Abstract
Human immunodeficiency virus type 1 (HIV-1) protease inhibitors have dramatically improved treatment options for HIV infection, but frequent dosing may impact adherence to highly active antiretroviral treatment regimens (HAART). Previous studies demonstrated that combined therapy with ritonavir and saquinavir allows a decrease in frequency of saquinavir dosing to twice daily. In this study, we evaluated the safety and pharmacokinetics of combining once-daily doses of the soft-gel capsule (SGC) formulation of saquinavir (saquinavir-SGC) and minidose ritonavir. Forty-four healthy HIV-negative volunteers were randomized into groups receiving once-daily doses of saquinavir-SGC (1,200 to 1,800 mg) plus ritonavir (100 to 200 mg) or a control group receiving only saquinavir-SGC (1,200 mg) three times daily. Saquinavir-SGC alone and saquinavir-SGC–ritonavir combinations were generally well tolerated, and there were no safety concerns. Addition of ritonavir (100 mg) to saquinavir-SGC (1,200 to 1,800 mg/day) increased the area under the concentration-time curve (AUC) for saquinavir severalfold, and the intersubject peak concentration in plasma and AUC variability were reduced compared to those achieved with saquinavir-SGC alone (3,600 mg/day), while trough saquinavir levels (24 h post-dose) were substantially higher than the 90% inhibitory concentration calculated from HIV-1 clinical isolates. Neither increasing the saquinavir-SGC dose to higher than 1,600 mg nor increasing ritonavir from 100 to 200 mg appeared to further enhance the AUC. These results suggest that an all once-daily HAART regimen, utilizing saquinavir-SGC plus a more tolerable low dose of ritonavir, may be feasible. Studies of once-daily saquinavir-SGC (1,600 mg) in combination with ritonavir (100 mg) in HIV-infected patients are underway.
The availability of highly active antiretroviral therapy (HAART), typically containing combinations of reverse transcriptase inhibitors and protease inhibitors, has dramatically improved the therapeutic options for patients with human immunodeficiency virus type 1 (HIV-1) infection. HIV-1 protease inhibitors are capable of rapidly suppressing viral replication to below the level of detection for many individuals. HAART regimens are associated with delayed progression to AIDS and decreased mortality when compared with less potent one- or two-drug reverse transcriptase inhibitor regimens, both in randomized studies (14, 15) and in clinical practice (25). However, the long-term success rate with HAART regimens has generally been lower in unselected populations than in controlled clinical trials (2, 21), suggesting the possibility that adherence to the complex medication regimens is better in the more rigid setting of a clinical trial. Indeed, all presently approved HIV-1 protease inhibitors are administered either two or three times daily, in order to maintain trough drug concentrations higher than the in vitro 90% inhibitory concentration for viral replication. This has been recommended both to maximize antiviral activity and to minimize the selection of drug-resistant viruses (reviewed by Flexner [11]). Thus, reducing the required number of medication doses per day is likely to improve patient adherence and may also contribute to more-prolonged viral load suppression during HAART.
HIV-1 protease inhibitors are subject to potentially significant drug-drug interactions, given that they undergo cytochrome P450-based metabolism in the gastrointestinal tract and liver. Such interactions may be beneficial when two protease inhibitors are administered simultaneously. For example, the potency of the original hard-gel capsule formulation of saquinavir (Invirase) was limited due to poor bioavailability (<4%) (11), which necessitated administering the drug at very high, inconvenient doses (e.g., 18 to 36 200-mg capsules per day) to achieve a level of antiviral activity comparable to that achieved with other approved HIV-1 protease inhibitors (29). Taking saquinavir along with a meal increases the area under the concentration-time curve (AUC) more than sixfold (11). Ritonavir demonstrates greater bioavailability (66 to 75%, with few or no effects related to food intake) and a high degree of potency when administered at the recommended dosage of 600 mg twice daily (6, 11, 22). However, many patients experience dose-limiting adverse effects, including gastrointestinal intolerance, headaches, and circumoral numbness. Saquinavir undergoes extensive first-pass metabolism by CYP3A in the gut wall and liver. While saquinavir at high concentrations has minimal inhibitory effects on cytochrome P450 3A, ritonavir is a very potent inhibitor of this isoenzyme, even at low doses (19; N. Buss, Abstr. 5th Conf. Retrovir. Opportunistic Infect., abstr. 354, 1998). Because the original hard-gel formulation of saquinavir has limited bioavailability as a result of metabolism by cytochrome P450 3A (10), combined administration has been utilized as a strategy to elevate saquinavir concentrations and improve virologic responses (1, 17). In clinical trials, the combination of saquinavir (400 mg to 800 mg) and ritonavir (400 mg to 600 mg), both administered twice daily, is a well-tolerated and potent regimen that is effective in both treatment-naïve and, at least in short-term evaluations, protease inhibitor-experienced patients (1, 7, 18, 26, 30). Saquinavir concentrations in serum during coadministration with ritonavir are increased compared with those achieved with higher and more frequent doses of saquinavir alone (17). Thus, saquinavir and ritonavir in combination demonstrated the potential for fewer doses and improved tolerability compared with either drug as the sole protease inhibitor in a HAART regimen.
A soft-gel capsule formulation of saquinavir (saquinavir-SGC; Fortovase) has recently become available. At the presently approved dosage of 1,200 mg (six 200-mg capsules) three times daily, saquinavir-SGC achieves potent therapeutic drug concentrations several times higher than the hard-gel capsule formulation and is well tolerated (3, 11, 13, 20, 23, 27). It has previously been shown that ritonavir (200 to 400 mg twice daily) profoundly decreases the oral clearance of saquinavir-SGC at the lower dosage of 800 mg twice daily (N. Buss, Abstr. 5th Conf. Retrovir. Opportunistic Infect.), leading to substantial increases in the area under the AUC, peak saquinavir concentrations in plasma (Cmax), and trough saquinavir concentrations in plasma (Cmin). In view of this unique pharmacokinetic interaction, the present trial was undertaken to further evaluate the safety and pharmacokinetics of ritonavir–saquinavir-SGC combinations at multiple doses compared with saquinavir-SGC alone. The study recruited HIV-negative volunteers because of the concern that potential undertreatment of HIV-infected patients could lead to selection of drug-resistant viral phenotypes.
(This work was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 26 to 29 September 1999 [M. S. Saag, M. Kilby, E. Ehrensing, N. A. Gizzi, P. Siemon-Hryczyk, N. Buss, and C. Y. Oo, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 330, 1999] and at the 7th European Conference on Clinical Aspects and Treatment of HIV Infection, Lisbon, Portugal, 23 to 27 October 1999 [M. S. Saag et al., abstr. 829].)
MATERIALS AND METHODS
Subject selection and screening.
Healthy male or female volunteers, aged 18 to 45 years and within 20% of ideal body weight, were eligible for enrollment in this study. Subjects were excluded if they had a history of significant drug hypersensitivity or substance abuse, positive serology for HIV infection or hepatitis B-hepatitis C surface antigen, or abnormal liver function tests. Subjects were also not eligible for enrollment if they had taken any prescription medications within 28 days of study commencement. Other exclusion criteria were a history of significant central nervous system, pulmonary, renal, cardiovascular, gastrointestinal, oncologic, or allergic disease or any major medical illness during the month prior to enrollment. All subjects gave written informed consent to participate in this study, which was approved by the University of Alabama at Birmingham (UAB) institutional review board.
Study design, dietary parameters, and dosing.
This study used an open-label, randomized, multiple-dose, parallel-group design and was performed at a single site (UAB 1917 Clinic). The original intention was to enroll 64 volunteers into the following eight dosage groups (8 subjects/group): group A, 1,200 mg of saquinavir-SGC (three times a day [t.i.d]); group B, 1,200 mg of saquinavir-SGC plus 100 mg of ritonavir (both once daily); group C, 1,600 mg of saquinavir-SGC plus 100 mg of ritonavir (both once daily); group D, 1,800 mg of saquinavir- SGC plus 100 mg of ritonavir (both once daily); group E, 1,200 mg of saquinavir-SGC plus 200 mg of ritonavir (both once daily); group F, 1,600 mg of saquinavir-SGC plus 200 mg of ritonavir (both once daily); group G, 1,800 mg of saquinavir-SGC plus 200 mg of ritonavir (both once daily); and group H, 2,400 mg of saquinavir-SGC plus 100 mg of ritonavir (both once daily).
The randomization schedule was designed to enroll volunteers into groups A through E first, following which an interim safety analysis and preliminary pharmacokinetic assessment was to be carried out, prior to enrollment of the remaining three groups. On the basis of this analysis, which demonstrated the trend towards no additional benefit from higher saquinavir-SGC or ritonavir dosages, no volunteers were enrolled and randomized for groups F through H.
Subjects received their assigned study medication orally for 13 days, starting with the evening of day 1. Those volunteers randomized to groups C and F were to receive an additional day of saquinavir-SGC–ritonavir dosing (day 14), during which a single 400-mg oral dose of didanosine would be given 30 min prior to dinner to evaluate the pharmacokinetic effects of this triple drug combination. This would allow an exploration of the proof-of-concept that didanosine (taken once daily) could be safely incorporated into the regimen, by testing the effects of a single didanosine dose in combination with a midlevel dose of 1,600 mg of saquinavir-SGC daily (combined with either 100 mg or 200 mg of ritonavir daily).
Volunteers were admitted to the research clinic on day 1 for overnight safety monitoring following the first dose of study medication. Thereafter (days 2 to 12), subjects returned to the clinic each evening for a standardized meal (900 kCal; 35% fats, 20% proteins, and 45% carbohydrates), following which the evening doses of the study medication(s) were administered (within 45 min of the start and within 15 min of the finish of the meal).
All doses of study medication for groups B through E were directly observed. For group A, evening doses of saquinavir-SGC were directly observed, while adherence to the remainder of medication doses was monitored by pill counts and diary records in which volunteers noted the start and finish times of their meals as well as the times of medication administration.
On the afternoon of day 13 of the study, all subjects returned to the research clinic and an intravenous line was placed for serial blood withdrawal for pharmacokinetics purposes. The subjects were discharged on the evening of day 14 without further study medications except that, as mentioned above, subjects in groups C and F were required to undergo an additional day of medication dosing. All volunteers returned to the clinic for a follow-up visit between days 19 and 24.
Safety assessments.
The safety and tolerability of study medications was evaluated throughout the study on the basis of clinical adverse experiences, vital signs, clinical laboratory results, physical examinations, and electrocardiogram recordings. The severity, duration, and potential relationship of any adverse events to study drugs were recorded. The AIDS Clinical Trials Group toxicity grading scale (9) was used to characterize abnormal laboratory values, physical findings, and signs and symptoms.
Blood collection and drug concentration assays.
Cmin were observed following the standardized evening meal and prior to administration of study drugs on days 2, 7, 11, and 12. Intensive pharmacokinetic assessment of drug concentrations was performed on day 13 before dosing and at 1, 2, 3, 4, 6, 8, 12, 18, and 24 h postdosing. For subjects randomized to group C or F, repeat pharmacokinetic assays were also planned for day 14.
Plasma ritonavir and saquinavir concentrations were assayed using a validated SCIEXIII-Plus API liquid chromatography extraction method with a mass spectrophotometric detection system. The method was validated for a range of 5.0 to 3,000 ng/ml for saquinavir and 10.0 to 6,000 ng/ml for ritonavir based on a 0.05-ml sample volume. Quantitation was performed using a weighted linear least squares regression line generated from spiked calibration standards (saquinavir, 5, 10, 20, 100, 500, 1,000, and 3,000 ng/ml and ritonavir, 10, 20, 40, 200, 1,000, 2,000 and 6,000 ng/ml in human heparinized plasma). The internal standard was reserpine (1 μg/ml), and quality control (QC) samples were human heparinized plasma samples spiked with saquinavir or ritonavir at 10, 100, and 1,000 ng/ml (saquinavir) or 20, 200, and 2,000 ng/ml (ritonavir). All test, calibration, internal, and QC samples were extracted with acetonitrile.
Data analysis.
This pilot study was designed to explore whether similar degrees of drug exposure (especially the AUC) could be achieved using protease inhibitor combination regimens that are more convenient than the presently approved regimen for saquinavir-SGC alone. The study design was not powered to enable formal statistical comparisons of the saquinavir exposure in each of the dose groups. Therefore, pharmacokinetic parameters were determined from the concentration-time curves and are presented descriptively. If comparable AUC values were suggested by these preliminary data, larger clinical studies in HIV-infected patients could then be developed to confirm these observations based on standard statistical considerations.
Preliminary data from regimens A to E indicated that study of the remaining regimens F to H was not necessary (see below). Therefore, data analysis for the regimens actually studied is described.
The three primary pharmacokinetic parameters of saquinavir-SGC and ritonavir were the Cmax, observed Cmin, and the AUC over 24 h (AUC0–24h) on day 13. For regimen A, AUC0–24h was estimated by multiplying AUC0–8h by a factor of three. The secondary pharmacokinetic parameters were the time to maximum concentration of drug in serum [day 13 or 14 as appropriate]; the Cmin on days 2, 7, 11 and 12; and the Cmax, Cmin, and AUC0–24h on day 14 for regimen C. The Cmin on days 2, 7, 11 and 12 was used to assess whether steady-state conditions were achieved. The differences between pharmacokinetic parameters on days 13 and 14 for regimen C was used to assess dideoxyinosine interaction.
RESULTS
Forty-eight subjects were screened for enrollment. Three subjects did not meet the entry criteria (due to a positive illicit drug screen, first-degree heart block, and glucose intolerance at the time of screening, respectively). An additional subject was randomized into group C but then dropped out of the study for personal reasons without ever receiving study medications. Thus, a total of 44 subjects (22 females and 22 males) received study medication and were evaluable. The majority (80%) of subjects were Caucasian. The mean demographics of evaluable subjects were as follows (standard deviations [SD] are in parentheses): age, 29 (7.0) years; body weight, 70 (14.2) kg; and height, 170 (7.6) cm.
Four subjects dropped out of the study while receiving study drugs. One subject (in group E) dropped out due to a non-study-related reason on day 5. One group C subject discontinued drugs on day 2 because of one episode of nausea and vomiting. One group D subject discontinued drugs on day 12 due to palpitations and anxiety, which were not clearly related to study drugs. Finally, an additional subject in Group C was not able to complete the additional pharmacokinetic evaluations (day 14) due to difficulties with maintaining venous access. Thus, 40 subjects (8 per dose group) completed the entire study, while one additional subject in group C completed 13 days of monitoring.
Safety and tolerability.
Saquinavir-SGC alone and saquinavir-SGC–ritonavir combinations were well tolerated, with no grade III or IV adverse events reported. The most commonly reported adverse effects included nausea, flatulence, headache, fatigue, diarrhea, and bloating (Table 1). There appeared to be no substantial difference in terms of the total number of adverse events per subject between those in the saquinavir-SGC–ritonavir combination groups and those who received saquinavir-SGC alone (7.9 and 6.4 events/subject, respectively). No clinically relevant alterations in laboratory safety parameters were noted during the study. In particular, fasting triglyceride and total low- and high-density lipoprotein (LDL and HDL, respectively) cholesterol concentrations remained stable (ranges were as follows: triglycerides, 72 to 140 mg/dl, total cholesterol, 162 to 197 mg/dl, LDL cholesterol, 91 to 118 mg/dl and HDL cholesterol, 44 to 55 mg/dl).
TABLE 1.
Summary of the most commonly reported adverse events following administration of saquinavir-SGC alone or in combination with ritonavir for 13 days in healthy HIV-negative volunteers
| Adverse event | No. of subjects reporting event who were in groupa:
|
||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Bloating | 4 | 2 | 1 | 2 | 5 |
| Constipation | 0 | 0 | 0 | 3 | 0 |
| Diarrhea | 0 | 3 | 4 | 4 | 4 |
| Fatigue | 6 | 7 | 3 | 3 | 5 |
| Flatulence | 5 | 4 | 5 | 3 | 5 |
| Headache | 4 | 4 | 5 | 4 | 4 |
| Irritability | 2 | 2 | 3 | 0 | 0 |
| Nausea | 5 | 5 | 5 | 6 | 5 |
Dosing for groups was as follows: for group A (n = 8), 1,200 mg of saquinavir-SGC three times a day; for group B (n = 8), 1,200 mg of saquinavir-SGC plus 100 mg of ritonavir (both once daily); for group C (n = 10), 1,600 mg of saquinavir-SGC plus 100 mg of ritonavir (both once daily); for group D (n = 9), 1,800 mg of saquinavir-SGC plus 100 mg of ritonavir (both once daily); and for group E (n = 9), 1,200 mg of saquinavir-SGC plus 200 mg of ritonavir (both once daily).
Assay performance.
Assay performance was acceptable. The correlation coefficient for saquinavir was 0.9953 or better, with a mean intercept and a slope of −0.0005 and 0.0009, respectively. Values for ritonavir were 0.9935 or better, with a mean intercept and a slope of 0.0002 and 0.0016, respectively. The coefficient of variation for assay precision ranged from 2.7 to 8.9% (absolute variation, 0.01 to 3.2%) for saquinavir and 2.9 to 7.7% (absolute variation, 0.17 to 3.5%) for ritonavir. The coefficient of variation for QC precision for saquinavir and ritonavir ranged from 9.5 to 18.1% (absolute variation, 0.01 to 2.3%) and 10.3 to 11.9% (absolute variation, 2.8 to 6.4%), respectively. The lower limit of quantitation was 5.0 ng/ml.
Pharmacokinetics.
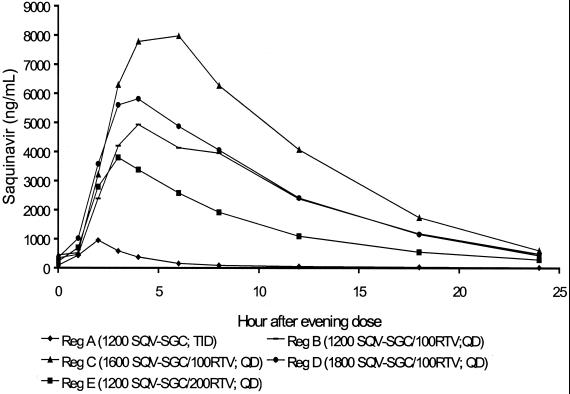
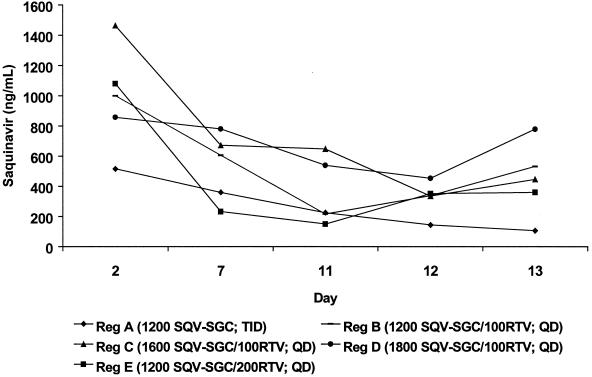
Figure 1 shows the mean plasma concentration-time curve for saquinavir over the 24-h postdosing period during steady-state conditions. Steady-state conditions were approached by volunteers in groups B through E by day 7. For unclear reasons, the trough saquinavir concentrations suggested a declining trend across all groups, including that receiving saquinavir-SGC alone (group A) (Fig. 2). This trend might have resulted from decreased adherence to the study regimens over time. However, this explanation is not supported by the documentation of directly observed therapy (groups B to E) or by the drug diaries regarding the unobserved doses or the pill counts of group A, all of which suggested strict adherence (data not shown). For groups B to E, these decreasing concentrations most likely result from ritonavir metabolic autoinduction, a previously recognized phenomenon that has prompted a dose-escalation strategy for the initiation of ritonavir in order to minimize initial high peak plasma drug concentrations prior to achieving steady-state conditions. A reduction in systemic ritonavir concentrations over time would be predicted to result in a reduction in saquinavir concentrations as well. This would not explain the apparent drug concentration reductions for group A; similar reductions have not been reported in other studies of saquinavir administered alone to healthy volunteers. Regardless, compared with saquinavir-SGC alone, the combination of ritonavir with saquinavir-SGC (groups B to E) resulted in elevated saquinavir concentrations in plasma throughout the course of the day (Fig. 1). Trough saquinavir concentrations in plasma were very similar for all four combination regimens and were substantially (around fivefold) higher than those observed in subjects who received saquinavir-SGC alone (Table 2). Compared with saquinavir-SGC alone (group A), the addition of ritonavir increased the mean AUC saquinavir for by three- to sevenfold (Table 2). This difference was most apparent for 1,600 mg of saquinavir-SGC in combination with 100 mg of ritonavir (group C). While there was a trend towards a dose-proportional increase (+27%) in the mean AUC for saquinavir-SGC dosages of up to 1,600 mg, no further increase was demonstrated at the higher dosage of 1,800 mg (group D). Similar findings were observed for the Cmax, with all saquinavir-SGC–ritonavir combination groups having higher Cmax than the saquinavir-SGC alone group (Table 2). Again, there was a dose-proportional increase (+24%) in the mean Cmax with an increase in dosage from 1,200 mg (group B) to 1,600 mg (group C), but a further increase in the mean Cmax was not shown by the higher-dosage group (group D).
FIG. 1.
Mean plasma saquinavir concentration-time profiles on day 13 for regimens A to E in healthy HIV-negative volunteers.
FIG. 2.
Mean saquinavir concentrations in plasma for groups A to E, measured on days 2, 7, 11, 12, and 13. SQV-SGC, saquinavir-SGC; RTV, ritonavir; TID, three times daily; QD, once daily; Reg, regimen.
TABLE 2.
Summary of saquinavir pharmacokinetic parameters following administration of saquinavir-SGC alone and in combination with ritonavir for 13 days in healthy HIV-negative volunteers
| Parameter | Value for groupa:
|
||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| AUC0–24hb | |||||
| Mean (SD) | 12,636 (13,452) | 63,789 (27,137) | 87,398 (42,670) | 69,745 (31,103) | 40,188 (23,022) |
| Geometric mean | 9,358 | 57,534 | 76,956 | 64,642 | 33,931 |
| Median (range) | 8,202 (3,813–45,108) | 69,101 (24,077–101,354) | 90,128 (27,274–144,789) | 60,318 (36,044–134,262) | 36,135 (8,563–86,033) |
| Cmax | |||||
| Mean (SD) | 1,334 (1,176) | 6,761 (3,116) | 8,890 (4,680) | 7,749 (2,342) | 4,698 (2,230) |
| Geometric mean | 1,039 | 6,037 | 7,947 | 7,480 | 4,116 |
| Median (range) | 862 (397–4000) | 6,760 (2,340–11,400) | 8,000 (4,160–17,700) | 6,790 (5,660–12,500) | 4,530 (1,190–7,800) |
| Cmin | |||||
| Mean (SD) | 116 (115) | 552 (293) | 608 (381) | 602 (596) | 470 (521) |
| Geometric mean | 89 | 484 | 490 | 427 | 281 |
| Median (range) | 79 (48–392) | 541 (201–1,080) | 594 (171–1,230) | 417 (87–2,000) | 275 (44–1,590) |
Dosing for groups was as follows: for group A (n = 8), 1,200 mg of saquinavir-SGC three times a day; for group B (n = 8), 1,200 mg of saquinavir-SGC plus 100 mg of ritonavir (both once daily); for group C (n = 10), 1,600 mg of saquinavir-SGC plus 100 mg of ritonavir (both once daily); for group D (n = 9), 1,800 mg of saquinavir-SGC plus 100 mg of ritonavir (both once daily); and for group E (n = 9), 1,200 mg of saquinavir-SGC plus 200 mg of ritonavir (both once daily).
For group A, the AUC0–24h was estimated by multiplying the AUC0–8h by a factor of three.
Increasing the dosage of ritonavir to 200 mg once daily (group E) did not lead to saquinavir exposure that was increased compared with that achieved with a 100-mg dosage in combination with an identical dosage of saquinavir-SGC (group B). In fact, mean AUC and Cmax were 37 and 30% lower, respectively, than the corresponding values in group B (Table 2). Increasing the dose of ritonavir from 100 mg to 200 mg appeared to result in an approximate dose-proportional increase in ritonavir exposure (Table 3).
TABLE 3.
Summary of ritonavir pharmacokinetic parameters following administration of ritonavir in combination with saquinavir-SGC for 13 days in healthy HIV-negative volunteers
| Parameter | Value for groupa:
|
|||
|---|---|---|---|---|
| A | C | D | E | |
| AUC0–24h | ||||
| Mean (SD) | 11,721 (5043) | 10,344 (7,061) | 7,879 (2,087) | 23,869 (11,774) |
| Geometric mean | 10,711 | 8,755 | 7,647 | 21,216 |
| Median (range) | 11,540 (5,794–17,872) | 9,871 (3,449–27,371) | 7,613 (5,151–11,544) | 19,968 (8,808–40,507) |
| Cmax | ||||
| Mean (SD) | 1,001 (411) | 1,019 (922) | 676 (139) | 2,268 (1,170) |
| Geometric mean | 925 | 796 | 663 | 2,028 |
| Median (range) | 1,011 (519–1,670) | 835 (268–3,360) | 671 (512–855) | 2,110 (982–4,610) |
| Cmin | ||||
| Mean (SD) | 92 (65) | 61 (45) | 74 (51) | 253 (250) |
| Geometric mean | 76 | 48 | 52 | 171 |
| Median (range) | 67 (31–216) | 47 (15–145) | 63 (5–145) | 189 (42–817) |
Dosing for groups was as follows: for group A (n = 8), 1,200 mg of saquinavir-SGC three times a day; for group C (n = 10), 1,600 mg of saquinavir-SGC plus 100 mg of ritonavir (both once daily); for group D (n = 9), 1,800 mg of saquinavir-SGC plus 100 mg of ritonavir (both once daily); and for group E (n = 9), 1,200 mg of saquinavir-SGC plus 200 mg of ritonavir (both once daily).
Eight of the subjects in group C extended study involvement by 1 day in order to evaluate whether the addition of a single 400-mg dose of didanosine, another potent once-daily antiretroviral agent, would alter the pharmacokinetics and safety of saquinavir-SGC–ritonavir (group F was also planned to include analysis of potential drug interactions with didanosine, though ultimately no enrollment was carried out, for reasons described above). Mean pharmacokinetics for saquinavir-SGC before (n = 9) and after (n = 8) the addition of didanosine were as follows: AUC, 87,398 ng · h/ml versus 59,698 ng · h/ml; Cmax, 8,890 ng/ml versus 6,670 ng/ml; and Cmin, 608 ng/ml versus 604 ng/ml. Corresponding values for ritonavir were as follows: AUC, 10,344 ng · h/ml versus 8,228 ng · h/ml; Cmax, 1,019 ng/ml versus 1,142 ng/ml; and Cmin, 60 ng/ml versus 56 ng/ml. Thus, while there was a suggestion that the addition of didanosine might slightly lower the saquinavir-SGC AUC, the degree of the effect would appear to be clinically insignificant relative to the boost in AUC provided by the combination of saquinavir-SGC at 1,600 mg daily and ritonavir at 100 mg daily in this limited analysis. Didanosine concentrations were not evaluated in this study. There was no substantial difference in clinical tolerability following the addition of once-daily didanosine to the study regimen.
DISCUSSION
Our hypothesis, based on the known pharmacokinetic interaction between saquinavir and ritonavir, was that a 100-mg dose of ritonavir would increase saquinavir concentrations in plasma sufficiently to enable once-daily administration of saquinavir-SGC, while avoiding the commonly encountered adverse events associated with higher doses of ritonavir. This approach would facilitate patient adherence, as it is clear that the number of doses per day influences the degree of patient adherence to complex drug regimens (5, 8). In particular, many patients appear to neglect the middle dose of the day because of interference with work schedules or irregular midday meals.
The results of this pilot study suggest the potential to achieve effective saquinavir exposure (AUC) with once-daily dosing by combining the drug with very low doses of ritonavir. While this preliminary study was not designed for formal statistical analysis, it is notable that the addition of 100 mg of ritonavir once daily appeared to increase saquinavir exposure as indicated by the (AUC) severalfold and reduce intersubject Cmax and AUC variability. Neither increasing the saquinavir-SGC dosage beyond 1,600 mg once daily nor increasing the dosage of ritonavir from 100 mg to 200 mg once daily resulted in further improvements in drug exposure. The combination of ≥1,200 mg of saquinavir-SGC and 100 mg of ritonavir resulted in trough saquinavir concentrations that were approximately 40 times higher than the in vitro 90% inhibitory concentration estimates reported for saquinavir (geometric mean, 16 nM or 10.5 ng/ml) (4). In view of the fact that this combination appeared to be advantageous from a variety of standpoints, and because further dose elevations of either drug appeared to provide no additional benefits, it was considered unnecessary to continue enrollment for the other dose groups initially planned for the study. While the saquinavir-SGC doses in groups B and D (1,200 mg and 1,800 mg, respectively) also demonstrated potential for once-daily administration, the favorable pharmacokinetic findings observed in group C prompted us to select this regimen 1,600 mg of saquinavir-SGC and 100 mg of ritonavir once daily) to pursue in further studies involving HIV-infected subjects.
The lack of an increase in saquinavir concentrations with further dose escalations of saquinavir-SGC (more than 1,600 mg) or ritonavir (more than 100 mg) is somewhat counterintuitive and may simply reflect random variability in this preliminary exploratory trial. One possible interpretation is that higher doses of ritonavir in the gut may interfere with absorption of saquinavir, which would counteract some of the effects on drug metabolism in the liver. It is also possible that saquinavir doses above 1,600 mg do not result in dose-related increases in concentration because of saturation in the ability to absorb further drug. While further evaluations of higher dose combinations would appear to be unnecessary, an explanation for this phenomenon based on the available data can only be speculative.
While several different dosage regimens of ritonavir and saquinavir in combination have proven effective for treatment of HIV infection, these studies have evaluated much higher dosages of ritonavir (daily dose of 800 to 1,200 mg) (1, 7, 18, 26, 30). Intolerance to ritonavir may be dose related, as supported by data that a total daily dose of 800 mg is associated with significantly fewer adverse events than a total daily dose of 1,200 mg (12, 28). Our clinical experience in the present investigation suggests that once-daily doses of 100 mg of ritonavir are very well tolerated. In this study, there was no substantial difference in the frequency of adverse events, including laboratory abnormalities, between subjects receiving saquinavir-SGC and ritonavir in combination and those receiving saquinavir-SGC alone.
This study was conducted with non-HIV-infected individuals because of theoretical concerns about the potential for suboptimal drug concentrations that could increase the risk of selection for drug-resistant HIV isolates. More studies are needed in order to evaluate the relative potential for resistance selection when doses are administered at less-frequent intervals (once a day) and when the regimen includes two different protease inhibitors. While boosting saquinavir concentrations would theoretically decrease the probability of saquinavir resistance, it is possible that including ritonavir at doses substantially lower than those recommended for antiviral effects (∼100 mg) would raise the risk for selecting HIV isolates with more broad cross-resistance to the class of HIV protease inhibitors.
Preliminary information from the present study also suggests that the addition of 400 mg of didanosine once daily, a dose that appears to be an equally effective alternative to the initially approved dosage of 200 mg twice daily (16, 24), does not significantly alter the pharmacokinetics of either protease inhibitor. These data, along with the increasing number of once-daily reverse transcriptase inhibitors (including efavirenz and potentially lamivudine and emtricitabine), increases the feasibility of developing HAART regimen with once-daily dosing for all drugs in the near future.
In conclusion, the results of this study suggest that combinations of saquinavir-SGC and minidose ritonavir raise the potential for once-daily administration of HAART regimens. Evaluation of the once-daily regimen of 1,600 mg of saquinavir-SGC and 100 mg of ritonavir, in combination with conveniently dosed reverse transcriptase inhibitors, is now underway in multicenter, randomized clinical trials involving HIV-infected patients.
ACKNOWLEDGMENTS
We thank the volunteers in this study for their participation, Karen Tamburello for coordinating clinical care, and Angie Chatwood and Sandy McKinnon for pharmaceutical assistance.
This study was supported by Roche Laboratories (NR15708B/M61026) and the UAB General Clinical Research Center (NCRR MO1 RR00032).
REFERENCES
- 1.Cameron D W, Japour A J, Xu Y, Hsu A, Mellors J, Farthing C, Cohen C, Poretz D, Markowitz M, Follansbee S, Angel J B, McMahon D, Ho D, Devanarayan V, Rode R, Salgo M P, Kempf D J, Granneman R, Leonard J M, Sun E. Ritonavir and saquinavir combination therapy for the treatment of HIV infection. AIDS. 1999;13:213–224. doi: 10.1097/00002030-199902040-00009. [DOI] [PubMed] [Google Scholar]
- 2.Clugh L A, D'Agata E D, Raffanti S, Haas D W. Factors that predict incomplete virological response to protease inhibitor-based antiretroviral therapy. Clin Infect Dis. 1999;29:75–84. doi: 10.1086/520185. [DOI] [PubMed] [Google Scholar]
- 3.Cohen Stuart J W T, Schuurman R, Burger D M, Koopman P P, Sprenger H G, Juttman J R, Richter C, Meenhorst P L, Hoetelmans R M W, Kroon F P, Bravenboer B, Hamann D, Boucher C A B, Borleffs J C C. Randomized trial comparing saquinavir soft gelatin capsules versus indinavir as part of triple therapy (CHEESE study) AIDS. 1999;13:F53–F58. doi: 10.1097/00002030-199905070-00001. [DOI] [PubMed] [Google Scholar]
- 4.Craig J C, Duncan I B, Hockley D, Grief C, Roberts N A, Mills J S. Antiviral properties of Ro 31-8959, an inhibitor of HIV proteinase. Antivir Res. 1991;16:295–305. doi: 10.1016/0166-3542(91)90045-s. [DOI] [PubMed] [Google Scholar]
- 5.Cramer J, Mattson R H, Prevey M L, Scheyer R D, Ouellette V L. How often is medication taken as prescribed? JAMA. 1989;261:3273–3277. [PubMed] [Google Scholar]
- 6.Danner S A, Carr A, Leonard J M, Lehman L M, Gudiol F, Gonzales J, Raventos A, Rubio R, Bouza E, Pintado V, Aguado A G, de Lomas J G, Delgado R, Borleffs J C C, Hsu A, Valdes J M, Boucher C A B, Cooper D A. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. N Engl J Med. 1995;333:1528–1533. doi: 10.1056/NEJM199512073332303. [DOI] [PubMed] [Google Scholar]
- 7.Deeks S G, Grant R M, Beatty G W, Horton C, Detmer J, Eastman S. Activity of a ritonavir plus saquinavir-containing regimen in patients with virologic evidence of indinavir or ritonavir failure. AIDS. 1998;12:F97–F102. doi: 10.1097/00002030-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Eisen S A, Miller D K, Woodward R S, Spitznagel E, Przybeck T R. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150:1881–1883. [PubMed] [Google Scholar]
- 9.Fischl M A, Stanley K, Collier A C, Arduino J M, Stein D S, Feinberg J E, Allan J D, Goldsmith J C, Powderly W G. Combination and montherapy with zidovudine and zalcitabine in patients with advanced HIV disease. Ann Intern Med. 1995;122:24–32. doi: 10.7326/0003-4819-122-1-199501010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Fitzsimmons M E, Collins J M. Selective biotransformation of the human immunodeficiency virus protease inhibitor saquinavir by human small-intestinal cytochrome P450-3A4: potential contribution to high first-pass metabolism. Drug Metab Dispos. 1997;25:256–266. [PubMed] [Google Scholar]
- 11.Flexner C. HIV-Protease Inhibitors. N Engl J Med. 1998;338:1281–1292. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 12.Gatti G, Di Biagio A, Casazza R, De Pascalis C, Bassetti M, Cruciani M, Vella S, Bassetti D. The relationship between ritonavir plasma levels and side-effects: implications for therapeutic drug monitoring. AIDS. 1999;13:2083–2089. doi: 10.1097/00002030-199910220-00011. [DOI] [PubMed] [Google Scholar]
- 13.Gill M J. Safety profile of soft gelatin formulation of saquinavir in combination with nucleosides in a broad patient population. NV15182 Study Team. AIDS. 1998;12:1400–1402. [PubMed] [Google Scholar]
- 14.Gulick R M, Mellors J, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, Emini E A, Chodakewitz J A. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 15.Hammer S M, Squires K E, Hughes M D, Grimes J M, Demeter L M, Currier J S, Eron J J, Jr, Feinberg J E, Balfour H H, Jr, Deyton L R, Chodakewitz J A, Fischl M A. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 16.Hoetelmans R M, van Heeswijk R P, Profijt M, Mulder J W, Meenhorst P L, Lange J M, Reiss P, Beijnen J H. Comparison of the plasma pharmacokinetics and renal clearance of didanosine during once and twice daily dosing in HIV-1 infected individuals. AIDS. 1998;12:F211–F216. doi: 10.1097/00002030-199817000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Kempf D J, Marsh K C, Kumar G, Rodriguez A D, Denissen J F, McDonald E, Kukulka M J, Hsu A, Granneman G R, Baroldi P A, Sun E, Pizzuti D, Plattner J J, Norbeck D W, Leonard J M. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob Agents Chemother. 1997;41:654–660. doi: 10.1128/aac.41.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirk O, Katzenstein T L, Gerstoft J, Mathiesen L, Nielsen H, Pedersen C, Lundgren J D. Combination therapy containing ritonavir plus saquinavir has superior short-term antiretroviral efficacy: a randomized trial. AIDS. 1999;13:F9–F16. doi: 10.1097/00002030-199901140-00002. [DOI] [PubMed] [Google Scholar]
- 19.Kumar G N, Dykstra J, Roberts E M, Jayanti V K, Hickman D, Uchic J, Yao Y, Surber B, Thomas S, Granneman G R. Potent inhibition of the cytochrome P-450 3A-mediated human microsomal metabolism of a novel HIV protease inhibitor by ritonavir: a positive drug-drug interaction. Drug Metab Dispos. 1999;27:902–908. [PubMed] [Google Scholar]
- 20.Lalezari J. Selecting the optimum dose for a new soft gelatin capsule formulation of saquinavir. NV15107 Study Group. J Acquir Immun Defic Syndr Hum Retrovirol. 1998;19:195–197. doi: 10.1097/00042560-199810010-00015. [DOI] [PubMed] [Google Scholar]
- 21.Lucas G M, Chaisson R E, Moore R D. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 22.Markowitz M, Saag M S, Powderly W G, Hurley A M, Hsu A, Valdes J M, Henry D, Sattler F, La Marca A, Leonard J M, Ho D D. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med. 1995;333:1534–1539. doi: 10.1056/NEJM199512073332204. [DOI] [PubMed] [Google Scholar]
- 23.Mitsuyasu R T, Skolnik P R, Cohen S R, Conway B, Gill M J, Jensen P C, Pulvirentl J J, Slater L N, Schooley R T, Thompson M A, Torres R A, Tsoukas C M. Activity of the soft gelatin formulation of saquinavir in combination therapy in antiretroviral naïve patients. NV15355 Study Team. AIDS. 1998;12:F103–F109. doi: 10.1097/00002030-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Mobley J E, Pollard R B, Schrader S, Adler M H, Kelleher T, McLaren C. Virological and immunological responses to once-daily dosing of didanosine in combination with stavudine. AI454-143 Team. AIDS. 1999;13:F87–F93. doi: 10.1097/00002030-199907300-00003. [DOI] [PubMed] [Google Scholar]
- 25.Palella F J, Jr, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman D J, Holmberg S D. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 26.Paredes R, Puig T, Arno A, Negredo E, Balague E M, Bonjoch A, Jou A, Tuldra A, Tural C C, Sirera G, Veny A, Romeu J, Ruiz L, Clotet B. High-dose saquinavir plus ritonavir: long-term efficacy in HIV-positive protease inhibitor-experienced patients and predictors of virologic response. J Acquir Immune Defic Syndr. 1999;22:132–138. doi: 10.1097/00126334-199910010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Perry C M, Noble S. Saquinavir soft-gel capsule formulation. A review of its use in patients with HIV infection. Drugs. 1998;55:461–486. doi: 10.2165/00003495-199855030-00014. [DOI] [PubMed] [Google Scholar]
- 28.Rublein J C, Eron J J, Jr, Butts J D, Raasch R H. Discontinuation rates for protease inhibitor regimens containing ritonavir 600 mg versus ritonavir 400 mg plus saquinavir 400 mg. Ann Pharmacother. 1999;33:899–905. doi: 10.1345/aph.18260. [DOI] [PubMed] [Google Scholar]
- 29.Schapiro J M, Winters M A, Stewart F, Efron B, Norris J, Kozal M J, Merigan T C. The effect of high dose saquinavir on viral load and CD4+ T cell counts in HIV-infected patients. Ann Intern Med. 1996;124:1039–1050. doi: 10.7326/0003-4819-124-12-199606150-00003. [DOI] [PubMed] [Google Scholar]
- 30.Tebas P, Patick A K, Kane E M, Klebert M K, Simpson J H, Erice A, Powderly W G, Henry K. Virologic responses to a ritonavir-saquinavir-containing regimen in patients who had previously failed nelfinavir. AIDS. 1999;13:F23–F28. doi: 10.1097/00002030-199902040-00002. [DOI] [PubMed] [Google Scholar]