Abstract
Cell therapies are an emerging treatment option for a variety of diseases, especially with the success of chimeric antigen receptor T-cell therapies. With 18 FDA-approved cell therapy products as of December 2020 and a growing number in clinical trials, standards for most aspects of the cell therapy lifecycle are well-established by professional organizations like AABB and FACT; however, there are limited standardized protocols regarding the day-of infusion. Infusions were observed at three academic medical centers in the United States, and the workflows were analyzed and compared based on factors including facility layout, product verification processes, cryobag design, timing restrictions, and use of electronic medical records. Based on this analysis, opportunities were identified for standardization and streamlining the infusion workflow which may help facilitate adoption of new and existing cell therapies at a wider range of hospitals.
Keywords: Cell therapy, infusion, standardization, cryopreservation
Introduction
The cell therapy industry has rapidly expanded in recent years, particularly due to the success of chimeric antigen receptor T cell therapy (CAR-T). Cell therapies are being tested for use in a wide variety of diseases, including neurological, liver, heart, and autoimmune diseases, as well as many types of cancer[1–10]. Between September 2017 and March 2018, the number of cancer cell therapies in clinical trials increased by 87%[11]. Globally, as of March 2020, there were 1483 agents in development (preclinical to marketed) for cancer therapies with the majority (58%) being CAR-T[12]. This is an increase of 472 overall and 290 for CAR-T. As of November 2020, there were almost 700 total cell therapies, including both gene-modified and non-gene-modified cells, in clinical trials[13]. As of December 2020 there are currently several approved cellular and gene therapy products worldwide: 18 in the United States and 8 in Europe[14, 15]. Cell therapy products have a unique supply chain compared to other medicines, including pharmaceuticals and other biologics. Cells must be collected from a donor, manipulated in a cell therapy lab, and then supplied to the patient. To maintain clinical efficacy, the viability and functionality of the cells must be preserved along the entire supply chain. Cryopreservation is one common method used to keep cells viable and functional while being transported between sites and at points in the manufacturing process. Once manufactured, some cell therapies are cryopreserved at the manufacturing site (company or cell therapy laboratory) and transported to the clinical site for thaw and administration. The process of product thawing and administration can also influence the quality of the cells infused and potentially the clinical efficacy of the product.
Recent efforts in the cell therapy field have involved improving and standardizing the collection of starting cells, the manufacturing process, biopreservation, and quality control and lot release testing, including cell potency assays[16–21]. However, day-of infusion practices have received little attention in the literature and standards from AABB (formerly the American Association of Blood Banks) and the Foundation for the Accreditation of Cellular Therapy (FACT) & the Joint Accreditation Committee – ISCT and EBMT (JACIE) surrounding the infusion workflow are vague[22, 23]. The few cell therapy standards that relate to infusions are product verification standards prior to infusion, label requirements, and the requirement of having Standard Operating Procedures for the administration of cell therapies for clinical staff.
The entire process of preparing and infusing a cell therapy product takes several hours and requires close communication between cell therapy technologists (CTTs) and nursing staff. The process begins by the CTT calling the patient’s nurse to ensure the patient’s status is stable and they are ready for the infusion. For products thawed on the floor, the CTT will wait to begin the thaw until receiving approval from the nurse and/or patient physician so that the product can be immediately infused once thawed.
As more patients are treated with either investigational or FDA-approved cell therapies, managing an increasing number of cell therapy products and patients will become complicated, especially when workflows vary between products. Well-designed and/or harmonized workflows can improve efficiency and reduce the potential for detrimental effects on the product[24]. This paper describes the processes utilized by three well-established and highly regarded academic facilities and reveals similarities, differences, and opportunities for improvement in workflow and delivery of treatment to patients.
Methods
Cell therapy infusions were observed at three different large academic medical centers in the United States: one in the Midwest, one in the South, and one in the Northeast (Table 1). Each center visited has a standard-of-care laboratory that processes hematopoietic stem cell transplants, other minimally manipulated products, licensed cell therapy products (e.g., Kymriah™, Yescarta®), as well as a novel biologics manufacturing group equipped to support investigational clinical trials. Additionally, each center had both pediatric and adult clinical programs and large transplant programs. At each center, workflows, infusions, and product handling were observed. Interviews were conducted with infusion and transplant nurses and cell therapy technologists on their training, handling of the products, interactions with other staff in the workflow, and pain points in the infusion workflow. Additionally, the standard operating procedures (SOPs) relevant to the infusion workflow were compared between the three centers Clinical contacts at cell therapy manufacturers were contacted and interviewed about the requirements and recommendations for centers on handling and infusing their product(s). These included discussion on the required training, infusion materials (bags, tubing), standard operating procedures, and auditing of the workflow.
Table 1:
Overview of the centers visited to shadow cell therapy infusion workflows.
| M Health Fairview / Molecular & Cellular Therapeutics | Baylor College of Medicine | Dana-Farber Cancer Institute | |
|---|---|---|---|
| Facility Label | A | B | C |
| Location | Minneapolis, Minnesota | Houston, Texas | Boston, Massachusetts |
| Cell Therapy Certifications | FACT, AABB | FACT, AABB | FACT, AABB |
| 2019 transplant volume (preserved, more than minimally manipulated) | 166 | 180 | 362 |
| Number of active investigational new drugs (INDs) | 20 | 50 | 20 |
Results
At each facility, 2–4 patient infusions were observed, most of which were cryopreserved products (Table 2). Facility A had an offsite cell processing lab while B and C were onsite on a different floor. Cryopreserved products were transported in a dry shipper via courier to the blood bank for facility A. Products were walked to the patient’s room in a liquid nitrogen dewar and a cooler with ice packs for facilities B and C respectively. Thawing was done close to the patient’s room at facilities A and B but was thawed in the onsite lab at C. All centers had three separate steps of product/patient verification. The average time of the verification step, after thawing, was 6 ± 3 minutes with no difference between the centers. Facilities A and B thawed the therapies in or just outside the patient’s room while facility C thawed in the cell therapy lab. The thaw to infusion was less than 20 minutes for A and B but more than 40 minutes on average for C. This was strongly reflective of the thawing location being in the cell therapy lab. However, products with an expiration or recommended infusion time of 30 minutes were still infused in time. The infusion workflow for fresh products was nearly identical to cryopreserved products. The differences were upstream of being received in the cell therapy lab and if manipulated, the products were infused within the recommended time after the final wash. One small volume T-cell therapy early clinical trials was supplied in a cryovial that was thawed and transferred to a syringe. The workflow was otherwise identical to other products.
Table 2:
Key details of cell therapy processing and administration in three different facilities.
| A: M Health Fairview / Molecular & Cellular Therapeutics | B: Baylor College of Medicine | C: Dana-Farber Cancer Institute | |
|---|---|---|---|
| Number of infusions observed | 4 patients / 5 bags / 3 product types | 2 patients / 5 bags / 2 product types | 4 patients / 5 bags / 3 product types |
| Cell therapy lab location | Offsite by approximately 15 minutes / 5 miles | Onsite, different floor | Onsite, different floor |
| Transportation from lab to floor | Courier driver, picked up by CTT in blood bank within hospital | CTT walks with cart with all equipment | CTT walks only product |
| Container transporting product | Dry shipper – vapor phase | Dewar – liquid phase | Cooler with ice packs |
| Thawing location | Bedside | Hallway outside patient room | Cell therapy lab |
| Thawing equipment | Water bath / Plasmatherm (GenesisBPS™) | Water bath | Water bath |
| Product verifications | 1). Label, electronic records by CTTs/pharmacist 2). Confirm electronic record by nurse 3). Patient ID by 2 nurses |
1). Label, paperwork by 2 CTTs/pharmacist 2). Confirm paperwork by physician 3). Patient ID by physician and a nurse |
1). Label, paperwork by CTTs/pharmacist 2). Confirm paperwork by nurse 3). Patient ID by 2 nurses |
| Time from thaw to infusion (num.bags) | 15 ± 5 mins (n = 5 bags) | 8 ± 3 (n = 5 bags) | 46 ± 21* (n =5 bags) |
Significant within p < 0.05 by a Student’s t test
Training
Training regarding infusions for cell therapy technologists (CTTs) was determined and managed by the center. At Facilities A and B, training involves performing infusions under supervision prior to performing them independently. At Facility A, CTTs are required to perform 5–8 infusions of any type of product under supervision. At facility C training involved instruction from the sponsor for sponsored products and shadowing by cell therapy staff, although, all processes have a primary technician with 2–3 verifiers including a supervisor. For FDA approved products requiring a REMS, a risk evaluation and mitigation strategy, special additional safety training hosted by the manufacturer is required. One or more staff members is trained and then trains other staff on site as needed.
Facility Location and Design
At all sites visited, cell products were stored in the cell therapy processing laboratory until the day of infusion. Each site supports internal novel cell therapy trials with on-site manufacturing of several IND products. For licensed products, the laboratories serve as shipping, receiving, and storage centers. As shown in Table 1, the location of the cell therapy processing lab impacts the infusion workflow. An onsite cell therapy laboratory facility streamlines the process and limits travel time; however, hospital space is limited. The design of a Bone Marrow Transplant (BMT) floor and the location of infusion also affects the infusion workflow. At Facility B, desks were located between each patient room, which created a workspace for the CTT to set up on-site procedures. There was a window between the room and the desk area, so the CTT could monitor the infusion process with nurses when multiple bags are required.
Procedure Timing
One of the major challenges with infusion of products is the often tenuous patient condition which can change quickly and delay the procedure from hours to potentially days. For example, an infusion cannot begin if the patient experiences serious medical complications, adversely responds to pre-medication, is hemodynamically or otherwise unstable. While these delays are rare, this complication makes timing and coordination challenging for cell therapy lab staff.
If there are multiple bags of product to be administered, one bag is thawed at a time and infused. If the patient responds negatively to the infusion, nursing staff will ensure the next bag is not thawed until the patient is treated. At the centers, the doctors, nurses, and cell therapy staff routinely review the status of patients on the floor and coordinate pre-infusion testing, such as busulfan level or T-cell count, and infusion times. Typically, a cell therapy coordinator places an order for the therapy, the physician or medical director approves the order the day before, cell therapy technicians prepare a cart and check in with the patient’s care providers a couple hours before the planned infusion time, and removes the product from storage if the therapy is approved to move forward.
Nursing Workflow
At each facility, there was varying degrees of electronic medical record (EMR) use; however, all facilities used paper for most of their documentation. Facilities B and C had a paper record that stayed with the product during the infusion on which nurses could indicate any infusion reactions, whereas Facility A documented this in the EMR. All patients were pre-medicated before the infusion. For patients receiving products cryopreserved with dimethyl sulfoxide (DMSO), patients were informed that they may experience an unusual taste (e.g., “creamed-corn” or “garlic”) in their mouth and all the facilities gave the patients hard candy for this intended to decrease nausea. Dimethyl sulfone, the major metabolite of DMSO, could sometimes be detected as a smell in the room. Facility C places a portable air filtration unit in the patient’s room to prevent the smell from lingering.
Product Verification
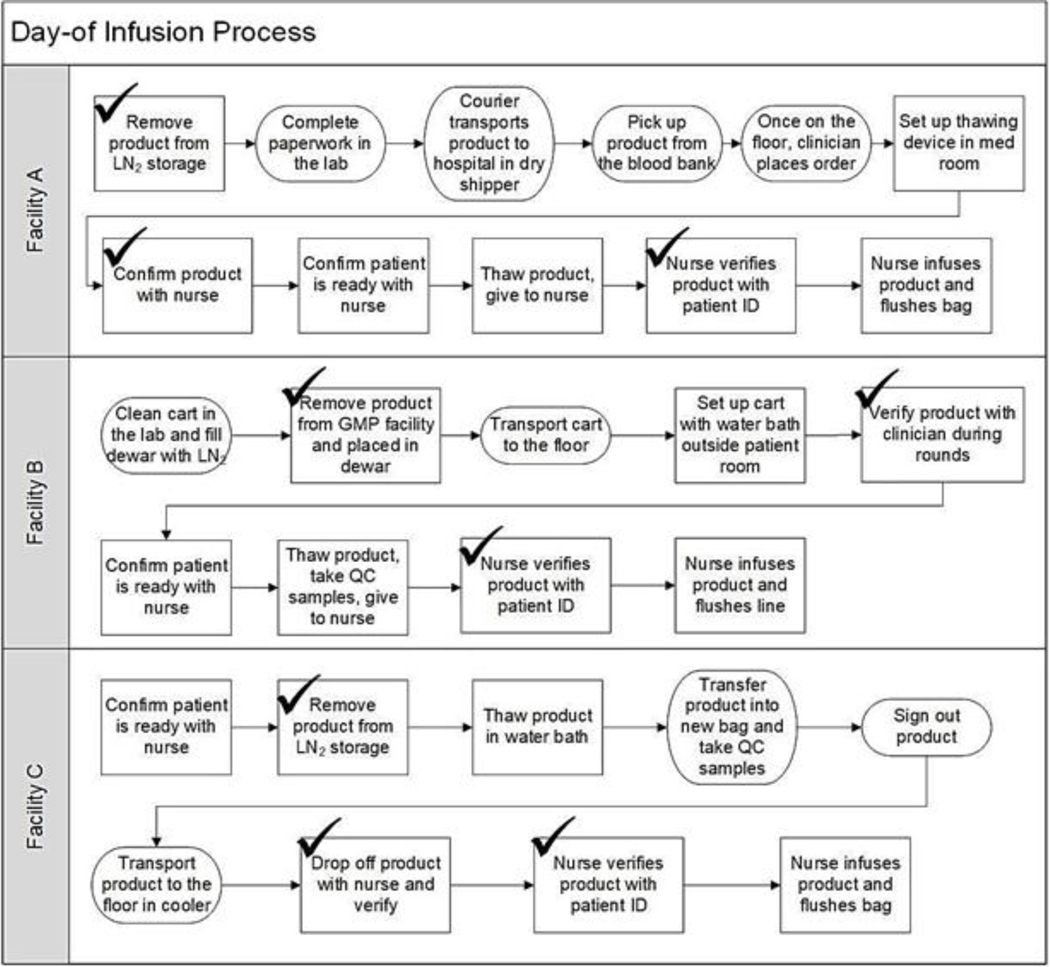
A critical step in the infusion process involves ensuring that the correct patient receives the correct product. Kymriah™ and Yescarta® require confirming the patient identity as the first step of product preparation. Verification of the product identity typically occurs at three steps, as shown in Figure 1, where the patient’s name, medical record number, birthdate, and product identification are confirmed. First, when the product is removed from the long-term cold storage tank, it is verified by two cell therapy technologists or a CTT and a pharmacist for a commercial product. It is verified again when it arrives on the hospital floor by both a CTT and either a physician or nurse, depending on hospital policy. At Facilities A and B, this step occurs prethaw, while at Facility C it occurs post-thaw since the product is already thawed when it arrives on the floor. The final check occurs in the patient room with two nurses confirming patient and product identities using paperwork at Facilities B and C or the electronic medical record (EMR) at Facility A. At all three sites observed, every verification was done manually, without barcode scanning.
Figure 1:
Workflow comparison across facilities. Checks indicate a step with product verification. Steps that differed significantly are round and steps that are similar are rectangles.
Thawing Location
Facilities A and B thawed product at the patient bedside. All facilities used a water bath for thawing products while Facility A used a Plasmatherm dry thawing device for cord blood and certain other products. At Facility C products were thawed in the laboratory. Products with a short stability period (e.g. 15 minutes) are thawed in a small lab close to the BMT floor. In the case of bag breakage, thawing in the laboratory provides the benefit of a sterile environment (biological safety cabinets) for salvaging the product by manually transferring it into a new bag. At each location, the CTT recorded the initial time of thawing and nursing recorded the time each product started and finished infusing. Measurement of the actual time to infusion for each product is critical (Table 2) as is development of a contingency plan to address a change in patient state or other circumstances resulting in unexpected delays.
Cryobags and Tubing
For products manufactured on-site, the cell therapy lab chooses the cryobag used and all three facilities used OriGen Biomedical CryoStore™ infusion bags while center C would then transfer hematopoietic progenitor and mononuclear cell products into a Fenwal TP bag after thawing. CAR-T products were kept in their original bags (50 mL) that have three ports with two sealed by the manufacturer. In some cases, the infusion bag was placed inside a second, sterile bag to protect the ports from contamination and in the rare case of a bag break. In other cases, the water bath was filled with sterile saline to collect the sample in case of a bag break. Spiking the bags was sometimes challenging and believed to be from the new supplier. Twisting the port aided in spiking but potentially results in the plastic coming off the bag into the solution so a large filter was added to prevent it moving down the line. Additionally, the spikes supplied were replaced with DMSO-resistant spikes for products containing DMSO.
Nurses faced some difficulties adding saline to flush the bag to ensure the entire product was administered pending the product bag design and if pre-supplied tubing was used. In some cases only the tubing lines were flushed or syringes were used to inject saline into the product bag. Some bags had ports suitable for flushing using saline-filled syringes whereas other bags required backflushing with a second bag containing saline connected by tubing. Kymriah™ and Yescarta® bags have 3 ports but 2 are sealed requiring a back flush or saline syringes to flush these bags. The amount of saline used to flush the bags of product varied between facilities and depended on the product sponsor requirements, typically ranging between 20 and 50 mL. The length of the tubing from the product to the patient’s port was considered in this calculation, as an adequate volume of saline must be used to completely flush the lines to ensure the patient receives the full therapeutic dose of the product.
Manufacturer and Sponsor Recommendations
Nine CAR-T companies were contacted regarding their recommendations and requirements for their product(s) regarding handling and infusion workflow and three responded. Water baths are the specified thawing method and/or by FACT requirements. Thawing location is not specified but having the shortest thaw to infusion time is strongly desired. Should this time go above the product expiration this, a deviation is recorded. Training by the manufacturer or sponsor on their product(s) is required. For large volumes, generally greater than 50 mL, transferring the product to another bag for infusion is not allowed. Smaller volumes may be transferred to a syringe, but this is usually specified in the instructions anyway. Tubing, bag spikes, and syringes (if applicable) used are as approved by the center. Flushing of the product bag(s) after infusion is required and the manufacturer/sponsor instructions should specify the how and the volume of the flush. A third required approval by the manufacturer/sponsor for a SOP revision involving their product(s) while the others did not require separate approval but were reviewed in their annual audit.
The centers were also asked about their interaction with manufacturers and their recommendations on workflow for the product they administer. Overall, the sites would follow their typical workflow for infusions after the protocols are reviewed by the sponsor in a site visit. Besides regulatory requirements and procedures covered in accreditation, other requirements were specific toward the therapy such as if a filter should be used, with what specifications, or if the product can or should not be transferred to another bag or a syringe. Of course, the sponsor has the ultimate decision on instructions for their product and the centers would work closely with them to meet their needs and follow all instructions provided.
Discussion
Infusion Workflow Analysis and Comparison
This study was limited by volume of cell therapies ongoing at each facility during the allowed study period. Some nurses indicated the time since their last cell therapy infusion were weeks and sometimes months even at these large centers. To expand the study, interviews with cell therapy technicians, infusion nurses, and cell therapy laboratory and medical directors were conducted and comparisons between the workflow standard operating procedures (SOPs) between the centers were made.
A comparison of cell therapy infusion SOPs revealed the differences observed at the centers were reflected in the SOPs with other differences dependent on the specific product type and the instructions provided by the manufacturer. As expected, the source of inter-institutional variability lies in the SOPs but the workflow is consistent within the center. Interviews with nurses, technicians, and cell therapy laboratory and medical directors also reflected these differences and of the results from shadowing therapies. This suggests guidance and standards would reduce this variability, although, not just for the centers themselves but for the manufacturers upstream of the overall workflow and in the studies performed in the development of the therapy.
The primary factor that affected workflow timing was the thawing location. If products are thawed in the cell therapy lab, an onsite lab is necessary to infuse products within the expiration time. However, if thawing is done close to the patients room an onsite lab is not necessary as seen by the time to infusion of facility A and B. The duration of product verifications was similar between the three centers. These verifications are necessary and while marginal time savings are possible, every minute is essential should an irregularity is identified. Setting up infusion was dependent on training, but the primary variation was the ease or difficulty of spiking the product bag or if tubing, if supplied, was difficult to use. The duration of infusion itself depends on the volume of the product, typically 50–100 mL. Product flushing was quite varied between the sites on if it was performed, how, with what volume, and if the product bag was easy to backflush. While factors such as spiking the bags, verification, labeling, and flushing did not have a significant impact on the time to infusion, they are worth discussing regarding workflow standards, guidance, and best practices for upcoming cell therapies.
Kymriah™ requires the product to be infused with 30 minutes of thawing, and certain novel products have infusion time limits of 15 minutes[25, 26]. The short duration between thawing and infusion make compliance challenging as the product must be transported to the patient bedside, verified, and then infused into the patient all within this short timeframe. Overall, 30 mins is a reasonable target but there is risk during transport if thawing is not done adjacent to room. There is additional risk of the product being held up during transportation if thawed a distance away from the patient room. Still, there was a sense of rush to reach this timepoint at all three centers. A 15 minute expiration time necessitates thawing by the patient room but is still risky given the time for product verifications after thawing and the time to infuse and flush the product itself. This assumes there are no verification, bag, tubing, or spiking issues with most of the thaw to infusion time being infusion rate itself. A 45 or 60 minute expiration time would greatly reduce risk during the infusion workflow but is likely an upper limit on maintaining a high level of cell function and viability after thawing. For multiple bags, the nursing staff will infuse the first bag and evaluate how the patient responds before asking the technologist to thaw the next bag, as is standard practice[27]. The effect of transient warming of removing the product from cryogenic storage to check the label was not evaluated. For products with short expiration times, this warming could be mitigated with an automated system that reads vial and bag labels within the cryogenic environment.
Training and Coordination
Cell therapy manufacturers may require specific training and professional certification prior to offering their product. This is required if a product has an FDA risk evaluation and mitigation strategy (REMS) such as Kymriah™ and Yescarta® [28, 29]. A REMS requires an authorized representative from the hospital or clinic to oversees REMS requirements and is responsible for training all staff involved in the administration of the product and establishing processes to ensure new staff are appropriately trained. While not every cell therapy product has a REMS, product sponsors may require similar training and request certain lab accreditation(s), such as AABB or FACT. However, centers must have their own training protocols with written procedures and competency evaluations that includes training from a REMS-trained staff for the appropriate products. An additional consideration for cell therapy products is the current low rate of infusions done per year. Even at these large centers, some nurses administered a cell therapy once a month or just a few times a year. This could be mitigated with dummy infusions of mock products while mimicking the multiple product verifications as deemed appropriate by the center.
As cell therapies rapidly evolve and more become FDA approved, infusion workflows and training for these workflows need to be developed. The unique nature of these therapies is evident with the introduction of an entire course for nurses to address the complexities of administering a cell therapy [30]. Other educational options used by different organizations for nurses include daily huddles, physician-led symposiums, and pairing of a clinical nurse specialist with the inpatient nurse[31]. Cell therapies are not covered in the traditional nursing curriculum, so cell therapy-specific training has been shown to improve preparedness[32]. Training can vary by hospital so the creation of standards for cell therapy education would ensure all nurses receive the same baseline knowledge. Even with additional training, these infusions are not as commonplace as other procedures, and depending on the level of institutional activity, continuing education may be necessary. Competency programs are critical, and some facilities have created nursing coordination roles to help with the enormous amount of logistics required by cell therapy treatments[33].
With the training of cell therapy lab staff, most do not have direct experience with more-than-minimally manipulated cell therapies. While infusion of minimally manipulated products is essentially the same as novel cell therapies, the companies or manufacturing academic sites typically have some unique requirements. In a 2015 survey by the Alliance for Harmonization of Cellular Therapy Accreditation (AHCTA), staff in cell processing labs are commonly trained by observing other staff perform procedures and then being observed themselves. Additional training programs utilized a yearly competency assessment and analysis of product quality during the assessment period[34]. Training for a licensed product or a product under IND for a multicenter investigational trial may start with similar approaches and coordination with the manufacturing group.
The protocols for staff training and coordination of infusion date and time is likely best handled by the center and their own risk-evaluation with the cell therapy lab and care unit or a greater cell therapy team in the center. This coordination is important for products handled offsite, infusion of multiple bags for one patient, and especially if the product thawed within a cell lab than close to the patient’s room. Typically, this included weekly meetings with the care providers and cell therapy lab, processing and approval of the therapy order with the proposed infusion date and time, inventory check and discussion the day before with the providers, lab, and patient, and floor-lab confirmation in the morning of which worked well. Previous studies have demonstrated that the establishment of a core cell therapy task force dedicated to managing administration of all cell therapy products within their center is highly valuable in managing workflow[35, 36]. H. Dave and colleagues recommended that the members of a core cell therapy team include physicians (e.g., primary care, BMT, oncology, and apheresis/transfusion medicine, if applicable), infusion and oncology floor nurses, trial coordinators, cell therapy technologists, pharmacists, and administrative support as needed. A defined group provides a means to proactively harmonize and optimize a cellular therapy program for an increasing number of patients and products. For commercial products, the pharmacist also becomes a significant member in the infusion workflow as these products, compared to clinical ones, require additional paperwork and verification by the pharmacist before release.
Facility Location and Design
The location of the cell therapy lab and thawing location cannot be standardized for existing centers. However, it is apparent that having space for thawing and handling the product adjacent to the patient’s room reduces risk and variation in the workflow. This should be of consideration for the design of new clinics and wings or additions to large centers. If thawed close to the patient’s room the location of the cell therapy lab does not need to be onsite. Infusions can then stay within the expiration time without having a large impact on the workflow besides including transportation time for the desired product arrival time.
Cryobags
The purpose of a cryobag is to contain product in both frozen and liquid states and allow for thaw in a uniform, repeatable manner without worry of sample loss. These bags are critical to the workflow as they are handled from the initial filling of the product at the site of manufacture through thawing and infusion, and they house the product label for tracking and verification. Bags come in many designs including varying volumes, ports, tubing sets, and connections. Cryobag design can influence the entire workflow, so careful consideration of the bag’s design can lead to a more harmonized workflow.
However, cryobags for products manufactured off-site at a company or other academic cell therapy laboratory may not be optimal for all clinical sites. The primary concerns are ensuring ease of spiking and flushing regarding workflow timing and complete delivery of the cells respectively. Spiking the infusion bag was sometimes a challenge causing delay in the workflow after thawing and appeared to be related to the specific model. Additionally, the tubing choice, sometimes supplied by the manufacturer/sponsor, and choice of spike for DMSO-resistance did affect workflow but was best handled and determined by the center. Flushing of the product bag is critical to ensuring complete delivery of the therapy and both Kymriah™ and Yescarta® indicate rinsing in the package inserts. [25, 37]. However, Kymriah™ specifies rinsing of the bag with 10–30 mL of normal saline delivered in a closed tubing system while Yescarta® specifies rinsing the tubing with normal saline. The discrepancy may from inter-institutional variability as to what to flush, how to flush, and how much saline to use especially for therapies that don’t specify this but also from the manufacturer as in what bag configuration is supplied. Since these products tend to be 50–100 mL, the volume left in the bag and/or line may be a significant fraction of the therapy. Flushing processes that went smoothly in some centers and product types and was challenging and caused difficulties in others, so the ease of this process depended on the exact bag and tubing used, as well as training of the nurses.
AABB provides a sample procedure for validating bags regarding freezing, thawing, and label readability, but it is designated as a guidance document[38]. Guidelines on the number of ports and product flushing methods can improve product administration and ensure complete dosage delivery, while still allowing for variation as one design may not be ideal for all therapies. Still, common choices or recommendations can simplify infusion training and flushing protocols, which are currently dependent on the bag and tubing used. The ease of flushing can be handled through the manufacture on the choice of bag configuration and the recommendation or standard of a separate port or procedure for flushing.
Product Verification
Product labeling standardization in cell therapies is already in effect. ISBT 128 is an established set of labeling guidelines for blood products that has more recently been utilized by cell therapies as well[39]. They provide a unique global identifier of cell therapy products, as well as barcode standards. These barcode standards would ideally be utilized so that cell therapies may be barcode scanned to the electronic medical record to reduce the time for verification and improve accuracy. It has been shown that barcode verification can reduce transfusion errors and should be considered for new cell therapy products as well[40]. However, the use electronic records are not universal nor is the use of barcode scanners. Given the technology of both EMRs and barcode scanners are not universal, this presents an additional barrier to adopting these for part of product verification. This is not meant to replace the product order, label, and patient verification but can act as one quick verification step to save time in the workflow reducing risk.
Harmonization and Benchmarking
Although there are standards on quality systems and product verification regarding cell therapies, there is limited attention on day-of-infusion workflow leading to inter-institutional variability in workflows. Many needs have been identified to address the variations mentioned by standards and harmonization related to infusion workflow. Harmonization can ease the adoption of more cell therapies enabling the treatment of patients at more locations while maximizing clinical efficacy and reducing the risks associated with infusion. Such harmonization, however, will have to balance standards with flexibility as the cell therapy field is continually evolving, improving, and growing.
Guidance and harmonization can be established and benchmarked to a similar field, such as blood banking. AABB has released 32 editions of standards for blood banking and transfusion services, as well as 9 editions for cell therapy services[22]. Still, there are challenges and uncertainty in establishing new transfusion services in hospitals and clinics, in particular with workflow, information technology, and education[41, 42]. To point out and discuss these challenges, the Standards Coordinating Body (SCB) for cell, gene, and regenerative medicine therapies released a perspective identifying twenty-two needed standards in cell therapy[43]. Needs identified by SCB that are relevant to the infusion workflow include universal labeling, transportation and packaging controls, chain of custody tracking, cryopreservation methods and processes, and safety training and education. Collaborative working groups between academia, industry, accreditation and regulatory bodies can address these needs and establish harmonization.
The processes and protocols for infusion of cell therapy products at the three centers were found to be mostly dependent on the hospital or clinic administering them. Some differences are attributed to variations in facility design and layout, cell therapy training, product verification processes, and electronic medical records. The cryobags, unless manufactured or obtained fresh on site, were dependent on the manufacturer as it is often specified to infuse the product from the bag supplied in. Generally, the center will follow their usual workflow, using their own materials, with slight modifications such as if a filter should be included or not as covered by sponsor provided training. Thawing methods were left up to the centers although with the request of a minimal thaw to infusion time and the thawing location was the primary source of inter-institutional variation. A minimal time is favored to keep the viability and efficacy of the cells at their maximal level. This is balanced, however, by the need for product verifications, thawing location, infusion setup, infusion rate, and product volume. An expiration time of 30 minutes still felt rushed to the nurses meaning 15 minutes is especially challenging to meet. Therefore, the evaluation of safety and efficacy for a range of times after thawing is essential to connect efficacy with logistics. The expiration time could be slightly extended, easing the workflow, while still maintaining a high level of integrity of the cell therapy. Therapies that still require a short thaw to infusion time may have challenges in their implementation to many centers and are at greater risk of recording workflow deviation on the infusion time reported. Regarding the product bag itself, often the product is meant to be infused from the bag it was supplied in and flushing is critical to ensure complete and consistent dose delivery, arguably, next most important workflow risk factor after thaw to infusion time. Given flushing was difficult for some products, consideration of the product cryobag design for ease of flushing while meeting other requirements is desirable. Verifications of the product are required although technology to do so is not yet harmonized although the degree of which technology is employed is also varied even between these centers. This would not greatly reduce the thaw to infusion time but can reduce risk in transfusion errors.
Additional guidance or standards for cell therapies in clinical trials on labels, patient and therapy verifications, post-thaw expiration time evaluation for academic sites, sponsors, and manufacturers may alleviate the variation and challenges experienced in the infusion workflow. All these considerations would ease the translation of these valuable therapies into clinics and to not place all the burden on the cell therapy center that already balances audits for each cell product, especially with the observed inter-institutional variation. While these may be helpful, such guidance and standards ought to remain flexible enough as this field is still evolving and different products may have special requirements for other factors in the workflow. A 30 minute expiration time after thawing, while still having a sense of rush and risk with workflow variations, is much preferred over 15 minutes. Therefore, designing studies to evaluate the product stability at these various times can reveal how constrained and risky or flexible the infusion workflow is at various centers and is highly recommended. It was shown the thawing location is the dominant factor in thaw to infusion time and is the shortest and least variable when thawed close to the patient’s room.
Establishment of additional guidance regarding infusions and standard harmonization by FACT and AABB could accelerate the adoption of new and breakthrough cell therapies for manufacturers and clinics. Relevant harmonization topics for cell therapies include cryobags, labeling, product chain of identity, electronic records, in-hospital workflow, and infusion setup. Without harmonization, as more cell therapy products are approved for clinical use the burden of managing them increases with the increasingly complex coordination of company regulation and varying workflows of each product. For example, approval for a hospital or clinic to administer cell therapy products Kymriah™ and Yescarta® requires routine auditing by the manufacturer[28, 29]. If new cell therapies are developed by multiple companies then the continued effort to offer all of them, via routine company audits, and managing varying protocols of each product, can become quite large. The National Marrow Donor Program (NMDP) recently developed the Quality System Audit Program to simplify the audit process for cell therapy companies and hospitals [44]. However, the program is still relatively new, and the benefits remain to be seen.
Conclusions
The infusion workflow for cellular therapies was shadowed at three large medical centers across the United States. A variety of cell types were observed with most of the products being cryopreserved and then thawed before infusion. Interviews with infusion nurses, cell laboratory technicians, and cell therapy medical directors were conducted, and comparisons of infusion workflow standard operating procedures (SOPs) compared. Differences between the SOPs were the primary source of workflow variation between the centers but workflow was consistent within each center. The thaw to infusion time was significantly different at one center and is due to the difference in thawing location being in the cell laboratory on a different floor compared to adjacent to the patient’s room. Other differences, while not significantly impacting the thaw to infusion time, included the method of product verification, flushing of the product bag, and degree of electronic medical record use. Product expiration times of 30 minutes felt rushed to the staff involved in the infusion but was achievable by the centers. Post-thaw studies of the product in the time range of 15 to 90 minutes are suggested to find a balance of the product safety and efficacy with the rush of workflow or reveal a logistical limitation in its use at some centers. Often, the centers must infuse the cells from the bag they are supplied in and troubles in spiking and flushing of the bags suggest the choice of cryobag configuration can impact the workflow and administration of the cell therapy. Manufacturers and therapy sponsors do have an impact on the workflow from product bag choice, product training, SOP revision approval, and audits. The current process of a cell therapy manufacturer directly auditing the center for use of its product will become an unsustainable practice as more therapies are approved. The complexity of the workflow and impact of upstream decisions on the product suggest harmonization between therapy manufacturers, study designers and sponsors, and centers administering the cell therapies is necessary to successfully translate these therapies to the patients in need of them. Guidance and standards for these factors, however, must be flexible enough to adapt to the growing and evolving field of cellular therapies.
Table 3:
Breakdown of products observed at the centers
| Product type | Clinical or commercial? | Percent observed | Preserved or fresh? | Expiration time |
|---|---|---|---|---|
| Stem cell | Clinical | 40 | Cryopreserved | 90 mins |
| Cord blood | Clinical | 20 | Cryopreserved | 60 mins |
| CAR-T | Commercial | 10 | Cryopreserved | 30 mins |
| Non CAR T-cell | Clinical | 10 | Cryopreserved | 15 mins |
| Bone marrow | Clinical | 10 | Cryopreserved | 4–6 hours |
| Bone marrow | Clinical | 10 | Fresh | 4–6 hours |
Acknowledgments
Funding
Research reported in this publication was supported by the National Institute of Health (R01EB023880 and R25HL128372). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Competing Interest
The authors have no commercial, proprietary or financial interest in the products or companies described in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vismara I, Papa S, Rossi F, Forloni G, Veglianese P. Current Options for Cell Therapy in Spinal Cord Injury. Trends Mol Med. 2017;23:831–49. [DOI] [PubMed] [Google Scholar]
- 2.Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strom S, Jorns C. Cell Therapy of Liver Disease: From Hepatocytes to Stem Cells. In: Principles of Regenerative Medicine. 3rd edition. Academic Press; 2019. p. 229–46. [Google Scholar]
- 4.Kahlon A, Vaidya G, Bolli R. Cell therapy for heart disease: current status and future directions. Minerva Cardioangiol. 2018;66:273–91. [DOI] [PubMed] [Google Scholar]
- 5.Műzes G, Sipos F. Issues and opportunities of stem cell therapy in autoimmune diseases. World J Stem Cells. 2019;11:212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunk PR, Bauer TW, Slingluff CL, Rahma OE. From bench to bedside a comprehensive review of pancreatic cancer immunotherapy. J Im munother Cancer. 2016;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rezvani K. Adoptive cell therapy using engineered natural killer cells. Bone Marrow Transplant. 2019;54:785–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, et al. Umbilical cord blood–derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016;127:1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthay MA, Thompson BT, Read EJ, McKenna DH, Liu KD, Calfee CS, et al. Therapeutic Potential of Mesenchymal Stem Cells for Severe Acute Lung Injury. Chest. 2010;138:965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koepsell SA, Miller JS, McKenna DH Jr. Natural killer cells: a review of manufacturing and clinical utility: MANUFACTURING AND CLINICAL UTILITY OF NK CELLS. Transfusion (Paris). 2013;53:404–10. [DOI] [PubMed] [Google Scholar]
- 11.Tang J, Hubbard-Lucey VM, Pearce L, O’Donnell-Tormey J, Shalabi A. The global landscape of cancer cell therapy. Nature Reviews Drug Discovery. 2018. doi: 10.1038/nrd.2018.74 [DOI] [PubMed] [Google Scholar]
- 12.Yu JX, Upadhaya S, Tatake R, Barkalow F, Hubbard-Lucey VM (2020) Cancer cell therapies: the clinical trial landscape. Nature Reviews Drug Discovery [DOI] [PubMed] [Google Scholar]
- 13.Alliance for Regenerative Medicine. ARM’s Q3 2020 Trend Talk. 2020. http://alliancerm.org/wp-content/uploads/2020/11/Q3-Report-Print-Version.pdf
- 14.US Food and Drug Administration C for BE and R. Approved Cellular and Gene Therapy Products. 2020. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapyproducts/approved-cellular-and-gene-therapy-products. Accessed Dec 2020.
- 15.Paul-Ehrlich-Institut FI for V and B. Gene Therapy Medicinal Products with a valid marketing authorisation. 2020. https://www.pei.de/EN/medicinal-products/atmp/gene-therapymedicinal-products/gene-therapy-node.html. Accessed Dec 2020.
- 16.Levine BL, Miskin J, Wonnacott K, Keir C. Global Manufacturing of CAR T Cell Therapy. Mol Ther Methods Clin Dev. 2017;4:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gniadek TJ, Garritsen HSP, Stroncek D, Szczepiorkowski ZM, McKenna DH. Optimal Storage Conditions for Apheresis Research (OSCAR): a Biomedical Excellence for Safer Transfusion (BEST) Collaborative study. Transfusion (Paris). 2018;58:461–9. [DOI] [PubMed] [Google Scholar]
- 19.Hubel A. Preservation of Cells: A Practical Manual. 1st edition. Wiley Blackwell; 2018. [Google Scholar]
- 20.Li R, Johnson R, Yu G, Mckenna DH, Hubel A. Preservation of cell-based immunotherapies for clinical trials. Cytotherapy. 2019;21:943–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Worsham DN, Reems J-A, Szczepiorkowski ZM, McKenna DH, Leemhuis T, Mathew AJ, et al. Clinical methods of cryopreservation for donor lymphocyte infusions vary in their ability to preserve functional T-cell subpopulations. Transfusion (Paris). 2017;57:1555–65. [DOI] [PubMed] [Google Scholar]
- 22.American Association of Blood Banks. Standards Programs. AABB. 2018. http://www.aabb.org/sa/standards/Pages/standards-programs.aspx. Accessed 6 Apr 2020. [Google Scholar]
- 23.FACT. FACT-JACIE Hematopoietic Cell Therapy Standards. http://www.factweb.org/forms/store/ProductFormPublic/sixth-edition-v6-1-fact-jacie-internationalstandards-for-hematopoietic-cellular-therapy-product-collection-processing-and-administrationfree-download. Accessed 17 Jun 2020.
- 24.Cain C, Haque S. Organizational Workflow and Its Impact on Work Quality. In: Hughes RG, editor. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. http://www.ncbi.nlm.nih.gov/books/NBK2638/. Accessed 6 Feb 2020. [PubMed] [Google Scholar]
- 25.Food and Drug Administration. Prescribing information - Kymriah. 2018. https://www.fda.gov/media/107296/download.
- 26.Wuchter P. Processing, cryopreserving, and controlling the quality of HSCs. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT Handbook. Cham: Springer International Publishing; 2019. p. 128–9. doi: 10.1007/978-3-030-02278-5_30 [DOI] [PubMed] [Google Scholar]
- 27.Areman E, Loper K, editors. Cellular therapy: principles, methods, and regulations. 2nd edition. Bethesda, MD: AABB Press; 2016. [Google Scholar]
- 28.U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) - Kymriah (tisagenlecleucel). 2019. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=368. Accessed 6 Apr 2020.
- 29.U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) - Yescarta (axicabtagene ciloleucel). 2019. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=375. Accessed 6 Apr 2020.
- 30.Winacoo A, Peterson J, Wheatley T. Implementing Immune Effector Cell Education for Nursing Staff. Biol Blood Marrow Transplant. 2019;25:S439. [Google Scholar]
- 31.Benedetti H, Latchford TM. Developing BMT Nurses to Care for Axicabtogene Ciloleucel (Yescarta) Chimeric Antigen Receptor T Cell Patients. Biol Blood Marrow Transplant. 2019;25:S438–9. [Google Scholar]
- 32.Hankewycz M. Evaluating Nursing Readiness and Competency for Direct Patient Care after Initiation of New Pediatric CAR-T Cell Therapy Program. Biol Blood Marrow Transplant. 2019;25:S82–3. [Google Scholar]
- 33.Hutnick E, Ruehle K, Gahres N. Shifting Gears from Transplant to CAR T: Creating a Cell Therapy Nurse Coordinator Role. Biol Blood Marrow Transplant. 2019;25:S82. [Google Scholar]
- 34.Keever-Taylor CA, Slaper-Cortenbach I, Celluzzi C, Loper K, Aljurf M, Schwartz J, et al. Training practices of cell processing laboratory staff: analysis of a survey by the Alliance for Harmonization of Cellular Therapy Accreditation. Cytotherapy. 2015;17:1831–44. [DOI] [PubMed] [Google Scholar]
- 35.Dave H, Jerkins L, Hanley PJ, Bollard CM, Jacobsohn D. Driving the CAR to the Bone Marrow Transplant Program. Curr Hematol Malig Rep. 2019;14:561–9. [DOI] [PubMed] [Google Scholar]
- 36.Perica K, Curran KJ, Brentjens RJ, Giralt SA. Building a CAR Garage: Preparing for the Delivery of Commercial CAR T Cell Products at Memorial Sloan Kettering Cancer Center. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2018;24:1135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Food and Drug Administration. Prescribing information - Yescarta. 2017. https://www.fda.gov/media/108377/download
- 38.American Association of Blood Banks. Sample Procedure: Validation of Cryopreservation Bags. http://www.aabb.org/aabbcct/Documents/validationsamplesop.pdf
- 39.ICCBBA. ISBT 128 Standard Labeling of Cellular Therapy Products. 2012. http://www.iccbba.org/uploads/6c/db/6cdba776ce2ba38a0c21776f2b7ee95b/ST-004-ISBT-128Standard-Labeling-of-Cellular-Therapy-Products-v1.2.0.pdf
- 40.Nuttall GA, Abenstein JP, Stubbs JR, Santrach P, Ereth MH, Johnson PM, et al. Computerized bar code-based blood identification systems and near-miss transfusion episodes and transfusion errors. Mayo Clin Proc. 2013;88:354–9. [DOI] [PubMed] [Google Scholar]
- 41.Murphy CH, Lim AY, Chua L, Shan H, Goodnough LT, Virk MS. Establishing a Satellite Transfusion Service Within an Academic Medical Center. Am J Clin Pathol. doi: 10.1093/ajcp/aqaa018. [DOI] [PubMed] [Google Scholar]
- 42.Algora M, Grabski G, Batac-Castro AL, Gibbs J, Chada N, Humieda S, et al. Challenges in Establishing a Transfusion Medicine Service: The Cleveland Clinic Abu Dhabi Experience. Arch Pathol Lab Med. 2018;142:1233–41. [DOI] [PubMed] [Google Scholar]
- 43.Standards Coordinating Body - Nexight Group. Community Perspectives: Needed Standards in Regenerative Medicine. 2020. https://static1.squarespace.com/static/58a331b0db29d63c7fb64528/t/5e42d0a2a67d2e7384d4b22a/1581437093995/NeededStandardsReportJanuary2020.pdf
- 44.Be The Match Bio Therapies. Be The Match BioTherapies® Launches Quality System Audit Program. Be The Match BioTherapies. 2018. https://bethematchbiotherapies.com/newsroom/be-the-match-biotherapies-announces-launchof-quality-system-audit-program/. Accessed 21 Jun 2020. [Google Scholar]