Abstract
Disuse osteoporosis describes a state of bone loss due to local skeletal unloading or systemic immobilization. This review will discuss advances in the field that have shed light on clinical observations, mechanistic insights and options for the treatment of disuse osteoporosis. Clinical settings of disuse osteoporosis include spinal cord injury, other neurological and neuromuscular disorders, immobilization after fractures and bed rest (real or modeled). Furthermore, spaceflight-induced bone loss represents a well-known adaptive process to microgravity. Clinical studies have outlined that immobilization leads to immediate bone loss in both the trabecular and cortical compartments accompanied by relatively increased bone resorption and decreased bone formation. The fact that the low bone formation state has been linked to high levels of the osteocyte-secreted protein sclerostin is one of the many findings that has brought matrix-embedded, mechanosensitive osteocytes into focus in the search for mechanistic principles. Previous basic research has primarily involved rodent models based on tail suspension, spaceflight and other immobilization methods, which have underlined the importance of osteocytes in the pathogenesis of disuse osteoporosis. Furthermore, molecular-based in vitro and in vivo approaches have revealed that osteocytes sense mechanical loading through mechanosensors that translate extracellular mechanical signals to intracellular biochemical signals and regulate gene expression. Osteocytic mechanosensors include the osteocyte cytoskeleton and dendritic processes within the lacuno-canalicular system (LCS), ion channels (e.g., Piezo1), extracellular matrix, primary cilia, focal adhesions (integrin-based) and hemichannels and gap junctions (connexin-based). Overall, disuse represents one of the major factors contributing to immediate bone loss and osteoporosis, and alterations in osteocytic pathways appear crucial to the bone loss associated with unloading.
Keywords: Immobilization, Unloading, Bone loss, Microstructure, Osteocyte
Introduction
Skeletal integrity is maintained by the process of bone remodeling, i.e., the balanced removal of old bone matrix by bone-resorbing osteoclasts and the deposition of new bone tissue by bone-forming osteoblasts [1]. Since the early work of Julius Wolff in 1892 [2], it has been well established that the skeleton represents a dynamic tissue undergoing adaptive changes in response to loading to better withstand these loads (“Wolff’s law”). There are several clinical examples of the anabolic response of bone to loading, one of them being the observation that the bone mass is markedly higher in the dominant arm than in the nondominant arm in tennis players [3]. In recent years, a number of essential molecular load-sensation pathways to build the optimal amount of bone required for adequate stability have been uncovered. Osteocytes, which represent terminally differentiated osteoblasts embedded into the bone matrix, have gained research attention due to their ability to sense loading and translate mechanical signals into biochemical signals to orchestrate bone remodeling [4]. The discovery of osteocyte-secreted proteins such as sclerostin has already led to new osteoporosis treatments [5, 6], and more recently, other molecular osteocytic and nonosteocytic proteins and nanostructures involved in the process of mechanosensation, such as the calcium channel Piezo1, were revealed [7–9].
Clinically, disuse represents one of the major risk factors for osteoporosis, a widespread, multifactorial disorder characterized by low bone mass, impaired bone quality and increased risk of fragility fractures [10]. It has been shown that disuse conditions are characterized by an unfavorable combination of high bone resorption and low bone formation [11], leading to immediate bone loss and ultimately to osteoporosis with increased fracture risk [12]. In contrast, skeletal loading leads to an anabolic bone response in terms of increased bone formation [13]. Physical activity represents an effective option to increase bone mineral density (BMD) [14], which is the reason that adequate exercise constitutes the most natural basic treatment for patients with osteoporosis.
The term “disuse osteoporosis” comprises various clinical situations of mechanical unloading or pathological immobilization associated with bone loss, including spinal cord injury [15], neuromuscular diseases [16], bed rest [17], spaceflight [18] and others. Relative unloading has also been discussed as an important factor contributing to the relevant bone loss observed in obese patients after bariatric surgery [19]. Bone loss during disuse or unloading occurs due to a lack of movement and muscular insertions, but it is assumed that lower mechanical stimulation in connection with non-weight-bearing, and especially microgravity, has additional negative effects on skeletal integrity. This is also the reason why bone microstructure studies often compare weight-bearing bones (such as the tibia) with non-weight-bearing bones (such as the radius). The interaction of muscle and bone (“crosstalk”) has opened up a whole new field of science, including both clinical studies on the bone status in sarcopenia in the elderly [20] and, at the basic research level, studies on the interaction of muscle and bone and the role of osteocytes in this relationship [21] (not discussed in detail in this review). This review will focus on recent clinical and molecular advances in understanding the specific effects of unloading on the skeleton. In particular, microstructural changes, mechanistic concepts and options for the treatment of disuse osteoporosis will be discussed.
Bone Loss under Disuse Conditions
Disuse-associated bone loss has been outlined in many studies, which documented the magnitude of bone loss in a variety of disuse conditions early on. Dual-energy X-ray absorptiometry (DXA), regularly performed for the proximal femur and lumbar spine, represents the most established method to quantify the areal BMD and to determine the diagnosis of osteoporosis or low bone mass based on T- and Z-scores, respectively. DXA studies have been widely performed in disuse conditions, with both cross-sectional and longitudinal study designs.
Spinal Cord Injury and Neurological/Neuromuscular Disorders
In patients with spinal cord injury, early studies have demonstrated that bone loss begins shortly after injury, with up to 2–4% bone loss per month in the sublesional skeletal sites during the first year [22]. A peak of increased bone resorption was reported at approximately three months after injury [23, 24]. While a steady bone metabolic state at approximately 60–70% of the original bone mass has been observed after 1.5–2 years [25], other studies have documented the persistence of increased bone resorption markers after similar follow-up periods [24]. While other neurological disorders, such as multiple sclerosis [26] and a variety of pediatric neuromuscular diseases [27, 28], are also associated with disuse-induced bone loss, it is likely that unloading is not the only factor causing skeletal deterioration and that other factors (neuronal, immunological, or vascular) also contribute to the observed bone loss. Nonetheless, the fact that the skeletal sites not affected by disuse (e.g., the upper extremities in spinal cord injury) are not affected by bone loss [24, 29] confirms the key role of the mechanical environment, although it is important to note that non-weight-bearing bones can also generally be affected by disuse-induced bone loss, such as in tetraplegia [29], or in the affected limbs in patients after stroke [30].
Bed Rest
To further address the magnitude and mechanisms of disuse-induced bone loss, a number of bed rest studies have been performed, with different approaches. Most studies also aimed to assess the recovery of bone mass in response to reambulation (reloading). In early studies, a similar amount of bone loss in the lumbar spine and hip of approximately 1% per month was detected in healthy males who had been followed up for 17 weeks [31]. Other bed rest studies in premenopausal women also found that the bone loss in the proximal femur was approximately 1% per month, but the bone loss in the lumbar spine was less severe [32]. It is interesting to note that weight-bearing, but not non-weight-bearing skeletal sites such as the forearm, showed significant bone loss during bed rest [33], which underlines the additional influence of weight-bearing. Our clinical experience in treating patients with skeletal disorders and osteoporosis supports the notion that disuse affects weight-bearing bones more severely than non-weight-bearing bones or the axial skeleton, such as the vertebral column (Fig. 1). Finally, bone loss has also been documented by DXA in elderly patients with disuse conditions, including patients who were bedridden or in a vegetative state, although these studies did not comment on the exact rate of bone loss per unit time [34, 35].
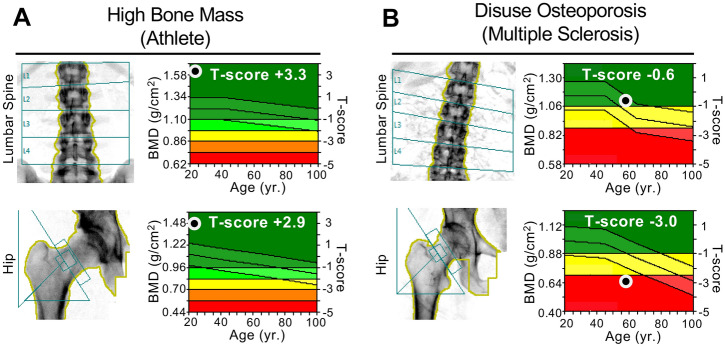
Fig. 1.
Clinical examples of two patients with high vs. low BMD in response to intense physical exercise vs. unloading. a DXA scans of a professional athlete (soccer player) with high BMD values in both the lumbar spine and hip. b DXA scans of an immobilized patient with multiple sclerosis showing low BMD values, which were more severe in the hip than the lumbar spine, meeting the World Health Organization’s (WHO) definition of osteoporosis in the hip (i.e., T-score ≤ − 2.5)
Spaceflight
In addition to bed rest, the loss of BMD has also been well documented in spaceflight studies. Here, the rate of bone loss ranged from 1.07–1.92% per month in the proximal femur and 0.51–0.68% per month in the spine [36], or in other studies, 1.4–1.5% per month in the proximal femur and 0.9% per month in the spine [37]. While microgravity-induced bone loss is positively associated with the duration of spaceflight, the lack of complete recovery or even persistence of bone loss after return to Earth is both unfavorable and not fully understood [18, 38].
Local Disuse Osteoporosis
Disuse osteoporosis includes not only systemic bone loss but also various conditions of local bone loss. In a previous study, magnetic resonance imaging (MRI) was performed in local disuse osteoporosis, where high signal intensity in the respective unloaded limbs was detected, indicating immediate changes following unloading [39]. In a cohort of patients after stroke, the affected lower extremities showed lower mineral and geometric characteristics than the unaffected upper extremities [40]. Rapid unilateral bone loss has been observed in patients after surgery [41] and after experimental unilateral limb suspension [42]. The bone loss observed after periods of reduced loading represents a strong risk factor for subsequent fractures [43]. These unilateral changes can be underlined by clinical examples, such as bone loss that is readily visible on CT images and measurable by DXA after fracture with subsequent immobilization (Fig. 2).
Fig. 2.
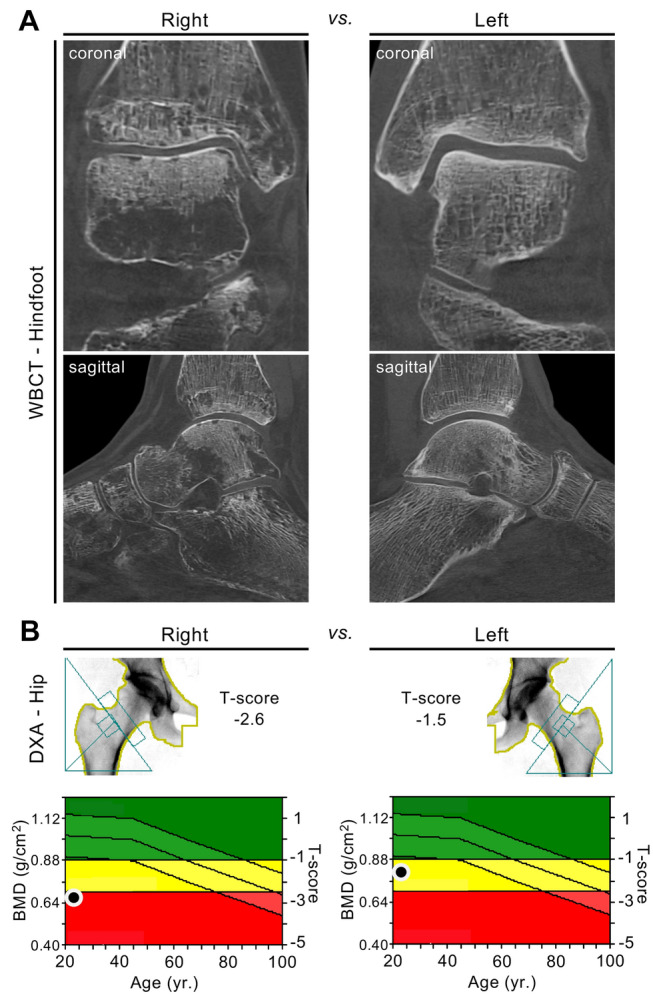
Local deterioration of bone mass after fracture and subsequent unilateral disuse of the right limb. a Cone-beam computed tomography (CBCT) images of the right foot compared to the left foot (coronal and sagittal reconstruction) in a 22-year-old female patient obtained eight months after suffering from an ankle fracture with subsequent unilateral unloading of the right limb. Focal osteolytic changes (often referred to as “local disuse osteopenia/osteoporosis”) are visible. b DXA scans demonstrating the differences in BMD between the right and left proximal femur (T-score − 2.6 vs. − 1.5). Bilateral HR-pQCT scans of the distal tibiae were also performed and showed cortical and trabecular bone loss syndrome on the unloaded side compared to the contralateral (loaded) side (cortical thickness − 28%, bone volume per tissue volume − 7%)
Laboratory Changes in Bone Turnover and Calcium Metabolism
In previous clinical studies focusing on laboratory changes in bone turnover, disuse or unloading was associated with increased bone turnover (primarily bone resorption) across different disuse conditions such as spinal cord injury [23, 24], vegetative state [35] or spaceflight [38]. While some studies, including bed rest [44] and spaceflight [38] studies, failed to detect any changes in bone formation, other bed rest studies found low bone formation marker levels accompanied by increased sclerostin levels [11, 45]. Patients with nonambulatory (i.e., immobilized) cerebral palsy showed higher sclerostin levels than patients with ambulatory cerebral palsy [46]. In contrast to these previous findings, one study found that sclerostin levels were lower in subjects with spinal cord injury who were immobilized in a wheelchair than in those who walked regularly [47]. Sclerostin represents a protein that is almost specifically produced by mechanosensitive osteocytes [5], which highlights the potential role of osteocytes in the pathogenesis of disuse osteoporosis [11, 45, 48]. Conversely, sclerostin levels were lower and bone formation was higher with higher physical activity [49]. Sclerostin inhibition prevented spinal cord injury-induced cancellous bone loss in a rodent spinal cord injury model [50]. However, the effects of sclerostin inhibition on bone quality in human patients suffering from disuse osteoporosis conditions have not yet been examined in detail. Since the sclerostin antibody romosozumab is now an established and approved treatment for severe postmenopausal osteoporosis [6], it will be of interest whether this drug is particularly effective in disuse osteoporosis or, more generally, how effective the drug is under different loading conditions.
Along with these previous findings on catabolic bone turnover dissociation following disuse, it is clear that the organism’s calcium metabolism is also impacted. Urinary calcium excretion was increased in patients during bed rest [45] and in individuals during spaceflight [51]. Furthermore, vitamin D deficiency was observed more frequently in immobilized patients in a vegetative state than in controls [35]. Interestingly, the high calcium loss observed during bed rest could not be counteracted by higher calcium intake [48]. These collective findings point to a specific pattern of high bone resorption and low bone formation during disuse, which is accompanied by calcium loss and, in most conditions, also vitamin D deficiency.
Fracture Healing
The impact of mechanical load on the process of fracture healing represents a complex issue that must be considered separately from the aspects of disuse-induced alterations in bone remodeling outlined in this review. Nonetheless, it is known that the compromised mechanical environment associated with immobilization and load-bearing restrictions in the course of operative or conservative fracture treatment may be followed by a vicious cycle of disuse-induced fracture healing problems. While direct bone healing is usually achieved by high stability and low interfragmentary movement (e.g., by compression plates), indirect bone healing via callus formation is induced by a low degree of stability and considerable interfragmentary movement (e.g., by internal or external splinting) [52]. Therefore, the most adequate balance between fracture stability and mobilization or load-bearing is directly influenced by the anatomical fracture localization and/or the surgical concept [53]. During indirect fracture healing, controlled loading such as cyclic compression of fractures led to enhanced callus formation [54], but optimal loading protocols (e.g., timing, strain) are still debated. In conditions of reduced mechanical loading, osteoanabolic teriparatide (PTH 1–34) treatment could be a promising option to improve fracture healing, as evidenced by mouse studies [55]. PTH administration together with loading across the fracture site was associated with further accelerated fracture healing, indicating a synergistic effect [56].
Microstructural Findings
To obtain more detailed data on the bone loss associated with disuse beyond DXA-based observations, volumetric and microstructural approaches, primarily peripheral quantitative computed tomography (pQCT) and, more recently, high-resolution peripheral quantitative computed tomography (HR-pQCT), have been introduced. The bone microstructural changes following immobilization have also been discussed in review articles [57], including those specifically discussing bone loss patterns in patients with spinal cord injury [58] and other neuromuscular disorders [59].
Previous pQCT studies with cross-sectional study designs have demonstrated negative associations between the duration of disuse and bone microstructural parameters in patients with spinal cord injury, where steady states were reached after 3–8 years and the total amount of bone loss ranged from 25–35% (diaphyses) to 50–60% (epiphyses) [60]. Only a few studies with longitudinal approaches, i.e., repeated measurements of the same parameters and in the same patients at standardized time intervals after disuse onset, are available. A selection of relevant longitudinal pQCT and HR-pQCT studies is presented in Table 1. Simulated (i.e., bed rest) or real microgravity studies are typically performed in young and healthy individuals. These conditions have been shown to lead to a pattern of both trabecular and cortical bone loss [37, 57]. During bed rest, it was reported that the relative bone loss in the tibia was larger in the cortical than the trabecular compartment [61]. Furthermore, relevant site specificity was demonstrated, with all tibial regions (epiphyseal to diaphyseal) depicting cortical loss, while considerable trabecular loss could only be detected in the proximal tibia epiphysis. However, a similar team of authors also observed greater trabecular loss in the distal tibial epiphysis in another bed rest study [62]. Greater trabecular than cortical loss was also detected after acute spinal cord injury [29]. More recently, HR-pQCT bed rest studies have confirmed that bone loss in both male and female individuals occurs primarily in weight-bearing bones such as the tibia [63, 64]. These studies demonstrated combined cortical and trabecular bone loss during bed rest.
Table 1.
Relevant studies on microstructural findings (pQCT, HR-pQCT) under real or modeled disuse osteoporosis conditions
| Study | Model/Sex | Method | Skeletal site | Disuse/Recovery | % Loss | % at Recovery |
|---|---|---|---|---|---|---|
| Rittweger et al. [61] | Bed rest/Male | pQCT |
Tibia, Patella, Femur |
35 days | BMC − 0.7 to − 3.2% (mostly cortical, epiphyseal) | n/a |
| Rittweger et al. [67] | Bed rest/Male | pQCT | Tibia, Radius | 56 days/360 days | Tib.BMC − 3.6% (dist. epiphysis) | Tib.BMC − 1.4% (dist. epiphysis) |
| Beller et al. [32] | Bed rest/Female | pQCT | Tibia, Radius | 43 days/360 days |
Tib.vBMD − 1.9% Rad.vBMD − 0.2% |
Tib.vBMD − 1.8% Rad.vBMD − 1.4% |
| Belavy et al. [63] | Bed rest/Male | HR-pQCT | Tibia, Radius | 59 days/720 days |
Tib.Ct.Th − 2.2% Tib.Tb.N − 4.2% Rad.Ct.Th + 1.0% Rad.Tb.N + 1.3% |
Tib.Ct.Th − 0.4% Tib.Tb.N − 3.7% Rad.Ct.Th + 1.2% Rad.Tb.N + 1.6% |
| Armbrecht et al. [64] | Bed rest/Female | HR-pQCT | Tibia, Radius | 43 days/360 days |
Tib.Ct.Th − 1.5% Tib.Tb.N − 1.4% Rad.Ct.Th − 0.1% Rad.Tb.N − 2.3% |
Tib.Ct.Th − 0.6% Tib.Tb.N − 3.1% Rad.Ct.Th − 0.4% Rad.Tb.N − 3.0% |
| Vico et al. [38] | Spaceflight/Male | HR-pQCT | Tibia, Radius | 4–6 mo./12 mo. |
Tib.Ct.Th − 3.5% Tib.Tb.N − 4.6% Rad.Ct.Th − 1.1% Rad.Tb.N + 4.9% |
Tib.Ct.Th ± 0% Tib.Tb.N − 4.6% Rad.Ct.Th − 4.5% Rad.Tb.N + 1.5% |
| Coupaud et al. [29] | Spinal cord injury/Male + Female | pQCT |
Tibia, Femur, Radius |
12 mo. |
Tib.Tb.BMD − 17.3 to – 22.2% Tib.Ct.BMD − 2.5 to – 2.6% Rad.Tt.BMD − 1.4% |
n/a |
| Rittweger et al. [42] | Unilateral limb suspension/Male | pQCT | Tibia | 24 days/90 days | Tib.BMC − 0.21 to – 0.6% | BMC − 0.52 to – 0.64% |
| Kazakia et al. [41] | Surgery/Male + Female | HR-pQCT | Tibia | 6 weeks/13 weeks |
Ct.Po + 16.1% Tb.N + 5.6% Tb.Th − 5.4% |
Ct.Po + 16.2% Tb.N recovered* Tb.Th recovered* |
A selection of relevant microstructural human studies with longitudinal data from 2000–2020 is presented. The table includes the magnitude of both bone loss and recovery (the displayed data are for the respective control groups, while the therapeutic effects of some studies are not displayed). The results are sorted by disease/model and year
Tib. tibial, Rad. radial, BMC bone mineral content, vBMD volumetric bone mineral density, Tt.BMD total bone mineral density, Ct.BMD cortical bone mineral density, Tb.BMD trabecular bone mineral density, Ct.Th cortical thickness, Tb.N trabecular number, Tb.Th trabecular thickness, Ct.Po cortical porosity, n/a not available/not assessed
*Not reported as numeric values
During spaceflight, cortical and trabecular bone loss was observed in the tibia [65]. Notably, trabecular bone showed both more severe and earlier bone loss than cortical bone, although a high degree of variability among the subjects was noted. There have also been QCT studies with measurements of the lumbar spine and hip, in which trabecular and cortical bone loss has been documented [37]. A recent HR-pQCT study in subjects before and after spaceflight showed that the loss of cortical bone was not only characterized by cortical thinning but also primarily by an increase in cortical porosity of 15% at the distal tibia [38]. Interestingly, a similar increase in cortical porosity (+16.1%) was also observed in the affected limbs of patients after 6 weeks of non-weight-bearing following surgery [41]. In summary, both trabecular and cortical compartments are affected by disuse, and while site and compartment differences exist among the disuse conditions, the most pronounced bone loss seems to occur in the epiphyses, with cortical porosity being the microstructural parameter that has shown the highest increases.
Recovery and Therapeutic Considerations
The recovery of bone mass in response to physical exercise programs, pharmacotherapy or both has been studied across different disuse and unloading models. Indeed, the most natural treatment for disuse osteoporosis is physical exercise or remobilization (loading) of the affected bones, although some of the disuse conditions per se do not allow remobilization. A recent systematic review found that the favorable effect of exercise on BMD in postmenopausal women is largely independent of the type of exercise [66]. Experiments on recovery from disuse conditions (mostly bed rest) often compare a study group with different exercise programs during or after immobilization with a control group (i.e., the study group without any additional exercise program). It is interesting to note that bed rest-induced bone loss continues in the first days/weeks after reambulation [61, 67]. In different control groups, the bone mass did not fully recover within the same time that the individuals were immobilized and, in most studies, even after long-term follow-up [32, 67] (Table 1). For instance, a remaining significant reduction in the tibial epiphyseal bone mineral content (BMC) was noted at approximately one year after reambulation [67]. While HR-pQCT bed rest studies have indicated a recovery of most microstructural parameters (including the cortical thickness or trabecular bone volume fraction) after mid-term follow-up, the trabecular number in the distal tibia remained significantly reduced after two years of follow-up in both male and female subjects [63, 64]. In individuals returning from space, the tibial cortical density recovered, while the cortical porosity or trabecular bone did not fully recover during the year after landing [38]. Increased cortical porosity after 6 weeks of non-weight-bearing did also not recover after 13 weeks of full weight-bearing [41].
Regarding the optimal exercise program, various studies with different protocols have been conducted, from which diverging findings regarding their success could be derived. In individuals with spinal cord injury, an overall positive and site-specific effect of functional electrical stimulation-induced cycling exercise on bone mass has been observed [68–70]. These studies also suggested a dose-dependent effect, with the best results after high-volume training [68]. To counteract bone loss during bed rest, the addition of whole-body vibration to high-load resistive exercise has been found to be effective in male subjects [67]. HR-pQCT studies have further confirmed these findings, with the combination of vibration and resistive exercise showing overall better preservation of cortical and trabecular parameters than resistive exercise alone [71]. In women, a previous pQCT study found that a combination of resistance and aerobic training did not provide any benefit compared to the control protocol [32]. In another study, this was also confirmed by HR-pQCT, where combined resistive and endurance exercise countermeasures were ineffective in preventing skeletal deterioration during disuse in women [64]. Such findings suggest a sex-specific effect in the skeletal response to loading, and the difference in the loading response may be attributed to sex hormones (estrogens and androgens) and their receptors [72]; however, the exact mechanisms are complex and remain partly unexplored. Of note, previous studies in non-immobilized patients examining the treatment effects of whole-body vibration on BMD have shown conflicting results with both positive and no effects [73, 74].
The fact that increased bone resorption plays a primary role in the development of disuse osteoporosis is supported by the findings of several previous therapeutic studies, in which antiresorptive treatment with bisphosphonates or the receptor activator of NF-κB ligand (RANKL) antibody denosumab attenuated bone loss [44, 75]. For example, intravenous bisphosphonates were partly effective in preserving bone mass in male subjects during bed rest [76]. Furthermore, denosumab treatment led to preserved bone mass in the knee region in patients with subacute spinal cord injury [75]. Compatible with the observation that chronic unloading also leads to low bone formation, the osteoanabolic agent teriparatide led to a solid increase in BMD in individuals with chronic spinal cord injury [77]. Interestingly, this increase was not augmented by additional vibration stimulation [77]. A previous study in postmenopausal, non-immobilized, osteoporotic women demonstrated a clinically relevant treatment effect in lumbar spine BMD with twelve months of whole-body vibration exercise and teriparatide compared to teriparatide alone [78].
Taken together, the treatment of disuse osteoporosis by combining bone-specific medications with physical exercise appears to be effective in at least partially counteracting the observed bone loss. In particular, the prompt increase in bone resorption markers after the onset of disuse [24] argues for the early use of anti-resorptive drugs such as denosumab. Individual exercise programs using vibration or electrical stimulation have shown positive, but sometimes inconsistent, results. The combination of bone anabolic drugs with physical exercise protocols appears to be promising but remains largely unexplored in conditions of disuse osteoporosis.
Basic Research on Skeletal Mechanotransduction
To decipher the mechanisms of disuse-induced bone loss, loading and unloading conditions have been modeled in a variety of human and animal studies. In one of our previous experimental human cadaveric studies analyzing the micromorphology of cortical bone, we found increased cortical porosity in long-term immobilization compared to controls and postmenopausal osteoporosis [79] (Fig. 3a), which supports previous clinical findings of a primary effect on cortical porosity after disuse [38]. Cortical deterioration was associated with lower osteocyte viability and impaired osteocyte connectivity [79] (Fig. 3b).
Fig. 3.
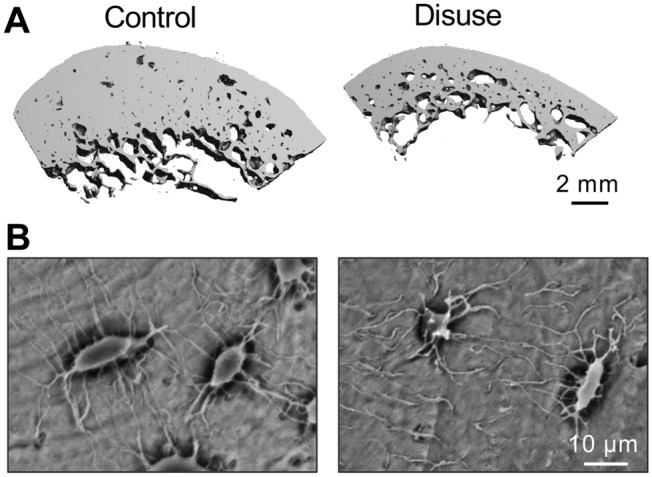
Micromorphological changes in cortical bone after immobilization. a µ-CT images demonstrating profound cortical changes, including a decrease in cortical thickness and an increase in cortical porosity, in individuals after long-term bed rest. b SEM images of acid-etched plastic-embedded cortical bone sections revealing decreased canalicular connectivity of osteocytes after immobilization, underlining the role of osteocytes in mechanotransduction. Images were obtained from representative samples obtained in the context of a previous study [79]
Regarding the evaluation of recovery or therapeutic effects in disuse osteoporosis, there have been many experimental studies taking advantage of animal models. One such study reported that different types of exercises (e.g., swimming, jumping, or vibration) applied to rats with immobilization-induced bone loss were equally efficient in preventing bone loss [80], thereby confirming clinical observations. Disuse in rats attenuated the bone anabolic response to teriparatide treatment [81]. In another experimental study in dogs, the cortical bone loss observed in disuse osteoporosis could only partially be counteracted by bisphosphonates, which indicates that the strong osteoclastogenic stimulus of disuse may not fully be antagonized by antiresorptive agents [82]. Together, these results suggest that mechanical loading is of critical importance in optimizing the treatment effects of bone-specific agents. Of note, an emerging model to study bone and its adaption to physical activity is the zebrafish [83]. Modification of the physical exercise intensity in a swim tunnel experiment led to rapid bone formation and increased bone volume and mineralization [84].
There have been a number of studies in rodents focusing on mechanical loading, bone remodeling and especially osteocyte mechanobiology. In mice, spaceflight led to both trabecular and cortical deterioration along with increased numbers of cortical empty osteocyte lacunae [85]. Early studies found evidence of osteocytic changes following immobilization, including increased osteocyte apoptosis [86]. More recently, it was demonstrated that osteocyte apoptosis following weightlessness (i.e., unloading by tail suspension) in mice triggers osteocytic RANKL production along with activation of both cortical and trabecular bone resorption [87]. While osteocyte apoptosis preceded the recruitment of osteoclastic cells and bone loss [88], another study by the same group found that inhibition of osteocyte apoptosis did not prevent unloading-induced bone loss although osteocytic RANKL production was sufficiently inhibited [89]. Tatsumi et al. developed a transgenic mouse model with the ablation of osteocytes through the injection of diphtheria toxin [90]. Importantly, these mice did not develop unloading-induced bone loss, underlining the osteocyte’s function in sensing mechanical loading and in the development of disuse osteoporosis. It is interesting to note that other models, including Botox-induced immobilization, were shown to lead to significant changes in bone mechanical and structural properties without affecting cortical osteocyte lacunar properties in rats [91]. A reason for these diverging findings, and especially the lack of osteocytic changes in the latter work, may be due to the almost nonexistent intracortical remodeling in the rats studied as well as the fact that osteocyte apoptosis was not studied [91]. Overall, it is likely that certain factors influence how osteocytes respond to unloading, for example, cortical vs. trabecular bone compartments, local loading history, and remodeling rates.
In the search for molecular mechanisms involved in the pathogenesis of disuse osteoporosis, several other important signaling pathways and proteins involved in the process of mechanically driven bone remodeling have been discovered (Fig. 4). Indeed, matrix-embedded osteocytes represent the skeletal cell type that most likely plays a major role during mechanotransduction, i.e., the process of converting external mechanical forces into biochemical responses. This is achieved by sensing local mechanical signals and responding to these signals both directly and indirectly [92]. Notably, both mechanically induced bone formation and disuse-induced bone loss and skeletal fragility may be mediated by osteocytes. Osteocytes are embedded in a lacuno-canalicular system (LCS), which becomes evident in acid-etched bone with subsequent scanning electron microscopy. Inside the LCS reside the osteocytes and the dendritic processes that originate from the osteocyte cell body. The osteocyte-secreted protein sclerostin was originally discovered through the identification of loss-of-function mutations in the SOST gene in individuals with the genetic high-bone-mass disorder sclerosteosis (van Buchem disease) [93]. Further research has demonstrated that increased sclerostin expression is primarily caused by mechanical unloading in mice, which leads to low bone formation [94]. While in vitro studies have revealed that disuse promotes osteocyte apoptosis and mechanical stimulation by fluid shear stress promotes osteocyte survival [95], it has further been demonstrated that increased sclerostin expression is specifically caused by unloading in osteocytic cells [96]. Translating these findings into therapeutic concepts, it is most likely that the recently approved sclerostin antibody romosozumab [6, 97] is especially effective in disuse osteoporosis, but the efficacy of romosozumab in association with conditions of disuse osteoporosis has to be investigated in future clinical studies.
Fig. 4.
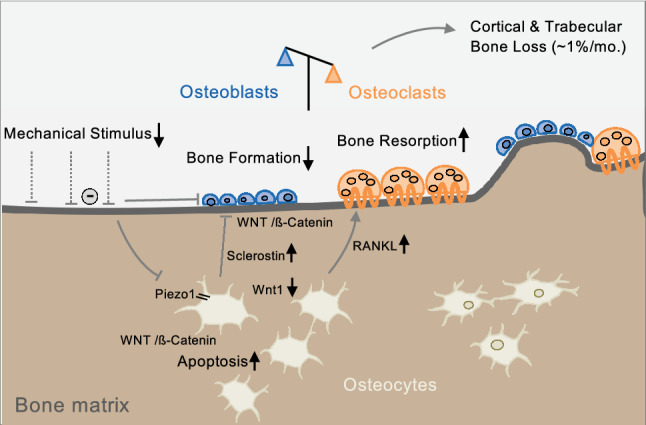
Schematic model of the molecular pathways involved in skeletal unloading. Mechanosensation in osteocytes is mediated by the lacuno-canalicular system (LCS) and via ion channels of the Piezo family (primarily Piezo1), among others. Inactivation of Piezo1 leads to decreased Wnt1 expression. Furthermore, sclerostin secretion by osteocytes is increased in response to unloading, which inhibits Wnt/β-catenin and results in suppressed osteoblast activity. Unloading also leads to increased osteocyte apoptosis (partly via Wnt/β-catenin signaling) and increased RANKL expression, promoting increased bone resorption
The mechanosensitive function of the skeleton was recently evidenced by the identification of mechanically activated ion channels of the Piezo family, specifically Piezo1 in osteoblasts and osteocytes [7, 8]. Interestingly, the deletion of Piezo1 reduced the expression of Wnt1 [7], which is a key regulator of bone formation [98]. By a systematic analysis of mice with inactivated Piezo proteins in different skeletal cell populations, our group confirmed that Piezo1 acts as a mechanosensor in osteocytes and demonstrated that it represents an essential osteogenic differentiation factor during endochondral bone formation [9]. The latter finding supports the assumption that various cell types are involved in mechanotransduction, which is highly relevant not only for bone remodeling but also for developmental and regenerative processes [99]. Together, these collective data suggest that Piezo1 plays a crucial role in mechanosensation and that Piezo channels could be a novel therapeutic target for osteoanabolic treatment in mechanical unloading-induced bone loss.
Another molecular example of musculoskeletal mechanotransduction, more specifically the close interaction between muscle and bone during this process, is the identification of the myokine irisin, a protein that is derived from muscle in response to exercise. Irisin injection increased cortical bone mass in mice, partly through suppression of sclerostin expression [100]. In this way, irisin prevented not only muscular atrophy but also the development of disuse osteoporosis accompanied by decreased osteocyte apoptosis [101]. In human subjects, circulating irisin levels were associated with the risk of osteoporotic fracture [102]. Beyond this associative evidence, there is currently no strong clinical evidence demonstrating that muscle or bone modulate each other’s tissue response to load changes.
Finally, the molecular mechanosensors in osteocytes are the subject of ongoing research, as recently discussed in a detailed review article [92]. Briefly summarized (and as partly outlined above), osteocytic mechanosensors include the osteocyte cytoskeleton and dendritic processes within the lacuno-canalicular system (LCS), as well as ion channels (e.g., Piezo1). However, the extracellular matrix (ECM), primary cilia, integrin-based focal adhesions and connexin-based intercellular junctions have also been identified to contribute to mechanotransduction in osteocytes. During skeletal development and mechanical stimulation, there is interactive communication between osteocytes and the ECM, which is mainly composed of proteoglycans, glycoproteins, and hyaluronic acid. One example highlighting the role of the ECM during the osteocyte mechanotransduction process is perlecan (HSPG2), a proteoglycan that is localized in the pericellular space of osteocytes within the LCS [103]. Importantly, perlecan-deficient mice showed dysfunctional bone formation in response to loading [104]. Primary cilia are solitary, rigid structures that span from the cell body into the extracellular space [105]. Interestingly, primary cilia translate extracellular mechanical stimuli into cellular responses via an ion-channel-independent mechanism [106]. In addition to these important structures responsible for mechanotransduction in osteocytes, integrin-based focal adhesions play an important role in this complex process. Integrins are transmembrane receptors with an extracellular and a cytoplasmic domain composed of an α and β subunit. Both in vitro and in vivo studies have revealed the involvement of the different subunits during mechanotransduction [107]. Finally, cellular communication via hemichannels and gap junctions, which are composed of the protein connexin, has been found to be essential during mechanotransduction. While multiple types of connexins are expressed in bone cells, Cx43 is the major candidate for mechanotransduction in osteocytes. The cell-specific deletion of Cx43 in osteoblasts and osteocytes led to an attenuated response to both loading and unloading [108, 109].
Concluding Remarks
Although a number of fundamental questions concerning the clinical, molecular and therapeutic aspects of disuse osteoporosis remain unanswered, the combined effort of both clinical and basic research has revealed the microstructural and mechanistic basis of the profound negative effects of disuse on skeletal integrity. Clinical studies with real or modeled unloading conditions, including spinal cord injury, limb suspension, bed rest and microgravity (spaceflight), have demonstrated that early bone loss is characterized by a relative increase in bone resorption compared to decreased bone formation. As a consequence, combined cortical and trabecular microstructural deterioration was noted in most studies, with subtle disease-, sex-, site- and compartment-specific differences that can be accurately imaged using high-resolution CT-based techniques such as HR-pQCT. In a broader sense, disuse osteoporosis also includes clinical conditions of bone loss in response to local skeletal unloading, such as after surgery or around implants. Furthermore, full and timely recovery from bone loss may not be achieved by loading, but bone loss may be counteracted by high-frequency physical exercise programs and bone-specific agents such as bisphosphonates/denosumab and teriparatide. The use of animal models has allowed the cellular and molecular mechanisms of disuse-induced bone loss to be elucidated. Matrix-embedded osteocytes have been identified as the primary mechanoresponsive cell type translating mechanical into biochemical signals to orchestrate mechanically induced bone formation and remodeling, which is in fact a regulatory process with high complexity also involving neighboring bone cells. Osteocyte-specific proteins such as sclerostin and Piezo1 are involved in this process of mechanotransduction, and new therapeutic options have already been introduced; however, their efficacy needs to be clarified in further experiments and clinical trials acknowledging the mechanical environment.
Acknowledgements
We apologize to the many researchers whose valuable contributions to disuse osteoporosis and mechanobiology were not included due to space restrictions. We thank Prof. Thorsten Schinke for critically reviewing the manuscript as well as Dr. Maximilian M. Delsmann and Dr. Julian Stürznickel for providing figure content.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflict of interest
Tim Rolvien and Michael Amling declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tim Rolvien, Email: t.rolvien@uke.de.
Michael Amling, Email: amling@uke.de.
References
- 1.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 2.Wolff J. Das Gesetz der Transformation der Knochen. Berlin: Hirschwald; 1892. [Google Scholar]
- 3.Huddleston AL, Rockwell D, Kulund DN, Harrison RB. Bone mass in lifetime tennis athletes. JAMA. 1980;244:1107–1109. doi: 10.1001/jama.1980.03310100025022. [DOI] [PubMed] [Google Scholar]
- 4.Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcif Tissue Int. 2014;94:5–24. doi: 10.1007/s00223-013-9790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 6.McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Han L, Nookaew I, Mannen E, Silva MJ, Almeida M, Xiong J. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife. 2019 doi: 10.7554/eLife.49631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun W, Chi S, Li Y, Ling S, Tan Y, Xu Y, Jiang F, Li J, Liu C, Zhong G, Cao D, Jin X, Zhao D, Gao X, Liu Z, Xiao B, Li Y. The mechanosensitive Piezo1 channel is required for bone formation. Elife. 2019 doi: 10.7554/eLife.47454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendrickx G, Fischer V, Liedert A, von Kroge S, Haffner-Luntzer M, Brylka L, Pawlus E, Schweizer M, Yorgan T, Baranowsky A, Rolvien T, Neven M, Schumacher U, Beech DJ, Amling M, Ignatius A, Schinke T. Piezo1 inactivation in chondrocytes impairs trabecular bone formation. J Bone Miner Res. 2021;36:369–384. doi: 10.1002/jbmr.4198. [DOI] [PubMed] [Google Scholar]
- 10.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, Pulvirenti I, Hawa G, Tringali G, Fiore CE. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab. 2010;95:2248–2253. doi: 10.1210/jc.2010-0067. [DOI] [PubMed] [Google Scholar]
- 12.Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36:790–796. doi: 10.1038/sj.sc.3100648. [DOI] [PubMed] [Google Scholar]
- 13.Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005;20:809–816. doi: 10.1359/JBMR.041222. [DOI] [PubMed] [Google Scholar]
- 14.Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, Harbour RT, Caldwell LM, Creed G. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD000333.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 2006;29:489–500. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iolascon G, Paoletta M, Liguori S, Curci C, Moretti A. Neuromuscular diseases and bone. Front Endocrinol. 2019;10:794. doi: 10.3389/fendo.2019.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res. 1998;13:1594–1601. doi: 10.1359/jbmr.1998.13.10.1594. [DOI] [PubMed] [Google Scholar]
- 18.Stavnichuk M, Mikolajewicz N, Corlett T, Morris M, Komarova SV. A systematic review and meta-analysis of bone loss in space travelers. NPJ Microgravity. 2020;6:13. doi: 10.1038/s41526-020-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagnon C, Schafer AL. Bone health after bariatric surgery. JBMR Plus. 2018;2:121–133. doi: 10.1002/jbm4.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanker J, Duque G. Osteosarcopenia: the path beyond controversy. Curr Osteoporos Rep. 2020;18:81–84. doi: 10.1007/s11914-020-00567-6. [DOI] [PubMed] [Google Scholar]
- 21.Maurel DB, Jahn K, Lara-Castillo N. Muscle-bone crosstalk: emerging opportunities for novel therapeutic approaches to treat musculoskeletal pathologies. Biomedicines. 2017;5(4):62. doi: 10.3390/biomedicines5040062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–677. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]
- 23.Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, McWhinney B, Hickman PE. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab. 1998;83:415–422. doi: 10.1210/jcem.83.2.4581. [DOI] [PubMed] [Google Scholar]
- 24.Maimoun L, Couret I, Mariano-Goulart D, Dupuy AM, Micallef JP, Peruchon E, Ohanna F, Cristol JP, Rossi M, Leroux JL. Changes in osteoprotegerin/RANKL system, bone mineral density, and bone biochemicals markers in patients with recent spinal cord injury. Calcif Tissue Int. 2005;76:404–411. doi: 10.1007/s00223-004-0048-6. [DOI] [PubMed] [Google Scholar]
- 25.Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C, Liotta FJ, Weinstein DA. Osteoporosis after spinal cord injury. J Orthop Res. 1992;10:371–378. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- 26.Terzi T, Terzi M, Tander B, Canturk F, Onar M. Changes in bone mineral density and bone metabolism markers in premenopausal women with multiple sclerosis and the relationship to clinical variables. J Clin Neurosci. 2010;17:1260–1264. doi: 10.1016/j.jocn.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 27.Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, Case LE, Cripe L, Hadjiyannakis S, Olson AK, Sheehan DW, Bolen J, Weber DR, Ward LM, Group DMDCCW Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17:347–361. doi: 10.1016/S1474-4422(18)30025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolvien T, Butscheidt S, Jeschke A, Neu A, Denecke J, Kubisch C, Meisler MH, Pueschel K, Barvencik F, Yorgan T, Oheim R, Schinke T, Amling M. Severe bone loss and multiple fractures in SCN8A-related epileptic encephalopathy. Bone. 2017;103:136–143. doi: 10.1016/j.bone.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 29.Coupaud S, McLean AN, Purcell M, Fraser MH, Allan DB. Decreases in bone mineral density at cortical and trabecular sites in the tibia and femur during the first year of spinal cord injury. Bone. 2015;74:69–75. doi: 10.1016/j.bone.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Ashe MC, Fehling P, Eng JJ, Khan KM, McKay HA. Bone geometric response to chronic disuse following stroke: a pQCT study. J Musculoskelet Neuronal Interact. 2006;6:226–233. [PubMed] [Google Scholar]
- 31.Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990;5:843–850. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- 32.Beller G, Belavy DL, Sun L, Armbrecht G, Alexandre C, Felsenberg D. WISE-2005: bed-rest induced changes in bone mineral density in women during 60 days simulated microgravity. Bone. 2011;49:858–866. doi: 10.1016/j.bone.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Spector ER, Smith SM, Sibonga JD. Skeletal effects of long-duration head-down bed rest. Aviat Space Environ Med. 2009;80:A23–28. doi: 10.3357/ASEM.BR02.2009. [DOI] [PubMed] [Google Scholar]
- 34.Aoki M, Kawahata H, Sotobayashi D, Yu H, Moriguchi A, Nakagami H, Ogihara T, Morishita R. Effect of angiotensin II receptor blocker, olmesartan, on turnover of bone metabolism in bedridden elderly hypertensive women with disuse syndrome. Geriatr Gerontol Int. 2015;15:1064–1072. doi: 10.1111/ggi.12406. [DOI] [PubMed] [Google Scholar]
- 35.Oppl B, Michitsch G, Misof B, Kudlacek S, Donis J, Klaushofer K, Zwerina J, Zwettler E. Low bone mineral density and fragility fractures in permanent vegetative state patients. J Bone Miner Res. 2014;29:1096–1100. doi: 10.1002/jbmr.2122. [DOI] [PubMed] [Google Scholar]
- 36.LeBlanc A, Schneider V, Shackelford L, West S, Oganov V, Bakulin A, Voronin L. Bone mineral and lean tissue loss after long duration space flight. J Musculoskelet Neuronal Interact. 2000;1:157–160. [PubMed] [Google Scholar]
- 37.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 38.Vico L, van Rietbergen B, Vilayphiou N, Linossier MT, Locrelle H, Normand M, Zouch M, Gerbaix M, Bonnet N, Novikov V, Thomas T, Vassilieva G. Cortical and trabecular bone microstructure did not recover at weight-bearing skeletal sites and progressively deteriorated at non-weight-bearing sites during the year following international space station missions. J Bone Miner Res. 2017;32:2010–2021. doi: 10.1002/jbmr.3188. [DOI] [PubMed] [Google Scholar]
- 39.de Abreu MR, Wesselly M, Chung CB, Resnick D. Bone marrow MR imaging findings in disuse osteoporosis. Skeletal Radiol. 2011;40:571–575. doi: 10.1007/s00256-010-1042-x. [DOI] [PubMed] [Google Scholar]
- 40.Pang MY, Ashe MC, Eng JJ. Muscle weakness, spasticity and disuse contribute to demineralization and geometric changes in the radius following chronic stroke. Osteoporos Int. 2007;18:1243–1252. doi: 10.1007/s00198-007-0372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kazakia GJ, Tjong W, Nirody JA, Burghardt AJ, Carballido-Gamio J, Patsch JM, Link T, Feeley BT, Ma CB. The influence of disuse on bone microstructure and mechanics assessed by HR-pQCT. Bone. 2014;63:132–140. doi: 10.1016/j.bone.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rittweger J, Winwood K, Seynnes O, de Boer M, Wilks D, Lea R, Rennie M, Narici M. Bone loss from the human distal tibia epiphysis during 24 days of unilateral lower limb suspension. J Physiol. 2006;577:331–337. doi: 10.1113/jphysiol.2006.115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jarvinen M, Kannus P. Injury of an extremity as a risk factor for the development of osteoporosis. J Bone Joint Surg Am. 1997;79:263–276. doi: 10.2106/00004623-199702000-00017. [DOI] [PubMed] [Google Scholar]
- 44.LeBlanc AD, Driscol TB, Shackelford LC, Evans HJ, Rianon NJ, Smith SM, Feeback DL, Lai D. Alendronate as an effective countermeasure to disuse induced bone loss. J Musculoskelet Neuronal Interact. 2002;2:335–343. [PubMed] [Google Scholar]
- 45.Spatz JM, Fields EE, Yu EW, Divieti Pajevic P, Bouxsein ML, Sibonga JD, Zwart SR, Smith SM. Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab. 2012;97:E1736–1740. doi: 10.1210/jc.2012-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin YK, Yoon YK, Chung KB, Rhee Y, Cho SR. Patients with non-ambulatory cerebral palsy have higher sclerostin levels and lower bone mineral density than patients with ambulatory cerebral palsy. Bone. 2017;103:302–307. doi: 10.1016/j.bone.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 47.Morse LR, Sudhakar S, Danilack V, Tun C, Lazzari A, Gagnon DR, Garshick E, Battaglino RA. Association between sclerostin and bone density in chronic spinal cord injury. J Bone Miner Res. 2012;27:352–359. doi: 10.1002/jbmr.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baecker N, Frings-Meuthen P, Smith SM, Heer M. Short-term high dietary calcium intake during bedrest has no effect on markers of bone turnover in healthy men. Nutrition. 2010;26:522–527. doi: 10.1016/j.nut.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Ardawi MS, Rouzi AA, Qari MH. Physical activity in relation to serum sclerostin, insulin-like growth factor-1, and bone turnover markers in healthy premenopausal women: a cross-sectional and a longitudinal study. J Clin Endocrinol Metab. 2012;97:3691–3699. doi: 10.1210/jc.2011-3361. [DOI] [PubMed] [Google Scholar]
- 50.Beggs LA, Ye F, Ghosh P, Beck DT, Conover CF, Balaez A, Miller JR, Phillips EG, Zheng N, Williams AA, Aguirre JI, Wronski TJ, Bose PK, Borst SE, Yarrow JF. Sclerostin inhibition prevents spinal cord injury-induced cancellous bone loss. J Bone Miner Res. 2015;30:681–689. doi: 10.1002/jbmr.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith SM, Wastney ME, Morukov BV, Larina IM, Nyquist LE, Abrams SA, Taran EN, Shih CY, Nillen JL, Davis-Street JE, Rice BL, Lane HW. Calcium metabolism before, during, and after a 3-mo spaceflight: kinetic and biochemical changes. Am J Physiol. 1999;277:R1–10. doi: 10.1111/j.1469-7793.1999.0001o.x. [DOI] [PubMed] [Google Scholar]
- 52.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 53.Kubiak EN, Beebe MJ, North K, Hitchcock R, Potter MQ. Early weight bearing after lower extremity fractures in adults. J Am Acad Orthop Surg. 2013;21:727–738. doi: 10.5435/00124635-201312000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Gardner MJ, van der Meulen MC, Demetrakopoulos D, Wright TM, Myers ER, Bostrom MP. In vivo cyclic axial compression affects bone healing in the mouse tibia. J Orthop Res. 2006;24:1679–1686. doi: 10.1002/jor.20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ellegaard M, Kringelbach T, Syberg S, Petersen S, Beck Jensen JE, Bruel A, Jorgensen NR, Schwarz P. The effect of PTH(1–34) on fracture healing during different loading conditions. J Bone Miner Res. 2013;28:2145–2155. doi: 10.1002/jbmr.1957. [DOI] [PubMed] [Google Scholar]
- 56.Gardner MJ, van der Meulen MC, Carson J, Zelken J, Ricciardi BF, Wright TM, Lane JM, Bostrom MP. Role of parathyroid hormone in the mechanosensitivity of fracture healing. J Orthop Res. 2007;25:1474–1480. doi: 10.1002/jor.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sievanen H. Immobilization and bone structure in humans. Arch Biochem Biophys. 2010;503:146–152. doi: 10.1016/j.abb.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 58.Cirnigliaro CM, Myslinski MJ, La Fountaine MF, Kirshblum SC, Forrest GF, Bauman WA. Bone loss at the distal femur and proximal tibia in persons with spinal cord injury: imaging approaches, risk of fracture, and potential treatment options. Osteoporos Int. 2017;28:747–765. doi: 10.1007/s00198-016-3798-x. [DOI] [PubMed] [Google Scholar]
- 59.Cervinka T, Giangregorio L, Sievanen H, Cheung AM, Craven BC. Peripheral quantitative computed tomography: review of evidence and recommendations for image acquisition, analysis, and reporting, among individuals with neurological impairment. J Clin Densitom. 2018;21:563–582. doi: 10.1016/j.jocd.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34:869–880. doi: 10.1016/j.bone.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Rittweger J, Simunic B, Bilancio G, De Santo NG, Cirillo M, Biolo G, Pisot R, Eiken O, Mekjavic IB, Narici M. Bone loss in the lower leg during 35 days of bed rest is predominantly from the cortical compartment. Bone. 2009;44:612–618. doi: 10.1016/j.bone.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Cervinka T, Rittweger J, Hyttinen J, Felsenberg D, Sievanen H. Anatomical sector analysis of load-bearing tibial bone structure during 90-day bed rest and 1-year recovery. Clin Physiol Funct Imaging. 2011;31:249–257. doi: 10.1111/j.1475-097X.2011.01009.x. [DOI] [PubMed] [Google Scholar]
- 63.Belavy DL, Beller G, Ritter Z, Felsenberg D. Bone structure and density via HR-pQCT in 60d bed-rest, 2-years recovery with and without countermeasures. J Musculoskelet Neuronal Interact. 2011;11:215–226. [PubMed] [Google Scholar]
- 64.Armbrecht G, Belavy DL, Backstrom M, Beller G, Alexandre C, Rizzoli R, Felsenberg D. Trabecular and cortical bone density and architecture in women after 60 days of bed rest using high-resolution pQCT: WISE 2005. J Bone Miner Res. 2011;26:2399–2410. doi: 10.1002/jbmr.482. [DOI] [PubMed] [Google Scholar]
- 65.Vico L, Collet P, Guignandon A, Lafage-Proust MH, Thomas T, Rehaillia M, Alexandre C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355:1607–1611. doi: 10.1016/S0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 66.Kemmler W, Shojaa M, Kohl M, von Stengel S. Effects of different types of exercise on bone mineral density in postmenopausal women: a systematic review and meta-analysis. Calcif Tissue Int. 2020;107:409–439. doi: 10.1007/s00223-020-00744-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rittweger J, Beller G, Armbrecht G, Mulder E, Buehring B, Gast U, Dimeo F, Schubert H, de Haan A, Stegeman DF, Schiessl H, Felsenberg D. Prevention of bone loss during 56 days of strict bed rest by side-alternating resistive vibration exercise. Bone. 2010;46:137–147. doi: 10.1016/j.bone.2009.08.051. [DOI] [PubMed] [Google Scholar]
- 68.Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, Donaldson NN, Eser P. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone. 2008;43:169–176. doi: 10.1016/j.bone.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 69.Mohr T, Podenphant J, Biering-Sorensen F, Galbo H, Thamsborg G, Kjaer M. Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int. 1997;61:22–25. doi: 10.1007/s002239900286. [DOI] [PubMed] [Google Scholar]
- 70.Coupaud S, Jack LP, Hunt KJ, Allan DB. Muscle and bone adaptations after treadmill training in incomplete Spinal cord injury: a case study using peripheral quantitative computed tomography. J Musculoskelet Neuronal Interact. 2009;9:288–297. [PubMed] [Google Scholar]
- 71.Belavy DL, Beller G, Armbrecht G, Perschel FH, Fitzner R, Bock O, Borst H, Degner C, Gast U, Felsenberg D. Evidence for an additional effect of whole-body vibration above resistive exercise alone in preventing bone loss during prolonged bed rest. Osteoporos Int. 2011;22:1581–1591. doi: 10.1007/s00198-010-1371-6. [DOI] [PubMed] [Google Scholar]
- 72.Saxon LK, Galea G, Meakin L, Price J, Lanyon LE. Estrogen receptors alpha and beta have different gender-dependent effects on the adaptive responses to load bearing in cancellous and cortical bone. Endocrinology. 2012;153:2254–2266. doi: 10.1210/en.2011-1977. [DOI] [PubMed] [Google Scholar]
- 73.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 74.Kiel DP, Hannan MT, Barton BA, Bouxsein ML, Sisson E, Lang T, Allaire B, Dewkett D, Carroll D, Magaziner J, Shane E, Leary ET, Zimmerman S, Rubin CT. Low-magnitude mechanical stimulation to improve bone density in persons of advanced age: a randomized, Placebo-Controlled trial. J Bone Miner Res. 2015;30:1319–1328. doi: 10.1002/jbmr.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cirnigliaro CM, La Fountaine MF, Parrott JS, Kirshblum SC, McKenna C, Sauer SJ, Shapses SA, Hao L, McClure IA, Hobson JC. Administration of denosumab preserves bone mineral density at the knee in persons with subacute spinal cord injury: findings from a randomized clinical trial. JBMR Plus. 2020 doi: 10.1002/jbm4.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rittweger J, Frost HM, Schiessl H, Ohshima H, Alkner B, Tesch P, Felsenberg D. Muscle atrophy and bone loss after 90 days' bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone. 2005;36:1019–1029. doi: 10.1016/j.bone.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 77.Edwards WB, Simonian N, Haider IT, Anschel AS, Chen D, Gordon KE, Gregory EK, Kim KH, Parachuri R, Troy KL, Schnitzer TJ. Effects of teriparatide and vibration on bone mass and bone strength in people with bone loss and spinal cord injury: a randomized, controlled trial. J Bone Miner Res. 2018;33:1729–1740. doi: 10.1002/jbmr.3525. [DOI] [PubMed] [Google Scholar]
- 78.Jepsen DB, Ryg J, Hansen S, Jorgensen NR, Gram J, Masud T. The combined effect of Parathyroid hormone (1–34) and whole-body Vibration exercise in the treatment of postmenopausal OSteoporosis (PaVOS study): a randomized controlled trial. Osteoporos Int. 2019;30:1827–1836. doi: 10.1007/s00198-019-05029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rolvien T, Milovanovic P, Schmidt FN, von Kroge S, Wolfel EM, Krause M, Wulff B, Puschel K, Ritchie RO, Amling M, Busse B. Long-term immobilization in elderly females causes a specific pattern of cortical bone and osteocyte deterioration different from postmenopausal osteoporosis. J Bone Miner Res. 2020;35:1343–1351. doi: 10.1002/jbmr.3970. [DOI] [PubMed] [Google Scholar]
- 80.Falcai MJ, Zamarioli A, Okubo R, de Paula FJ, Volpon JB. The osteogenic effects of swimming, jumping, and vibration on the protection of bone quality from disuse bone loss. Scand J Med Sci Sports. 2015;25:390–397. doi: 10.1111/sms.12240. [DOI] [PubMed] [Google Scholar]
- 81.Turner RT, Lotinun S, Hefferan TE, Morey-Holton E. Disuse in adult male rats attenuates the bone anabolic response to a therapeutic dose of parathyroid hormone. J Appl Physiol. 1985;101:881–886. doi: 10.1152/japplphysiol.01622.2005. [DOI] [PubMed] [Google Scholar]
- 82.Li CY, Price C, Delisser K, Nasser P, Laudier D, Clement M, Jepsen KJ, Schaffler MB. Long-term disuse osteoporosis seems less sensitive to bisphosphonate treatment than other osteoporosis. J Bone Miner Res. 2005;20:117–124. doi: 10.1359/JBMR.041010. [DOI] [PubMed] [Google Scholar]
- 83.Busse B, Galloway JL, Gray RS, Harris MP, Kwon RY. Zebrafish: an emerging model for orthopedic research. J Orthop Res. 2020;38:925–936. doi: 10.1002/jor.24539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suniaga S, Rolvien T, Vom Scheidt A, Fiedler IAK, Bale HA, Huysseune A, Witten PE, Amling M, Busse B. Increased mechanical loading through controlled swimming exercise induces bone formation and mineralization in adult zebrafish. Sci Rep. 2018;8:3646. doi: 10.1038/s41598-018-21776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gerbaix M, Gnyubkin V, Farlay D, Olivier C, Ammann P, Courbon G, Laroche N, Genthial R, Follet H, Peyrin F, Shenkman B, Gauquelin-Koch G, Vico L. One-month spaceflight compromises the bone microstructure, tissue-level mechanical properties, osteocyte survival and lacunae volume in mature mice skeletons. Sci Rep. 2017;7:2659. doi: 10.1038/s41598-017-03014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krempien B, Manegold C, Ritz E, Bommer J. The influence of immobilization on osteocyte morphology: osteocyte differential count and electron microscopical studies. Virchows Arch A Pathol Anat Histol. 1976;370:55–68. doi: 10.1007/BF00427310. [DOI] [PubMed] [Google Scholar]
- 87.Cabahug-Zuckerman P, Frikha-Benayed D, Majeska RJ, Tuthill A, Yakar S, Judex S, Schaffler MB. Osteocyte apoptosis caused by Hindlimb unloading is required to trigger osteocyte RANKL production and subsequent resorption of cortical and trabecular bone in mice femurs. J Bone Miner Res. 2016;31:1356–1365. doi: 10.1002/jbmr.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006;21:605–615. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- 89.Plotkin LI, Gortazar AR, Davis HM, Condon KW, Gabilondo H, Maycas M, Allen MR, Bellido T. Inhibition of osteocyte apoptosis prevents the increase in osteocytic receptor activator of nuclear factor kappaB ligand (RANKL) but does not stop bone resorption or the loss of bone induced by unloading. J Biol Chem. 2015;290:18934–18942. doi: 10.1074/jbc.M115.642090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 91.Bach-Gansmo FL, Wittig NK, Bruel A, Thomsen JS, Birkedal H. Immobilization and long-term recovery results in large changes in bone structure and strength but no corresponding alterations of osteocyte lacunar properties. Bone. 2016;91:139–147. doi: 10.1016/j.bone.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 92.Qin L, Liu W, Cao H, Xiao G. Molecular mechanosensors in osteocytes. Bone Res. 2020;8:23. doi: 10.1038/s41413-020-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/β-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 95.Bakker A, Klein-Nulend J, Burger E. Shear stress inhibits while disuse promotes osteocyte apoptosis. Biochem Biophys Res Commun. 2004;320:1163–1168. doi: 10.1016/j.bbrc.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 96.Spatz JM, Wein MN, Gooi JH, Qu Y, Garr JL, Liu S, Barry KJ, Uda Y, Lai F, Dedic C, Balcells-Camps M, Kronenberg HM, Babij P, Pajevic PD. The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro. J Biol Chem. 2015;290:16744–16758. doi: 10.1074/jbc.M114.628313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417–1427. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 98.Luther J, Yorgan TA, Rolvien T, Ulsamer L, Koehne T, Liao NN, Keller D, Vollersen N, Teufel S, Neven M, Peters S, Schweizer M, Trumpp A, Rosigkeit S, Bockamp E, Mundlos S, Kornak U, Oheim R, Amling M, Schinke T, David JP. Wnt1 is an Lrp5-independent bone-anabolic Wnt ligand. Sci Transl Med. 2018;10:eaau7137. doi: 10.1126/scitranslmed.aau7137. [DOI] [PubMed] [Google Scholar]
- 99.Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017;18:728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, Lu P, Sartini L, Di Comite M, Mori G, Di Benedetto A, Brunetti G, Yuen T, Sun L, Reseland JE, Colucci S, New MI, Zaidi M, Cinti S, Grano M. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A. 2015;112:12157–12162. doi: 10.1073/pnas.1516622112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Storlino G, Colaianni G, Sanesi L, Lippo L, Brunetti G, Errede M, Colucci S, Passeri G, Grano M. Irisin prevents disuse-induced osteocyte apoptosis. J Bone Miner Res. 2020;35:766–775. doi: 10.1002/jbmr.3944. [DOI] [PubMed] [Google Scholar]
- 102.Anastasilakis AD, Polyzos SA, Makras P, Gkiomisi A, Bisbinas I, Katsarou A, Filippaios A, Mantzoros CS. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos Int. 2014;25:1633–1642. doi: 10.1007/s00198-014-2673-x. [DOI] [PubMed] [Google Scholar]
- 103.Thompson WR, Modla S, Grindel BJ, Czymmek KJ, Kirn-Safran CB, Wang L, Duncan RL, Farach-Carson MC. Perlecan/Hspg2 deficiency alters the pericellular space of the lacunocanalicular system surrounding osteocytic processes in cortical bone. J Bone Miner Res. 2011;26:618–629. doi: 10.1002/jbmr.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang B, Lai X, Price C, Thompson WR, Li W, Quabili TR, Tseng WJ, Liu XS, Zhang H, Pan J, Kirn-Safran CB, Farach-Carson MC, Wang L. Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J Bone Miner Res. 2014;29:878–891. doi: 10.1002/jbmr.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Temiyasathit S, Jacobs CR. Osteocyte primary cilium and its role in bone mechanotransduction. Ann N Y Acad Sci. 2010;1192:422–428. doi: 10.1111/j.1749-6632.2009.05243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104:13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cabahug-Zuckerman P, Stout RF, Jr, Majeska RJ, Thi MM, Spray DC, Weinbaum S, Schaffler MB. Potential role for a specialized beta3 integrin-based structure on osteocyte processes in bone mechanosensation. J Orthop Res. 2018;36:642–652. doi: 10.1002/jor.23792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grimston SK, Brodt MD, Silva MJ, Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1) J Bone Miner Res. 2008;23:879–886. doi: 10.1359/jbmr.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lloyd SA, Lewis GS, Zhang Y, Paul EM, Donahue HJ. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J Bone Miner Res. 2012;27:2359–2372. doi: 10.1002/jbmr.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]