Abstract
Background
Over the previous two decades, there have been significant advancements in the treatment of laryngeal cancer. While survival of head and neck cancer has improved over time, the temporal trend of laryngeal cancer survival is an area of controversy.
Methods
77,527 patients with laryngeal cancer treated with curative intent in the United States from 2004–2016 were identified in the National Cancer Database. Relative and observed survival were assessed for temporal trends. Multinomial logistic regression investigated the relationship between AJCC stage and increasing calendar year.
Results
There was no significant improvement in two- or five-year observed (OS) or relative survival (RS). Five-year RS ranged from 61.72% to 63.97% and OS from 54.26% to 56.52%. With each increasing year, proportion of stage IV disease increased, with risk of having stage IV disease at the time of diagnosis increasing 2.2% annually (aOR 1.022, 1.017-1.028, p<0.001). This was driven by a 4.7% yearly increase in N2 disease (aOR 1.047, 1.041-1.053, p<0.001) and annually increasing T3 disease by 1.2% (aOR 1.012, 1.007-1.018, p<0.001) and T4 disease by 1.2% (aOR 1.012, 1.005-1.018, p<0.001).
Conclusion
Despite advances in the field, laryngeal cancer survival in the United States is not improving over time. This may be due to an increase in the proportion of stage IV disease, driven primarily by increasing nodal disease. To achieve survival improvement commensurate with scientific and technological advances, efforts should be made to diagnose and treat laryngeal cancer at earlier stages to prevent further stage migration.
Introduction
The past two decades have brought significant treatment advancements in the management of laryngeal cancer. RTOG 91-11 demonstrated concurrent chemoradiation as the most effective nonsurgical modality for advanced laryngeal cancer.1 Patient selection for organ preservation treatment has significantly evolved, including the development of bioselection protocols.2,3 Furthermore, intensity modulated radiation therapy (IMRT) has resulted in improved treatment outcomes while limiting treatment toxicity.4-6 Expanding use of transoral robotic surgery and transoral laser surgery has allowed for greater ability to perform primary surgery via minimally invasive techniques for appropriate tumors.7,8 There has also been renewed emphasis on primary surgical treatment for T4 tumors and nonfunctional larynges.2,9
Implementation of these advances should have theoretically resulted in incremental survival improvement; however, it remains unknown whether survival of laryngeal cancer in the United States (U.S.) is improving or remaining stagnant. In 2006, Hoffman et al. reported worsening survival trends in patients with laryngeal cancer treated in the U.S. from 1985–2001. This sparked great controversy as to the etiology of this decrease in survival and whether this correlated with a shift toward organ preservation treatment protocols.10,11 Indeed, a prior study of the Surveillance, Epidemiology, and End Results database has shown improved survival in advanced stage laryngeal cancer for those treated surgically.12 Given shifting treatment paradigms and technological advancements in the last 15 years, a contemporary analysis of survival and demographic trends in the United States since 2006 is warranted to better understand gaps and variations in care. This study provides an epidemiologic analysis of laryngeal cancer in the United States by examining trends in survival and stage that coincide with a shift in treatment paradigms in the field of laryngeal cancer.
Materials and Methods
Data Source
The NCDB is a national hospital-based cancer registry which collects high-quality and standardized demographic, cancer, treatment, and outcome related information from Committee on Cancer (CoC) accredited facilities. Since 1985 it has accrued >34 million cancer cases and currently represents data from >70% of all incident cancer cases yearly.13 The Ohio State University Institutional Review Board deemed this study exempt from review.
Inclusion and Exclusion Criteria
We queried the NCDB for all laryngeal cancer cases from 2004 to 2016. In accordance with site groupings from the American Joint Committee on Cancer (AJCC) 8th edition staging, the following International Classification of Diseases-O-3 subsites were included: C32.0 (glottis), C32.1 (supraglottis), C32.2 (subglottis), C32.8 (overlapping lesion of larynx), C32.9 (larynx, NOS). As consistent with AJCC site guidelines and previous NCDB studies of laryngeal squamous cell carcinoma, C10.1 (laryngeal surface of epiglottis) was included as a supraglottic site.14,15 We included only patients with one reported cancer diagnosis (sequence number 00). The cancer diagnosis was restricted to those with invasive malignant behavior (behavior=3), and squamous cell carcinoma histology (histology codes 8070, 8071, 8072, 8074). Patients under the age of 18 years and those with distant metastatic (M1) disease were excluded.
Demographic Variable Definitions
Demographic variables analyzed included age at diagnosis, sex, race, urban vs rural residence, geographic region, insurance, median income quartile, and percent without high school degree quartile. Sex was categorized as male or female. Race was categorized as White, Black, and Other, which included Hispanic, Asian-American, American-Indian, other, and missing or unknown. Median income quartile was based on the most recent available US census data. Percent without a high school degree was divided into quartiles (≥17.6%, 10.9-17.5%, 6.3-10.8%, and <6.3%) by zip-code. Insurance type was categorized into not insured, private insurance, and public insurance groups based on Facility Oncology Registry Data Standards (FORDS) codes. The geographic region variable was created from facility location based on United States Census grouping: Northeast (New England, Middle Atlantic), Midwest (East North Central, West North Central), South (South Atlantic, East South Central, West South Central), West (Pacific, Mountain).
Staging Definitions
TNM tumor information and AJCC clinical stage group were extracted directly from the NCDB. The NCDB records stage group based on the AJCC staging manual in effect at the year of diagnosis. For the purposes of this study, AJCC 6th edition was in effect from 2004-2009 and the 7th edition from 2010-2014. No significant changes in TNM or clinical group stage were made between the 6th and 7th editions for larynx cancer. Pathologic stage was used when patients received surgery, and clinical stage was used when pathologic stage was not available.
Statistical Analysis
Patient characteristics were summarized using descriptive statistics including median and interquartile range for continuous variables and frequencies for categorical variables. Observed and relative survival rates were estimated based on a publicly available and previously validated algorithm.16 Observed survival was defined as the probability of surviving for a specified time interval based from the study population. This method yields results identical to the estimates obtained from the Kaplan–Meier method. Relative survival was defined as the ratio of the proportion of observed survivors in the cohort against the expected survival of the U.S. population with the same sex, age and year at which the diagnosis was coded. Two- and five-year observed and relative survival rates were presented graphically to depict trends across the study period. The time period 2004-2015 was used for two-year survival and 2004-2011 for five-year survival toto allow for adequate follow-up time.
Proportions of T, N, and AJCC stage groups or primary sites were also presented graphically to depict trends across the study period (2004-2016). Multinomial logistic regression was used to examine the trend of T, N, and AJCC stage (compared to reference groups) across the study period. To further examine the associations between year of diagnosis and stage, the AJCC stages were grouped into two groups (stage IV vs all other stages). Logistic regression models were used to explore associations between stage IV disease and demographic or clinical variables. Multivariable logistic regression was used to examine the associations between year of diagnosis and stage IV disease controlling for all the potential confounders having p<0.05 from the bivariate analysis. Adjusted odds ratios (aOR) and 95% confidence intervals (CI) are reported. To explore the association between year of diagnosis and demographic or clinical variables, multinomial logistic regression was used to assess bivariate associations between year of diagnosis with categorical variables while linear regression was used to assess bivariate associations between year of diagnosis with continuous variables. All p-values <0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9 (SAS Institute Inc., Cary, NC, USA).
Results
Study Population
From the study period of 2004 to 2016, query of the NCDB revealed 77,527 cases of laryngeal squamous cell carcinoma. The majority of cases were diagnosed in either the southern (41.7%) or midwestern (26.6%) United States. Stage I (n=28,676, 37.0%) and stage IV (n=21,768, 28.0%) disease represented the most common diagnoses followed by stage III (n=14,761) and II (n=12,322). Table 1 presents a summary of the demographic characteristics of the study population by stage. The majority of cases were in men (78.7%) and those of Caucasian descent (82.5%). Great circle distance, defined as the number of miles between a patient’s residence and the hospital reporting the case, was greatest for stage IV disease (11.5 miles). Those with stage IV disease represented the highest proportion of uninsured patients (8.9%). Additionally, those with stage IV disease represented the subgroup with the highest proportion of patients residing in counties in the lowest quartile of median income (30.2%) and high-school degree attainment (30.9%).
Table 1:
Overall Demographics
| Tumor Stage; N (%) | |||||
|---|---|---|---|---|---|
| Patient Variable | Stage I (N=28676) |
Stage II (N=12322) |
Stage III (N=14761) |
Stage IV (N=21768) |
Total (N=77527) |
| Age (years); Median [IQR] | 65 [57, 74] | 63 [56, 71] | 62 [54, 70] | 60 [54, 67] | 63 [55, 71] |
| Great Circle Distance (mi.); Median [IQR] | 9.7 [4.4, 22.3] | 10.2 [4.3, 24.8] | 10.9 [4.4, 27.7] | 11.5 [4.4, 30.6] | 10.4 [4.4, 25.8] |
| Sex | |||||
| Male | 23533 (82.1%) | 9478 (76.9%) | 11125 (75.4%) | 16852 (77.4%) | 60988 (78.7%) |
| Female | 5143 (17.9%) | 2844 (23.1%) | 3636 (24.6%) | 4916 (22.6%) | 16539 (21.3%) |
| Race | |||||
| White | 24780 (86.4%) | 10261 (83.3%) | 11889 (80.5%) | 17050 (78.3%) | 63980 (82.5%) |
| Black | 2893 (10.1%) | 1698 (13.8%) | 2406 (16.3%) | 4012 (18.4%) | 11009 (14.2%) |
| Other | 640 (2.2%) | 227 (1.8%) | 320 (2.2%) | 480 (2.2%) | 1667 (2.2%) |
| Charlson-Deyo Score | |||||
| 0 | 22480 (78.4%) | 8893 (72.2%) | 10006 (67.8%) | 14665 (67.4%) | 56044 (72.3%) |
| 1 | 4586 (16%) | 2391 (19.4%) | 3252 (22%) | 5052 (23.2%) | 15281 (19.7%) |
| 2 | 1136 (4%) | 737 (6%) | 1065 (7.2%) | 1443 (6.6%) | 4381 (5.7%) |
| ≥3 | 474 (1.7%) | 301 (2.4%) | 438 (3%) | 608 (2.8%) | 1821 (2.3%) |
| Urban vs. Rural | |||||
| Non-metropolitan | 4950 (17.3%) | 2532 (20.5%) | 3041 (20.6%) | 4350 (20%) | 14873 (19.2%) |
| Metropolitan | 23020 (80.3%) | 9521 (77.3%) | 11388 (77.1%) | 16930 (77.8%) | 60859 (78.5%) |
| Insurance Type | |||||
| Uninsured | 856 (3%) | 636 (5.2%) | 1056 (7.2%) | 1943 (8.9%) | 4491 (5.8%) |
| Private Insurance | 11261 (39.3%) | 4162 (33.8%) | 4479 (30.3%) | 6323 (29%) | 26225 (33.8%) |
| Public Insurance | 15923 (55.5%) | 7190 (58.4%) | 8839 (59.9%) | 12828 (58.9%) | 44780 (57.8%) |
| Percent with No High School Degree | |||||
| ≥17.6% | 6186 (21.6%) | 3356 (27.2%) | 4248 (28.8%) | 6732 (30.9%) | 20522 (26.5%) |
| 10.9-17.5% | 7895 (27.5%) | 3730 (30.3%) | 4554 (30.9%) | 6705 (30.8%) | 22884 (29.5%) |
| 6.3-10.8% | 7901 (27.6%) | 3093 (25.1%) | 3640 (24.7%) | 5055 (23.2%) | 19689 (25.4%) |
| <6.3% | 6217 (21.7%) | 1954 (15.9%) | 2091 (14.2%) | 2946 (13.5%) | 13208 (17%) |
| Median Income | |||||
| <$40,227 | 5955 (20.8%) | 3267 (26.5%) | 4230 (28.7%) | 6582 (30.2%) | 20034 (25.8%) |
| $40,227-50,353 | 6615 (23.1%) | 3184 (25.8%) | 3807 (25.8%) | 5577 (25.6%) | 19183 (24.7%) |
| $50,354-63,332 | 6606 (23%) | 2714 (22%) | 3155 (21.4%) | 4625 (21.2%) | 17100 (22.1%) |
| ≥$63,333 | 8957 (31.2%) | 2937 (23.8%) | 3303 (22.4%) | 4591 (21.1%) | 19788 (25.5%) |
| Facility Location | |||||
| Northeast | 6012 (21%) | 2338 (19%) | 2837 (19.2%) | 4006 (18.4%) | 15193 (19.6%) |
| Midwest | 7587 (26.5%) | 3287 (26.7%) | 4021 (27.2%) | 5757 (26.4%) | 20652 (26.6%) |
| South | 11397 (39.7%) | 5219 (42.4%) | 6219 (42.1%) | 9458 (43.4%) | 32293 (41.7%) |
| West | 3233 (11.3%) | 1331 (10.8%) | 1488 (10.1%) | 2295 (10.5%) | 8347 (10.8%) |
Survival
When analyzed by year from 2004-2015, patients with laryngeal cancer showed no significant annual change in overall survival. Figure 1a shows two-year and five-year observed (OS) and relative survival (RS), which showed no trend toward improvement or worsening survival during the study period. From 2004 to 2015, two-year RS remained stagnant in the 76 to 78% range aside from two peaks in 2012 (79.9%) and 2014 (80.1%). Survival values, along with 95% confidence intervals (CI) are presented in Supplementary Table 1. The 95% CI in 2004 (75.46%-77.85%) and 2015 (76.62%-79.18%) overlap, demonstrating the lack of statistically significant improvement. This overlap of CIs remains true for all years aside from two outlier years in 2012 and 2014. A similar trend is reflected in the two-year OS. Five-year survival also remained stable during the study period: RS ranged from 61.72% to 63.97% with overlap of 95% CI for all years studied. Likewise, five-year OS ranged from 54.26% to 56.52% with overlap of 95% CI throughout the study period. Similar to overall survival, survival by stage did not undergo significant changes during the study period (Figure 1b).
Figure 1.
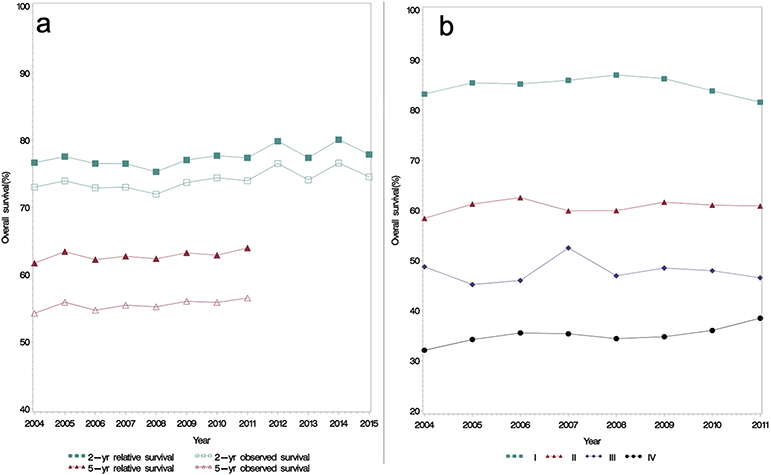
Survival Trends in Laryngeal Cancer in the United States
a: Two-year relative and observed survival from 2004-2015 shows no significant improvement in survival during the study period. b: Five-year relative and observed survival from 2004-2011 shows similar lack of survival improvement.
b: Five-year relative survival was stable across all stages during the study period.
Observed survival was defined as the probability of surviving for a specified time interval based from the study population. Relative survival was defined as the ratio of the proportion of observed survivors in the cohort against age, sex, and gender matched cohort from the United States population.
Stage distribution and disease specific trends
To investigate the lack of demonstrable survival improvement during the study period, temporal trends in disease-specific characteristics were analyzed. Notably, this showed a notable increase in stage IV disease during the study period with a corresponding decrease in stage I disease (Figure 2). Stage IV disease represented 24.4% (n=1385/5667) of all diagnoses in 2004 and 31.1% (n=2014/6487) in 2016. Concurrently, the proportion of stage I disease (38.0%, n=2174/5667 to 36.0%, n=2052/6487) and stage II disease (17.4%, n=1163/5667 to 13.8%, n=954/6487) both decreased from 2004 to 2016. There were no significant trends in disease subsite distribution (Figure 3c). Further analysis of disease specific characteristics revealed subtle changes in T stage distribution (Figure 3a) and a clear trend (p<0.001) of increasing N2 disease with a reciprocal decrease in N0 disease (Figure 3b). In 2004, N2 disease comprised 13.3% (n=716) of diagnoses versus 20.9% (n=1376) in 2016 and this trend was seen in both clinical and pathologic N-stage (Supplementary Figure 1).
Figure 2.
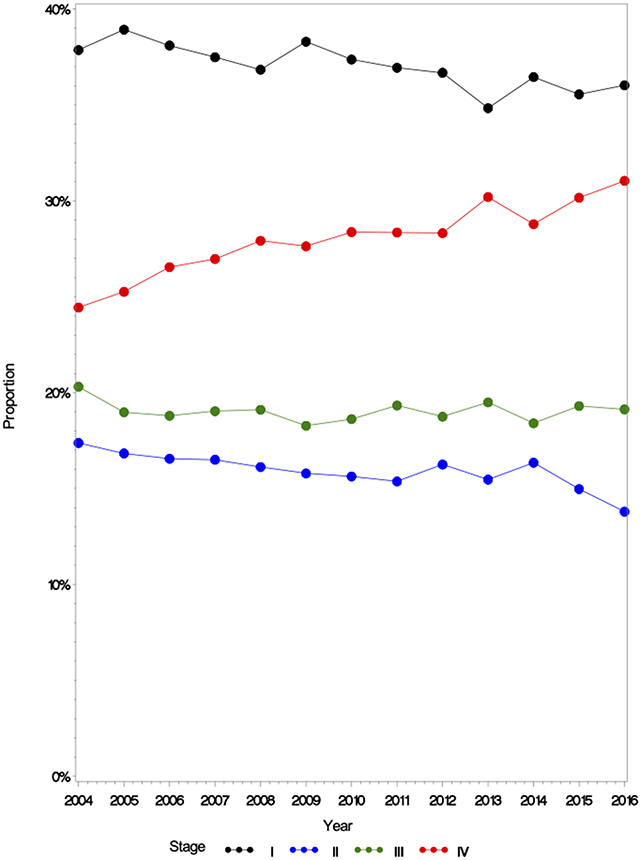
AJCC Overall Stage Distribution in Laryngeal Cancer in the United States from 2004-2016
Stage IV disease increased across the study period with reciprocal decreases in Stage I, II and III disease. Stage IV disease increased from 24.4% of all diagnoses in 2004 to 31.1% in 2016.
Figure 3.
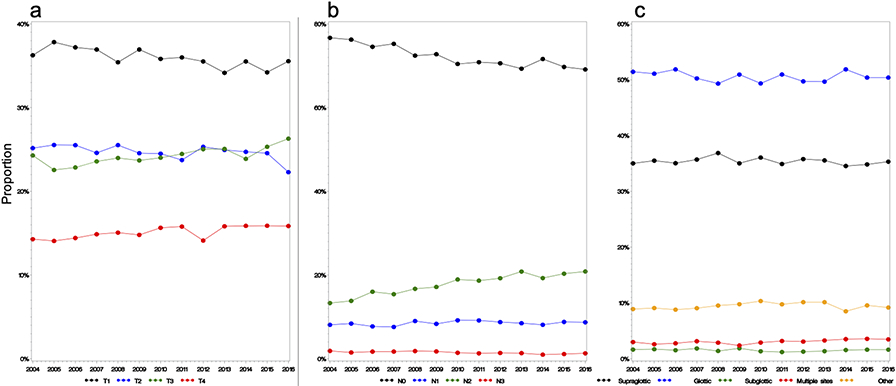
T and N Stage Distribution and Subsite Distribution from 2004–2016
a: Trends in T stage distribution. A subtle increase in T3 and T4 disease were observed. b: Trends in N stage distribution–a significant increase in N2 disease with a decrease in N0 disease. c: Trends in subsite distribution–no trends were noted.
Table 2 shows results of a multinomial logistic regression investigating the relationship between AJCC, T, and N stage and increasing year. Adjusted ORs shown are controlled for the following demographic variables: age, sex, race, Charlson-Deyo score, facility location, high school degree quartile, insurance status, and distance from hospital. Correcting for these variables, compared to the odds of having stage I disease, each progressing year conferred an additional 2.2% increased odds of having stage IV disease at the time of diagnosis (adjusted OR 1.022, 95%CI: 1.017-1.028, p<0.001). Additionally, each progressing year increased the odds of having T3 (adjusted OR 1.012, 95% CI: 1.007-1.018, p<0.001) and T4 (adjusted OR 1.012, 95% CI 1.005-1.018, p<0.001) disease by 1.2% when compared to the odds of having T1. Simultaneously, the risk of N2 disease increased by 4.7% annually (adjusted OR 1.047, 95% CI: 1.041-1.053, p<0.001) when compared to the odds of having N0 disease. The annual odds of N1 disease relative to N0 disease increased by 1.4% (adjusted OR 1.014, 95% CI: 1.006-1.021, p<0.001).
Table 2:
Multinomial Logistic Regression per Year by T, N, and Overall AJCC Stage
| Stage | OR | 95% CI | p-Value | aOR | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| T Stage | ||||||
| T1 | Ref | - | - | - | - | - |
| T2 | 1.000 | (0.995, 1.005) | 0.99 | 0.998 | (0.992, 1.003) | 0.41 |
| T3 | 1.015 | (1.009, 1.020) | <.01 | 1.012 | (1.007, 1.018) | <.01 |
| T4 | 1.015 | (1.009, 1.022) | <.01 | 1.012 | (1.005, 1.018) | <.01 |
| N Stage | ||||||
| N0 | Ref | - | - | - | - | - |
| N1 | 1.015 | (1.008, 1.022) | <.01 | 1.014 | (1.006, 1.021) | <.01 |
| N2 | 1.044 | (1.039, 1.050) | <.01 | 1.047 | (1.041, 1.053) | <.01 |
| N3 | 0.969 | (0.954, 0.984) | <.01 | 0.973 | (0.957, 0.989) | <.01 |
| AJCC Stage | ||||||
| I | Ref | - | - | - | - | - |
| II | 0.994 | (0.989, 1.000) | 0.05 | 0.992 | (0.986, 0.998) | 0.01 |
| III | 1.005 | (1.000, 1.010) | 0.06 | 1.002 | (0.996, 1.008) | 0.50 |
| IV | 1.023 | (1.019, 1.028) | <.01 | 1.022 | (1.017, 1.028) | <.01 |
OR - Odds Ratio ; CI - confidence interval ; aOR - Adjusted Odds Ratio
Associations with Stage IV disease
To further study the stage migration toward increasing stage IV disease, logistic regression was performed to determine factors associated with stage IV disease during the entire study period. Increasing age was negatively associated with stage IV disease (OR 0.961, 95% CI 0.959–0.963, p<0.001). Black race was also associated with increased odds of stage IV disease (OR 1.330, 95% CI: 1.287–1.395, p<0.001). Socioeconomic factors including lack of insurance, residence in a zip code with ≥17.6% of residents without a high-school diploma, and increasing distance from treatment hospital were also all associated with higher odds of stage IV disease (Table 3). To further assess for any underlying change in demographic trends that may have played a role, we explored the bivariate associations with year of diagnosis as the explanatory variable and demographic or clinical variables as the dependent variables (Table 4). With each increasing year of diagnosis, the average age of diagnosis rose by 0.09 years (p<0.001), and those categorized as ‘Other race’ represented an increasing proportion of diagnoses (OR 1.055, 95%CI:1.042-1.069, p<0.001) compared to White race. Additionally, the proportion of subjects with a higher Charlson-Deyo comorbidity score increased with increasing year of diagnosis, most markedly for scores ≥3 (OR 1.091, 95%CI: 1.078-1.105, p<0.001). Finally, those diagnosed with laryngeal cancer were increasingly insured through public health insurance programs (OR 1.037, 95%CI: 1.028, 1.045, p<0.001) compared to not insured.
Table 3:
Multivariate Associations with Stage IV Disease
| Variable | Odds Ratio | 95% CI |
p- value |
|---|---|---|---|
| Year of Diagnosis | 1.024 | (1.019, 1.028) | <.01 |
| Age at Diagnosis | 0.961 | (0.959, 0.963) | <.01 |
| Sex | |||
| Female vs. Male | 1.049 | (1.007, 1.093) | 0.02 |
| Race | |||
| Black vs. White | 1.330 | (1.269, 1.395) | <.01 |
| Other vs. White | 1.126 | (1.004, 1.263) | 0.04 |
| Charlson-Deyo Score | |||
| 1 vs 0 | 1.442 | (1.383, 1.503) | <.01 |
| 2 vs 0 | 1.469 | (1.370, 1.576) | <.01 |
| 3 vs 0 | 1.425 | (1.283, 1.583) | <.01 |
| Metropolitan vs. Non-Metropolitan Residence | 1.027 | (0.983, 1.073) | 0.23 |
| Distance to hospital (100-mile steps) | 1.062 | (1.042, 1.083) | <.01 |
| Insurance | |||
| Private vs Not Insured | 0.487 | (0.454, 0.522) | <.01 |
| Public vs. Not Insured | 0.780 | (0.728, 0.835) | <.01 |
| Percent with No High School Degree | |||
| 10.9-17.5% vs ≥17.6% | 0.911 | (0.872, 0.952) | <.01 |
| 6.3-10.8% vs ≥17.6% | 0.829 | (0.790, 0.870) | <.01 |
| <6.3% vs ≥17.6% | 0.737 | (0.700, 0.780) | <.01 |
| Treatment Facility Location | |||
| Midwest vs. Northeast | 1.001 | (0.951, 1.053) | 0.98 |
| South vs. Northeast | 0.942 | (0.898, 0.989) | 0.02 |
| West vs. Northeast | 1.062 | (0.996, 1.133) | 0.07 |
Table 4:
Bivariate analysis between year of diagnosis and other covariates
| Variable | Odds Ratio/Beta Value β |
95% CI | p-Value |
|---|---|---|---|
| Age | 0.09 | (0.07, 0.11) | <0.001 |
| Sex | |||
| Male | Ref | - | 0.83 |
| Female | 1 | (0.995, 1.004) | |
| Race | |||
| White | Ref | - | <.0001 |
| Black | 1.003 | (0.998, 1.009) | |
| Other | 1.055 | (1.042, 1.069) | |
| Charlson-Deyo Score | |||
| 0 | Ref | - | <.0001 |
| 1 | 1.029 | (1.024, 1.034) | |
| 2 | 1.046 | (1.038, 1.055) | |
| ≥3 | 1.091 | (1.078, 1.105) | |
| Residence Area | |||
| Nonmetropolitan | Ref | - | 0.6714 |
| Metropolitan | 0.999 | (0.994, 1.004) | |
| Insurance | |||
| Not Insured | Ref | - | <.0001 |
| Private Insurance | 1.007 | (0.999, 1.015) | |
| Public Insurance | 1.037 | (1.028, 1.045) | |
| Percent with No High School Degree | |||
| ≥17.6% | Ref | - | 0.05 |
| 10.9-17.5% | 1.001 | (0.996, 1.006) | |
| 6.3-10.8% | 1.004 | (0.999, 1.009) | |
| <6.3% | 0.996 | (0.990, 1.002) | |
| Median Income | |||
| <$40,227 | Ref | - | 0.8891 |
| $40,227-50,353 | 1 | (0.995, 1.005) | |
| $50,354-63,332 | 1.002 | (0.996, 1.007) | |
| ≥$63,333 | 1 | (0.995, 1.005) | |
| Treatment Facility Location | |||
| Northeast | Ref | - | 0.4189 |
| Midwest | 1.003 | (0.998, 1.008) | |
| South | 1.001 | (0.996, 1.006) | |
| West | 0.998 | (0.991, 1.005) | |
| Distance to Hospital | −0.07β | (−0.23, 0.1) | 0.4614 |
Value for association between age and year of diagnosis presented as a beta-coefficient since age is continuous. Values for all other variables presented as odds ratios since they are categorical.
Discussion
This paper represents the first update on laryngeal cancer epidemiologic trends in the United States since 2006, where Hoffman et al noted a decrease in five-year OS and RS from 1985-2001.14 In this study, we show that despite major treatment advances, there has been a lack of improvement in two-and five-year OS and RS from 2004-2016. Most striking, and partially explaining the lack of survival improvement, is stage migration towards an increasing proportion of stage IV disease from 2004–2016. Notably, evidence suggests the proportion of stage IV disease has been increasing since 1980. In a survey of acute care hospitals from 1980-1992, Shah et al noted a slight increase in stage IV disease from 18.7% to 20.5% when comparing diagnoses from 1980-1985 versus 1990-1992.17 Similarly, Hoffman et al identified a clear uptrend in stage IV disease from 1985 to 2001 in a study of the NCDB.14 However, the early years included in this study were plagued by a high percentage of entries with unknown stage–as high as 43% in 1985. As the NCDB matured, the percentage of cases with missing stage decreased, falling and remaining below 5% after 1995.14 From that point onwards, the proportion of stage IV disease remained relatively stable. In 2001, the final year included in their study, Hoffman et al found stage IV disease to account for 24% of new diagnoses. In 2004, the first year included in the present study, stage IV disease accounted for 24.4% of cases, rising to represent nearly a third of all new laryngeal cancer diagnoses by 2016.
This change in stage distribution appears to be driven mostly by increasing regional nodal disease, as evidenced by an increasing proportion of N2 disease at the time of diagnosis. To a lesser degree, increasing proportions of T3 and T4 disease are also contributing (Table 2). Although the AJCC staging manual did undergo a transition from the 6th to the 7th edition during the study period, no changes in tumor or regional nodal staging were made. It is unclear why the degree of nodal disease increased during the study period. While supraglottic cancers are more likely to result in advanced nodal disease, no trend in subsite distribution was identified. However, it is possible that increasing utilization of advanced imaging technology may lead to upstaging.
In their survey of Committee on Cancer facilities, Shah et al showed an increase in the use of computed tomography (CT) scan from 1980 to 1992.17 Correspondingly, Champion and Piccirillo demonstrated TNM upstaging in 15 of 90 patients with laryngeal SCC undergoing pre-therapeutic CT from 1995 to 1997.18 However, rates of CT scan usage are not stored in the NCDB. Similarly, usage of positron emission tomography (PET) is not recorded in the NCDB, but there has been a significant increase in usage of PET-CT since Medicare began reimbursing for its use in 2001.19 With increasing PET-CT utilization, it is possible regional nodal metastases are being identified more frequently. However, comparison of PET-CT versus CT or magnetic resonance imaging (MRI) in laryngeal cancer has shown equivalent sensitivity for the identification of nodal metastases.20 Further supporting the idea that advances in imaging cannot completely explain the increasing nodal disease is the finding that pathologic N2 disease is also increasing. It has also been shown that surgical treatment, allowing for pathologic staging, changes stage in up to 17% of patients, with 74% of those patients being upstaged.21 With increasing rates of organ preservation therapy, this study suggests patients are more likely to be incorrectly down-staged. If true, this would lead to decreasing rates of advanced stage disease, the opposite of our finding. Finally, PET-CT has been shown to be more sensitive for the identification of distant metastases, thereby driving changes in trend distribution in lung cancer.22 The exclusion of those with M1 disease in our study group excludes this as an explanation of the stage migration identified here.
Other organic factors such as delay in diagnosis or socioeconomic factors may also play a role in stage migration. While delay in diagnosis is not stored in the NCDB and is difficult to accurately ascertain, other studies have used time-to-treatment initiation (TTI) as a proxy. Unsurprisingly, increasing TTI has been shown to cause pathologic T, N and group upstaging in head and neck cancer.23 The increasing time to diagnosis theory may be further supported by the association of several demographic factors with stage IV disease, including non-White race, lack of insurance, increasing distance to treating hospital, and residence in poorly educated or low-income zip codes. Poverty, non-white race, low education levels, and lack of insurance have all been associated with lack of access to healthcare and worse outcomes in colon, esophageal, pancreatic, and liver cancers.24 This finding has been attributed to several factors, including lack of insurance, under-utilization of high-volume treatment centers, and longer TTI in lower socioeconomic classes.25 Lower socioeconomic status has been associated with poor health literacy, making treatment decisions in complex cancer care increasingly difficult potentially delaying care. Our study further suggests lower socioeconomic class may contribute to higher stage at the time of diagnosis in laryngeal cancer.
To determine whether a shift in demographics and socioeconomic factors had an effect on the observed stage migration, we also performed analysis of demographic trends. This showed those falling in the Other category of racial background increased during the study period while also being associated with stage IV disease. Additionally, Charlson-Deyo comorbidity score increased during the study period and was also associated with stage IV disease. The higher degree of comorbidities in laryngeal cancer patients may also contribute to the lack of improvement in survival. However, some demographic trends identified moved in the opposite direction from those found to be associated with stage IV disease. Specifically, while younger age was associated with stage IV disease, the study population increased in age. Further, while lack of insurance was a risk factor for stage IV disease, an increasing proportion of the study population obtained insurance through public insurance programs as opposed to remaining uninsured. This suggests that factors unable to be examined through the NCDB, such as smoking rates and delay in diagnosis may be the strongest socioeconomic contributors to the stage migration identified here.
Notably, although those of Black race did not represent an increasing proportion of the study population (Table 5), Black race was one of the strongest demographic factors associated with stage IV disease (Table 4). This race related disparity has been previously demonstrated in other head and neck cancer subsites including the larynx.26-28 In an institutional study of 87 Black patients and 261 matched White patients, Black patients with laryngeal cancer were more likely to present with advanced stage disease, even after adjusting for socioeconomic and insurance differences.28 Overall, Black patients with head and neck cancer are also more likely to experience delays in treatment initiation, potentially due to higher rates of unemployment and less social support (higher rates of single marital status).26
The current study carries limitations intrinsic to the NCDB itself. While the NCDB is a well-validated and reliable database, it is subject to selection, information, and recall bias. Specifically pertaining to this study, lack of smoking data precluded investigation of trends regarding this critical risk factor. Large multi-institutional studies may be better suited to elucidate the effect smoking, delay in diagnosis, and changes in surgical or histopathologic techniques may have on stage migration.
Encouragingly, the lack of survival improvement despite improvements in diagnostic and treatment protocols and technology can also be viewed in a positive light: despite increasing proportions of stage IV disease, survival has actually flattened rather than declined further as previously described. While this may be due to shifting trends in treatment modalities, especially for advanced stage disease, such analyses were beyond the scope of the current study. The data shown here suggest relatively simple interventions may help further improve survival. Social determinants of health, can at times, be more important than many of the treatments provided in healthcare. Laryngeal cancer is no different. Patients with poor social determinants of health have lower access to care which leads to presentation with more advanced disease. Over the last several decades interventions and policies to improve these inequities have failed to improve outcomes in laryngeal cancer for certain subsets of the population. We therefore recommend developing screening programs, refining referral patterns, and developing community outreach programs, especially aimed towards Black patients.
Conclusion
Despite significant advances in the management of laryngeal cancer, survival for laryngeal cancer has not improved over the last fifteen years in the United States. There is a clear trend towards an increasing proportion of Stage IV disease with an accompanying decrease in stage I disease. This change appears to be driven primarily by increasing nodal disease. Each progressive year from 2004-2016 resulted in a 2% increased risk of being stage IV at time of treatment. Efforts should be made to diagnose and treat laryngeal cancer at earlier stages in order to prevent further stage migration and achieve incremental survival improvements commensurate with scientific and technological advances.
Supplementary Material
Supplemental Figure 1. Pathologic N Stage Distribution
Pathologic N2 disease increased from 2004 through 2013 before decreasing from 2014 to 2016. N1 disease showed a similar trend while N0 disease decreased from 2004 through 2015 before increasing in 2016.
Synopsis.
While advances in treatment protocols were developed in the early 2000s, survival of laryngeal cancer has not improved from 2004-2016. Concurrently, stage migration toward greater proportions of stage IV disease has occurred. Reversal of this is critical to optimize outcomes.
Footnotes
The authors have no disclosures.
References
- 1.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317 [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Ismaila N, Lewin JS, et al. Use of Larynx-Preservation Strategies in the Treatment of Laryngeal Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;36(11):1143–1169. doi: 10.1200/JCO.2017.75.7385 [DOI] [PubMed] [Google Scholar]
- 3.Wolf GT, Bellile E, Eisbruch A, et al. Survival rates using individualized bioselection treatment methods in patients with advanced laryngeal cancer. JAMA Otolaryngol Neck Surg. 2017;143(4):355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Susko MS, Lazar AA, Dhar S, et al. Improved Tumor Control Related to Radiotherapy Technological Development for Hypopharyngeal Cancer. Laryngoscope. 2021;131(2):E452–E458. doi: 10.1002/lary.28726 [DOI] [PubMed] [Google Scholar]
- 5.Daly ME, Le Q-T, Jain AK, et al. Intensity-modulated radiotherapy for locally advanced cancers of the larynx and hypopharynx. Head Neck. 2011;33(1):103–111. doi: 10.1002/hed.21406 [DOI] [PubMed] [Google Scholar]
- 6.Kouloulias V, Thalassinou S, Platoni K, et al. The Treatment Outcome and Radiation-Induced Toxicity for Patients with Head and Neck Carcinoma in the IMRT Era: A Systematic Review with Dosimetric and Clinical Parameters. Lee T-F, ed. Biomed Res Int. 2013;2013:401261. doi: 10.1155/2013/401261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozer E, Alvarez B, Kakarala K, Durmus K, Teknos TN, Carrau RL. Clinical outcomes of transoral robotic supraglottic laryngectomy. Head Neck. 2013;35(8):1158–1161. doi: 10.1002/hed.23101 [DOI] [PubMed] [Google Scholar]
- 8.Dziegielewski PT, Kang SY, Ozer E. Transoral robotic surgery (TORS) for laryngeal and hypopharyngeal cancers. J Surg Oncol. 2015;112(7):702–706. doi: 10.1002/jso.24002 [DOI] [PubMed] [Google Scholar]
- 9.Forastiere AA, Maor M, Weber RS, et al. Long-term results of Intergroup RTOG 91–11: A phase III trial to preserve the larynx—Induction cisplatin/5-FU and radiation therapy versus concurrent cisplatin and radiation therapy versus radiation therapy. J Clin Oncol. 2006;24(18_suppl):5517. doi: 10.1200/jco.2006.24.18_suppl.5517 [DOI] [Google Scholar]
- 10.Olsen KD. Reexamining the treatment of advanced laryngeal cancer. Head Neck. 2010;32(1):1–7. doi: 10.1002/hed.21294 [DOI] [PubMed] [Google Scholar]
- 11.Wolf GT. Reexamining the treatment of advanced laryngeal cancer: the VA laryngeal cancer study revisited. Head Neck. 2010;32(1):7–14. doi: 10.1002/hed.21296 [DOI] [PubMed] [Google Scholar]
- 12.Megwalu UC, Sikora AG. Survival outcomes in advanced laryngeal cancer. JAMA Otolaryngol - Head Neck Surg. 2014;140(9):855–860. doi: 10.1001/jamaoto.2014.1671 [DOI] [PubMed] [Google Scholar]
- 13.Boffa DJ, Rosen JE, Mallin K, et al. Using the national cancer database for outcomes research a review. JAMA Oncol. 2017;3(12):1722–1728. doi: 10.1001/jamaoncol.2016.6905 [DOI] [PubMed] [Google Scholar]
- 14.Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: Changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9 SUPPL. 2):1–13. doi: 10.1097/01.mlg.0000236095.97947.26 [DOI] [PubMed] [Google Scholar]
- 15.Huang SH, O’Sullivan B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr Treat Options Oncol. 2017;18(7):40. doi: 10.1007/s11864-017-0484-y [DOI] [PubMed] [Google Scholar]
- 16.Dickman PW, Hills M. Estimating and modelling relative survival. stata J. 2013;vv(ii):1–25. [Google Scholar]
- 17.Shah JP, Karnell LH, Huffman HT, et al. Patterns of care for cancer of the larynx in the United States. Arch Otolaryngol - Head Neck Surg. 1997;123(5):475–483. doi: 10.1001/archotol.1997.01900050021002 [DOI] [PubMed] [Google Scholar]
- 18.Champion GA, Piccirillo JF. The impact of computed tomography on pretherapeutic staging in patients with laryngeal cancer: Demonstration of the will Rogers’ phenomenon. Head Neck. 2004;26(11):972–976. doi: 10.1002/hed.20071 [DOI] [PubMed] [Google Scholar]
- 19.Hillner BE, Tosteson AN, Song Y, et al. Growth in the Use of PET for Six Cancer Types After Coverage by Medicare: Additive or Replacement? J Am Coll Radiol. 2012;9(1):33–41. doi: 10.1016/j.jacr.2011.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatar G, Cermik TF, Karagoz Y, et al. The value of whole-body contrast-enhanced 18F-FDG PET/CT imaging in the diagnosis and staging of patients with laryngeal carcinoma. Nucl Med Commun. 2018;39(4). https://journals.lww.com/nuclearmedicinecomm/Fulltext/2018/04000/The_value_of_whole_body_contrast_enhanced_18F_FDG.9.aspx. [DOI] [PubMed] [Google Scholar]
- 21.Contrera KJ, Hair BB, Prendes B, et al. Clinical Versus Pathologic Laryngeal Cancer Staging and the Impact of Stage Change on Outcomes. Laryngoscope. 2020. doi: 10.1002/lary.28924 [DOI] [PubMed] [Google Scholar]
- 22.Morgensztern D, Ng SH, Gao F, Govindan R. Trends in Stage Distribution for Patients with Non-small Cell Lung Cancer: A National Cancer Database Survey. J Thorac Oncol. 2010;5(1):29–33. doi: 10.1097/JTO.0b013e3181c5920c [DOI] [PubMed] [Google Scholar]
- 23.Xiao R, Ward MC, Yang K, et al. Increased pathologic upstaging with rising time to treatment initiation for head and neck cancer: A mechanism for increased mortality. Cancer. 2018;124(7):1400–1414. doi: 10.1002/cncr.31213 [DOI] [PubMed] [Google Scholar]
- 24.Wasif N, Etzioni D, Habermann EB, et al. Racial and Socioeconomic Differences in the Use of High-Volume Commission on Cancer-Accredited Hospitals for Cancer Surgery in the United States. Ann Surg Oncol. 2018;25(5):1116–1125. doi: 10.1245/s10434-018-6374-0 [DOI] [PubMed] [Google Scholar]
- 25.Kompelli AR, Li H, Neskey DM. Impact of Delay in Treatment Initiation on Overall Survival in Laryngeal Cancers. Otolaryngol neck Surg Off J Am Acad Otolaryngol Neck Surg. 2019;160(4):651–657. doi: 10.1177/0194599818803330 [DOI] [PubMed] [Google Scholar]
- 26.Naghavi AO, Echevarria MI, Strom TJ, et al. Treatment delays, race, and outcomes in head and neck cancer. Cancer Epidemiol. 2016;45:18–25. doi: 10.1016/j.canep.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 27.Mahal BA, Inverso G, Aizer AA, Bruce Donoff R, Chuang S-K. Impact of African–American race on presentation, treatment, and survival of head and neck cancer. Oral Oncol. 2014;50(12):1177–1181. doi: 10.1016/j.oraloncology.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 28.Ragin CC, Langevin SM, Marzouk M, Grandis J, Taioli E. Determinants of head and neck cancer survival by race. Head Neck. 2011;33(8):1092–1098. doi: 10.1002/hed.21584 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Pathologic N Stage Distribution
Pathologic N2 disease increased from 2004 through 2013 before decreasing from 2014 to 2016. N1 disease showed a similar trend while N0 disease decreased from 2004 through 2015 before increasing in 2016.


