Abstract
Anion channels and connexin hemichannels are permeable to amino acid neurotransmitters. It is hypothesized that these conductive pathways release GABA, thereby influencing ambient GABA levels and tonic GABAergic inhibition. To investigate this, we measured the effects of anion channel/hemichannel antagonists on tonic GABA currents of rat hippocampal neurons. In contrast to predictions, blockade of anion channels and hemichannels with NPPB potentiated tonic GABA currents of neurons in culture and acute hippocampal slices. In contrast, the anion channel/hemichannel antagonist carbenoxolone (CBX) inhibited tonic currents. These findings could result from alterations of ambient GABA concentration or direct effects on GABAA receptors. To test for effects on GABAA receptors, we measured currents evoked by exogenous GABA. Coapplication of NPPB with GABA potentiated GABA-evoked currents. CBX dose-dependently inhibited GABA-evoked currents. These results are consistent with direct effects of NPPB and CBX on GABAA receptors. GABA release from hippocampal cell cultures was directly measured using HPLC. Inhibition of anion channels with NPPB or CBX did not affect GABA release from cultured hippocampal neurons. NPPB reduced GABA release from pure astrocytic cultures by 21%, but the total GABA release from astrocytes was small compared to that of mixed cultures. These data indicate that drugs commonly used to antagonize anion channels and connexin hemichannels may affect tonic currents via direct effects on GABAA receptors and have negligible effects on ambient GABA concentrations. Interpretation of experiments using NPPB or CBX should include consideration of their effects on tonic GABA currents.
Keywords: tonic inhibition, GABAA receptor, ambient GABA, seizure
Introduction
Ambient GABA levels in the extracellular space of brain are a key factor determining the baseline activation of high-affinity extrasynaptic GABAA receptors. Tonic inhibition resulting from activation of extrasynaptic GABAA receptors impacts neuronal and network behavior, and affects many physiological and pathophysiological processes (Farrant and Nusser, 2005; Maguire et al., 2005; Martin et al., 2010). Several factors are believed to influence ambient GABA, including vesicular GABA release at synapses (Glykys and Mody, 2007) and both uptake and release by GABA transporters (Richerson and Wu, 2003; Ransom et al., 2013). In the cerebellum, another mechanism regulating ambient GABA levels (and tonic inhibition) is diffusion of GABA through glial anion channels (Bestrophin 1 anion channel) (Lee et al., 2010). Other conductive pathways permeable to amino acids (i.e. anion channels and connexin hemichannels) in both neurons and glia may also affect ambient GABA levels (Basarsky et al., 1999; Ye et al., 2003). The contribution of anion channel/connexin hemichannels to ambient GABA levels and tonic inhibition in the hippocampus is not known.
We hypothesized that antagonizing GABA-permeable conductive pathways (i.e. anion channels or connexin hemichannels) would lead to reductions in ambient GABA, causing a reduction of tonic GABA current amplitude. To investigate this, we measured tonic currents from cultured hippocampal neurons. In contrast to our expectations, NPPB, an antagonist of both anion channels and connexin hemichannels (Ye et al., 2009), increased endogenous tonic currents. NPPB also enhanced currents produced by exogenous GABA. We present results that support the conclusion that NPPB enhances tonic currents of hippocampal neurons via direct effects on GABAA receptors rather than alterations in ambient GABA concentrations, similar to findings in cerebellar granule cells (Diaz et al., 2011). Our results also show that another anion channel/connexin hemichannel antagonist (carbenoxolone) has direct effects on GABAA receptors.
Materials and Methods
Cell culture
Primary hippocampal cell cultures were prepared as previously described (Gaspary et al., 1998). In brief, 0–2 day old Sprague-Dawley rat pups of both sexes were decapitated and the hippocampi were dissected. The tissue was minced in sterile-filtered, HEPES-buffered solution and then treated with a digestion solution containing papain (10 U/ml), 0.5 mM EDTA, and cysteine (0.2 mg/ml) for 15 minutes. The enzyme-treated tissue was triturated in complete Minimum Essential Medium (MEM), trypsin inhibitor (1.5 mg/ml), and bovine serum albumin (1.5 mg/ml). Triturated cells were added to culture media (MEM/10%FBS) and plated directly on polyornithine-coated round glass coverslips (Erie Scientific, Portsmouth, NH, USA) in 12 well culture dishes at a density of 2.5–5 × 105 cells/ml. After 1 hour, media was changed to 70% MEM/30% Neurobasal with B27 supplement (Gibco, Carlsbad, CA, USA). Cells were maintained in an incubator (Forma Scientific, model 3110, Marietta, OH) with a humidified environment containing 5% CO2 in room air at 37°C. Media was changed on day 5–6 to Neurobasal Medium with B27 supplement and 1 μM cytosine arabinoside (Ara-C), followed by half medium changes (without Ara-C) every 7 days.
Acute brain slices
Hippocampal brain slices were prepared from 4–6 week old Sprague-Dawley rats of both sexes. Rats were anesthetized with 4% isoflurane, decapitated, and the brain dissected free. Transverse slices were made from hippocampus with a thickness of 300 μm. Slices were cut and stored in a solution containing (in mM): 125 NaCl, 3 KCl, 26 NaHCO3, 1.2 NaH2PO4, 0.5 CaCl2, 4 MgCl2, 20 dextrose, and 1 kynurenic acid. Solutions were continuously gassed with 95% O2/5% CO2. Slices were allowed to recover at room temparture for >1 hour prior to recording.
Electrophysiology
Conventional whole-cell patch clamp techniques were used to record membrane currents from neurons aged 14–60 days in vitro. Cultured cells were visualized with an Axiovert 200 inverted microscope with DIC optics (Carl Zeiss Inc., Thornwood, NY). Dentate gyrus granule cells in brain slices were visualized with an Axioskop 2 upright microscope with fixed stage (Carl Zeiss Inc., Thornwood, NY). Recordings were made at room temperature (typically 23°C) using a Multiclamp 700B or Axopatch 200B amplifier, a Digidata 1200 series A-D converter, and pCLAMP 10 software (Molecular Devices, Redwood City, CA). Data were acquired at 2–5 kHz and low-pass filtered at 1 kHz. Series resistance and whole-cell capacitance were determined and compensated 50–70% online; whole-cell capacitance was recorded as the compensation value from the front of the amplifier. Drug applications were made via a microperfusion device (SF-77B, Warner Instruments, Hamden, CT) connected to manifolds that allowed up to 5 test solutions to be applied in a single experiment. Flow through the manifolds was controlled with electronic solenoid valves (Parker Hannifin Corporation, General Valve Division, Cleveland, OH) and step application of test solutions was controlled by pCLAMP software. The microperfusion device produced 90% solution exchange times of 50–55 ms across open pipette tips. Local application of bicuculline (30 μM) in acute brain slices was made by pressure ejection from a patch pipette containing (in mM): 150 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 10 dextrose, and 10 HEPES with pH adjusted to 7.4 with NaOH. A Picospritzer II was used for pressure ejection (General Valve Corporation). The recording chamber (RC-26, Warner Instruments, Hamden, CT) had a volume of ~0.3 ml and was continuously superfused at a rate of ~0.5 ml/min with bath solution that contained (in mM): 134 NaCl, 3 KCl, 1.4 NaH2PO4, 24 NaHCO3, 10 Dextrose, 2 MgCl2, 2 CaCl2, 1–3 Kynurenic acid, and 0.001 CGP 55845. The pH was 7.35–7.4 when bubbled with 95% O2/5% CO2. Osmolarity was adjusted to 305 mOsm with H2O. Patch electrodes were made with borosilicate glass without filament (593400, A-M Systems, Carlsborg, WA) using a micropipette puller (P-97; Sutter Instruments, Novato, CA). Pipettes had resistances of 2.5 to 3.5 MΩ when filled with an intracellular solution containing (in mM): 125 CsCl, 10 QX-314 chloride salt, 10 HEPES, 1 EGTA (pH corrected to 7.25 with CsOH). Osmolarity was adjusted to 275–285 mOsm with H2O as needed. All chemicals were purchased from Sigma except QX-314 (Alomone Labs, Tel Aviv, Israel). Data acquisition was begun 3–5 minutes after establishing a whole-cell recording. Experiments were performed at room temperature, typically 23°C.
High Performance Liquid Chromatography (HPLC)
GABA and glutamate release from cultured neurons and astrocytes were quantified by HPLC. The cultures were prepared from rat hippocampus and grown to confluency (typically 2 weeks) for HPLC experiments. Amino acids were separated and measured as previously described (Ye et al., 2001), using an Empower HPLC system from Waters (Milford, MA) with some minor modifications. Briefly, samples were centrifuged at 13,000 g for 3 min and supernatants were pre-column derivatized with o-phthalialdehyde (OPA, Sigma, St. Louis, MO) and injected by a 717 plus autosampler, separated through a C18 reverse-phase Adsorbosphere OPA-HR column (Alltech, Deerfield, IL). Amino acids were detected by a 2475 scanning fluorescent detector excited at 338 nm with emission detected at 450 nm. Gradient elution was powered by Waters 1525 binary pumps with mobile phase A (pH 5.90) consisting of 25 mM sodium acetate, dioxane and isopropanol in a mixture of (v/v) 95.6/0.4/4.0 and mobile phase B a mixture of methanol, dioxane and isopropanol (v/v, 97/1.5/1.5). Chromatography was analyzed with Empower software with external standards.
The amount of amino acid release was calculated from the volume of the solution and normalized to the protein content of the cultures. To measure protein contents and cytoplasmic amino acid contents, cultured cells were dissolved in 0.1 M NaOH and then neutralized with HCl. Aliquots were used to measure protein concentration using the Bio-Rad protein assay kit or to measure amino acid concentration by HPLC.
Analysis
Data analysis was performed with Clampfit (pCLAMP 10, Molecular Devices, Mountain View CA) and Origin (v6.1, Microcal Software, Northhampton, MA) software. Tonic current amplitudes were measured as the difference in mean holding current in the absence and presence of the GABAA receptor antagonist bicuculline methiodide (10–20 μM). The mean current values were obtained from Gaussian fits to all-point current amplitude histograms. Histograms were constructed from 2–10 seconds of data with a bin width of 1 pA. Gaussian fits were performed using a Levenberg-Marquardt curve-fitting algorithm provided in Clampfit software.
Data values are presented as mean ± standard error of mean (SEM) and all error bars represent SEM. Statistical analyses were performed using Microsoft Excel (Bellevue, WA). A two-tailed, paired or homoscedastic Students t-test was used with a p-value of 0.05 considered as significant. Where appropriate the actual p-values are reported.
Results
NPPB potentiated tonic GABA currents in cultured hippocampal neurons
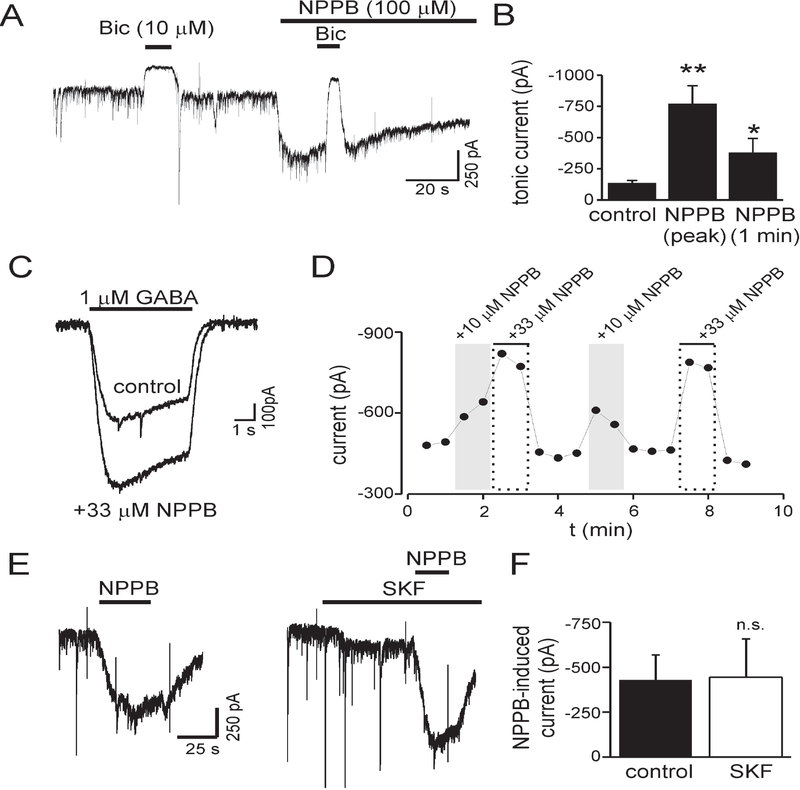
We measured tonic GABA currents in cultured hippocampal neurons to assess a contribution of anion channels/connexin hemichannels to ambient GABA concentrations. Because anion channels/hemichannels are conductive pathways permeable to GABA, we hypothesized that NPPB (which inhibits both anion channels and hemichannels) would reduce ambient GABA concentration and tonic current amplitude. We measured tonic currents as the change in holding current produced by application of bicuculline (Bic, 10 μM) at baseline and then during NPPB (100 μM) application (Fig. 1A). Contrary to our hypothesis, NPPB induced an inward current that was inhibited by Bic, indicating that NPPB was increasing GABAA receptor mediated tonic currents. NPPB-induced currents reached their peak in 13 ± 3 s (n = 9). On average, NPPB increased tonic currents by 424 ± 127% (n = 5, p<0.01) (Fig. 1B). Tonic currents in the continued presence of NPPB diminished over time but remained above baseline values after 1 minute of exposure (381 ± 113% increase, n = 5, p<0.05) (Fig. 1B).
Figure 1.
The anion channel and hemichannel antagonist NPPB increased tonic GABA currents and currents evoked with exogenous GABA. A: Membrane current during application of NPPB (100 μM). Tonic currents, measured as change in holding current produced by bicuculline (Bic), were increased by NPPB. Vm = −60 mV. B: Mean tonic current amplitude under control conditions and in the presence of NPPB (n = 5). The increase in tonic current produced by NPPB decayed over time but remained above baseline values after 1 minute. C: GABA-evoked currents were potentiated by coapplication of NPPB with GABA. Displayed currents are average of 4–10 measurements. D: Time course for experiment in panel C. E: NPPB-induced currents before and during GAT1 inhibition with SKF89976a (SKF, 30 μM). F: Mean NPPB-induced currents before and during SKF application (n = 4, n.s.). NPPB effects were independent of GAT1 function. * - p<0.05, ** - p<0.01, n.s. – nonsignificant.
NPPB could increase tonic currents by elevating ambient GABA concentration. If this were the case, application of exogenous GABA is expected to occlude the effects of NPPB. To test this, we measured currents evoked with exogenous GABA (1 μM) and then co-applied exogenous GABA with NPPB (10 or 33 μM) (Fig. 1C, D). Coapplication of NPPB enhanced currents produced by exogenous GABA. On average, 10 μM or 33 μM NPPB increased GABA-evoked currents by 49 ± 22% and 86 ± 22%, respectively (n = 4, p <0.05 for 10 μM and p<0.01 for 33 μM). The dose dependent enhancement was also significant (10 μM vs. 33 μM, p<0.01). These results suggest that NPPB did not increase tonic currents by elevating ambient [GABA] but rather via potentiation of GABAA receptors.
Inhibition of GABA transporters has the potential to increase ambient GABA levels (and GABA concentrations in the presence of exogenous GABA). To assess whether NPPB effects involved inhibition of GABA transporter type 1 (GAT1), we measured NPPB-induced currents before and during bath application of the GAT1 antagonist SKF89976a (SKF, 30 μM) (Fig. 1E–F). NPPB-induced currents were not significantly affected by SKF (n = 3, p = 0.85), indicating that NPPB effects were independent of GAT1 function.
NPPB potentiated tonic GABA currents in dentate gyrus granule cells of acute hippocampal slices
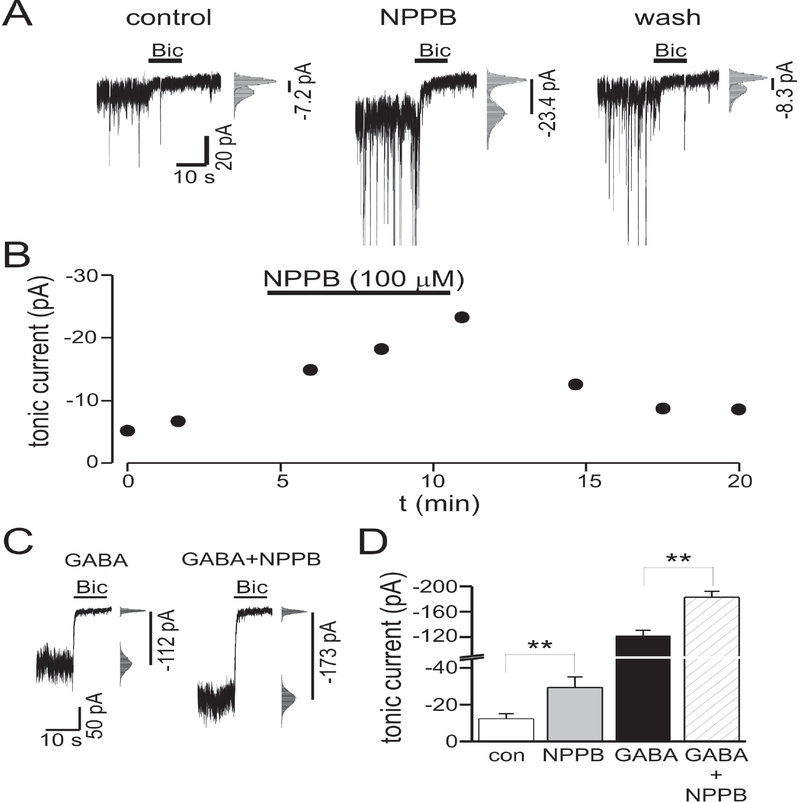
Because prior work in acute brain slices has shown that NPPB reduces tonic currents of cerebellar granule cells (Lee et al., 2010), we wished to confirm our results from cultured neurons in acute hippocampal brain slices. Tonic currents of dentate gyrus granule cells were measured by focal pressure ejection of bicuculline (30 μM). Similar to results in cell culture, NPPB (100 μM) reversibly increased tonic current amplitude by 157 ± 41 % (n = 5, p < 0.05) (Fig. 2A, B). To confirm that the enhancement of tonic currents in acute brain slices was not caused by increases in ambient GABA concentrations, we measured the effects of NPPB on tonic currents in the presence of exogenous GABA. If NPPB were increasing tonic currents solely by elevating ambient GABA, then increasing tonic currents with exogenous GABA, beyond that seen with NPPB alone, should occlude NPPB effects. Bath application of exogenous GABA (10 μM) increased tonic currents from –12 ± 3 pA to –122 ± 5 pA (n = 4, p < 0.01). In the presence of exogenous GABA, NPPB still increased tonic currents by 50 ± 10 % (n = 4, p < 0.05) (Fig. 2C, D). Although the percent increase of tonic current amplitude produced by NPPB was smaller in the presence of exogenous GABA (157 % increase with NPPB alone vs. 50 % increase with exogenous GABA, p < 0.05), this may have been due to a saturation effect, and these results suggest that NPPB can enhance tonic currents via effects on GABAA receptors themselves, independent of any effect on ambient GABA concentration. The effects of other drugs that modulate GABAA receptors can be influenced by GABA concentration; for example, the enhancement of GABAA currents by propofol is occluded in the presence of higher GABA concentrations (Houston et al., 2012).
Figure 2.
NPPB increased tonic currents of dentate gyrus granule cells in acute hippocampal brain slices. A: Tonic currents under control conditions, during NPPB (100 μM) application, and after wash out of NPPB. All points histograms and Gaussian fits used to determine tonic current amplitudes [i.e. change in holding current produced by bicuculline (Bic)] are shown to right of each trace. Tonic current amplitudes are indicated by vertical lines. B: Time course for experiment in panel A. C: Tonic currents in presence of exogenous GABA (10 μM) before and during NPPB application. D: Mean tonic current amplitude under control conditions, in the presence of NPPB, exogenous GABA (GABA), and GABA + NPPB (n = 5 cells for NPPB alone, n = 3 cells with exogenous GABA). ** - p<0.01.
The connexin hemichannel antagonist carbenoxolone (CBX) inhibited GABA currents
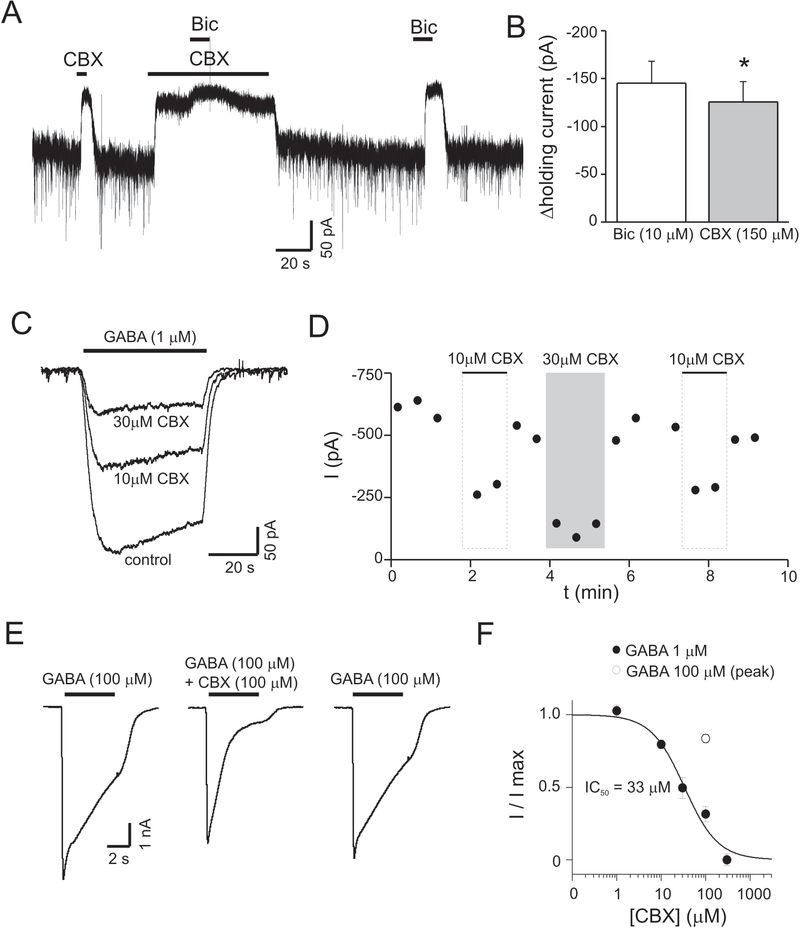
Carbenoxolone (CBX) is a commonly used antagonist of gap junctions and also inhibits connexin hemichannels and anion channels (Ye et al., 2009). We determined the effects of CBX on tonic currents in cultured hippocampal neurons. CBX (150 μM) reduced holding currents, similar to the effects of Bic. In the presence of CBX, Bic produced only a small additional reduction in holding current (Fig. 3A). The change in holding current produced by CBX (150 μM) was 86 ± 4 % of that produced by Bic (n = 5, p < 0.05) (Fig. 3B). CBX effects could be caused by changes in ambient GABA or via direct effects on GABAA receptors themselves. To evaluate the latter, we measured currents evoked with exogenous GABA during coapplication of CBX (1–300 μM). Coapplication of CBX with exogenous GABA (1 μM) dose-dependently inhibited currents (Fig. 3 C–D), indicating that CBX reduced tonic currents via effects on GABAA receptors rather than by lowering ambient GABA concentrations. Peak currents evoked with higher concentrations of exogenous GABA (100 μM) were inhibited by CBX (100 μM) to a lesser extent than currents produced by 1 μM GABA (16 ± 3 %inhibition with 100 μM GABA vs. 69 ± 4 % inhibition with 1 μM GABA) (p<0.01, n = 8 cells for 1 μM GABA, n = 4 cells for 100 μM GABA). This result is consistent with competitive antagonism of GABAA receptors by CBX (Fig. 3E–F).
Figure 3.
The hemichannel antagonist carbenoxolone (CBX) inhibited tonic GABA currents and currents evoked with exogenous GABA. A: Membrane current during application of CBX (100 μM) and bicuculline (Bic). CBX caused near complete inhibition of the Bic-sensitive tonic current. B: Mean change in holding current produced by Bic or CBX (150 μM) (n = 5). On average, the change in holding current produced by 150 μM CBX was 87% of that produced by Bic. C: Currents evoked with exogenous GABA (1 μM) or coapplication of GABA+CBX (concentrations indicated in figure). D: Time course for experiment illustrated in panel C. E: Currents evoked with higher concentrations of exogenous GABA (100 μM). Peak currents due to 100 μM GABA were minimally affected by CBX compared to the inhibition seen with 1 μM GABA, suggesting CBX acts as a competitive antagonist at GABAA receptors. Currents at the end of GABA applications for both 1 μM and 10 μM GABA were similarly inhibited by CBX (see text). F: Dose response curve for CBX inhibition of GABA-evoked currents. GABA currents were measured at their peak [1 μM, filled circles, n = 4–8 cells at each CBX concentration; 100 μM (peak current), open circle, n = 4].
HPLC measurements of GABA release from cultured hippocampal cells
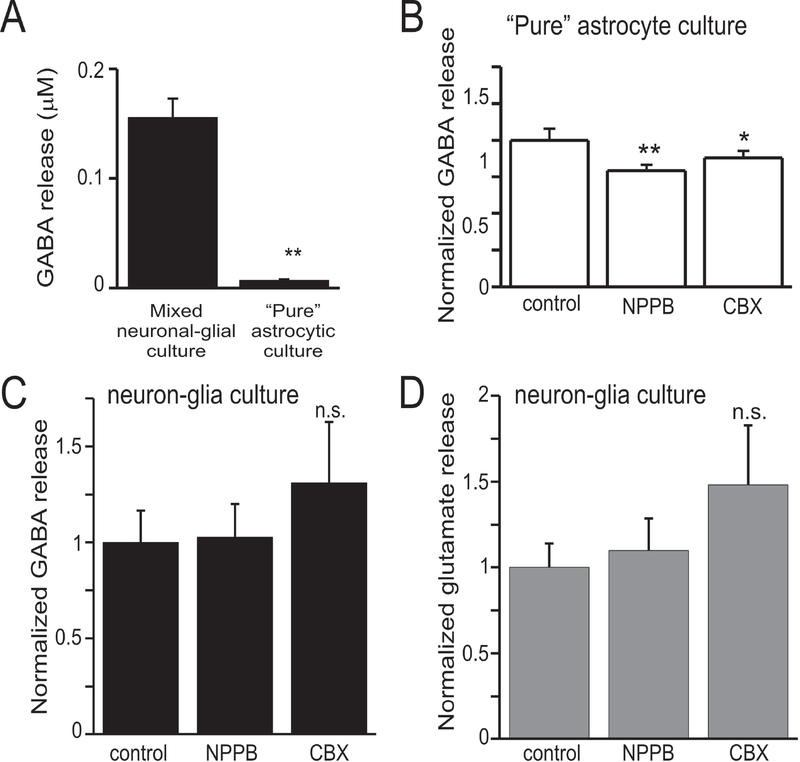
Our approach to assess GABA release via anion channels/connexin hemichannels by measuring tonic GABA currents was complicated by direct effects of NPPB and CBX on GABAA receptors. To determine the contribution of conductive pathways (i.e. anion channels/hemichannels) to GABA release from cultured hippocampal cells grown to confluency, we used HPLC to directly measure [GABA]. We were particularly interested in GABA release from astrocytes because both anion channels and connexin hemichannels in these cells previously have been shown to release amino acid neurotransmitters (Basarsky et al., 1999; Ye et al., 2003; Lee et al., 2010). GABA release was determined as the mean GABA concentration measured by HPLC from 6–12 culture wells under each condition. GABA release from mixed neuron-glia cultures was 21-fold greater than that from “pure” astrocyte cultures (Fig. 4A). This suggests that GABA release from astrocytes is small compared to that from neurons. NPPB (100 μM) and CBX (100 μM) significantly reduced GABA release from cultured hippocampal astrocytes by 21 % (p<0.001) and 12% (p<0.05), respectively (Fig. 4B). Neither NPPB or CBX affected GABA nor glutamate release from mixed neuronal-glia cultures (Fig. 4C–D).
Figure 4.
GABA release from “pure” astrocyte cell culture, but not mixed neuron-glia cell culture, was reduced by anion channel inhibition. A: Total GABA release measured by HPLC from mixed neuron-glia cell culture and “pure” astrocyte cell culture. Astrocytic GABA release was less than 5% of that seen from mixed neuron-glia cell culture. B: Effects of anion channel and connexin hemichannel antagonists on GABA release from astrocyte cell culture. NPPB (100 μM) and CBX (150 μM) inhibited astrocytic GABA release by 21% and 12%, respectively. n-octanol did not affect astrocytic GABA release. C: Effects of anion channel and connexin hemichannel antagonists on GABA release from mixed neuron-glia cell culture. NPPB and CBX did not affect GABA release from mixed cultures. Octanol caused a large increase in GABA release. D: Glutamate release from mixed neuron-glia cell culture. Octanol also increased glutamate release from neuron cultures, the mechanism of this is unknown but is suspected to be a non-specific, toxic effect. Data represent measurements from 6–12 culture wells under each condition. * - p<0.05, ** - p<0.01, n.s. – nonsignificant.
Discussion
We measured the effects of anion channel/connexin hemichannel antagonists on tonic GABA currents to assess the contribution of anion channels/connexin hemichannels to ambient GABA levels in hippocampal cell culture and hippocampal slices. It was hypothesized that if GABA release via these conductive pathways significantly contributed to ambient GABA levels, blocking this release would be reflected in a reduction of tonic current amplitude. Both anion channel/hemichannel antagonists tested (NPPB, CBX) affected tonic GABA currents. However, our data showed that these drugs affected currents caused by endogenous GABA as well as exogenous GABA. These results indicate that these effects were caused, at least in part, by direct modulatory actions on extrasynaptic GABAA receptors independent of changes in ambient GABA levels. The finding that three commonly used antagonists of anion channels/hemichannels modulate GABAA receptor function complicated our strategy to evaluate GABA release via conductive pathways.
NPPB effects on tonic GABA currents
Intracellular [GABA] is much higher than extracellular [GABA] (Richerson and Wu, 2003). Because of this, GABA-permeable anion channels and connexin hemichannels could provide a pathway for GABA efflux and contribute to ambient GABA levels. NPPB antagonizes several types of anion channels, including volume-activated channels (Ransom et al., 2001) and bestrophin 1 (Lee et al., 2010). NPPB also antagonizes connexin hemichannels (Eskandari et al., 2002; Ye et al., 2009). Thus, if these GABA-permeable conductive pathways contributed significantly to ambient GABA levels we predicted that broad inhibition with NPPB would cause a reduction in ambient GABA and tonic current amplitude. In contrast, we found that NPPB increased tonic current amplitude. Our data also show that NPPB dose-dependently increased currents evoked with exogenous GABA, indicating that the effect was independent of GABA release or changes in ambient [GABA]. NPPB enhanced tonic currents of both cultured neurons and dentate gyrus granule cells of acute brain slices, indicating that the effect was not an artifact of cell culture preparation. Antagonism of GABA transporters by NPPB could increase ambient GABA and enhance tonic currents but this is unlikely because NPPB effects were unaltered by GAT1 inhibition with SKF89976a. We cannot exclude an effect of NPPB on other GABA transporters such as GAT3, but this is unlikely because GAT3 inhibition alone has negligible effects on ambient GABA and tonic currents in hippocampal neurons (Kersanté et al., 2013).
The effects of NPPB on GABA release from cultured hippocampal cells was directly measured using HPLC. Because release of amino acid neurotransmitters by anion channels/connexin hemichannels has been well-documented to occur from astrocytes (Basarsky et al., 1999; Ye et al., 2003; Lee et al., 2010), we were particularly interested in determining NPPB effects on GABA release from astrocytes. NPPB caused a small but significant reduction (21%) of GABA release from cultured hippocampal astrocytes. These data confirm channel-mediated release of GABA from astrocytes, but the total GABA release from astrocytes was small compared to that of mixed neuron-glia cultures, approximately 5% of the value of mixed cultures. Although our quantification of GABA release was not normalized to cell number or protein expression, our data on GABA release (measured from confluent cell cultures) suggests that the contribution of channel-mediated GABA release from astrocytes to ambient GABA levels is minimal in our preparation. . GABA release from mixed neuronal-glia cultures was unaffected by NPPB, supporting our conclusion that enhancement of GABA currents produced by NPPB is independent of changes in ambient GABA.
CBX effects on tonic GABA currents
CBX is commonly used to antagonize gap junctional intercellular communication, connexin hemichannels, and anion channels (Ye et al., 2009). Our data show that CBX reduced tonic GABA currents in cultured hippocampal neurons. This finding could indicate that GABA release from connexin hemichannels contributes to ambient GABA levels, but currents evoked with exogenous GABA were also inhibited by CBX. The dose-dependent antagonism of exogenous GABA currents by CBX is consistent with a direct effect on GABAA receptors themselves. The reduced inhibition of currents produced with 100 μM GABA compared to currents produced with 1 μM GABA suggest CBX functions as a competitive antagonist of GABAA receptors. Prior studies have shown that evoked IPSCs (responses mediated by synaptic GABAA receptors) are inhibited by CBX (Tovar et al., 2009). Our results indicate that extrasynaptic GABAA receptors mediating tonic currents are also sensitive to CBX. We did not observe any effects of CBX on GABA release from mixed neuron-glia cultures but CBX produced a small (12%) reduction of GABA release from “pure” astrocyte cultures. However, as noted above, because the total GABA release from astrocytes is small compared to that of neuron-glia cultures, experiments with CBX suggest that connexin hemichannels/anion channels contribute minimally to ambient GABA levels in our preparation. NPPB and carbenoxolone both inhibit anion channels and hemichannels yet their effects on tonic currents were opposite to each other; this provides additional support that anion channel/hemichannel antagonism was not responsible for the observed changes in GABA currents.
Significance of our results
Ambient GABA levels are a key determinant of tonic inhibition. The factors controlling ambient GABA levels include vesicular GABA release at synapses, both uptake and release from GABA transporters, and anion channel mediated release (Glykys and Mody, 2007; Lee et al., 2010; Ransom et al., 2013). Although NPPB has been shown to reduce tonic currents in cerebellar granule cells (Lee et al., 2010), other authors have reported potentiation of tonic currents in cultured cerebellar granule cells by NPPB (Diaz et al., 2011), similar to our results. Our data indicate that the effect of NPPB on tonic currents was caused by direct action on GABAA receptors rather than by alterations of ambient GABA. The contribution of NPPB-sensitive conductive pathways (i.e. anion channels or connexin hemichannels) to ambient GABA is likely minimal in our preparation.
Of potentially greater importance is the recognition that all three of the antagonists tested in this study can directly modulate extrasynaptic GABAA receptors. Our data suggest that these drugs, by altering tonic inhibition, will influence cellular and network excitability, independent of any actions on anion channels or connexin hemichannels. These drugs are likely to modulate many types of GABAA receptors independent of subunit composition because NPPB enhances tonic currents in both cerebellar granule cells (that are believed to be mediated by α6- and δ-subunit containing receptors) and tonic currents in dentate gyrus granule cells (that are believed to be mediated by α4- and δ-subunit containing receptors) (Farrant and Nusser, 2005; Diaz et al., 2011). Additionally, CBX inhibits both tonic currents and inhibitory postsynaptic currents in dentate gyrus granule cells, responses mediated by δ- or γ-subunit containing receptors, respectively (Tovar et al., 2009). Modulation of tonic GABA currents should be considered when interpreting experiments that use NPPB or CBX.
The classic studies of Ransom and Barker on pentobarbital enhancement of postsynaptic GABAA responses introduced the concept that GABAA receptors are subject to pharmacological modulation (Ransom and Barker, 1975, 1976). Since that time GABAA receptors have been shown to be dynamic pharmacological targets that are modulated by a wide range of molecules including benzodiazepines, high molecular weight alcohols, neurosteroids, and general anesthetics (Kurata et al., 1999; Meera et al., 2009; Houston et al., 2012). Our study adds NPPB and CBX to the list of drugs that can modulate GABAA receptor function.
Future Directions
Our results do not support a significant contribution of anion channels to ambient GABA levels in the hippocampus, although anion channels are reported to importantly influence ambient GABA in the cerebellum (Lee et al., 2010). This raises the possibility that regulation of ambient GABA levels has important differences across different brain regions or even differs across regions of a single neuroanatomical structure. For example, ambient GABA levels in hippocampus have been suggested to be higher in stratum radiatum than in the pyramidal cell layer due to laminar differences in uptake (and release) (Semyanov et al., 2003). Additionally it is not known whether anion channel mediated GABA release from neurons or glia is more prominent under some physiological or pathophysiological conditions or if this release is augmented by drugs that increase cytosolic GABA concentrations (e.g.. vigabatrin). Our results indicate that future experiments examining these issues should rely on non-pharmacologic approaches to inhibit anion channel/hemichannel function because of the confounding effects of antagonist drugs on GABAA receptors (i.e. knockout models or anti-sense treatment).
Acknowledgements:
Work was supported by a VA Career Development Award (CBR, 1IK2BX001307-01A1), VA Merit Review awards to CBR (1 IO1BX002745-01A1) and WJS (101BX000386), and by NINDS (GBR, R01NS43288).
References
- Basarsky TA, Feighan D, MacVicar BA (1999) Glutamate release through volume-activated channels during spreading depression. Journal of Neuroscience 19:6439–6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MR, Wadleigh A, Hughes BA, Woodward JJ, Valenzuela CF (2011) Bestrophin1 channels are insensitive to ethanol and do not mediate tonic GABAergic currents in cerebellar granule cells. Frontiers in Neuropharmacology 5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD (2002) Inhibition of gap junction hemichannels by chloride channel blockers. Journal of Membrane Biology 185:93–102. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nature Reviews Neuroscience 6:215–229. [DOI] [PubMed] [Google Scholar]
- Gaspary HL, Wang W, Richerson GB (1998) Carrier-mediated GABA release activates GABA receptors on hippocampal neurons. Journal of Neurophysiology 80:270–281. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I (2007) The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. Journal of Physiology 582:1163–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston CM, McGee TP, MacKenzie G, Troyano-Cuturi K, Rodriguez PM, Kutsarova E, Diamanti E, Hosie A, Franks NP, Brickley SG (2012) Are extrasynaptic GABAA receptors important targets for sedative/hypnotic drugs? Journal of Neuroscience 32:3887–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersanté F, Rowley SCS, Pavlov I, Gutièrrez-Mecinas M, Semyanov A, Reul JMHM, Walker MC, Linthorst ACE (2013) A functional role for both γ-aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus. The Journal of Physiology 591:2429–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata Y, Marszalec W, Yeh JZ, Narahashi T (1999) Agonist and potentiation actions of n-octanol on gamma-aminobutyric acid type A receptors. Molecular Pharmacology 55:1011–1019. [DOI] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ (2010) Channel-mediated tonic GABA release from glia. Science 330:790–796. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I (2005) Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature Neuroscience 8:797–804. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Zurek AA, MacDonald JF, Roder JC, Jackson MF, Orser BA (2010) alpha5 GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory. Journal of Neuroscience 30:5269–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Olsen RW, Otis TS, Wallner M (2009) Etomidate, propofol and the neurosteroid THDOC increase the GABA efficacy of recombinant α4β3δ and α4β3 GABAA receptors expressed in HEK cells. Neuropharmacology 56:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom BR, Barker JL (1975) Pentobarbital modulates transmitter effects on mouse spinal neurones grown in tissue culture. Nature 254:703–705. [DOI] [PubMed] [Google Scholar]
- Ransom BR, Barker JL (1976) Pentobarbital selectively enhances GABA-mediated post-synaptic inhibition in tissue cultured mouse spinal neurons. Brain Research 114:530–535. [DOI] [PubMed] [Google Scholar]
- Ransom CB, O’Neal JT, Sontheimer H (2001) Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. Journal of Neuroscience 21:7674–7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom CB, Tao W, Wu Y, Spain WJ, Richerson GB (2013) Rapid regulation of tonic GABA currents in cultured rat hippocampal neurons. Journal of Neurophysiology 109:803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB, Wu Y (2003) Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. Journal of Neurophysiology 90:1363–1374. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM (2003) GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nature Neuroscience 6:484–490. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Maher BJ, Westbrook GL (2009) Direct actions of carbenoxolone on synaptic transmission and neuronal membrane properties. Journal of Neurophysiology 102:974–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Ransom BR, Sontheimer H (2001) (1R,3S)-1-Aminocyclopentane-1,3-dicarboxylic acid (RS-ACPD) reduces intracellular glutamate levels in astrocytes. J Neurochem 79:756–766. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR (2003) Functional hemichannels in astrocytes: a novel mechanism of glutamate release. Journal of Neuroscience 23:3588–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Oberheim N, Kettenmann H, Ransom BR (2009) Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. GLIA 57:258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]