomnia Disorder under commonly experienced stressful situations.
Mood disturbance was measured using PROMIS Depression and Anxiety Short Form [48]. Both are brief 8-item measures of emotional distress and were used to measure symptoms of depression and anxiety.
PROMIS Instrumental and Emotional Support Short Forms [49] are 4-item instruments that measure social supports, and were used as covariates.
Analysis
Descriptive statistics are used to describe the proportion of participants meeting each DSISD diagnostic status at each time point (Aim 1). To compare characteristics in sleep and mental health among the three diagnostic statuses (Aim 2), we used a linear mixed-effects model to predict each sleep and mental health outcome using random intercept and DSISD diagnostic status as categorical fixed effect predictors. Intervention group allocation, age, instrumental and emotional support, and mental health history (“no history of diagnosis,” “past diagnosis,” and “currently diagnosed”) were adjusted for. These mixed-effects models included all available data over all time points and estimated an overall difference between diagnostic statuses after adjusting for covariates. We also examined differences in demographic variables (education, ethnicity, employment status, and mental health history) between diagnostic statuses using chi-square tests of independence. Effect sizes were also tested using Cramer’s V and Cohen’s d. Data were analyzed using R 4.0.2 [50]. All statistical significance was determined based on two-tailed p value at .05.
Results
The sample consisted of 163 generally healthy nulliparous females (age M ± SD = 33.35 ± 3.42 years, gestation at enrollment M ± SD = 27.59 ± 1.42 weeks). Rates of missing data were low: among the 158 women who started intervention, 65.8% completed all seven assessments, and 95.6% completed five or more assessments. Analyses below include all 163 randomized participants who provided baseline data.
A total of 944 interviews and 1009 questionnaires were collected across seven time-points. The majority of the sample had completed at least university undergraduate education (89.0%), were Caucasian (87.1%) or Asian (11.0%), worked fulltime (77.3%), had a household income ≥130k AUD (58.3%), and were married/in a de facto relationship (96.3%). Participant demographics and descriptive statistics are presented in Table 2. For complete descriptive statistics, see primary outcome paper [23].
Table 2.
Descriptive statistics of baseline sample characteristics
| N = 163 | Low Complaints | Perinatal Sleep Disruption | Insomnia Disorder | P | |
|---|---|---|---|---|---|
| Age in years, M (SD) | 33.35 (3.42) | 33.20 (3.67) | 32.50 (2.68) | 34.87 (2.53) | .028* |
| Race | |||||
| Caucasian, n (%) | 142 (87.1) | 91 (82.7) | 25 (92.6) | 26 (100.0) | .092 |
| Asian, n (%) | 18(11.0) | 17 (15.5) | 1 (3.7) | 0 (0.0) | |
| Other, n (%) | 3 (1.8) | 2 (1.8) | 1 (3.7) | 0 (0.0) | |
| Marital status | |||||
| Married/De facto, n (%) | 157 (96.3) | 107 (97.3) | 25 (92.6) | 25 (96.2) | .557 |
| Never married, n (%) | 6 (3.7) | 3 (2.7) | 2 (7.4) | 1 (3.8) | |
| Employment | |||||
| Not working, n (%) | 6 (3.7) | 3 (2.7) | 2 (7.4) | 1 (3.8) | .620 |
| Working part time, n (%) | 31 (19.0) | 19 (17.3) | 5 (18.5) | 7 (26.9) | |
| Working fulltime, n (%) | 126 (77.3) | 88 (80.0) | 20 (74.1) | 18 (69.2) | |
| Education | |||||
| Less than Bachelor, n (%) | 18 (11.0) | 15 (13.6) | 3(11.1) | 0 (0.0) | .230 |
| Bachelor, n (%) | 56 (34.4) | 35 (31.8) | 12 (44.4) | 9 (34.6) | |
| Postgraduate, n (%) | 89 (54.6) | 60 (54.5) | 12 (44.4) | 17 (65.4) | |
| Mental health history† | |||||
| None, n (%) | 120 (73.6) | 88 (80.0) | 19 (70.4) | 13 (50.0) | .013* |
| Past not current, n (%) | 40 (24.5) | 21 (19.1) | 8 (29.6) | 11 (42.3) | |
| Current, n (%) | 3 (1.8) | 1 (0.9) | 0 (0.0) | 2 (7.7) | |
| FIRST, M (SD) | 21.25 (5.46) | 20.22 (5.26) | 21.74 (5.30) | 25.12 (4.77) | .001* |
†Established using structured clinical interview.
FIRST, Ford Insomnia Response to Stress Test.
*P <. 05. M (mean) and SD (standard deviation) are presented for continuous variables, and n (%) are presented for categorical variables.
Participants with Insomnia Disorder, Perinatal Sleep Disruption, and Low Complaints had similar baseline characteristics, except that participants with Insomnia Disorder were older (p = .028), scored higher on FIRST (p < .001), and were more likely to report past (42.3%) or current (7.7%) mental health diagnoses (p = .013) compared to Perinatal Sleep Disruption and Low Complaints (see Table 2).
Differences in sleep outcomes between the CBT sleep intervention and control conditions were reported in the primary outcome paper of the trial [23]. Briefly, compared to the control condition, receiving CBT for sleep was associated with lower Insomnia Disorder symptom severity, sleep disturbance, and sleep-related impairment at the pregnancy endpoint (T2), and at 24 months postpartum (T7). Group differences across the first postpartum year were nonsignificant. Minimal differences in Insomnia Disorder diagnosis rates were observed postpartum between the CBT sleep intervention and control condition, as seen in Supplementary Table S2.
The percentage of women who met criteria for one of the three statuses (Insomnia Disorder, Perinatal Sleep Disruption, or Low Complaints) at each time point is presented in Table 3.
Table 3.
Rates of Insomnia Disorder, Perinatal Sleep Disruption, and Low Complaints with and without considering sleep opportunity
| Time | Low Complaints | Perinatal Sleep Disruption | Insomnia Disorder (meet DSM-5 criteria) |
“Insomnia Disorder” if the DSM-5 sleep opportunity criteria were not considered |
|---|---|---|---|---|
| T1 (28–30 weeks pregnancy) | 110 (67.48) | 27 (16.56) | 26 (15.95) | 53 (32.52) |
| T2 (35–36 weeks pregnancy) | 79 (62.70) | 22 (17.46) | 25 (19.84) | 47 (37.30) |
| T3 (1.5 months postpartum) | 87 (67.97) | 26 (20.31) | 15 (11.72) | 41 (32.03) |
| T4 (3 months postpartum) | 114 (78.62) | 19 (13.10) | 12 (8.28) | 31 (21.38) |
| T5 (6 m postpartum) | 90 (59.60) | 45 (29.80) | 16 (10.60) | 61 (40.40) |
| T6 (12 months postpartum) | 89 (78.07) | 19 (16.67) | 6 (5.26) | 25 (21.93) |
| T7 (24 months postpartum) | 87 (74.36) | 22 (18.80) | 8 (6.84) | 30 (25.64) |
n (%) are presented for each diagnostic status; see Table 1 for diagnostic status definitions.
Overall, by 2 years postpartum, 70 participants (42.9%) met Insomnia Disorder criteria at least once during the observational period. At baseline of 28–30 weeks’ gestation (T1), 16.0% participants met Insomnia Disorder criteria. From T1 to T2 (35–36 weeks’ gestation), the number of participants meeting Insomnia Disorder criteria in the CBT condition reduced from 15 (18.5%) to 8 (13.1%), while the control condition increased from 11 (13.4%) to 17 (26.2%). During the postpartum time points, Insomnia Disorder rates did not differ significantly between treatment conditions [22] (see Supplementary Table S2 for diagnosis rates between conditions) and were lower compared to the third trimester. As seen in Table 3, the lowest rates of Insomnia Disorder occurred at 1 (T6, 5.3%) and 2 (T7, 6.8%) years postpartum, followed by 3 months postpartum (T4, 8.3%), while rates at 1.5 (T3, 11.7%) and 6 (10.6%) months postpartum were somewhat higher in comparison.
The proportion of women experiencing Perinatal Sleep Disruption was 16.6% and 17.5% during the early and late third trimester. During the first 6 months postpartum, except for T4 (13.1%), the rate of Perinatal Sleep Disruption was higher compared to late pregnancy (20.3% for T3, 29.8% for T5). At 1 and 2 years postpartum, however, the rate of Perinatal Sleep Disruption was comparable to that of late pregnancy (16.7% and 18.8% for T6 and T8, respectively).
If the DSM-5 sleep opportunity criteria were not considered, some women with Perinatal Sleep Disruption would also have been diagnosed with “Insomnia Disorder,” yielding rates that are approximately 2–4 times higher than actual Insomnia Disorder rates across all time-points. Specifically, the estimated rates would have ranged from 32.5% to 37.3% during pregnancy and from 21.4% to 40.4% postpartum (see the right column in Table 3).
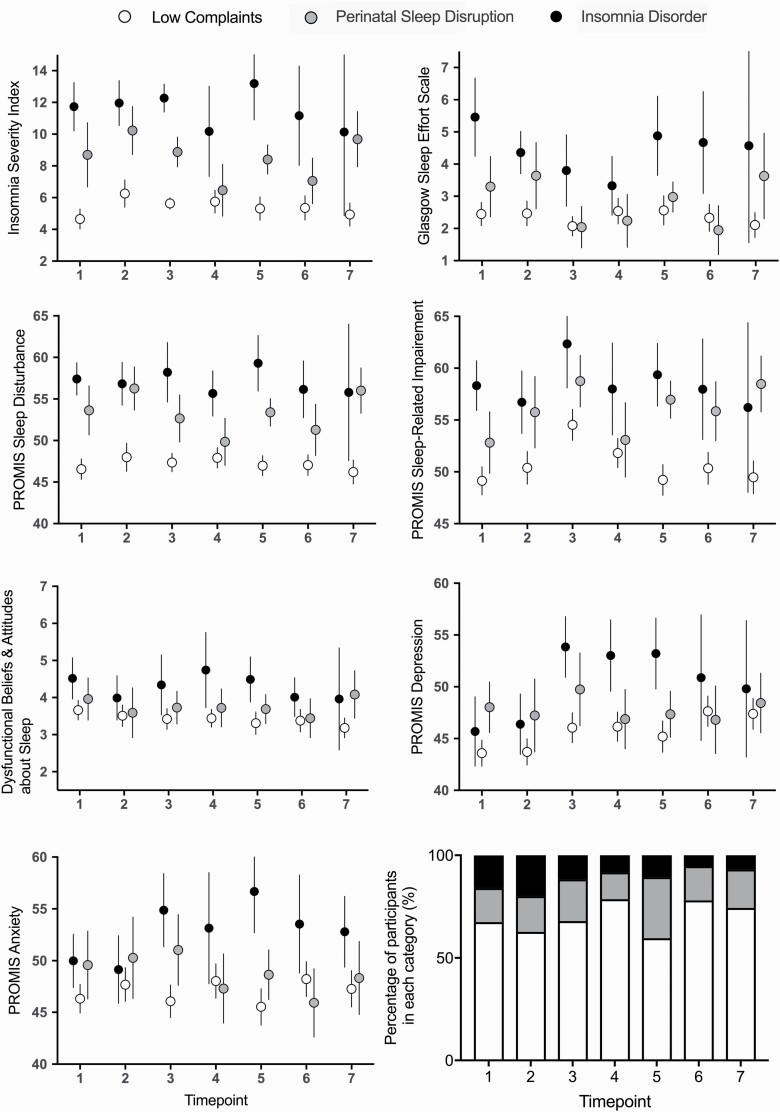
Descriptive statistics for sleep and mental health outcomes at each time point for each diagnostic status are presented in Figure 1 and Supplementary Table S1. Briefly, mean ISI scores exceeded 10 across all time points for Insomnia Disorder status (range: 10.14–13.19). Mean ISI scores for Sleep Disruption and Low Complaints ranged 6.47–10.23 and 4.65–6.26, respectively. At baseline, the mean ISI scores were 11.73 for Insomnia Disorder, 8.70 for Sleep Disruption, and 4.65 for Low Complaints.
Figure 1.
Mean and 95% confidence intervals for the Insomnia Severity Index, Glasgow Sleep Effort Scale, Dysfunctional Beliefs and Attitudes about Sleep Scale, PROMIS Sleep Disturbance, Sleep-Related Impairment, Depression, and Anxiety. The proportion of participants meeting Low Complaints, Perinatal Sleep Disruption, and Insomnia Disorder criteria based on structured clinical interview are presented (see Table 1 for definitions). T1 = 28–30 weeks gestation; T2 = 35–36 weeks gestation; T3 = 1.5 months postpartum; T4 = 3 months postpartum; T5 = 6 months postpartum; T6 = 12 months postpartum; T7 = 24 months postpartum.
Linear mixed-effects models examining how diagnostic status predicted sleep and mental health outcomes controlling for covariates are summarized in Table 4.
Table 4.
Linear mixed-effects model for sleep and mental health outcomes
| Insomnia Disorder versus Low Complaints | Perinatal Sleep Disruption versus Low Complaints | Insomnia Disorder versus Perinatal Sleep Disruption | |
|---|---|---|---|
| Insomnia Severity Index | 5.30 [4.59 to 6.02], <.001*** | 2.63 [2.05 to 3.21], <.001*** | 2.68 [1.86 to 3.50], <.001*** |
| PROMIS Sleep Disturbance | 8.19 [6.97 to 9.42], <.001*** | 5.31 [4.32 to 6.31], <.001*** | 2.88 [1.47 to 4.29], <.001*** |
| PROMIS Sleep-Related Impairment | 5.31 [4.00 to 6.62], <.001*** | 3.77 [2.70 to 4.83], <.001*** | 1.55 [0.05 to 3.05], .044* |
| Glasgow Sleep Effort Scale | 1.67 [1.32 to 2.03], <.001*** | 0.41 [0.12 to 0.70], .005** | 1.26 [0.86 to 1.67], <.001*** |
| Dysfunctional Beliefs and Attitudes about Sleep | 0.55 [0.35 to 0.76], <.001*** | 0.14 [−0.03 to 0.30], .108 | 0.42[0.19 to 0.65], <.001*** |
| PROMIS Depression | 1.53 [0.33 to 2.73], .013* | 1.43 [0.46 to 2.41], .004** | 0.09 [−1.28 to 1.47], .895 |
| PROMIS Anxiety | 1.82 [0.57 to 3.08], .004** | 0.72 [−0.30 to 1.73], .166 | 1.11 [−0.32 to 2.53], .128 |
Unstandardized coefficients [95% Confidence Intervals], p values are presented; Intervention group allocation, age, instrumental and emotional support, and mental health history were controlled for.
*p < .05 **p < .01 ***p < .001.
Compared to both Perinatal Sleep Disruption and Low Complaints status, Insomnia Disorder status was associated with significantly higher insomnia symptoms, sleep disturbance, sleep-related impairment, sleep effort, and DBAS (all p values < .05). The model-estimated coefficients (i.e. mean differences between groups after controlling for covariates, presented on the original scale) between Insomnia Disorder and Low Complaints were also practically meaningful. For example, compared to Low Complaints, Insomnia Disorder status was associated with higher PROMIS Sleep Disturbance and Sleep-Related Impairment scores, with mean differences of 8.19 and 5.31 (i.e. 0.82 and 0.53 SD), respectively (see the unstandardized estimate and 95% confidence intervals in Table 4). Practically speaking, a T-score of 55 is in the 69th percentile, and a T-score 8.19 less than 55 (i.e. 46.81) is in the 38th percentile, demonstrating meaningful differences between scores. Similarly, compared to Low Complaints, Perinatal Sleep Disruption was associated with significantly higher symptoms of Insomnia Disorder, sleep disturbance, sleep-related impairment, and sleep effort (all p values < .01), but levels of DBAS were comparable (p > .05).
No statistically significant differences were observed between Perinatal Sleep Disruption and Insomnia Disorder for symptoms of depression and anxiety (p values > .05). Compared to Low Complaints, Insomnia Disorder was associated with significantly higher symptoms of depression (p = .013) and anxiety (p = .004), while Perinatal Sleep Disruption was associated with significantly higher symptoms of depression (p = .004) but not anxiety (p = .166). These covariate-adjusted differences are relatively small in size, ranging from 1.43 to 1.82 (see Table 4).
Discussion
This study used gold standard structured interviews to assess Insomnia Disorder longitudinally across the perinatal transition, from late pregnancy to 2 years postpartum. The rates of “Insomnia Disorder” would be substantially inflated if sleep opportunity criteria were not considered in assessing Insomnia Disorder during pregnancy and early parenthood. Both Insomnia Disorder and Perinatal Sleep Disruption were associated with significant sleep complaints and sleep-related impairments, but they were comparable on symptoms of depression and anxiety.
Assessing sleep opportunity: an essential diagnostic ingredient
Rates of Insomnia Disorder and Perinatal Sleep Disruption were both high during pregnancy, ranging from 16.0% to 19.8% for Insomnia Disorder and 16.6% to 17.5% for Perinatal Sleep Disruption. During the postpartum, the proportion of participants experiencing Perinatal Sleep Disruption exceeded rates of Insomnia Disorder at all time-points. Average scores on the ISI were consistently >10 for the Insomnia Disorder status only. Although this is comparable to the cutoff of 10 to detect Insomnia Disorder in a community sample [50], establishing a specific cutoff for the perinatal population is not feasible in this study and requires future study.
The rates of Insomnia Disorder in this study are higher than the 6%–10% reported in the general population [7] but substantially lower than estimates derived from self-report questionnaires (54%–62%) [1, 2, 5, 25]. This is unlikely due to lower Insomnia Disorder symptoms in the study sample, as the average ISI score in this study is comparable to that from other community samples of females during pregnancy [6, 51]. But rather, structured clinical interviews enabled us to differentiate Insomnia Disorder from Perinatal Sleep Disruption and avoid misdiagnosing the latter as Insomnia Disorder. Indeed, when the sleep opportunity criteria were not considered, rates of Insomnia Disorder would have been approximately 2–4 times higher than actual rates, comparable to rates in studies using self-report questionnaires.
While self-report measures provide meaningful information concerning Insomnia Disorder symptoms, distress, and impairment, many commonly used self-report measures (e.g. BIS [52], Women’s Health Initiative Insomnia Rating Scale [53]) do not evaluate the context in which sleep complaints occur (e.g. physical discomfort, going to the bathroom, attending to baby). This leaves the perinatal periods a vulnerable time for misdiagnosis of sleep problems.
Both Insomnia Disorder and Perinatal Sleep Disruption were associated with adverse outcomes
As would be expected and consistent with the existing literature, Insomnia Disorder was associated with significant sleep disturbance, sleep-related impairment, sleep effort, and DBAS during pregnancy and postpartum [37, 54, 55]. We found that the same was also true for those with Perinatal Sleep Disruption, except DBAS, suggesting that in the absence of Insomnia Disorder, disruptions to sleep caused by perinatal factors can cause considerable sleep concerns and sleep-related impairments. It is worth noting that Insomnia Disorder, but not Perinatal Sleep Disruption, was associated with higher DBAS. It is possible that unhelpful thoughts and beliefs about sleep are perpetuating factors for Insomnia but not sleep disruption.
Further, both Insomnia Disorder and Perinatal Sleep Disruption were associated with significantly greater symptoms of depression than Low Complaint status. Although the size of the differences was relatively small, data from this study extends existing knowledge linking Insomnia Disorder symptoms and adverse mental health outcomes in the perinatal periods [5, 6, 25, 27] by demonstrating that Perinatal Sleep Disruption, in the absence of Insomnia Disorder, is also detrimental to mood. Therefore, both deserve clinical attention in their own right in the perinatal period.
Limitations
A number of limitations need to be considered when interpreting results. Participants were first-time parents, mostly university-educated, and in stable relationships, potentially limiting the generalizability of these findings. Symptoms of depression and anxiety were relatively low in this community sample, and findings may not generalize to clinical samples. Although this study utilized a large community sample, rates of Insomnia Disorder were low, especially during the postpartum period. To further understand the nature of perinatal Insomnia Disorder, future research should oversample individuals at risk for Insomnia Disorder (e.g. those seeking help for poor sleep). The study was carried out in the context of a clinical trial with half of the sample-receiving a sleep intervention. The frequency of Insomnia Disorder at post-randomization time-points is not suitable for estimating the overall prevalence of Insomnia Disorder postpartum due to sleep intervention received in half of the sample. Because our recruitment materials focused on wellbeing, rather than Insomnia symptoms, our observed rate of Insomnia Disorder at baseline is likely a good estimate of the prevalence of Insomnia Disorder during late pregnancy in a community sample of nulliparous females. Parity has been shown to have an inconsistent effect on parental sleep, with some studies reporting more night awakenings for multiparas, and others reporting poorer objective sleep in nulliparas [56]. Therefore, rates of Insomnia Disorder/Sleep Disruption in this sample cannot be extrapolated to multiparous populations.
Conclusion and practical implications
Insomnia Disorder and Perinatal Sleep Disruption are rarely differentiated in the perinatal sleep literature, limiting our understanding of the characteristics that are unique to each sleep concern. This study demonstrated that establishing whether sleep complaints occur despite “adequate sleep opportunity” was essential to prevent over-diagnosing Insomnia Disorder. Further, both Insomnia Disorder and Perinatal Sleep Disruption were associated with adverse sleep and mental health outcomes, highlighting the importance of not over-looking Sleep Disruption and providing evidence-based support for both Insomnia and Sleep Disruption. These findings have implications for both the assessment and treatment of sleep complaints among women in the perinatal periods.
From an assessment perspective, a binary diagnostic system (e.g. Insomnia Disorder vs. No Insomnia Disorder) is likely inadequate during the perinatal period when sleep problems have heterogeneous causes. In choosing sleep measures for the perinatal periods, we advocate for measures that are well developed, validated, and normed for the general population (rather than specific measures for the perinatal periods). This way, sleep characteristics quantified during the perinatal transition can be understood in broader contexts across the lifespan, and be compared with diverse populations. However, when administering commonly used instruments (e.g. ISI, BIS), additional information related to the context of sleep could be sought to help distinguish between Insomnia Disorder and Perinatal Sleep Disruption. For example, asking individuals to rate sleep complaints “while baby is sleeping” as suggested by Swanson et al. [57], and adding qualitative items (e.g. “Sleep difficulties can occur for a variety of reasons. Please tell us about any physical, psychological, or environmental factors that may be interfering with your current sleep.”). Where possible, clinical interviews should be utilized to assess the contexts where sleep complaints occur. Questions that assess sleep opportunity, such as “Do you still experience difficulty sleeping when you are relatively free of pain and discomfort, and you don’t have to go to the bathroom?,” are essential for clinicians and researchers attempting to differentiate between these two common sleep problems. Although Insomnia and Sleep Disruption need to be distinguished to prevent over-diagnosis of Insomnia, it is important not to overlook or dismiss Sleep Disruption during the perinatal period, as both Insomnia and Sleep Disruption are associated with daytime impairment and distress.
From a treatment perspective, we advocate for providing intervention and support that are informed by an accurate assessment/diagnosis (see above). CBT-I is increasingly used in perinatal populations [21, 22, 58], and RCTs report promising findings regarding Insomnia Disorder symptom severity and remissions rates [21, 59]. Interventions for Perinatal Sleep Disruption are limited, particularly during pregnancy [51, 60, 61]. Given that some components of CBT-I may be unfavorable in the context of Perinatal Sleep Disruption (e.g. sleep restriction), future research may consider adapting CBT-I for women with Perinatal Sleep Disruption, with a greater emphasis on reducing psychological/physiological reactivity to sleep-disrupting factors, providing psychoeducation on helpful napping behaviors, and fatigue management strategies. An alternative direction is for interventions to focus on infant sleep as a primary source of sleep disruption. Parents may benefit from information on infant sleep development and strategies that promote infant sleep during early postpartum, as a majority of infant sleep interventions do not commence until the infant is approaching 6 months old [62–65]. Therefore, future perinatal sleep interventions should (1) carefully differentiate between Insomnia Disorder and Perinatal Sleep Disruption to ensure the presenting concerns are appropriately addressed, and (2) investigate efficacious interventions for women experiencing Sleep Disruption in pregnancy and/or the first 6 months postpartum.
Together, these findings highlight the importance of screening women in pregnancy and postpartum for various sleep complaints, and carefully considering sleep opportunities to distinguish between Insomnia and other sleep concerns (e.g. sleepiness/fatigue). Developing clear and standardized terminology will allow researchers and clinicians to understand the heterogeneous nature of sleep disturbance during the perinatal periods, and facilitate the development and delivery of interventions that appropriately address each complaint.
Supplementary Material
Acknowledgments
The authors would like to thank all participants for generously donating their time to this project. This study utilized data from a randomized controlled trial, and we thank Kaye Dyson and Elisabeth Gasparini for supporting recruitment, and (alphabetical) Laura Astbury, Cassandra Fong, Dr. Catherine Fulgoni, Anthony Hand, Ashley Lam, Sarah Samuel, Isabelle Smith, Emma Thompson, and Sumedha Verma for assistance in collecting data used in this manuscript.
Contributor Information
Nina Quin, Turner Institute for Brain and Mental Health, School of Psychological Sciences, Faculty of Medicine, Nursing and Health Sciences, Monash University, Melbourne, VIC, Australia; Women’s Mental Health Service, Royal Women’s Hospital, Parkville, VIC, Australia.
Jin Joo Lee, Turner Institute for Brain and Mental Health, School of Psychological Sciences, Faculty of Medicine, Nursing and Health Sciences, Monash University, Melbourne, VIC, Australia; Women’s Mental Health Service, Royal Women’s Hospital, Parkville, VIC, Australia.
Donna M Pinnington, Turner Institute for Brain and Mental Health, School of Psychological Sciences, Faculty of Medicine, Nursing and Health Sciences, Monash University, Melbourne, VIC, Australia; Women’s Mental Health Service, Royal Women’s Hospital, Parkville, VIC, Australia.
Louise Newman, Department of Psychiatry, University of Melbourne, Melbourne, VIC, Australia.
Rachel Manber, Department of Psychiatry and Behavioral Sciences, Stanford University, Palo Alto, CA, USA.
Bei Bei, Turner Institute for Brain and Mental Health, School of Psychological Sciences, Faculty of Medicine, Nursing and Health Sciences, Monash University, Melbourne, VIC, Australia; Women’s Mental Health Service, Royal Women’s Hospital, Parkville, VIC, Australia.
Disclosure Statements
Financial Disclosure: Data collection was supported by Rob Pierce Grant-in-Aid and Helen Bearpark Scholarship from Australasian Sleep Association, Strategic Grant Scheme from Faculty of Medicine, Nursing and Health Sciences, Monash University, and the Royal Women’s Hospital Foundation. Intervention materials were adapted from those developed via a National Institute of Health R01 grant (NR013662). BB is supported by National Health and Medical Research Council Fellowships (APP1140299), and DMP and NQ by Australian Postgraduate Awards by the Department of Education and Training. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Nonfinancial Disclosure: The authors declare no conflicts of interest; there is no off-label or investigational use.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Sivertsen B, et al. Trajectories of maternal sleep problems before and after childbirth: a longitudinal population-based study. BMC Pregnancy Childbirth. 2015;15:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Facco FL, et al. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115(1):77–83. [DOI] [PubMed] [Google Scholar]
- 3. Fernández-Alonso AM, et al. Factors related to insomnia and sleepiness in the late third trimester of pregnancy. Arch Gynecol Obstet. 2012;286(1):55–61. [DOI] [PubMed] [Google Scholar]
- 4. Austin MP. Antenatal screening and early intervention for “perinatal” distress, depression and anxiety: where to from here? Arch Womens Ment Health. 2004;7(1):1–6. [DOI] [PubMed] [Google Scholar]
- 5. Dørheim SK, et al. Insomnia and depressive symptoms in late pregnancy: a population-based study. Behav Sleep Med. 2012;10(3):152–166. [DOI] [PubMed] [Google Scholar]
- 6. Manber R, et al. Factors associated with clinically significant insomnia among pregnant low-income Latinas. J Womens Health (Larchmt). 2013;22(8):694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washington, DC: American Psychiatric Association Publishing; 2013. [Google Scholar]
- 8. Mindell JA, et al. Sleep patterns and sleep disturbances across pregnancy. Sleep Med. 2015;16(4):483–488. [DOI] [PubMed] [Google Scholar]
- 9. Schweiger MS. Sleep disturbance in pregnancy. A subjective survey. Am J Obstet Gynecol. 1972;114(7):879–882. [DOI] [PubMed] [Google Scholar]
- 10. Gay CL, et al. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs. 2004;5(4):311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee KA, et al. Evaluating insomnia during pregnancy and postpartum. In: Attarian HP, ed. Sleep Disorders in Women. Springer; 2006:185–198. doi: 10.1007/978-1-59745-115-4_15 [DOI] [Google Scholar]
- 12. Stremler R, et al. Postpartum period and early motherhood. In: Principles and Practice of Sleep Medicine. Philadelphia, PA: Elsevier; 2017:1547–1552.e1544. [Google Scholar]
- 13. Bonnet MH, et al. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med Rev. 2003;7(4):297–310. [DOI] [PubMed] [Google Scholar]
- 14. Baratte-Beebe KR, et al. Sources of midsleep awakenings in childbearing women. Clin Nurs Res. 1999;8(4):386–397. [DOI] [PubMed] [Google Scholar]
- 15. Sedov ID, et al. Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res. 2021;30(1):e13207. [DOI] [PubMed] [Google Scholar]
- 16. Felder JN, et al. Sleep disorder diagnosis during pregnancy and risk of preterm birth. Obstet Gynecol. 2017;130(3):573–581. [DOI] [PubMed] [Google Scholar]
- 17. Abbott SM, et al. Sleep disorders in perinatal women. Best Pract Res Clin Obstet Gynaecol. 2014;28(1):159–168. [DOI] [PubMed] [Google Scholar]
- 18. Nodine PM, et al. Common sleep disorders: management strategies and pregnancy outcomes. J Midwifery Womens Health. 2013;58(4):368–377. [DOI] [PubMed] [Google Scholar]
- 19. Guerreiro da Silva JB, et al. Acupuncture for insomnia in pregnancy—a prospective, quasi-randomised, controlled study. Acupunct Med. 2005;23(2):47–51. [DOI] [PubMed] [Google Scholar]
- 20. Kızılırmak A, et al. Insomnia in pregnancy and factors related to insomnia. ScientificWorldJournal. 2012;2012:197093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manber R, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 2019;133(5):911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bei B, et al. Improving maternal sleep via a scalable cognitive behavioural intervention: findings from a randomised controlled trial from pregnancy to two years postpartum. Psychol Med. 2021:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bei B, et al. Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep. 2010;33(4):531–538. doi: 10.1093/sleep/33.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Emamian F, et al. Link between insomnia and perinatal depressive symptoms: a meta-analysis. J Sleep Res. 2019;28(6):e12858. [DOI] [PubMed] [Google Scholar]
- 25. Osnes RS, et al. Insomnia late in pregnancy is associated with perinatal anxiety: a longitudinal cohort study. J Affect Disord. 2019;248:155–165. [DOI] [PubMed] [Google Scholar]
- 26. Piteo AM, et al. Postnatal depression mediates the relationship between infant and maternal sleep disruption and family dysfunction. Early Hum Dev. 2013;89(2):69–74. [DOI] [PubMed] [Google Scholar]
- 27. Swanson LM, et al. Relationships among depression, anxiety, and insomnia symptoms in perinatal women seeking mental health treatment. J Womens Health (Larchmt). 2011;20(4):553–558. [DOI] [PubMed] [Google Scholar]
- 28. Tikotzky L. Postpartum maternal sleep, maternal depressive symptoms and self-perceived mother-infant emotional relationship. Behav Sleep Med. 2016;14(1):5–22. [DOI] [PubMed] [Google Scholar]
- 29. Tomfohr LM, et al. Trajectories of sleep quality and associations with mood during the perinatal period. Sleep. 2015;38(8):1237–1245. doi: 10.5665/sleep.4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou H, et al. Poor sleep quality of third trimester exacerbates the risk of experiencing postnatal depression. Psychol Health Med. 2018;25(2):229–238. [DOI] [PubMed] [Google Scholar]
- 31. Filtness AJ, et al. Longitudinal change in sleep and daytime sleepiness in postpartum women. PLoS One. 2014;9(7):e103513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rychnovsky J, et al. The relationship between sleep characteristics and fatigue in healthy postpartum women. Womens Health Issues. 2009;19(1):38–44. [DOI] [PubMed] [Google Scholar]
- 33. Tsai SY, et al. Cross-sectional and longitudinal associations between sleep and health-related quality of life in pregnant women: a prospective observational study. Int J Nurs Stud. 2016;56:45–53. [DOI] [PubMed] [Google Scholar]
- 34. Bei B, et al. A scalable cognitive behavioural program to promote healthy sleep during pregnancy and postpartum periods: protocol of a randomised controlled trial (the SEED project). BMC Pregnancy Childbirth. 2019;19(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Edinger JD. Is it time to step up to stepped care with our cognitive-behavioral insomnia therapies? Sleep. 2009;32(12):1539–1541. doi: 10.1093/sleep/32.12.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sheehan D, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatry. 1997;12(5):232–241. [Google Scholar]
- 37. Buysse DJ, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33(6):781–792. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ameringer S, et al. Psychometric evaluation of the patient-reported outcomes measurement information system fatigue-short form across diverse populations. Nurs Res. 2016;65(4):279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blackwell CK, et al. Developing a common metric for depression across adulthood: linking PROMIS depression with the Edinburgh Postnatal Depression Scale. Psychol Assess. 2021;33(7):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lundsberg LS, et al. Clinical validation of PROMIS global short form in pregnancy. Appl Res Qual Life. 2018;13(1):89–103. [Google Scholar]
- 41. Thomas KA, et al. Sleep, depression, and fatigue in late postpartum. MCN Am J Matern Child Nurs. 2016;41(2):104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bastien CH, et al. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 43. Morin CM, et al. Insomnia: Psychological Assessment and Management. New York: Guilford Press; 1993. [Google Scholar]
- 44. Morin CM, et al. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16). Sleep. 2007;30(11):1547–1554. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Broomfield NM, et al. Towards a valid, reliable measure of sleep effort. J Sleep Res. 2005;14(4):401–407. [DOI] [PubMed] [Google Scholar]
- 46. Drake C, et al. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27(2):285–291. [DOI] [PubMed] [Google Scholar]
- 47. Pilkonis PA, et al. ; PROMIS Cooperative Group. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18(3):263–283. doi: 10.1093/sleep/27.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hahn EA, et al. ; PROMIS Cooperative Group. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol. 2014;33(5):490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. R Core Team. R: A Language and Environment for Statistical Computing; 2020. Retrieved from http://www.R-project.org. Accessed 2021.
- 50. Morin CM, et al. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kempler L, et al. A brief sleep focused psychoeducation program for sleep-related outcomes in new mothers: a randomised controlled trial. Sleep. 2020;43(11). doi: 10.1093/sleep/zsaa101. [DOI] [PubMed] [Google Scholar]
- 52. Pallesen S, et al. A new scale for measuring insomnia: the Bergen Insomnia Scale. Percept Mot Skills. 2008;107(3):691–706. [DOI] [PubMed] [Google Scholar]
- 53. Levine DW, et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):137–148. [DOI] [PubMed] [Google Scholar]
- 54. Morin CM, et al. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychol Aging. 1993;8(3):463–467. [DOI] [PubMed] [Google Scholar]
- 55. Espie CA, et al. The attention-intention-effort pathway in the development of psychophysiologic insomnia: a theoretical review. Sleep Med Rev. 2006;10(4):215–245. [DOI] [PubMed] [Google Scholar]
- 56. Bei B, et al. Sleep and mood during pregnancy and the postpartum period. Sleep Med Clin. 2015;10(1):25–33. [DOI] [PubMed] [Google Scholar]
- 57. Swanson LM, et al. An open pilot of cognitive-behavioral therapy for insomnia in women with postpartum depression. Behav Sleep Med. 2013;11(4):297–307. [DOI] [PubMed] [Google Scholar]
- 58. Verma S, et al. Cognitive behavioural therapy and light dark therapy for maternal postpartum insomnia symptoms: protocol of a parallel-group randomised controlled efficacy trial. Front Glob Womens Health. 2020;1:591677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Felder JN, et al. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psychiatry. 2020;77(5):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bacaro V, et al. Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev. 2020;50:101234. [DOI] [PubMed] [Google Scholar]
- 61. Carroll JE, et al. Maternal sleep in pregnancy and postpartum part II: biomechanisms and intervention strategies. Curr Psychiatry Rep. 2019;21(3):19. [DOI] [PubMed] [Google Scholar]
- 62. Hall WA, et al. A randomized controlled trial of an intervention for infants’ behavioral sleep problems. BMC Pediatr. 2015;15:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hiscock H, et al. Improving infant sleep and maternal mental health: a cluster randomised trial. Arch Dis Child. 2007;92(11):952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hiscock H, et al. Preventing early infant sleep and crying problems and postnatal depression: a randomized trial. Pediatrics. 2014;133(2):e346–e354. [DOI] [PubMed] [Google Scholar]
- 65. Hiscock H, et al. Randomised controlled trial of behavioural infant sleep intervention to improve infant sleep and maternal mood. BMJ. 2002;324(7345):1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.