Abstract
Background
To study whether the association between the CD4/CD8 ratio variation over time and the development of clinical outcomes vary in late presenters (CD4 count < 350/µL or AIDS event at enrolment) or advanced presenters (CD4 count < 200/µL or AIDS event at enrolment).
Methods
We included ART-naïve adults from the Cohort of the Spanish HIV/AIDS Research Network (CoRIS) enrolled between January 2004 up to November 2018 and with at least 6 months of follow-up. We used extended Cox proportional hazard models to estimate the hazard ratios (HRs) for the association between CD4/CD8 ratio over time and a composite endpoint of the occurrence of the first AIDS event, first serious non-AIDS event or overall mortality occurring from 6 months after enrolment. HRs in non-late, late and advanced presenters were obtained by including an interaction term between late presentation status and CD4/CD8 ratio over time.
Results
Of 10,018 participants, 55.6% were late presenters and 26.5% were advanced presenters. Compared with CD4/CD8 ratio > 0.4, CD4/CD8 ratio ≤ 0.4 over time was associated with an increased risk of experiencing the composite endpoint in non-late (HR 1.90; 95%CI 1.48, 2.43), late (HR 1.94; 1.46, 2.57) and advanced presenters (HR 1.72; 1.26, 2.34). Similarly, CD4/CD8 ratio ≤ 0.4 over time was associated with a higher risk of developing an AIDS event (HR 3.31; 2.23, 4.93 in non-late; HR 2.75; 1.78, 4.27 in late and HR 2.25; 1.34, 3.76 in advanced presenters) or serious non-AIDS event (HR 1.39; 0.96, 2.02 in non-late, HR 1.62; 1.10, 2.40 in late and HR 1.49; 0.97, 2.29 in advanced presenters) as well as with a higher risk of overall mortality (HR 1.49; 0.92, 2.41 in non-late, HR 1.80; 1.04, 3.11 in late and HR 1.61; 0.92, 2.83 in advanced presenters) compared to CD4/CD8 > 0.4, regardless of the late presentation status.
Conclusions
A low CD4/CD8 measured over time is associated with increased risk of morbidity and mortality in people living with HIV independently of their late presentation status. These data support the prognostic role of CD4/CD8 over time and can help defining a subgroup of patients who need closer monitoring to avoid comorbidities.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-022-07352-z.
Keywords: Late presentation, CD4/CD8, Clinical outcomes, AIDS events, Serious non-AIDS events, Mortality
Background
Mortality in people living with HIV (PLWH) has decreased since the advent of highly active antiretroviral treatment (ART) [1]. Nonetheless comorbidities are increasing [2, 3] and they are particularly high in those who present late for diagnosis [4, 5].
In this scenario, efforts have been made to find prognostic markers of clinical events [6]. The CD4+ T cells/CD8+ T cells ratio (CD4/CD8 ratio) has shown an association with both AIDS and non-AIDS events in virologically suppressed individuals [7]. Particularly, a CD4/CD8 ratio ≤ 0.4 has been found to be associated with T cell activation, innate immune activation and an immunosenescent phenotype, and to predict serious non-AIDS events [8, 9], even in virologically suppressed persons with absolute CD4 count > 500/µL.
Although mortality and morbidity are especially high in late presenters [4, 10] the potential prognostic role of the CD4/CD8 ratio has not been studied yet in this population. Our aim is to evaluate whether the variation in CD4/CD8 ratio over time is associated with the development of AIDS and non-AIDS events and mortality in participants from the cohort of the Spanish HIV/AIDS Research Network (CoRIS) and to study whether these associations vary with late presentation and advanced disease.
Methods
Study design
CoRIS is an open, prospective multicentre cohort of persons with confirmed HIV infection, naïve to ART at study entry, recruited in 46 centres from 13 Autonomous Regions in Spain from 2004-onwards, as described in detail in [11]. CoRIS Study Groups are listed in Additional file 1.
Study population
We included CoRIS participants recruited from January 2004 to November 2018, age ≥ 18 years, with available information on late presentation at enrolment, at least 6 months of follow up, at least one determination of CD4 count and CD8 count from the 6th month after enrolment, and information on the clinical outcomes of the composite endpoint. Individuals who were monitored in the three centres not providing data on non-AIDS events were excluded.
Definitions of late presentation
Late presenters were participants with CD4 count below 350/µL and/or with an AIDS event occurred before the 24th week after enrolment regardless of the CD4 cell count, both conditions met before ART initiation. Advanced presenters were participants with CD4 count below 200/µL and/or with an AIDS event occurred before the 24th week after enrolment regardless of the CD4 cell count, both conditions met before ART initiation [12]. By definition, all advanced presenters were also late presenters. We considered as non-late presenters participants with CD4 count greater than 350/µL and no AIDS events.
Health outcomes
The primary endpoint was clinical progression, defined as a composite endpoint of the occurrence of the first AIDS or serious non-AIDS event or death from any cause occurred from 6 months after enrolment. As serious non-AIDS event we considered any of the following conditions: cardiovascular events (myocardial infarction, angina, heart disease, transient ischemic attack, reversible ischemic deficit, stroke and peripheral arteriopathy or death from cardiovascular disease), renal events (end-stage renal disease, initiation of dialysis or renal transplantation, or death from renal disease), liver events (ascites, digestive haemorrhage due to oesophageal varices, hepatic encephalopathy, liver transplantation, or death from liver disease), non-AIDS malignancy or death from non-AIDS malignancy, and infectious-related deaths.
Statistical methods
We classified the participants as having low (≤ 0.4) or high (> 0.4) CD4/CD8 ratio: the cut-off at 0.4 correspond to the first quartile of the overtime distribution of CD4/CD8 ratio and was chosen based on its association with serious non-AIDS events [8]. Since both the CD4 and the CD8 count and their ratio vary over time and its variation may affect the risk of developing a clinical outcome, we modelled the CD4/CD8 ratio as a time-varying covariate. We estimated the hazard ratios (HRs) and 95% confidence intervals (CI) for the association with the clinical outcomes with extended Cox proportional hazard models [13].
Briefly, the follow-up period for each person was broken up into subintervals, corresponding to the CD4 and CD8 measurements. The time-varying covariates values and the endpoint were updated at each subinterval up to the occurrence of the first event or the censoring time. We performed two sets of analysis: one set including non-late and late presenters and another one including non-late and advanced presenters. To evaluate whether the association between the CD4/CD8 ratio and the clinical outcomes varied with the late presentation status, we included an interaction term between late presentation and over time CD4/CD8 ratio in the multivariable Cox models, which allowed us to obtain HRs for the association of interest in non-late presenters, late presenters and advanced presenters.
Multivariable models were adjusted for age (< 50, ≥ 50 years) and CD4 count (< 500, ≥ 500/µL), that were both modelled as time-varying covariates, a combined variable of gender and HIV transmission category (men who have sex with men, injection drug use, heterosexual women, heterosexual men, other/unknown), educational level (no education or primary education alone, secondary education, university, other/unknown), region of origin (Europe, Sub-Saharan Africa, Latin America, other/unknown) and presence of hepatitis C virus antibodies (no, yes or unknown), presence of hepatitis B surface antigen (no, yes or unknown) and viral load (< 10,000, 10,000–100,000, ≥ 100,000 copies/mL, unknown) at enrolment.
All statistical analyses were performed using R version 4.0 [14] and extended Cox regression models were estimated with the survival package [13, 15].
Sensitivity analyses
To account for the role of viral suppression (viral load < 50 copies mL) in the association between the CD4/CD8 ratio and the clinical outcomes, we included the viral load as a time-varying variable (< 50, ≥ 50 copies/mL). Further, in order to evaluate whether early ART initiation modify the associations under study, we repeated the analyses after stratification for timing of ART initiation (< 1 year vs ≥ 1 year after the enrolment).
Results
During the study period, 10,018 participants met the inclusion criteria. Out of them, 5574 (55.6%) were non-late presenters, 4444 (44.4%) were late presenters and 2659 (26.5%) were advanced presenters (Table 1).
Table 1.
Characteristics at enrolment of non-late presenters, late presenters and advanced presenters
| All participants, n = 10,018 | Non-late presenters, n = 5574, 55.6% | Late presenters, n = 4444, 44.4% |
Advanced presenters, n = 2659, 26.5% |
|
|---|---|---|---|---|
| Age (years) | ||||
| Median (1st, 3rd quartile) | 35.5 (29.1 43.1) | 33. 4 (27.7 40.4) | 38.6 (31.4 46.1) | 40.2 (33.4 47.6) |
| < 30 | 2469 (24.6%) | 1696 (30.4%) | 773 (17.4%) | 327 (12.3%) |
| 30–49 | 6290 (62.8%) | 3422 (61.4%) | 2868 (64.5%) | 1742 (65.5%) |
| ≥ 50 | 1259 (12.6%) | 456 (8.2%) | 803 (18.1%) | 590 (22.2%) |
| Transmission group (n (%)) | ||||
| MSM | 6071 (60.6%) | 3910 (70.1%) | 2161 (48.6%) | 1120 (42.1%) |
| IDU | 707 (7.1%) | 280 (5.0%) | 427 (9.6%) | 295 (11.1%) |
| Heterosexual women | 1334 (13.3%) | 616 (11.1%) | 718 (16.2%) | 451 (17.0%) |
| Heterosexual men | 1560 (15.6%) | 633 (11.4%) | 927 (20.9%) | 645 (24.3%) |
| Other/unknown | 346 (3.5%) | 135 (2.4%) | 211 (4.7%) | 148 (5.6%) |
| Educational level (n (%)) | ||||
| Primary education or less | 1390 (13.9%) | 574 (10.3%) | 816 (18.4%) | 546 (20.5%) |
| Secondary education | 4598 (45.9%) | 2648 (47.5%) | 1950 (43.9%) | 1185 (44.6%) |
| University | 2395 (23.9%) | 1548 (27.8%) | 847 (19.1%) | 421 (15.8%) |
| Other/unknown | 1635 (16.3%) | 804 (14.4%) | 831 (18.7%) | 507 (19.1%) |
| Region of origin (n (%)) | ||||
| Europe | 7498 (74.8%) | 4307 (77.3%) | 3191 (71.8%) | 1918 (72.1%) |
| Sub-Saharan Africa | 439 (4.4%) | 169 (3.0%) | 270 (6.1%) | 173 (6.5%) |
| Latin America | 1875 (18.7%) | 992 (17.8%) | 883 (19.9%) | 495 (18.6%) |
| Other/unknown | 206 (2.1%) | 106 (1.9%) | 100 (2.3%) | 73 (2.7%) |
| CD4 count, cells/µL | ||||
| Median (1st; 3rd quartile) | 400 (215 600) | 566 (450 739) | 189 (75 275) | 93 (38 162) |
| < 200 | 2293 (22.9%) | 0 (0.0%) | 2293 (51.6%) | 2293 (86.2%) |
| 200–350 | 1943 (19.4%) | 0 (0.0%) | 1943 (43.7%) | 158 (5.9%) |
| 350–500 | 2061 (20.6%) | 2007 (36.0%) | 54 (1.2%) | 54 (2.0%) |
| ≥ 500 | 3614 (36.1%) | 3567 (64.0%) | 47 (1.1%) | 47 (1.8%) |
| Unknown | 107 (1.1%) | 0 (0.0%) | 107 (2.4%) | 107 (4.0%) |
| Nadir CD4 count, cells/µL | ||||
| Median (1st; 3rd quartile), | 351 (192 520) | 499 (407 644) | 179 (72 263) | 89 (36 157) |
| < 200 | 2235 (22.3%) | 29 (0.5%) | 2206 (49.6%) | 2100 (79.0%) |
| 200–500 | 4016 (40.1%) | 2309 (41.4%) | 1707 (38.4%) | 179 (6.7%) |
| ≥ 500 | 2369 (23.6%) | 2335 (41.9%) | 34 (0.8%) | 34 (1.3%) |
| Unknown | 1398 (14.0%) | 901 (16.2%) | 497 (11.2%) | 346 (13.0%) |
| CD4/CD8 ratio, Median (1st; 3rd quartile) | 0.42 (0.22, 0.66) | 0.58 (0.41, 0.81) | 0.21 (0.11, 0.36) | 0.14 (0.07, 0.23) |
| ≤ 0.4 | 3841 (38.3%) | 1107 (19.9%) | 2734 (61.5%) | 1798 (67.6%) |
| > 0.4 | 4225 (42.2%) | 3529 (63.3%) | 696 (15.7%) | 184 (6.9%) |
| Unknown | 1952 (19.5%) | 938 (16.8%) | 1014 (22.8%) | 677 (25.5%) |
| Viral load (n (%)), copies/mL | ||||
| < 10,000 | 1627 (16.2%) | 1252 (22.5%) | 375 (8.4%) | 149 (5.6%) |
| 10,000–100,000 | 3751 (37.4%) | 2315 (41.5%) | 1436 (32.3%) | 652 (24.5%) |
| > 100,000 | 3135 (31.3%) | 1076 (19.3%) | 2059 (46.3%) | 1460 (54.9%) |
| Unknown | 1505 (15.0%) | 931 (16.7%) | 574 (12.9%) | 398 (15.0%) |
| Aids diagnosis (n (%)) | 1339 (13.4%) | 0 (0.0%) | 1339 (30.1%) | 1339 (50.4%) |
| Hepatitis C virus antibodies (n (%)) | ||||
| No | 8021 (80.1%) | 4615 (82.8%) | 3406 (76.6%) | 1982 (74.5%) |
| Yes | 975 (9.7%) | 410 (7.4%) | 565 (12.7%) | 373 (14.0%) |
| Unknown | 1022 (10.2%) | 549 (9.8%) | 473 (10.6%) | 304 (11.4%) |
| Hepatitis B virus surface antigen (n (%)) | ||||
| No | 7719 (77.1%) | 4117 (73.9%) | 3602 (81.1%) | 2129 (80.1%) |
| Yes | 325 (3.2%) | 158 (2.8%) | 167 (3.8%) | 112 (4.2%) |
| Unknown | 1974 (19.7%) | 1299 (23.3%) | 675 (15.2%) | 418 (15.7%) |
All p-values for the comparisons between late and non-late presenters were < 0.001. All p-values for the comparisons between advanced and non-late presenters were < 0.001
MSM men who have sex with men, IDU injection drug user
Late presenters and advanced presenters were more likely than non-late presenters to have acquired HIV infection through heterosexual contact or injections, to have a lower level of education, to be from sub-Saharan Africa or Latin America, and to have enrolled at an older age and with a higher viral load (Table 1).
Dynamic of CD4/CD8 ratio by late presentation status
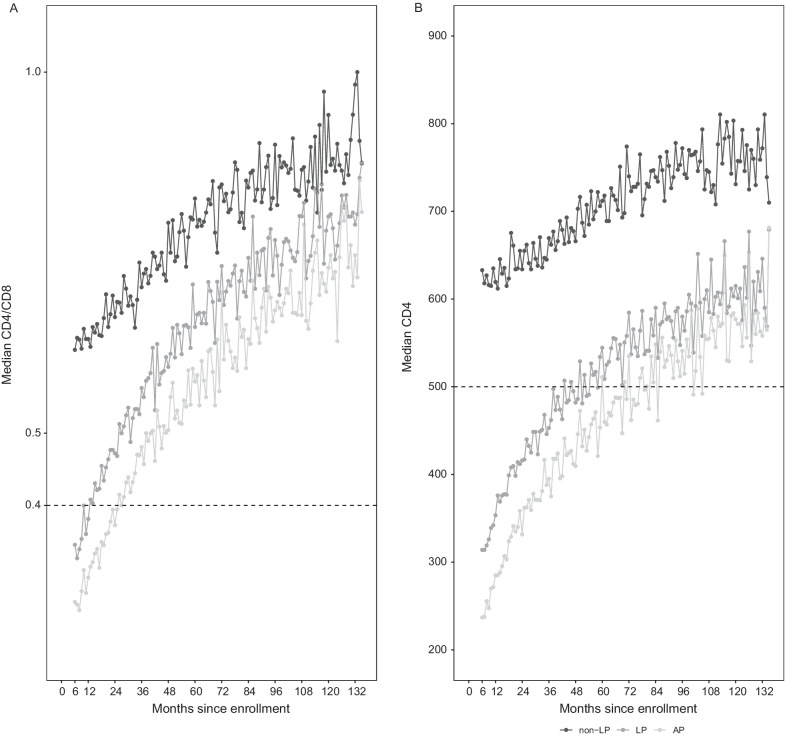
Median CD4/CD8 ratio and CD4 count increased over time in the three groups. While non-late presenters had a median CD4/CD8 ratio above the threshold of 0.4 from 6 months after enrolment, late presenters reached it after 12 months and advanced presenters after 24 months (Fig. 1A, B).
Fig. 1.
Median CD4/CD8 (A) and CD4 count (B) over time according to late presentation status. non-LP non-late presenters, LP late presenters; AP advanced presenters
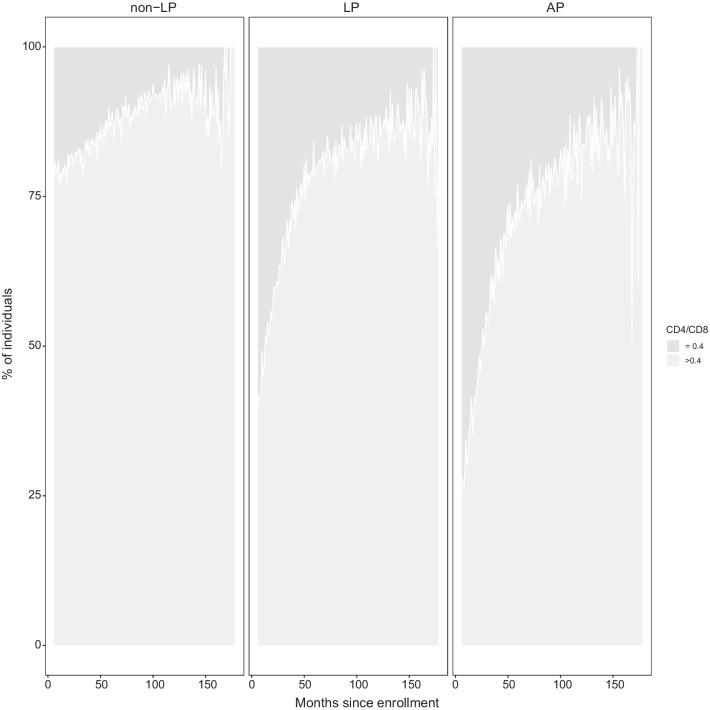
The percentage of individuals with CD4/CD8 ≤ 0.4 was lower in non-late presenters than in late and advanced presenters during the entire follow-up. More than half of the individuals with late presentation or advanced disease had CD4/CD8 ratio ≤ 0.4 at enrolment and over time, with the highest proportion observed in advanced presenters (Fig. 2).
Fig. 2.
Percentage of individuals with CD4/CD8 ratio below or above 0.4 over time in non-late, late and advanced presenters. non-LP non-late presenters, LP late presenters; AP advanced presenters
Impact of dynamic of CD4/CD8 ratio on clinical outcomes (overall and by late presentation status)
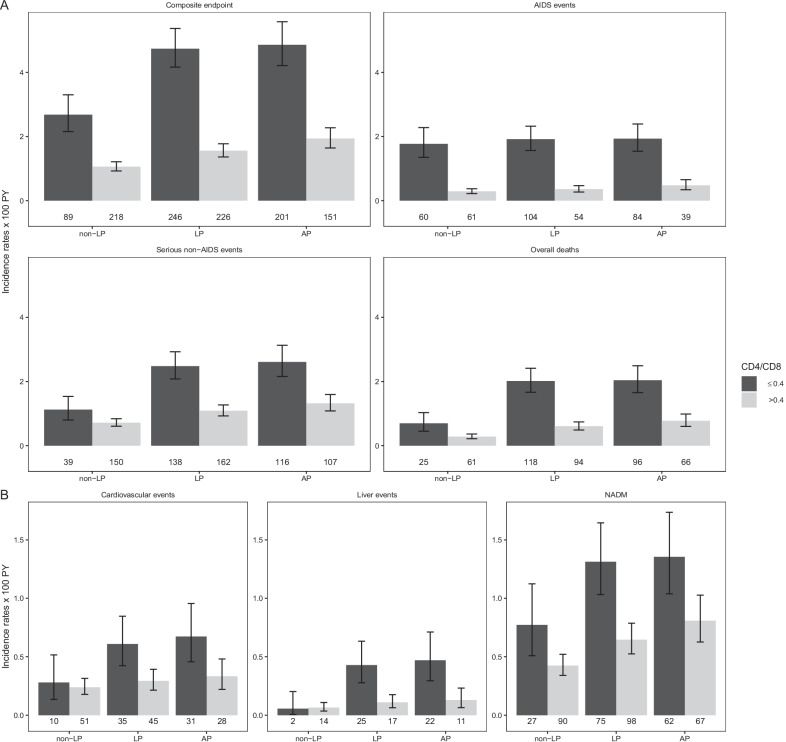
Regardless of their late presentation status at enrolment, low CD4/CD8 ratio over time was associated with an increased incidence of the composite endpoint and of each of its components (Fig. 3).
Fig. 3.
Incidence rates of the clinical outcomes of interest according to CD4/CD8 ratio over time in in non-late, late and advanced presenters. The figures below each bar correspond to the number of events. non-LP non-late presenters, LP late presenters; AP advanced presenters; NADM non-AIDS defining malignancies
As shown in Table 2, compared with CD4/CD8 ratio > 0.4, CD4/CD8 ratio ≤ 0.4 was associated with an increased risk of clinical progression in non-late (HR 1.90; 95%CI 1.48, 2.43), late (HR 1.94; 95%CI 1.46, 2.57), and advanced presenters (HR 1.72; 95%CI 1.26, 2.34). In general, after adjustment for a wide range of covariates that included the CD4 count measured over time, the adjusted HRs were attenuated compared with the unadjusted estimates.
Table 2.
Association between CD4/CD8 ratio over time and clinical outcomes in non-late, late and advanced presenters
| Non-late presenters, CD4/CD8 ≤ 0.4 vs CD4/CD8 > 0.4 |
p-value | Late presenters, CD4/CD8 ≤ 0.4 vs CD4/CD8 > 0.4 |
p-value | Interaction p-value LP vs non-LP |
Advanced presenters, CD4/CD8 ≤ 0.4 vs CD4/CD8 > 0.4 |
p-value | Interaction p-value AP vs non-LP |
||
|---|---|---|---|---|---|---|---|---|---|
| Composite endpoint | Unadjusted HR (95%CI) | 2.57 (1.97, 3.35) | < 0.001 | 3.11 (2.33, 4.17) | < 0.001 | 2.55 (1.88, 3.45) | < 0.001 | ||
| Adjusted HR (95%CI) | 1.90 (1.48, 2.43) | < 0.001 | 1.94 (1.46, 2.57) | < 0.001 | 0.869 | 1.72 (1.26, 2.34) | < 0.001 | 0.598 | |
| AIDS events | Unadjusted HR (95%CI) | 5.80 (3.82, 8.81) | < 0.001 | 4.63 (2.93, 7.33) | < 0.001 | 3.39 (1.99, 5.77) | < 0.001 | ||
| Adjusted HR (95%CI) | 3.31 (2.23, 4.93) | < 0.001 | 2.75 (1.78, 4.27) | < 0.001 | 0.365 | 2.25 (1.34, 3.76) | 0.002 | 0.139 | |
| Serious Non-AIDS events | Unadjusted HR(95%CI) | 1.62 (1.16, 2.28) | 0.005 | 2.45 (1.69, 3.55) | < 0.001 | 2.12 (1.41, 3.20) | < 0.001 | ||
| Adjusted HR (95%CI) | 1.39 (0.96, 2.02) | 0.079 | 1.62 (1.10, 2.40) | 0.016 | 0.423 | 1.49 (0.97, 2.29) | 0.069 | 0.640 | |
| Cardiovascular events | Unadjusted HR(95%CI) | 1.25 (0.57, 2.73) | 0.570 | 2.44 (0.94, 6.35) | 0.067 | 2.50 (0.88, 7.14) | 0.087 | ||
| Adjusted HR(95%CI) | 1.33 (0.64, 2.76) | 0.444 | 1.82 (0.72, 4.61) | 0.208 | 0.511 | 1.85 (0.67, 5.12) | 0.234 | 0.425 | |
| Liver events | Unadjusted HR (95%CI) | 0.84 (0.22, 3.18) | 0.792 | 3.54 (0.81, 15.51) | 0.093 | 3.36 (0.65, 17.44) | 0.149 | ||
| Adjusted HR (95%CI) | 0.42 (0.12, 1.50) | 0.183 | 1.54 (0.37, 6.34) | 0.565 | 0.059 | 1.60 (0.33, 7.71) | 0.556 | 0.078 | |
| Neoplasms | Unadjusted HR (95%CI) | 1.92 (1.34, 2.75) | < 0.001 | 2.27 (1.52, 3.40) | < 0.001 | 1.90 (1.25, 2.88) | 0.003 | ||
| Adjusted HR (95%CI) | 1.61 (1.02, 2.56) | 0.043 | 1.56 (0.97, 2.50) | 0.066 | 0.867 | 0.64 (0.04, 11.32) | 0.193 | 0.500 | |
| Overall mortality | Unadjusted HR (95%CI) | 2.63 (1.57, 4.39) | < 0.001 | 3.85 (2.15, 6.91) | < 0.001 | 3.09 (1.69, 5.64) | < 0.001 | ||
| Adjusted HR (95%CI) | 1.49 (0.92, 2.41) | 0.103 | 1.80 (1.04, 3.11) | 0.036 | 0.511 | 1.61 (0.92, 2.83) | 0.095 | 0.734 |
CI confidence interval; HR hazard ratio; LP late presenters; AP advanced presenters
aHR (CI 95%) were estimated with extended Cox regression models adjusted for age (< 50, ≥ 50 years) and CD4 count (< 500, ≥ 500/µL), both modelled as time-varying covariates, a combined variable of gender and HIV transmission category (MSM, IDU, heterosexual women, heterosexual men, other/unknown), educational level (no studies or primary education, secondary education, university, other/unknown), region of origin (Europe, Sub-Saharan Africa, Latin America, other/unknown), presence of hepatitis C virus antibodies (no, yes or unknown), presence of hepatitis B surface antigen (no, yes or unknown) and viral load (< 10,000, 10,000–100,000, ≥ 100,000 copies/mL, unknown) at enrolment, and with an interaction term between CD4/CD8 ratio over time and late or advanced presentation
The risk of developing an AIDS event was three times higher for individuals with CD4/CD8 ratio ≤ 0.4 compared to those with CD4/CD8 ratio > 0.4 among non-late presenters and twice as high in both late and advanced presenters. The adjusted HRs for serious non-AIDS events, comparing CD4/CD8 ratio ≤ 0.4 versus CD4/CD8 ratio > 0.4, varied from 1.39 (95%CI 0.96, 2.02) in non-late to 1.62 (95%CI 1.10, 2.40) in late, and 1.49 (95%CI 0.97, 2.29) in advanced presenters. These estimates suggested that CD4/CD8 ratio ≤ 0.4 could be associated with serious non-AIDS events. However, in non-late presenters as well as in advanced presenters, possible HRs that are highly compatible with our data, given our model, ranged from 0.96 and 0.97 (essentially no association) to 2.02 and 2.29 (a relatively strong association). Similar considerations can also be extrapolated from the results on specific serious non-AIDS events.
Mortality associated with CD4/CD8 ratio ≤ 0.4 was 49% higher in non-late, 80% higher in late and 61% higher in advanced presenters. There was no evidence that the association between CD4/CD8 ratio and the clinical outcomes differed between non-late and late presenters (all p-values for interaction > 0.5) or between non-late and advanced presenters (all p-values for interaction > 0.1).
Overall, compared with CD4/CD8 ratio > 0.4, CD4/CD8 ratio ≤ 0.4 doubled the risk of experiencing the composite endpoint (HR 2.00; 95%CI 1.63, 2.45), with a three-fold increased risk of an AIDS event (3.05; 95%CI 2.25, 4.12), and it was also associated with an increased risk of serious non-AIDS event (1.58; 95%CI 1.27, 1.97), and overall mortality (1.62; 95%CI 1.32, 1.98). CD4/CD8 ratio ≤ 0.4 was also associated with increased risk of some specific serious non-AIDS events, such as cardiovascular (1.89; 95%CI 1.19, 3.00) and non-AIDS defining malignancies (1.52; 95%CI 1.10, 2.12).
Sensitivity analysis
The analysis including viral load as time-varying variable was consistent with the main results (data not shown). In the sensitivity analysis stratified by the timing of ART initiation, the association between low CD4/CD8 ratio with clinical outcomes was confirmed, regardless on ART initiation time. In those starting ART within the 1st year since the enrolment, the HRs for the association between the CD4/CD8 and the composite endpoint was 2.34 (95%CI 1.42, 3.85, n of events = 99) in non-late, 1.86 (95%CI 1.09, 3.17, n = 507) in late and 1.75 (95%CI 1.28, 2.40, n = 399) in advanced presenters. In participants starting ART 1 year after the enrolment or later, the corresponding HRs were 1.72 (95%CI 1.23, 2.41, n = 239) for non-late, 2.43 (95%CI 1.31, 4.52, n = 54) for late and 1.03 (95%CI 0.29, 3.64, n = 27) for advanced presenters.
Discussion
In this observational study, we found that PLWH with a CD4/CD8 ratio ≤ 0.4 over time are at a greater risk of clinical progression regardless their late presentation status. This corroborates the role of the CD4/CD8 ratio as prognostic marker of clinical outcomes in PLWH no matter how they present at diagnosis. Although this marker has previously shown an association with the development of AIDS- and non-AIDS-related events in some cohorts of PLWH [8, 9, 16–18] to the best of our knowledge this is the first study evaluating its role among late and advanced presenters and assessing whether its clinical impact is the same among the non-late presenters.
Certainly, late presentation status was found to have a negative impact on both the absolute CD4 cell count and CD4/CD8 ratio recovery, as observed in other studies [18, 19]. Besides, we found that the risk of clinical progression, and specifically of AIDS events, was higher when the CD4/CD8 ratio was ≤ 0.4 over time for both non-late and late or advanced presenters. However, the negative impact of a CD4/CD8 ≤ 0.4 ratio was also detected among non-late presenters. It is worth highlighting that we did not focus on CD4/CD8 ratio values at enrolment or at ART initiation, but on CD4/CD8 ratio values measured over time. We also observed that the negative impact of the low CD4/CD8 ratio did not depend on ART initiation timing or on viral response, suggesting that the prognostic role of the CD4/CD8 ratio may be independent on when the treatment is initiated and on its effectiveness in terms of viral suppression.
The adjusted models suggested an increased risk of serious non-AIDS events associated with low CD4/CD8 ratio. This association was observed regardless of late presentation status, although it was of lower magnitude than the one observed for the AIDS-related events and in non-late and advanced presenters was quite imprecise. Among non-AIDS events, we observed increased risk of malignancies, cardiovascular and liver events, though with large and imprecise confidence intervals, probably due to the low number of events in each category. Our results are in discordance with those of two previous studies. Castilho et al. [20] observed no association between baseline CD4/CD8 ratio and risk of non-AIDS events in a cohort of virologically suppressed HIV-positive adults, independently of the CD4+ cell count. These results are not directly comparable with ours, as the authors did not include time-varying CD4/CD8 ratio as predictor and they estimated HRs for 0.1 increase in the CD4/CD8 ratio. Using similar methods to those in our study, Hema et al. [21] did find inconclusive estimates of the association between time-varying CD4/CD8 ratio ≥ 0.5 (vs CD4/CD8 < 0.5) and serious non-AIDS events considered overall, when controlling for time-varying CD4 count. The discrepancy with our results may depend on the different definition of non-AIDS events, while, in line with our findings, the authors observed that low CD4/CD8 ratio was associated with increased risk of non-AIDS defining cancers.
The higher risk of non-AIDS events found in our study could be explained by increased inflammation and immunoactivation, that have been already identified as potential mechanisms for both atherosclerosis [22] and malignancies [23] in PLWH. Thus, our findings bring to light the presence of those phenomena specifically in individuals with low CD4/CD8 ratio [8]. Some authors have in fact reported a lower CD4/CD8 ratio in PLWH diagnosed with a cardiovascular event when compared to those without [9, 20]. Besides, earlier studies describe an association between the inversion of the CD4/CD8 ratio and surrogate markers of vascular disease, such as the carotid intima-media thickness [24, 25] and arterial stiffness [24]. In terms of neoplasms, Serrano et al. [9] found lower CD4/CD8 ratio in participants diagnosed with non-AIDS defining malignancies in comparison with those without a cancer diagnosis. A longer follow-up of our cohort it is desirable as it will allow us to observe more events and therefore make more reliable estimates to confirm the impact of low CD4/CD8 ratio on clinical outcomes.
We acknowledge that our analysis is not adjusted for some potential confounder factors, such as smoking status. Nevertheless, other studies showed higher risk of lung cancer associated with low CD4/CD8 ratio even after accounting for smoking status [26, 27]. As further limitation, we did not consider the effect of ART regimens, which may vary according to initial CD4 count. We also could not classify as late or non-late presenters those who did not have CD4 count or AIDS events available before starting treatment, although they accounted for only 3% of the entire cohort. Finally, although cohort participants were followed for an extended period, their young age may decrease our ability to evaluate outcomes which develop only over the long-term and later in life.
Conclusions
In conclusion, a low CD4/CD8 ratio measured over time is associated with an increased risk of morbidity and mortality in PLWH independently of their late presentation status. These preliminary findings support the prognostic role of variation of CD4/CD8 ratio over time in this highly vulnerable subpopulation and can help define the subgroup of service users who may need closer monitoring to avoid comorbidities, thus optimizing the follow-up and the use of healthcare resources.
Supplementary Information
Additional file 1. Title of data: Cohort of the Spanish HIV/AIDS Research Network (CoRIS). Description of data: Centres and investigators involved in CoRIS.
Acknowledgements
This study would not have been possible without the collaboration of all patients, medical and nursery staff and data mangers who have taken part in the Project.
Abbreviations
- ART
Active antiretroviral treatment
- CD4/CD8ratio
CD4+ T cells/CD8+ T cells ratio
- CI
Confidence intervals
- CoRIS
Cohort of the Spanish HIV/AIDS Research Network
- HR
Hazard ratio
- PLWH
People living with HIV
Author contributions
All authors were involved in the setting up of the cohort and contributed to its design. All authors were involved in data collection. LDD and MR drafted the paper, under the supervision of IJ. MR planned and realized the statistical analyses, OB contributed to the study design and the manuscript writing, RR, JAI, SM and IJ planned the study. LLC, JP, DP, JO, DF reviewed the manuscript. All authors read and approved the final manuscript.
Funding
The RIS cohort (CoRIS) is supported by the Instituto de Salud Carlos III through the Red Temática de Investigación Cooperativa en Sida (RD06/006, RD12/0017/0018 and RD16/0002/0006) as part of the Plan Nacional R+D+I and cofinanced by ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER)”. This study has received funding from Gilead Sciences. The funding body did not participate in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The CoRIS cohort was approved by the Research Ethic Committee of the Gregorio Marañón Hospital and the study was approved by the Research Ethic Committee of the “12 de Octubre” Hospital (Number 14/080). All the patients signed informed consent to participate in the CoRIS cohort.
Consent for publication
Not applicable.
Competing interests
MR has received a grant from Gilead Science. IJ has received teaching fees from Viiv and advisory fees from Gilead Science. SM has been involved in speaking activities and has received grants for research from Gilead, Janssen Cilag, Merck Sharp&Dohme, and ViiV Healthcare. RR reports personal fees from ViiV Healthcare and Merck Sharp & Dohme; grants and personal fees from Gilead Sciences and Janssen Cilag. OB reports payments for lectures by Gilead Sciences and ViiV Healthcare, and non-financial support for national conference and national HIV courses from ViiV Healthcare and Merck Sharp and Dohme, outside the submitted work. LD has received honoraria for lectures by Gilead Sciences and ViiV Healthcare, and non-financial support for HIV conferences and courses from Gilead, ViiV Healthcare and Merck Sharp and Dohme, outside the submitted work. All other authors declare that they have no conflict of interest related to this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lourdes Domínguez-Domínguez, Marta Rava, José Antonio Iribarren, and Santiago Moreno have equal contributions.
References
- 1.Trickey A, May MT, Vehreschild JJ, Obel N, Gill MJ, Crane HM, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–e356. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53(11):1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 3.Maciel RA, Klück HM, Durand M, Sprinz E. Comorbidity is more common and occurs earlier in persons living with HIV than in HIV-uninfected matched controls, aged 50 years and older: a cross-sectional study. Int J Infect Dis. 2018;2019(70):30–35. doi: 10.1016/j.ijid.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Mocroft A, Lundgren JD, Sabin ML, d’Arminio-Monforte A, Brockmeyer N, Casabona J, et al. Risk factors and outcomes for late presentation for HIV-positive persons in Europe: results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE). PLoS Med. 2013;10(9):e1001510. [DOI] [PMC free article] [PubMed]
- 5.Rava M, Domínguez-Domínguez L, Bisbal O, López-Cortés LF, Busca C, Antela A, et al. Late presentation for HIV remains a major health issue in Spain: results from a multicenter cohort study, 2004–2018. Andrei G, editor. PLoS ONE. 2021;16(4):e0249864. [DOI] [PMC free article] [PubMed]
- 6.Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis. 2016;214(Suppl 2):S44–50. doi: 10.1093/infdis/jiw275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu W, Mehraj V, Vyboh K, Cao W, Li T, Routy JP. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc. 2015;18(1)20052. [DOI] [PMC free article] [PubMed]
- 8.Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ t cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014;10(5):e1004078. [DOI] [PMC free article] [PubMed]
- 9.Serrano-Villar S, Pérez-Elías MJ, Dronda F, Casado JL, Moreno A, Royuela A, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS ONE. 2014;9(1):e85798. [DOI] [PMC free article] [PubMed]
- 10.The Strategies for Management of Antiretorviral Therapy (SMART) Study Group. Major clinical outcomes in antiretroviral therapy (ART)–naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–44. [DOI] [PubMed]
- 11.Caro-Murillo AM, Castilla J, Pérez-Hoyos S, Miró JM, Podzamczer D, Rubio R, et al. Spanish cohort of naïve HIV-infected patients (CoRIS): rationale, organization and initial results. Enferm Infecc Microbiol Clin. 2007;25(1):23–31. doi: 10.1157/13096749. [DOI] [PubMed] [Google Scholar]
- 12.Antinori A, Coenen T, Costagiola D, Dedes N, Ellefson M, Gatell J, et al. Late presentation of HIV infection: a consensus definition. HIV Med. 2011;12(1):61–64. doi: 10.1111/j.1468-1293.2010.00857.x. [DOI] [PubMed] [Google Scholar]
- 13.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model (statistics for biology and health) New York: Springer; 2000. [Google Scholar]
- 14.R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna; 2019.
- 15.Therneau TM. A package for survival analysis in R. R package version 3.2-13. 2021.
- 16.Mussini C, Lorenzini P, Cozzi-Lepri A, Lapadula G, Marchetti G, Nicastri E, et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV. 2015;2(3):e98–106. doi: 10.1016/S2352-3018(15)00006-5. [DOI] [PubMed] [Google Scholar]
- 17.Han WM, Apornpong T, Kerr SJ, Hiransuthikul A, Gatechompol S, Do T, et al. CD4/CD8 ratio normalization rates and low ratio as prognostic marker for non-AIDS defining events among long-term virologically suppressed people living with HIV. AIDS Res Ther. 2018;15(1):13. doi: 10.1186/s12981-018-0200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okhai H, Vivancos-Gallego MJ, Hill T, Sabin CA. CD4+:CD8+ T cell ratio normalization and the development of AIDS events in people with HIV starting antiretroviral therapy. AIDS Res Hum Retrovir. 2020 doi: 10.1089/aid.2020.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mutoh Y, Nishijima T, Inaba Y, Tanaka N, Kikuchi Y, Gatanaga H, et al. Incomplete recovery of CD4 cell count, CD4 percentage, and CD4/CD8 ratio in patients with human immunodeficiency virus infection and suppressed viremia during long-term antiretroviral therapy. Clin Infect Dis. 2018;67(6):927–933. doi: 10.1093/cid/ciy176. [DOI] [PubMed] [Google Scholar]
- 20.Castilho JL, Shepherd BE, Koethe J, Turner M, Bebawy S, Logan J, et al. CD4+/CD8+ ratio, age, and risk of serious noncommunicable diseases in HIV-infected adults on antiretroviral therapy. AIDS. 2016;30(6):899–908. doi: 10.1097/QAD.0000000000001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hema MN, Ferry T, Dupon M, Cuzin L, Verdon R, Thiébaut R, et al. Low CD4/CD8 ratio is associated with non AIDS-defining cancers in patients on antiretroviral therapy: ANRS CO8 (Aproco/Copilote) prospective cohort study. Apetrei C, editor. PLoS ONE. 2016;11(8):e0161594. [DOI] [PMC free article] [PubMed]
- 22.Kearns A, Gordon J, Burdo TH, Qin X. HIV-1-associated atherosclerosis: unraveling the missing link. J Am Coll Cardiol. 2017;69(25):3084–3098. doi: 10.1016/j.jacc.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva Neto MM, Brites C, Borges ÁH. Cancer during HIV infection. APMIS. 2020;128(2):121–128. doi: 10.1111/apm.13020. [DOI] [PubMed] [Google Scholar]
- 24.Serrano-Villar S, Moreno S, Fuentes-Ferrer M, Sánchez-Marcos C, Ávila M, Sainz T, et al. The CD4:CD8 ratio is associated with markers of age-associated disease in virally suppressed HIV-infected patients with immunological recovery. HIV Med. 2014;15:40–49. doi: 10.1111/hiv.12081. [DOI] [PubMed] [Google Scholar]
- 25.Bernal-Morell E, Serrano-Cabeza J, Muñoz Á, Marín I, Masiá M, Gutiérrez F, et al. The CD4/CD8 ratio is inversely associated with cIMT progression in HIV infected patients on antiretroviral treatment. AIDS Res Hum Retrovir. 2016;32(7):648–653. doi: 10.1089/aid.2015.0385. [DOI] [PubMed] [Google Scholar]
- 26.Clifford GM, Lise M, Franceschi S, Egger M, Bouchardy C, Korol D, et al. Lung cancer in the Swiss HIV Cohort Study: role of smoking, immunodeficiency and pulmonary infection. Br J Cancer. 2012;106:447–452. doi: 10.1038/bjc.2011.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sigel K, Wisnivesky J, Crothers K, Gordon K, Brown ST, Rimland D, et al. Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. Lancet HIV. 2017;4(2):e67–73. doi: 10.1016/S2352-3018(16)30215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Title of data: Cohort of the Spanish HIV/AIDS Research Network (CoRIS). Description of data: Centres and investigators involved in CoRIS.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.