Abstract
We compared four doses of amphotericin B lipid complex (ABLC) with three doses of fluconazole in temporarily neutropenic mice in a murine model of disseminated candidiasis due to four different isolates of Candida tropicalis. The mice were infected with a 90% lethal dose of four strains of C. tropicalis for which the fluconazole MICs ranged from 1 to >125 mg/liter 3 days after receiving 200 mg of cyclophosphamide/kg of body weight. Treatment was started 18 h after infection and lasted for 7 days. ABLC (1, 2, 5, and 10 mg/kg) was administered once a day intravenously, fluconazole was administered by oral gavage once daily (25 and 50 mg/kg/day) or twice daily (125 mg/kg). MICs determined in five different ways with 24- and 48-h endpoints were also compared. The overall survival rates were controls, 14%; fluconazole, 64%; and ABLC, 82%. Treatment with ABLC at 2 to 10 mg/kg increased survival compared to controls (P = <0.0001) and was also superior to fluconazole at 25 and 50 mg/kg (P = 0.006). In the fluconazole-resistant C. tropicalis model (MIC, 128 μg/ml), ABLC at 2 to 10 mg/kg was superior to fluconazole at 250 mg/kg and ABLC at 10 mg/kg was superior to all fluconazole doses (P = <0.05). Fluconazole at 250 mg/kg daily was superior to both 25 and 50 mg/kg at reducing mortality with most isolates. ABLC was superior to fluconazole (P = <0.01), and fluconazole at 250 mg/kg was superior to fluconazole at both 25 and 50 mg/kg (P = 0.02) in all models at reducing C. tropicalis counts in the kidneys. Neither drug consistently sterilized the brain or kidneys. A 48-h endpoint reading with the NCCLS susceptibility testing microtiter variation overestimates resistance to fluconazole. ABLC is an effective treatment for fluconazole-resistant C. tropicalis at all doses tested.
The incidence of candidal bloodstream infections has increased dramatically over the last 3 decades, and they are now a commonplace complication of many surgical and medical therapies. Recently, there has been a huge increase in the frequency of non-albicans candidemia (1, 17, 20). Increases in Candida tropicalis (4 to 24%) have been noted particularly from blood cultures from leukemic, oncology, and intensive-care unit patients.
Amphotericin B lipid complex (ABLC) consists of the antifungal agent amphotericin B complexed to two phospholipids in a 1:1 drug-to-lipid ratio. ABLC has been shown to be better tolerated than conventional amphotericin B therapy, particularly with regard to nephrotoxicity (21, 22).
Resistance to antifungal drugs among pathogenic yeasts is increasingly being recognized, particularly with the azole group of drugs (2). Recently, C. tropicalis resistance to fluconazole (FLU) (48%) has been highlighted as a problem in the North West of England, with treatment failures leading to fatal outcomes (6). However, the optimal method for susceptibility testing of C. tropicalis is uncertain, with some authors recommending a 24-h endpoint (14), but there is no consensus on other susceptibility testing parameters. The present broth macrodilution methodology recommended by the NCCLS (National Committee for Clinical Laboratory Standards) is cumbersome and is not applicable to the routine clinical setting. In this study, therefore, we compared two broth microdilution methods, that of NCCLS and the EUCAST (European Committee for Standardisation of Antibiotic Susceptibility Testing) proposed standard, with one broth macrodilution method using high-resolution (HR) medium and one plate method, E-test on RPMI agar, of MIC measurement.
In this study, we tested ABLC in an immunocompromised-mouse model (10) of disseminated candidiasis against FLU-resistant and FLU-susceptible C. tropicalis and compared its efficacy to that of FLU.
MATERIALS AND METHODS
Three clinical isolates of C. tropicalis from Hope Hospital and one isolate from the American Type Culture Collection (ATCC 750) were used for this study. C. tropicalis FA1572 was isolated from a throat swab, FA2317 was isolated from the sputum of an intensive-care unit patient, and FA2542 was isolated from a tracheal aspirate of an intensive-care unit patient. The strains were maintained on slopes of Oxoid Sabouraud dextrose agar (Unipath Limited, Basingstoke, England) supplemented with 0.05 g of chloramphenicol/liter. Long-term storage was at −70°C in nutrient broth (Unipath Limited) supplemented with 15% glycerol (Sigma-Aldrich, Poole, Dorset, United Kingdom).
In vitro susceptibility testing against FLU.
Four methods were compared and are summarized in Table 1.
TABLE 1.
Summary of in vitro susceptibility methods against FLU
| Parameter | Value
|
||||
|---|---|---|---|---|---|
| NCCLS | EUCAST proposed | HR medium | E-Test
|
||
| RPMI-MOPS | RPMI-phosphate | ||||
| Format | Microplate | Microplate | Test tube | Agar plate | Agar plate |
| Medium | RPMI | RPMI-glucose | HR medium | RPMI-MOPS | RPMI-phosphate |
| Inoculum | 5 × 102–2.5 × 103/ml | 1 × 105–5 × 105/ml | 104/ml | McFarland 0.5 | McFarland 0.5 |
| Temperature (°C) | 37 | 37 | 37 | 37 | 37 |
| Reading time (h) | 24 and 48 | 24 and 48 | 24 and 48 | 24 and 48 | 24 and 48 |
| Endpoint determination | Spectrophotometer | Spectrophotometer | Eye | Eye | Eye |
| Endpoint inhibition | 80% | 50% | 80% | See technical guide 4b | See technical guide 4b |
(i) NCCLS M27A method using the microtiter variation.
The in vitro susceptibility results of the isolates were tested on three occasions using the NCCLS M27-A broth microdilution method (9). The stock suspension of the organism (0.5 McFarland standard) was diluted 1:100 in saline followed by a 1:20 dilution in RPMI 1640 broth (Sigma-Aldrich). The organisms were added to previously prepared dilutions of FLU in the range from 0.03 to 128 μg/ml in a microdilution plate and incubated at 37°C. The plates were read on a Molecular Devices (Menlo Park, Calif.) Thermomax microplate reader at 490 nm after 24- and 48-h incubations using an 80% reduction in the optical density endpoint.
(ii) EUCAST method in development.
The EUCAST method (J. L. Rodriguez-Tudela, personal communication) is also a broth microdilution method, but it uses an inoculum of 1 × 105 to 5 × 105 organisms/ml prepared spectrophotometrically and then diluted in RPMI plus 2% glucose (Sigma-Aldrich) buffered with morpholinepropanesulfonic acid (MOPS) (Sigma-Aldrich). One hundred microliters of the organism suspension is then added to 100 μl of fluconazole diluted in RPMI plus 2% glucose (final range, 0.5 to 128 μg/liter). The plates were incubated at 37°C and read at 24 and 48 h at 490 nm on a Thermomax microplate reader with a 50% reduction in the optical density endpoint.
(iii) HR broth macrodilution method.
For the HR broth macrodilution method (6), inoculum concentrations of 1.2 × 104 organisms/ml were prepared in HR medium (Unipath Limited), and 0.9 ml of this suspension was added to 0.1 ml of FLU (range, 1.25 to 1,280 μg/ml) in sterile test tubes (final concentration of organisms, 104/ml; final range of FLU, 0.125 to 128 μg/ml). The tubes were mixed well and then incubated at 37°C and read at 24 and 48 h. After being vortexed, the tubes were read by eye, with an 80% reduction in growth taken as the endpoint.
(iv) E-test sensitivities.
For the E-test (E-test technical guide 4b), 90-mm-diameter plates containing RPMI agar to a depth of 4 mm were prepared. The RPMI agar was buffered with MOPS or phosphate buffers, and 0.5 McFarland standard inocula were applied to the agar surface with a cotton swab and then allowed to dry. E-test strips (AB Biodisc, Solna, Sweden) were then applied to the surface. The plates were incubated at 37°C and read at 24 and 48 h. The MIC was read as the point where growth touched the strip. When a diffuse growth of microcolonies within the zone of inhibition was observed, the endpoint was selected at the point of 80% inhibition of growth touching the strip.
In vitro susceptibility testing against amphotericin B: antibiotic medium 3 microdilution method.
Inoculum concentrations of 2 × 103 organisms/ml were prepared in antibiotic medium 3 (Difco, Detroit, Mich.) (13), and 100 μl of the suspension was added to 100 μl of amphotericin B (range, 0.015 to 16 μg/ml) in a sterile microdilution plate (final concentration of organisms, 103/ml; final range of amphotericin B, 0.0075 to 8 μg/ml). The plates were mixed well and then incubated in a moist chamber at 37°C and read after 48 h of incubation at 490 nm on a Thermomax microplate reader with an 80% reduction in the optical density endpoint.
Animal models. (i) Animals.
Male CD1 mice 5 to 6 weeks old and weighing between 22 and 25 g were purchased from Charles River UK Ltd. (Margate, Kent, United Kingdom). The mice were virus free and were allowed free access to food and water. The mice were randomized into groups of 10.
(ii) Immunosuppression.
Cyclophosphamide (Sigma-Aldrich) was administered intravenously via the lateral tail vein to all animals at a dose of 200 mg/kg of body weight. A state of profound neutropenia was achieved 3 days after administration and lasted for at least 4 days (3).
(iii) Preparation of inoculum.
For each experiment, an isolate was thawed and then incubated overnight on Sabouraud dextrose agar (Unipath Limited). One colony was transferred into 25 ml of Sabouraud dextrose broth (Unipath Limited). The broth was incubated on an orbital mixer for 8 h at 37°C and then centrifuged to pellet the organisms. The cells were washed in saline and then resuspended in saline, and their density was adjusted using their optical density at 490 nm.
(iv) Infection of mice.
Prior to each experiment, inoculum-finding studies for each isolate were performed using intravenous injections of 0.15 ml of a range of yeast densities. The 90% lethal dose (LD90) was defined as the inoculum of the organism which caused 90% mortality 10 days postinfection. The inocula for the isolates were as follows: ATCC 750, 7.5 × 106/ml; FA1572, 3.5 × 106/ml; FA2542, 3 × 106/ml; and FA2317, 6.5 × 106/ml. These inocula were used in the study. Mice were infected with 0.15 ml of the desired inoculum on day 0 via the lateral tail vein. Postinfection viability counts were performed to ensure the correct inoculum had been given (all were within 5% of expected values).
(v) Antifungal therapy.
ABLC (The Liposome Company Inc., Hammersmith, London, United Kingdom) was gently resuspended according to the manufacturer's instructions and then diluted in sterile 5% glucose (Baxter Healthcare, Norfolk, United Kingdom) to provide ABLC at 1, 2, 5, and 10 mg/kg. FLU (Pfizer Ltd., Sandwich, Kent, United Kingdom) was diluted in sterile saline plus 0.03% Noble agar (Unipath Limited) to provide doses of 25, 50, and 125 mg/kg. Both drugs were prepared immediately before use. All doses of ABLC were given via intravenous injection (0.1 ml) into the lateral tail vein once daily. FLU was administered by gavage (0.125 ml) either once daily for the 25- and 50-mg/kg doses or twice daily for the 250-mg/kg dose (two doses of 125 mg/kg at 12-h intervals). All treatments started 18 h after infection and continued for 7 days postinfection. Control mice were infected but received no active treatment. One group received 5% glucose intravenously, and the second received saline plus 0.03% agar by gavage. Mice unable to reach the feeder or in severe distress were euthanized.
On day 11 of the experiment, all surviving mice were culled. The brains, kidneys, livers, and lungs were removed and transferred into 2 ml of sterile phosphate-buffered saline (BDH, Poole, Dorset, United Kingdom). The organs were homogenized in a tissue grinder (Polytron, Kinematica AG, Lucerne, Switzerland) for approximately 15 to 30 s and then diluted 10−1, 10−2, and 10−3. One hundred microliters each of the neat and diluted suspensions was then transferred to Sabouraud dextrose agar (Unipath Limited), and the liquid was spread over the surfaces of the plates. The plates were incubated at 37°C in a moist atmosphere and examined daily for 5 days. Colony counts were recorded from all plates that showed growth.
(vi) FLU pharmacokinetics.
Blood samples were collected from a separate group of mice by cardiac puncture to determine the pharmacokinetics of the FLU treatment (all levels were collected in duplicate). In this study, all mice were immunosuppressed with 200 mg of cyclophosphamide/kg administered 4 days before the first dose of FLU (as in the C. tropicalis models), but they were uninfected. Samples were collected in plain tubes and allowed to clot at room temperature. Serum was then removed and stored at −20°C until it was analyzed. Samples were thawed and analyzed as a batch in bioassays using RPMI MOPS agar and the Candida kefyr San Antonio strain (Technical note on bioassay of fluconazole and other antifungal agents, Pfizer Central Research, Sandwich, Kent, United Kingdom).
(vii) Statistical analysis.
Mortality and culture data were analyzed using the Mann-Whitney U test or the Kruskall-Wallis test if the Mann-Whitney test was not possible (i.e., if all values were identical in one group). Two-sided P values are given. Mice which died before day 10 were assumed to have organ colony counts at least as high as the highest counts in surviving mice in the calculation of culture result statistics. All data analysis was performed using the computer package Arcus Quik Stat (Addison Wesley Longman Ltd.). Two-sided probability values are quoted in the text.
RESULTS
In vitro FLU susceptibility data.
The isolates were selected to include one unequivocally FLU-resistant strain (FA1572), one unequivocally susceptible strain (FA2317), and two strains with variable results.
In vitro susceptibility data is shown in Table 2. The final interpretations of the in vitro susceptibility data were as follows. FA1572 was resistant by all methods (MIC, 64 to >256 μg/ml), FA2317 was susceptible by all methods (MIC, 0.5 to 4 μg/ml), and both FA2542 and ATCC 750 had trailing endpoints in the NCCLS test at 48 h but were susceptible by the other methods.
TABLE 2.
FLU MICs for four isolates of C. tropicalis determined by five different methods
| Isolate | MIC (μg/ml)
|
Interpretatione
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCCLSa
|
EUCASTb
|
HR mediume
|
E-Testd
|
|||||||||
| RPMI MOPS
|
RPMI Phos
|
|||||||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | In vitro | In vivo | |
| FA1572 | 64 | 128 | 128 | 128 | 64 | 64 | >256 | >256 | >256 | >256 | R | R |
| FA2317 | 1 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.75 | 1 | 2 | 4 | S | S |
| FA2542 | 0.25 | >128f | 0.25 | 0.25 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 1.5 | S | S |
| ATCC 750 | 0.5 | 8f | 0.25 | 2 | 0.5 | 1 | 0.5 | 0.5 | 0.75 | 2 | S | S |
NCCLS M27A protocol.
EUCAST method in final development.
HR broth method.
RPMI, RPMI 1640 agar; Phos, phosphate buffer.
R, resistant; S, susceptible.
Trailing endpoint (a gradual reduction in amount of growth in wells with increasing drug concentration rather than reduction occurring over one or two wells as in a normally susceptible isolate). The MIC is recorded as the drug concentration at which >80% inhibition is achieved.
In vitro amphotericin B susceptibility data.
Susceptibilities were determined on at least three occasions using an antibiotic medium 3 microdilution method (13). All strains were susceptible to amphotericin B at the following MICs: C. tropicalis FA1572 and ATCC 750, 0.015 μg/ml, and FA2317 and FA2542, 0.03 μg/ml.
In vivo mortality data.
The mortality in each experiment is shown in Fig. 1 to 4 and Tables 3 to 7. Control mice had mortalities of 60 to 100% in all models, demonstrating the very high mortality of this model with no active intervention. In all models, the animals became very sick within 24 h of infection, showing reduced mobility and a hunched appearance. Many animals never recovered from this morbidity and were culled when they were no longer able to reach food and water. After this initial period of severe morbidity, the condition of the mice gradually improved, and most mice surviving to day 11 showed no signs of severe disease. The LD90 of ATCC 750 was slightly higher than those of other strains and caused a relatively rapid 100% mortality (in 3 days). Marginally lower infecting doses of this strain caused much lower and unpredictable mortality (data not shown). The efficacy of ABLC at 10 mg/kg was demonstrated in all models, with 80 to 100% of the mice surviving. Slightly reduced survival rates were seen for ABLC at 5 and 2 mg/kg, with both having overall survival rates of 70 to 100%. ABLC at 1 mg/kg was less effective (30 to 90% survival) in all models, but the differential between 10 and 1 mg/kg varied substantially. The efficacy of FLU at 250 mg/kg/day was variable (40 to 100% survival), and these results were affected by what appeared to be drug toxicity in the ATCC 750 model. FLU at 50 and 25 mg/kg/day produced variable survival rates (30 to 80%), and these rates were dependent on the strain causing infection.
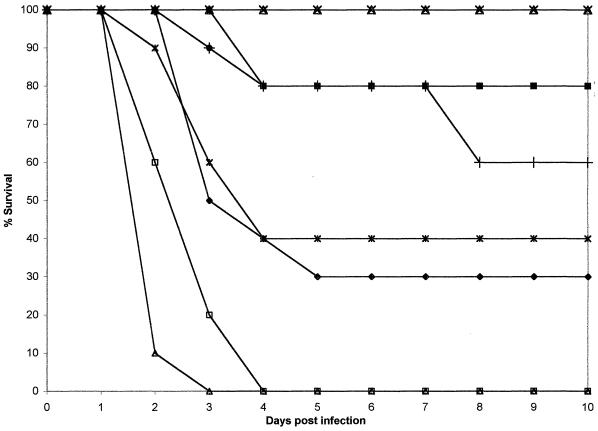
FIG. 1.
Plot of cumulative mortality against time in a murine model against C. tropicalis FA1572. ×, ABLC at 10 mg/kg; ▴, ABLC at 5.0 mg/kg; ■, ABLC at 2.0 mg/kg; ⧫, ABLC at 1.0 mg/kg; ✠, FLU at 250 mg/kg; ●, FLU at 50 mg/kg; ✚, FLU at 25 mg/kg, □, agar control; ▵, 5% glucose control.
FIG. 4.
Plot of cumulative mortality against time in a murine model against C. tropicalis ATCC 750. ×, ABLC at 10 mg/kg; ▴, ABLC at 5.0 mg/kg; ■, ABLC at 2.0 mg/kg; ⧫, ABLC at 1.0 mg/kg; ✠, FLU at 250 mg/kg; ●, FLU at 50 mg/kg; ✚, FLU at 25 mg/kg; □, agar control; ▵, 5% glucose control.
TABLE 3.
Geometric means of organ culture counts for C. tropicalis FA1572
| Treatment | Survivors/no. in group (%) | Sterilizeda | Mean countb
|
|||
|---|---|---|---|---|---|---|
| Brain | Kidneys | Liver | Lungs | |||
| ABLC (10 mg/kg) | 10/10 (100) | 2 | NDd | 5 | 2 | 0 |
| ABLC (5 mg/kg) | 7/9 (88) | 0 | ND | 2.7 × 102 | 40 | 0 |
| ABLC (2 mg/kg) | 10/10 (100) | 0 | ND | 7.9 × 102 | 20 | 0 |
| ABLC (1 mg/kg) | 5/10 (50) | 0 | 1.8 × 102 | 1.9 × 104 | 3.6 × 103 | 0 |
| FLU (250 mg/kg) | 8/10 (80) | 0 | 20 | 1.8 × 104 | 3.7 × 102 | 0 |
| FLU (50 mg/kg) | 4/9 (45) | 0 | 1.1 × 104 | 3.0 × 105 | 1.1 × 104 | 50 |
| FLU (25 mg/kg) | 4/10 (40) | 0 | 8.8 × 103 | 1.0 × 106 | 3.3 × 103 | 1.3 × 102 |
| Agar-glucosec | 0/20 (0) | 0 | ||||
Sterilized, number of mice in which all cultures were negative.
All counts are expressed as CFU/ml of organ homogenate.
Control groups combined (n = 20).
ND, not determined.
TABLE 7.
Probability values (two sided) of mortality in models
| Inferior regime | Dose (mg/kg) | Probabilitya with superior regime of:
|
||
|---|---|---|---|---|
| ABLC (10 mg/kg) | ABLC (5 mg/kg) | FLU (250 mg/kg) | ||
| FLU | 250 | NS/NS/NS/0.002 | NS/NS/NS/0.002 | |
| FLU | 50 | 0.04/NS/NS/NS | NS/NS/NS/NS | NS/NS/NS/0.007 |
| FLU | 25 | 0.01/NS/NS/NS | NS/NS/NS/NS | NS/NS/NS/NS |
| ABLC | 5 | NS/NS/NS/NS | NS/NS/NS/0.002b | |
| ABLC | 2 | NS/NS/NS/NS | NS/NS/NS/NS | NS/NS/NS/0.002b |
| ABLC | 1 | 0.009/NS/0.004/0.0009 | NS/NS/NS/0.0009 | NS/NS/NS/NS |
| Controls | <0.0001/NS/<0.0001/<0.0001 | 0.003/NS/0.006/<0.0001 | 0.003/NS/<0.0001/0.008 | |
Probabilities are shown as follows: FA1572/FA2317/FA2542/ATCC 750. NS, not significant.
ABLC at 5 and 2 mg/kg was superior to FLU at 250 mg/kg/day in the ATCC 750 model.
In the FA1572 model treated with ABLC at doses from 2 to 10 mg/kg, treatment was effective (87.5 to 100% survival), but a lower dose was less so (50% survival). Treatment with FLU was most successful with 250 mg/kg/day, but only 40% survival was achieved with 25 or 50 mg/kg/day. The data are consistent with a partially resistant, intermediate, or susceptible dose-dependent MIC interpretation. However, serum drug concentrations of 50 mg/kg/day were relatively high by human standards.
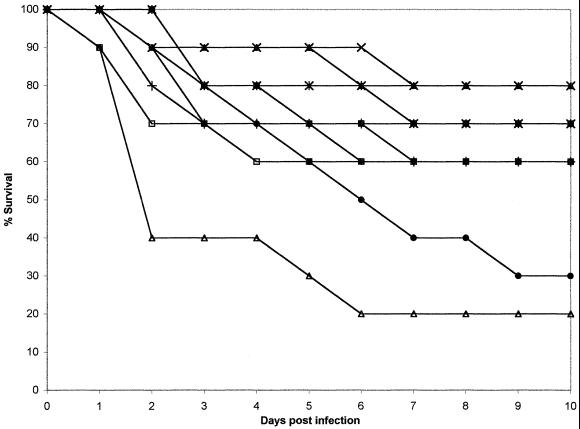
In the FA2317 model, 40% of the control mice survived with no active treatment. Survival after all doses of ABLC was between 60 and 80%. Treatment with FLU at 250 and 25 mg/kg/day produced 60 to 70% survival (only 30% of the mice survived after treatment with FLU at 50 mg/kg/day. These data are consistent with a susceptible MIC interpretation.
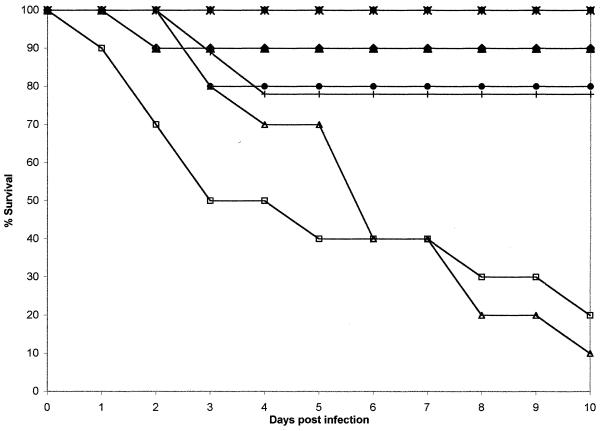
In the FA2542 model only 15% of the control mice survived. All ABLC regimes produced a survival rate between 90 and 100%. FLU at 250 mg/kg/day produced 100% survival, which was not significantly superior to the 25- and 50-mg/kg/day doses (78 to 80% survival). These data are consistent with a susceptible MIC interpretation.
In the ATCC 750 model, rapid mortality occurred with no active treatment (all controls had died by day 3). Treatment with ABLC at 2 to 10 mg/kg allowed 80 to 100% survival, but treatment with ABLC at 1 mg/kg/day was less effective, with only 30% survival. FLU treatment at 250 mg/kg/day appeared toxic, as only 40% of the mice survived, whereas with 25 and 50 mg/kg/day, 60 to 70% of the mice survived. Therefore, once again these data are consistent with a susceptible MIC interpretation. The reason for the FLU toxicity at 250 mg/kg/day in this model is uncertain, but it was notable that in the early stages of infection with this strain, the mice were particularly severely affected and stopped eating and drinking. It is likely that the combination of high drug levels and severe dehydration caused increased mortality, but this is speculative. No toxicity was noted with 250 mg of FLU/kg/day in immunosuppressed but uninfected mice.
Organ culture data.
Geometric mean colony counts of the brain, kidneys, liver, and lungs are shown in Tables 3 to 6.
TABLE 6.
Geometric means of organ culture counts for C. tropicalis ATCC 750
| Treatment | Survivors/ no. in group (%) | Sterilizeda | Mean countb
|
|||
|---|---|---|---|---|---|---|
| Brain | Kidneys | Liver | Lungs | |||
| ABLC (10 mg/kg) | 10/10 (100) | 0 | 29 | 3 × 102 | <1 | 0 |
| ABLC (5 mg/kg) | 10/10 (100) | 0 | 23 | 3.7 × 102 | <1 | 0 |
| ABLC (2 mg/kg) | 8/10 (80) | 0 | 47 | 4.9 × 103 | 1 | 0 |
| ABLC (1 mg/kg) | 3/10 (30) | 0 | 247 | 2.2 × 105 | 81 | 0 |
| FLU (250 mg/kg) | 4/10 (40) | 0 | 24 | 1.3 × 105 | 13 | 0 |
| FLU (50 mg/kg) | 9/10 (90) | 0 | 42 | 4.2 × 105 | <1 | 0 |
| FLU (25 mg/kg) | 6/10 (60) | 0 | 143 | 4.2 × 105 | 24 | 0 |
| Agar-glucosec | 0/20 (0) | 0 | ||||
Sterilized, number of mice in which all cultures were negative.
All counts are expressed as CFU/ml of organ homogenate.
Control groups combined (n = 20).
In the FLU-resistant model, C. tropicalis FA1572, the organ colony counts were significantly lower in the kidneys and the livers of the group receiving ABLC at 10 mg/kg than in those of mice receiving all Flu treatments and the group receiving ABLC at 1 mg/kg (P = 0.02). In the same model, the kidneys had lower colony counts in the groups receiving ABLC at 2 and 5 mg/kg than in any of the FLU groups (P = <0.03). Liver colony counts after treatment with ABLC at 10 mg/kg/day were significantly lower than after all FLU treatments (P = <0.02), but after ABLC treatment at 2 or 5 mg/kg, the counts were significantly lower than only those receiving FLU at 25 and 50 mg/kg/day (P = <0.04). Counts for the brains of the group receiving ABLC at 2 to 10 mg/kg were not available.
In the model with isolate FA2317, the kidney colony counts in the group receiving ABLC at 2 to 10 mg/kg were not significantly lower than those in the group receiving FLU at 250 mg/kg but were significantly lower than those in the groups receiving FLU at 50 and 25 mg/kg (P = <0.015). All treatments other than ABLC at 1 mg/kg and FLU at 50 mg/kg/day lowered liver colony counts to a level superior to that in controls. ABLC at 2 to 10 mg/kg and FLU at 250 and 25 mg/kg/day significantly lowered brain colony counts.
With isolate C. tropicalis FA2542, the kidney colony counts were significantly lower in the groups receiving ABLC at 2 to 10 mg/kg than in those receiving all FLU regimes (P = <0.015). The brain culture results after treatment with FLU at 250 mg/kg/day were significantly superior to those of all other groups (P = <0.016). The other treatment groups were superior to that receiving ABLC at 1 mg/kg/day, and all active-treatment groups were superior to the control regimes (P = <0.01). All of the treatment regimes significantly lowered liver colony counts in comparison to those of controls.
In the ATCC 750 model, the kidney colony counts were significantly lower in the groups receiving ABLC at 2 to 10 mg/kg than in those receiving all FLU regimes (P = <0.01). The group receiving ABLC at 5 to 10 mg/kg had significantly lower liver colony counts than the groups receiving FLU at 250 and 25 mg/kg/day (P = <0.02) but not the group receiving 50 mg/kg/day. The brain culture results in all the treatment groups did not show significant differences, but the counts in the group receiving FLU of 250 mg/kg/day were numerically lower.
Serum FLU concentrations.
Mouse serum FLU concentrations are shown in Fig. 5. In the first 24 h, a dose-dependent serum concentration was seen, with maximum concentrations of drug in the serum of 74, 21, and 9 μg/ml for the 250-, 50-, and 25-mg/kg/day doses, respectively. The maximum concentrations were maintained for both the 50- and 25-mg/kg/day doses over the 5-day assay period, whereas a gradual reduction was seen with the 250-mg/kg/day dose.
FIG. 5.
Plot of serum FLU level against time in murine models. ×, FLU at 250 mg/kg; ●, FLU at 50 mg/kg; +, FLU at 25 mg/kg.
DISCUSSION
C. tropicalis is one of the three most commonly isolated non-albicans Candida species (NAC) and accounts for 4 to 25% of those isolated (and 20 to 45% of NAC isolated from blood cultures). Equally importantly, C. tropicalis also produces higher overall mortality (33 to 90%) than Candida albicans or other NAC (5, 17) regardless of therapy. Breakthrough candidemias while patients are on treatment or prophylaxis have an even worse prognosis. C. tropicalis was long considered universally susceptible to FLU, but over the last few years, rapid development of resistance to FLU has been recorded (the MICs for up to 20% of isolates were 16 μg/ml) (12, 15). As amphotericin B is also relatively ineffective in the clinical setting, new approaches to therapy are urgently required.
Substantial efforts by a large number of investigators have resulted in reproducible and meaningful susceptibility testing methods for FLU against C. albicans (9; EUCAST proposed standard; J. L. Rodriguez-Tudela, personal communication). Although there are ongoing discussions about appropriate breakpoints, a broad assumption has been made that the same methods used for C. albicans can be used for all NAC. Recently published work has suggested that a 24-h reading of the MIC is superior to a 48-h endpoint (7). As in our work, the authors used carefully controlled conditions pertaining to animal models. We have also been concerned about the validity of susceptibility test results for C. tropicalis, having documented an apparent rise from 0 to 80% of FLU resistance (MIC ≥ 25 μg/ml) in our intensive-care unit (6; L. A. Joseph, C. B. Moore, D. Law, D. Thornton, B. Bowles, and D. W. Denning, Abstr. 4th Congr. Eur. Confed. Med. Mycol., abstr. P91, 1998). We selected four isolates to test, one resistant, one susceptible, and two with intermediate or variable MICs depending on the test method. We used a wide dose range of FLU yielding serum concentrations at and above those found in patients. For example, the peak dose in our model after the 250-mg/kg/day regime was 74 mg/liter, whereas peak levels in human sera rarely exceed 20 mg/liter. Likewise, the ABLC dose range encompassed all those used in humans, typically 5 mg/kg.
The model with FA1572 showed substantial dose dependency with FLU, suggesting that it might be possible to treat an infection by this strain with extremely high doses of FLU. Assuming our susceptible isolate (FA2317) is truly susceptible (all in vitro data suggest that it is), FLU is slightly more effective than controls at reducing mortality, but it is still not very effective, even though the model was less acute than the other three. It is notable that this is the only isolate yielding a consistent pulmonary infection virtually untouched by therapy. Strains FA2542 and ATCC 750 are best classified as susceptible, although a lesser degree of dose dependency was seen. It should be noted that some FLU toxicity occurred in the high-dose regime in the treatment of ATCC 750 which was not seen with other isolates.
A variety of in vitro testing formats have been examined in this study, and it is clear that for some strains (FA1572 and FA2317) any of these methods read at 24 h would correctly predict the outcome of an animal treatment model. Unfortunately, some strains do not have as clear an endpoint because of a trailing phenomenon (gradual reduction of growth over a series of wells). This produces severe problems with the NCCLS M27A methodology, which requires an 80% reduction of growth at 48 h. This is not always achieved. We would therefore recommend adopting the EUCAST standard for C. tropicalis, as the RPMI with glucose plus a heavy initial inoculum produces more luxuriant growth after 24 h of incubation and a 50% cutoff avoids problems with a trailing endpoint. It may be that a 24-h endpoint with the NCCLS microtiter variation would be as good for C. tropicalis, but in a clinical setting, selecting different endpoints for different species is problematic.
The E-test also correctly predicted the in vivo response, but this test was difficult to read due to the production of small colonies within the zone in the strains which demonstrated a trailing phenomenon. If strains do not demonstrate this phenomenon (and most do not), the E-test can be used to reliably predict the MIC. Other groups have compared the MICs in the E-test and the NCCLS broth microdilution methods against large numbers of C. albicans and C. tropicalis isolates and also found the method easy to use and interpret (11, 16, 19, 20). Furthermore, it has also been reported that if small or poorly thriving, less pigmented colonies (trailing endpoints) within the E-test zone are ignored, the results are in good agreement with those of broth dilution (11).
ABLC consists of the antifungal agent amphotericin B complexed with two phospholipids. It has been shown to have impressive activity against Candida spp. in vitro (4) and against C. albicans in vivo (2, 8, 18). Few data have been published about its activity against FLU-resistant Candida spp. strains in vivo, and no data are available about its activity against FLU-resistant strains of C. tropicalis in vivo. The present study compared the activity of ABLC with that of FLU in four mouse models of invasive candidiasis.
The activity of ABLC at 10 mg/kg daily was superior to that of any of the FLU regimes in all models. ABLC at 2 to 10 mg/kg daily was the only treatment to significantly improve survival rates compared to those with no active treatment in the FA1572 FLU-resistant model. A clear dose response was demonstrable with ABLC, with 10 mg/kg being superior to all other doses and 5 mg/kg being significantly superior to controls. The combined data from all models demonstrate the dose dependency of ABLC in these infections, with 1 mg/kg being substantially less effective than higher doses. This dose dependency is not seen with FLU. There are numerical differences between the response rates after treatment with ABLC at 2 to 10 mg/kg, but these differences do not reach statistical significance; it is possible that the differences would become significant if larger groups were examined.
Although there are dangers in extrapolating to the clinical setting, the data presented here suggest that the maximum tolerated dose of ABLC should be used in the treatment of C. tropicalis infections and that it should be as effective against FLU-resistant isolates as against FLU-susceptible isolates.
FIG. 2.
Plot of cumulative mortality against time in a murine model against C. tropicalis FA2317. ×, ABLC at 10 mg/kg; ▴, ABLC at 5.0 mg/kg; ■, ABLC at 2.0 mg/kg; ⧫, ABLC at 1.0 mg/kg; ✠, FLU at 250 mg/kg; ●, FLU at 50 mg/kg; ✚, FLU at 25 mg/kg; □, agar control; ▵, 5% glucose control.
FIG. 3.
Plot of cumulative mortality against time in a murine model against C. tropicalis FA2542. ×, ABLC at 10 mg/kg; ▴, ABLC at 5.0 mg/kg; ■, ABLC at 2.0 mg/kg; ⧫, ABLC at 1.0 mg/kg; ✠, FLU at 250 mg/kg; ●, FLU at 50 mg/kg; ✚, FLU at 25 mg/kg; □, agar control; ▵, 5% glucose control.
TABLE 4.
Geometric means of organ culture counts for C. tropicalis 2317
| Treatment | Survivors/no. in group (%) | Sterilizeda | Mean countb
|
|||
|---|---|---|---|---|---|---|
| Brain | Kidneys | Liver | Lungs | |||
| ABLC (10 mg/kg) | 8/10 (80) | 0 | 1.5 × 102 | 1.2 × 103 | 12 | 2 |
| ABLC (5 mg/kg) | 8/10 (80) | 0 | 5.6 × 102 | 1.9 × 103 | 7 | 2 |
| ABLC (2 mg/kg) | 7/10 (70) | 0 | 5.8 × 102 | 6.1 × 103 | 1.2 × 102 | 3 |
| ABLC (1 mg/kg) | 6/10 (60) | 0 | 2.5 × 103 | 1.1 × 104 | 2.7 × 102 | 4 |
| FLU (250 mg/kg) | 7/10 (70) | 0 | 2.8 × 102 | 2.6 × 103 | 49 | 3 |
| FLU (50 mg/kg) | 3/10 (30) | 0 | 5.5 × 103 | 2.2 × 105 | 8.3 × 102 | 17 |
| FLU (25 mg/kg) | 6/10 (60) | 0 | 1.6 × 103 | 9.5 × 104 | 30 | 6 |
| Agar-glucosec | 8/20 (40) | 0 | 2.0 × 104 | 3.4 × 105 | 2.7 × 103 | 31 |
Sterilized, number of mice in which all cultures were negative.
All counts are expressed as CFU/ml of organ homogenate.
Control groups combined (n = 20).
TABLE 5.
Geometric means of organ culture counts for C. tropicalis FA2542
| Treatment | Survivors/no. in group (%) | Sterilizeda | Mean countb
|
|||
|---|---|---|---|---|---|---|
| Brain | Kidneys | Liver | Lungs | |||
| ABLC (10 mg/kg) | 10/10 (100) | 0 | 70 | 70 | 2 | 0 |
| ABLC (5 mg/kg) | 9/10 (90) | 0 | 1.3 × 102 | 3.8 × 102 | 3 | 0 |
| ABLC (2 mg/kg) | 9/9 (100) | 0 | 3.9 × 102 | 4.7 × 102 | 5 | 0 |
| ABLC (1 mg/kg) | 9/10 (90) | 0 | 9.0 × 102 | 1.9 × 103 | 2.3 × 102 | 0 |
| FLU (250 mg/kg) | 10/10 (100) | 0 | 6 | 8.8 × 103 | 2 | 0 |
| FLU (50 mg/kg) | 8/10 (80) | 0 | 2.0 × 102 | 1.4 × 105 | 60 | 0 |
| FLU (25 mg/kg) | 7/9 (78) | 0 | 1.8 × 102 | 7.9 × 104 | 44 | 0 |
| Agar-glucosec | 3/20 (15) | 0 | 8.8 × 103 | 3.3 × 105 | 4.5 × 104 | 0 |
Sterilized, number of mice in which all cultures were negative.
All counts are expressed as CFU/ml of organ homogenate.
Control groups combined (n = 20).
REFERENCES
- 1.Abi-Said D, Anaissie E, Uzun O, Raad I, Pinzcowski H, Vartivarian S. The epidemiology of hematogenous candidiasis caused by different Candida species Clin. Infect Dis. 1997;24:1122–1128. doi: 10.1086/513663. [DOI] [PubMed] [Google Scholar]
- 2.Clark J M, Whitney R R, Olsen S J, George R J, Swerdel M R, Kunselman L, Bonner D P. Amphotericin B lipid complex therapy of experimental fungal infections in mice. Antimicrob Agents Chemother. 1991;35:615–621. doi: 10.1128/aac.35.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denning D W, Hall L, Jackson M, Hollis S. Efficacy of D0870 compared with those of itraconazole and amphotericin B in two murine models of invasive aspergillosis. Antimicrob Agents Chemother. 1995;39:1809–1814. doi: 10.1128/aac.39.8.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson E M, Ojwang J O, Szekely A, Wallace T L, Warnock D W. Comparison of in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four amphotericin B formulations. Antimicrob Agents Chemother. 1998;42:1412–1416. doi: 10.1128/aac.42.6.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kremery V. Candidaemia in cancer patients: risk factors and outcomes in 140 episodes from a single cancer institution. Acta Chemother. 1999;1:133–145. [Google Scholar]
- 6.Law D, Moore C B, Joseph L A, Keaney M G, Denning D W. High incidence of antifungal drug resistance in Candida tropicalis. Int J Antimicrob Agents. 1996;7:241–245. doi: 10.1016/s0924-8579(96)00328-7. [DOI] [PubMed] [Google Scholar]
- 7.Marr K A, Rustad T R, Rex J H, White T C. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob Agents Chemother. 1999;43:1383–1386. doi: 10.1128/aac.43.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitsutake K, Kohno S, Miyazaki Y, Noda T, Miyazaki H, Miyazaki T, Kaku M, Koga H, Hara K. In vitro and in vivo antifungal activities of liposomal amphotericin B, and amphotericin B lipid complex. Mycopathologia. 1994;128:13–17. doi: 10.1007/BF01104273. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 10.Oakley K L, Morrissey G, Denning D W. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1504–1507. doi: 10.1128/aac.41.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaller M A, Messer S A, Bolmstrom A. Evaluation of Etest for determining in vitro susceptibility of yeast isolates to amphotericin B. Diagn Microbiol Infect Dis. 1998;32:223–227. doi: 10.1016/s0732-8893(98)00120-5. [DOI] [PubMed] [Google Scholar]
- 12.Powderly W G, Kobayashi G S, Herzig G P, Medoff G. Amphotericin B-resistant yeast infection in severely immunocompromised patients. Am J Med. 1988;84:826–832. doi: 10.1016/0002-9343(88)90059-9. [DOI] [PubMed] [Google Scholar]
- 13.Rex J H, Cooper C R, Jr, Merz W G, Galgiani J N, Anaissie E J. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob Agents Chemother. 1995;39:906–909. doi: 10.1128/aac.39.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rex J H, Nelson P W, Paetznick V, Lozano C, Espinel I A, Anaissie E. Optimizing the correlation between results of testing in vitro and therapeutic outcomes in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 1998;42:129–134. doi: 10.1128/aac.42.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rex J H, Pfaller M A, Barry A L, Nelson P W, Webb C D. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Antimicrob Agents Chemother. 1995;39:40–44. doi: 10.1128/aac.39.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sewell D L, Pfaller M A, Barry A L. Comparison of broth macrodilution, broth microdilution, and E test antifungal susceptibility tests for fluconazole. J Clin Microbiol. 1994;32:2099–2102. doi: 10.1128/jcm.32.9.2099-2102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slavin M A, Osborne B, Adams R, Levenstein M J, Schoch H G, Feldman A R, Meyers J D, Bowden R A. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation—a prospective, randomized, double-blind study. J Infect Dis. 1995;171:1545–1552. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 18.Swenson C E, Perkins W R, Roberts P, Ahmad I, Stevens R, Stevens D A, Janoff A S. In vitro and in vivo antifungal activity of amphotericin B lipid complex: are phospholipases important? Antimicrob Agents Chemother. 1998;42:767–771. doi: 10.1128/aac.42.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tortorano A M, Viviani M A, Barchiesi F, Arzeni D, Rigoni A L, Cogliati M, Compagnucci P, Scalise G. Comparison of three methods for testing azole susceptibilities of Candida albicans strains isolated sequentially from oral cavities of AIDS patients. J Clin Microbiol. 1998;36:1578–1583. doi: 10.1128/jcm.36.6.1578-1583.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van-Eldere J, Joosten L, Verhaeghe V, Surmont I. Fluconazole and amphotericin B antifungal susceptibility testing by National Committee for Clinical Laboratory Standards broth macrodilution method compared with E-test and semiautomated broth microdilution test. J Clin Microbiol. 1996;34:842–847. doi: 10.1128/jcm.34.4.842-847.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh T J, Hiemenz J W, Seibel N L, Perfect J R, Horwith G, Lee L, Silber J L, DiNubile M J, Reboli A, Bow E, Lister J, Anaissie E J. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis. 1998;26:1383–1396. doi: 10.1086/516353. [DOI] [PubMed] [Google Scholar]
- 22.Walsh T J, Whitcomb P, Piscitelli S, Figg W D, Hill S, Chanock S J, Jarosinski P, Gupta R, Pizzo P A. Safety, tolerance, and pharmacokinetics of amphotericin B lipid complex in children with hepatosplenic candidiasis. Antimicrob Agents Chemother. 1997;41:1944–1948. doi: 10.1128/aac.41.9.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]