Abstract
Primary care clinicians have a vital role to play in the diagnosis and management of patients with major depressive disorder (MDD). This includes screening for MDD as well as identifying other possible psychiatric disorders including bipolar disorder and/or other comorbidities. Once MDD is confirmed, partnering with patients in the shared decision-making process while considering different treatment options and best management of MDD over the course of their illness is recommended. Vortioxetine has been approved for the treatment of adults with MDD since 2013, and subsequent US label updates indicate that vortioxetine may be particularly beneficial for specific populations of patients with MDD, including those with treatment-emergent sexual dysfunction and patients experiencing certain cognitive symptoms. Given these recent label updates, this prescribing guide for vortioxetine aims to provide clear and practical guidance for primary care clinicians on the safe and effective use of vortioxetine for the treatment of MDD, including how to identify appropriate patients for treatment.
Keywords: antidepressant, measurement-guided care, shared decision-making
Plain Language Summary
Diagnosis and management of major depressive disorder (MDD) in a primary care setting
Primary care clinicians need practical guidance for the diagnosis and management of MDD, which is a major health concern in the United States.
A variety of tools suitable for clinical practice can support primary care clinicians in screening depressive symptoms and suicide risk, distinguishing MDD from other mood disorders (eg, bipolar disorder) and monitoring for disease outcomes over time.
Shared decision-making between the primary care clinician and patient can help improve patient education and comfort, providing an opportunity for the clinician to address any stigma associated with MDD in a nonjudgmental way.
Distinguishing characteristics of vortioxetine
Vortioxetine is a serotonin modulator and stimulator approved for the treatment of MDD in adult patients aged 18 years or older.
In addition, vortioxetine has demonstrated efficacy as maintenance therapy for the prevention of MDD relapse and/or recurrence. New data have recently demonstrated that long-term continuation of treatment with vortioxetine delays recurrence of depressive episodes compared with placebo.
Subsequent updates to the vortioxetine US label support its use in patients with treatment-emergent sexual dysfunction and those experiencing certain cognitive symptoms.
Importantly, vortioxetine has demonstrated good tolerability, with no dose adjustments required for patients with renal or hepatic impairment.
Introduction
With major depressive disorder (MDD) recognized as a major mental health concern in the US population,1,2 screening for depression is now recommended as part of routine primary care. A vast number of reports in the literature have documented the devastating impact of MDD and associated suicide on the individual, family, and society as a whole. Primary care clinicians (PCCs) are at the front lines of diagnosing and managing patients who suffer from depression. For this reason, education and practical guidance for PCCs on the screening and management of individuals at risk for MDD and suicide are needed.
Screening for MDD, Suicide Risk, and Measurement-Guided Care
The United States Preventive Services Task Force (USPSTF) 2016 recommendations for screening for MDD include, but are not limited to, a comprehensive list of high-risk individuals: women, young and middle-aged adults, non-White persons, persons who are undereducated or who have lower socioeconomic status, previously married, or unemployed, or those who have chronic illnesses or other mental health disorders, or a family history of psychiatric disorders.3 Assessment of drug and alcohol abuse may also provide insight into individuals at risk for depression and suicide, given the rates of suicide by overdose and unintentional overdose with associated deaths.4 Alcohol and/or drug abuse or dependency may also contribute to poor response to treatment of MDD.5
Use of validated self-administered screening tools, such as the 9-item Patient Health Questionnaire (PHQ-9), is an important standard of care that not only helps in the diagnosis of individuals with MDD but also guides management and monitoring of symptoms.6,7 Measurement-guided care refers to a systematic approach to treatment for clinical practice that includes routine use of validated symptom scales and side effect measurements. These tools should be brief, either self-administered or easy to administer as measurement instruments. Guidance can then be provided for clinicians on when and how to modify medication doses at critical decision points.8,9 Table 1 provides an overview of some of the key screening and monitoring tools for MDD, suicide risk, and differential diagnosis. Examples of screening instruments for MDD recommended by the USPSTF include the PHQ in various forms and the Hospital Anxiety and Depression Scale in adults, the Geriatric Depression Scale in older adults, and the Edinburgh Postnatal Depression Scale in postpartum and pregnant women.3 The PHQ-9 and the Columbia-Suicide Severity Rating Scale may be particularly useful in primary care settings.10,11 In addition, the US National Strategy for Suicide Prevention recommends the use of suicide-prediction tools, and the European Psychiatric Association endorses using these tools as adjuncts to an individual psychiatric assessment.12
Table 1.
Details of Select Patient Screening Tools for Depression and Suicide That are Suited to Clinical Practice
| Patient Screening Tool | Details |
|---|---|
| Patient Health Questionnaire-9 (PHQ-9) | ● A brief self-administered questionnaire for mental disorders scoring each of the 9-DSM-IV criteria as “0” (not at all) to “3” (nearly every day) ● Higher scores indicate increasing severity of depression ● It takes approximately 2 minutes to complete10,56 |
| Hospital Anxiety and Depression Scale (HADS) | ● Designed to be a simple and reliable tool for the evaluation of anxiety and depression in clinical practice ● It takes only 2–5 minutes to complete ● Seven items each for depression and anxiety, with a score of 8–10 being indicative of the presence of depression or anxiety in the patient57 |
| Geriatric Depression Scale (GDS) | ● Long-form version is a brief 30-item questionnaire in which participants are asked to respond by answering yes or no in reference to how they felt over the past week ● A short-form version consisting of 15 questions that can be completed in 5–7 minutes is also available ● The most extensively tested and used depression scale in the elderly population ● Increasing scores indicate increasing severity58,59 |
| Edinburgh Postnatal Depression Scale | ● A 10-item self-completed questionnaire that can be completed in 5 minutes, with simple scoring to determine depression in new mothers60 |
| Columbia-Suicide Severity Rating Scale (C-SSRS) | ● Quantifies the severity of suicidal ideation and behavior in clinical and research settings ● Measures 4 constructs including severity of ideation, intensity of ideation, behavior, and lethality ● The questionnaire should take between 5 and 8 minutes to complete11,61 |
| Mood Disorders Questionnaire (MDQ) | ● Frequently used screening questionnaire for BPD that can be completed in 5 minutes in primary care settings ● Includes 13 questions plus items that assess clustering of symptoms and degree of functional impairment ● Patients who screen positive on the MDQ should undergo a full clinical evaluation for BPD15,16 |
| Rapid Mood Screener (RMS) | ● Utilizes easily understood terminology to screen for manic symptoms and BPD risk factors in less than 2 minutes ● Can be used during or outside of a clinical visit (eg, online, waiting room, via electronic medical record system)15 |
Abbreviations: BPD, bipolar disorder; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition.
MDD is truly a diagnosis of exclusion. It is imperative to rule out bipolar disorder (BPD) to make a diagnosis of MDD.13 It has been estimated that as many as 1 in every 4 to 5 patients presenting with a major depressive episode may eventually transition to BPD.14 This may be in part because patients with BPD who present for treatment are more likely to present with depressive symptoms than with symptoms of mania. Two screening tools in particular, the Rapid Mood Screener (RMS) and Mood Disorders Questionnaire (MDQ) are suitable for screening BPD in primary care settings.15 Patients who screen positive on the RMS or MDQ should undergo a full clinical evaluation for BPD.16
Suicidal ideation and behavior is implicated in several neuropsychiatric disorders, including borderline personality disorder, schizophrenia, and BPD.17,18 Suicide risk is an important consideration for patients with MDD who initiate treatment with antidepressants, as antidepressants as a class have been associated with an increased risk of suicidal thoughts and behaviors in certain age groups.19 As an example, when given to individuals with BPD as monotherapy, antidepressants can precipitate an unintended response, such as no response, poor response, or antidepressant misadventure, which can precipitate hypomania and mania, subsequently leading to worsening of disease and heightened suicide risk.20,21 Treatments including anti-anxiety medications and opioid-based analgesics have been associated with an increased suicide risk in certain patients, although caution should be exercised when examining associations and drawing conclusions about causality from observational data.22 Thus, suicide screening is important for all patients receiving treatment with antidepressant therapy, and assessment of suicidal ideation and behaviors should be a routine part of treatment monitoring.4,18 Of note, denial of suicidal ideation would appear to be more common than previously acknowledged, thus presenting a formidable challenge for clinicians, since denial, by itself, is an inadequate indicator of suicide risk.18 The USPSTF is currently updating its recommendations on screening patients for suicide.23 In terms of timing and frequency of assessment, it is considered ideal for PCCs to have 3 contacts with the patient within 12 weeks after the initial identification of a depressed patient, though visit frequency must be optimized based on individual patient needs. The PHQ-9 should be administered during follow-up visits to monitor patient progress and to assess treatment response, keeping in mind that item 9 on the PHQ-9 asks about suicidality.24
Of relevance to clinical practice for the monitoring of side effects related to treatment is the Frequency, Intensity, and Burden of Side Effects Rating (FIBSER) Scale, which is a brief self-report scale developed to document these domains of side effects from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) prospective clinical trial. In that clinical program, which enrolled over 4000 patients, the FIBSER was demonstrated to be a reliable and valid measure of the 3 domains of side effects in a depressed population and is brief enough to be well-suited to clinical practice.25 Another simple self-report measure, the Antidepressant Side-Effect Checklist, is a reliable scale for the assessment of side effects related to antidepressant therapy. It measures 21 adverse reactions, including dry mouth, drowsiness, and difficulty sleeping.26
While these screening and monitoring tools can provide a helpful standardized way to evaluate patients for depression and depressive symptoms in clinical practice, consideration of individual patient characteristics, including comorbidities and the use of concomitant medications, can help guide treatment selection and improve the chance of positive treatment outcomes.27
Shared Decision-Making in Primary Care Settings: Collaboration Between the Clinician and Patient
In primary care settings, shared decision-making between the clinician and patient can improve patient education and comfort; this should include educating the patient about treatment options and taking into consideration any concerns the patient may have about treatment.28 Key concerns expressed by patients receiving long-term (3–15 years) treatment for MDD included weight gain, withdrawal effects, and sexual dysfunction.29 In addition to providing the patient with an opportunity to address treatment issues and concerns, the PCC has an opportunity to address any associated stigma and discrimination via psychoeducation in a validating, nonjudgmental manner. Legitimizing depression as a chronic illness has been shown to increase the patient’s willingness to receive appropriate care.30 Patient resources from established mental health associations and foundations may also help newly diagnosed patients with MDD feel supported, improve their education on the condition, and minimize their isolation. These support groups allow patients to discuss their conditions and foster the shared decision-making process by offering a platform for provider-patient discussions. Table 2 provides a list of selected patient support resources and groups from various mental health foundations.
Table 2.
A Selection of Support Groups for Patients with MDD
| Name | Details |
|---|---|
| Anxiety & Depression Association of America | ● Has a support group directory54 ● https://adaa.org/find-help/support |
| Depression and Bipolar Support Alliance | ● Provides information on support for specialized groups, including military veterans and caregivers55 ● https://www.dbsalliance.org/support/chapters-and-support-groups/online-support-groups/ ● DBSAlliance.org also has a screening center that includes self-rated scales and educational resources for patients and families supporting loved ones and friends with mood disorders |
| Mental Health America | ● Large discussion community on a wide range of topics related to depression and anxiety62 ● https://www.mhanational.org/find-support-groups |
| 7 Cups | ● Offers free 24/7 one-on-one chat with volunteer listeners63 ● https://www.7cups.com |
| National Alliance on Mental Illness (NAMI) | ● Follows a structured model to ensure that patients have an opportunity to be heard and receive the support they need64 ● https://nami.org/Support-Education/Support-Groups/NAMI-Connection |
Abbreviation: MDD, major depressive disorder.
Beyond the patient-provider relationship, seeking permission to involve family members and friends in psychoeducation and treatment can be important for fostering a positive therapeutic relationship.30 Information from family members, friends, and other supporters may help confirm the differential diagnosis of MDD, BPD, and suicidality; however, caution should be advised, as input from certain family members or friends may be unhelpful, and must always be engaged with the consent and endorsement of the patient.30,31
Current treatment options for patients with MDD include psychotherapy and pharmacotherapy, alone or in combination. Involvement of patients in the shared decision-making process while considering these treatment options is critical for long-term adherence and improvement of MDD. Common pharmacological options include selective serotonin reuptake inhibitors (SSRIs), serotonin (5-HT) transporter (SERT) inhibitors, tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs).30 Among these options is vortioxetine, a second-generation multimodal serotonergic agent, approved in the US for the treatment of MDD in adult patients since 2013 (Table 3).19 The mechanism of antidepressant effect of vortioxetine is not well understood, but it is thought to act via serotonin reuptake inhibition and serotonin receptor modulation.19,32
Table 3.
Key Features of the Vortioxetine Clinical Profile
| Vortioxetine Clinical Profile: Key Features | |
|---|---|
| Indication | ● Treatment of MDD in adults aged ≥18 years |
| Approval year | ● 2013 |
| Suitable for specific populations? | ● Elderly patients with MDD (aged ≥64 years) ● Patients with MDD experiencing TESD on SSRIs ● Patients with MDD experiencing impairments in certain aspects of cognition |
| Suitable as a maintenance therapy? | ● Yes |
| Approved for children? | ● No |
| Suitable in pregnancy and breastfeeding? | ● Caution advised; use in third trimester may increase risk of persistent pulmonary hypertension and withdrawal in newborn ● No data are available on human lactation, but vortioxetine was present in the milk of animals when studied |
| Dose | ● Starting dose is 10 mg/day orally once daily, increased to 20 mg/day or decreased to 5 mg/day as tolerated |
| Necessary to take with food? | ● No |
| Time to onset of antidepressant effects? | ● Approximately 2 weeks, with the full antidepressant effect observed after 4 weeks or longer in some cases |
| Abrupt discontinuation OK? | ● Yes, although 15 mg/day and 20 mg/day doses should be reduced to 10 mg/day for 1 week prior to full discontinuation due to risk of AEs |
| Most common adverse reactions (≥5%) | ● Nausea, constipation, vomiting |
| Contraindications | ● Hypersensitivity to vortioxetine or any of its components ● MAOIs (allow 14 days to elapse before switch to vortioxetine or 21 days after stopping vortioxetine to start MAOIs) |
| Dose adjustments for renal or hepatic impairment? | ● No |
| Effects on weight? | ● No |
| Black box warning? | ● Suicidal thoughts and behaviors (observed in pediatric and young adult patients) |
| Warnings and precautions | ● Serotonin syndrome ● Risk of bleeding ● Activation of mania ● Angle closure glaucoma ● Hyponatremia |
| Drug interactions | ● MAOIs, other serotonergic drugs ● Strong inhibitors of CYP2D6 (such as bupropion, fluoxetine, paroxetine, quinidine; reduce dose of vortioxetine by half) ● Strong inducers of CYP (such as rifampin, carbamazepine, phenytoin) ● Drugs that interfere with hemostasis (antiplatelets and anticoagulants, including aspirin, heparin, and warfarin) ● Drugs highly bound to plasma protein (warfarin) |
Note: Data from TRINTELLIX (vortioxetine) [package insert].19
Abbreviations: AEs, adverse events; MAOIs, monoamine oxidase inhibitors; MDD, major depressive disorder; SSRI, selective serotonin reuptake inhibitors; TESD, treatment-emergent sexual dysfunction.
Vortioxetine Clinical Profile
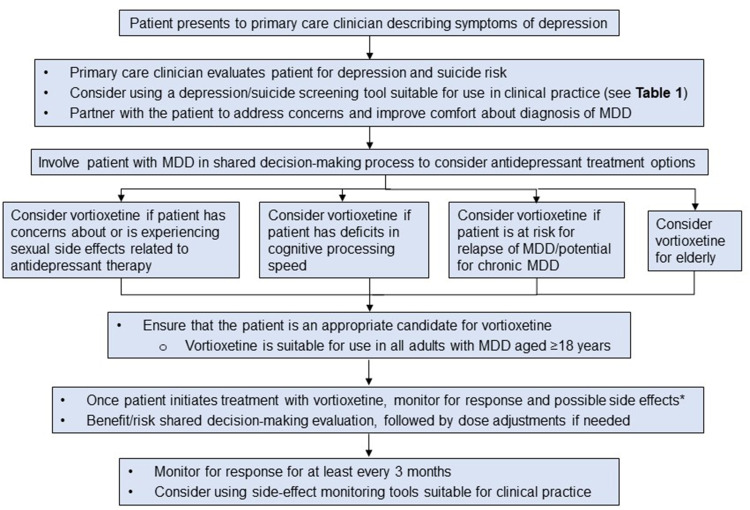
Recent label updates for vortioxetine include new relapse-prevention data and improvements in patients with treatment-emergent sexual dysfunction (TESD) and those experiencing symptoms related to cognitive processing speed. Here, we provide practical guidance for the implementation of vortioxetine in the management of MDD in primary care settings, with case vignettes (hypothetical examples) highlighting patients who may be particularly suitable for treatment with vortioxetine. In addition, the management of potential adverse events (AEs) with vortioxetine and the need for a shared decision-making process to evaluate benefit-risk ratio is also addressed. Figure 1 provides an overview of how patients with MDD may be screened, diagnosed, and monitored in primary care settings, and includes considerations for selecting vortioxetine to treat MDD.
Figure 1.
Screening, decision-making algorithm for vortioxetine, and monitoring patients with MDD. *Nausea, constipation, vomiting, diarrhea, dry mouth, and dizziness.18
Note: These are commonly reported adverse reactions with serotonergic antidepressants.29
Abbreviation: MDD, major depressive disorder.
Six 6- to 8-Week Studies Supported the Approval of Vortioxetine for the Treatment of MDD
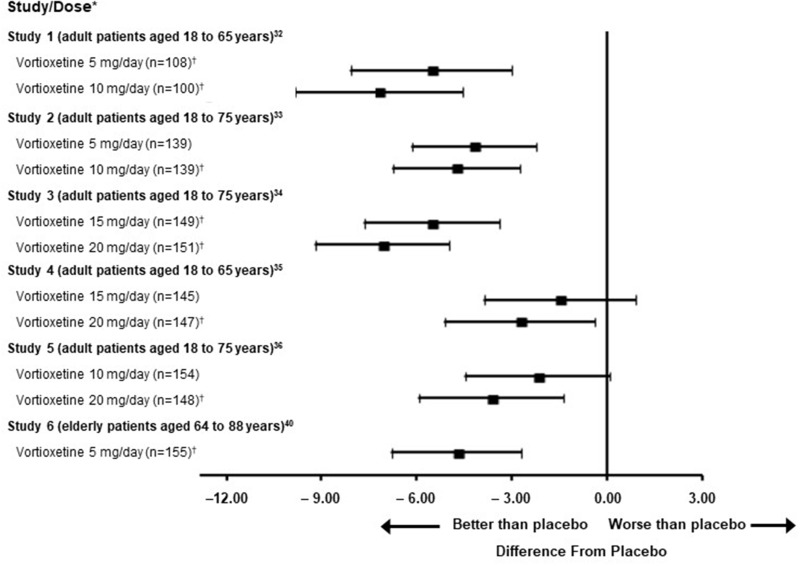
Vortioxetine was approved for the treatment of MDD in adult patients based on six 6- to 8-week randomized, double-blind, placebo-controlled, fixed-dose studies including 1 study in the elderly (Figure 2); and 1 maintenance study in adult inpatients and outpatients who met the Diagnostic and Statistical Manual of Mental Disorders IV Text Revision criteria for MDD.19,33–37 In the placebo-controlled studies, patients were randomized to receive vortioxetine 5 mg, 10 mg, 15 mg, 20 mg, or placebo once daily. The primary efficacy measures were the Hamilton Depression Scale-24 or the Montgomery-Åsberg Depression Rating Scale (MADRS) total score (Table 4).19,33–37 In each of these studies, at least 1 dose group of vortioxetine was superior to placebo in improvement of depressive symptoms as measured by mean change from baseline to endpoint visit on the primary efficacy measurement used in each study.19,33–37 The initial antidepressant effect of vortioxetine was generally observed starting at week 2 and increased in subsequent weeks, with the full antidepressant effect of vortioxetine not observed until week 4 or later.19,37
Figure 2.
Difference from placebo in LS mean change from baseline in MADRS total score at week 6 or week 8 in patients treated with vortioxetine in 6 clinical studies. Reprinted from TRINTELLIX (vortioxetine) [package insert].19 *Point estimates and unadjusted 95% confidence intervals are from MMRM analysis. In Study 1, the analysis was performed at week 6, and in others at week 8. The primary efficacy measure was based on MADRS score, except for Studies 2 and 6 (HAMD-24 scores). †Doses that are statistically significantly superior to placebo after adjusting for multiplicity.
Abbreviations: HAMD, Hamilton Depression Scale; LS, least-squares; MADRS, Montgomery-Åsberg Depression Rating Scale; MMRM, mixed model for repeated measures.
Table 4.
Selected Scales and Tests Used in the Clinical Studies for Vortioxetine
| Name | Details |
|---|---|
| Hamilton Depression Scale (HAMD-24) | ● A clinician-administered depression assessment scale65 ● Primary endpoint in a clinical study of vortioxetine in patients with MDD aged 18–75 years19 |
| Montgomery-Åsberg Depression Rating Scale (MADRS) | ● A widely used scale in clinical trials of antidepressant therapies administered by a clinician and designed to be sensitive to change66 ● Primary endpoint in 4 clinical studies of vortioxetine in patients with MDD aged 18–75 years19 ● Also used in maintenance study of vortioxetine treatment up to 64 weeks19 |
| Clinical Global Impression of Improvement (CGI-I) scale | ● Clinician’s assessment of the patient’s global functioning prior to and after treatment initiation41,42 ● Used in the clinical trial of vortioxetine in elderly patients with MDD19 |
| Changes in Sexual Functioning Questionnaire Short-Form (CSFQ-14) | ● Designed to measure illness and medication-related changes in sexual functioning, and consists of 14 items measuring sexual function as a total score19,37 |
| Arizona Sexual Experiences Scale (ASEX) | ● Validated measure for the identification of sexual side effects67 ● Used in placebo-controlled trials of vortioxetine19 |
| Digit Symbol Substitution Test (DSST) | ● Neuropsychological test that specifically measures processing speed68 ● Used in the clinical trial of vortioxetine to evaluate cognitive processing speed19 |
Abbreviation: MDD, major depressive disorder.
Vortioxetine Has Demonstrated Efficacy as a Maintenance Therapy for the Prevention of MDD Relapse
Vortioxetine is effective as a maintenance therapy for MDD over the long term and may be suitable for patients who are at risk of relapse of MDD.19,38 This was evaluated in a double-blind, randomized, placebo-controlled study, with an open-label, fixed-dose period of 12 weeks followed by a double-blind, fixed-dose, placebo-controlled period of 24–64 weeks. Participants in remission at weeks 10 and 12 (as defined by MADRS total score ≤10) who received vortioxetine during the double-blind period had significantly longer time to relapse (defined as MADRS total score ≥22 or an insufficient therapeutic response) compared with participants who received placebo during the double-blind period.19,38
In a US-based relapse prevention study of 580 patients in stabilized remission after open-label treatment with 10 mg vortioxetine, randomization to 5, 10, or 20 mg vortioxetine led to a statistically significant longer time to recurrence of depressive episodes compared with placebo.19,39 Importantly, patients achieving remission on the 10 mg/day dose of vortioxetine in the acute phase maintained remission and treatment tolerability during the double-blind phase across the approved dose range.39 These data were part of a subsequent label update for vortioxetine.
Vortioxetine is a Suitable Treatment for MDD in Older Patients Aged 64 to 88 Years
Vortioxetine may be a suitable treatment for older patients with depression, with a lifetime prevalence of 14.4% in individuals aged ≥65 years, based on a recent large-scale study conducted in the US.40 In a randomized, double-blind, placebo-controlled, fixed-dose study of vortioxetine in patients aged 64 to 88 years with MDD, vortioxetine was superior to placebo on the Clinical Global Impression of Improvement scale, which is a clinician’s assessment of the patient’s global functioning prior to and after treatment initiation (Figure 2, Table 4).19,41,42
Use of Vortioxetine in Patients with TESD
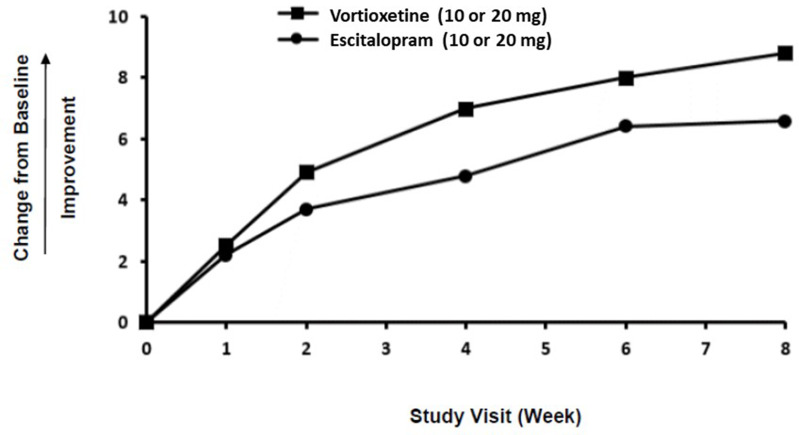
TESD is a key concern of patients with MDD and frequently a reason for early self-discontinuation of antidepressant therapy.29,43 As many as 73% of patients receiving treatment for depression express concern about sexual problems as a side effect of treatment.29,43 Therefore, it is important to routinely inquire about possible sexual side effects of antidepressant treatment. Based on results from 2 clinical studies, vortioxetine may be a treatment of choice in managing depressive disorders when sexual functioning is a concern.19,44 The effect of vortioxetine on TESD induced by prior SSRI treatment in MDD patients whose depressive symptoms were adequately treated was evaluated in an 8-week, randomized, double-blind, active-controlled (escitalopram), flexible-dose study.19,45 The allowed prior monotherapy treatments (before switch to vortioxetine) included citalopram, paroxetine, or sertraline, and participants were required to have been on a stable dosage for at least 8 weeks.45 Improvement in TESD (induced by prior SSRI treatment) in participants who switched to vortioxetine was superior to the improvement observed in participants who switched to escitalopram after 8 weeks of treatment, while both drugs maintained the patients’ prior antidepressant response (Figure 3).19,45
Figure 3.
Effect of vortioxetine compared with escitalopram on sexual functioning in adults with MDD experiencing SSRI-induced sexual dysfunction. The graph shows the change from baseline in CSFQ-14 total score during the double-blind treatment period. Reprinted from TRINTELLIX (vortioxetine) [package insert].19
Abbreviations: CSFQ, Changes in Sexual Functioning Short-Form; MDD, major depressive disorder; SSRI, selective serotonin reuptake inhibitor.
Of note, vortioxetine 10 mg (but not 20 mg) showed statistically significantly less TESD in healthy individuals compared with paroxetine 20 mg and placebo, highlighting the benefit of vortioxetine without the confounding effects of MDD.44 Lower doses of vortioxetine produced an incidence of TESD comparable with placebo. Thus, vortioxetine is a suitable antidepressant therapy for patients experiencing TESD, and the dose of vortioxetine may be lowered if patients experience side effects of a sexual nature or any other side effects. (Box 1).
Box 1.
Case Vignette 1
Switching from SSRI to Vortioxetine Because of TESD |
A 28-year-old woman with a 12-month history of MDD who has been effectively treated with an SSRI begins to experience diminished sexual functioning. Specifically, she reports loss of interest in sex and reduced ability to have an orgasm. She has a long-term partner and no other health concerns of note. Her PCC discusses the extent of the patient’s sexual dysfunction with her and decides to switch her from her current SSRI to vortioxetine 10 mg for 1 week, followed by an increase to 20 mg. On initial follow-up 1 month later, the patient reports a mild improvement in symptoms of TESD and good tolerability of vortioxetine. At another follow-up 1 month later, the patient reports a good response to treatment, with increased interest in sex and improvement in ability to have an orgasm. This is consistent with her responses on the Changes in Sexual Functioning Short-Form, which the PCC asks her to complete during her follow-up. The patient reports mild nausea and dizziness at this visit, but otherwise reports good tolerability. Her PCC asks to see her again in 3 months. |
Use of Vortioxetine in Individuals with MDD and Symptoms Related to Cognitive Processing Speed
Treating cognitive symptoms such as slowed processing speed, impaired learning and memory, as well as worsened executive function, decision-making, and response control can improve both functioning and performance in patients with MDD.46 Patients treated with vortioxetine have shown improvements in processing speed, which is an aspect of cognitive function that can be impaired in MDD. Subjective complaints of cognitive deficits are prevalent in MDD, with deficits in processing speed being one of the most replicated cognitive symptoms reported.46 Cognitive processing speed may affect a person’s ability to perceive, recognize, and appropriately interpret emotions, and deficits in these aspects of cognition have the potential to impair coping abilities and interfere with a patient’s normal functioning and outcomes.47 Data from 2 clinical studies suggest that vortioxetine may be a suitable treatment for patients with MDD who experience symptoms related to processing speed. Two 8-week randomized, double-blind, placebo-controlled studies were conducted to evaluate the effect of vortioxetine as measured by the Digit Symbol Substitution Test (DSST) during the treatment of acute MDD. The DSST is a neuropsychological test that specifically measures processing speed.19,36 In both studies, patients in the vortioxetine group had a statistically significant greater improvement in the number of correct responses on the DSST; depressed mood as assessed by change from baseline in MADRS total score also improved in both studies.19,36,48(Box 2).
Box 2.
Case Vignette 2
Vortioxetine for Elderly Patient with Cognitive Issues |
A 74-year-old man recently diagnosed with MDD complains of having difficulty concentrating. Comorbidities include high blood pressure and a history of asthma; concurrent medications are lisinopril and a bronchodilator. As part of the clinical workup, Alzheimer’s disease was ruled out, and a Mini-Mental State Exam was performed, with a resulting score of 29/30. After a discussion with the patient about his treatment options, the patient was prescribed vortioxetine at the full dose, as dose reductions are not required based on age. However, due to the age of the patient, his PCC monitored for signs and symptoms of hyponatremia, such as nausea, confusion, and headache, as elderly patients on antidepressants are at increased risk for this. At a follow-up visit 1 month later, the patient was tolerating vortioxetine well and did not report any signs or symptoms of hyponatremia but did report feeling nauseous at times and having dry mouth. At a 3-month follow-up visit, the patient reported improvement in symptoms of depression and ability to concentrate. |
Vortioxetine Has a Favorable Benefit-Risk Profile, with Good Tolerability and No Dose Adjustments Required for Patients with Renal or Hepatic Impairment
Vortioxetine was well tolerated in clinical trials; nausea, constipation, and vomiting were the most common AEs associated with vortioxetine.19 Frequency of nausea with vortioxetine was dose-dependent, and usually considered mild or moderate in intensity, with a median duration of 2 weeks.19,49
No clinically significant differences in the exposure of vortioxetine were observed based on age, sex, ethnicity, or renal or hepatic function, and thus, no dose adjustments are required for patients with hepatic or renal impairment (Table 3); however, other dose adjustments may be required as reviewed in the next section.19,50
Vortioxetine is not approved for the treatment of children and adolescents aged <18 years.19 However, like other antidepressants, vortioxetine carries a boxed warning for suicide risk because of an observed increase in suicidal thoughts and behaviors in pediatric and young adult patients taking antidepressants.19 Of note, vortioxetine was not included in the original meta-analysis that led to the requirement for the boxed warning for antidepressants by the US Food and Drug Administration Advisory Committee; however, this labeling requirement was mandated for all antidepressants.51,52 In fact, a recent post hoc, pooled analysis of short- and long-term clinical trials for vortioxetine did not find an increased risk of suicidality in adults with MDD.51 As a general rule, however, all patients should be monitored closely for worsening and emergence of suicidal thoughts and behaviors.19
Dosing and Initiation of Vortioxetine Treatment, Drug Interactions, and Timing Considerations for Discontinuation
The recommended starting dose of vortioxetine is 10 mg administered orally once daily without regard to meals. Dosage should then be increased to 20 mg per day, as tolerated, or decreased to 5 mg once daily for improved tolerability in patients at risk of AEs.19 Drugs such as monoamine oxidase inhibitors, other serotonergic drugs, strong inhibitors of CYP2D6, strong CYP inducers, drugs that interfere with hemostasis, and drugs that are strongly bound to plasma protein may have clinically important interactions with vortioxetine (Table 3).19,50
Unlike other antidepressant therapies that require tapering, vortioxetine can be discontinued abruptly. However, abrupt discontinuation of the 15 mg/day and 20 mg/day dosages of vortioxetine is associated with adverse reactions such as headache and muscle tension; therefore, it is recommended that dosages of 15 mg/day or 20 mg/day be reduced to 10 mg/day for 1 week prior to full discontinuation if possible.19
Conclusions
Primary care clinicians have a vital role to play in the proper diagnosis and management of patients with MDD. Shared decision-making, as well as early and repeated follow-up visits with appropriate monitoring for AEs and suicidality, symptom improvement, and treatment adherence is recommended.4,7–9,27,28,30 This includes screening for MDD, ensuring the accuracy of the MDD diagnosis by excluding BPD as well as partnering with patients to provide them with the appropriate treatment options.15 Understanding each patient’s symptoms and treatment goals are important for the practitioner to determine the suitability of the antidepressant treatment. Involvement of patients in the decision-making process while evaluating different treatment options is critical for long-term adherence and remission of MDD.7,28,30,39 Discussion about treatment options, duration of treatment, likelihood of recurrent depression (after expected remission of MDD), as well as recommendation of mental health resources and support groups for newly diagnosed patients with MDD may also help patients feel more supported as they begin their treatment.7,29,53–55
One of the most troubling AEs for patients on antidepressant therapy is TESD.29,43 Thus, having a focused conversation with patients while discussing sexual effects of different treatment options is very important. Vortioxetine is a suitable acute and long-term treatment for adult patients with MDD and may be especially useful for patients experiencing sexual dysfunction associated with their current antidepressants. In addition, vortioxetine can also be a preferred treatment for patients with MDD who experience symptoms related to impaired cognition. Increasing the speed of cognitive processing has recently been established as another beneficial attribute for vortioxetine and is now expressed as such in the package label. Patients who are treated with vortioxetine for MDD should be monitored for response to treatment and for resolution of suicidality, with the goal of achieving and maintaining remission.7,24,38,39 Many AEs associated with antidepressants can be evaluated with approved screening tools (eg, FIBSER), and patients should be monitored and involved in the decision-making process to improve medication adherence and plan for any dose adjustments, if needed.4,7,24,27 There should be a discussion about the duration of successful antidepressant therapy and referral to a mental health specialist, as psychotherapy may help patients better understand stressors that may trigger or exacerbate their depressive symptoms. These considerations often increase the chance of successful treatment outcomes for patients with MDD.
Acknowledgments
Under direction of the authors, medical writing assistance was provided by Katherine Price, PhD, on behalf of Syneos Health Medical Communications, LLC. Takeda Pharmaceuticals U.S.A. and H. Lundbeck A/S provided funding to Syneos Health for support in writing this manuscript.
Funding Statement
This article was funded by Takeda Pharmaceuticals U.S.A., Inc., and H. Lundbeck A/S.
Data Sharing Statement
Not applicable for review article.
Ethics Approval
Not applicable for review article.
Author Contributions
All authors contributed to the conception and design of this article, including literature review and interpretation, as well as drafting and critically revising the manuscript for important intellectual content. All authors gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
C. Brendan Montano has received consulting fees from AbbVie, Acadia, Alkermes, Arbor, Eisai, Neos, Novo Nordisk, Otsuka, Sunovion, Supernus, and Takeda, and has also received payment/honoraria from AbbVie, Arbor, Eisai, Otsuka, and Takeda. W. Clay Jackson has received consulting fees and payment/honoraria from AbbVie, Alkermes, Genentech, Otsuka, and Sunovion; has received honoraria from AbbVie, Alkermes, Genentech, Otsuka, and Sunovion for attending meetings and/or travel; and has participated on a data safety monitoring board or advisory board for AbbVie, Alkermes, Genentech, Otsuka, and Sunovion. Denise Vanacore has received payments/honoraria from AbbVie. Richard Weisler has received grants or contracts from Allergan, Alkermes, Astellas, Axsome Therapeutics, Ironshore, Janssen, Lundbeck, Major League Baseball (MLB), Neos Therapeutics, Otsuka America Pharmaceuticals, Sirtsei Pharmaceuticals, Shire, Supernus Pharmaceuticals, Takeda, and Validus. The authors report no other conflicts of interest in this work.
References
- 1.Villarroel MA, Terlizzi EP. Symptoms of depression among adults: United States, 2019. NCHS Data Brief. 2020;379:1–8. [PubMed] [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on drug use and health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville MD: Center for Behavioral Health Statistics and Quality. Substance Abuse and Mental Health Services Administration; 2017. [Google Scholar]
- 3.United States Preventive Services Task Force. Screening for depression in adults: screening. Clinical summary; 2016. Available from: https://uspreventiveservicestaskforce.org/uspstf/recommendation/depression-in-adults-screening. Accessed February 22, 2021.
- 4.Ng CWM, How CH, Ng YP. Depression in primary care: assessing suicide risk. Singapore Med J. 2017;58(2):72–77. doi: 10.11622/smedj.2017006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayo Clinic [website]. Antidepressants and alcohol. What’s the concern? Available from: https://www.mayoclinic.org/diseases-conditions/depression/expert-answers/antidepressants-and-alcohol/faq-20058231. Accessed April 4, 2021.
- 6.Akincigil A, Matthews EB. National rates and patterns of depression screening in primary care: results from 2012 and 2013. Psychiatr Serv. 2017;68(7):660–666. doi: 10.1176/appi.ps.201600096 [DOI] [PubMed] [Google Scholar]
- 7.Guo T, Xiang YT, Xioa L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatr. 2015;172(10):1004–1013. doi: 10.1176/appi.ajp.2015.14050652 [DOI] [PubMed] [Google Scholar]
- 8.Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439–1445. doi: 10.1176/ps.2009.60.11.1439 [DOI] [PubMed] [Google Scholar]
- 9.Trivedi MH, Rush AJ, Gaynes BN, et al. Maximizing the adequacy of medication treatment in controlled trials and clinical practice: STAR*D measurement-based care. Neuropsychopharmacology. 2007;32(12):2479–2487. doi: 10.1038/sj.npp.1301390 [DOI] [PubMed] [Google Scholar]
- 10.Kroenke K, Spitzer RL, Williams JB. The PHQ-9. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posner K, Brown GK, Stanley B, et al. The Columbia–suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fazel S, Runeson B, Ropper AH. Suicide. N Engl J Med. 2020;382(3):266–274. doi: 10.1056/NEJMra1902944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung G, Deng Y, Zhao Q, et al. Distinguishing bipolar and major depressive disorders by brain structural morphometry: a pilot study. BMC Psychiatry. 2015;15:298. doi: 10.1186/s12888-015-0685-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratheesh A, Davey C, Hetrick S, et al. A systematic review and meta-analysis of prospective transition from major depression to bipolar disorder. Acta Psychiatr Scand. 2017;135(4):273–284. doi: 10.1111/acps.12686 [DOI] [PubMed] [Google Scholar]
- 15.McIntyre RS, Patel MD, Masand PS, et al. The Rapid Mood Screener (RMS): a novel and pragmatic screener for bipolar I disorder. Curr Med Res Opin. 2021;37(1):135–144. doi: 10.1080/03007995.2020.1860358 [DOI] [PubMed] [Google Scholar]
- 16.Hirschfeld RMA. The mood disorder questionnaire: a simple, patient-rated screening instrument for bipolar disorder. Prim Care Companion J Clin Psychiatry. 2002;4(1):9–11. doi: 10.4088/pcc.v04n0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orsolini L, Latini R, Pomili M, et al. Understanding the complex of suicide in depression: from research to clinics. Psych Invest. 2020;17(3):207–221. doi: 10.30773/pi.2019.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obegi JH. How common is recent denial of suicidal ideation among ideators, attempters, and suicide decedents? A literature review. Gen Hosp Psychiatry. 2021;72:92–95. doi: 10.1016/j.genhosppsych.2021.07.009 [DOI] [PubMed] [Google Scholar]
- 19.TRINTELLIX (vortioxetine) [package insert]. Lexington, MA: Takeda Pharmaceuticals America, Inc.; 2021. [Google Scholar]
- 20.Gitlin MJ. Antidepressants in bipolar depression: an enduring controversy. Int J Bipolar Disord. 2018;6(1):25. doi: 10.1186/s40345-018-0133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McElroy SL, Kotwal R, Kaneria R, Keck PE. Antidepressants and suicidal behavior in bipolar disorder. Bipolar Disord. 2006;8(5 Pt 2):596–617. doi: 10.1111/j.1399-5618.2006.00348.x [DOI] [PubMed] [Google Scholar]
- 22.Gibbons R, Hur K, Lavigne J, Wang J, Mann JJ. Medications and suicide: high dimensional empirical Bayes screening (iDEAS). Harvard Data Sci Rev. 2019;12. doi: 10.1162/99608f92.6fdaa9de [DOI] [Google Scholar]
- 23.United States Preventive Services Task Force. Screening for depression, anxiety, and suicide risk in adults, including pregnant and postpartum persons; 2020. Available from: https://uspreventiveservicestaskforce.org/uspstf/draft-update-summary/screening-depression-anxiety-suicide-risk-adults. Accessed February 22, 2021.
- 24.DeJesus RS, Vickers KS, Melin GJ, Williams MD. A system-based approach to depression management in primary care using the patient health questionnaire-9. Mayo Clin Proc. 2007;82(11):1395–1402. doi: 10.4065/82.11.1395 [DOI] [PubMed] [Google Scholar]
- 25.Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA. Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract. 2006;12(2):71–79. doi: 10.1097/00131746-200603000-00002 [DOI] [PubMed] [Google Scholar]
- 26.Uher R, Farmer A, Henigsberg N, et al. Adverse reactions to antidepressants. Br J Psychiatry. 2009;195(3):202–210. doi: 10.1192/bjp.bp.108.061960 [DOI] [PubMed] [Google Scholar]
- 27.Weihs K, Wert JM. A primary care focus on the treatment of patients with major depressive disorder. Am J Med Sci. 2011;342(4):324–330. doi: 10.1097/MAJ.0b013e318210ff56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeBlanc A, Herrin J, Williams MD, et al. Shared decision making for antidepressants in primary care: a cluster randomized trial. JAMA Intern Med. 2015;175(11):1761–1770. doi: 10.1001/jamainternmed.2015.5214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cartwright C, Gibson K, Read J, Cowan O, Dehar T. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401–1407. doi: 10.2147/PPA.S110632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng CWM, How CH, Ng YP. Managing depression in primary care. Singapore Med J. 2017;58(8):459–466. doi: 10.11622/smedj.2017080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffiths KM, Crisp DA, Barney L, Reid R. Seeking help for depression from family and friends: a qualitative analysis of perceived advantages and disadvantages. BMC Psychiatry. 2011;11:196. doi: 10.1186/1471-244X-11-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther. 2015;145:43–57. doi: 10.1016/j.pharmthera.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 33.Alvarez E, Perez V, Dragheim M, Loft H, Artigas F. A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15(5):589–600. doi: 10.1017/S1461145711001027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henigsberg N, Mahableshwarkar AR, Jacobsen P, Chen K, Thase ME. A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry. 2012;73(7):953–959. doi: 10.4088/JCP.11m07470 [DOI] [PubMed] [Google Scholar]
- 35.Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138–149. doi: 10.1097/YIC.0000000000000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahableshwarkar AR, Zajecka J, Jacobson W, Chan W, Keefe R. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology. 2015;40(8):2025–2037. doi: 10.1038/npp.2015.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobsen PL, Mahableshwarkar AR, Serenko M, Chan S, Trivedi MH. A randomized, double-blind, placebo-controlled study of the efficacy and safety of vortioxetine 10 mg and 20 mg in adults with major depressive disorder. J Clin Psychiatry. 2015;76(5):575–582. doi: 10.4088/JCP.14m09335 [DOI] [PubMed] [Google Scholar]
- 38.Boulenger JP, Loft H, Florea I. A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol. 2012;26(11):1408–1416. doi: 10.1177/0269881112441866 [DOI] [PubMed] [Google Scholar]
- 39.Thase ME, Hanson E, Jacobsen PL, et al. Efficacy and safety of vortioxetine (5, 10, and 20 mg) in relapse prevention: results of a randomized, double-blind, placebo-controlled, Phase 4 study in adults with major depressive disorder (MDD). Virtual poster presented at 2020 American Society for Clinical Psychopharmacology; May 29−30; 2020. [Google Scholar]
- 40.Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346. doi: 10.1001/jamapsychiatry.2017.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215–223. doi: 10.1097/YIC.0b013e3283542457 [DOI] [PubMed] [Google Scholar]
- 42.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry. 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobsen PL, Nomikos GG, Zhong W, Cutler AI, Affinito H, Clayton A. Clinical implications of directly switching antidepressants in well-treated depressed patients with treatment-emergent sexual dysfunction: a comparison between vortioxetine and escitalopram. CNS Spectr. 2020;25(1):50–63. doi: 10.1017/S1092852919000750 [DOI] [PubMed] [Google Scholar]
- 44.Jacobsen PL, Zhong W, Nomikos G, Clayton A. Paroxetine, but not vortioxetine, impairs sexual functioning compared with placebo in healthy adults: a randomized, controlled trial. J Sex Med. 2019;16(10):1638–1649. doi: 10.1016/j.jsxm.2019.06.018 [DOI] [PubMed] [Google Scholar]
- 45.Jacobsen PL, Mahableshwarkar AR, Chen Y, Chrones L, Clayton AH. Effect of vortioxetine vs. escitalopram on sexual functioning in adults with well-treated major depressive disorder experiencing SSRI-induced sexual dysfunction. J Sex Med. 2015;12(10):2036–2048. doi: 10.1111/jsm.12980 [DOI] [PubMed] [Google Scholar]
- 46.McIntyre RS, Cha DS, Socynska JK, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515–527. doi: 10.1002/da.22063 [DOI] [PubMed] [Google Scholar]
- 47.Kwasi Ahorsu D, Tsang HWH. Do people with depression always have decreased cognitive processing speed? Evidence through electrophysiological lens. Neuropsychiatry. 2018;8(4):1227–1231. [Google Scholar]
- 48.McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557–1567. doi: 10.1017/S1461145714000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baldwin DS, Chrones L, Florea I, et al. The safety and tolerability of vortioxetine: analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol. 2016;30(3):242–252. doi: 10.1177/0269881116628440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen G, Højer AM, Areberg J, Nomikos G. Vortioxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2018;57(6):673–686. doi: 10.1007/s40262-017-0612-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahableshwarkar AR, Affinito J, Heldbo Reines E, Xu J, Nomikos G, Jacobsen PL. Suicidal ideation and behavior in adults with major depressive disorder treated with vortioxetine: post hoc pooled analyses of randomized, placebo-controlled, short-term and open-label, long-term extension trials. CNS Spectr. 2020;25(3):352–362. doi: 10.1017/S109285291900097X [DOI] [PubMed] [Google Scholar]
- 52.Stone M, Laughren T, Jones ML, et al. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ. 2009;339:b2880. doi: 10.1136/bmj.b2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157(2):229–233. doi: 10.1176/appi.ajp.157.2.229 [DOI] [PubMed] [Google Scholar]
- 54.Support groups. Anxiety and Depression Association of America (ADAA) [website]. Available from: https://adaa.org/find-help/support/support-groups. Accessed February 23, 2021.
- 55.Online support groups. Depression and Bipolar Support Alliance (DBSA) [website]. Available from: https://www.dbsalliance.org/support/chapters-and-support-groups/online-support-groups/. Accessed February 23, 2021.
- 56.ChecKIT Series. PAR Staff. Administration and scoring of the patient health questionnaire-9 (PHQ-9) [technical supplement]; 2020. Available from: https://www.parinc.com/Portals/0/Webuploads/samplerpts/ChecKIT%20Series_PHQ-9_Tech%20Supp%20Paper.pdf. Accessed December 13, 2021.
- 57.Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1:1–29. doi: 10.1186/1477-7525-1-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yesavage J, Brink T, Rose T, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 59.Greenberg SA. The Geriatric Depression Scale (GDS). Try this. Best practices in nursing care to older adults. Available from: https://wwwoundcare.ca/Uploads/ContentDocuments/Geriatric%20Depression%20Scale.pdf. Accessed April 4, 2021.
- 60.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. 1987;150(6):782–786. doi: 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- 61.Núñez D, Arias V, Méndez-Bustos P, Fresno A. Is a brief self-report version of the Columbia severity scale useful for screening suicidal ideation in Chilean adolescents? Compr Psychiatry. 2019;88:39–48. doi: 10.1016/j.comppsych.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 62.Mental Health America (MHA) [website]. Available from: https://www.inspire.com/groups/mental-health-america/topic/depression/. Accessed February 23, 2021.
- 63.7 Cups [website]. Online therapy and free counseling. Available from: https://www.7cups.com/. Accessed February 23, 2021.
- 64.NAMI connection. National Alliance on Mental Illness (NAMI) [website]. Available from: https://nami.org/Support-Education/Support-Groups/NAMI-Connection. Accessed February 23, 2021.
- 65.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Montgomery S, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- 67.McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26(1):25–40. doi: 10.1080/009262300278623 [DOI] [PubMed] [Google Scholar]
- 68.Jaeger J. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol. 2018;38(5):513–519. doi: 10.1097/JCP.0000000000000941 [DOI] [PMC free article] [PubMed] [Google Scholar]