Abstract
The goal of the present study was to examine the ability of cytochrome P450-2C9 (CYP2C9) to activate cyclophosphamide (CPA) and elicit tumor cell death. A CYP2C9-deficient human lymphoblastoid cell line (AHH-1 cells) and a derivative cell line (H2C9 cells) stably transfected with a cDNA encoding CYP2C9 were used. The catalytic activity present in cell lines was examined by measuring the conversion of diclofenac, a CYP2C9-specific substrate, to its 4′-hydroxy metabolite by high-pressure liquid chromatography. Initial rate plots were constructed and the maximal rate of formation (Vmax) and the Michaelis-Menten constant (Km) for diclofenac metabolism were determined. Cytotoxicity was studied by exposing the cells to 0.01 to 4 mM CPA in the presence or absence of sulfaphenazole, a CYP2C9-specific inhibitor. Cell survival was quantitated by determination of the level of tritiated thymidine incorporation. H2C9 cells quickly metabolized diclofenac, indicating the presence of high levels of CYP2C9. Kinetic experiments demonstrated a Vmax and Km of 0.62 ± 0.012 pmol/min/106 cells and 6.16 ± 0.62 μM, respectively, for diclofenac metabolism. Diclofenac 4′-hydroxylase activity was undetectable in AHH-1 cells. H2C9 cells were more sensitive to the cytotoxic effects of CPA (50% inhibitory concentration [IC50], 0.80 ± 0.03 mM) than AHH-1 cells (IC50, 4.07 ± 0.35 mM). The cytotoxicity (IC50, 1.99 ± 0.14 mM) of CPA to H2C9 cells was blocked by sulfaphenazole, demonstrating that the chemosensitivity of these cells is a consequence of intracellular prodrug activation. H2C9 cells mediated a bystander killing effect for CYP2C9-negative PPC-1 cells, reducing the IC50 of CPA from about 14 to 3.62 ± 0.73 mM in PPC-1 cells when they were cocultured with H2C9 cells. These results suggest that the enzyme-prodrug system of CYP2C9 and CPA may be an effective combination for gene-directed enzyme prodrug therapy. Ongoing studies are examining the utility of this system for use in prostate cancer cells.
One of the major problems associated with cancer chemotherapy is a lack of selectivity that leads to harmful side effects in normal tissues. An attractive concept for improving the selectivity of cancer chemotherapy is tumor-specific activation of noncytotoxic prodrugs to active drugs by either endogenous or specifically introduced exogenous enzymes (W. F. Anderson, Editorial, Hum. Gene Ther. 5:1–2, 1994). Gene-directed enzyme prodrug therapy (GDEPT) is one such therapeutically attractive strategy (8, 15). In GDEPT, a drug susceptibility gene (or “suicide gene”) is delivered to the tumor in a form that directs tumor-specific expression of an enzyme capable of drug activation. A nontoxic prodrug can then be administered and converted to a cytotoxic drug intratumorally by the action of the expressed enzyme. Since the enzyme is now expressed at a higher level (or exclusively) in the tumor, cytotoxic drug concentrations will be increased in the tumor and tumor cell killing should be enhanced. Theoretically, such an approach should enhance the therapeutic indices of chemotherapeutic agents by minimizing systemic toxicity (8). This approach is also attractive because the administration of the prodrug may result not only in the death of the recipient cell but also in the death of surrounding cells, a process often referred to as a “bystander effect” (10). Several methods for delivery of genes to the target tumor have been proposed, including methods that use retroviruses, adenoviruses, and adeno-associated viruses and physical methods those that use liposomes (1).
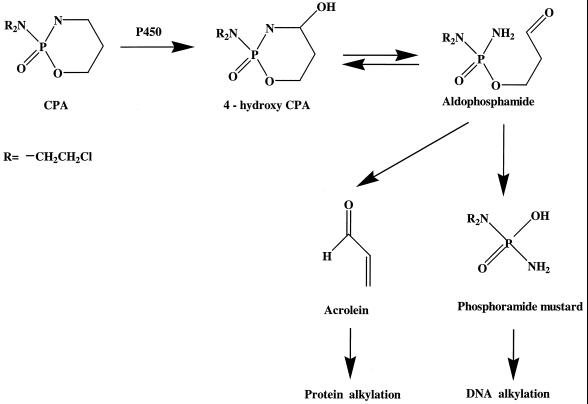
Cyclophosphamide (CPA) is a cell cycle-independent alkylating agent and is widely used in the clinical management of a variety of human malignancies (16). CPA is a therapeutically inactive prodrug that normally requires bioactivation by liver cytochrome P450 (CYP) to exert its antitumor function in patients (Fig. 1). The primary 4-hydroxy metabolite is formed in the liver and equilibrates with the ring-opened aldophosphamide. This intermediate spontaneously decomposes to yield acrolein and phosphoramide mustard. The phosphoramide mustard exhibits the DNA cross-linking and cytotoxic effects associated with the parent drug (20). The specific CYP enzymes designated CYP2B6 (2), CYP3A4 (2), CYP2C9 (3), and CYP2C18 (3) contribute to the metabolism of CPA in the human liver.
FIG. 1.
Pathways of cytochrome P450-catalyzed CPA metabolism.
Transduction of tumor cells with a CPA-activating CYP gene may sensitize the tumor cells to CPA by a mechanism that involves direct, intratumoral prodrug activation, increasing CPA activation and improving the therapeutic index. The present paper evaluates the feasibility of this approach and suggests that the enzyme-prodrug system of CYP2C9 and CPA may be an effective combination for GDEPT in patients with prostate cancer.
MATERIALS AND METHODS
Chemicals.
CPA, sulfaphenazole, diclofenac, isoxicam, alpha-naphthoflavone, and all other reagents were purchased from Sigma Chemical Co. (St. Louis, Mo.). 4′-Hydroxydiclofenac was purchased from Gentest Co. (Woburn, Mass.). Tienilic acid was kindly provided by Daniel Mansuy (Universite Rene Descartes, Paris, France). All materials were used as received from the manufacturer.
Cell lines.
A human lymphoblastoid cell line (AHH-1 cells) that does not express the CYP2C9 enzyme was grown as a suspension culture in RPMI 1640 medium containing 2 mM l-glutamine supplemented with 9% horse serum. AHH-1 cells stably transfected with a cDNA encoding CYP2C9 (18) (hereafter referred to as H2C9 cells) were grown under identical conditions with additional 2 mM l-histidinol to maintain selection. The AHH-1 and H2C9 cell lines were purchased from Gentest Co. Human prostate cancer cell lines (PPC-1, LNCaP, TSU, PC3, and DU145 cells) and rat prostate cancer cell lines (MLL and G cells) were grown in RPMI 1640 medium containing 2 mM l-glutamine supplemented with 10% fetal bovine serum. The cells were maintained in a humidified 5% CO2–95% air atmosphere at 37°C.
Western blot analysis.
The cells were extracted and microsome proteins were isolated as instructed by the manufacturer (Gentest Co.). Microsome protein from prostate cancer cells including PPC-1, DU145, LNCaP, TSU, PC3, G, and MLL cells (30 μg), H2C9 cells (5 μg), and human control liver microsomes (4 μg) were loaded onto 10% polyacrylamide gels and subjected to gel electrophoresis in the presence of sodium dodecyl sulfate. The proteins were then transferred electrophoretically to a nitrocellulose membrane (Bio-Rad, Hercules, Calif.). The membrane was treated with blocking solution (15% nonfat milk and 0.02% sodium azide in phosphate-buffered saline) overnight at 4°C. The membrane was incubated for 1 h at room temperature with rabbit anti-human CYP2C9 polyclonal antibody (Oxford Biomedical Research, Inc., Oxford, Mich.). The membrane was washed with blocking solution and incubated with goat anti-rabbit antibody coupled with peroxidase (ECL kit; Amersham Life Science, Little Chalfont, England) for 1 h at room temperature, and enhanced chemiluminescence detection was performed as instructed by the manufacturer.
Cytotoxicity assay.
AHH-1 and H2C9 cells were plated in 96-well microtiter plates at a density of 2,500 cells/well, and the plates were incubated at 37°C. Six hours later, the cells were treated with different concentrations (0.01 to 4 mM) of CPA. The cells were preincubated with or without 100 μM sulfaphenazole, a CYP2C9-specific inhibitor (13, 17), 2 h before treatment with CPA. Cultures were incubated for an additional 2 days at 37°C. [3H]thymidine (1 μCi/well) was added to the cells for the final 18 h of incubation. The cells were then harvested onto filter paper with a 12-well harvester (Brandel M-12; Biomedical Research and Development Laboratories Inc., Gaithersburg, Md.). The filter paper disks were removed from the cell harvester and were placed in glass vials containing 5 ml of EcoLite Plus scintillation cocktail (ICN Biomedical, Costa Mesa, Calif.). Radioactivity was counted in a liquid scintillation counter (model L56800; Beckman Instruments, Inc., Fullerton, Calif.). Ten micromolar alpha-naphthoflavone, a potent inhibitor of CYP1A1 (7), was also used in an attempt to protect AHH-1 cells from CPA cytotoxicity. Tritiated thymidine incorporation as a percentage of that for the control (no drug treatment) was plotted versus the CPA concentration, and the CPA concentration required to inhibit thymidine incorporation by 50% (IC50) was calculated as described below.
Bystander effect.
PPC-1 cells were plated in 96-well plates at a density of 1,000 cells/well, and the plates were incubated at 37°C. Fifteen hours later, H2C9 cells were added at a density of 104 cells/well. After another 4 h of incubation, the cells were treated with different concentrations (8 μM to 3.2 mM) of CPA. The cells were incubated for an additional 54 h and then washed once with fresh RPMI 1640 medium to remove the suspended H2C9 cells. Incubation was continued for 18 h at 37°C in RPMI 1640 medium containing [3H]thymidine (1 μCi/well). The cells were detached by treatment with 0.25% trypsin without EDTA and harvested onto filter paper with the cell harvester, and the radioactivity was determined as described above for the cytotoxicity assays. PPC-1 cells treated with different concentrations of CPA in the absence of H2C9 cells were used as a control. All experiments were performed in triplicate.
CYP2C9 activity in whole cells.
Diclofenac 4′-hydroxylation was determined at different incubation times (5 to 120 min) and different total cell concentrations (1.25 × 106 to 2 × 107 cells/ml) to establish the linear range for 4′-hydroxydiclofenac formation. AHH-1 or H2C9 cells were grown and maintained in 75-cm2 culture flasks as described above. On the morning of the experiment, cells were centrifuged at 1,000 × g for 5 min in a refrigerated centrifuge (Centra-MP4R; International Equipment Company, Needham, Mass.). Cell pellets were resuspended in phosphate-buffered saline, centrifuged again, and then resuspended in 0.1 M Tris buffer (pH 7.5) at a density of 107 cells/ml. An aliquot (100 μl) of the cell suspension was mixed with 100 μl of a buffered solution containing diclofenac (final concentration range, 1 μM to 1 mM). The drug-containing cell suspension was vortexed and incubated at 37°C in a water bath for 45 min. The reactions were stopped by adding 40 μl of ice-cold 6% glacial acetic acid in acetonitrile and 20 μl of 50 μg of isoxicam per ml (as an internal standard). The mixture was vortexed and centrifuged at about 500 × g for 2 min in a microcentrifuge (HSC10K Speedfuge; Savant Instruments Inc., Farmingdale, N.Y.). The supernatant was transferred to a small tube, and a 150-μl aliquot was used for quantitation. A high-pressure liquid chromatography method for 4′-hydroxydiclofenac and isoxicam was developed on the basis of the method of Leemann et al. (11). Briefly, samples were injected directly onto a C18 reversed-phase column (3.9 by 150 mm; particle size 3 μm; Nova-Pak column; Waters Corp., Milford, Mass.). The mobile phase (0.5% formic acid in a 40:60 mixture of acetonitrile and deionized water) was delivered at 1.0 ml/min (model 510; Waters Corp.), and the column effluent was monitored with a variable-wavelength UV detector (model 481; Waters Corp.) set at 280 nm. The peak heights of the analytes were measured with commercially available software (MULTICHROM program; University of Tennessee, Memphis). The retention times for 4′-hydroxydiclofenac and isoxicam were 5.8 and 9.5 min, respectively. Diclofenac eluted at 18.5 min and did not interfere with quantitation of the other analytes. Calibration curves for 4′-hydroxydiclofenac were linear (r2 > 0.99) for concentrations ranging from 0.5 to 3.0 nmol/ml. The intraday assay precision (percent coefficient of variation) ranged from 11.3% for the lowest standard to 5.7% for the highest standard. The interday coefficient of variation ranged from 6.6% for the lowest standard to 5.2% for the highest standard. Kinetic parameters describing the rate of metabolism were determined from plots of the initial rate of 4′-hydroxydiclofenac formation versus the diclofenac concentration. Specifically, the maximal rate of 4′-hydroxydiclofenac formation (Vmax) and the Michaelis-Menten constant (Km) were calculated as described below.
Data analysis and statistics.
IC50s for the cytotoxicity experiments were determined by computer fitting the experimental data to the equation E = Emax · [1 − Cn/(Cn + IC50n)] with NONLIN nonlinear regression software (Pharsight Corporation, Mountain View, Calif.), where E represented cell survival in the absence of CPA, IC50 represented the concentration of CPA necessary to elicit a 50% decrease in [3H]thymidine incorporation, C represented the concentration of CPA, and n represented the curve slope factor. Kinetic parameters (Vmax and Km) for diclofenac hydroxylation were determined with the same software by fitting the experimental data to the equation V = Vmax · C/(Km + C), where V was the experimentally determined rate at each substrate concentration, and C was the initial concentration of diclofenac in the incubated material. Statistical comparisons were made at a 5% level of significance by analysis of variance or the t test, as appropriate.
RESULTS
Kinetic analysis CYP2C9 activity in whole cells.
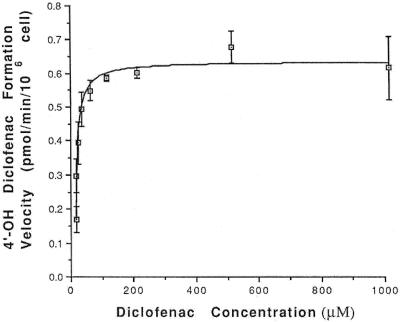
Diclofenac is a known substrate for CYP2C9 (11). We examined diclofenac hydroxylation in AHH-1 and H2C9 cells to confirm their metabolic activities with regard to CYP2C9. Preliminary experiments showed that 4′-hydroxydiclofenac formation was linear for up to 45 min in incubations containing up to 5 × 106 cells/ml. H2C9 cells quickly metabolized diclofenac, indicating the presence of high levels of CYP2C9. The formation of 4′-hydroxydiclofenac was totally blocked by sulfaphenazole (data not shown). The reaction exhibited single-enzyme Michaelis-Menten kinetics for diclofenac concentrations up to 1 mM. Mean ± standard error (SE) Vmax and Km values were 0.62 ± 0.012 pmol/min/106 cells and 6.16 ± 0.62 μM, respectively (Fig. 2). These values are consistent with earlier studies and show that CYP2C9 is an enzyme with a low Km for both diclofenac (11) and cyclophosphamide (2). Diclofenac 4′-hydroxylase activity was undetectable in AHH-1 cells, indicating no detectable CYP2C9 enzyme activity.
FIG. 2.
4′-Hydroxydiclofenac (4′-OH diclofenac) formation was measured at different concentrations of diclofenac in the presence of 106 H2C9 whole cells. Km and Vmax were analyzed by nonlinear regression with the NONLIN program. The line represents nonlinear least-squares regression of the mean experimental data. Error bars represent the standard deviation of the mean from the results of quadruplicate experiments.
Expression of CYP2C9 gene in human prostate cancer cells.
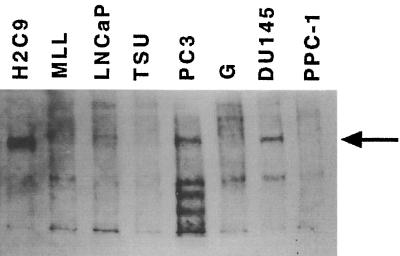
The expression of CYP2C9 in H2C9 cells and in prostate cancer cell lines was determined by Western blot analysis (Fig. 3). Human liver (LB174) microsomes (data not shown) showed the most abundant levels of CYP2C9 expression. CYP2C9 expression was undetectable in the PPC-1 cell line but was easily detectable in H2C9 cells (∼20% of the level of expression by human liver microsomes). Among the prostate cancer cells examined, PC-3 cells had the richest levels of CYP2C9 expression (∼8% of the level of expression by human liver cells). The MLL and LNCaP cell lines showed minor levels of CYP2C9 expression (∼1% of the level of expression) by human liver cells), and TSU and G cell lines demonstrated less than 1% of the level of CYP2C9 expression observed in H2C9 cells. PPC-1 cells, which had undetectable CYP2C9 expression, were chosen for further studies.
FIG. 3.
Immunodetection of CYP2C9 in a series of human prostate cell lines and in the H2C9 cell line that stably express CYP2C9. Microsomal protein prepared from cultured cell lines were electrophoresed on 10% polyacrylamide gels with sodium dodecyl sulfate, transferred to a nitrocellulose membrane, and probed with rabbit anti-human CYP2C9 polyclonal antibody.
Effect of CPA on cultured AHH-1 and H2C9 cells.
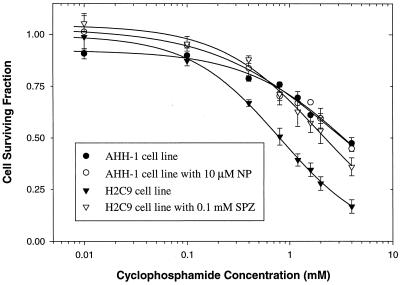
CYP2C9-positive cells (H2C9 cells) and CYP2C9-negative cells (AHH-1 cells) were cultured with various concentrations of CPA. The growth of H2C9 cells was inhibited in a concentration-dependent manner by CPA, with an IC50 of 0.80 ± 0.03 mM (mean ± SE) (Fig. 4). CPA was significantly less cytotoxic to the non-CYP2C9-expressing parental control cells AHH-1 (IC50, 4.07 ± 0.35 mM [mean ± SE]). This finding is consistent with the requirement of P450 metabolism to convert CPA to cytotoxic metabolites. The CYP-dependent nature of this cytotoxicity was confirmed in experiments with the CYP2C9-specific inhibitor sulfaphenazole, which nearly eliminated the cytotoxicity of CPA toward H2C9 cells (IC50, 1.99 ± 0.14 mM [mean ± SE]) (Fig. 4). In control experiments (data not shown), sulfaphenazole had no effect on the cytotoxicity of CPA to parental AHH-1 cells. The ability of sulfaphenazole to protect H2C9 cells from CPA cytotoxicity demonstrated that the presence of a catalytically active CYP2C9 enzyme could be used to elicit CPA chemosensitivity. However, tienilic acid, a mechanism-based inhibitor of CYP2C9 (12), did not influence the cytotoxicity of CPA (data not shown). High CPA concentrations led to decreased cell survival for both AHH-1 and H2C9 cells. The facts that this cytotoxicity occurred in non-CYP2C9-expressing AHH-1 cells and that it could not be completely blocked by sulfaphenazole suggested that other oxidative enzymes were contributing to CPA cytotoxicity in these cells.
FIG. 4.
Cytotoxicity of CPA in human lymphoblastoid cell line (AHH-1 cells) and derivative cell line (H2C9 cells) expressing CYP2C9. The effect of CPA on cell survival was expressed as survival fraction (i.e., cell number in plates with drug as a fraction of the cell number in plates without drug). Alpha-naphthoflavone (NP; final concentration, 10 μM), a potent inhibitor of CYP1A1, and sulfaphenazole (SPZ; final concentration, 1 mM), a CYP2C9-specific inhibitor, were added to assess the effects of these cytochromes on CPA cytotoxicity. Sulfaphenazole blocked the cytotoxicity of CPA, while alpha-naphthoflavone had no effect. Lines represent nonlinear least-squares regression of the mean experimental data. Error bars represent the standard deviation of the mean from the results of triplicate experiments.
Both AHH-1 and H2C9 cells express CYP1A1 (4, 5). We also tested whether CYP1A1 can activate CPA. AHH-1 cells were treated with CPA in the absence or presence of 10 μM alpha-naphthoflavone, a potent inhibitor of CY1A1. As shown in Fig. 4, alpha-naphthoflavone did not block the cytotoxicity of CPA to AHH-1 cells (IC50, 2.99 ± 0.34 mM [mean ± SE]). These results indicate that CYP1A1 is not responsible for CPA activation and suggest that other oxidative enzymes exist in these cells.
Bystander effect of H2C9 cells.
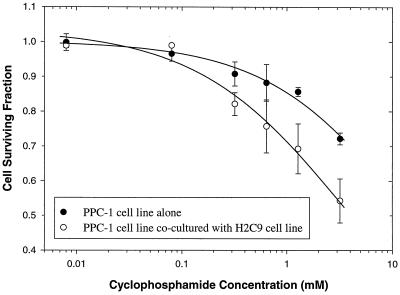
To test whether H2C9 cells mediate a CPA-dependent bystander killing effect, CYP2C9-negative prostate cancer cells (PPC-1) in a monolayer culture and CYP2C9-positive cells (H2C9) in a suspension culture were cocultured. The mixed culture was then treated with CPA as described above. As shown in Fig. 5, H2C9 cells chemosensitized the adjacent CYP2C9-negative PPC-1 cells. PPC-1 cells exhibited growth inhibition following treatment with CPA in the presence of H2C9 cells (IC50, 3.63 ± 0.73 mM [mean ± SE]). However, the majority (∼80%) of PPC-1 cells survived in the absence of H2C9 cells. The extrapolated estimate of IC50 (the highest CPA concentration examined in this experiment was 3.2 mM) for PPC-1 cells treated with CPA (with no H2C9 cells present) was 14.4 ± 5.1 mM (mean ± SE), suggesting that the presence of H2C9 cells enhanced CPA-mediated cytotoxicity to PPC-1 cells by approximately fourfold. H2C9 cells did not inhibit the growth of PPC-1 cells when both cell lines were cocultured in the absence of CPA (data not shown). It is important that we used a bystander cell (H2C9 cell)-to-tumor cell (PPC-1 cell) ratio of 10:1 for these studies. In experiments with a single concentration of CPA (4 mM) and a 1:1 or 10:1 bystander cell:tumor cell ratio, the mean ± standard deviation cell survivals were 67% ± 19% and 43% ± 16%, respectively. These results indicate that CYP2C9-positive cells confer a moderate bystander killing effect on adjacent CYP2C9-negative cells by a mechanism that involves CYP2C9 enzyme activity.
FIG. 5.
CYP2C9-expressing cells (H2C9 cells) mediate a bystander effect toward CYP2C9-negative cells (PPC-1 cells) in the presence of CPA. PPC-1 cells were treated with CPA in the absence or presence of H2C9 cells. After 2 days of coincubation, H2C9 cells were removed and PPC-1 cell survival was determined. Lines represent nonlinear least-squares regression of the mean experimental data. Error bars represent the standard deviation of the mean from the results of triplicate experiments.
DISCUSSION
The primary goals of this study were (i) to evaluate whether expression of CYP2C9 in human lymphoblastoid cells would sensitize the cells to CPA and (ii) to establish whether adjacent, non-CYP-containing prostate cancer cells become drug sensitive via a bystander effect in the presence of CPA.
We found that a human lymphoblastoid cell line stably transfected with CYP2C9 was sensitized to CPA treatment in vitro. This chemosensitization was completely blocked by a CYP2C9-specific inhibitor, sulfaphenazole, demonstrating that this chemosensitization is the result of CPA activation by CYP2C9. The control cell line used in this study, AHH-1, is a human lymphoblastoid cell line that contains native CYP1A1 activity. This experiment also showed that CPA inhibited the growth of AHH-1 cells in a concentration-dependent manner. However, alpha-naphthoflavone, a potent inhibitor of CYP1A1, was unable to block this cytotoxicity. This suggested that CYP1A1 was not responsible for CPA activation in AHH-1 cells and that other as yet unidentified oxidases may contribute to the cytotoxicity of CPA. Sulfaphenazole, a specific competitive inhibitor of CYP2C9, was able to fully block the activity of CYP2C9. Although tienilic acid was previously shown to be a mechanism-based inhibitor for CYP2C9 (12), it was unable to block the activity of CYP2C9 in our studies. One possible explanation may be that tienilic acid (pKa = 6.0) would be expected to be partially ionized in cell medium and thus less capable of diffusion across the cell membrane.
Chang et al. (2) previously reported that CYP2C9 could catalyze CPA oxidation. This oxidative activation was characterized by a low Km value compared to those for CYP2A6, CYP2B6, and CYP3A4. A high substrate affinity (i.e., a low Km) could be of advantage when the substrate concentration is low, as may be the case during in vivo studies. Chang et al. (3) also showed that CYP2C9 had a lower in vitro intrinsic clearance (i.e., a lower Vmax/Km ratio) than CYP2C18. On the basis of in vitro intrinsic clearances, CYP2C18 was the most efficient of the four CYP2C subfamily members in activating CPA. However, it is important that the apparent Km of CYP2C18 (∼3 mM) was approximately sixfold larger than the apparent Km of CYP2C9 (∼0.5 mM) in their studies. This is of particular importance when one considers that the concentrations of CPA in the plasma of cancer patients typically range from 0.1 to 0.7 mM after the administration of standard doses (i.e., much lower than the Km of CYP2C18) (19). Hence, CYP2C9 is likely to exhibit greater catalytic activity in this situation and may be the first choice for GDEPT. The ability of CYP2C9 to promote tumor cell death in our experiments corroborates this hypothesis.
We observed that CYP2C9-positive cells (H2C9 cells) conferred a bystander killing effect onto CYP2C9-negative prostate cancer cells (PPC-1 cells). The bystander effect has great therapeutic significance because only a subset of a tumor cell population can be expected to effectively express the drug susceptibility gene in a practical setting (4, 6). The limitation that all tumor cells must express the drug susceptibility gene to eradicate a tumor can be overcome by this bystander effect. There are several mechanisms by which the bystander effect may occur, including the release of apoptotic vesicles, the presence of gap junctions, or drug diffusion. Gap junctions for the bystander killing effect may involve intracellular transfer of activated soluble cytotoxic metabolic drug through cell-cell contact (14). Apoptotic vesicles could transfer the cytotoxic metabolite of the prodrug or the enzyme itself to the nearby tumor cells, which can phagocytose apoptotic vesicles (6). Cell-cell contact is not required for the release of apoptotic vesicles. In our experiments, H2C9 cells and PPC-1 cells were not physically separated. However, the cytotoxic effects of CPA on PPC-1 cells cocultured with H2C9 cells indicate that CYP2C9 is able to activate CPA and elicit tumor cell death in cells not expressing the drug susceptibility enzyme. It is important that a significant fraction of tumor cells must express the drug susceptibility gene for it to be useful in a practical setting. Studies with a 1:1 bystander cell:tumor cell ratio also demonstrated CPA-mediated cytotoxicity, indicating that smaller fractions of tumor cells expressing the drug susceptibility gene may be needed to elicit the desired effect. However, CPA-mediated killing of drug-activating (i.e., CYP2C9-expressing) cells during CPA treatment would serve to limit subsequent drug activation and increase the need for CYP2C9-expressing cells. The bystander effect mechanism of the CYP2C9-CPA system for GDEPT needs further investigation.
Jounaidi et al. (9) recently showed that rat 9L gliosarcoma cells expressing CYP2B6 exhibited the highest sensitivity to CPA. Because CYP2C9 had a lower efficiency for sensitization of the rat tumor cells, the investigators concluded that CYP2B6 was the gene of choice for CPA-based P450 gene therapy. However, CYP2C9 may prove to be useful in some instances. Our experiments confirm that CYP2C9 can directly sensitize tumor cells to CPA and demonstrate for the first time that the enzyme-prodrug system of CYP2C9 and CPA can elicit a potent cytotoxic bystander effect in prostate cancer cells. These data, coupled with the lack of CYP2C9 expression in prostate cancer cell lines, indicate that the enzyme-prodrug system of CYP2C9 and CPA may be an effective combination for GDEPT of prostate cancer.
ACKNOWLEDGMENTS
These studies were supported by a grant to the University of Tennessee Prostate Cancer Research Group from the St. Francis of Assisi Foundation, Memphis, Tenn.
REFERENCES
- 1.Brenner M K. Gene transfer and therapeutic drug monitoring. Ther Drug Monit. 1996;18:322–327. doi: 10.1097/00007691-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Chang T K H, Weber G F, Crespi C L, Waxman D J. Differential activation of cyclophosphamide and ifosphamide by cytochromes P450 2B and 3A in human liver microsomes. Cancer Res. 1993;53:5629–5637. [PubMed] [Google Scholar]
- 3.Chang T K H, Yu L, Goldstein J A, Waxman D J. Identification of polymorphically expressed CYP2C19 and the wild-type CYP2C9-ILE359 allele as low-Km catalysts of cyclophosphamide and ifosfamide activation. Pharmacogenetics. 1997;7:211–221. doi: 10.1097/00008571-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Crespi C L, Altman J F, Marletta M A. Xenobiotic metabolism and mutation in a human lymphoblastoid cell line. Chem Biol Interact. 1985;53:257–272. doi: 10.1016/s0009-2797(85)80103-4. [DOI] [PubMed] [Google Scholar]
- 5.Crespi C L, Thilly W G. Assay for gene mutation in human lymphoblast line, AHH-1, competent for xenobiotic metabolism. Mutat Res. 1984;128:221–230. doi: 10.1016/0027-5107(84)90110-6. [DOI] [PubMed] [Google Scholar]
- 6.Freeman S M, Abbound C N, Whartenby K A, Packman C H, Koeplin D S, Moolten F L, Abraham G N. The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–5283. [PubMed] [Google Scholar]
- 7.Gurtoo H L, Parker N B, Paigen B, Havens M B, Minowadw J, Freedman H J. Induction, inhibition, and some enzymological properties of aryl hydrocarbon hydroxylase in fresh mitogen-activated human. Cancer Res. 1979;39:4620–4629. [PubMed] [Google Scholar]
- 8.Huber B E, Richards C A, Krenitsky T A. Retroviral-mediated gene therapy for the treatment of hepatocellular carcinoma: an innovative approach for cancer therapy. Proc Natl Acad Sci USA. 1991;88:8039–8043. doi: 10.1073/pnas.88.18.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jounaidi Y, Hecht J E D, Waxman D J. Retroviral transfer of human cytochrome P450 genes for oxazaphosphorine-based cancer gene therapy. Cancer Res. 1998;58:4391–4401. [PubMed] [Google Scholar]
- 10.Kolberg R. Gene therapist test puzzling “bystander effect.”. NIH Res. 1992;4:68–74. [Google Scholar]
- 11.Leemann T, Transon C, Dayer P. Cytochrome P450TB (CYP2C): a major monooxygenase catalyzing diclofenac 4′-hydroxylation in human liver. Life Sci. 1993;52:29–34. doi: 10.1016/0024-3205(93)90285-b. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Garcia M P, Dansette P M, Mansuy D. Thiophene derivatives as new mechanism-based inhibitors of cytochromes P-450: inactivation of yeast-expressed human liver cytochrome P-450 2C9 by tienilic acid. Biochemistry. 1994;33:166–175. doi: 10.1021/bi00167a022. [DOI] [PubMed] [Google Scholar]
- 13.Mancy A, Dijols S, Poli S, Guengerich F P, Mansuy D. Interaction of sulfaphenazole derivatives with human liver cytochromes P450 2C: molecular origin of the specific inhibitory effects of sulfaphenazole on CYP2C9 and consequences for the substrate binding site topology of CYP2C9. Biochemistry. 1996;35:16205–16212. doi: 10.1021/bi961950t. [DOI] [PubMed] [Google Scholar]
- 14.Moolten F L. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. [PubMed] [Google Scholar]
- 15.Moolten F L. Drug sensitivity (“suicide”) gene for selective cancer chemotherapy. Cancer Gene Ther. 1994;1:279–287. [PubMed] [Google Scholar]
- 16.Moore M J. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 1991;20:194–208. doi: 10.2165/00003088-199120030-00002. [DOI] [PubMed] [Google Scholar]
- 17.Pond S M, Brikett D J, Wade D N. Mechanism of inhibition of tolbutamide metabolism: phenylbutazone, oxyphenbutazone, sulfaphenazole. Clin Pharmacol Ther. 1977;22:573–579. doi: 10.1002/cpt1977225part1573. [DOI] [PubMed] [Google Scholar]
- 18.Rettie A E, Wienkers L C, Gonzalez F J, Trager W F, Korzekwa K R. Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9. Pharmacogenetics. 1994;4:39–42. doi: 10.1097/00008571-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Schuler U, Waidelich P, Kolb H, Wagner T, Ehninger G. Pharmacokinetics and metabolism of cyclophosphamide administered after total body irradiation of bone marrow transplant recipients. Eur J Clin Pharmacol. 1991;40:521–523. doi: 10.1007/BF00315233. [DOI] [PubMed] [Google Scholar]
- 20.Sladek N E. Metabolism of oxazaphosphorines. Pharmacol Ther. 1988;37:301–355. doi: 10.1016/0163-7258(88)90004-6. [DOI] [PubMed] [Google Scholar]