SUMMARY
Drugs of abuse induce persistent remodeling of reward circuit function – a process thought to underlie the emergence of drug craving and relapse to drug use. However, how circuit-specific, drug-induced molecular and cellular plasticity can have distributed effects on the mesolimbic dopamine reward system to facilitate relapse to drug use is not fully elucidated. Here, we demonstrate that dopamine receptor D3 (DRD3)-dependent plasticity in the ventral pallidum (VP) drives potentiation of dopamine release in the nucleus accumbens during relapse to cocaine seeking after abstinence. We show that two distinct VP DRD3+ neuronal populations projecting to either the lateral habenula (LHb) or the ventral tegmental area (VTA) display different patterns of activity during drug seeking following abstinence from cocaine self-administration, and that selective suppression of elevated activity or DRD3 signaling in the LHb-projecting population reduces drug seeking. Together, our results uncover how circuit-specific DRD3-mediated plasticity contributes to the process of drug relapse.
Keywords: Dopamine receptor D3, cocaine, drug self-administration, drug relapse, ventral pallidum, lateral habenula, neuronal circuits, dopamine sensor, fiber photometry, optogenetics
eTOC blurb
Pribiag et al. show that ventral pallidum dopamine receptor D3 signaling regulates drug seeking following prolonged abstinence from cocaine self-administration, via activation of a subpopulation of neurons projecting to the lateral habenula. This regulation feeds back to influence dopamine release in the lateral shell of the nucleus accumbens during drug seeking.
INTRODUCTION
Drug addiction is a chronic, often life-long disorder characterized by high rates of relapse to drug use despite successful abstinence periods (Dong et al., 2017). Through repeated potentiation of the mesolimbic dopamine (DA) system, addictive drugs produce potent and long-lasting changes in gene expression, synapse function and overall dynamics of neural circuits that control motivated behaviors (Luscher and Malenka, 2011; Nestler and Luscher, 2019; Volkow and Boyle, 2018). How this remodeling process is coordinated across different subcircuits of the brain’s reward system to mediate relapse to drug use is not well understood.
As an anatomical hub of the ventral basal ganglia circuitry, the ventral pallidum (VP) encodes reward-related information (Ahrens et al., 2016; Fujimoto et al., 2019; Ottenheimer et al., 2020; Richard et al., 2016; Root et al., 2015; Smith et al., 2009; Tachibana and Hikosaka, 2012) and participates in the development of addictive behaviors in response to cocaine and other drugs of abuse (Creed et al., 2016; Farrell et al., 2019; Heinsbroek et al., 2020; Mahler et al., 2014; Pardo-Garcia et al., 2019; Root et al., 2015). VP neurons display altered firing patterns in response to self-administered cocaine (Root et al., 2012) and exhibit synaptic adaptations following repeated cocaine exposure (Creed et al., 2016; Heinsbroek et al., 2017; Kupchik et al., 2014). Reducing the activity of VP neurons during relapse sessions reduces drug seeking in animals previously trained to self-administer cocaine or other drugs of abuse (Farrell et al., 2019; Mahler et al., 2014; McFarland and Kalivas, 2001; Prasad and McNally, 2016; Prasad et al., 2020; Root et al., 2015), underlining a critical role for the VP in mediating drug relapse behaviors. However, how molecular factors mediate drug-induced plasticity in the VP to differentially influence downstream structures participating in relapse to drug use is not known. In addition, while extracellular DA levels are known to rise in the VP during cocaine self-administration (Sizemore et al., 2000), the role of VP DA signaling in mediating the development of drug relapse behavior remains unexplored.
DA receptor signaling is critical to driving synaptic plasticity and drug-seeking behavior that occurs following exposure to drugs of abuse (Baik, 2013; Bossert et al., 2013; Crombag et al., 2002; Luscher and Malenka, 2011). In particular, DA receptor D3 (DRD3) antagonism has shown promise as a feasible therapeutic strategy for addiction treatment (Chen et al., 2014; Galaj et al., 2018; Song et al., 2014; You et al., 2019). Compared to D1 (DRD1) and D2 (DRD2) DA receptors, DRD3 has a higher affinity for dopamine (Richtand, 2006; Robinson et al., 1994; Sokoloff et al., 1990), suggesting that it may serve as a readout of tonic DA levels and/or play a prominent role in regions that receive sparse DAergic innervation. DRD3 is preferentially expressed in areas of the brain involved in motivation, reward and emotion, including the VP, ventral striatum, and lateral septum (Gurevich and Joyce, 1999; Shin et al., 2018; Sokoloff and Le Foll, 2017; Stanwood et al., 2000). DRD3 expression is elevated in several brain areas in response to psychostimulant drugs such as cocaine and amphetamine, both in animal models of addiction (Bahi et al., 2005; Le Foll et al., 2005; Neisewander et al., 2004) and in human addicts (Boileau et al., 2012; Payer et al., 2014; Staley and Mash, 1996). However, the specific contribution of DRD3 signaling to drug-mediated plasticity within neural circuits that drive motivated behaviors remains unknown. Elucidating a circuit-specific role for DRD3 may help unlock the therapeutic potential of DRD3 antagonism as applied to drug abuse.
Here, we undertook a multi-faceted approach to understand how DRD3 signaling, neuronal projection-specific activity, and DA release are coordinated by the VP to drive relapse to cocaine seeking. Using a drug relapse assay consisting of cocaine self-administration followed by a two-week period of forced abstinence and a drug-seeking test, we found that DRD3 expression in the mouse VP drives relapse to cocaine seeking via context-dependent augmented activity of VP DRD3+ neurons. Knock-down of DRD3 expression in the VP reduces activity of the lateral ventral tegmental area (latVTA) and DA release in the lateral shell of the nucleus accumbens (NAc latSh) during relapse to cocaine seeking, suggesting that the change in activity of VP DRD3+ neurons controls drug relapse by influencing the DAergic output of the VTA. Detailed circuit analyses of VP DRD3+ neuronal projections to the lateral habenula (LHb) and the VTA revealed that while both projections are capable of elevating NAc latSh DA release when optogenetically stimulated, only LHb-projecting VP DRD3+ neurons display tonically-elevated activity during relapse to cocaine seeking. Moreover, inhibiting the VP DRD3+ projection to the LHb, either optogenetically or chemogenetically, or reducing DRD3 signaling in the VP to LHb projection, suppressed cocaine seeking behavior following prolonged abstinence, whereas analogous manipulations of the VP DRD3+ projection to the VTA did not suppress cocaine seeking. Our results provide a circuit-level understanding of VP DRD3 signaling in driving relapse to drug use, and more specifically, how activity of a molecularly-defined output of the VP to the LHb can serve as an effective target in the treatment of drug relapse.
RESULTS
Abstinence from cocaine exposure upregulates DRD3 expression in the VP
The VP expresses DRD1, DRD2 and DRD3 (Beckstead et al., 1988; Contreras et al., 1987; Mansour et al., 1990; Richfield et al., 1989; Tziortzi et al., 2011), and receives DAergic innervation from the VTA (Del-Fava et al., 2007; Klitenick et al., 1992; Taylor et al., 2014). To assess the impact of cocaine exposure on DA receptor signaling in the VP, we measured DRD1, DRD2 and DRD3 mRNA levels using quantitative PCR (qPCR), following passive or self-administered cocaine exposure. Mice injected with cocaine for 5 days (15 mg/kg/day, i.p.) showed a significant elevation of DRD3 mRNA in the VP, but only after a drug-free period of 10 days (Figures 1A and S1A). Similarly, mice that self-administered cocaine for ten days showed a significant elevation of DRD3 mRNA in the VP after 2 weeks of home-cage abstinence (Figure 1B), and a slight trend towards increased expression following 1 day of abstinence (Figure S1B). Thus, we hypothesized that VP DRD3 may participate in the remodelling of gene expression and circuit function that occurs during prolonged withdrawal from cocaine exposure (Dong et al., 2017; Luscher and Malenka, 2011; Massart et al., 2015; Walker et al., 2018), and that VP DRD3 signaling may be functionally involved in driving relapse to cocaine seeking after prolonged abstinence.
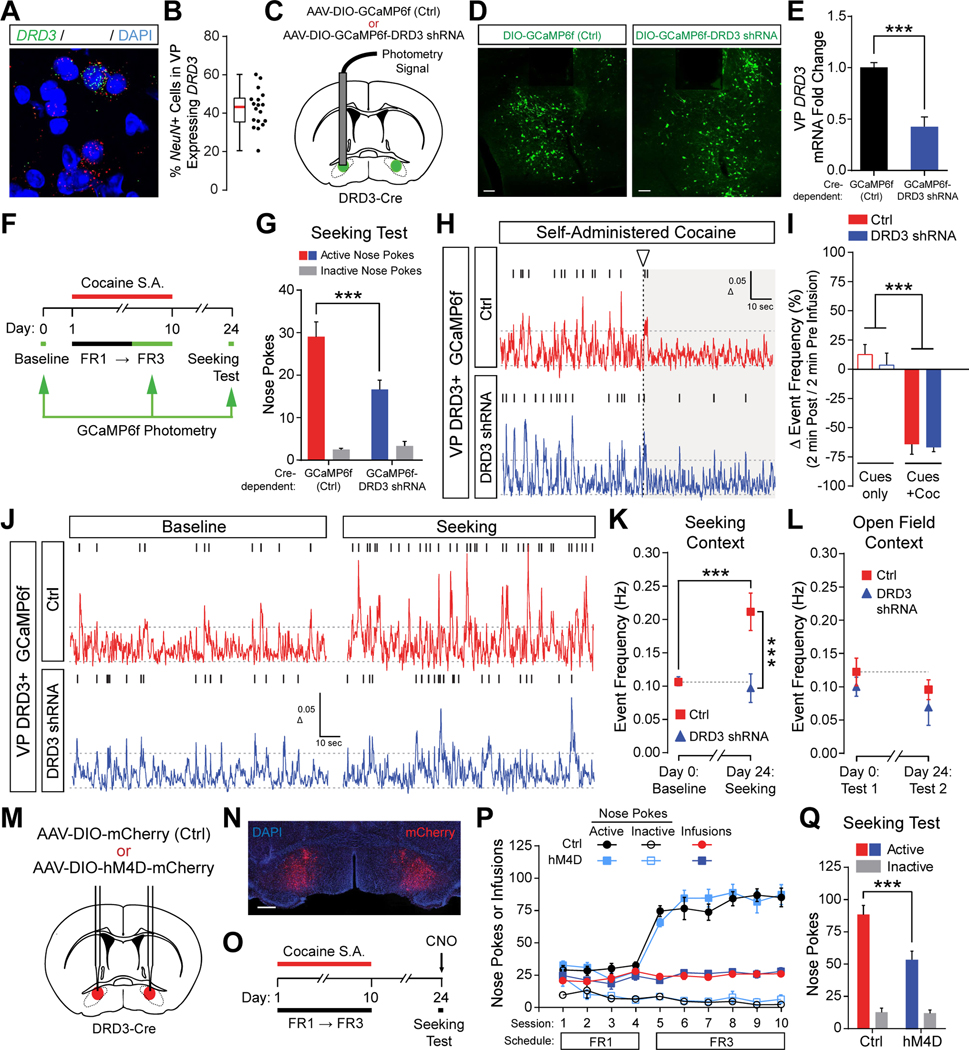
Figure 1. VP DRD3 drives relapse to cocaine seeking.
(A and B) Non-contingent (I.P.)(A) or self-administered (S.A.)(B) cocaine selectively elevated DRD3 expression in the VP following prolonged abstinence. For (A), Two-way repeated measures (RM) ANOVA, Sidak’s post hoc test, ** p < 0.01; n = 11, 12 mice for Sal, Coc. For (B), Two-way RM ANOVA, Sidak’s post hoc test, ** p < 0.01; n = 9,12 mice for Sal, Coc. (C) Selective and robust knock-down of DRD3 expression in the VP by AAV-mediated delivery of DRD3-targeting shRNA. Two-way RM ANOVA, Sidak’s post hoc test, *** p < 0.001; n = 6 mice each for Ctrl, DRD3 shRNA.
(C) Selective and robust knock-down of DRD3 expression in the VP by AAV-mediated delivery of DRD3-targeting shRNA. Two-way RM ANOVA, Sidak’s post hoc test, *** p < 0.001; n = 6 mice each for Ctrl, DRD3 shRNA.
(D) Experimental schedule of cocaine S.A. experiments.
(E and F) Schematic of bilateral stereotaxic injection of control (Ctrl), DRD3 shRNA, and DRD3 rescue AAVs into the VP (E), and corresponding representative images of EGFP expression (F). Scale bar, 250 μm for all images. L, lateral; D, dorsal.
(G) Nose pokes and cocaine infusions during FR1, FR3 and PR of cocaine S.A. n = 17, 17, 16 mice for Ctrl, DRD3 shRNA, DRD3 Rescue.
(H) Active and inactive nose pokes during the Day 25 seeking test (2 hrs), corresponding to mice in (G). Active nose pokes were significantly reduced by VP DRD3 KD, and restored by re-expression of DRD3. Two-way RM ANOVA, Tukey’s post hoc test, *** p < 0.001; n = 16, 17, 16 mice for Ctrl, DRD3 shRNA, DRD3 Rescue.
Error bars indicate ±SEM for all.
See Table S2 for detailed statistics.
See also Figure S1.
VP DRD3 signaling mediates relapse to cocaine seeking
To understand the role of VP DRD3 signaling in the development of cocaine seeking behavior, we used viral-mediated delivery of shRNA to robustly knock down (KD) DRD3 expression in the VP, without affecting DRD1 or DRD2 expression (Figure 1C). We applied this manipulation, along with a rescue construct co-expressing DRD3 shRNA and a KD-resistant DRD3 mutant (Shin et al., 2018), to mice that went on to self-administer cocaine for 11 days, followed by 2 weeks of home-cage abstinence, and a seeking test performed under extinction conditions on day 25 (Figures 1D to F). DRD3 KD in the VP did not alter nose poke rates under fixed-ratio 1 (FR1), FR3 or progressive ratio (PR) schedules in either male or female mice (Figures 1G, S1F, S1H). In contrast, following 2 weeks of abstinence, the number of drug-seeking nose pokes performed on the side previously paired with cocaine delivery (i.e. active nose pokes) was markedly reduced in both male and female mice expressing DRD3 shRNA in the VP (Figures 1H, S1G, and S1I). Conversely, rescue of DRD3 expression fully restored drug-seeking behavior without affecting consummatory behavior (Figures 1G, 1H, and S1F to S1I). Analogous results were obtained using cocaine-induced conditioned place preference (CPP), with mice expressing DRD3 shRNA in the VP showing significantly reduced place preference after a 10 day drug-free period (Figures S1C to S1E). However, VP DRD3 KD mice did not display significantly altered weight, locomotor activity or open field anxiety (Figures S1J and S1K). Moreover, VP DRD3 KD mice did not show differences in the consummatory or seeking phases of a sugar pellet self-administration paradigm that mimicked our cocaine self-administration paradigm (Figures S1L to S1N), indicating that the ability to recall an operant task and the motivation to seek a palatable reward remained intact. Taken together, these results support the notion that DRD3 signaling in the VP is a critical and specific mediator of relapse to cocaine seeking following prolonged abstinence.
The VP contains a heterogeneous mix of cell types defined by neurotransmitter identity and other molecular markers (Root et al., 2015). Using fluorescent in situ hybridization we found that approximately 42.2±2.3% of NeuN-expressing cells in the VP co-express DRD3 (Figures 2A and 2B), indicating that a substantial fraction of VP neurons are likely to signal through DRD3. To understand the extent to which DRD3 signaling in the VP occurs in conjunction with other DA receptors, we quantified the proportion of DRD3+ VP cells co-expressing DRD1 or DRD2, and found that 40.9±3.6% of these cells express neither DRD1 nor DRD2 (Figures S2A and S2B). In contrast, in the NAc medial core/shell, we found that all DRD3+ cells co-express DRD1 and/or DRD2, with a large majority (78.7±1.5%) co-expressing DRD1 (Figures S2C and S2D). These findings suggest the presence of a distinct DRD3 signaling landscape in the VP as compared to the NAc medial core/shell. In addition, we found that 68.0±2.2% of VP DRD3+ cells express VGAT, 23.9±2.2% express VGluT2, and 6.2±0.8% express ChAT (Figures S2E to S2H), suggesting a primarily inhibitory output to downstream structures.
Figure 2. DRD3-dependent augmented activity in the VP drives relapse to cocaine seeking.
(A and B) Sample image of RNA in situ hybridization (A), and corresponding quantification of DRD3+ cells as a percentage of NeuN+ cells in the VP (B). Filled arrowheads show double-positive cells, empty arrowheads show NeuN+ only cells. n = 18 images. Scale bar, 20 μm.
(C) Schematic of bilateral viral expression (green) of Cre-dependent GCaMP6f or GCaMP6f-DRD3 shRNA in the VP of DRD3-Cre mice, with unilateral optic fiber implant for photometry.
(D) Sample images of Cre-dependent GCaMP6f and GCaMP6f-DRD3 shRNA expression in the VP, with optic fiber implants. Scale bars, 100 μm. L, lateral; D, dorsal.
(E) Robust knock-down of DRD3 expression in the VP of DRD3-Cre mice by Cre-dependent GCaMP6f-DRD3 shRNA. Two-tailed t-test, *** p < 0.001, n = 7 mice each for Ctrl, DRD3 shRNA.
(F) Experimental schedule of cocaine S.A. experiments with GCaMP6f photometry recording sessions (green arrows) at Day 0 (Baseline), Days 6–10 (FR3), and Day 24 (Seeking Test).
(G) Active and inactive nose pokes during the Day 24 seeking test (20 min), corresponding to mice in (C-K). Two-way RM ANOVA, Sidak’s post hoc test, *** p < 0.001; n = 9, 10 mice for Ctrl, DRD3 shRNA.
(H) Sample photometry traces from Ctrl and DRD3 shRNA GCaMP6f constructs expressed in VP DRD3+ neurons. Arrowhead and vertical dotted line indicate the start of a self-administered cocaine infusion. Tick marks above traces indicate threshold-detected events.
(I) Self-administered cocaine infusion decreased activity of VP DRD3+ neurons (Cues + Coc), as compared to self-administered cocaine delivery-associated cues presented in the absence of drug infusion (Cues only). Two-way RM ANOVA, main effectcocaine *** p < 0.0001; n = 5, 6 mice for Ctrl, DRD3 shRNA.
(J) Sample VP DRD3+ GCaMP6f photometry traces during Baseline vs Seeking Test sessions, comparing Ctrl and DRD3 shRNA mice. Tick marks above traces indicate threshold-detected events.
(K) DRD3-dependent increase in activity of VP DRD3+ neurons during cocaine seeking. Two-way RM ANOVA, Sidak’s post hoc tests, *** p < 0.001; n = 9, 10 mice for Ctrl, DRD3 shRNA.
(L)Unaltered VP DRD3+ neuron activity in a drug-free open field context for mice that self-administered cocaine. n = 3, 4 mice for Ctrl, DRD3 shRNA.
(M) Schematic of bilateral viral expression (red) of Cre-dependent mCherry or hM4D-mCherry in the VP of DRD3-Cre mice.
(N) Sample image of bilateral Cre-dependent mCherry expression in the VP of DRD3-Cre mice. Scale bar, 500 μm.
(O) Experimental schedule of cocaine S.A. experiments with CNO administration prior to the Seeking Test on day 24.
(P) Cocaine self-administration data corresponding to experiments described in (M,O,Q). n = 8 mice each for Ctrl, hM4D.
(Q) hM4D-dependent reduction of VP DRD3+ neuron activity reduces cocaine seeking. Two-way RM ANOVA, Sidak’s post hoc test, *** p < 0.001; n = 8 mice each for Ctrl, hM4D.
Error bars indicate ±SEM for all.
See Table S2 for detailed statistics.
See also Figures S2, S3 and S4.
Context- and DRD3-dependent augmented activity of VP DRD3+ neurons drives relapse to cocaine seeking
To understand how the activity of DRD3-expressing neurons in VP is regulated by cocaine, relapse to cocaine seeking, and DRD3 signaling, we virally-expressed Cre-dependent GCaMP6f or GCaMP6fDRD3 shRNA in the VP of DRD3-Cre mice, and used fiber photometry to perform in vivo Ca2+ imaging (Figures 2C, 2D, S3K and S3L). We validated DRD3-Cre mice as faithfully recapitulating endogenous DRD3 expression in the VP (Figures S2I and S2J), and observed a robust knock-down of DRD3 expression in the VP using our Cre-dependent GCaMP6f-DRD3 shRNA construct (Figure 2E). This approach allowed us to measure the activity of VP DRD3+ neurons with knocked-down DRD3 expression, while also recapitulating the behavioral effect of reduced cocaine seeking following 2 weeks of abstinence from cocaine self-administration (Figures 2F and2G).
We found that self-administered intravenous cocaine infusion profoundly reduced the frequency of Ca2+ transient events observed during a 2 min period following drug delivery, suggesting a decrease in the average neuronal activity of VP DRD3+ neurons (Figures 2H and 2I). However, when the tone-light cues accompanying successful schedule completion were delivered in the absence of cocaine infusion, the overall Ca2+ event frequency of VP DRD3+ neurons was not suppressed (Cues only; Figure 2I). Furthermore, these measurements were not significantly altered by VP DRD3 KD, suggesting that DRD3 signaling does not overtly regulate the acute pharmacological effects of cocaine on VP DRD3+ neuronal activity.
In contrast, the frequency of Ca2+ events was strongly elevated during the seeking test performed following 2 weeks of home-cage abstinence (Day 24), as compared to baseline (Day 0) measurements taken in the operant box one day prior to the first self-administration session (Figures 2J and 2K). This effect was observed only upon re-exposure to the drug-taking operant context during the seeking test, and not in a drug-neutral open field context (Figure 2L) or in drug-naive mice (Figures S3E and S3F). Crucially, DRD3 KD completely abolished the elevated activity observed during cocaine seeking (Figure 2J and 2K), indicating that emergence of drug salience-encoding activity in the VP requires DRD3 signaling.
To understand the dynamics of VP DRD3+ activity associated with drug-seeking nose pokes, we quantified the Area Under the Curve (AUC) of GCaMP6f signal epochs between −1 sec and +3 sec surrounding nose pokes (Figures S3A to S3C). Control mice had significantly higher active nose poke AUC values compared to VP DRD3 KD mice, as well as higher AUC values associated with active nose pokes compared to inactive nose pokes (Figure S3C). However, when plotting the difference in Ca2+ event frequency between the baseline (Day 0) and the seeking session (Day 24) as a function of the number of active nose pokes performed during the seeking session, we observed that for the majority of control mice the increase in Ca2+ events was largely unaccounted for by the number of active nose pokes (Figure S3D, Ctrl points above the identity line), suggesting that other aspects of the drug-associated context of the operant box may be strongly evoking VP DRD3+ activity. Furthermore, we performed CPP experiments with VP DRD3+ GCaMP6f photometry, which also showed that VP DRD3+ neuronal activity was significantly elevated in cocaine-treated control mice as compared to VP DRD3 KD mice and saline-treated mice, but only during the preference test that followed a 10-day drug-free period (Figures S3G to S3J). Together, these results suggest that prolonged abstinence elevates VP DRD3+ neuronal activity encoding drug-related contextual salience, in a DRD3-dependent manner.
In ex vivo patch clamp experiments following 2 weeks of abstinence from cocaine self-administration, VP DRD3+ neurons showed a DRD3-dependent elevation of intrinsic excitability at high input current steps, increased excitation to inhibition ratio (E:I), and higher resting membrane potential (Figures S4A to S4I), suggesting that VP DRD3+ neurons are primed to augment their response to drug-related inputs following prolonged abstinence.
To determine whether the activity of VP DRD3+ neurons contributes to cocaine seeking behavior we bilaterally expressed Cre-dependent Gi-coupled DREADD (hM4D) receptors in the VP of DRD3-Cre mice (Figures 2M and 2N), trained mice to self-administer cocaine for 10 days (Figure 2P), and injected clozapine-n-oxide (CNO) 30 min prior to the seeking test on day 24 (Figure 2O). The mice expressing hM4D in VP DRD3+ neurons showed significantly reduced active nose poking following CNO injection on day 24 (Figure 2Q). However, hM4D-mediated suppression of VP DRD3+ activity on Day 8 of the consummatory phase did not affect self-administration behavior (Figures S4J to S4L). In addition, CNO injection did not significantly alter locomotor activity or open field anxiety in hM4D mice (Figures S4M and S4N). Thus, taken together, our results suggest that DRD3 signaling-induced increase in VP DRD3+ neuronal activity drives the process of relapse to cocaine seeking after prolonged abstinence by contributing to the development of contextually-specific augmented activity in the VP.
VP DRD3-mediated potentiation of DA release in the NAc latSh during relapse to cocaine seeking
VTA DA neuronal activity and NAc DA release are elevated during cocaine seeking and functionally contribute to relapse to cocaine seeking (Mahler et al., 2019; Phillips et al., 2003; Saunders et al., 2013; Solecki et al., 2019). Moreover, distinct VTA activity and NAc DA release profiles (Liu et al., 2020), as well as distinct functional roles (Bossert et al., 2007; Liu et al., 2020), have been documented across the lateral and medial aspects of the mesolimbic pathway during context-dependent drug relapse. To assess whether VP DRD3 KD may reduce relapse to cocaine seeking by reducing VTA activity relative to control mice seeking cocaine, we quantified c-Fos expression in the latVTA and medial VTA at one hour following the drug seeking test (Figures 3A and 3B). Strikingly, the rostral latVTA – which projects to the NAc latSh (Yang et al., 2018) – showed a pronounced decrease in c-Fos+ cells in VP DRD3 KD mice following the seeking test (Figure 3B). This suggests that activity of NAc latSh-projecting VTA neurons, potentially including DAergic neurons, may be reduced during cocaine seeking in mice with DRD3 KD in the VP.
Figure 3. VP DRD3-mediated potentiation of DA release in the NAc latSh during relapse to cocaine seeking.
(A)Representative images of c-Fos immunostaining in the VTA of mice that underwent the post-abstinence cocaine seeking test prior to perfusion, with AAV-mediated bilateral expression of Ctrl or DRD3 shRNA constructs in the VP. Medial VTA (mVTA), lateral VTA (latVTA). Scale bar, 250 μm.
(B) Quantification of the number of c-Fos+ cells in the medial and lateral VTA along anterior-posterior (A.-P.) coordinates, from mice corresponding to (A). Two-way ANOVA, Sidak’s post hoc test, ** p < 0.01 for latVTA A.P. −2.92 Ctrl vs DRD3 shRNA; n = 4 to 6 images per group.
(C) Schematic of unilateral AAV-GRABDA2m expression in the NAc latSh, and bilateral AAV-GRABDA2m plus AAV-DRD3 shRNA in the VP, with corresponding optic fibers for photometry.
(D)Sample images of GRABDA2m and mCherry expression in the NAc latSh and VP. Scale bars, 200 μm. L, lateral; D, dorsal.
(E) Experimental schedule of cocaine S.A. experiments with GRABDA2m photometry signal collection points (green arrows) at Day 0 (Baseline), Days 6–10 (FR3), and Day 24 (Seeking Test).
(F) Averaged GRABDA2m photometry signals from NAc latSh (top) and VP (bottom) of Ctrl and VP DRD3 shRNA mice, in response to a self-administered cocaine infusion (arrowhead and vertical dotted line).
(G to I) DA levels in the NAc latSh and VP were indistinguishable between Ctrl and VP DRD3 shRNA following a self-administered cocaine infusion. n = 8, 7 mice for Ctrl, DRD3 shRNA.
(J) Representative traces of NAc latSh GRABDA2m photometry during Baseline (Day 0) and Seeking Test (Day 24) sessions. Tick marks above traces indicate threshold-detected events.
(K) Ctrl mice seeking cocaine have a significantly higher number of DA release events in the NAc latSh as compared to their Baseline event frequency, as well as compared to VP DRD3 shRNA mice seeking cocaine or Ctrl mice that self-administered saline. Two-way RM ANOVA, Sidak’s post hoc tests comparing Baseline vs Seeking days, *** p < 0.001; Newman-Keuls post hoc tests comparing treatments, * p < 0.05; n = 6, 10, 9 mice for Ctrl:Sal, Ctrl:Coc, DRD3 shRNA:Coc.
Error bars or shading indicate ±SEM for all.
See Table S2 for detailed statistics.
See also Figure S5.
To measure DA levels in the NAc latSh and the VP, we used fiber photometry to perform in vivo DA imaging using the genetically-encoded DA sensor GRABDA2m (Sun et al., 2020b) (Figures 3C to 3E, S5J and S5K). We first compared the effects of a self-administered intravenous cocaine infusion on DA levels in the NAc latSh vs. the VP. In both control and VP DRD3 KD mice, we found that a self-administered cocaine infusion caused DA levels to rapidly rise and decay in the NAc latSh, whereas VP DA levels rose comparatively slowly but persisted at high levels for far longer (Figures 3F to 3I). This suggests a sustained level of DA receptor signaling in the VP during cocaine self-administration sessions, which may cause pronounced effects on local neuronal plasticity.
Throughout GRABDA2m imaging sessions, we observed frequent transient events in the NAc latSh signal. During the seeking test, we expected a substantial fraction of these events to represent motivational drive and reward expectation previously associated with the cocaine self-administration context during the consummatory phase (de Jong et al., 2019; du Hoffmann and Nicola, 2014; Phillips et al., 2003; Roitman et al., 2004). We quantified the average frequency of DA transient events occurring during the pre-drug baseline (Day 0) and seeking (Day 24) imaging sessions, in control and VP DRD3 KD mice that had previously self-administered saline or cocaine (Figure 3J). Whereas the frequency of DA transients in the NAc latSh of control mice was significantly elevated during the seeking test as compared to the pre-drug baseline, VP DRD3 KD mice seeking cocaine, as well as saline controls, did not show a significant elevation in the frequency of DA transients during the seeking test (Figures 3J and 3K).
To assess NAc latSh DA release associated with cocaine-seeking nose pokes, we quantified AUC for GRABDA2m signal epochs between −1 sec and +3 sec surrounding nose pokes (Figures S5A to S5D). We divided our analysis into two parts, separating the first 50% of nose pokes from the second 50% of nose pokes performed by each animal during the seeking test, as we expected a gradual devaluation of active nose pokes over the course of the seeking test. Similar to VP DRD3+ GCaMP6f recordings, control mice had higher AUC values associated with active nose pokes compared to inactive nose pokes; however, these effects were only statistically significant for the first 50% of nose pokes performed during the seeking session (Figure S5C, 0–50, nose poke type main effect). In addition, when analyzing active nose poked-related events aggregated from all subjects (using subject ID as a covariate), control mice had higher active nose poke GRABDA2m AUC values compared to VP DRD3 KD mice, for the first 50% of active nose pokes performed (Figure S5D, 0–50). Analogous analyses of GRABDA2m measurements from the VP indicated similar but less robust trends compared to the NAc latSh (Figures S5E to Figure S5H). Furthermore, as for VP DRD3+ GCaMP6f recordings, for control mice that self-administered cocaine we found that the increase in number of DA release events occurring across the seeking session (Day 24) as compared to the pre-drug baseline (Day 0) was generally larger than the number of active nose pokes performed during the seeking session (Figure S5I, Ctrl points above the identity line), suggesting that elevated DA release in the NAc latSh is not simply a function of active nose pokes performed. Taken together, this set of results strongly suggests that DRD3-dependent signaling in the VP contributes to elevating accumbal DA release during relapse to cocaine seeking.
VP DRD3+ neuronal projections to the LHb and VTA are predominantly inhibitory
To understand the circuit-level mechanisms through which VP DRD3+ neurons control DA release in the NAc we probed the anatomical and functional properties of VP DRD3+ neuronal projections to the LHb and VTA, as these two targets have been previously shown to control reward-related behaviors (Faget et al., 2018; Knowland et al., 2017; Stephenson-Jones et al., 2020). VP DRD3+ neurons send non-collateralizing, synapse-forming projections to the LHb and the VTA (Figures S6A to S6F, and Figures 4A to 4D). Whole cell patch clamp recordings from the LHb and VTA revealed large ChR2evoked GABAA receptor-mediated responses in both target structures, with small and infrequent contributions from AMPA receptor-mediated currents (Figures 4E to 4H). Connectivity was higher in the LHb (72.2%) versus the VTA (53.2%), suggesting greater control over LHb output. The LHb is known to provide excitatory input to GABAergic neurons of the VTA and RMTg (Araki et al., 1988; Brinschwitz et al., 2010), which in turn inhibit VTA DA neurons (Hong et al., 2011; Ji and Shepard, 2007; Lammel et al., 2012). To determine whether VP DRD3+ neurons connect to this circuit, we recorded VP DRD3+ ChR2-evoked inhibitory post-synaptic currents (IPSCs) from LHb neurons labelled by red retrobeads injected in the VTA or RMTg (Fig. 4I and 4J). Both connectivity and the amplitude of ChR2-evoked IPSC responses were greater for LHb neurons projecting to the RMTg (Figures 4K and 4L), suggesting that LHb-projecting VP DRD3+ neurons may disinhibit VTA DA neurons primarily via RMTg GABAergic neurons.
Figure 4. Characterization of VP DRD3+ projections to the LHb and VTA.
(A and B) Cre-dependent AAV-mRuby2/synaptophysin-EGFP was stereotaxically injected in the VP of DRD3-Cre mice to visualize synaptic terminals in the LHb and VTA. Scale bar, 100 μm. L, lateral; D, dorsal.
(C and D) Representative images showing a high density of VP DRD3+ synaptic terminals in the LHb and VTA. Scale bars: 100 μm for left panels, 30 μm for right (zoom) panels.
(E) Schematic of Cre-dependent ChR2 expression in the VP of DRD3-Cre mice to characterize connectivity to the LHb and VTA using whole-cell patch clamp electrophysiology.
(F and G) Large IPSCs and some small EPSCs were evoked by stimulating ChR2-expressing VP DRD3+ terminals in the LHb or VTA. Bar graphs show average IPSC and EPSC amplitudes for connected cells. Pie charts indicate % connectivity for each type of response. n = 26/36, 25/47 connected/total cells patched from LHb, VTA. PTX, picrotoxin, GABAAR antagonist; NBQX, AMPA/Kainate receptor antagonist.
(H) Scatter plot of ChR2-evoked IPSC vs EPSC amplitudes for VP DRD3+ neurons projecting to the LHb or VTA.
(I) Schematic for experiments assessing connectivity of VP DRD3+ neurons to LHb neurons projecting to the RMTg or VTA; Cre-dependent ChR2 expression in the VP of DRD3-Cre mice, with red retrobeads injected in the RMTg or VTA.
(J) ChR2-EYFP-expressing terminals and red retrobeads in the LHb, corresponding to experiments in (I), (K), (L). Scale bar: 100 μm for both images.
(K) IPSC connectivity of VP DRD3+ neurons to LHb neurons targeting the RMTg or VTA. Connected/Total cells.
(L) ChR2-evoked IPSC amplitudes corresponding to connected cells in (K). Mann-Whitney two-tailed U test, ** p = 0.002.
(M) Schematic showing procedure for collecting cytosolic contents from single VTA neurons receiving monosynaptic inputs from VP DRD3+ neurons, followed by single-cell reverse transcriptase polymerase chain reaction (scRT-PCR).
(N) Sample images of scRT-PCR products for VTA cell type markers: TH, tyrosine hydroxylase; DAT, dopamine transporter; VGLUT2, vesicular glutamate transporter 2; GAD1&2, GAD1/2, glutamic acid decarboxylase 1 and 2, 1 or 2; VGAT, vesicular GABA transporter. Cell type “A” was defined as DAergic-like based on expression of both TH and DAT; cell type “B” was defined as glutamatergic-like based on expression of VGLUT2 and absence of GAD1&2, and VGAT; cell type “C” was defined as GABAergic-like based on expression of GAD1 or 2, and absence of VGLUT2 and DAT.
(O) Total number of cells recorded and genetically characterized for each of the three cell types presented in (N), (P), (Q).
(P) % of VTA cell types “A”, “B” and “C” receiving IPSC input from VP DRD3+ neurons. Connected/Total cells.
(Q) ChR2-evoked IPSC amplitudes corresponding to connected cells in (P).
Error bars indicate ±SEM for all.
See Table S2 for detailed statistics.
See also Figure S6.
To characterize the cell-type specific connectivity of VP DRD3+ neurons sending direct projections to the VTA, we used single-cell reverse-transcriptase PCR (scRT-PCR) to identify genetic markers of cells receiving ChR2-evoked IPSCs directly from VP DRD3+ neurons (Figures 4M and 4N). We categorized patched VTA cells as either putatively DAergic (Type A), glutamatergic (Type B), or GABAergic (Type C), and found substantial overlap between Type A and Type B cells (Figures 4N and 4O). Putatively GABAergic VTA cells (Type C) received approximately half as many inhibitory inputs from VP DRD3+ neurons as putatively dopaminergic (DAergic) (Type A) and glutamatergic (Type B) VTA cells (23.8% versus 45.5% and 50.0%, respectively), but IPSC amplitudes were similar across all three categories (Figures 4P and 4Q). Therefore, VTA-projecting VP DRD3+ neurons may have the capacity to excite or inhibit DAergic neurons depending on which subsets of cells are activated and/or depending on their firing pattern. On the other hand, LHb-projecting VP DRD3+ neurons are likely to inhibit LHb neurons projecting to GABAergic midbrain neurons, thereby disinhibiting mesoaccumbal DA neurons (Barrot et al., 2012; Brown et al., 2017; Lammel et al., 2012).
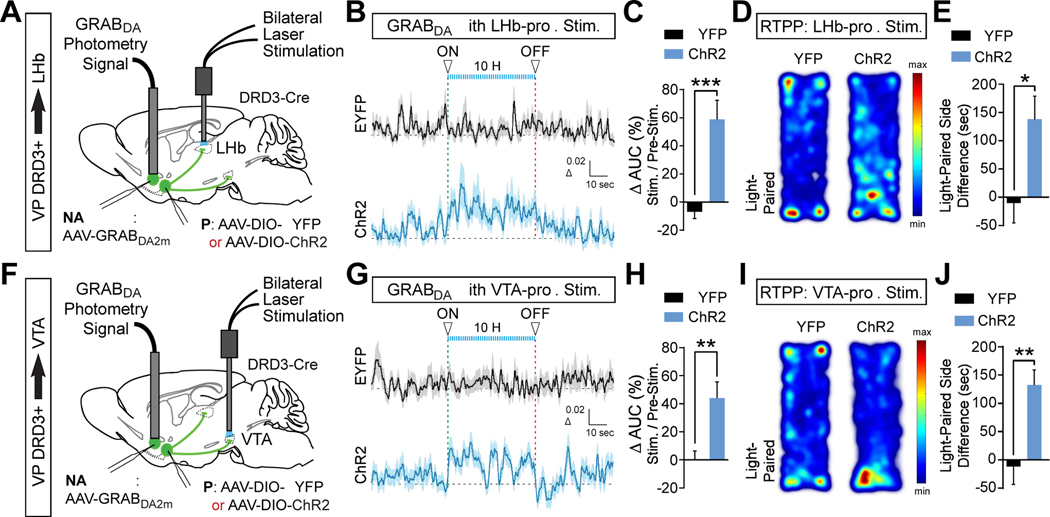
Optogenetic stimulation of VP DRD3+ neuronal projections to the LHb and VTA causes DA release in the NAc latSh and appetitive behavior
To assess the ability of distinct VP DRD3+ neuronal projections to alter accumbal DA release, we monitored GRABDA2m activation in the NAc latSh in response to ChR2-mediated stimulation of VP DRD3+ neuronal projections to the LHb or VTA (Figures 5A, 5F, S7A to S7C, and S7F to S7H). We found that stimulation of both LHb- and VTA-projecting VP DRD3+ neurons elevated DA release in the NAc latSh during stimulation periods (Figures 5B, 5C, 5G, 5H, S7D and S7E), suggesting that concerted, artificial activation of either projection can drive appetitive behavior. Indeed, contextually-paired stimulation of either LHb- or VTA-projecting VP DRD3+ neurons drove real-time place preference (Figures 5D, 5E, 5I, and 5J).
Figure 5. VP DRD3+ projections to the LHb and VTA control NAc latSh DA release and appetitive behavior.
(A and F) Cre-dependent ChR2 or EYFP were expressed in the VP of DRD3-Cre mice and projections to the LHb (A) or VTA (F) were optogenetically stimulated while GRABDA2m was monitored in the NAc latSh.
(B and C) DA release in the NAc latSh was elevated while stimulating VP DRD3+ terminals in the LHb. Analysis of covariance (ANCOVA) using subject ID (mouse identity) as a covariate, *** p < 0.001; n = 24, 25 trials for EYFP, ChR2, from 4 mice each. Analysis by subject presented in Figure S7D.
(D and E) Real-time place preference was induced by optogenetic stimulation of VP DRD3+ terminals in the LHb. Mann-Whitney two-tailed U test, * p = 0.045; n = 8, 5 mice for EYFP, ChR2.
(G and H) DA release in the NAc latSh was elevated while stimulating VP DRD3+ terminals in the VTA. ANCOVA using subject ID as a covariate, ** p = 0.003 for treatment comparison; n = 28, 34 trials for EYFP, ChR2, from 4 mice each. Analysis by subject presented in Figure S7E.
(I and J) Real-time place preference was induced by optogenetic stimulation of VP DRD3+ terminals in the VTA. Mann-Whitney two-tailed U test, ** p = 0.002; n = 7, 11 mice for EYFP, ChR2.
Error bars indicate ±SEM for all.
See Table S2 for detailed statistics.
See also Figure S7.
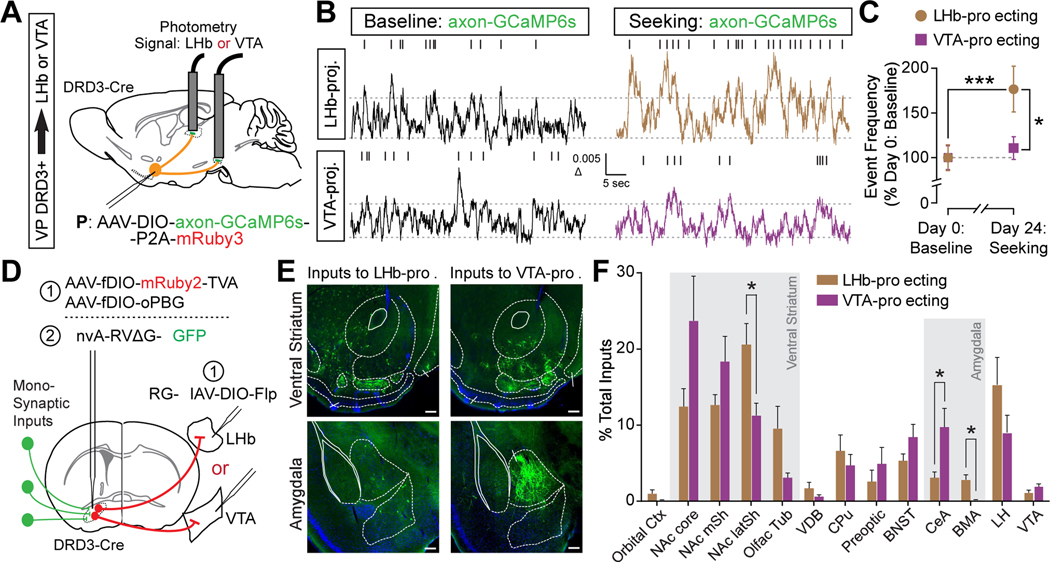
Activity of VP DRD3+ axonal terminals in the LHb is augmented during relapse to cocaine seeking.
To understand the relative contributions of LHb-projecting vs VTA-projecting VP DRD3+ neurons to relapse to cocaine seeking after abstinence, we first directly monitored the activity of each projection at baseline (Day 0) and during the cocaine seeking test (Day 24) using viral-mediated delivery of axon-targeting GCaMP6s to VP DRD3+ neurons (Broussard et al., 2018), and fiber photometric measurements of axonal Ca2+ signals from each target structure (Figure 6A, S7I and S7J). The overall frequency of Ca2+ signal transients in axonal terminals of VP DRD3+ neurons in the LHb was significantly elevated during the post-abstinence seeking test as compared to baseline levels, whereas Ca2+ signals in axonal terminals of VP DRD3+ neurons in the VTA did not differ significantly between the two sessions (Figures 6B and 6C). Interestingly, unlike our findings from VP DRD3+ GCaMP6f recordings, AUC for the −1 sec to +3 sec time window surrounding nose pokes was not significantly different between active and inactive nose pokes for either the LHb or VTA terminals (Figure S7K to S7M), suggesting that active nose poke-related transients may preferentially occur in subpopulations of VP DRD3+ neurons projecting to other targets (eg. NAc, mediodorsal thalamus (MD), lateral hypothalamus (LH)). Together, our results suggest that LHb-projecting VP DRD3+ neurons are selectively and tonically activated throughout the cocaine-seeking session, and that this activity may be disinhibiting mesoaccumbal DA release to invigorate cocaine-seeking behavior.
Figure 6. Augmented activity of VP DRD3+ axonal terminals in the LHb during relapse to cocaine seeking.
(A) Cre-dependent axon-targeting GCaMP6s was expressed in the VP of DRD3-Cre mice and fiber photometry signals were collected from VP DRD3+ terminals in the LHb and VTA.
(B) Representative photometry signal traces collected from VP DRD3+ axonal terminals in the LHb (top panels) or VTA (bottom panels) at baseline (left panels) or during post-abstinence cocaine seeking (Day 24) (right panels). Tick marks above traces indicate threshold-detected events.
(C) Activity of VP DRD3+ axonal terminals in the LHb was significantly elevated during the seeking test compared to their baseline levels, as well as compared to the activity of VP DRD3+ axonal terminals in the VTA. Two-way RM ANOVA, Sidak’s post hoc tests, * p < 0.05, *** p < 0.001; n = 7 mice each for LHb, VTA.
(D) Schematic of viral strategy to identify monosynaptic inputs to LHb- or VTA-projecting VP DRD3+ neurons. DRD3-Cre mice were (1) injected with Flp-dependent TVA and oPBG-expressing viruses in the VP, and Cre-dependent Flp recombinase-expressing retrograde equine infectious anemia virus (RGEIAV-DIO-Flp) in the LHb or VTA; (2) three weeks later, EnvA-pseudotyped rabies virus expressing EGFP was injected in the VP.
(E) Representative images of monosynaptic inputs to LHb- and VTA-projecting VP DRD3+ neurons. Scale bars, 200 μm. L, lateral; D, dorsal.
(F) Quantification of brain-wide inputs to LHb- and VTA-projecting VP DRD3+ neurons. Mann-Whitney two-tailed U tests, * p < 0.05 for NAc latSh, CeA, BMA, n = 5, 4 hemispheres each.
Orbital Ctx, orbital cortex; NAc core, nucleus accumbens core; NAc mSh, nucleus accumbens medial shell; NAc latSh, nucleus accumbens lateral shell; Olfac Tub, olfactory tubercle; VDB, vertical diagonal band; CPu, caudate putamen; Preoptic, preoptic area; BNST, bed nucleus of the stria terminalis; CeA, central amygdala; BMA, basomedial amygdala; LH, lateral hypothalamus; VTA, ventral tegmental area.
Error bars indicate ±SEM for all.
See Table S2 for detailed statistics.
See also Figure S7.
The difference between LHb- and VTA-projecting VP DRD3+ neuronal activity during post-abstinence seeking (Day 24) may be caused by various factors, including the relative contributions of brain-wide anatomical inputs to each projection branch. Indeed, using monosynaptic rabies tracing combined with target-specific retrograde viral expression of Cre-dependent Flp recombinase (RG-EIAV-DIO-Flp)(Knowland et al., 2017; Lilascharoen et al., 2021)(Figure 6D), we uncovered significant differences in inputs from the NAc latSh, central amygdala (CeA), and basomedial amygdala (BMA)(Figures 6E and 6F), supporting a distinction between the functional roles of LHb- and VTA-projecting VP DRD3+ neurons.
Activity and DRD3 expression in LHb-projecting VP DRD3+ neurons is necessary to drive relapse to cocaine seeking
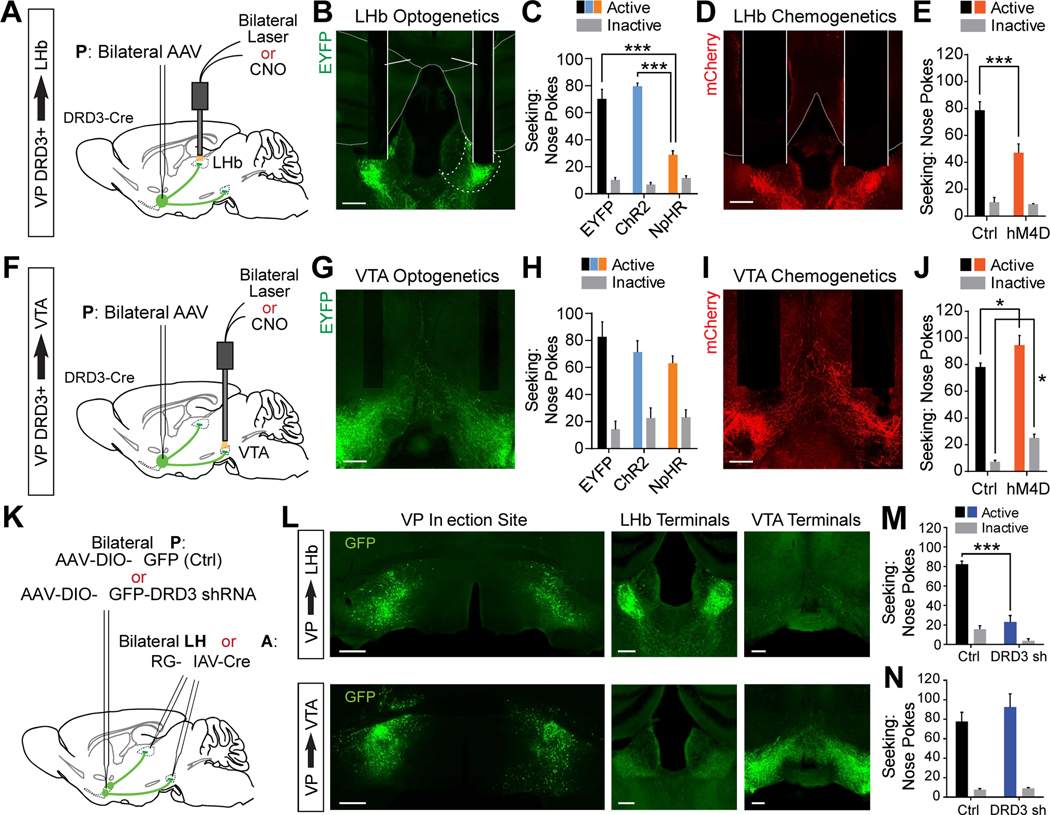
To further address the relative functional contributions of LHb- and VTA-projecting VP DRD3+ neurons during relapse to cocaine seeking, we first optogenetically or chemogenetically manipulated terminal activity of VP DRD3+ neurons during the post-abstinence seeking test (Day 24) using bilateral optic fibers or drug-infusion cannulae positioned above each target structure (Figures 7A, 7F, S8A and S8B). We found that inhibiting VP DRD3+ neuronal terminals in the LHb, either episodically with Cre-dependent NpHR3.0, or throughout the seeking test with Cre-dependent hM4D, significantly reduced the number of active nose pokes performed during the seeking test, whereas episodic ChR2-mediated activation of this projection had no effect on nose poke number (Figures 7B to 7E). Conversely, optogenetically activating or inhibiting VP DRD3+ terminals in the VTA did not significantly alter nose pokes performed during the seeking session, whereas hM4D-mediated inhibition throughout the seeking test slightly elevated the number of both active and inactive nose pokes performed, suggesting a small increase either in overall locomotor activity or motivated drug seeking when using uninterrupted inhibition of activity throughout the session (Figures 7G to 7J).
Figure 7. Activity and DRD3 signaling in the LHb-projecting branch of VP DRD3+ neurons is necessary to drive relapse to cocaine seeking.
(A, B and D) Cre-dependent AAVs were injected bilaterally in the VP of DRD3-Cre mice, and bilateral optic or drug infusion cannulae were implanted over the LHb. Scale bars, 200 μm. L, lateral; D, dorsal for all.
(C and E) Mice seeking cocaine following two weeks of abstinence performed fewer active nose pokes if VP DRD3+ terminals in the LHb were inhibited with NpHR (C) or with hM4D (E). Two-way RM ANOVA for optogenetic treatment, Tukey’s post hoc tests, *** p < 0.001; n = 7, 4, 7 mice for EYFP, ChR2, NpHR. Two-way RM ANOVA for chemogenetic treatment, Tukey’s post hoc test, *** p < 0.001; n = 4, 6 mice for Ctrl, hM4D.
(F, G and I) Cre-dependent AAVs were injected bilaterally in the VP of DRD3-Cre mice and bilateral optic or drug infusion cannulae were implanted over the VTA. Scale bars, 200 μm. L, lateral; D, dorsal for all.
(H and J) Optogenetically stimulating or inhibiting VP DRD3+ terminals in the VTA did not affect cocaine seeking (H), whereas chemogenetically inhibiting VP DRD3+ terminals in the VTA with hM4D significantly elevated both active and inactive nose pokes (J). n = 5, 6, 7 mice for EYFP, ChR2, NpHR. Two-way RM ANOVA for chemogenetic treatment, Tukey’s post hoc tests, * p < 0.05; n = 5, 5 mice for Ctrl, hM4D.
(K) Schematic for experiments in (L), (M), and (N). Cre-dependent EGFP (Ctrl) or DRD3 shRNA were injected bilaterally in the VP of wild-type mice and Cre recombinase-expressing RG-EIAV was injected in the LHb or VTA to selectively knock down DRD3 expression in LHb- or VTA-projecting VP neurons.
(L) Representative images of EGFP expression in LHb-projecting (top panels) or VTA-projecting (bottom panels) VP neurons, showing minimal collateralization between the two projections. Scale bars, 500 μm for VP injection site, 200 μm for LHb and VTA terminals. L, lateral; D, dorsal for all.
(M) Knock-down of DRD3 expression in LHb-projecting VP neurons decreases cocaine seeking following two weeks of abstinence from cocaine self-administration (Day 24). Two-way RM ANOVA, Sidak’s post hoc test, *** p < 0.001; n = 3 mice each for Ctrl, DRD3 shRNA.
(N) As in (M), but for VTA-projecting VP neurons. n = 3 mice each for Ctrl, DRD3 shRNA.
Error bars indicate ±SEM for all.
See Table S2 for detailed statistics.
See also Figure S8.
Secondly, we assessed cocaine-seeking behavior in groups of mice with DRD3 expression knocked-down in either the LHb- or the VTA-projecting subpopulations of VP neurons. We achieved this by using a Cre-dependent DRD3 knock-down construct virally-expressed in the VP, combined with a retrograde Cre-expressing virus (RG-EIAV-Cre)(Knowland et al., 2017; Lilascharoen et al., 2021) injected either in the LHb or the VTA (Figure 7K and 7L). Consistent with previous findings for VP PV+ neurons (Knowland et al., 2017) and our findings for VP DRD3+ neurons (Figure S6), we found that LHb-projecting and VTA-projecting VP neurons form distinct subpopulations that show minimal axonal cross-collateralization (Figure 7L), enabling us to separately manipulate DRD3 expression in each projection branch. Strikingly, DRD3 KD in LHb-projecting VP neurons reduced the number of nose pokes performed during the post-abstinence seeking test (Day 24), while DRD3 KD in VTA-projecting VP neurons had no effect. Thus, on the whole, our findings support the conclusion that augmented activity of LHb-projecting VP DRD3+ neurons by cocaine-associated contextual cues drives relapse to cocaine-seeking behavior, and that DRD3-dependent plasticity within the LHb-projecting branch of the VP plays a critical role in driving cocaine-seeking behavior.
DISCUSSION
Identifying the factors that sustain long-lasting vulnerability to drug relapse is pivotal to designing therapeutics for addictive disorders. Our model incorporates a two-week period of forced abstinence in the home cage, which is aimed at modeling periods of involuntary drug unavailability in human addiction (Farrell et al., 2018; Reiner et al., 2019; Venniro et al., 2016). In this study we provide a molecular and circuit-level understanding of how repeated use of cocaine can modify the activity of a subset of neurons in the VP to influence DA release in the NAc and motivate drug seeking upon delayed re-exposure to the self-administration context.
DRD3 expression in the VP as a critical factor driving relapse to cocaine seeking
We found that prolonged abstinence from passive or self-administered cocaine elevates DRD3 expression in the VP. This increase parallels gene expression (Freeman et al., 2010; Walker et al., 2018) and DNA methylation (Massart et al., 2015) changes observed in several brain regions, specifically following long (> 10 days) periods of abstinence from cocaine self-administration, but not following short (1 day) abstinence. Increased DRD3 expression may be driving augmented activity of VP neurons during relapse, possibly through suppression of inhibitory neurotransmission (Chen et al., 2006; Shin et al., 2018; Swant et al., 2008), or an increase in excitability resembling the effects of DRD2 overexpression (Cazorla et al., 2012).
Pharmacological antagonism of DRD3 signaling has been shown to attenuate various forms of relapse to drug seeking (Andreoli et al., 2003; Galaj et al., 2015; Sokoloff and Le Foll, 2017; Vorel et al., 2002; Xi and Gardner, 2007; Xi et al., 2013). Here, we used a highly-specific approach to studying DRD3 function during relapse by selectively knocking down DRD3 expression in the VP – an anatomical reward hub not previously studied in the context of DRD3 function. Consistent with systemic administration of DRD3 antagonists, we found decreased drug seeking in mice with DRD3 KD in the VP. However, whereas systemic DRD3 antagonism reduces cocaine self-administration under high ratio and PR schedules (Xi and Gardner, 2007; Xi et al., 2005), we found that VP DRD3 KD did not significantly impact FR3 or PR performance, suggesting that VP DRD3-mediated plasticity either plays a larger role under conditions when reinforcement is absent, or it impacts the development of incubation of cocaine craving (Pickens et al., 2011). Our observations that CPP score and VP DRD3+ neuronal activity in the CPP paradigm are only significantly affected by DRD3 KD after 10 days of abstinence suggest that prolonged abstinence enhances the contribution of VP DRD3 signaling to cocaine seeking.
DRD3 controls augmented activity of VP neurons during relapse
To understand how DRD3 KD in the VP reduces cocaine seeking after abstinence, we explored how DRD3 signaling in the VP impacts neural activity and plasticity. Using in vivo Ca2+ imaging we found that activity of DRD3-expressing VP neurons is elevated during the drug-deprived cocaine seeking state, decreased during the cocaine-intoxicated state, and unaltered in a context never paired with cocaine. Recent findings show a similar increase in the firing rate of VP GABAergic neurons during cue-induced reinstatement of cocaine seeking (Heinsbroek et al., 2020). Augmented activity of VP DRD3+ neurons during cocaine seeking, as well as increased net excitatory drive to these neurons during the abstinence period, were all vitally dependent on DRD3 expression. Mimicking the effects of DRD3 KD by chemogenetically inhibiting VP DRD3+ neuronal activity also resulted in suppressed drug seeking. Together, these findings support the notion that VP DRD3+ neurons encode cocaine craving and drive cocaine seeking in the drug-associated environment, in a manner dependent on DRD3-mediated plasticity. How might cocaine induce plasticity in the VP via DRD3 signaling? We observed a large and prolonged increase in DA release in the VP in response to intravenous cocaine infusion. The decay of DA release in the VP displayed a longer half-life than in the NAc latSh, possibly due to lower DRD2-mediated autoregulation of DA release (Mengual and Pickel, 2002) and/or lower dopamine transporter expression (Stout et al., 2016) in the VP as compared to striatal areas. Thus, despite sparse DAergic innervation of the VP as compared to the NAc (Adrover et al., 2014; Papathanou et al., 2019), DA may play an important local signaling role in the VP in response drugs of abuse, such as cocaine, that cause long-lasting elevation of DA. DRD3 may be especially important given its higher affinity for DA (Richtand, 2006; Robinson et al., 1994; Sokoloff et al., 1990).
Which direct synaptic inputs are modulating VP DRD3+ neuronal activity? Prior work has shown that DRD1-expressing NAc medium spiny neurons (D1 MSNs) increase their activity in response to passively-administered cocaine and immediately prior to entry into a drug-associated context, whereas DRD2-expressing NAc medium spiny neurons (D2 MSNs) correspondingly decrease their activity (Calipari et al., 2016). As VP DRD3+ neurons receive a large fraction of their inputs from the ventral striatum, likely from an equal mix of D1 and D2 MSNs (Creed et al., 2016; Knowland et al., 2017), it is possible that elevated D1 MSN-mediated inhibition dominates during the cocaine-intoxicated state, whereas reduced D2 MSN-mediated inhibition during seeking disinhibits VP DRD3+ neurons. Inputs from the VTA are also a probable source of modulation, as glutamatergic VTA neurons synapse onto and activate VP neurons (Yoo et al., 2016), and VTA neuron firing is acutely suppressed by cocaine (Calipari et al., 2017).
Distributed control of NAc latSh DA release via pallido-habenular DRD3+ neurons
Because knock-down of DRD3 in the VP blocked augmented activity of VP DRD3+ neurons in the cocaine-seeking context, we expected a distributed effect of VP DRD3 KD on neuronal activity in other structures of the reward circuitry, including the VTA. Indeed, we observed reduced activation of neurons in the latVTA and reduced DA release in the NAc latSh of VP DRD3 KD mice during the seeking test. This suggests that VP DRD3+ neurons regulate the reciprocal circuit between latVTA DA neurons and NAc latSh MSNs – a circuit that is known to drive appetitive behavior (Yang et al., 2018) and that has been associated with relapse behavior (Bossert et al., 2007; Liu et al., 2020). DA release in the NAc is crucial for expression of cocaine-seeking behavior (Mahler et al., 2019; Phillips et al., 2003; Saunders et al., 2013; Solecki et al., 2019), and generally plays an important role in motivational vigor, reinforcement learning, and reward processing (Hamid et al., 2016; Salamone and Correa, 2012; Schultz, 2016; Watabe-Uchida et al., 2017). We therefore sought to understand how VP DRD3+ neuronal activity regulates DA release in the NAc latSh by dissecting the circuitry connecting VP DRD3+ neurons to the VTA. We compared the direct projection to the VTA with an indirect connection to the VTA sent via the LHb. We found that both LHb-projecting and VTA-projecting VP DRD3+ neurons were primarily GABAergic, and capable of eliciting DA release in the NAc latSh and real-time place preference when optogenetically stimulated. The LHb is largely devoid of GABAergic interneurons (Brinschwitz et al., 2010) and sends excitatory projections to inhibitory GABAergic neurons in the VTA and the rostromedial tegmental nucleus (RMTg) (Araki et al., 1988; Brinschwitz et al., 2010), thereby inhibiting VTA DA neurons (Hong et al., 2011; Ji and Shepard, 2007; Lammel et al., 2012). We found that VP DRD3+ neurons preferentially inhibited RMTg-projecting LHb neurons. Therefore, we suspect that activation of pallido-habenular VP DRD3+ neurons suppresses LHb activity, which reduces activity in GABAergic RMTg neurons, and thereby disinhibits VTA DA neurons, leading to increased DA release in the NAc latSh. On other hand, because we observed that VTA-projecting VP DRD3+ neurons synapse onto both DAergic and GABAergic neurons in the VTA, it is unclear precisely how these predominantly inhibitory VP DRD3+ neurons elicit DA release in the NAc latSh. It appears that strong and concerted activation of VP DRD3+ projections to the VTA using ChR2 results in a net disinhibition of VTA DA neurons. However, natural forms of activity may select for subsets of VP DRD3+ neurons projecting only to VTA DA neurons or only to VTA GABA neurons during specific behaviors, which could result in net excitation or inhibition of VTA DA neurons.
Our axonal fiber photometry measurements showed that LHb-projecting VP DRD3+ neurons tonically elevate their activity during drug seeking after abstinence, whereas the VTA-projecting branch does not. Thus, elevated activity inferred from our somatic measurements of VP DRD3+ neurons (Figure 2) would be expected to contain the elevated activity of neurons projecting to the LHb, and possibly other subpopulations not explored in this study, such as those projecting to the NAc, MD, or LH. Compared to the VTA-projecting population, LHb-projecting VP DRD3+ neurons received more direct input from the NAc latSh and the BMA, but less input from the CeA (Figure 6). DA is released in the Nac latSh in response to reward and reward-predictive cues (de Jong et al., 2019; Liu et al., 2020), and inhibition of the Nac latSh to VP projection regulates motivational attraction to reward-paired cues (Smedley et al., 2019), suggesting that reward-encoding information from the NAc is preferentially conveyed to the LHb-projecting VP DRD3+ subpopulation. Inactivation of the CeA reduces cocaine self-administration (Warlow et al., 2017) and context-dependent relapse to cocaine seeking (Pelloux et al., 2018), while inhibition of glutamatergic inputs to the CeA reduces relapse after voluntary abstinence from cocaine self-administration (Venniro et al., 2017). Given that CeA neurons are predominantly GABAergic (Adams et al., 2018; Gungor et al., 2018), these findings imply that CeA inputs are preferentially inhibiting VTA-projecting VP DRD3+ neurons to facilitate relapse to cocaine seeking. Enhancing this inhibition would be expected to augment cocaine seeking, which is consistent with our observation that chemogenetically inhibiting VTA-projecting VP DRD3+ neurons slightly increased seeking. We note that prior findings implicating the VP to VTA projection in driving relapse to cocaine seeking (Mahler et al., 2014) used a different model of relapse in rats and did not specifically manipulate DRD3expressing neurons, but likewise did not observe a reduction in relapse behavior with inhibition of caudal VP terminals in the VTA, suggesting that relapse model, species, cell-type, and anatomical specificity may be important considerations for the VTA-projecting subpopulation.
DA-driven plasticity in the VP
In addition to anatomical input to the VP from VTA DAergic neurons (Klitenick et al., 1992) and regulation of VP DA release by drugs of abuse (Sizemore et al., 2000; Stout et al., 2016), functional evidence suggests a role for VP DA signaling in regulating local neuronal activity (Clark and Bracci, 2018; Maslowski-Cobuzzi and Napier, 1994; Mitrovic and Napier, 2002) and behavioral responses to drugs of abuse (Gong et al., 1996, 1997). Using GRABDA2m we observed a robust increase in DA level in the VP in response to self-administered intravenous cocaine infusion, but unlike in the NAc latSh, spontaneous DA transients were rare, preventing us from performing a threshold-based event analysis throughout the seeking session. Our analysis of nose poke-related GRABDA2m events in the VP during seeking did not find conclusive differences between active and inactive nose pokes, but warrants further investigation to understand the role of VP DA signaling during relapse, as well as in relation to DA-driven plasticity occurring during the consummatory and abstinence phases. Differentiating between DRD3-dependent plasticity occurring during the consummatory phase versus the role of elevated DRD3 expression during incubation of cocaine craving remains an open question, as does the heterogeneity of DA receptor expression in the VP. The latter point may be especially relevant in understanding why DRD3 KD restricted to LHb-projecting VP neurons was effective at reducing seeking, while DRD3 KD in the VTA projection was not.
Circuit model for role of DRD3-expressing VP neurons in cocaine seeking
In summary, our findings explain how DRD3-mediated plasticity in the VP can drive downstream changes in mesoaccumbal DA release during relapse to cocaine seeking through a projection-defined subpopulation of neurons. We propose a circuit model (Figure S8C) detailing how re-exposure to the drug-taking context elevates activity in the pallido-habenular branch of DRD3-expressing VP neurons to inhibit the LHb, which in turn disinhibits VTA DA neurons to drive release of DA in the NAc latSh. Elevated activity in this loop interlinking the NAc, VP, LHb and midbrain, may sustain an appetitive motivational state that drives cocaine seeking (Ikemoto et al., 2015). Conversely, if DRD3-mediated plasticity in the LHb-projecting branch of the VP is absent, elevated activity cannot be sustained as effectively in this loop, and drug seeking is diminished. Thus, our findings suggest that DRD3 antagonism or suppression of activity aimed at the ventral pallido-habenular branch of the reward circuitry may be an effective intervention for chronic, relapsing-remitting drug abuse.
STAR METHODS (Summary)
RESOURCES AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Byung Kook Lim (bklim@ucsd.edu).
Materials availability
DNA constructs and viruses generated by the authors will be distributed to other research investigators upon request.
Data and code availability
The data and custom code that support the findings from this study are available from the Lead Contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
C57BL/6J mice were obtained from The Jackson Laboratory. DRD3-Cre mice were obtained from the Gene Expression Nervous System Atlas (GENSAT, Founder line: KI196) and crossed to C57BL/6J mice for several generations prior to use. Adult male and female mice (~8–14 weeks old), housed on a 12h light/dark cycle were used for all experiments. Mice were generally housed in groups of 2–4 per cage, except for experiments involving drug infusion cannula implants, in which case they were housed individually post stereotaxic surgery. All experiments involving the use of animals were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, San Diego.
METHOD DETAILS
Plasmid and virus generation
AAV vector plasmids were generated using standard molecular cloning methods or were obtained from external sources. Origins and cloning of pAAV-EF1α-EGFP, pAAV-EF1α-EmGFP-DRD3 shRNA, pAAV-EF1α-DRD3(T1182G)-EmGFP-DRD3 shRNA (knock-down with rescue mutant), pAAV- EF1α-DIO-EGFP, pAAV-EF1α-DIO-EmGFP-DRD3 shRNA, and pAAV-EF1α-DIO-GCaMP6f were described previously (Shin et al., 2018). Construction and validation of shRNA targeting DRD3 was described previously (Shin et al., 2018), and contained the targeting sequence 5’-TTCTTCTTGACTCACGTTCTT-3’. pAAV-EF1α-mCherry-DRD3 shRNA and pAAV-EF1α-DIO-GCaMP6f-DRD3 shRNA were generated based on pAAV-EF1α-EmGFP-DRD3 shRNA and pAAV-EF1α-DIO-EmGFP-DRD3 shRNA, respectively. pAAV-hSyn-GRABDA2m was a gift from Dr. Yulong Li (Sun et al., 2020b). Origins and cloning of pAAV-EF1α-fDIO-EGFP, pAAV-EF1α-fDIO-mRuby-2TVA, pAAV-EF1α-fDIO-oPBG, pAAV-EF1α-DIO-mCherry, pAAV-hSyn-DIO-hM4D(Gi)-mCherry, pAAV-sSyn-DIO-mRuby2-T2A-Synaptophysin-eGFP, pAAV-EF1α-DIO-hChR2(H134R)-EYFP, and pAAV-EF1α-DIO-eNpHR3.0-EYFP were described previously (Knowland et al., 2017). pAAV-hSyn-DIO-axon-GCaMP6s-P2A-mRuby3 was a gift from Dr. Lin Tian (Broussard et al., 2018).
All AAV viruses used in this study were produced in-lab, as previously described (Knowland et al., 2017; Lilascharoen et al., 2021; Lim et al., 2012; Shin et al., 2018). Briefly, adenoassociated viruses (AAVs) were produced by transfection of 293 cells with three plasmids: (1) an AAV vector plasmid expressing the target construct (pAAV), (2) an AAV helper plasmid (pHELPER; Agilent), (3) and AAV-rep-cap helper plasmid (pRC-DJ, gift from M. Kay). Viral particles were isolated from cell lysates using iodixanol step-gradient ultracentrifugation and subsequently concentrated using a 100-kDa molecular mass cutoff ultrafiltration device (Millipore). Genomic titer was determined using real-time quantitative PCR and diluted in PBS to a working concentration of approximately 1012 viral particles/mL.
Production of EnvA-pseudotyped, glycoprotein-deleted rabies virus expressing EGFP (EnvA-RVΔG-eGFP) was described previously (Knowland et al., 2017; Lilascharoen et al., 2021; Shin et al., 2018) and based on a published protocol (Osakada and Callaway, 2013). Briefly, B7GG cells were used to generate non-pseudotyped RVΔG-eGFP, which was then pseudotyped by transducing BHK-EnVA cells.
Retrograde equine infectious anemia virus expressing Cre-dependent Flp recombinase (RG-EIAV-DIO-Flp) or Cre recombinase (RG-EIAV-Cre) was generated using a modified version of a published protocol (Cetin and Callaway, 2014), as described previously (Knowland et al., 2017; Lilascharoen et al., 2021). Briefly, HEK293T cells were transfected with three plasmids: (1) an EIAV genomic vector (pEIAV-CAG-DIO-Flp or pEIAV-CAG-Cre), (2) a helper packaging plasmid (pEV53B; a gift from John Olsen), and (3) a pseudotyping plasmid-encoding fusion protein, (FuG-B2; a gift from Kazuto Kobayashi). Viral particles were harvested from the media by centrifugation, and the resulting pellet was reconstituted with PBS and immediately stored at −80°C.
Surgeries
Stereotaxic injections
Mice were anesthetized either with a mixture of ketamine (100 mg/kg) and dexmedetomidine (0.5 mg/kg) or with isoflurane (5% for induction, 1–2% for maintenance; Somnosuite, Kent Scientific), and then placed in a stereotaxic apparatus (David Kopf Instruments). During surgery and recovery, mice were kept on heating pads to maintain body temperature. Viral injections of 150–300 nl were delivered unilaterally or bilaterally using a Hamilton microsyringe connected to borosilicate glass pipettes, at an infusion rate of 80–100 nl/min controlled by a syringe pump (PHD Ultra, Harvard Apparatus). Pre-emptive analgesia was provided by a subcutaneous injection of buprenorphine (0.1 mg/kg) or sustained-release buprenorphine (0.5–1.0 mg/kg). Post-operative analgesia was provided by additional injections of buprenorphine (spaced 12 hrs apart) or the long-lasting (72 hrs) effect of sustained-release buprenorphine. For fiber photometry, optogenetic, and local chemogenetic activation experiments, optic fibers or drug-delivery cannulae were chronically implanted above the target region following viral injection, and secured to the skull with adhesive cement (C&B Metabond, Parkell). Incisions were closed with absorbable sutures (Oasis) and sterile tissue adhesive (Vetbond, 3M).
Stereotaxic coordinates were derived from Franklin and Paxino’s Mouse Brain Atlas in Stereotaxic Coordinates (Franklin and Paxinos, 2008), and were as follows relative to Bregma (in mm): VP [anteroposterior (AP), +0.55; mediolateral (ML), +/−1.60; dorsoventral (DV), −5.00], NAc latSh (AP, +1.50; ML, +/− 1.60; DV, −4.40), LHb (AP, −1.00; ML, +/−0.50; DV, −2.80), VTA (AP, −3.00; ML, +/0.60; DV, −4.70), RMTg (AP, −4.20; ML, +/−0.30; DV, −4.40). Optic fibers and drug infusion cannulae were placed ~0.2–0.4 mm dorsal to DV coordinates. Based on histological validations, actual injection/implant sites were determined to correspond to locations approximately 0.40 mm posterior on the atlas as compared to the AP coordinates above.
For viral-mediated knock-down of DRD3 experiments, AAV-EGFP (control), AAV-EmGFP-DRD3 shRNA (knock-down), AAV-DRD3(T1182G)-EmGFP-DRD3 shRNA (knock-down with rescue mutant)(Shin et al., 2018), AAV-mCherry-DRD3 shRNA (knock-down), AAV-DIO-EGFP (Cre-dependent control for patch clamp electrophysiology), AAV-DIO-EmGFP-DRD3 shRNA (Cre-dependent knock-down for patch clamp electrophysiology and projection-specific knock-down), AAVDIO-GCaMP6f (control for photometry), or AAV-DIO-GCaMP6f-DRD3 shRNA (knock-down for photometry) were injected bilaterally in the VP of wild-type mice. Mice recovered for 2 weeks prior to subsequent jugular vein catheter surgery or 3 weeks prior to the start of behavioral experiments.
For identification of synaptic puncta in target regions of VP DRD3+ neurons, AAV-DIO-mRuby2-Synaptophysin-EGFP was injected unilaterally in the VP of DRD3-Cre mice; after ~8 weeks of viral expression brains were processed for histology. We used an intersectional strategy to assess the extent to which VP DRD3+ neurons collateralize to different target regions: AAV-fDIO-EGFP was unilaterally injected in the VP, and RG-EIAV-DIO-Flp was injected in the LHb or VTA of DRD3-Cre mice; histological analyses followed after 3–4 weeks of viral expression.
For projection-defined monosynaptic rabies tracing of inputs to VP DRD3+ neurons, a 1:1 mixture of AAV-fDIO-mRuby2-TVA and AAV-fDIO-oPBG were injected in the VP, and RG-EIAV-DIO-Flp was injected into the ipsilateral LHb or VTA of DRD3-Cre mice. After 3 weeks of viral expression, the same mice were injected in the VP with EnvA-RVΔG-eGFP. After an additional 6–10 days of expression, mice were euthanized and brains were processed for histological analyses.
For calcium imaging of DRD3+ neurons in the VP, AAV-DIO-GCaMP6f or AAV-DIO-GCaMP6f-DRD3 shRNA was bilaterally injected in the VP of DRD3-Cre- mice and a photometry optic fiber (400μm diameter, 0.48 NA, Doric Lenses) was inserted unilaterally just above the VP. For dopamine imaging in the NAc latSh and VP during self-administration experiments, AAV-GRABDA2m was injected unilaterally in the NAc latSh and a 1:1 mixture of AAV-GRABDA2m and AAV-mCherry-DRD3 shRNA was injected bilaterally in the VP of wild-type mice. One photometry optic fiber was inserted just above the site of virus injection in the NAc latSh while a second photometry optic fiber was inserted just above the contralateral VP. For dopamine imaging in the NAc latSh during optogenetic stimulation of VP DRD3+ neuronal terminals in the LHb or VTA, AAV-GRABDA2m was injected unilaterally in the NAc latSh and AAV-DIO-EYFP or AAV-DIO-ChR2-EYFP was injected bilaterally in the VP of DRD3-Cre mice. A photometry optic fiber was inserted just above the site of virus injection in the NAc latSh, and bilateral optogenetic optic fiber implants (200 μm diameter, 0.22 NA, Doric Lenses) were inserted just above the LHb or VTA. For axonal calcium imaging in the LHb and VTA, AAV-DIO-axon-GCaMP6s-P2A-mRuby3 was injected unilaterally in the VP of DRD3-Cre mice, and a photometry optic fiber was inserted above the ipsilateral LHb or VTA. Mice recovered for 2 weeks prior to subsequent jugular vein catheter surgery or 3 weeks prior to the start of behavioral experiments.
For chemogenetics experiments, AAV-DIO-hM4D(Gi)-mCherry was injected bilaterally in the VP of DRD3-Cre mice. For control of terminal activity in the LHb or VTA, bilateral drug-infusion cannulae (26 gauge, InVivo1) were inserted just above the LHb or VTA. Mice recovered for 2 weeks prior to subsequent jugular vein catheter surgery.
For optogenetic manipulations during cocaine seeking, DRD3-Cre mice were bilaterally injected with either AAV-DIO-EYFP, AAV-DIO-ChR2-EYFP or AAV-DIO-eNpHR3.0-EYFP, and dual fiber-optic cannulae (200 μm fiber diameter, 0.22 NA, Doric Lenses) were inserted just above the LHb or VTA. Mice recovered for 2 weeks prior to subsequent jugular vein catheter surgery. For ChR2-assisted circuit mapping using patch clamp electrophysiology, AAV-DIO-ChR2-EYFP was injected bilaterally in the VP of DRD3-Cre mice and ex vivo patch clamp recordings were performed after 3–4 weeks of viral expression.
Jugular vein catheterization
Mice were anesthetized with isoflurane (5% for induction, 1–2% for maintenance; Somnosuite, Kent Scientific), and placed on a warming pad throughout the surgery. The right jugular vein was isolated and a silicone catheter (2 French tip size, Access Technologies) pre-filled with heparin (10 USP/mL, BD) was inserted to a depth of ~1.2 cm toward the heart. Sutures were tied around the catheter to secure it to the vein. The other end of the catheter was attached to a mouse vascular access button (22 gauge, Instech) positioned in the interscapular area. Incisions were closed with absorbable sutures (Oasis) and sterile tissue adhesive (Vetbond, 3M). Analgesia was provided as for stereotaxic surgeries. Catheters were flushed with Heparin (100 USP/mL, BD) within a few hours of recovery from anesthesia. Mice recovered for ~1 week prior to the start of cocaine self-administration sessions.
Behavioral procedures
Cocaine self-administration
Cocaine self-administration experiments were conducted using standard mouse operant chambers (Med-Associates) equipped with two illuminated nose pokes, a syringe pump and a vascular access button tether kit (Instech). The ‘active’ nose poke was paired with delivery of cocaine, whereas the ‘inactive’ nose poke had no consequence. Operant chambers were dark (house light off) during all sessions. Days 1–4 were conducted on a fixed-ratio 1 (FR1) schedule, and ays 5–10 were conducted on an FR3 schedule, with one session performed each day. FR sessions lasted either: (i) 2 hrs for days 1-10 (for experiments presented in Figures 1D to 1H, and S1F to S1I), or (ii) 2 hrs for day 1 and 3 hrs for days 2–10 (for all other experiments involving cocaine self-administration). Prior to the start of the first session, mice were food-deprived overnight (12–16 hrs) to increase exploratory behavior. Some experiments (Figures 1D to 1H, and S1F, S1H) included a progressive ratio (PR) session on Day 11, with 30 min given to reach an exponentially-increasing requirement for the next infusion. Successful completion of the schedule during FR and PR sessions simultaneously resulted in: one infusion of cocaine, a 0.5 sec sound cue, a 10 sec illumination of the ‘active’ nose poke, and a 10 sec time-out period during which ‘active’ nose pokes were recorded but did not contribute to delivery of the next infusion. Cocaine solution (Sigma-Aldrich, 0.75 mg/mL, dissolved in 0.9% saline) was delivered at a rate of ~18 μl/sec, with each infusion delivering 1.0 mg/kg intravenously via the implanted vascular access button. One ‘priming’ infusion of ~72 μl was delivered at the start of each session. For mice self-administering saline instead of cocaine, infusion volumes were equivalent. Catheters were flushed with heparin solution (100 USP/mL, BD) prior to and after the completion of each session. Catheter patency was assessed by: (i) low resistance to catheter flushing prior to the start of each session, (ii) presence of cocaine-elicited hyperlocomotor behavior at the end of each session, and (iii) infusion of sodium brevital solution (5 mg/kg) via the vascular access button to observe a rapid anesthesia response (loss of righting reflex within 5 sec). Mice with poor catheter patency typically displayed a decaying nose-poking rate across days, and were removed from the study. Following the completion of all cocaine self-administration sessions, mice remained in their home cages for 2 weeks (forced abstinence). This was followed by a seeking test during which mice were reintroduced to the operant chamber, and nose pokes were recorded but had no consequence (extinction conditions). The seeking test lasted 20 min for fiber photometry experiments and 2 hrs for all other experiments.
For quantitative PCR experiments, cocaine self-administration was performed as described above in the first paragraph, but the seeking test was omitted. Mice were euthanized and brain tissue was microdissected after 1 or 14 days of home-cage abstinence. Brain tissue processing and quantitative PCR were performed as described in a subsection below.
For immunostaining of c-Fos experiments, cocaine self-administration was performed as described above in the first paragraph, and mice were euthanized ~60 min after the end of the 2hr seeking test. Brain tissue was processed as described in the histology section below.
For fiber photometry experiments, cocaine self-administration was performed as described above in the first paragraph, with some modifications. Fiber photometry measurements were made using a custom-built sCMOS camera-based set-up, described in a subsection below. Baseline photometry measurements were acquired while the (drug-naive) mouse explored the drug self-administration operant box for 10 min on Day 0 (the day prior to the first FR1 session), with only the photometry patch cord attached to the mouse; nose poking had no consequence during this session. During days 6-10, prior to the 3 hr FR3 self-administration session, fiber photometry measurements were performed on a subset of mice, during short self-administration sessions, as follows: (i) the mouse did not receive an initial priming infusion, (ii) the first FR3 completion resulted in cue delivery only (no cocaine), (iii) the second FR3 completion resulted in cue and cocaine (1.0 mg/kg) delivery, (iv) drug was infused via a hand-held syringe to prevent the photometry patch cord and drug infusion tubing from tangling, (v) recordings proceeded for at least 2 min after the infusion, following which the mouse was returned to the home cage, (vi) visualization of the operant chamber using a near-infrared camera (Basler) facilitated these experiments. During the 20 min seeking test, only the photometry patch cord was attached to the mouse.
For experiments involving chemogenetic manipulation of the entire VP DRD3+ population, cocaine self-administration was performed as described above in the first paragraph, and clozapine-n-oxide (CNO) (0.1 mg/kg) was injected intraperitoneally (I.P.) ~30 min prior to the start of the seeking test (for Figures 2O to 2Q) or the Day 8 FR3 session (Figures S4J to S4L). For chemogenetic control of VP DRD3+ terminals in the LHb and VTA, CNO (1 mM, dissolved in ACSF, 0.2 μl/side for LHb, 0.5 μl/side for VTA) was delivered at a rate of 0.1 μl/min using a pair of Hamilton syringes connected to the chronically-implanted drug infusion cannulae, ~10 min prior to the start of the 2 hr seeking test.
For patch clamp electrophysiology experiments, mice self-administered saline or cocaine for 10 days, as described above in the first paragraph. Following 12 to 16 days of home-cage abstinence, the seeking test was omitted, and mice were euthanized for ex vivo patch clamp electrophysiology, as described in the patch clamp slice electrophysiology section below.
For optogenetic experiments, cocaine self-administration was performed as described above in the first paragraph. Prior to the seeking test, mice were habituated to moving freely in an open field context with the optic fiber patch cord (Doric Lenses) attached to the optical fiber implant. Laser light (OEM Laser Systems) was split bilaterally through a 1×2 rotary joint (Doric Lenses). Laser power, measured with a light power meter (Thorlabs) prior to each experiment, was set to 10–15 mW bilaterally at the tip of the patch cord. During the seeking test, laser stimulation (473nm, 10 Hz, 5ms pulses for EYFP or ChR2; 593 nm, continuous for EYFP or NpHR3.0) was delivered throughout the session using an optogenetic TTL pulse generator (Doric Lenses), for alternating 10 min light ON/10 min light OFF periods, starting with a light ON period.
For projection-specific DRD3 knock-down experiments, cocaine self-administration and the seeking test were performed exactly as described above in the first paragraph of the cocaine self-administration subsection.
Sugar pellet self-administration
Sugar pellet self-administration experiments were conducted in the same operant chambers as cocaine self-administration experiments, equipped with two illuminated nose pokes and a food pellet dispenser (Med-Associates). Mice were mildly food deprived to ~90% of free-feeding body weight throughout the experiment. The ‘active’ nose poke was paired with delivery of a 20 mg sugar pellet (Bio-Serv), whereas the ‘inactive’ nose poke had no consequence. Operant chambers were dark (house light off) during all sessions. Days 1–4 were conducted on a FR1 schedule, and days 5–10 were conducted on an FR3 schedule, with one session performed each day. FR sessions lasted 1 hr each. The PR session on Day 11 had a 30 min time-out period to reach an exponentially-increasing requirement for delivery of the next pellet. Successful completion of the schedule during FR and PR sessions simultaneously resulted in: delivery of one sugar pellet, a 0.5 sec sound cue, a 10 sec illumination of the ‘active’ nose poke, and a 10 sec time-out period during which ‘active’ nose pokes were recorded but did not contribute to delivery of the next pellet. Following the completion of all 11 sugar self-administration sessions, mice remained in their home cages for a further two weeks. This was followed by a 1hr seeking test during which mice were reintroduced to the operant chamber and nose pokes were recorded but had no consequence (extinction conditions).
GRABDA photometry with optogenetic stimulation
Mice were connected to fiber photometry and optogenetic patch cords (Doric Lenses) and placed in a clean cage with fresh bedding. Mice habituated for ~10 min to the environment prior to data acquisition. Fiber photometry measurements of GRABDA2m expressed in the NAc latSh were made using an open source Python-based set-up (Akam and Walton, 2019), described in a subsection below. Optogenetic stimulation (473nm, 10 Hz, 5ms pulses, laser power 10–15 mW) was delivered for periods of 50 seconds, with a minimum of 60 seconds in between stimulation trials. Six or more stimulation trials were performed for each animal, over the course of at least 2 separate sessions.
Real-time place preference
Mice were connected to an optogenetic patch cord delivering light bilaterally via a 1×2 rotary joint (Doric Lenses). Mice were placed in a rectangular arena (L: 67.5, W:24, H: 50 cm), which had a middle section with a clear plexiglass floor (11.5 × 24 cm), and two sections on opposite sides (28 × 24 cm each) which were distinguished by wall pattern (checkered vs striped) and floor texture (smooth vs rough). Behavior was monitored using a camera (Logitech) and video tracking software (Biobserve Viewer or Noldus Ethovision XT). Mice explored the entire arena for 20 min without optical stimulation. Based on this initial habituation session, the side with lower preference was assigned as the light-paired side. Another 20 min session followed, during which presence inside the 28 × 24 cm light-paired side resulted in optogenetic stimulation (473nm, 10 Hz, 5ms pulses, laser power 10–15 mW). In some experiments, a third 20 min session followed, during which optogenetic stimulation was switched to the opposite 28 × 24 cm side. To obtain the ‘light-paired side difference’ value, the amount of time spent in the light-paired side during the habituation session was subtracted from the amount of time spent on the light-paired side during the stimulation session (i.e., light-paired side difference = stim. – hab.). In cases where a second stimulation session was performed, the amount of time spent in the light-paired side during the second stimulation session was subtracted from the amount of time spent on that same side during the first stimulation session, and the average of the two light-paired side differences was calculated [i.e., light-paired side difference = (stim. 1 – hab. + stim. 2 – stim. 1)/2].
Conditioned place preference (CPP)
Experiments were conducted in a three-chambered rectangular arena (L: 67.5, W:24, H: 50 cm) with a middle chamber (11.5 × 24 cm) and two chambers on opposite sides (28 × 24 cm each) separable by two removable vertical inserts. The two chambers on each side differed in terms wall pattern (checkered vs striped) and floor texture (smooth vs rough). On day 1, the mouse freely explored the open arena for 20 min (habituation). On days 2–4, a morning session and an afternoon session were carried out. On day 2, the mouse was injected with saline (I.P.) in the morning session and immediately confined to one side for 15 min, while in the afternoon session, the mouse was injected with cocaine (15 mg/kg, I.P.) and immediately confined to the opposite side for 15 min. On days 3 and 4, the procedure was repeated as on day 2, with cocaine/saline and saline/cocaine administered in the morning/afternoon sessions, respectively. The cocaine-paired side was chosen randomly for each mouse. On days 5 (CPP Test 1) and 14 (CPP Test 2), the mouse freely explored the open arena again for 20 min. The amount of time spent in each chamber was tracked during the habituation and CPP Tests using a camera (Logitech) and video tracking software (Biobserve Viewer or Noldus Ethovision XT). The amount of time spent in the cocaine-paired side during the baseline session was subtracted from the amount of time spent on the same side during the CPP tests. For CPP experiments involving fiber photometry measurements, the behavioral procedures were identical, but mice were connected to a photometry patch cord on days 1, 5 and 14. Photometry data was collected and analyzed as described in a subsection below.
Open field test
Mice were placed in an open field arena (44 × 44 × 44 cm) and the total distance travelled was measured for 10 min (Biobserve). The percent time spent in the centre was determined using a 20 × 20 cm zone in the centre of the arena.
Fiber photometry data acquisition
Cocaine self-administration and CPP
A fiber photometry patch cord (400 μm, 0.48 NA, Doric Lenses) was coupled to the optic fiber implant using bronze sleeves (Doric Lenses) which allowed for rotational motion. Fiber photometry data was collected using a custom-built set-up with light path as follows: a 405nm/470nm/550nm LED light source (SPECTRA X, Lumencor) delivered light through an excitation filter (Thorlabs, FB410–10 and FB470–10 and M565F1), a dichroic mirror (Semrock, FF495-Di02–25 ×36) and a 20X objective (Nikon, CFI Plan Apo Lambda) connected to the optic fiber patch cord; emission light passed back through the patch cord, 20X objective, and across the dichroic mirror, on its way to an (optional) image-splitting optics unit (W-VIEW GEMINI, Hamamatsu) that separated green and red emission light. Inside the image splitter, a 560nm dichroic mirror (Chroma, T560lpxr-UF2–26 × 28 × 2 mm) separated the emission into two channels, each of which was additionally filtered by a 600–637nm (Semrock, FF01–600/37–25) or a 520–535nm emission filter (Semrock, FF01–520/35–25) and then projected onto the sCMOS camera (Orca-Flash4.0, Hamamatsu). The light source was adjusted for each mouse to generate an emission signal hovering around 20–40% of sCMOS sensor saturation (~40-150 μW). Images were acquired at 20 Hz using HC-Image (Hamamatsu) and converted into a string of numbers (signal) using ImageJ. For self-administration experiments, HCImage acquisition and the MED-PC operant behavior program (Med-Associates) were started simultaneously; nose poke and infusion time stamps recorded by MED-PC were used to locate events in the photometry signal. GCaMP6f and GRABDA2m were excited with 470 nm light. Excitation with 405 nm light (isosbestic point) for GCaMP6f and GRABDA2m was performed in separate sessions; based on these recordings, contributions from motion artifacts were estimated to be much smaller than activity- or DA release-driven transients (i.e. generally < 5% of peak transient amplitudes). Axon-GCaMP6s-P2A-mRuby3 was excited with simultaneous 470/550nm light, and image-splitting optics (W-VIEW GEMINI, Hamamatsu) separated green (GCaMP6s) and red (mRuby3) emission light into two distinct areas of the camera’s field of view, allowing for separation of GCaMP6s and mRuby3 signals.
GRABDA photometry with optogenetic stimulation
Mice were connected to the photometry rig using a fiber photometry patch cord and bronze sleeve, as described above for self-administration experiments. GRABDA2m signals were collected using an open source, Python-based photometry rig (pyPhotometry) (Akam and Walton, 2019). Briefly, the system used 470 nm and 405 nm LEDs (Doric Lenses), a Micropython Pyboard with 2 analog and 2 digital inputs, and a photoreceiver (Doric Lenses). A graphical user interface was used to control acquisition, visualize signals and record data. Time-division multiplexed illumination allowed for interleaved acquisition of GRABDA2m fluorescence evoked by 470 nm (DA-dependent) and 405 nm (DA-independent) excitation light. Optogenetic laser stimulation of ChR2 was controlled using an optogenetics TTL pulse generator (Doric Lenses) which also sent TTL pulses to one of the digital inputs on the Micropython Pyboard, allowing for precise identification of optogenetic simulation epochs.
Patch clamp slice electrophysiology
Mice were euthanized using isofluorane inhalation and coronal brain slices (300 μm) containing either the VP, LHb or VTA were prepared using a vibratome (Leica VT1200S), in a solution containing (in mM): 110 choline chloride, 25 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 7 MgCl2, 25 glucose, 0.5 CaCl2, 11.6 ascorbic acid, and 3.1 pyruvic acid, saturated with 95% O2/5% CO2. Slices recovered at 30°C for 20 min, and subsequently at room temperature in a solution containing (in mM): 118 NaCl, 26 NaHCO3, 11 glucose, 15 HEPES, 2.5 KCl, 1.25 NaH2PO4, 2 pyruvic acid, 0.4 ascorbic acid, 2 CaCl2, and 1 MgCl2, saturated with 95% O2/5% CO2. Following > 1.5 hr of recovery, slices were transferred to the electrophysiology rig’s recording chamber, which was perfused with artificial cerebrospinal fluid (ACSF) containing (in mM): 119 NaCl, 26.2 NaHCO3, 11 glucose, 2.5 KCl, 1 NaH2PO4, 2.5 CaCl2, and 1.3 MgCl2, saturated with 95% O2/5% CO2, maintained at a temperature of 30±1°C and flowing at a rate of 2 mL/min. Cell soma were visualized using differential interference microscopy using 4X or 40X objectives (Olympus BX61WI). All patch clamp recordings were performed in the whole cell configuration using borosilicate glass pipettes (3–4 MΩ). Liquid junction potentials were left uncompensated. Signals were amplified and filtered (2 kHz) using an Axopatch 700B amplifier, sampled at 10 kHz using a Digidata 1550, and recorded with Clampex 10.4 (Molecular Devices). Analyses were performed offline using Clampfit 10.4 (Molecular Devices). Only cells with stable access resistance of < 25 MΩ throughout the recording period were included in the analysis.
For ChR2-assisted circuit mapping of VP DRD3+ inputs to the LHb and VTA, recordings were performed following 3–4 weeks of Cre-dependent ChR2 virus expression in the VP of DRD3-Cre mice. For recordings of LHb cells projecting to the RMTg or VTA, red retrobeads (Lumafluor) were stereotaxically injected in the RMTg or VTA in the same surgery as ChR2 injections; during recordings, LHb cells containing red retrobeads were visualized using epifluorescence and selectively patched. ACSF contained 0.5 μM tetrodotoxin (TTX, Tocris) and 100 μM 4-aminopyridine (4-AP, Sigma) to isolate monosynaptic responses (Petreanu et al., 2009). Recording pipettes were filled with (in mM): 115 CsMeSO3, 1.5 MgCl2, 1 EGTA, 10 HEPES, 4 Mg-ATP, 0.3 Na3-GTP, 10 Na-phosphocreatine, 2 QX-314, and 10 BAPTA-Cs4. Excitatory to inhibitory ratio (E:I Ratio) recordings were performed in voltage clamp mode and calculated as the peak synaptic response at Vh = −70 mV divided by the peak synaptic response at Vh = 0 mV. ChR2 terminals in the LHb or VTA were activated by an LED light source (SPECTRA X, Lumencor) delivering blue light (473 nm, 5 ms pulses, ~10 mW) via the 40X objective. For each cell, 10–15 sweeps of 15 sec duration were collected at each Vh for analysis, with one blue light pulse delivered at the start of each sweep. For some recordings, after obtaining E:I ratio data, ACSF containing 10 μM NBQX and/or 100 μM picrotoxin (PTX) (in addition to 0.5 μM TTX and 100 μM 4-AP) was washed onto the cells to determine whether the ChR2-evoked EPSCs or IPSCs were mediated by AMPA receptors and GABA(A) receptors, respectively. In all cases, EPSC or IPSC amplitude was reduced > 95% by washing on NBQX or PTX, respectively.
For studies of plasticity of VP DRD3+ neurons following abstinence from saline/cocaine self-administration, recordings were performed after 12–16 days of home-cage abstinence following the last cocaine or saline self-administration session. Cells expressing Cre-dependent EGFP or Cre-dependent EGFP-DRD3 shRNA in the VP of DRD3-Cre mice were visualized using epifluorescence and selectively patched. Current clamp recordings were performed in ACSF containing 10 μM NBQX and 100 μM PTX to block synaptic transmission. For current clamp recordings, recording pipettes were filled with (in mM): 125 K-gluconate, 4 NaCl, 10 HEPES, 0.5 EGTA, 20 KCl, 4 Mg-ATP, 0.3 Na3-GTP, and 10 Na-phosphocreatine. To measure firing frequency in response to current injection, cells were brought to rest at −65 mV, and +10 pA increments were applied in 500 ms steps. E:I ratio recordings were performed in voltage clamp, in plain ACSF, as for ChR2-assisted circuit mapping, but using electrical stimulation of the slice instead of blue light.
For mapping of VTA cell types receiving VP DRD3+ neuronal inputs using single-cell RT-PCR, recordings were performed following 3–4 weeks of Cre-dependent ChR2 virus expression in the VP of DRD3-Cre mice. Due to the predominantly GABAergic VP DRD3+ neuronal input to the VTA, we focused our analysis on cells with inhibitory ChR2-mediated responses. Recording pipettes were filled with (in mM): 125 CsCl, 8 NaCl, 0.6 EGTA, 10 HEPES, 4 Mg-ATP, 0.3 Na3-GTP, 10 Na-phosphocreatine, and 2 QX-314. Recordings were performed in voltage clamp mode in the presence of 5 μM NBQX at Vh = −70 mV. ChR2-mediated responses were evoked with blue light stimulation, as described above. At the end of the recording, negative pressure was applied to the pipette to draw in the cytoplasmic contents of the recorded cell; the pipette tip was then crushed into the bottom of a tube containing ddH2O and RNAse inhibitor.
Single-cell RT-PCR
Cytoplasmic contents of each recorded cell were collected in separate tubes containing 1.5 μl ddH2O and 1.0 μl RNAse inhibitor. Samples were immediately stored at −80°C for subsequent batch processing. Samples were reverse transcribed using the SuperScript IV First-strand cDNA synthesis kit (Thermo Fisher Scientific). Target sequences were amplified from the cDNA by a multiplex PCR (mPCR) reaction with wide-spanning primers (~350–500 bp product size) followed by a nested PCR (nPCR) reaction (~250–300 bp product). Products were separated and visualized on 2% agarose gels. The targets amplified were: tyrosine hydroxylase (TH) (mPCR-F: 5’-CCGAGCCAGACTGCTGCCAC-3’, mPCR-R: 5’-ACCCCCTCTAAGGAGCGCCG-3’; nPCR-F: 5’-TGGTTCACTGTGGAGTTTGGGCT-3’, nPCR-R: 5’-GGGCGCTGGATACGAGAGGC −3’), dopamine transporter (DAT) (mPCR-F: 5’-CAGCCTGCCTGGTGCTGGTC-3’, mPCR-R: 5’AGACAGCGGGAGTGTGGCGA-3’; nPCR-F: 5’-CACAGCCCTGCTCCTGCGTG-3’, nPCR-R: 5’-GCTTCTGTGCCATGTACCCCAGG-3’), vesicular GABA transporter (VGAT) (mPCR-F: 5’-ACTGCGACGATCTCGACTTT-3’, mPCR-R: 5’-TGAGGAACAACCCCAGGTAG-3’; nPCR-F: 5’- GCCAGGGCCTGCAGATGGAC-3’, nPCR-R: 5’-CGCCGTGGAGGATGGCGTAG-3’), vesicular glutamate transporter 2 (VGLUT2) (mPCR-F: 5’-GGCTACTCTCATACTAGAGGG-3’, mPCR-R: 5’-GCACAAATTCTTCCTTTTTCTC-3’; nPCR-F: 5’-TTGGCACGCTGTCGGGGATG-3’, nPCR-R: 5’-TTCTCCTGTGAGGTAGCACCGT-3’), glutamate decarboxylase 1 (GAD1) (mPCR-F: 5’-CACAGGTCACCCTCGATTTT-3’, mPCR-R: 5’-TCTATGCCGCTGAGTTTGTG-3’; nPCR-F: 5’-GGGCTGCGCTTGGCTTTGGA-3’, nPCR-R: 5’-TGAGCAGTCCACCACCCCAGG-3’), glutamate decarboxylase 2 (GAD2) (mPCR-F: 5’-CAGCCTTAGGGATTGGAACA-3’, mPCR-R: 5’-ACCCAGTAGTCCCCTTTGCT-3’; nPCR-F: 5’-GTTCCTTTCCTGGTGAGTGC-3’, nPCR-R: 5’-TGCATCAGTCCCTCCTCTCT-3’), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (mPCR-F: 5’-ACTCCACTCACGGCAAATTC-3’, mPCR-R: 5’- CACATTGGGGGTAGGAACAC-3’; nPCR-F: 5’-TCTCCGCCCCTTCTGCCGAT-3’, nPCR-R: 5’-CCACAGCCTTGGCAGCACCA-3’). The scRT-PCR primers listed above are also listed in Table S1.
Quantitative Real-Time PCR
Mice were euthanized using isofluorane inhalation, and coronal brain slices (300 μm) containing the VP were prepared using a vibratome (Leica VT1200S), in standard ACSF solution containing (in mM): 119 NaCl, 26.2 NaHCO3, 11 glucose, 2.5 KCl, 1 NaH2PO4, 2.5 CaCl2, and 1.3 MgCl2, saturated with 95% O2/5% CO2. The VP was microdissected bilaterally, immediately frozen on dry ice and then transferred to −80°C for subsequent batch processing. RNA was extracted using the Hybrid-R RNA purification kit (GeneAll Biotechnology). Purified RNA was reverse transcribed using the SuperScript IV First-strand cDNA synthesis kit (Thermo Fisher Scientific). Target sequences were amplified from the cDNA using the TaqMan Gene Expression Assay Kit (Thermo Fisher Scientific) and the ViiA 7 Real-Time PCR System (Thermo Fisher Scientific). Taqman probes were obtained from Thermo Fisher Scientific and were as follows: DRD1 (Mm02620146_s1), DRD2 (Mm00438545_m1), DRD3 (Mm00432887_m1), GAPDH (Mm99999915_g1). mRNA expression level for each sample was calculated using 2-ΔCt, where Ct was the cycle threshold for each reaction, ΔCt = Ct (gene of interest) – Ct (GAPDH) (Schmittgen and Livak, 2008). Fold change was calculated by normalizing the value of each sample to the mean of the control samples.
Fluorescent in situ hybridization (FISH)
Mice were euthanized using isofluorane inhalation, perfused with ice-cold ACSF, and brains were quickly extracted and placed in an isopentane bath chilled with dry ice and 70% EtOH. Coronal slices (20 μm) were sectioned using a cryostat (Leica CM3050S) and mounted on slides. Tissue processing and FISH were performed using the RNAScope Fluorescent Multiplex Assay (Advanced Cell Diagnostics), using the following probes: Drd1a (406491-C2), Drd2 (406501-C3), Drd3 (447721), Slc17a6 (VGluT2, 319171-C2), Slc32a1 (VGAT, 319191-C3), NeuN (Rbfox3) (313311-C2), Chat (408731-C2), Cre recombinase (312281-C2). Slides were imaged using a confocal microscope (Olympus FluoView FV1200). Several images for each anatomical area were acquired at 30X magnification, from several slices/mouse, from at least 2 mice. Quantifications were performed using the ‘cell counter’ function in Fiji (ImageJ). Cells were considered positive for a given marker if a cluster of ≥ 3 puncta surrounded the soma.
Histology and immunohistochemistry
Mice were perfused intracardially with 4% paraformaldehyde/PBS and post-fixed at 4°C overnight. For validation of GCaMP6f expression, 10 mM CaCl2 was added to the perfusion solution to activate the sensor and thereby improve visualization for imaging. Coronal sections (60μm) were sliced using a vibratome (Leica VT1000). Samples that were not immunostained were mounted directly on slides, with a DAPI counterstain. For immunohistochemistry, free-floating sections were incubated with primary antibody overnight in a PBS solution containing 10% horse serum, 0.2% bovine serum albumin, 0.5% Triton X-100, at 4°C. Sections were then washed in PBS, incubated with Alexa Fluor-conjugated secondary antibodies (1:500, Thermo Fisher Scientific) for 1hr at room temperature, washed in PBS, and then mounted on slides with DAPI counterstain medium (Fluoromount-G, Thermo Fisher Scientific). Primary antibodies were: anti-GFP to visualize GRABDA2m expression (1:2000, #A10262, Thermo Fisher Scientific), and anti-c-Fos (1:5000, #2550, Cell Signaling Technology). Slides were imaged using an automated slide scanner (Olympus VS120) or a confocal microscope (Olympus FluoView FV1200). C-Fos images were quantified by applying equal thresholds to all images and using the ‘analyze particles’ function in ImageJ. The location of viral expression and cannula implant tracts were examined for all mice following the completion of behavioral experiments; mice with off-target expression and/or off-target implant tip location were excluded from final analyses.
QUANTIFICATION AND STATISTICAL ANALYSIS
Fiber photometry
Cocaine self-administration and CPP experiments
Signals were analyzed with Clampfit 10.4 (Molecular Devices) and MATLAB R2015b (MathWorks), and plotted with MATLAB. For analyses of GCaMP6f and GRABDA2m comparing event frequency between Baseline (Day 0) and Seeking Test (Day 24) sessions, each animal performed only 1 trial for each of the two sessions, and signals from the entire duration of the sessions were analyzed as follows: (i) raw signals were adjusted to have a flat baseline using Clampfit, in a manner blind to the experimental condition; (ii) the baseline-adjusted signal was then converted to ΔF/F by dividing it by the mean of its raw signal; (iii) a value of 4 times the mean absolute deviation (4*MAD) of the Baseline (Day 0) session was used as a threshold for detecting events in both the Baseline (Day 0) and Seeking Test (Day 24) traces, with a value of 95% of 4*MAD used to re-arm event detection. The same approach was used to analyze data from CPP experiments comparing the habituation session with CPP Tests 1 and 2. For plots of the difference in total # of GCaMP6f or GRABDA2m thresholded events between the Seeking and Baseline sessions (Figures S3D and S5I), the total # of thresholded events in the Baseline session was estimated by doubling the # of quantified events, to account for the fact that Baseline sessions were only 10 min long whereas Seeking Test sessions were 20 min long. For analyses of GCaMP6f event frequency change within the same session (i.e. in response to cocaine infusion), events were detected as above, and the event frequency during the 2 min after cocaine infusion was compared to the event frequency during the 2 min preceding cocaine infusion. Analyses of axon-GCaMP6s signals were performed in a similar manner to GCaMP6f analyses, but ΔF/F values were obtained by normalizing the green emission signal (axon-GCaMP6s, Ca2+-dependent) to the simultaneously-acquired red emission signal (mRuby3, Ca2+-independent), which served to minimize the impact of motion artifacts. Analysis of axon-GCaMP6s signals was performed by normalizing the data for each group to Day 0, in order to account for any initial differences in event frequency between VP DRD3+ terminals in the LHb versus the VTA.
GRABDA2m responses to cocaine infusion, as well as GCaMP6f and GRABDA2m nose poke-related events during the Seeking Test (Day 24), were analyzed by focusing on signal epochs time-locked to nose pokes, using custom MATLAB scripts. Nose poke and infusion time stamps recorded by MED-PC were used to extract epochs (i.e. nose poke-related events) from the ΔF/F photometry signal. For GRABDA2m responses to cocaine infusion, 1–2 trials were performed for each mouse; Area Under the Curve (AUC, 0–50sec relative to nose poke time stamp), time to peak, and half-life were determined for each trial, averaged across trials for each mouse, and then analyzed by group, with 1 data point representing each mouse. For nose poke-related events extracted from the Seeking Test, AUC (−1 to +3 sec relative to nose poke time stamp) of GCaMP6f or GRABDA2m signals was determined for all inactive nose pokes, but only for active nose pokes preceded by a period of >30 sec without active nose pokes, in order to avoid ambiguity in the baseline signal leading up to an active nose poke. For the GCaMP6f seeking nose poke-related analyses (Figures S3B, S3C, S7K to S7M), AUC values were averaged for each mouse and then analyzed by group, with 1 data point representing each mouse. For the GRABDA2m seeking nose poke-related analyses, AUC values were either collapsed by subject as for GCaMP6f analyses (Figures S5B, S5C, S5F, S5G), or pooled from all subjects (Figures S5D, S5H) and statistically analyzed using analysis of covariance (ANCOVA) with subject ID as a covariate. Furthermore, this data was separated into the 1st 50% and the 2nd 50% of active nose pokes performed during the seeking session, in order to assess the impact of cocaine omission on DA release.
GRABDA photometry with optogenetic stimulation
ΔF/F traces were obtained by normalizing the 470 nm (DA-dependent) signal to the 405 nm (DA-independent) signal. Custom MATLAB scripts were used to extract the stimulation and pre-stimulation epochs, and quantify AUC across trials and subjects. AUC was quantified instead of threshold-based event frequency because the predominant change observed was a shift in the signal baseline. Each optogenetic stimulation epoch lasted 50 sec and its AUC was compared to the AUC of the 50 sec epoch immediately preceding stimulation. AUC values were either collapsed by subject as for GCaMP6f analyses (Figures S7D, S7E), or pooled from all subjects (Figures 5C, 5H) and statistically analyzed using ANCOVA with subject ID as a covariate.
Inputs to VP DRD3+ neurons
Sample fixation and slides were processed as described above in the histology section. Coronal sections were quantified at 120 μm intervals, approximately between AP +2.96 and −4.24 mm (Franklin and Paxinos, 2008). GFP-expressing inputs were counted using the ‘cell counter’ function in Fiji (ImageJ), and data were calculated as a percentage of the total number of GFP-expressing cells observed brain-wide. Inputs located around the VP injection site were not included in the total number of inputs. Anatomical regions that accounted for <1% of total inputs were not presented in the graph.
VP DRD3+ axonal projections collateralization
Sample fixation and slides were processed as described above in the histology section. Coronal sections were quantified at ~120 μm intervals using ImageJ by applying equal thresholds to all images and measuring the thresholded area contained in a 250 × 250 pixel zone for each target. In this manner, the density of EGFP-expressing fibers could be assessed for each major VP DRD3+ anatomical target. Data were presented as a percentage of the thresholded area observed in the LHb or VTA (i.e. the site of RG-EIAV-DIO-Flp injection).
Statistics
Statistical details are located in the figure legends and Table S2. Statistical analyses were performed with GraphPad Prism 6 or MATLAB R2015b. Final samples sizes were estimated using power analysis for a subset of behavioral experiments. Comparisons between two groups with normally distributed data were made using the two-tailed, unpaired Student’s t-test. To compare two groups with non-normal distributions or in cases where sample size was too small to determine normality, the MannWhitney test was used for unpaired data, and the Wilcoxon signed-rank test was used for paired data. The D’Agostino-Pearson test or the Shapiro-Wilk test were used to assess distribution normality. Analysis of covariance (ANCOVA) was performed for a subset of fiber photometry analyses in which events or trials were pooled from all subjects in order to increase statistical power; the ‘separate lines’ model of the ‘aoctool’ function in MATLAB was used, with subject ID (mouse identity) as a covariate. Differences across more than two groups were analyzed with either: (1) a one-way analysis of variance (ANOVA) for data with normal distribution and one independent variable; (2) the Kruskal-Wallis test for data with non-normal distribution and one independent variable; (3) a two-way ANOVA for data with two independent variables; or (4) a two-way repeated measures (RM) ANOVA for data with two independent variables and multiple measurements from the same subject. ANOVAs were followed by post-hoc tests with multiple comparisons correction, as indicated in the figure legends. Statistical outliers were identified using the ROUT test (Motulsky and Brown, 2006) and removed from analyses. All data are presented as mean ± standard error of the mean (SEM).
Supplementary Material
Table S2. Detailed statistical information. Related to Figures 1, 2, 3, 4, 5, 6, 7, S1, S3, S4, S5, S7.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-GFP | Thermo Fisher Scientific | A110262; RRID: AB_2534023 |
| anti-c-Fos | Cell Signaling Technology | 2250; RRID: AB_2247211 |
| Bacterial and Virus Strains | ||
| EnvA-pseudotyped, glycoprotein-deleted rabies virus; EnvA-RVΔG-eGFP | Byung Kook Lim Lab | N/A |
| Pseudotyped equine infectious anemia virus; RG-EIAV-DIO-Flp and RG-EIAV-Cre | Byung Kook Lim Lab | N/A |
| Adeno-associated virus-DJ; various | Byung Kook Lim Lab | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Clozapine N-oxide | Enzo Life Sciences | BML-NS105-0025 |
| NBQX | Tocris | 0373 |
| QX-314 | Tocris | 2313 |
| Picrotoxin | Sigma | P1675 |
| Cocaine hydrochloride | Sigma | C5776 |
| Heparin | BD | 306424 |
| Dustless precision pellets, sugar | Bio-Serv | F05550 |
| Tetrodotoxin | Tocris | 1078 |
| 4-Aminopyridine | Sigma | 275875 |
| Red retrobeads | Lumafluor | R170 |
| BAPTA, Tetracesium Salt, cell impermeant | Thermo Fisher Scientific | B1212 |
| Fluoromount-G | Thermo Fisher Scientific | 00-4959-52 |
| Critical Commercial Assays | ||
| Hybrid-R RNA purification kit | GeneAll | |
| SuperScript IV First-strand cDNA synthesis kit | Thermo Fisher Scientific | 18091200 |
| TaqMan Universal Master Mix II, with UNG | Thermo Fisher Scientific | 4440038 |
| RNAscope Fluorescent Multiplex Reagent Kit | Advanced Cell Diagnostics | 320850 |
| Taqman probe: DRD1 | Mm02620146_s1 | |
| Taqman probe: DRD2 | Thermo Fisher Scientific | Mm00438545_m1 |
| Taqman probe: DRD3 | Thermo Fisher Scientific | Mm00432887_m1 |
| Taqman probe: GAPDH | Thermo Fisher Scientific | Mm99999915_g1 |
| RNAscope probe: DRD1a | Advanced Cell Diagnostics | 406491-C2 |
| RNAscope probe: DRD2 | Advanced Cell Diagnostics | 406501-C3 |
| RNAscope probe: DRD3 | Advanced Cell Diagnostics | 447721 |
| RNAscope probe: SLC17A6 | Advanced Cell Diagnostics | 319171-C2 |
| RNAscope probe: SLC32A1 | Advanced Cell Diagnostics | 319191-C3 |
| RNAscope probe: RBFOX3 | Advanced Cell Diagnostics | 313311-C2 |
| RNAscope probe: CHAT | Advanced Cell Diagnostics | 408731-C2 |
| RNAscope probe: CRE recombinase | Advanced Cell Diagnostics | 312281-C2 |
| Experimental Models: Cell Lines | ||
| AAV-293 cells | Agilent | 240073 |
| HEK293T cells | ATCC | CRL-3216 |
| B7GG cells | Ed Callaway Lab; (Osakada and Callaway, 2013) | N/A |
| BHK-EnvA cells | Ed Callaway Lab; (Osakada and Callaway, 2013) | N/A |
| Experimental Models: Organisms/Strains | ||
| Mouse: DRD3-Cre: B6.FVB(Cg)-Tg(Drd3-cre)KI196Gsat/Mmucd | GENSAT | Stock#: 036968-UCD; RRID:MMRRC_036968-UCD |
| Mouse: C57BL/6J | The Jackson Laboratory | JAX: 000664; RRID: IMSR_JAX:000664 |
| Oligonucleotides | ||
| DRD3 shRNA targeting sequence: 5’- TTCTTCTTGACTCACGTTCTT-3’ | (Shin et al., 2018) | N/A |
| Single cell RT-PCR primers | See Table S1 | N/A |
| Recombinant DNA | ||
| pAAV-EF1α-EGFP | (Shin et al., 2018) | N/A |
| pAAV-EF1α-EmGFP-DRD3 shRNA | N/A | |
| pAAV-EF1α-DRD3(T1182G)-EmGFP-DRD3 shRNA | (Shin et al., 2018) | N/A |
| pAAV-EF1α-DIO-EGFP | (Shin et al., 2018) | N/A |
| pAAV-EF1α-DIO-EmGFP-DRD3 shRNA | (Shin et al., 2018) | N/A |
| pAAV-EF1α-DIO-GCaMP6f | (Shin et al., 2018) | N/A |
| pAAV-EF1α-mCherry-DRD3 shRNA | This paper | N/A |
| pAAV-EF1α-DIO-GCaMP6f-DRD3 shRNA | This paper | N/A |
| pAAV-hSyn-GRABDA2m | (Sun et al., 2020a) | N/A |
| pAAV-EF1α-fDIO-EGFP | (Knowland et al., 2017) | N/A |
| pAAV-EF1α-fDIO-mRuby2-TVA | (Knowland et al., 2017) | N/A |
| pAAV-EF1α-fDIO-oPBG | (Knowland et al., 2017) | N/A |
| pAAV-EF1α-DIO-mCherry | (Knowland et al., 2017) | N/A |
| pAAV-hSyn-DIO-hM4D(Gi)-mCherry | (Krashes et al., 2011) | Addgene plasmid # 44362 |
| pAAV-sSyn-DIO-mRuby2-T2A-Synaptophysin-eGFP | (Knowland et al., 2017) | N/A |
| pAAV-EF1α-DIO-hChR2(H134R)-EYFP | Gift from Karl Deisseroth | Addgene plasmid # 20298 |
| pAAV-EF1α-DIO-eNpHR3.0-EYFP | (Gradinaru et al., 2010) | Addgene plasmid # 26966 |
| pAAV-hSyn-FLEX-axon-GCaMP6s-P2A-mRuby3 | (Broussard et al., 2018) | Addgene plasmid # 112008 |
| Software and Algorithms | ||
| Illustrator CS4 | Adobe | https://www.adobe.com/ |
| Photoshop CS4 | Adobe | https://www.adobe.com/ |
| GraphPad Prism 6 | GraphPad Software | |
| MATLAB R2015b | Mathworks | https://www.mathworks.com/products/matlab.html |
| FIJI (ImageJ) | NIH | https://imagej.net/Welcome |
| Viewer II | Biobserve | http://www.biobserve.com/behavioralresearch/products/viewer/ |
| EthoVision XT 13 | Noldus | https://www.noldus.com/ |
| Clampfit 10.4 | Molecular Devices | https://www.moleculardevices.com/products/axon-patchclamp-system/acquisition-and-analysis-software/pclampsoftware-suite |
| Clampex 10.4 | https://www.moleculardevices.com/products/axon-patchclamp-system/acquisition-and-analysis-software/pclampsoftware-suite | |
| MATLAB scripts for analyzing photometry data | This paper | https://github.com/VP-DRD3/Photometry-Matlab-Code |
| MED-PC IV | Med Associates Inc | https://www.med-associates.com/tag/med-pc-iv/ |
| MED-PC IV code for operant behaviors | This paper | https://github.com/VP-DRD3/MED-PC-Code |
| pyPhotometry | (Akam and Walton, 2019) | https://pyphotometry.readthedocs.io/en/latest/userguide/graphical-user-interface/ |
| OTPG8 TTL pulse generator | Doric Lenses | https://neuro.doriclenses.com/products/doricneuroscience-studio |
| HCImage | Hamamatsu | https://hcimage.com/ |
| Other | ||
| Mouse vascular access button | Instech Laboratories | VAB62BS/22 |
| Mouse jugular vein catheter | Access Technologies | AT-MJVC-0612A |
| Mono fiber-optic cannulae for fiber photometry | Doric Lenses | MFC_400/430-0.48-[length]mm_MF1.25_FLT |
| Dual fiber-optic cannulae for optogenetic stimulation | Doric Lenses | DFC_200/240-0.22_[length]mm_GS[spacing]_FLT |
| Bilateral infusions: guide cannulae | InVivo1 (P1 Technologies) | C235GS-5-[spacing]/SPC GUIDE 26GA DBL (5MM PED) |
| Bilateral infusions: infusion cannulae | InVivo1 (P1 Technologies) | C235IS-5/SPC INTERNAL 33GA DBL |
| Bilateral infusions: dummy cannulae | InVivo1 (P1 Technologies) | C235DCS-5/SPC DUMMY DBL |
| Infusion cannulae dust caps | InVivo1 (P1 Technologies) | 303DC/1 DUST CAP |
Highlights.
DRD3-mediated plasticity in the VP drives post-abstinence cocaine seeking behavior
VP DRD3 signaling regulates dopamine release in the NAc latSh during seeking
VP DRD3+ projections to the LHb and VTA display differing activity during seeking
DRD3 signaling/activity of LHb-projecting VP DRD3+ neurons drives seeking behavior
ACKNOWLEDGEMENTS
We thank Dr. Shannan McClain for technical assistance, Dr. Daniel Knowland for helpful discussions, Dr. Sung Han for assistance with pyPhotometry, and Dr. Adrian Lozada and Dr. Christie Fowler for advice on jugular vein catheter surgeries and self-administration protocols. This work was supported by NIH grants R01MH108594, R01DA049787, and U01MH114829. H.P was supported by a Canadian Institutes of Health Research (CIHR) postdoctoral fellowship. S.S. was supported by a Tobacco-related disease research program (TRDRP) postdoctoral fellowship. E.H.W. was supported by an NIH T32 training grant and an American Heart Association Predoctoral Fellowship.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing intere
SUPPLEMENTAL INFORMATION
Supplemental information consists of Figures S1–S8 and Tables S1–2.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adam JM., Pe H., Sandova DA., Seele RJ., Chan RB., Liberle SD., and Olso DP. (2018). Liraglutide Modulates Appetite and Body Weight Through Glucagon-Like Peptide 1 Receptor-Expressing Glutamatergic Neurons. Diabetes 67, 1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrover MF, Shin JH, and Alvarez VA (2014). Glutamate and dopamine transmission from midbrain dopamine neurons share similar release properties but are differentially affected by cocaine. J Neurosci 34, 3183–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens AM, Meyer PJ, Ferguson LM, Robinson TE, and Aldridge JW (2016). Neural Activity in the Ventral Pallidum Encodes Variation in the Incentive Value of a Reward Cue. J Neurosci 36, 7957–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akam T, and Walton ME (2019). pyPhotometry: Open source Python based hardware and software for fiber photometry data acquisition. Sci Rep 9, 3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, and Heidbreder CA (2003). Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology 28, 1272–1280. [DOI] [PubMed] [Google Scholar]
- Araki M, McGeer PL, and Kimura H. (1988). The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res 441, 319–330. [DOI] [PubMed] [Google Scholar]
- Bahi A, Boyer F, Bussard G, and Dreyer JL (2005). Silencing dopamine D3-receptors in the nucleus accumbens shell in vivo induces changes in cocaine-induced hyperlocomotion. Eur J Neurosci 21, 3415–3426. [DOI] [PubMed] [Google Scholar]
- Baik JH (2013). Dopamine signaling in reward-related behaviors. Front Neural Circuits 7, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Sesack SR, Georges F, Pistis M, Hong S, and Jhou TC (2012). Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. J Neurosci 32, 14094–14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM, Wooten GF, and Trugman JM (1988). Distribution of D1 and D2 dopamine receptors in the basal ganglia of the cat determined by quantitative autoradiography. J Comp Neurol 268, 131–145. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, Wilkins D, Selby P, George TP, Zack M, et al. (2012). Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydronaphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci 32, 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, and Shaham Y. (2013). The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 229, 453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, and Shaham Y. (2007). Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci 27, 12655–12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinschwitz K, Dittgen A, Madai VI, Lommel R, Geisler S, and Veh RW (2010). Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience 168, 463–476. [DOI] [PubMed] [Google Scholar]
- Broussard GJ, Liang Y, Fridman M, Unger EK, Meng G, Xiao X, Ji N, Petreanu L, and Tian L. (2018). In vivo measurement of afferent activity with axon-specific calcium imaging. Nat Neurosci 21, 12721280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Palacorolla H, Brady D, Riegger K, Elmer GI, and Shepard PD (2017). Habenula-Induced Inhibition of Midbrain Dopamine Neurons Is Diminished by Lesions of the Rostromedial Tegmental Nucleus. J Neurosci 37, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Pena CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, et al. (2016). In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci U S A 113, 2726–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES., Juarez B., Morel C., Walker DM., Cahill ME., Ribeiro E., Roman-Ortiz C., Ramakrishnan C., Deisseroth K., Han MH., et al. (2017). Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun 8, 13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla M, Shegda M, Ramesh B, Harrison NL, and Kellendonk C. (2012). Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels. J Neurosci 32, 2398–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin A, and Callaway EM (2014). Optical control of retrogradely infected neurons using drug-regulated “TLoop” lentiviral vectors. J Neurophysiol 111, 2150–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Kittler JT, Moss SJ, and Yan Z. (2006). Dopamine D3 receptors regulate GABAA receptor function through a phospho-dependent endocytosis mechanism in nucleus accumbens. J Neurosci 26, 25132521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Song R, Yang RF, Wu N, and Li J. (2014). A novel dopamine D3 receptor antagonist YQA14 inhibits methamphetamine self-administration and relapse to drug-seeking behaviour in rats. Eur J Pharmacol 743, 126–132. [DOI] [PubMed] [Google Scholar]
- Clark M, and Bracci E. (2018). Dichotomous Dopaminergic Control of Ventral Pallidum Neurons. Front Cell Neurosci 12, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras PC, Quirion R, Gehlert DR, Contreras ML, and O’Donohue TL (1987). Autoradiographic distribution of non-dopaminergic binding sites labeled by [3H]haloperidol in rat brain. Neurosci Lett 75, 133140. [DOI] [PubMed] [Google Scholar]
- Creed M, Ntamati NR, Chandra R, Lobo MK, and Luscher C. (2016). Convergence of Reinforcing and Anhedonic Cocaine Effects in the Ventral Pallidum. Neuron 92, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, and Shaham Y. (2002). Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology 27, 1006–1015. [DOI] [PubMed] [Google Scholar]
- de Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim CK, Tian L, Deisseroth K, and Lammel S. (2019). A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron 101, 133–151 e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del-Fava F, Hasue RH, Ferreira JG, and Shammah-Lagnado SJ (2007). Efferent connections of the rostral linear nucleus of the ventral tegmental area in the rat. Neuroscience 145, 1059–1076. [DOI] [PubMed] [Google Scholar]
- Dong Y, Taylor JR, Wolf ME, and Shaham Y. (2017). Circuit and Synaptic Plasticity Mechanisms of Drug Relapse. J Neurosci 37, 10867–10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Hoffmann J, and Nicola SM (2014). Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J Neurosci 34, 14349–14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faget L., Zell V., Souter E., McPherson A., Ressler R., Gutierrez-Reed N., Yoo JH., Dulcis D., and Hnasko TS. (2018). Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum. Nat Commun 9, 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MR, Ruiz CM, Castillo E, Faget L, Khanbijian C, Liu S, Schoch H, Rojas G, Huerta MY, Hnasko TS, et al. (2019). Ventral pallidum is essential for cocaine relapse after voluntary abstinence in rats. Neuropsychopharmacology 44, 2174–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MR, Schoch H, and Mahler SV (2018). Modeling cocaine relapse in rodents: Behavioral considerations and circuit mechanisms. Prog Neuropsychopharmacol Biol Psychiatry 87, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KB, and Paxinos G. (2008). The Mouse Brain in Stereotaxic Coordinates, Third Edition. Academic Press, 1–350. [Google Scholar]
- Freeman WM, Lull ME, Patel KM, Brucklacher RM, Morgan D, Roberts DC, and Vrana KE (2010). Gene expression changes in the medial prefrontal cortex and nucleus accumbens following abstinence from cocaine self-administration. BMC Neurosci 11, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto A, Hori Y, Nagai Y, Kikuchi E, Oyama K, Suhara T, and Minamimoto T. (2019). Signaling Incentive and Drive in the Primate Ventral Pallidum for Motivational Control of Goal-Directed Action. J Neurosci 39, 1793–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj E, Ewing S, and Ranaldi R. (2018). Dopamine D1 and D3 receptor polypharmacology as a potential treatment approach for substance use disorder. Neurosci Biobehav Rev 89, 13–28. [DOI] [PubMed] [Google Scholar]
- Galaj E, Manuszak M, Babic S, Ananthan S, and Ranaldi R. (2015). The selective dopamine D3 receptor antagonist, SR 21502, reduces cue-induced reinstatement of heroin seeking and heroin conditioned place preference in rats. Drug Alcohol Depend 156, 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Neill D, and Justice JB Jr. (1996). Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Res 707, 64–74. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill D, and Justice JB Jr. (1997). 6-Hydroxydopamine lesion of ventral pallidum blocks acquisition of place preference conditioning to cocaine. Brain Res 754, 103–112. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, and Deisseroth K. (2010). Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor NZ, Yamamoto R, and Pare D. (2018). Glutamatergic and gabaergic ventral BNST neurons differ in their physiological properties and responsiveness to noradrenaline. Neuropsychopharmacology 43, 2126–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, and Joyce JN (1999). Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology 20, 60–80. [DOI] [PubMed] [Google Scholar]
- Hamid AA., Pettibone JR., Mabrouk OS., Hetrick VL., Schmidt R., Vander Weele CM., Kennedy RT., Aragona BJ., and Berke JD. (2016). Mesolimbic dopamine signals the value of work. Nat Neurosci 19, 117126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek JA, Bobadilla AC, Dereschewitz E, Assali A, Chalhoub RM, Cowan CW, and Kalivas PW (2020). Opposing Regulation of Cocaine Seeking by Glutamate and GABA Neurons in the Ventral Pallidum. Cell Rep 30, 2018–2027 e2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek JA, Neuhofer DN, Griffin WC 3rd, Siegel GS, Bobadilla AC, Kupchik YM, and Kalivas PW (2017). Loss of Plasticity in the D2-Accumbens Pallidal Pathway Promotes Cocaine Seeking. J Neurosci 37, 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, and Hikosaka O. (2011). Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci 31, 11457–11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Yang C, and Tan A. (2015). Basal ganglia circuit loops, dopamine and motivation: A review and enquiry. Behav Brain Res 290, 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, and Shepard PD (2007). Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci 27, 6923–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitenick MA, Deutch AY, Churchill L, and Kalivas PW (1992). Topography and functional role of dopaminergic projections from the ventral mesencephalic tegmentum to the ventral pallidum. Neuroscience 50, 371–386. [DOI] [PubMed] [Google Scholar]
- Knowland D, Lilascharoen V, Pacia CP, Shin S, Wang EH, and Lim BK (2017). Distinct Ventral Pallidal Neural Populations Mediate Separate Symptoms of Depression. Cell 170, 284–297 e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, and Lowell BB (2011). Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121, 1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Scofield MD, Rice KC, Cheng K, Roques BP, and Kalivas PW (2014). Cocaine dysregulates opioid gating of GABA neurotransmission in the ventral pallidum. J Neurosci 34, 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, and Malenka RC (2012). Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, and Sokoloff P. (2005). A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. Neuroreport 16, 175–178. [DOI] [PubMed] [Google Scholar]
- Lilascharoen V, Wang EH, Do N, Pate SC, Tran AN, Yoon CD, Choi JH, Wang XY, Pribiag H, Park YG, et al. (2021). Divergent pallidal pathways underlying distinct Parkinsonian behavioral deficits. Nat Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, and Malenka RC (2012). Anhedonia requires MC4Rmediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jean-Richard-Dit-Bressel P., Yau JO., Willing A., Prasad AA., Power JM., Killcross S., Clifford CWG., and McNally GP. (2020). The Mesolimbic Dopamine Activity Signatures of Relapse to Alcohol-Seeking. J Neurosci 40, 6409–6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, and Malenka RC (2011). Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69, 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Brodnik ZD, Cox BM, Buchta WC, Bentzley BS, Quintanilla J, Cope ZA, Lin EC, Riedy MD, Scofield MD, et al. (2019). Chemogenetic Manipulations of Ventral Tegmental Area Dopamine Neurons Reveal Multifaceted Roles in Cocaine Abuse. J Neurosci 39, 503–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, and Aston-Jones G. (2014). Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci 17, 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Bunzow JR, Civelli O, Akil H, and Watson SJ (1990). Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J Neurosci 10, 2587–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski-Cobuzzi RJ, and Napier TC (1994). Activation of dopaminergic neurons modulates ventral pallidal responses evoked by amygdala stimulation. Neuroscience 62, 1103–1119. [DOI] [PubMed] [Google Scholar]
- Massart R, Barnea R, Dikshtein Y, Suderman M, Meir O, Hallett M, Kennedy P, Nestler EJ, Szyf M, and Yadid G. (2015). Role of DNA methylation in the nucleus accumbens in incubation of cocaine craving. J Neurosci 35, 8042–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, and Kalivas PW (2001). The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21, 8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengual E, and Pickel VM (2002). Ultrastructural immunocytochemical localization of the dopamine D2 receptor and tyrosine hydroxylase in the rat ventral pallidum. Synapse 43, 151–162. [DOI] [PubMed] [Google Scholar]
- Mitrovic I, and Napier TC (2002). Mu and kappa opioid agonists modulate ventral tegmental area input to the ventral pallidum. Eur J Neurosci 15, 257–268. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ, and Brown RE (2006). Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 7, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, Tran-Nguyen LT, Weber SM, Coffey GP, and Joyce JN (2004). Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharmacology 29, 14791487. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, and Luscher C. (2019). The Molecular Basis of Drug Addiction: Linking Epigenetic to Synaptic and Circuit Mechanisms. Neuron 102, 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, and Callaway EM (2013). Design and generation of recombinant rabies virus vectors. Nat Protoc 8, 1583–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenheimer DJ, Bari BA, Sutlief E, Fraser KM, Kim TH, Richard JM, Cohen JY, and Janak PH (2020). A quantitative reward prediction error signal in the ventral pallidum. Nat Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathanou M, Dumas S, Pettersson H, Olson L, and Wallen-Mackenzie A. (2019). Off-Target Effects in Transgenic Mice: Characterization of Dopamine Transporter (DAT)-Cre Transgenic Mouse Lines Exposes Multiple Non-Dopaminergic Neuronal Clusters Available for Selective Targeting within Limbic Neurocircuitry. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Garcia TR, Garcia-Keller C, Penaloza T, Richie CT, Pickel J, Hope BT, Harvey BK, Kalivas PW, and Heinsbroek JA (2019). Ventral Pallidum Is the Primary Target for Accumbens D1 Projections Driving Cocaine Seeking. J Neurosci 39, 2041–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE., Behzadi A., Kish SJ., Houle S., Wilson AA., Rusjan PM., Tong J., Selby P., George TP., McCluskey T., et al. (2014). Heightened D3 dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [11C]-+-PHNO. Neuropsychopharmacology 39, 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Minier-Toribio A, Hoots JK, Bossert JM, and Shaham Y. (2018). Opposite Effects of Basolateral Amygdala Inactivation on Context-Induced Relapse to Cocaine Seeking after Extinction versus Punishment. J Neurosci 38, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, and Svoboda K. (2009). The subcellular organization of neocortical excitatory connections. Nature 457, 1142–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, and Carelli RM (2003). Subsecond dopamine release promotes cocaine seeking. Nature 422, 614–618. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, and Shaham Y. (2011). Neurobiology of the incubation of drug craving. Trends Neurosci 34, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AA, and McNally GP (2016). Ventral Pallidum Output Pathways in Context-Induced Reinstatement of Alcohol Seeking. J Neurosci 36, 11716–11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AA, Xie C, Chaichim C, Nguyen JH, McClusky HE, Killcross S, Power JM, and McNally GP (2020). Complementary Roles for Ventral Pallidum Cell Types and Their Projections in Relapse. J Neurosci 40, 880–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, and Shaham Y. (2019). Relapse to opioid seeking in rat models: behavior, pharmacology and circuits. Neuropsychopharmacology 44, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Ambroggi F, Janak PH, and Fields HL (2016). Ventral Pallidum Neurons Encode Incentive Value and Promote Cue-Elicited Instrumental Actions. Neuron 90, 1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, and Young AB (1989). Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience 30, 767–777. [DOI] [PubMed] [Google Scholar]
- Richtand NM (2006). Behavioral sensitization, alternative splicing, and d3 dopamine receptor-mediated inhibitory function. Neuropsychopharmacology 31, 2368–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SW, Jarvie KR, and Caron MG (1994). High affinity agonist binding to the dopamine D3 receptor: chimeric receptors delineate a role for intracellular domains. Mol Pharmacol 46, 352–356. [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, and Carelli RM (2004). Dopamine operates as a subsecond modulator of food seeking. J Neurosci 24, 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Fabbricatore AT, Pawlak AP, Barker DJ, Ma S, and West MO (2012). Slow phasic and tonic activity of ventral pallidal neurons during cocaine self-administration. Synapse 66, 106–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Melendez RI, Zaborszky L, and Napier TC (2015). The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol 130, 29–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, and Correa M. (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron 76, 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, and Robinson TE (2013). Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci 33, 13989–14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, and Livak KJ (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Schultz W. (2016). Dopamine reward prediction-error signalling: a two-component response. Nat Rev Neurosci 17, 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Pribiag H, Lilascharoen V, Knowland D, Wang XY, and Lim BK (2018). Drd3 Signaling in the Lateral Septum Mediates Early Life Stress-Induced Social Dysfunction. Neuron 97, 195–208 e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore GM., Co C., and Smith JE. (2000). Ventral pallidal extracellular fluid levels of dopamine, serotonin, gamma amino butyric acid, and glutamate during cocaine self-administration in rats. Psychopharmacology (Berl) 150, 391–398. [DOI] [PubMed] [Google Scholar]
- Smedley EB, DiLeo A, and Smith KS (2019). Circuit directionality for motivation: Lateral accumbens-pallidum, but not pallidum-accumbens, connections regulate motivational attraction to reward cues. Neurobiol Learn Mem 162, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, and Berridge KC (2009). Ventral pallidum roles in reward and motivation. Behav Brain Res 196, 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, and Schwartz JC (1990). Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347, 146–151. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, and Le Foll B. (2017). The dopamine D3 receptor, a quarter century later. Eur J Neurosci 45, 2–19. [DOI] [PubMed] [Google Scholar]
- Solecki W, Wilczkowski M, Pradel K, Karwowska K, Kielbinski M, Drwiega G, Zajda K, Blasiak T, Soltys Z, Rajfur Z, et al. (2019). Effects of brief inhibition of the ventral tegmental area dopamine neurons on the cocaine seeking during abstinence. Addict Biol, e12826. [DOI] [PubMed] [Google Scholar]
- Song R, Bi GH, Zhang HY, Yang RF, Gardner EL, Li J, and Xi ZX (2014). Blockade of D3 receptors by YQA14 inhibits cocaine’s rewarding effects and relapse to drug-seeking behavior in rats. Neuropharmacology 77, 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, and Mash DC (1996). Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci 16, 6100–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Artymyshyn RP, Kung MP, Kung HF, Lucki I, and McGonigle P. (2000). Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [(125)I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther 295, 1223–1231. [PubMed] [Google Scholar]
- Stephenson-Jones M, Bravo-Rivera C, Ahrens S, Furlan A, Xiao X, Fernandes-Henriques C, and Li B. (2020). Opposing Contributions of GABAergic and Glutamatergic Ventral Pallidal Neurons to Motivational Behaviors. Neuron 105, 921–933 e925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout KA., Dunn AR., Lohr KM., Alter SP., Cliburn RA., Guillot TS., and Miller GW. (2016). Selective Enhancement of Dopamine Release in the Ventral Pallidum of Methamphetamine-Sensitized Mice. ACS Chem Neurosci 7, 1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Zhou J, Dai B, Qian T, Zeng J, Li X, Zhuo Y, Zhang Y, Tan K, Feng J, et al. (2020a). New and improved GRAB fluorescent sensors for monitoring dopaminergic activity in vivo. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Zhou J, Dai B, Qian T, Zeng J, Li X, Zhuo Y, Zhang Y, Wang Y, Qian C, et al. (2020b). Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat Methods 17, 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swant J, Stramiello M, and Wagner JJ (2008). Postsynaptic dopamine D3 receptor modulation of evoked IPSCs via GABA(A) receptor endocytosis in rat hippocampus. Hippocampus 18, 492–502. [DOI] [PubMed] [Google Scholar]
- Tachibana Y, and Hikosaka O. (2012). The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron 76, 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, and Picciotto MR (2014). GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J Comp Neurol 522, 3308–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, and Gunn RN (2011). Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage 54, 264–277. [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, and Shaham Y. (2016). Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res 224, 25–52. [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, Cifani C, Marchant NJ, Yizhar O, Bossert JM, et al. (2017). The Anterior Insular Cortex-->Central Amygdala Glutamatergic Pathway Is Critical to Relapse after Contingency Management. Neuron 96, 414–427 e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, and Boyle M. (2018). Neuroscience of Addiction: Relevance to Prevention and Treatment. Am J Psychiatry 175, 729–740. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR Jr., Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, and Gardner EL (2002). Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci 22, 9595–9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Cates HM, Loh YE, Purushothaman I, Ramakrishnan A, Cahill KM, Lardner CK, Godino A, Kronman HG, Rabkin J, et al. (2018). Cocaine Self-administration Alters Transcriptome-wide Responses in the Brain’s Reward Circuitry. Biol Psychiatry 84, 867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warlow SM, Robinson MJF, and Berridge KC (2017). Optogenetic Central Amygdala Stimulation Intensifies and Narrows Motivation for Cocaine. J Neurosci 37, 8330–8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Eshel N, and Uchida N. (2017). Neural Circuitry of Reward Prediction Error. Annu Rev Neurosci 40, 373–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, and Gardner EL (2007). Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev 13, 240–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX., Gilbert JG., Pak AC., Ashby CR Jr., Heidbreder CA., and Gardner EL. (2005). Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progress-iveratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci 21, 34273438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Li X, Li J, Peng XQ, Song R, Gaal J, and Gardner EL (2013). Blockade of dopamine D3 receptors in the nucleus accumbens and central amygdala inhibits incubation of cocaine craving in rats. Addict Biol 18, 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, de Jong JW, Tak Y, Peck J, Bateup HS, and Lammel S. (2018). Nucleus Accumbens Subnuclei Regulate Motivated Behavior via Direct Inhibition and Disinhibition of VTA Dopamine Subpopulations. Neuron 97, 434–449 e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JH, Zell V, Gutierrez-Reed N, Wu J, Ressler R, Shenasa MA, Johnson AB, Fife KH, Faget L, and Hnasko TS (2016). Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat Commun 7, 13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Bi GH, Galaj E, Kumar V, Cao J, Gadiano A, Rais R, Slusher BS, Gardner EL, Xi ZX, et al. (2019). Dopamine D3R antagonist VK4–116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects. Neuropsychopharmacology 44, 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2. Detailed statistical information. Related to Figures 1, 2, 3, 4, 5, 6, 7, S1, S3, S4, S5, S7.
Data Availability Statement
The data and custom code that support the findings from this study are available from the Lead Contact upon request.