Abstract
Given the paradigm of anti-insulin resistance in therapies for metabolic syndrome, there has been considerable interest in adiponectin (APN), an adipocyte-derived sensitizer of insulin receptor signaling. In contrast to hypoadiponectinemia in metabolic syndrome, evidence suggests that Alzheimer’s disease (AD) and other diseases, including chronic heart failure (CHF) and chronic kidney disease (CKD), are characterized by hyperadiponectinemia as well as the APN/obesity paradoxes, indicating that a decrease in APN might also be beneficial for these diseases. Thus, distinct from metabolic syndrome, it is anticipated that APN receptor antagonists rather than agonists might be effective in therapy for some chronic diseases.
Introduction
The increasing prevalence of aging-related disorders, including neurodegeneration, cardiovascular disease (CVD), sarcopenia, osteoporosis, and immunological disorders, is associated with expansion of the older population worldwide [1]. Given that metabolic disorders, such as type 2 diabetes mellitus (T2DM), are linked to these diseases, anti-insulin resistance has become a paradigm of therapy [1]. This is particularly important for aging-associated neurodegenerative diseases, including AD and Parkinson’s disease (PD), for which no therapies are available [2]. Insulin, glucagon like peptide-1 receptor agonists, and inhibitors of dipeptidyl peptidase 4 have been studied [3–5] with promising results in small-scale trials [3,4], but the results require validation in larger clinical studies.
APN is a multifunctional adipocytokine that is also referred to as GBP-28, apM1, AdipoQ, and Acrp30 [6]. It is produced predominantly in adipose tissues, in which it is synthesized as a 28-kDa monomer, followed by post-translational modifications, such as glycosylation and oligomerization, to different molecular weight multimers [6–8]. APN is involved in diverse biological processes, including sensitization of the insulin receptor signaling pathway, suppression of inflammation, and stimulation of mitochondria biogenesis [6]. At the cellular level, the effects of APN are mediated through activation of transmembrane receptors, AdipoR1 and -R2, and their downstream signaling pathways. In the nervous system, this signaling is mediated by molecules such as AMP-activated protein kinase and peroxisome proliferator-activated receptor γ, which have essential roles in insulin sensitivity and oxidative metabolism, whereas other kinases, such as p38 MAP kinase and GSK-3β, are involved in neurogenesis and suppression of neurodegeneration [6,8].
It is well characterized that APN is beneficial for metabolic syndrome and related diseases. Similarly, it is generally believed that APN might be protective in other types of disease, including cardiovascular and neurodegenerative diseases [9]. However, accumulating evidence suggests that APN is not simply a protective molecule. Indeed, APN might exacerbate various chronic diseases in advanced stages, including CHF and CKD [8,10]. Furthermore, a recent prospective cohort study showed that hyperadiponectinemia was correlated with cognitive deficits and amyloid deposits in older patients, suggesting that APN is a risk factor for AD [11]. In this context, here we discuss the role of altered serum APN levels in some chronic diseases and propose a novel therapy strategy using antagonists of APR receptors for such disorders, which is distinct from the current strategy using APN agonists for the therapy of metabolic syndrome.
Hypoadiponectinemia in metabolic syndrome
APN belongs structurally to the complement 1q family and is found at high concentrations (>0.01% of total protein) in the serum of healthy individuals [6]. Given the abundance of APN in plasma, it is assumed that alterations in plasma levels of APN might affect various aspects of health and disease. Indeed, hypoadiponectinemia is observed in metabolic disorders, such as T2DM, obesity, dyslipidemia, hypertension, and atherosclerosis, and is inversely correlated with the severity of the disease (Fig. 1) [6]. In addition, hypoadiponectinemia is associated with risks of obesity-linked diseases, including osteoporosis, depression, hyperuricemia, sleep apnea, and visceral diseases, such as nonalcoholic fatty liver disease, gastritis/gastroesophageal reflux disease, inflammatory bowel disease, and pancreatitis (Fig. 1) [10]. Hypoadiponectinemia has also been found in endometrial cancer, postmenopausal breast cancer, and leukemia, colon, gastric, and prostate cancers (Fig. 1), and APN might have antimetabolic, antiangiogenesis, and antitumor activities in cancers [6,11]. Moreover, in osteoporosis, which is common in postmenopausal women, hypoadiponectinemia might contribute to the activation of osteoclasts and suppression of osteoblasts and osteocytes [12,13]. Finally, psychiatric diseases, such as depression and post-traumatic stress disorder, are also associated with hypoadiponectinemia [14,15]. Obesity is linked to these diseases, but not to hypoadiponectinemia, which suggests that obesity is not be the cause of hypoadiponectinemia in patients with psychiatric diseases.
FIGURE 1.
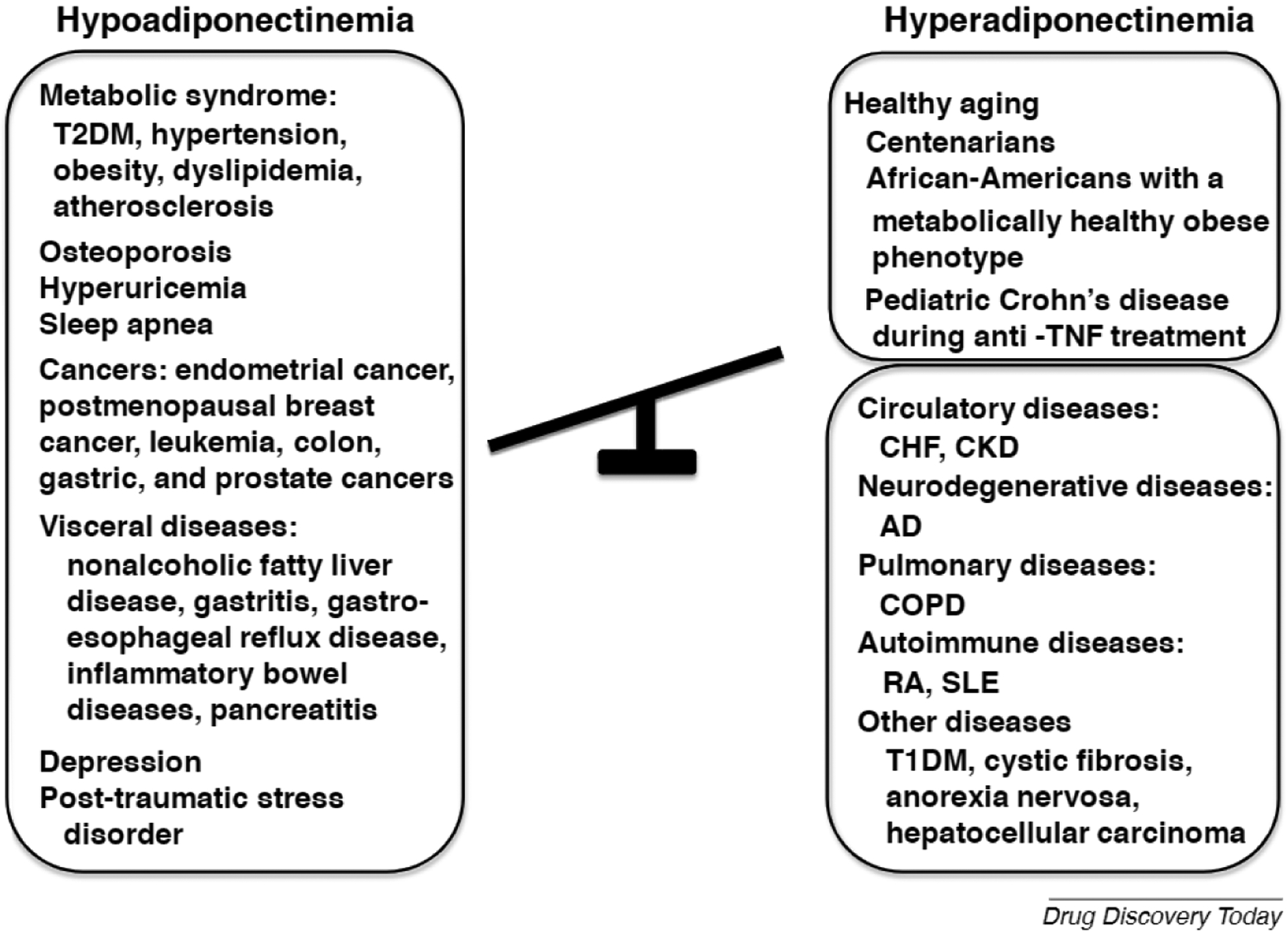
Alteration of plasma adiponectin (APN) in healthy and diseased conditions. Hypoadiponectinemia is associated with metabolic disorders, such as type 2 diabetes mellitus (T2DM), hypertension, obesity, dyslipidemia and atherosclerosis; obesity-related disorders, including cancer, osteoporosis, hyperuricemia, sleep apnea; and visceral diseases, such as nonalcoholic fatty liver disease, gastritis and gastroesophageal reflux disease, inflammatory bowel diseases, and pancreatitis. Hypoadiponectinemia is also observed in psychiatric diseases, such as depression and post-traumatic stress disorder. By contrast, hyperadiponectinemia occurs in healthy people, including centenarians and African-Americans with a metabolically healthy obese phenotype, and during therapy for Crohn’s disease. However, hyperadiponectinemia is also identified in disease conditions, such as circulatory diseases, including chronic heart failure (CHF) and chronic kidney disease (CKD); neurodegenerative diseases, such as Alzheimer’s disease (AD); and pulmonary diseases, such as chronic obstructive pulmonary disease (COPD), autoimmune diseases; rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), and other diseases, including type 1 diabetes mellitus (T1DM), cystic fibrosis, anorexia nervosa, and hepatocellular carcinoma.
Given that APN signaling is required for the activity of the insulin receptor signaling pathway [6], it naturally follows that loss of APN function might contribute to insulin resistance, leading to metabolic disorders. Hypoadiponectinemia is likely to be the result of the decreased production and secretion of APN in adipose tissue, and expression of APN mRNA in adipocytes might be affected by polymorphisms in the gene encoding APN [6] and by cadherin 13 genotypes [16]. Alternatively, expression of APN in adipocytes might be suppressed by environmental factors and lifestyle patterns, such as a high-fat diet and lack of exercise [6]. Expression of APN in adipocytes might also be decreased under hypoxic conditions induced by obesity [17]. Hypoxia might also activate macrophages to produce inflammatory cytokines, such as TNFα and IL-6, which could inhibit the local production of APN in adipose tissue [18]. Moreover, there are marked gender differences in the distribution of APN [19], with women having higher circulating levels of high-molecular-weight isoforms, because of the effect of steroid hormones [19].
Hyperadiponectinemia in a healthy state
Given that APN can ameliorate metabolic syndrome and related disorders, increased levels of plasma APN might be beneficial for health and longevity. Consistent with this view, hyperadiponectinemia has been found in disease prevention during aging [20]. Specifically, centenarians, a model of healthy aging [21], have few cardiovascular risk factors and a low prevalence of diabetes mellitus, carotid atherosclerotic plaques, and dementia, which could be associated with high levels of circulating APN (Fig. 1) [9]. Notably, APN activated PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1, indicating that APN might be involved in the regulation of mitochondria-related longevity [9]. Thus, it is likely that APN might be beneficial for aging-related diseases. Curiously, it has also been shown that paradoxical hyperadiponectinemia is associated with a metabolically healthy obese phenotype in African-Americans with high high-density lipoprotein cholesterol, and low insulin, triglyceride, and glucose (Fig. 1) [22]. Furthermore, a recent study found hyperadiponectinemia during therapy for pediatric Crohn’s disease [23], indicating that hyperadiponectinemia might cure inflammation in Crohn’s disease (Fig. 1). Taken together, these findings suggest, but do not prove, that hyperadiponectinemia could counteract obesity and contribute to health, leading to a prolonged life span.
Hyperadiponectinemia in disease conditions
Other evidence indicates that hyperadiponectinemia does not necessarily always imply a healthy outcome. Indeed, a recent study suggested that hyperadiponectinemia occurs in various diseases. Given that the risk for AD and vascular dementia is increased in metabolic dysfunction, plasma levels of APN might be reduced in AD. However, several studies have described increased levels of plasma APN in AD (Fig. 1) [8,24]. In particular, the Framingham Heart Study in a prospective cohort (N = 840, mean age 72 years) showed that elevated APN was a predictor for all-cause dementia and AD [24]. Furthermore, the Mayo Clinic Study of Aging in a large prospective cohort (N = 535, age ≥70 years without dementia) showed that levels of plasma APN were elevated, accompanied by amyloid deposits and cognitive deficits [25]. Studies of autopsied brains from patients with AD were consistent with these reports, because it was found that expression of APN was upregulated and associated with accumulation in neurofibrillary tangles in the AD brain (Fig. 2a) [26]. Collectively, these results suggest that APN is a risk factor for AD. Notably, hyperadiponectinemia has similarly, but inconsistently, been described for patients with PD. In support of this possibility, it was found that APN accumulates in Lewy bodies of brains from patients with α-synucleinopathies, such as PD and dementia with Lewy bodies (DLB) (Fig. 2c) [27].
FIGURE 2.
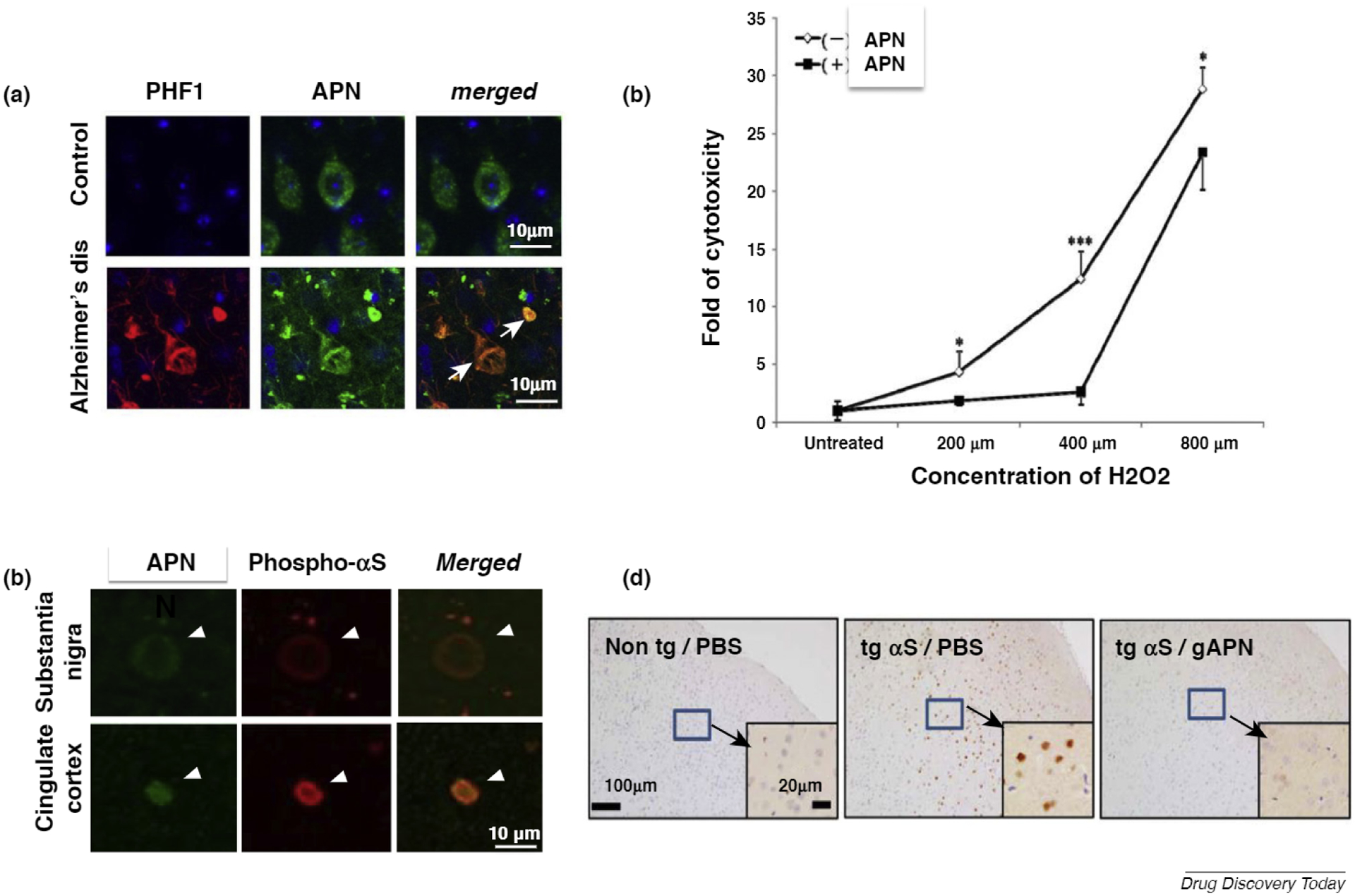
Adiponectin (APN) paradox in Alzheimer’s disease (AD), (a) Immunofluorescence of APN and tau. In autopsied AD brains, but not in controls, immunoreactivity of APN converged intracellularly with that of tau (PHF). (b) By contrast, a cellular study showed that APN was protective against oxidative stress-induced cytotoxicity in Aβ neurotoxicity in SH-SY5Y neuroblastoma cells expressing the Swedish mutant of amyloid precursor protein (APP). (c) Immunofluorescence of APN and phosphor-αS. In autopsied brains from patients with dementia with Lewy bodies (DLB), immunoreactivity of APN converged with that of phosphor αS in Lewy bodies, (d) Representative immunohistochemical images of the cortex stained with anti-pαS. αS transgenic mice and their nontransgenic littermates were treated with globular APN or PBS for 3 months, followed by various assessments. Insets are shown at a higher magnification for the cortex. Reproduced from Refs [26] (a), [36] (b), and [27] (c,d).
Similarly, circulating APN levels are increased in proportion to the disease severity of CHF and might be centrally involved in CHF-associated metabolic failure and muscle wasting [28]. Likewise, end-stage CKD, such as polycystic kidney and renal cell carcinoma, is characterized by increased cardiovascular risk associated with hyperadiponectinemia. Therefore, common mechanisms might underlie hyperadiponectinemia in CHF and CKD because cardiovascular complications remain the main cause of mortality in the CKD population [29]. In addition, chronic obstructive pulmonary disease (COPD) is characterized by cachexia and increased mortality because of respiratory failure, which is associated with elevated levels of circulating APN [30].
Hyperadiponectinemia might also associate with chronic autoimmune diseases, including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) [31]. In addition, hyperadiponectinemia has been implicated in a variety of diseases, including type I diabetes mellitus (T1DM), cystic fibrosis, anorexia nervosa, and hepatocellular carcinoma [31–33].
Potential mechanism of hyperadiponectinemia
One could predict that multiple mechanisms might contribute to hyperadiponectinemia. First, plasma APN might be upregulated because of decreased expression and/or decreased activity of APN receptor signaling; namely, APN resistance. Similar to insulin and leptin resistances, APN resistance might be caused by various mechanisms, including dysregulation of signal transduction, endoplasmic reticulum (ER) stress, and inflammation, and reduced expression of receptors AdipoR1 and -R2 [8]. Second, upregulation of plasma APN might reflect compensatory feedback in response to reduced activity of insulin/IGF-1 receptor signaling pathways, given that the network of these pathways is crucial in various biological systems, including the nervous and cardiovascular systems [8]. Third, based on the previous observation that APN accumulated in cytoplasmic inclusion bodies, including neurofibrillary tangles [26] and Lewy bodies [27], it is probable that hyperadiponectinemia is partially attributable to impairment of degradation systems, such as ubiquitin proteasomes and autophagy. Fourth, it is possible that some genetic factors, including single nucleotide polymorphisms, might contribute to the high expression of APN. Finally, APN might be ectopically produced. In support of this notion, production of APN was shown in extra-adipose tissue, such as osteoblast and vascular smooth muscle cells [34,35]. Thus, further studies are warranted to address this critical issue. In particular, APN receptor signaling has not been examined in terms of pathologies such as cardiovascular dysfunction and neurodegeneration.
The disease-stimulatory effects of hyperadiponectinemia in patients contradict a previous view based on studies of preclinical models showing that APN might be beneficial for AD [8]. Indeed, despite the action of APN as a risk factor in patients with AD, APN is protective against oxidative stress-induced cytotoxicity in Aβ neurotoxicity associated with the Swedish mutation of amyloid precursor protein (APP) (Fig. 2b) [34]. Furthermore, osmotin, a plant homolog of APN, attenuates Aβ42-induced neurotoxicity and tau hyperphosphorylation in the mouse hippocampus [37], and APN-knockout mice develop an AD-like pathology during aging, suggesting that loss of APN function leads to AD [35]. Thus, the widening gap between findings in preclinical models of APN and those from clinical observations in patients with neurodegenerative diseases result in the so-called ‘APN paradox’ [39]. Similarly, APN was shown to ameliorate neuropathological features, such as protein aggregation and impaired motor activity, in a mouse model of α-synucleinopathies (Fig. 2d) [27]. The relationship between APN and AD is clearly not fully established, and further studies are needed to determine whether this might also be the case for PD.
The APN paradox has already been recognized in various chronic wasting disorders. Although APN is protective for cardiomyocyte and vascular cell function [40], and is antiatherogenic in animal models [41], hyperadiponectinemia is correlated with the severity of circulatory diseases, such as CHF and CKD [28,29]. Furthermore, the APN paradox might also be involved in COPD. Despite the protective effect of APN on bronchial epithelial cells [42], hyperadiponectinemia is associated with COPD [30]. Further studies are warranted to determine whether the APN paradox is involved in other diseases with hyperadiponectinemia.
The mechanism by which high levels of APN impair the normal functions of the cells is unclear. Although many studies support the beneficial effects of APN on metabolic syndrome, some have demonstrated proinflammatory and proapoptotic actions of APN [31,43]. Therefore, we speculate that similar mechanisms might be involved in the impairment of cell function by APN, underlying the APN paradox.
Indeed, the discrepancy between findings from preclinical models and those from clinical observations is central to various aspects of disease biology, including that of neurodegenerative disease [44]. To the best of our knowledge, the APN paradox has been observed in patients, but not in animal models. Hypothetically, the late stages of various chronic disorders might behave in different ways in patients and experimental animals, especially given the extended postreproductive aging period in humans compared with animals [45]. In addition, a mutually nonexclusive possibility is that late-stage chronic diseases become prolonged in humans by virtue of sustained medical care in the absence of radical treatments to cure such conditions. Thus, late-stage chronic disease might exhibit a distinct human-specific pathology.
The obesity paradox and some chronic disease
An ‘obesity paradox’ or ‘reverse epidemiology’ of cardiovascular risk has also been observed in cancer, AIDS, and RA, in addition to the disorders with an APN paradox (CHF, CKD, and COPD), in which morbidity and mortality in the end stage are mitigated in obese patients [46,47]. It predictably follows that AD might also be associated with the obesity paradox. In support, a retrospective cohort study from the United Kingdom Clinical Practice Research Datalink recently showed that obesity exerts a protective effect on dementia [48]. Interestingly, the obesity paradox has also been observed in metabolic disorders, such as T2DM, hypertension, and CVD [49–51]. Given that serum levels of APN are decreased in obesity, it is predicted that the toxicity of APN might also be reduced. Thus, the APN paradox and the obesity paradox conceptually overlap to a significant degree. Thus, because the obesity paradox is observed in many disease types, the overlap between these two paradoxical phenomena could be a common feature of advanced stages of chronic disorders.
A novel therapeutic strategy based on the APN and obesity paradoxes
As shown above, the relationship of the serum APN concentration with health and disease states shows an inverted-U correlation, suggesting that moderate concentrations of serum APN are beneficial (Fig. 3). Given that both hypo- and hyperadiponectinemia might promote chronic disease states, both should be targeted therapeutically.
FIGURE 3.
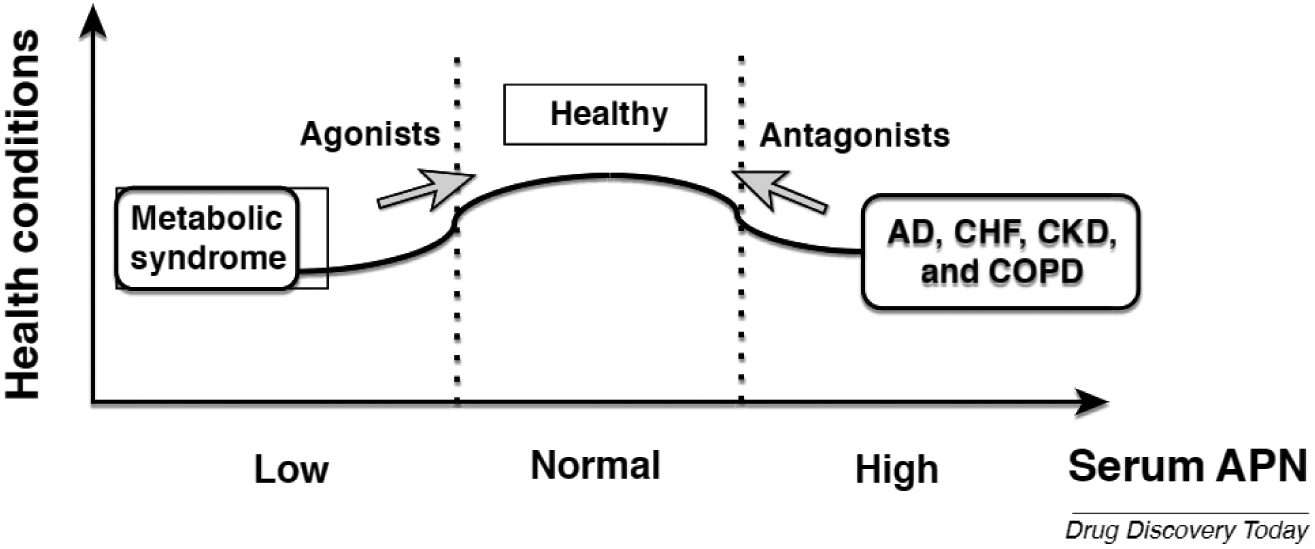
A therapeutic strategy based on disease-specific adiponectin (APN) actions. Metabolic syndrome, such as obesity and type 2 diabetes mellitus (T2DM), are featured with hypoadiponectinemia. In this context, agonists of APN receptors have been considered for the therapy of the metabolic disorders. However, various chronic diseases, including Alzheimer’s disease (AD), chronic heart failure (CHF), chronic kidney disease (CKD), and chronic obstructive pulmonary disease (COPD), are associated with hyperadiponectinemia and the APN and obesity paradoxes. Therefore, antagonism of the APN receptor signaling pathway could be effective for therapeutic purposes. Arrows indicate the therapeutic strategy using either agonists or antagonists.
Given that loss of function of APN might associate with metabolic syndrome, stimulating the APN receptor using an agonist could be effective for disease prevention (Fig. 3). Indeed, a small-molecule APN receptor agonist, adipoRon, has been shown to be beneficial in animal models of T2DM and obesity [53]. By contrast, hyperadiponectinemia might be a risk factor of AD and other chronic diseases, in which APN gain of function could be mitigated by suppression of APN receptor signaling (Fig. 3). Based on a previous report showing that serum APN was increased in both AD and mild cognitive impairment, a prodromal stage of AD [54], it is assumed that APN receptor antagonists might be initiated from the early stage of the disease. Collectively, the differential use of APN receptor agonists and antagonists depending on the types of disease might be important.
Notably, there are few reports on APN receptor antagonists compared with the extensive studies on agonists. However, recent advances in computer modeling software (e.g., docking methods) should allow easier identification of APN receptor antagonists for clinical application [55,56]. In this context, molecular docking methods have recently been applied for G-protein-coupled receptors (GPCRs) [57]. Given that adipo-R1 and -R2 are GPCRs [58], antagonists of APN receptors should be identifiable without difficulty.
Given that serum APN levels are expected to shift from hypo- to hyperadiponectinemia during disease progression, serum APN might also be a promising disease biomarker. Many studies are underway to identify and use biomarkers for diagnosis and assessment of therapeutic effects in AD. These include a variety of neuroimaging candidate markers, such as hippocampus and entorhinal cortex volumes, basal forebrain nuclei, cortical thickness, deformation-based and voxel-based morphometry, and structural and effective connectivity [59]. Cerebrospinal fluid (CSF) might also be promising as a source of biomarkers, such as Aβ42, BACE1, and total-/phospho-tau [59]. However, there are still no good candidates identified in blood and, thus, APN might be an attractive target in this respect.
Conceivably, therapeutic compounds based on the APN paradox might not only be effective for aging-associated chronic disorders, but also for other conditions, especially for cancers. For instance, it is generally believed that metabolic syndrome is a risk factor for obesity-liked cancers [60]. By contrast, it is firmly established that hormonal alterations, such as hyperinsulinism and elevated insulin-like growth factor levels, are associated with breast cancer [60]. Furthermore, increased APN might contribute to hepatocellular carcinoma at least in part through its activation of AKT signaling [33]. Given that APN sensitizes the insulin receptor signaling pathway associated with activation of AKT signaling, it is intriguing to consider that a similar dual strategy of APN receptor stimulation and inhibition can be applied to malignancies.
However, for balance, our proposed therapeutic strategy might also have several disadvantages. Most notably, any form of APN antagonist therapy must ‘trade off’ any derived benefits against the protective effects of APN on other cell types. Thus, APN signaling blockade might instead promote metabolic dysfunction and cancer. Second, current preclinical disease paradigms might be incomplete because they have not reproduced the APN paradox. Lastly, the simplest of APN biological interactions in AD are still poorly understood, especially the differential roles of APN oligomers and of the heterogeneous APN receptors, Adipo-R1 and -R2, in addition to T-cadherin on the cell membrane [58,61].
Concluding remarks
Following the disappointing outcomes of Aβ immunotherapy trials for AD [62], the current prevailing concept is that disease-modifying therapy must be initiated from the presymptomatic stage of the disease because of the presumed incurability of chronic diseases once they become symptomatic [63]. Regardless, treatment should ideally be as aggressively as possible even in the advanced stage. In this context, we propose a novel concept of APN-related therapy, in which antagonist-driven inhibition of the APN receptor signaling pathway might further ameliorate disease pathology and symptoms (Fig. 3). Hopefully, therapeutic outcomes could be monitored and directed by measuring serum APN as a biomarker. This is important because no good biomarkers are presently available for assessment of therapeutic effects in AD. Furthermore, a similar dual strategy of stimulation and inhibition of the APN receptor might also be of benefit for the treatment of cancer and other chronic diseases that involve the obesity paradox. However, the benefit-risk ‘trade-offs’ with other diseases, the absence of good preclinical disease models of the APN paradox, and the lack of a fundamental understanding of APN activity under pathological conditions remain challenges in APN-based dual therapy. Further investigations are required to obtain a better understanding of the pathological roles of APN signaling in chronic disorders, such as AD, CHF and CKD, toward fulfilling the promise of APN-related drug discovery.
Acknowledgment
This work was supported in part by KAKENHI from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
References
- 1.Takamatasu YHG et al. (2017) Combined immunotherapy with ‘anti-insulin resistance therapy’ as a novel therapeutic strategy against neurodegenerative diseases. NPJ Parkinson’s Dis 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selkoe DJ and Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med 8, 595–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craft S (2009) The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch. Neurol 66, 300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aviles-Olmos I et al. (2013) Exenatide and the treatment of patients with Parkinson’s disease. J. Clin. Invest 123, 2730–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groeneveld ON et al. (2016) Potentials of incretin-based therapies in dementia and stroke in type 2 diabetes mellitus. J. Diabetes Investig 7, 5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamauchi T and Kadowaki T (2013) Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab 17, 185–196 [DOI] [PubMed] [Google Scholar]
- 7.Waki H et al. (2003) Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J. Biol. Chem 278, 40352–40363 [DOI] [PubMed] [Google Scholar]
- 8.Waragai M et al. (2017) Importance of adiponectin activity in the pathogenesis of Alzheimer’s disease. Ann. Clin. Transl. Neurol 4, 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwabu M et al. (2015) Adiponectin/adiponectin receptor in disease and aging. NPJ Aging Mech. Dis 1, 15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishida K et al. (2014) Adiponectin as a routine clinical biomarker. Best Pract. Res. Clin. Endocrinol. Metab 28, 119–130 [DOI] [PubMed] [Google Scholar]
- 11.Wennberg AM et al. (2016) Serum adiponectin levels, neuroimaging, and cognition in the Mayo Clinic Study of Aging. J. Alzheimers Dis 53, 573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X and Wang Y (2011) Adiponectin and breast cancer. Med. Oncol 28, 1288–1295 [DOI] [PubMed] [Google Scholar]
- 13.Lubkowska A et al. (2014) Adiponectin as a biomarker of osteoporosis in postmenopausal women: controversies. Dis. Markers 2014, 975178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy B et al. (2016) Molecular mechanisms of obesity-induced osteoporosis and muscle atrophy. Front. Physiol 7, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho AF et al. (2014) Adipokines as emerging depression biomarkers: a systematic review and meta-analysis. J. Psychiatr. Res 59, 28–37 [DOI] [PubMed] [Google Scholar]
- 16.Na KS et al. (2017) Decreased plasma adiponectin among male firefighters with symptoms of post-traumatic stress disorder. J. Affect. Disord 221, 254–258 [DOI] [PubMed] [Google Scholar]
- 17.Teng MS et al. (2015) Association of CDH13 genotypes/haplotypes with circulating adiponectin levels, metabolic syndrome, and related metabolic phenotypes: the role of the suppression effect. PLoS One 10, e0122664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldi S et al. (2010) Fat-free mass change after nutritional rehabilitation in weight losing COPD: role of insulin, C-reactive protein and tissue hypoxia. Int. J. Chron. Obstruct. Pulmon. Dis 5, 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang K and Ratke J (2009) Leptin and adiponectin: new players in the field of tumor cell and leukocyte migration. Cell Commun. Signal 7, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishizawa H et al. (2002) Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 51, 2734–2741 [DOI] [PubMed] [Google Scholar]
- 21.Adamczak M et al. (2005) Ageing and plasma adiponectin concentration in apparently healthy males and females. Clin. Endocrinol 62, 114–118 [DOI] [PubMed] [Google Scholar]
- 22.Franceschi C and Bonafe M (2003) Centenarians as a model for healthy aging. Biochem. Soc. Trans 31, 457–461 [DOI] [PubMed] [Google Scholar]
- 23.Doumatey AP et al. (2012) Paradoxical hyperadiponectinemia is associated with the metabolically healthy obese (MHO) phenotype in African Americans. J. Endocrinol. Metab 2, 51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frivolt K et al. (2017) Hyperadiponectinemia during infliximab induction therapy in pediatric Crohn’s disease. J. Pediatr. Gastroenterol. Nutr [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.van Himbergen TM et al. (2012) Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and alzheimer disease: results from the Framingham Heart Study. Arch. Neurol 69, 594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waragai M et al. (2016) Possible involvement of adiponectin, the anti-diabetes molecule, in the pathogenesis of Alzheimer’s disease. J. Alzheimer’s Dis 52, 1453–1459 [DOI] [PubMed] [Google Scholar]
- 27.Sekiyama K et al. (2014) Disease-modifying effect of adiponectin in model of alpha-synucleinopathies. Ann. Clin. Transl. Neurol 1, 479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sente T et al. (2016) Adiponectin resistance in skeletal muscle: pathophysiological implications in chronic heart failure. J. Cachexia Sarcopenia Muscle 7, 261–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markaki A et al. (2016) Adiponectin and end-stage renal disease. Hormones 15, 345–354 [DOI] [PubMed] [Google Scholar]
- 30.Yoshikawa T and Kanazawa H (2012) Association of plasma adiponectin levels with cellular hydration state measured using bioelectrical impedance analysis in patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis 7, 515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fantuzzi G (2008) Adiponectin and inflammation: consensus and controversy. J. Allergy Clin. Immunol 121, 326–330 [DOI] [PubMed] [Google Scholar]
- 32.Diez JJ and Iglesias P (2003) The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol 148, 293–300 [DOI] [PubMed] [Google Scholar]
- 33.Wang SN et al. (2014) Increased adiponectin associated with poor survival in hepatocellular carcinoma. J. Gastroenterol 49, 1342–1351 [DOI] [PubMed] [Google Scholar]
- 34.Berner HS et al. (2004) Adiponectin and its receptors are expressed in bone-forming cells. Bone 35, 842–849 [DOI] [PubMed] [Google Scholar]
- 35.Ebner A et al. (2013) Secretion of adiponectin from mouse aorta and its role in cold storage?induced vascular dysfunction. Basic Res. Cardiol 108, 390. [DOI] [PubMed] [Google Scholar]
- 36.Chan KH et al. (2012) Adiponectin is protective against oxidative stress induced cytotoxicity in amyloid-beta neurotoxicity. PLoS One 7, e52354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali T et al. (2015) Osmotin attenuates amyloid beta-induced memory impairment, tau phosphorylation and neurodegeneration in the mouse hippocampus. Sci. Rep 5, 11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodward L et al. (2017) Unravelling the adiponectin paradox: novel roles of adiponectin in the regulation of cardiovascular disease. Br. J. Pharmacol 174, 4007–4020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schram K and Sweeney G (2008) Implications of myocardial matrix remodeling by adipokines in obesity-related heart failure. Trends Cardiovasc. Med 18, 199–205 [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi T et al. (2003) Dual roles of adiponectin/Acrp30 in vivo as an anti-diabetic and anti-atherogenic adipokine. Curr. Drug Targets Immune Endocr. Metabol. Disord 3, 243–254 [DOI] [PubMed] [Google Scholar]
- 42.Cheng MY et al. (2016) Different forms of adiponectin reduce the apoptotic and damaging effect of cigarette smoke extract on human bronchial epithelial cells. Exp. Ther. Med 12, 4168–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Y and Chen X (2010) Effect of adiponectin on apoptosis: proapoptosis or antiapoptosis? Biofactors 36, 179–186 [DOI] [PubMed] [Google Scholar]
- 44.Sekiyama K et al. (2016) Insight into the dissociation of behavior from histology in synucleinopathies and in related neurodegenerative diseases. J. Alzheimers Dis 52, 831–841 [DOI] [PubMed] [Google Scholar]
- 45.Aime C et al. (2017) Grandmothering and cognitive resources are required for the emergence of menopause and extensive post-reproductive lifespan. PLoS Comput. Biol 13, e1005631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalantar-Zadeh K et al. (2007) Risk factor paradox in wasting diseases. Curr. Opin. Clin. Nutr. Metab. Care 10, 433–442 [DOI] [PubMed] [Google Scholar]
- 47.Park J et al. (2014) Obesity paradox in end-stage kidney disease patients. Prog. Cardiovasc. Dis 56, 415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qizilbash N et al. (2015) BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol 3, 431–436 [DOI] [PubMed] [Google Scholar]
- 49.Kwon Y et al. (2017) Body mass index-related mortality in patients with type 2 diabetes and heterogeneity in obesity paradox studies: a dose-response meta-analysis. PLoS One 12, e0168247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esler M et al. (2018) Obesity paradox in hypertension: is this because sympathetic activation in obesity-hypertension lakes a benign form? Hypertension 71, 22–33 [DOI] [PubMed] [Google Scholar]
- 51.Ortega FB et al. (2016) Obesity and cardiovascular disease. Circ. Res 118, 1752–1770 [DOI] [PubMed] [Google Scholar]
- 53.Okada-Iwabu M et al. (2013) A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503, 493–499 [DOI] [PubMed] [Google Scholar]
- 54.Une K et al. (2011) Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer’s disease. Eur. J. Neurol 18, 1006–1009 [DOI] [PubMed] [Google Scholar]
- 55.Jasamai M et al. (2015) Molecular docking study on platelet-activating factor antagonistic activity of bioactive compounds isolated from Guttiferae and Ardisia species. Nat. Prod. Res 29, 1055–1058 [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z et al. (2013) Inverse antagonist activities of parabens on human oestrogen-related receptor gamma (ERRgamma): in vitro and in silico studies. Toxicol. Appl. Pharmacol 270, 16–22 [DOI] [PubMed] [Google Scholar]
- 57.Bartuzi D et al. (2017) Recent advances and applications of molecular docking to G protein-coupled receptors. Molecules 22 pii: E340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamauchi T et al. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769 [DOI] [PubMed] [Google Scholar]
- 59.Hampel H et al. (2008) Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimer’s Dement 4, 38–48 [DOI] [PubMed] [Google Scholar]
- 60.Engin A (2017) Obesity-associated breast cancer: analysis of risk factors. Adv. Exp. Med. Biol 960, 571–606 [DOI] [PubMed] [Google Scholar]
- 61.Thundyil J et al. (2012) Adiponectin receptor signalling in the brain. Br. J. Pharmacol 165, 313–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sacks CA et al. (2017) The failure of solanezumab - how the FDA saved taxpayers billions. N. Engl. J. Med 376, 1706–1708 [DOI] [PubMed] [Google Scholar]
- 63.Anon (2017) Solanezumab: too late in mild Alzheimer’s disease? Lancet Neurol 16, 97. [DOI] [PubMed] [Google Scholar]


