Abstract
Background
We systematically survey respiratory and gastrointestinal infections of viral origin in samples sent to our university hospital institute in Marseille, southern France. Here, we evaluated whether the measures implemented to fight COVID-19 had an effect on the dynamics of viral respiratory or gastrointestinal infections.
Methods
We analysed PCR performed and positive for the diagnoses of viral respiratory and gastrointestinal infections over five years (January 2017-February 2021). Data were collected from our epidemiological surveillance system (MIDaS). Dates and contents of French measures against SARS-CoV-2 were collected from: https://www.gouvernement.fr/info-coronavirus/les-actions-du-gouvernement.
Results
Over the 2017-2021 period, 990,364 analyses were carried out for respiratory infections not including SARS-CoV-2, 510,671 for SARS-CoV-2 and 27,719 for gastrointestinal infections. During winter 2020–2021, when the most restrictive lockdown measures were in place in France, a marked decrease of infections with influenza viruses (one case versus 1,839-1,850 cases during 2017-2020 cold seasons) and with the RSV (56 cases versus 988-1,196 cases during 2017-2020 cold seasons) was observed, demonstrating the relative effectiveness of these measures on their occurrence. SARS-CoV-2 incidence seemed far less affected. Rhinoviruses, parainfluenza 3 virus, and the coronavirus NL63 remained at comparable levels. Also, the norovirus winter season positivity rates decreased continuously and significantly over time from 9.3% in 2017–2018 to 2.0% in 2020–2021.
Conclusion
The measures taken to control COVID-19 were effective against lower respiratory tract infections viruses and gastroenteritis agents, but not on the agents of the common winter cold and SARS-CoV-2. This suggests that more specific measures to prevent COVID-19 and upper respiratory tract infections need to be discovered to limit the spread of this epidemic.
Key words: Coronavirus, Respiratory infections, Gastrointestinal infections, SARS-CoV-2, MIDaS, Epidemic, Surveillance
1. BACKGROUND
In December 2019, a new coronavirus named SARS-CoV-2 (for severe acute respiratory syndrome coronavirus 2) emerged in Wuhan, Hubei region, China. It spread rapidly to the rest of the world and was declared a pandemic in March 2020 [1]. As of February 09, 2022 402,064,265 SARS-CoV-2 cases and 5,768,927 patient deaths from COVID-19 (for coronavirus disease 2019) were reported [2]. SARS-CoV-2 variants have emerged since summer of 2020 [3,4] and have each determined an epidemic of variable intensity and duration. These variants have been revealed to be associated with differences regarding viral loads, transmissibility, and clinical severity and they have been involved in various degrees of escape to immunity elicited by vaccination or infection [5], [6], [7], [8]. The dynamics of SARS-CoV-2 epidemics at national and global scales proved to be unpredictable.
In order to reduce the spread of SARS-CoV-2, the French government decided to take several health and social measures. This initially involved repeated risk prevention messages on the use of protective measures including regularly hand washing with soap or alcohol-based hand gel, social distancing of 1.5 meter between individuals, and wearing a mask [9]. These measures had already been used in prevention campaigns for other viruses, particularly respiratory viruses such as influenza [10,11]. More restrictive measures on movement were also taken, with the implementation of a number of lockdowns and curfews (Decree No. 2020–260; Decree No. 2020–1310) [12,13]. Thus, in addition to the fight against COVID-19, these measures may also be effective at controlling other communicable respiratory and digestive diseases.
At the Hospital University Institute Méditerranée Infection (IHU-MI), the activity of the virology and microbiology laboratory is monitored by a collection and surveillance system known as MIDaS (for Mediterranée Infection Data Warehousing and Surveillance) [14,15]. This system enables us to monitor respiratory and digestive virus infections on a weekly basis, and has included COVID-19 since its emergence in France [16]. The objective of this study was to analyse the epidemiological curves of respiratory and gastrointestinal viruses since the emergence of SARS-CoV-2 and to evaluate if they changed under the measures implemented against COVID-19 in France by comparing them during cold seasons over the past five years.
2. MATERIALS AND METHODS
2.1. Surveillance system
Since 2003, the activity of our clinical microbiology laboratory has involved massive and unbiased monitoring of all clinical samples received for testing bacteria, viruses, parasites and microscopic fungi [16,17]. This followed recommendations from one of the authors (DR) [18] made to the French government in 2003 to set up surveillance systems of any abnormal events related to infectious diseases based on laboratory data, including through syndromic surveillance. Our laboratory is the single one to diagnose infections for all public university hospitals (Assistance Publique - Hôpitaux de Marseille (AP-HM)) in Marseille, which have a total of 3,288 beds with nearly 125,000 admissions and one million consultations per year. Our laboratory conducts approximately eight million tests every year.
Since 2013 when the IHU-MI was established, our surveillance tools have expanded further and have improved through our unique MIDaS (for Mediterranée Infection Data Warehousing and Surveillance) collection and surveillance system, which consists of five sub-systems [14]. We systematically collect all laboratory data (samples, tests, positive diagnoses) from the Nexlab laboratory management system. All microbiological analysis results (sample identification, requesting clinical department, date of sampling, analysis, result, antibiotic susceptibility testing, antibiotic resistance phenotype, bacterial co-identifications) and patient information (anonymised patient identification, age, sex, home postal code, anonymised hospital stay identification, date of stay within a department, death) are then deposited in a dedicated data warehouse. All clinical samples, tests and infectious agents are monitored on a weekly basis throughout the year. MIDaS automatically detects any aberrations in the statistical signal using the cumulative sum control chart (CUSUM) algorithm and triggers alarms [19]. These alarms are discussed during a weekly epidemiological staff meeting, which includes epidemiologists, biologists, and infectiologists.
Respiratory and gastrointestinal samples and infectious agents are some of the items surveyed. Respiratory and gastrointestinal viruses are diagnosed in our laboratory using commercial or in-house real-time Polymerase Chain Reaction (qPCR) tests and adopting a syndromic approach using multiplex or simplex tests. They included influenza A and B viruses, respiratory syncytial virus (RSV), rhinoviruses, enteroviruses, adenoviruses, metapneumovirus, endemic coronaviruses (HCoV-OC43, -NL63, -E229 and -HKU1), parainfluenza viruses 1 to 4 (HPIV1 to HPIV4) and SARS-CoV-2, over a period of time from January 2017 to February 2021. For the detection of SARS-CoV-2 RNA, we used in house qPCR procedures previously described [20]. To detect the other respiratory viruses, we used the FTD Respiratory pathogens 21 (Fast Track Diagnosis, Luxembourg), the Biofire FilmArray Respiratory panel 2 plus (BioMérieux, Marcy-l'Etoile, France), the Respiratory Multi-Well System r-gene (BioMérieux), or the GeneXpert Xpert Flu/RSV (Cepheid, Sunnyvale, CA, USA) assays [21].
Data on diagnoses of influenza A and B viruses were also collected from a private clinical microbiology and virology laboratory through the PACASurvE (for the Provence Alpes-Côte d'Azur Surveillance Epidemiological System) network that extents our surveillance system to private medical biology laboratories located in the Provence Alpes-Côte d'Azur French region that includes Marseille [22]. These diagnoses were reached by an immunochromatographic assay in 2017 and then by the GeneXpert Flu/RSV assay between 2018 and 2021.
The gastrointestinal viruses diagnosed included adenoviruses, rotaviruses, sapoviruses, noroviruses and astroviruses. The tests were performed using the Fast Track Diagnosis viral gastroenteritis pathogens assay (Fast Track Diagnosis).
2.2. Statistical analyses
In order to better understand the evolution of respiratory and gastrointestinal virus infections over time, the proportion of positive results between October and the end of February were compared for each virus for the 2017–2018, 2018–2019, 2019–2020 and 2020–2021 seasons. These evolutions were analysed using the log-linear model, and the Fisher and Chi-square tests for point comparisons with a two-tailed statistical significance threshold of 0.05. Statistical analyses were done using R version 4.1 [23].
2.3. Government measures and policies
Measures taken by the French government in the fight against the spread of SARS-CoV-2 and dates when these measures were implemented were collected from the government website (https://www.gouvernement.fr/info-coronavirus/les-actions-du-gouvernement).
3. RESULTS
3.1. Diagnoses of respiratory viral infections at IHU-MI from 2017 to 2021
Over a period of five years (January 2017 to February 2021), 990,364 analyses were performed for common respiratory viruses, with 37,915 positive results. Most of these cases were due to influenza viruses (influenza A virus, 6,544; influenza B viruses, 2,459) followed by rhinoviruses (7,379), RSVs (3,846), adenoviruses (1,991), metapneumoviruses (1,482), enteroviruses (790), HCoV HKU1 (424), HCoV NL63 (421), HCoV OC43 (227), HCoV E229 (87), HPIV3 (340), HPIV4 (68), HPIV2 (18) and HPIV4 (9) (Table 1 ).
Table 1.
Tests performed and positive for PCR detection of respiratory viruses in 2017, 2018, 2019, 2020 and 2021 at Hospital University Institute Méditerranée Infection.
| 2017 |
2018 |
2019 |
2020 |
2021 |
Total 2017-2021 |
2017 |
2018 |
2019 |
2020 |
2021 |
Total2017-2021 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N | N | N | N | N | % | N | % | N | % | N | % | N | % | N | % | |||
| Adenovirus | 5,656 | 14,881 | 17,636 | 32,237 | 5,283 | 75,693 | 160 | 2.8 | 449 | 3.0 | 652 | 3.7 | 600 | 1.9 | 130 | 2.5 | 1,991 | 2.6 | |
| Common coronaviruses | 2,395 | 3,773 | 8,211 | 32,237 | 5,283 | 51,899 | 70 | 2.9 | 110 | 2.9 | 231 | 2.8 | 998 | 3.1 | 276 | 5.2 | 1,685 | 3.2 | |
| HCoV 229E | 2,395 | 3,773 | 8,211 | 11,739 | 5,283 | 31,401 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 84 | 0.7 | 3 | 0.1 | 87 | 0.3 | |
| HCoV HKU1 | 2,395 | 3,773 | 8,211 | 11,736 | 5,283 | 31,398 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 423 | 3.6 | 1 | 0.0 | 424 | 1.4 | |
| HCoV NL63 | 3,791 | 3,773 | 8,211 | 11,739 | 5,283 | 32,797 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 252 | 2.1 | 169 | 3.2 | 421 | 1.3 | |
| HCoV OC43 | 9,007 | 3,773 | 8,211 | 11,74 | 5,283 | 38,014 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 175 | 1.5 | 102 | 1.9 | 277 | 0.7 | |
| Enterovirus | 4,362 | 8,933 | 17,649 | 32,237 | 5,283 | 68,464 | 36 | 0.8 | 164 | 1.8 | 279 | 1.6 | 307 | 1.0 | 4 | 0.1 | 790 | 1.2 | |
| Influenza virus | 12,992 | 14,856 | 17,844 | 32,237 | 5,283 | 83,212 | 1,737 | 13.4 | 1,708 | 11.5 | 2,427 | 13.6 | 3,119 | 9.7 | 0 | 0.0 | 8,991 | 10.8 | |
| Influenza A virus | 12,608 | 14,859 | 17,847 | 32,237 | 5,283 | 82,834 | 1,525 | 12.1 | 936 | 6.3 | 2,397 | 13.4 | 1,686 | 5.2 | 0 | 0.0 | 6,544 | 7.9 | |
| Influenza B virus | 13,088 | 14,858 | 17,847 | 32,237 | 5,283 | 83,313 | 222 | 1.7 | 772 | 5.2 | 30 | 0.2 | 1,435 | 4.5 | 0 | 0.0 | 2,459 | 3.0 | |
| Metapneumovirus | 7,654 | 14,75 | 17,622 | 32,237 | 5,283 | 77,546 | 230 | 3.0 | 325 | 2.2 | 445 | 2.5 | 462 | 1.4 | 20 | 0.4 | 1,482 | 1.9 | |
| Human parainfluenza virus | 9,007 | 3,771 | 8,268 | 32,237 | 5,283 | 58,566 | 15 | 0.2 | 200 | 5.3 | 438 | 5.3 | 129 | 0.4 | 322 | 6.1 | 1,104 | 1.9 | |
| HPIV1 | 3,791 | 3,771 | 8,268 | 9,268 | 5,283 | 30,381 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 9 | 0.1 | 0 | 0.0 | 9 | 0.0 | |
| HPIV2 | 3,791 | 3,771 | 8,268 | 9,268 | 5,283 | 30,381 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 17 | 0.2 | 1 | 0.0 | 18 | 0.1 | |
| HPIV3 | 2,395 | 3,771 | 8,268 | 9,268 | 5,283 | 28,985 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 41 | 0.4 | 299 | 5.7 | 340 | 1.2 | |
| HPIV4 | 2,395 | 3,771 | 8,268 | 9,268 | 5,283 | 28,985 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 48 | 0.5 | 20 | 0.4 | 68 | 0.2 | |
| Rhinovirus | 4,305 | 14,057 | 17,637 | 32,237 | 5,283 | 73,519 | 401 | 9.3 | 1,771 | 12.6 | 2,264 | 12.8 | 2,494 | 7.7 | 449 | 8.5 | 7,379 | 10.0 | |
| Respiratory syncytial virus | 12,756 | 14,849 | 17,851 | 32,237 | 5,283 | 82,976 | 923 | 7.2 | 1,024 | 6.9 | 1,347 | 7.5 | 498 | 1.5 | 54 | 1.0 | 3,846 | 4.6 | |
| SARS-CoV-2 | 0 | 0 | 0 | 420,120 | 90,551 | 510,671 | 0 | 0 | 0 | 0 | 0 | 0 | 26,723 | 6.4 | 8,236 | 9.1 | 34,959 | 6.8 | |
HCoV, human coronavirus; HPIV, human parainfluenza virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
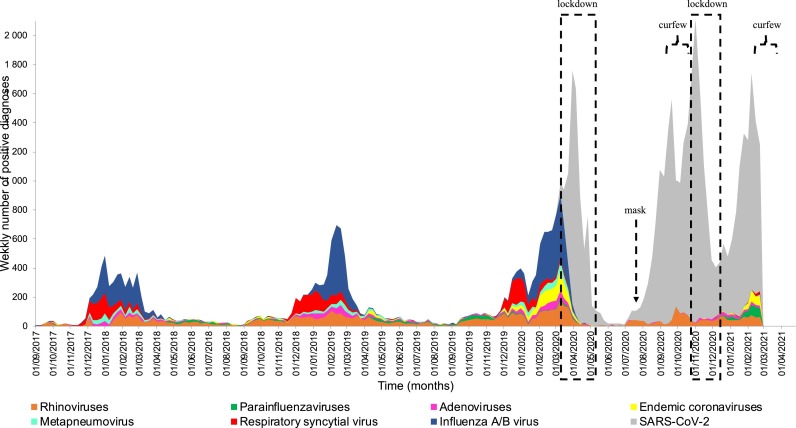
Slight yearly variations were observed from 2017 to 2019 with regards to the respective prevalence of these viruses (Fig. 1 ). In 2017, the influenza A virus was the most frequently identified respiratory viral agent (12.1%), followed by rhinovirus (9.3%) and RSV (7.2%). In the same year, 1.7% of samples tested for influenza B virus were positive for this agent. In 2018, the rhinovirus was the most commonly diagnosed (12.6%), compared to 6.9% for RSV, 6.3% for influenza A virus and 5.2% for influenza B virus. 2019 was comparable to 2017 in terms of the ranking of respiratory viruses, although the proportions of respiratory viruses’ diagnoses were higher in 2019. The intensity of the epidemic peak for each of these respiratory viruses therefore changed over the years, as did the date upon which they appeared (Fig. 1).
Fig. 1.
– Respiratory virus infections diagnosed at Hospital University Institute Méditerranée Infection in 2017-2021. Actions taken by the government are indicated by a dotted square for lockdowns, an arrow for the obligation to wear a mask in enclosed spaces, and a brace symbol for curfews.
Since February 2020, 510,671 samples have been analysed for SARS-CoV-2 and 34,959 tested positive (6.8%). Of 420,120 samples tested for SARS-CoV-2 in 2020, 6.4% (N = 26,723) were positive while in 2021, out of 90,551 samples, 9.1% were positive. The government introduced several restrictive measures in an attempt to mitigate the spread of SARS-CoV-2 and to control the epidemic as effectively as possible. A first lockdown was imposed between 17 March 2020 and 11 May 2020, recommendations have been in place on wearing masks in enclosed spaces (particularly in the workplace) since 20 July 2020, a curfew was introduced between 8:00 pm and 6:00 am between 17 October 2020 and 28 October 2020, a second lockdown took place between 29 October 2020 and 15 December 2020, and a new curfew was introduced on 16 January 2021 from 6:00 pm to 6:00 am. In addition to these actions, individual preventive measures have also been recommended, including hand washing with soap or alcohol-based hand gel, a distance of 1.5 metres between individuals and the promotion of remote working. In 2020, the proportion of positive tests dramatically decreased to 7.7% for rhinoviruses, 5.2% for influenza A virus, 4.5% for influenza B virus and 1.5% for RSV. This was also the case for the first two months of 2021, where no cases of influenza A or B were observed. In the first two months of 2021, the most frequently diagnosed virus was SARS-CoV-2 (9.1%), followed by rhinoviruses (8.5%), parainfluenza viruses (6.1%, mainly HPIV3: 5.7%) and endemic coronaviruses (5.2%, mainly HCoV NL63: 3.2%). The same results regarding influenza A and B viruses were observed from a private clinical microbiology and virology laboratory through the PACASurvE network (Table 2 ).
Table 2.
– Results for diagnoses of influenza A virus and influenza B virus by year for a private clinical microbiology and virology laboratory through the PACASurvE (for Provence Alpes-Côte d'Azur Surveillance Epidemiological System) network.
| Year | Number of samples tested | Influenza A virus diagnoses | Influenza B virus diagnoses | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| 2017 | 547 | 67 | 12.2 | 2 | 0.4 |
| 2018 | 1,111 | 63 | 5.7 | 29 | 2.6 |
| 2019 | 2,410 | 681 | 28.3 | 32 | 1.3 |
| 2020 | 2,625 | 500 | 19.0 | 357 | 13.6 |
| 2021 | 106 | 0 | 0.0 | 1 | 0.9 |
3.2. Comparison of winter seasons for respiratory viral infections
In order to avoid the Simpson effect, which is the presence of second order interactions between all factors that inverse statistical relations when data are pooled [24], we compared results during cold seasons (from October to mid-February). Over the last four such seasons, the most significant variations were observed for influenza A virus, with a positivity rate of 11.3% of the 9,819 tested samples (N= 1,106 cases) during the 2017–2018 winter season, which increased to 18.6% of the 10,973 tested samples (N= 2,042 cases) during the 2018–2019 season, dropped to 9.6% of the 11,711 tested samples (N= 1,125 cases) in 2019–2020 and accounted for 0% of the 8,786 tested samples in 2020–2021 (Fig. 1, Table 3 ). As of 24 February 2021, no cases of influenza A virus had been diagnosed during the 2020–2021 winter season. Influenza B virus was also absent for the 2020–2021 winter season, although this had already been observed in 2018–2019. RSV also showed a considerable decrease in the proportion of positive cases, reaching 0.6% (56 cases in 2020–2021 compared to between 9.4-10.9% (N= 988-1,196 cases) in the other three cold periods (p-value <0.001). Metapneumovirus and enterovirus had a less marked decrease (N= 21 and 9 in 2020–2021 vs N= 339 and 375 in 2019–2020, respectively; p-value < 0.001). The adenovirus positivity rate has remained relatively constant over time, at about 3% (p-value > 0.05), as was the case for endemic coronaviruses in 2017–2018 and 2018–2019. A significant decrease was nevertheless observed in 2020–2021 (p-value < 0.001). Rhinovirus exhibited a significantly higher positivity rate in 2020–2021 (12.9%) compared to 2017–2019 and 2018–2019 (9.9% and 10.9% respectively, p-value < 0.001). The positivity rate of the HPIV3 parainfluenza virus increased from 0.1% (N = 4) in 2019–2020 to 3.7% (N = 324) in 2020–2021 (p-value < 0.001).
Table 3.
- Tests performed and positive for PCR detection of respiratory viruses, during the same cold months in 2017–2018, 2018–2019, 2019–2020 and 2020–2021
| Viruses |
Tests |
Positive |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017-2018 |
2018-2019 |
2019-2020 |
2020-2021 |
2017-2018 |
2018-2019 |
2019-2020 |
2020-2021 |
|||||||||
| N | N | N | N | N | % | p value | N | % | p value | N | % | p value | N | % | ||
| Adenovirus | 8,876 | 10,831 | 11,687 | 8,786 | 262 | 3.0 | 0.51 | 416 | 3.8 | 0.006 | 355 | 3.0 | 0.74 | 274 | 3.1 | |
| Common coronaviruses | 387 | 1,123 | 11,556 | 8,786 | 13 | 3.4 | 0.97 | 37 | 3.3 | 0.96 | 617 | 5.3 | <0.001 | 292 | 3.3 | |
| HCoV 229E | 387 | 1,123 | 6,357 | 8,786 | 0 | 0.0 | 1 | 0 | 0.0 | 1 | 31 | 0.5 | <0.001 | 5 | 0.1 | |
| HCoV HKU1 | 387 | 1,123 | 6,357 | 8,786 | 0 | 0.0 | 1 | 0 | 0.0 | 1 | 236 | 3.7 | <0.001 | 1 | 0.0 | |
| HCoV NL63 | 387 | 1,123 | 6,357 | 8,786 | 0 | 0.0 | 0.006 | 0 | 0.0 | <0.001 | 114 | 1.8 | 0.49 | 171 | 1.9 | |
| HCoV OC43 | 657 | 1,123 | 6,357 | 8,786 | 0 | 0.0 | 0.003 | 0 | 0.0 | <0.001 | 56 | 0.9 | 0.02 | 114 | 1.3 | |
| Enterovirus | 900 | 10,83 | 11,688 | 8,786 | 13 | 1.4 | <0.001 | 153 | 1.4 | <0.001 | 375 | 3.2 | <0.001 | 9 | 0.1 | |
| Influenza virus | 9,819 | 10,973 | 11,711 | 8,786 | 1,839 | 18.7 | <0.001 | 2,044 | 18.6 | <0.001 | 1,85 | 15.8 | <0.001 | 1 | 0.0 | |
| Influenza A virus | 9,819 | 10,973 | 11,711 | 8,786 | 1,106 | 11.3 | <0.001 | 2,042 | 18.6 | <0.001 | 1,125 | 9.6 | <0.001 | 0 | 0.0 | |
| Influenza B virus | 9,819 | 10,973 | 11,711 | 8,786 | 743 | 7.6 | <0.001 | 2 | 0.0 | 1 | 727 | 6.2 | <0.001 | 1 | 0.0 | |
| Metapneumovirus | 8,873 | 10,83 | 11,687 | 8,786 | 315 | 3.6 | <0.001 | 258 | 2.4 | <0.001 | 339 | 2.9 | <0.001 | 21 | 0.2 | |
| Human parainfluenza virus | 657 | 1,127 | 11,605 | 8,786 | 4 | 0.6 | <0.001 | 42 | 3.7 | 0.68 | 226 | 1.9 | <0.001 | 350 | 4.0 | |
| HPIV1 | 387 | 1,127 | 6,001 | 8,786 | 0 | 0.0 | 1 | 0 | 0.0 | 1 | 2 | 0.0 | 0.16 | 0 | 0.0 | |
| HPIV2 | 387 | 1,127 | 6,001 | 8,786 | 0 | 0.0 | 1 | 0 | 0.0 | 1 | 4 | 0.1 | 0.17 | 1 | 0.0 | |
| HPIV3 | 387 | 1,127 | 6,001 | 8,786 | 0 | 0.0 | <0.001 | 0 | 0.0 | <0.001 | 4 | 0.1 | <0.001 | 324 | 3.7 | |
| HPIV4 | 387 | 1,127 | 6,001 | 8,786 | 0 | 0.0 | 0.62 | 0 | 0.0 | 0.1 | 14 | 0.2 | 0.73 | 23 | 0.3 | |
| Rhinovirus | 5,15 | 10,833 | 11,683 | 8,786 | 511 | 9.9 | <0.001 | 1,194 | 11.0 | <0.001 | 1,42 | 12.2 | 0.11 | 1,134 | 12.9 | |
| Respiratory syncytial virus | 9,912 | 10,973 | 11,707 | 8,786 | 988 | 10.0 | <0.001 | 1,196 | 10.9 | <0.001 | 1,104 | 9.4 | <0.001 | 56 | 0.6 | |
| SARS-CoV-2 | 0 | 0 | 5,628 | 244,310 | 0 | 0.0 | - | 0 | 0.0 | - | 0 | 0.0 | - | 20748 | 8.5 | |
HCoV, human coronavirus; HPIV, human parainfluenza virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
3.3. Total gastrointestinal viral infections at IHU-MI in 2017–2021
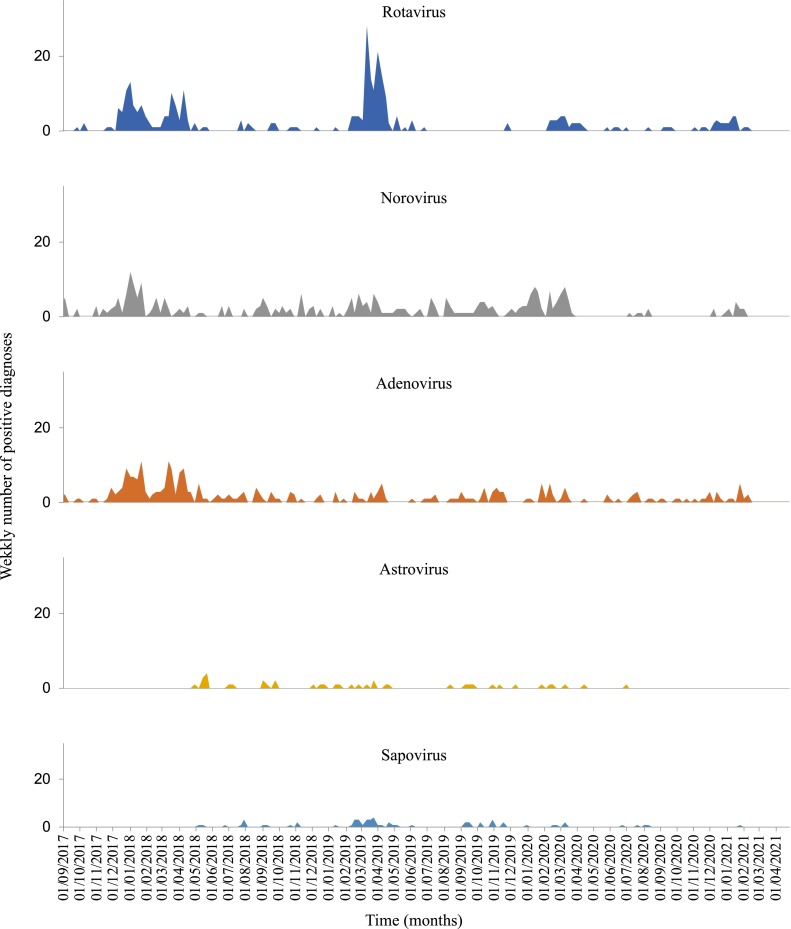
Between 2017 and 2021, 27,719 tests were performed resulting in approximately 1,098 diagnoses of gastrointestinal infections (Table 4 ). Rotavirus (5.6% for 6,612 samples analysed) was the most frequently diagnosed gastrointestinal virus over the study period, followed by adenovirus (5.2% for 6,227 samples analysed) and norovirus (4.2% for 7,791 samples analysed). As was previously observed for respiratory viruses, the intensity of the epidemic peak as well as the date of its onset varied over the years (Fig. 2 ). In 2017, 2018 and 2020, adenovirus was the most frequently identified virus (5.0%, 7.6% and 4.1% respectively) while in 2019, rotavirus (8.6%) was the virus most commonly identified.
Table 4.
- Tests performed and positive for PCR detection of gastrointestinal viruses in 2017, 2018, 2019, 2020 and 2021 at Hospital University Institute Méditerranée Infection.
| Viruses | Tests | Positive | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | Total 2017-2021 | 2017 | 2018 | 2019 | 2020 | 2021 | Total 2017-2021 | ||||||||
| N | N | N | N | N | N | N | % | N | % | N | % | N | % | N | % | N | % | ||
| Adenovirus | 1,674 | 1,662 | 1,47 | 1,166 | 255 | 6,227 | 83 | 5.0 | 127 | 7.6 | 56 | 3.8 | 48 | 4.1 | 10 | 3.9 | 324 | 5.2 | |
| Astrovirus | 0 | 886 | 1,458 | 1,146 | 255 | 3,745 | 0 | 0.0 | 18 | 2.0 | 16 | 1.1 | 6 | 0.5 | 0 | 0.0 | 40 | 1.1 | |
| Norovirus | 1,386 | 1,666 | 2,368 | 2,116 | 255 | 7,791 | 60 | 4.3 | 91 | 5.5 | 101 | 4.3 | 63 | 3.0 | 10 | 3.9 | 325 | 4.2 | |
| Rotavirus | 1,64 | 1,662 | 1,471 | 1,184 | 255 | 6,212 | 78 | 4.8 | 88 | 5.3 | 127 | 8.6 | 45 | 3.8 | 12 | 4.7 | 350 | 5.6 | |
| Sapovirus | 0 | 886 | 1,46 | 1,143 | 255 | 3,744 | 0 | 0.0 | 12 | 1.4 | 38 | 2.6 | 8 | 0.7 | 1 | 0.4 | 59 | 1.6 | |
Fig. 2.
– Gastrointestinal virus diagnosis between October 2017 and February 2021at Hospital University Institute Méditerranée Infection.Comparison of winter seasons for gastrointestinal viral infections
In the first two months of 2021, of the 255 samples analysed, rotavirus was again the most frequently identified virus (N = 12, 4.7%) followed by norovirus (N = 10, 3.9%) and adenovirus (N = 10, 3.9%).
The overall positivity rate of gastrointestinal infections decreased significantly over time during the winter seasons (Table 5 ). Notably, the norovirus winter season positivity rates decreased continuously and significantly over time (2017–2018: 9.3%; 2018–2019: 8.4%; 2019–2020: 5.5%; 2020–2021: 2.0%). In contrast, adenovirus and rotavirus showed stable positivity rates between 2018–2019 (3.8% and 2.4% respectively) and 2020–2021 winter seasons (3.4% and 3.6% respectively) (Fig. 2).
Table 5.
- Tests performed and positive for PCR detection of gastrointestinal viruses, during the same cold months in 2017–2018, 2018–2019, 2019–2020 and 2020–2021.
| Viruses | Tests | Positive | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017-2018 | 2018-2019 | 2019-2020 | 2020-2021 | 2017-2018 | 2018-2019 | 2019-2020 | 2020-2021 | |||||||||
| N | N | N | N | N | % | p value | N | % | p value | N | % | p value | N | % | ||
| Adenovirus | 661 | 369 | 380 | 642 | 61 | 9.2 | <0.001 | 14 | 3.8 | 0.8 | 31 | 8.2 | 0.001 | 22 | 3.4 | |
| Astrovirus | 0 | 369 | 368 | 619 | 0 | 0.0 | - | 6 | 1.6 | 0.003 | 5 | 1.4 | 0.01 | 0 | 0.0 | |
| Norovirus | 636 | 369 | 1026 | 664 | 59 | 9.3 | <0.001 | 31 | 8.4 | <0.001 | 56 | 5.5 | <0.001 | 13 | 2.0 | |
| Rotavirus | 661 | 369 | 380 | 661 | 64 | 9.7 | <0.001 | 9 | 2.4 | 0.3 | 5 | 1.3 | 0.03 | 24 | 3.6 | |
| Sapovirus | 0 | 369 | 369 | 619 | 0 | 0.0 | - | 5 | 1.4 | 0.03 | 8 | 2.2 | 0.002 | 1 | 0.2 | |
4. DISCUSSION
In this study, the systematic monitoring of our microbiology and virology laboratory activity has enabled us to identify changes in the epidemiology of respiratory and gastro-intestinal viral communicable diseases during the spread of a new emerging virus, SARS-CoV-2.
These data show that the epidemiology of infection with SARS-CoV-2 is not at all similar to that of other respiratory infections. As observed in other countries and in France, flu viruses have decreased dramatically [25], [26], [27]. It should be noted that the number of infections by endemic coronaviruses and rhinoviruses does not seem to be particularly affected by the preventive measures taken and may have, in common with COVID-19, modes of transmission that are different from those of influenza viruses, RSV and the other respiratory viruses studied. Curiously, in our region, a higher number of parainfluenza virus 3 (HPIV3) were observed. One of the explanations for these epidemiological figures could be that the viruses experiencing a decrease in their incidence are most often involved in pneumonia, while, conversely, the agents responsible for nasal infections and for causing colds, such as endemic coronaviruses or HPIV3, remain constant. Measures to control COVID-19 would then prevent pneumonia, and gastroenteritis. From this hypothesis, it would be interesting to study the nasal and pneumonic forms in COVID-19 patients and assess their evolution in time.
The impact of measures to control COVID-19 probably played a major role in these epidemiological changes [28]. These measures included both repeated recommendations on risk prevention measures such as hand washing with soap or alcohol-based hand gel, disinfecting surfaces, and social distancing, but also actions which were legally enforced, including wearing masks and the implementation of lockdown or curfews [9,29]. Hand washing and disinfection was probably the main factor having an impact upon the usual respiratory and gastrointestinal viral infections [30], and have been key elements of influenza prevention campaigns for several years [11]. It is not clear from the literature that lockdown measures and other social control measures have really had an impact on the spread of SARS-CoV-2 or on other respiratory infections [31]. For example, Sweden has issued very few social control measures while other countries such as France have implemented relatively strong measures without significant differences in the number of cases or mortality [32].
The lack of effectiveness of these measures on the COVID-19 epidemic raises several questions. The first is the existence of infection outbreaks in animals which are distinct from outbreaks in humans. It has been demonstrated that the emergence of new variants could be promoted by the intensive captive breeding of certain animals such as mink, which are likely to contaminate humans by being potentially more contagious or more pathogenic for humans [34], [35], [36]. Furthermore, it seems likely that a certain number of treatments, including serotherapy with hyper-human sera and antivirals such as remdesivir, can promote the appearance of mutations [37].
Strong reductions in the incidence of some but not all respiratory viruses, and of viral agents of gastrointestinal infections have been also reported in several countries worldwide. This has been particularly noticed for influenza virus infections [28]. Tanislav and Kostev reported fewer non-SARS-CoV-2 respiratory tract infections and gastrointestinal infections during the SARS-CoV-2 pandemic [38]. They collected data from 994 general practitioners and 192 pediatricians in Germany and compared the prevalence of these infections between April 2020-March 2021 and April 2019-March 2020. Substantial falls (-71% for general practices and -90% for paediatrician practices) were observed for influenza virus infections, which was accompanied by a 40% fall of intestinal infections for general practices. Agca et al. reported, in a study on 319 nasopharyngeal samples in Turkey, a 7.5-fold reduction of the proportion of positive testing for influenza virus during March 2020-February 2021 compared to the previous year [2.3% (n= 9 cases) versus 17.3% (133), respectively] [39]. A significant reduction was also observed for other respiratory viruses including RSV but not for rhinoviruses/enteroviruses and metapneumovirus. Ippolito et al. also reported in Italy a strong decrease during the SARS-CoV-2 pandemic of the diagnoses of several seasonal respiratory viruses among hospitalized children younger than two years [40]. Indeed, the number of positive tests was 80% lower during the September 2020-February 2021 period compared to between the same periods of years 2019-2020 and 2018-2019, with a disappearance of influenza viruses and RSV as well as disappearance or strong decreases of other respiratory viruses except rhinoviruses and endemic coronaviruses. In the Southern hemisphere, Yeoh et al., reported decreases by 99% and 98% of diagnoses of influenza viruses and RSV, respectively, in children in Western Australian through winter 2020 [41]. In Singapore, influenza virus positivity rate decreased by 64% during weeks 5–9 of 2020 compared with the preceding years [42].
The present study has several limitations. We focused on the epidemiology of respiratory and gastrointestinal viruses only over the last four cold seasons in our institute. We analyzed here a limited number of seasons but notwithstanding these data further support the unpredictability of the epidemiology of these viruses. We observed considerable variations from season to season throughout the respiratory virus epidemic period regarding the predominant viruses, the time of emergence and duration of winter epidemics, the level of incidence reached at the epidemic peaks, and the time at which this peak occurred [15]. Also, we acknowledge that estimating hospitalization pattern in the typical pre-SARS-CoV-2 season may be the subject of large random variation. Here, we have not analyzed the impact of respiratory viruses on hospital admissions, but their impact on hospital mortality has been the subject of previous studies [15,33]. Finally, the present work has been conducted in a single institution, and the results could therefore display local specificities.
In conclusion, this study confirms that it is futile to try to make predictions about a disease for which the level of knowledge is limited [43]. The course of the epidemic over the past year was unpredictable and could not be integrated into any predictive models. Caution should be taken when using such models. Furthermore, this leads to the search for different modes of transmission of most respiratory diseases, as had already been mentioned in relation to SARS-CoV, where infections were retrospectively detected at a significant distance from the heart of the SARS-CoV outbreak, with no reasonable explanation [44]. Broad epidemiological surveillance of respiratory and gastrointestinal infections should be pursued in the future, as many changes occur during this pandemic among which public health policies and population behaviours including mask wearing or social distancing [31]. Also, in France, the issue of carriage and transmission by domestic pets has not been resolved and should be the subject of intense research to really understand the reservoirs, transmission and epidemiology of this very atypical virus. A new study on the 2021-2022 winter season should be carried out to better understand the epidemiology of these respiratory and gastrointestinal viruses and the impact of barrier measures on the spread of new SARS-CoV-2 variants with different transmissibiliy [45].
Author contributions
Conceived and designed the study: DR.; Collected data or/and performed experiments: AGG, LK, CB, JPC.; Analysed and interpreted data: AGG, CD, PC, PG, HC and DR.; Wrote the manuscript: AGG, PC, PG and DR.; All authors read and approved the final manuscript.
Ethics
All data have been generated as part of the routine work at Assistance Publique-Hôpitaux de Marseille (Marseille university hospitals), and this study results from routine standard clinical management. The study was approved by the ethical committee of the University Hospital Institute Méditerranée Infection (N°: 2020-029 and 2022-015). Access to the patients’ biological and registry data issued from the hospital information system was approved by the data protection committee of Assistance Publique-Hôpitaux de Marseille (APHM) and was recorded in the European General Data Protection Regulation registry under number RGPD/APHM 2019-73.
Funding
This work was supported by the French Government under the “Investments for the Future” programme managed by the National Agency for Research (ANR), Méditerranée-Infection 10-IAHU-03, and was also supported by Région Provence Alpes Côte d'Azur and European funding FEDER PRIMMI (Fonds Européen de Développement Régional - Plateformes de Recherche et d'Innovation Mutualisées Méditerranée Infection), FEDER PA 0000320 PRIMMI.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
References
- 1.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemey P., Ruktanonchai N., Hong S.L., et al. Untangling introductions and persistence in COVID-19 resurgence in Europe. Nature. 2021;595:713–717. doi: 10.1038/s41586-021-03754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colson P, Fournier PE, Chaudet H, et al. Analysis of SARS-CoV-2 variants from 24,181 patients exemplifies the role of globalization and zoonosis in pandemics. Front. Microbiol. 2022;7 doi: 10.3389/fmicb.2021.786233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021 Jul;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Lai S, Gao GF, Shi W. The emergence, genomic diversity and global spread of SARS-CoV-2. Nature. 2021;600:408–418. doi: 10.1038/s41586-021-04188-6. [DOI] [PubMed] [Google Scholar]
- 7.Davis C, Logan N, Tyson G, et al. Reduced neutralisation of the Delta (B.1.617.2) SARS-CoV-2 variant of concern following vaccination. PLoS Pathog. 2021;2 doi: 10.1371/journal.ppat.1010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dao TL, Hoang VT, Colson P, et al. SARS-CoV-2 infectivity and severity of COVID-19 according to SARS-CoV-2 variants: Current evidence. J. Clin. Med. 2021;10:2635. doi: 10.3390/jcm10122635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Information coronavirus . February 25, 2021. Les bons gestes à adopter, affiche – mesures barrières.https://www.gouvernement.fr/sites/default/files/coronavirus-mesures_barrieres.pdf Retrievedfrom. [Google Scholar]
- 10.Canini L., Andréoletti L., Ferrari P, et al. Surgical mask to prevent influenza transmission in households: a cluster randomized trial. PloS ONE. 2010;5:e13998. doi: 10.1371/journal.pone.0013998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santé Publique France . Sept 2017. Prévenir la grippe saisonnière.https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/documents/depliant-flyer/prevenir-la-grippe-saisonniere-septembre-2017 Retrieved February 25, 2021, from. [Google Scholar]
- 12.Decree No. 2020-260 of 16 March 2020 introducing regulations on movement in the context of the fight against the spread of COVID-19, https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000041728476.
- 13.Decree No. 2020-1310 of 29 October 2020 prescribing general measures required to combat the COVID-19 epidemic in the context of the health emergency situation. https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000042475143?r=9BtQcTAF3G.
- 14.Kaba L., Giraud-Gatineau A., Jimeno MT., et al. Consequences of the COVID-19 Outbreak Lockdown on Non-Viral Infectious Agents as Reported by a Laboratory-Based Surveillance System at the IHU Méditerranée Infection, Marseille, France. J. Clin. Med. 2021;10:3210. doi: 10.3390/jcm10153210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colson P., Giraud-Gatineau A., Fournier P.E., et al. IHU Preprint; 2021. Epidemiological surveillance of respiratory viral infections at IHU Méditerranée Infection and its application to SARS-CoV-2. Preprint. [DOI] [Google Scholar]
- 16.Abat C., Chaudet H., Colson P., et al. Real-time microbiology laboratory surveillance system to detect abnormal events and emerging infections, Marseille, France. Emerg. Infect. Dis. 2015;21:1302–1310. doi: 10.3201/eid2108.141419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colson P., Rolain J.M., Abat C., et al. EPIMIC: A Simple homemade computer program for real-time EPIdemiological surveillance and alert based on MICrobiological Data. PloS ONE. 2015;10 doi: 10.1371/journal.pone.0144178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D. Raoult. Rapport de mission. http://ifr48 timone univ-mrs fr/files/Documents-Raoult/bioterrorisme2003 pdf 2003Available from: URL: http://ifr48.timone.univ-mrs.fr/files/Documents-Raoult/bioterrorisme2003.pdf.
- 19.Salmon M., Schumacher D., Höhle M. Monitoring Count Time Series in R: Aberration Detection in Public Health Surveillance. Journal of Statistical Software. 2016;70:1–3. doi: 10.18637/jss.v070.i10. [DOI] [Google Scholar]
- 20.Amrane S., Tissot-Dupont H., Doudier B., et al. Rapid viral diagnosis and ambulatory management of suspected COVID-19 cases presenting at the infectious diseases referral hospital in Marseille, France, January 31st to March 1st, 2020: A respiratory virus snapshot. Travel Med. Infect. Dis. 2020;36:10163. doi: 10.1016/j.tmaid.2020.101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boschi C., Hoang V.T., Giraud-Gatineau A., et al. Coinfections with SARS-CoV-2 and other respiratory viruses in Southeastern France: A matter of sampling time. J. Med. Virol. 2021;93:1878–1881. doi: 10.1002/jmv.26692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huart M., Bedubourg G., Abat C., Colson P., et al. Implementation and initial analysis of a laboratory-based weekly biosurveillance system, Provence-Alpes-Côte d’Azur, France. Emerg. Infect. Dis. 2017;23:582–589. doi: 10.3201/eid2304.161399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/2019.
- 24.Chu K.H., Brown N.J., Pelecanos A., Brown A.F. Simpson's paradox: A statistician's case study. Emerg. Med. Australas. 2018;30:431–433. doi: 10.1111/1742-6723.12943. [DOI] [PubMed] [Google Scholar]
- 25.February 25, 2021. Bulletin épidémiologique grippe, semaine 7. Saison 2020-2021.https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/grippe/documents/bulletin-national/bulletin-epidemiologique-grippe-semaine-7.-saison-2020-2021 Retrievedfrom. [Google Scholar]
- 26.Jones N. How COVID-19 is changing the cold and flu season. Nature. 2020;588:388–390. doi: 10.1038/d41586-020-03519-3. [DOI] [PubMed] [Google Scholar]
- 27.Olsen S.J., Azziz-Baumgartner E., Budd A.P., et al. Decreased influenza activity during the COVID-19 pandemic - United States, Australia, Chile, and South Africa, 2020. Morb. Mortal. Wkly Rep. 2020;69:1305–1309. doi: 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Servick K. COVID-19 measures also suppress flu-for now. Science. 2020;371:224. doi: 10.1126/science.371.6526.224. [DOI] [PubMed] [Google Scholar]
- 29.Decree No. 2020-860 of 10 July 2020 prescribing general measures required to fight against the COVID-19 epidemic in regions where the health emergency has ended and those in which it has been prolonged. https://www.legifrance.gouv.fr/loda/id/JORFTEXT000042105897/2020-07-11#JORFTEXT000042105897.
- 30.Zuin M., Rigatelli G., Zuliani G., Roncon L. COVID-19 restrictive measures are changing the flu season in Italy. Minerva Med. 2021;10 doi: 10.23736/S0026-4806.21.07359-6. 2021 Feb 8 Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Bendavid E., Oh C., Bhattacharya J., Ioannidis J. Assessing mandatory stay-at-home and business closure effects on the spread of COVID-19. Eur. J. Clin. Invest. 2021;51(4):e13484. doi: 10.1111/eci.13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Larochelambert Q., Marc A., Antero J., et al. Covid-19 mortality: A matter of vulnerability among nations facing lmited margins of adaptation. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.604339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giraud-Gatineau A., Colson P., Jimeno M.T., et al. Comparison of mortality associated with respiratory viral infections between December 2019 and March 2020 with that of the previous year in Southeastern France. Int. J. Infect. Dis. 2020;96:154–156. doi: 10.1016/j.ijid.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassanin A., Grandcolas P., Veron G. Covid-19: natural or anthropic origin ? Mammalia. 2021;85:1–7. doi: 10.1515/mammalia-2020-0044. [DOI] [Google Scholar]
- 35.Patterson E.I., Elia G., Grassi A., et al. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nat. Comm. 2020;11:6231. doi: 10.1038/s41467-020-20097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenollar F., Mediannikov O., Maurin M., et al. Mink, SARS-CoV-2, and the human-animal interface. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.663815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colson P., Devaux C.A., Lagier J.C., Gautret P., Raoult D. A possible role of remdesivir and plasma therapy in the selective sweep and emergence of new SARS-CoV-2 variants. J. Clin. Med. 2021;10:3276. doi: 10.3390/jcm10153276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanislav C., Kostev K. Fewer non-COVID-19 respiratory tract infections and gastrointestinal infections during the COVID-19 pandemic. J. Med.Virol. 2022;94:298–302. doi: 10.1002/jmv.27321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agca H., Akalin H., Saglik I., Hacimustafaoglu M., Celebi S., Ener B. Changing epidemiology of influenza and other respiratory viruses in the first year of COVID-19 pandemic. J. Infect. Public Health. 2021;14:1186–1190. doi: 10.1016/j.jiph.2021.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Ippolito G., La Vecchia A., Umbrello G., et al. Disappearance of seasonal respiratory viruses in children under two years old during COVID-19 pandemic: A monocentric retrospective study in Milan, Italy. Front. Pediatr. 2021;9 doi: 10.3389/fped.2021.721005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeoh D.K., Foley D.A., Minney-Smith C.A., et al. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin. Infect. Dis. 2021;72:2199–2202. doi: 10.1093/cid/ciaa1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soo R.J.J., Chiew C.J., Ma S., Pung R., Lee V. Decreased influenza incidence under COVID-19 control measures, Singapore. Emerg. Infect. Dis. 2020;26:1933–1935. doi: 10.1016/j.tmaid.2021.102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jewell N.P., Lewnard J.A., Jewell B.L. Predictive mathematical models of the COVID-19 pandemic: Underlying principles and value of projections. JAMA. 2020;323:1893–1894. doi: 10.1001/jama.2020.6585. [DOI] [PubMed] [Google Scholar]
- 44.Christian M.D., Poutanen S.M., Loutfy M.R., et al. Severe acute respiratory syndrome. Clin. Infect. Dis. 2004;38:1420–1427. doi: 10.1086/420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Del Rio C., Omer S.B., Malani P.N. Winter of Omicron - The evolving COVID-19 pandemic. JAMA. 2021;327:319–320. doi: 10.1001/jama.2021.24315. [DOI] [PubMed] [Google Scholar]