Abstract
Background:
Closed system drug-transfer devices (CSTD) allow the reconstitution of hazardous drugs into infusion bags, while preserving the sterility of the product and preventing the escape of liquids and aerosols into the environment. Air-cleaning technology CSTD is based on an activated carbon filter and a membrane which enable maintaining the drug sterile by filtration of air entering the vial during pressure equalization.
Objective:
The study aimed to investigate if an air-cleaning CSTD can prevent liquid viral contamination by human coronavirus OC43 (HCoV-OC43).
Methods:
Chemfort™ CSTD with (intact) or without (control) a Toxi-Guard system was used to transfer liquids between an IV bag and an empty vial (a total of 5 liquid transfers) inside a sealed glove box contaminated by HCoV-OC43 aerosols. In addition, the vial adaptor was challenged by direct spray of HCoV-OC43 solution on the septum and filter areas. HCoV-OC43 RNA was extracted from samples of the transferred liquid and compared between the devices with or without a Toxi-Guard system.
Results:
Use of a CSTD with the Toxi-Guard system resulted in non-detectable cycle threshold (CT) values, indicative of no detectable HCoV-OC43RNA in the transferred liquid, even when the septa and filter areas were directly sprayed with HCoV-OC43 stock solution. In contrast, use of the CSTD with no Toxi-Guard system resulted in a detectable CT value of the transferred liquid.
Conclusions:
Using Chemfort CSTD with integral Toxi-Guard technology can prevent the introduction of microbial and airborne contaminants into the fluid path, thus potentially protecting patients from infection.
Keywords: COVID-19, Drug preparation, Viral contamination
INTRODUCTION
Microbiologically contaminated drug products carry a clinical risk to patients.1-5 Most pharmaceutical facilities have specific procedures in place for controlling contamination by bacteria and fungi. Viral infection can be highly pathogenic, and often there are no effective treatments available. The findings of a recent survey demonstrated that 45% of 20 companies that completed the virus contamination assay reported experiencing at least one virus contamination event between 1985 and 2018.6 Prevention of contaminations is the only way to keep cell cultures for research, development, and the biotech industry free of viruses.7
Closed system transfer devices (CSTD) allow the reconstitution of liquid or pre-dissolved powder drugs into infusion bags, flexible bottles, or syringes, while preserving the sterility of the product, preventing both the entry of contaminants into the system and the escape of the hazardous drug, in whatever form it may exist, into the surrounding environment.8,9 Currently, there are two main technologies for CSTDs: One is based on a physical barrier, where a balloon or a closed chamber holds the air that is exhausted from a vial during drug reconstitution, and the other group of devices uses an air-cleaning system, where the exhausted air leaves the device, but the drug vapor is caught by a filter.10
Chemfort™ CSTD (Simplivia Healthcare Ltd., Kiryat Shmona, Israel;marketed as OnGuard^2 in the USA) is an air-cleaning technology CSTD, using a novel system (Toxi-Guard®) consisting of a hydrophobic filtration membrane with 0.2μm pores and an activated carbon filter. Sterility of the drug in the vial is maintained by filtration of air entering the vial during pressure equalization through a hydrophobic acrylic copolymer membrane with a pore size of 0.2 micron.10,11 The Chemfort CSTD with the Toxi-Guard technology is presented in Figure 1.
Figure 1.

Chemfort CSTD with Toxi-Guard System. Vial adaptor, which firmly connects to any standard vial (left), equipped with the Toxi-Guard system with a 100% activated carbon drug adsorbing matrix (middle) and a 0.2 micron hydrophobic and oleophobic membrane (right), serving together as an effective sterile, particulate, and toxic drug vapor barrier.
Carbon-based materials are known as the most used adsorbents for water and air treatment and are also largely used in virus removal. A recent review analyzed the interaction between viruses and carbon-based systems, among other solutions, regarding efficiency of virus adsorption.12 The authors found that activated carbon adsorbed virus more efficiently than other types of carbon materials. For the specific Toxi-Guard activated carbon system used in our study, laboratory testing performed by the UK’s Health Protection Agency (HPA) using MS-2 coliphage revealed moderate capture level (~10%) of the virus tested. Furthermore, the carbon layer was also shown to have a unique ability to deactivate viruses at a rate of up to 93% without chemical intervention.13 These protective effects of the activated carbon layer enhance the viral filtration efficiency of the 0.2μm membrane. Therefore, both protective layers of the Toxi-Guard system are active against airborne viruses and prevent the risk of virus penetration into the vial.
In this study, we examined the ability of Chemfort CSTD with the Toxi-Guard technology to prevent liquid contamination by human coronavirus OC43 (HCoV-OC43) from the outer environment.
METHODS
We compared the efficacy of preventing liquid contamination by an outer environment containing aerosols of human coronavirus of Chemfort CSTD with a vial adaptor in 2 devices: An intact Toxi-Guard vial adaptor versus a non-Toxi-Guard vial adaptor, i.e., a vial adaptor lacking the protective plastic covering and without the activated carbon filter and the sterilizing membrane. Both tested devices are depicted in Figure 2.
Figure 2.
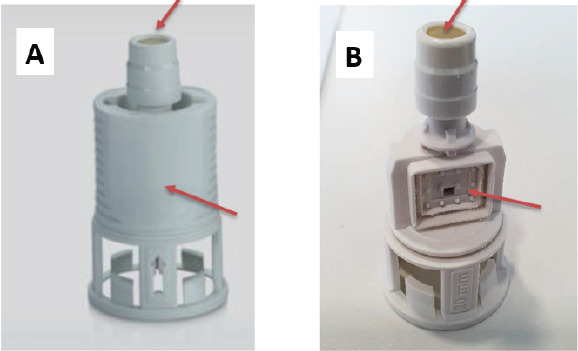
Tested CSTDs with challenged areas. CSTD were either intact vial adaptor (A) or vial adaptor lacking both the sterilizing membrane and activated carbon system (B). The challenged areas sprayed directly with HCoV-OC43 stock solution are indicated by red arrows
The test was performed inside a sealed glove box which was placed in a biological safety laminar flow cabinet. The glove box environment was aerosolized with either sterile growth medium (negative control; NC) or with a known titer of HCoV-OC43 stock solution (5x106 PFU/ml), which was verified twice by a cell plaque assay. The aerosol, comprising 1-5-µm aerosol droplets, was generated by a nebulizer (Air Pro, Medic Spa, China).
The CSTDs were handled as described in the device’s instructions for use (IFU) unless otherwise specified, i.e., before connecting the CSTD adaptors to their corresponding items, they were disinfected at the septum areas of all system adaptors with a 70% isopropanol pad (Aplicare Inc., Meriden, CT, USA).Prior to each test, all CSTD adaptors were applied to their corresponding items inside the laminar hood, i.e., the bag adaptor was applied to an intravenous (IV) bag, the vial adaptor was applied to a vial and the syringe adaptor to a syringe.
All test articles were inserted into the glove box and the following procedure was performed: a sample of 10 ml of sterile saline was aspirated from the IV bag using a 10-ml syringe equipped with a syringe adaptor and transferred into the glass vial equipped with a vial adaptor. The syringe adaptor was disconnected from the vial adaptor, and the vial was mixed well to mimic proper drug reconstitution. Subsequently, the syringe adaptor was reconnected to the vial adaptor and the sample was transferred from the vial back into the IV bag, via bag adaptor. The syringe adaptor was then disconnected from the bag adaptor, and the IV bag was mixed well. Finally, 10 ml from the IV bag were aspirated into the syringe, which was then disinfected with ethanol before being removed from the glove box. A scheme of the trial procedure is depicted in Figure 3.
Figure 3.

Using Chemfort CSTD inside glove box. This procedure mimics actual drug reconstitution and preparation done by pharmacists for IV administration in clinics and hospitals
A total of 13 CSTDs were used in the study as follows. One CSTD was used according to the IFU in a glove box that was aerosolized by a sterile medium (NC). Twelve other CSTDs were used in a contaminated environment containing aerosolized HCoV-OC43 under the following conditions: (1) Three CSTDs were used with the Toxi-Guard filter according to the IFU, i.e., the septum was disinfected before every connection. (2) Three CSTDs were used with the Toxi-Guard filter but prior to liquid transfers were challenged by spraying HCoV-OC43 stock solution directly onto the outer surfaces of the septum and in the filter area, and then used according to the IFU (i.e., the septum was disinfected before every connection). (3) Three CSTDs without the Toxi-Guard were challenged prior to liquid transfers by spraying HCoV-OC43 stock solution, and then used according to the IFU. (4) Three CSTDs without the Toxi-Guard were challenged prior to liquid transfers by spraying HCoV-OC43 stock solution and were then not disinfected at the septum area before every connection.
In addition to sampling the liquid transferred through the CSTD, the air inside the glove box was sampled using an ACD-200 Bobcat Dry Filter Air Sampler (InnovaPrep, Drexel, MO, USA). Air samples were taken (1) at the beginning of the test, after aerosolizing the box with sterile medium (NC), (2) before initiating the liquid sampling using the CSTDs and after aerosolizing the glove box with HCoV-OC43 stock solution, (3) after sampling all CSTDs while the box was aerosolized continuously with virus stock. Two additional air samplings were carried out before (PC II) and after (end-point II) the testing of sampling group 4.
Viral RNA extraction
After sampling, viral RNA was extracted from each sample using the MagCore viral nucleic acid extraction kit (RBC Bioscience, New Taipei City, Taiwan) according to the manufacturer’s instructions. RNA extraction was performed in triplicate samples from each CSTD sampled. Following RNA purification, 30µl from each triplicate of each Chemfort sample set were combined into one tube to generate a total of 12 RNA samples.
Complementary DNA preparation and quantitative PCR
Complementary DNA (cDNA) was synthesized from each pool of extracted RNA sample using the Hy RT PCR Kit (Hylabs, Rehovot, Israel). Briefly, 12.4µl from each RNA sample were used for both the test samples and the no reverse transcriptase (-RT) controls. The cDNA and -RT samples were each diluted 1:2 and then used as templates for quantitative polymerase chain reaction (qPCR), in a mix containing specific primers for HCoV-OC43 (forward:5’-ATTGTCGATCGGGACCCAAG-3’;reverse:5’-TGTGCGCGAAGTAGATCTGG-3’)and platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, Waltham, MA, USA). The specificity of the HCoV-OC43 qPCR specific primers was demonstrated by using the cDNA of influenza A virus H1N1 as a template with the HCoV-OC43 specific primers, which resulted in high cycle threshold (CT; see explanation below)values or no signal at all (38.26, 36.93 and ‘ND’). As positive control, H1N1 cDNA was targeted by its corresponding specific primers (Forward: 5’-CTTCTAACCG AGGTCGAAACGTA-3’; reverse: 5’-GGTGACAGGATTGGTCTTGTCTTTA-3’), which resulted in CTvalues of 24.13, 24.21 and 24.51, respectively.
qPCR was performed using the CFX96 instrument (Bio-Rad, CA, USA) as follows:
Fluorophore: SYBR green
| Fluorophore: SYBR green | ||
|---|---|---|
| Temperature | Time | |
| 95°C | 3min | |
| 95°C | 25sec | X45 cycles |
| 57°C | 30sec | |
| 72°C | 20sec | |
- Plate read following each cycle
- Melt curve (65-95°C) + plate read
Determination of cycle threshold values using HCoV-OC43 standard curve
The cycle threshold (CT) is the qPCR cycle during which the fluorescence of the amplified target has crossed the threshold value, i.e., where the PCR product starts to accumulate and becomes detectable in the assay. Therefore, the lower the CT value is, the higher the target nucleic acid concentration is, and accordingly, the higher the CT value is, the lower the target nucleic acid concentration is. A HCoV-OC43 stock solution at a concentration of 5x106 plaque forming units (PFU)/ml was used for generating a standard curve. Six 10-fold dilutions were prepared to obtain the different standard curve samples, ranging from 5 to 5x105 PFU/ml. RNA was extracted from each dilution sample and analyzed by qPCR, as described above, for the samples obtained from the Chemfort devices. The cycle threshold value for each dilution sample was determined and plotted against the PFU/ml value.
The HCoV-OC43 standard curve is presented in Figure 4. The range for detected extracted viral RNA was 5x105 to5PFU/ml. NC values of viral RNA were 39 (determined as 5 PFU/ml according to the HCoV-OC43 standard curve) and were defined as the lower limit of detection/lower limit of quantification.
Figure 4.

HCoV-OC43 standard curve. A HCoV-OC43 stock titer used for generating the standard curve samples was: 5x106 plaque forming units (PFU)/ml. Six 10-fold dilutions were prepared to obtain the different standard curve samples. The cycle threshold (CT) value was determined by qPCR as described in the Methods section. A negative control containing no virus sample was also used. The obtained standard curve equation is y = -3.429x + 38.889. The range for detected extracted viral RNA was 5x105 to 5PFU/ml
Statistical analysis
CFX Manager 2.1 software package (Bio-Rad, CA, USA) was used for data analysis.
RESULTS
Air sampling
When the glove box was aerosolized with sterile medium (negative control), no viral RNA was detected in the air. However, when the box was aerosolized with the HCoV-OC43 stock solution (positive control), the air samples showed low CT values, indicative of the presence of viral RNA corresponding roughly to 35000-130000 PFU/ml. The air sampling results for negative and positive control for the glove box, before and after the CSTDs were tested, are presented in Table 1.
Table 1.
qPCR CT results of air samples filters
| Filter sample | CT value (PFU/ml) | ||
|---|---|---|---|
| Replicate | |||
| 1 | 2 | 3 | |
| Negative control1 | 39.96 (0.5) | ND | ND |
| Positive control2 | 21.47 (120000) | 21.35 (130000) | 21.41 (120000) |
| Endpoint3 | 23.29 (35000) | 23.25 (36000) | 23.28 (36000) |
| Positive control II | 22.89 (46000) | 22.72 (52000) | 22.70 (53000) |
| Endpoint II | 22.64 (55000) | 22.62 (55000) | 22.60 (56000) |
Negative control: filter of air sampled from box aerosolized with sterile medium
Positive control: filter of air sampled from box aerosolized with HCoV-OC43 stock solution
Endpoint: filter of air sampled from box aerosolized with HCoV-OC43 stock after the test of CSTDs was complete
CT=cycle threshold, ND=not detected, PFU=plaque forming units
Liquid sample transition
The results of HCoV-OC43 RNA detection in samples obtained using Chemfort vial adaptors(with or without Toxi-Guard) under various conditions in different sample groups are presented in Table 2. When liquid samples were transferred using the adaptors containing the Toxi-Guard system and septa were disinfected with isopropanol according to the IFU, there was no evidence of viral RNA traces, even when the vial adaptors were directly sprayed with an HCoV-OC43 stock solution (Table 2, sample groups 1 and 2). In contrast, liquid samples that were transferred by the same procedure using vial adaptors not containing the Toxi-Guard filter that were also challenged by spraying HCoV-OC43 stock solution, were found to be positive for viral RNAranging between 0.9 and 8.6 PFU/ml (Table 2, sample groups 3 and 4). Notably, absence of the Toxi-Guard system, resulted in detection ofviral RNA in the liquid samples regardless of whether the vial adaptor at the septum area was disinfected.
Table 2.
Detection of HCoV-OC43RNA in samples obtained using Chemfort vial adaptors (with or without Toxi- Guard) under various conditions
| Group1 | Sample description | Biological repeat | CT value (PFU/ml) | ||
|---|---|---|---|---|---|
| Replicate number | |||||
| 1 | 2 | 3 | |||
| Negative control1 | Disinfected2 Toxi-Guard vial adaptor (negative control) | 1 | ND | ND | ND |
| 1 | Disinfected2 Toxi-Guard vial adaptor |
1 | ND | ND | ND |
| 2 | ND | ND | ND | ||
| 3 | ND | ND | ND | ||
| 2 | Challenged3 and disinfected Toxi-Guard vial adaptor | 1 | ND | ND | ND |
| 2 | ND | ND | ND | ||
| 3 | ND | ND | ND | ||
| 3 | Challenged and disinfected2 vial adaptor Toxi-Guard-less4 |
1 | ND | 38.86 (1.0) | ND |
| 2 | 35.90 (7.5) | 35.68 (8.6) | 35.80 (8.0) | ||
| 3 | ND | ND | 38.19 (1.6) | ||
| 4 | Challenged vial adaptor Toxi-Guard-less4 |
I | ND | ND | 38.10 (1.7) |
| 2 | ND | ND | ND | ||
| 3 | 39.03 (0.9) | 36.12 (6.4) | 37.16 (3.2) | ||
Groups 1-4 were used in an HCoV-OC43 aerosolized box, while the Negative Control group was used in a sterile medium aerosolized box.
“Disinfected”: the septa areas only were disinfected with isopropanol pads before every connection of Chemfort device according to the product’s instructions for use.
“Challenged”: the septa and filter areas were directly sprayed with 1.5 ml of HCoV-OC43 stock solution.
“Toxi-Guard-less”: vial adaptor lacking the protective plastic cover as well as the sterilizing membrane and the activated carbonfilter (i.e., the Toxi-Guard)
CT=cycle threshold, ND=not detected, PFU=plaque forming units
DISCUSSION
The benefits of CSTD systems in preventing exposure to hazardous drugs, such as antineoplastic agents and antibiotics are well established.14-17 With regards to preventing contamination of the drug preparation, CSTDs have been recently shown to prevent contamination by Gram-positive bacteria, Gram-negative bacteria, yeast, and mold.18 In addition, it was found that vials punctured in ISO5 conditions with a CSTD demonstrated a low frequency of microbial contamination.19 However, viruses are often more difficult to detect than other microbial contaminants.7 As far as we know, our study is the first to demonstrate that CSTD with an air-cleaning technology can prevent ingress of external coronavirus and airborne contaminants into liquids. In this study, CSTDs were used to transfer liquid between an IV bag and an empty vial and back to the bag, which mimics drug reconstitution and preparation for IV administration in clinics and hospitals, as routinely performed by pharmacists (Figure 3). Therefore, the results of this study, demonstrating that CSTDs with air-cleaning technology can prevent internal viral contamination, are highly important for clinical facilities, where patients can be exposed to different hazardous threats.
The results of our study demonstrate the efficacy of Chemfort CSTD in preventing viral penetration, as shown by air sample results (Table 1). The generation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) bioaerosols plays an important role in the COVID-19 pandemic. Genetic materials of coronaviruses have been detected in air in several studies.20-26 SARS-CoV-2 was shown to retain infectivity and virion integrity for up to 16 hours in respirable-sized aerosols.27 According to Lee, the minimum size of a respiratory particle that can contain SARS-CoV-2 is approximately 4.7μm.28 The minimum size of the particles can decrease due to the evaporation of water on the particle surfaces. Moreover, high viral loads can decrease the minimum size of respiratory particles containing SARS-CoV-2, thereby increasing the probability of aerosol generation of the viruses. The particle size of the coronavirus used in this study, HCoV-OC43, is 0.8-0.12 µm smaller than the particle size of SARS-CoV-2.29,30
In our study, we investigated the Chemfort CSTD, which is based on air-cleaning technology (Chemfort/OnGuard^2). As mentioned, there are also devices are based on a physical barrier technology, where a balloon or a closed chamber are used to hold the air during drug reconstitution. It may be that our results may not be relevant for physical-barrier CSTDs, which may act differently when exposed to viral contamination during drug reconstitution and preparation.
CONCLUSIONS
In conclusion, Chemfort CSTD with the integral Toxi-Guard system was found effective in preventing viral contamination of liquids transferred by the device. During the COVID-19 epidemic, use of a CSTD with an activated carbon system can be of importance to protect already vulnerable patients from further complications due to viral infection.
ACKNOWLEDGEMENTS
The authors would like to thank Sharon Furman-Assaf, PhD, for her assistance in manuscript preparation.
Contributor Information
Maya Amichay, Hy Laboratories Ltd., Rehovot, Israel. maya@hylabs.co.il.
Ortal Shimon, Hy Laboratories Ltd., Rehovot, Israel. ortal@hylabs.co.il.
Eitan Raveh, Simplivia Healthcare Ltd., Kiryat Shmona, Israel. eitan.raveh@simplivia.com.
CONFLICTS OF INTEREST
Maya Amichay and Ortal Shimon declare no conflict of interest relating to the material presented in this article. Eitan Raveh is employed by Simplivia Healthcare Ltd.
FUNDING
Funding for this project was provided by Simplivia Healthcare Ltd, manufacturer of the CSTD studied.
References
- 1.Hernandez-Ramos I, Gaitan-Meza J, Garcia-Gaitan E, et al. Extrinsic contamination of intravenous infusates administered to hospitalized children in Mexico. Pediatr Infect Dis J. 2000;19:8–90. doi: 10.1097/00006454-200009000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Macias AE, de Leon SP, Huertas M, et al. Endemic infusate contamination and related bacteremia. Am J Infect Control. 2008;36:48–53. doi: 10.1016/j.ajic.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Macias AE, Huertas M, de Leon SP, et al. Contamination of intravenous fluids:a continuing cause of hospital bacteremia. Am J Infect Control. 2010;38:217–21. doi: 10.1016/j.ajic.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Muller AE, Huisman I, Roos PJ, et al. Outbreak of severe sepsis due to contaminated propofol:lessons to learn. J Hosp Infect. 2010;76:225–30. doi: 10.1016/j.jhin.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Vonberg RP, Gastmeier P. Hospital-acquired infections related to contaminated substances. J Hosp Infect. 2007;65:15–23. doi: 10.1016/j.jhin.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Barone PW, Wiebe ME, Leung JC, et al. Viral contamination in biologic manufacture and implications for emerging therapies. Nature Biotechnology. 2010;38:563–72. doi: 10.1038/s41587-020-0507-2. [DOI] [PubMed] [Google Scholar]
- 7.Merten OW. Virus contaminations of cell cultures - A biotechnological view. Cytotechnology. 2002;39:91–116. doi: 10.1023/A:1022969101804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besheer A, Burton L, Galas RJ, et al. An Industry Perspective on Compatibility Assessment of Closed System Drug-Transfer Devices for Biologics. J Pharm Sci. 2021;110:610–4. doi: 10.1016/j.xphs.2020.10.047. [DOI] [PubMed] [Google Scholar]
- 9.NIOSH. Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings. 2004 [Google Scholar]
- 10.Levin G, Sessink PJ. Validation of chemotherapy drug vapor containment of an air cleaning closed-system drug transfer device. J Oncol Pharm Pract. :10781552211030682. doi: 10.1177/10781552211030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sessink PJM, Nyulasi T, Haraldsson ELM, et al. Reduction of Contamination with Antibiotics on Surfaces and in Environmental Air in Three European Hospitals Following Implementation of a Closed-System Drug Transfer Device. Ann Work Expo Health. 2019;63:459–67. doi: 10.1093/annweh/wxz010. [DOI] [PubMed] [Google Scholar]
- 12.Sellaoui L, Badawi M, Monari A, et al. Make it clean, make it safe:A review on virus elimination via adsorption. Chemical engineering journal (Lausanne, Switzerland :1996) 2021;412:128682. doi: 10.1016/j.cej.2021.128682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Health Protection Agency, UK Antiviral Testing Of FlexzorbTM. data on file. 2009 [Google Scholar]
- 14.Bartel SB, Tyler TG, Power LA. Multicenter evaluation of a new closed system drug-transfer device in reducing surface contamination by antineoplastic hazardous drugs. Am J Health Syst Pharm. 2018;75:199–211. doi: 10.2146/ajhp160948. [DOI] [PubMed] [Google Scholar]
- 15.Harrison BR, Peters BG, Bing MR. Comparison of surface contamination with cyclophosphamide and fluorouracil using a closed-system drug transfer device versus standard preparation techniques. Am J Health Syst Pharm. 2006;63:1736–44. doi: 10.2146/ajhp050258. [DOI] [PubMed] [Google Scholar]
- 16.Sessink PJ, Connor TH, Jorgenson JA, et al. Reduction in surface contamination with antineoplastic drugs in 22 hospital pharmacies in the US following implementation of a closed-system drug transfer device. Journal of Oncology Pharmacy Practice. 2011;17:39–48. doi: 10.1177/1078155210361431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valero S, Lopez-Briz E, Vila N, et al. Pre and post intervention study of antiblastic drugs contamination surface levels at a Pharmacy Department Compounding Area using a closed system drug transfer device and a decontamination process. Regul Toxicol Pharmacol. 2018:95. doi: 10.1016/j.yrtph.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Mills A, Yousef M. Sterility testing using a closed system transfer device in oncology medication compounding:a novel method for testing partially used vials. Drugs &Therapy Perspectives. 2021;37:206–11. doi: 10.1007/s40267-021-00823-4. [DOI] [Google Scholar]
- 19.Soubieux A, Tanguay C, Bussieres JF. Review of studies examining microbial contamination of vials used for preparations done with closed-system drug transfer devices. Eur J Hosp Pharm. 2021;28:65–70. doi: 10.1136/ejhpharm-2019-001913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azhar EI, Hashem AM, El-Kafrawy SA, et al. Detection of the Middle East respiratory syndrome coronavirus genome in an air sample originating from a camel barn owned by an infected patient. mBio. 2014;5:e01450–14. doi: 10.1128/mBio.01450-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chia PY, Coleman KK, Tan YK, et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun. 2020;11:2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583–91. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lednicky JA, Shankar SN, Elbadry MA, et al. Collection of SARS-CoV-2 Virus from the Air of a Clinic Within a University Student Health Care Center and Analyses of the Viral Genomic Sequence. Aerosol Air Qual Res. 2020;20:1167–71. doi: 10.4209/aaqr.2020.02.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–60. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 25.Santarpia JL, Rivera DN, Herrera VL, et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Scientific Reports. 2020;10:12732. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson NM, Norton A, Young FP, et al. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers:a narrative review. Anaesthesia. 2020;75:1086–95. doi: 10.1111/anae.15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fears AC, Klimstra WB, Duprex P, et al. Persistence of Severe Acute Respiratory Syndrome Coronavirus 2 in Aerosol Suspensions. Emerg Infect Dis. 2020:26. doi: 10.3201/eid2609.201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee BU. Minimum Sizes of Respiratory Particles Carrying SARS-CoV-2 and the Possibility of Aerosol Generation. Int J Environ Res Public Health. 2020:17. doi: 10.3390/ijerph17196960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu DX, Liang JQ, Fung TS. nHuman Coronavirus-229E, -OC43 -NL63 and -HKU1 (Coronaviridae) Encyclopedia of Virology. 2021:428–40. doi: 10.1016/B978-0-12-809633-8.21501-X. [DOI] [Google Scholar]
- 30.Vijgen L, Keyaerts E, Moes E, et al. Complete genomic sequence of human coronavirus OC43:molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J Virol. 2005;79:1595–604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


