Abstract
Objective:
Attentional deficits following degeneration of brain cholinergic systems contribute to gait–balance deficits in Parkinson disease (PD). As a step toward assessing whether α4β2* nicotinic acetylcholine receptor (nAChR) stimulation improves gait–balance function, we assessed target engagement of the α4β2* nAChR partial agonist varenicline.
Methods:
Nondemented PD participants with cholinergic deficits were identified with [18F]fluoroethoxybenzovesamicol positron emission tomography (PET). α4β2* nAChR occupancy after subacute oral varenicline treatment was measured with [18F]flubatine PET. With a dose selected from the nAChR occupancy experiment, varenicline effects on gait, balance, and cognition were assessed in a double-masked placebo-controlled crossover study. Primary endpoints were normal pace gait speed and a measure of postural stability.
Results:
Varenicline doses (0.25mg per day, 0.25mg twice daily [b.i.d.], 0.5mg b.i.d., and 1.0mg b.i.d.) produced 60 to 70% receptor occupancy. We selected 0.5mg orally b.i.d for the crossover study. Thirty-three participants completed the crossover study with excellent tolerability. Varenicline had no significant impact on the postural stability measure and caused slower normal pace gait speed. Varenicline narrowed the difference in normal pace gait speed between dual task and no dual task gait conditions, reduced dual task cost, and improved sustained attention test performance. We obtained identical conclusions in 28 participants with treatment compliance confirmed by plasma varenicline measurements.
Interpretation:
Varenicline occupied α4β2* nicotinic acetylcholine receptors, was tolerated well, enhanced attention, and altered gait performance. These results are consistent with target engagement. α4β2* agonists may be worth further evaluation for mitigation of gait and balance disorders in PD.
Dopamine replacement therapy (DRT)-refractory gait and balance disorders are among the most morbid aspects of Parkinson disease (PD). Gait deficits, including postural instability and freezing, worsen with disease progression and substantially increase fall risk. Falls are a significant source of morbidity in PD patients, with a relatively high rate of serious falls leading to fractures and hospitalizations, precipitation of nursing home placement, and increased mortality associated with falls.1–4
The DRT-refractory nature of gait and postural deficits in PD indicates involvement of non-dopaminergic systems. Considerable evidence suggests that DRT-resistant gait and balance disorders are associated with degeneration of central nervous system (CNS) cholinergic projection systems.5–13 Fall risk in PD is likely to be increased by the conjunction of striatal dopaminergic denervation and degeneration of cholinergic neurons of the basal forebrain corticopetal complex (BFCC) and pedunculopontine–laterodorsal tegmental complex (PPN-LDT). The best-defined role of the BFCC is in attention, with suggestions that PPN-LDT cholinergic neurons play a role in alertness.12,14,15 Preclinical experiments indicate that as BFCC neurons are lost, gait–balance dysfunction may increase markedly as BFCC cholinergic deficits unmask the full impacts of striatal dopaminergic deficits.12,16 This model is consistent with results of dual task paradigm experiments, in PD and control participants, indicating that impaired attention is associated with worsening gait–balance functions and increased fall risk.17
Cholinergic neurotransmission is mediated by both G-protein–coupled receptors and ionotropic nicotinic acetylcholine receptors (nAChRs). The predominant CNS nicotinic receptor is the α4β2* nAChR (*potential other subunits).18 Stimulation of cortical α4β2* receptors plays an important role in attention, and this is likely the mechanism by which nicotine enhances attention. In the setting of BFCC projection degeneration, pharmacologic stimulation of α4β2* nAChRs might improve attention and mitigate gait–balance deficits.
Varenicline (VCN) is a potent (Ki = 0.4nM) α4β2* nAChR partial agonist (efficacy = 45%) used widely for tobacco abuse cessation.19,20 VCN has an excellent safety record and favorable pharmacokinetic features.20–23 To initiate exploration of the potential of VCN to improve DRT-resistant gait–balance deficits, we performed a target engagement study of VCN in PD participants with neocortical cholinergic deficits. We assessed target engagement along 2 dimensions: VCN binding to brain α4β2* nAChRs; and VCN effects on laboratory-based measures of gait, balance, and cognitive functions.
Whereas there is abundant literature characterizing gait, balance, and fall risk in PD and in normal aging, there are no laboratory-based measures predicting intervention outcomes. In the absence of measures with predictive validity, measures linked to pathophysiologic mechanisms are more likely to be adequate indices of target engagement. In secondary–exploratory analyses, we studied both objective measures of postural sway and gait speed using body-worn inertial sensors and cognitive outcome measures, including a cognitive measure specifically related to disrupted attentional and cholinergic functions.
Materials and Methods
Regulatory Compliance
Informed consent was obtained from all participants according to the Declaration of Helsinki. This study was approved by the University of Michigan Medical School Institutional Review Board. An Investigational New Drug application waiver for VCN study was obtained from the US Food and Drug Administration. This study was registered at ClinicalTrials.gov (NCT04403399, NCT02933372).
Participant Selection
PD participants were recruited from a larger cohort characterized with [18F]fluoroethoxybenzovesamicol ([18F]FEOBV) positron emission tomography (PET).9 The vesicular acetylcholine transporter ligand [18eF]FEOBV was used to determine the magnitudes of cortical cholinergic terminal deficits. All participants met the International Parkinson and Movement Disorder Society clinical diagnostic criteria for PD. All underwent [11C] dihydrotetrabenazine PET to confirm the presence of characteristic putaminal nigrostriatal dopaminergic terminal deficits. No enrolled participant was demented, using drugs or supplements with cholinergic properties, or using tobacco products. Because of anecdotal reports of worsening mood disorders, adverse ethanol interactions, and myocardial infarctions, we excluded individuals with an active mood disorder (Geriatric Depression Scale [GDS] > 5 and evidence of recent, worsening mood), alcohol use disorder (Alcohol Use Disorder Identification Test score > 7 for those older than 65 years; >8 for those 65 years and younger), and active cardiovascular disease. Participants were counseled to avoid alcoholic beverages during study participation. Only participants with cortical cholinergic deficits were enrolled. In PD, the occipital cortex has highest vulnerability for cholinergic transporter losses compared to other brain regions.24 Hypocholinergic status was defined as falling within the lower tertile of occipital cortical [18F]FEOBV binding in normal older adults. Participants were maintained on stable DRT regimens throughout these experiments. To ensure that there was not a marked difference between VCN interaction with α4β2* nAChRs in PD participant and control brains, we performed a more limited dose–response experiment in normal participants. Age-matched control participants without clinical evidence of parkinsonism or other neurologic disorders, and not using any cholinergic agents or tobacco products, were studied.
VCN Occupancy of α4β2* nAChRs Study
VCN occupancy of α4β2* nAChRs was assessed with ascending doses of VCN and the selective α4β2* nAChR PET ligand [18F] flubatine.25 PD and control participants in the dose response–receptor occupancy study were treated with oral (p.o.) VCN for 10 days with ascending dose schedules. Higher dosing cohorts were begun after the previous dosing cohort completed its scheduled treatment and follow-up. Doses chosen were 0.25mg per day, 0.25mg twice daily (b.i.d.), 0.5mg b.i.d., and 1.0mg b.i.d. The clinically used VCN dose is 1.0mg b.i.d. Participants received an initial 0.25mg dose following confirmation of eligibility and baseline evaluations and were monitored for 4 hours. In participants scheduled for higher VCN doses, the total daily dose was escalated over the next 2 days, followed by 8 days of stable daily VCN dose. α4β2* nAChR agonists may induce nAChR expression, and it is possible that receptor density may not be stable during α4β2* nAChR agonist exposure.26 As we cannot measure absolute receptor density, but only relative receptor occupancy, the conventional strategy of imaging participants before and at the end of a drug exposure period might result in underestimation of receptor occupancy. To address this issue, we imaged participants at the end of their drug exposure periods and again after 5 days (~5 half-lives) of washout from drug exposure. [18F]Flubatine was synthesized as described previously.27 Participants were scanned on a Biograph TruePoint Model 1094 (Siemens, Erlangen, Germany), using a dynamic acquisition of 18 frames over 90 minutes (4 × 0.5 minutes, 3 × 1 minutes, 2 × 2.5 minutes, 2 × 5 minutes, 7 × 10 minutes). α4β2* nAChR occupancy was estimated by comparing [18F]flubatine standardized uptake values (SUVs) on and off VCN. SUVs were calculated as (B – D) / (B – ND), where B is the SUV of the “baseline” scan (off VCN), D is the SUV of the “drug” scan (on VCN), and ND is estimated from the non-displaceable SUV. ND is calculated from the x-intercept of a regression of (B – D) on D.
Crossover Study
Following selection of a study dose from the α4β2* nAChR occupancy experiment in PD participants (see Results below), we completed a double-masked, placebo-controlled crossover study to assess VCN effects on measures of gait, balance, and cognition (Fig 1). Participants completing the initial receptor occupancy study were eligible to enroll in this experiment. Participants were randomized 1:1 to 1 of 2 treatment sequences: placebo followed by VCN 0.5mg b.i.d., or VCN followed by placebo. A statistician prepared the randomization list using permuted blocks with random block sizes. The list with randomization number and treatment allocation was sent to the research pharmacy, and a blinded list of randomization numbers was sent to the study coordinator. After patient consent was completed and eligibility was confirmed, the coordinator assigned the next randomization number to the participant, and sent a prescription with participant ID and randomization number to the research pharmacist who dispensed the appropriate study medication. To mask drug, VCN pills or placebo were encapsulated in gelatin sheaths. Participants received an initial 0.25mg dose or equivalent placebo following baseline evaluations and were monitored for 4 hours after initial study medication administration with total daily dose or equivalent placebo escalated over the next 2 days.
FIGURE 1:
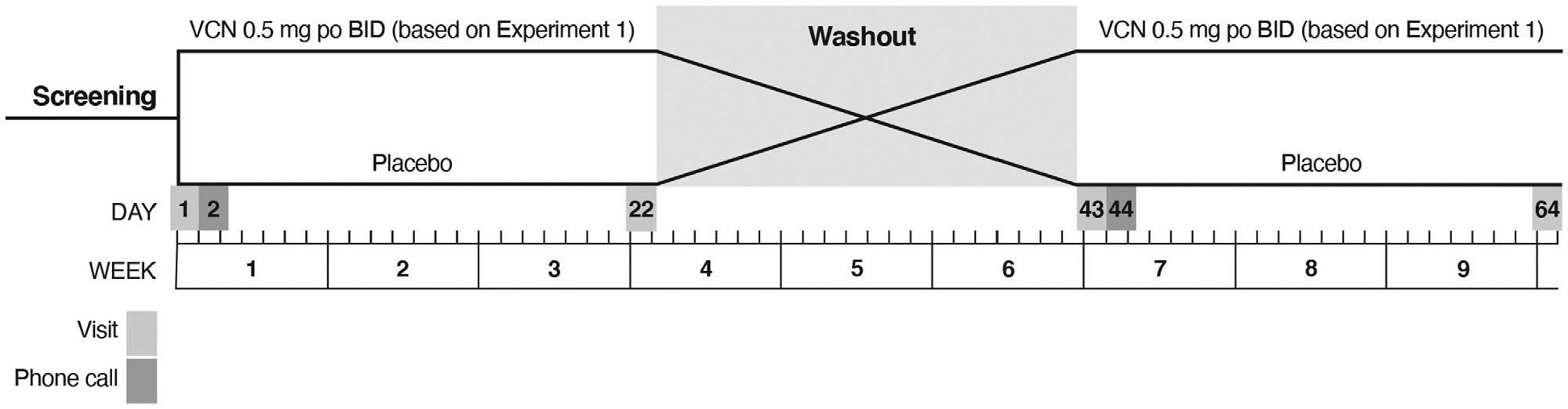
Design of crossover study. BID = twice daily; po = by mouth; VCN = varenicline.
Treatment periods were 3 weeks in duration and interrupted by 3-week washout periods. Participants underwent a standard evaluation at baseline, at the end of the first treatment period, at the end of the washout period–beginning of the second treatment period, and at the end of the second treatment period (see Fig 1). Outcome measures at the end of the VCN and placebo treatment periods were compared to assess VCN effects. The standard evaluation was a battery of motor, cognitive, and behavioral measures (see below). We a priori selected a measure of gait performance, normal pace gait speed, and a measure of postural stability, Mancini et al’s JERK, as coprimary endpoints.28,29 JERK is the time-based derivative of lower trunk accelerations during standing spontaneous sway. JERK was chosen because it tracks postural instability in PD.29 Normal pace gait speed was chosen because prior studies indicated that neocortical cholinergic denervation is associated with slower gait speed in PD.8 We hypothesized that VCN-treated participants would ambulate faster and that VCN treatment would reduce JERK. Gait analysis was performed on an 8m GAITRite pressure-sensitive walkway (CIR Systems, Peekskill, NY), and standard parameters were analyzed using ProtoKinetics Movement Analysis Software (GAITRite version 5.09C; ProtoKinetics, Havertown, PA). Gait assessments were repeated with a dual-task protocol in which participants counted backward by three starting at a random number (<100) provided by the examiner. Postural stability was assessed with the Ambulatory Parkinson’s Disease Monitoring (APDM) wearable sensor system (APDM Wearable Technologies, Portland, OR) using the iSWAY protocol, with participants standing on a foam pad with eyes open and eyes closed. Standard postural measures, including JERK, were assessed and calculated using the manufacturer’s software (Mobility Lab Version 1). We used the APDM system’s iTUG (Timed Up and Go) protocol to collect additional exploratory data. For cognition, we used a general cognitive measure, the Montreal Cognitive Assessment (MoCA), and selected tests to examine major cognitive domains, focusing on attention and executive function.
Motor Assessments
Motor assessments included Movement Disorder Society Unified Parkinson’s Disease Rating Scale, part III (MDS-UPDRS III; “on” state), MDS-UPDRS III postural instability and gait subscore (PIGD) score (sum of items 3.1, 3.9–3.13), gait speed (normal pace), gait speed (fast pace), gait speed (normal pace–dual task), gait speed (fast pace–dual task), and the postural stability measures mean sway velocity, JERK, root mean square sway distance (RMS), and double support time during gait. To assess the effects of attentional loading, normal pace and fast pace gait were performed under dual task conditions. Differences in gait speed between dual task and no dual task conditions are a measure of the attentional burden imposed by the dual task. To assess the effects of VCN on this aspect of gait performance, we compared the differences between no dual task and dual task gait speed between VCN and placebo treatment periods. We also computed the dual task cost (DTC; dual task gait speed minus no dual task gait speed divided by no dual task gait speed multiplied by 100), a standard metric of the attentional burden imposed by distractors during gait performance.30
Cognitive Assessments
Cognitive assessments included MoCA, Wechsler Adult Intelligence Scale–III Digit Symbol modalities test, California Verbal Learning Test (CVLT) short-term memory test; CVLT long-term memory test, CVLT recognition test, Delis–Kaplan Executive Function System (D-KEFS) Stroop III, D-KEFS sorting total, D-KEFS verbal fluency letters total, D-KEFS verbal fluency animals, D-KEFS Trail Making Test 4, and Judgment of Line Orientation test. We assessed attentional function with the Sustained Attention Test (SAT), established to reflect CNS cholinergic systems function in humans.31–33 The SAT is performed with 2 conditions: without (SAT) and with a distractor (dSAT). SAT and dSAT results are reported as the vigilance index, a measure that corrects estimates of accurate detection with penalties for false detections and not confounded by errors of omission.34
Behavioral Assessments
Behavioral assessments included GDS and Columbia Suicide Severity Rating Scale (C-SSRS).
Treatment Compliance Monitoring
To assess compliance, we measured plasma VCN levels at the ends of treatment periods. VCN concentrations were assessed by the University of Michigan College of Pharmacy Pharmacokinetics Core. Plasma samples were deproteinated with acetonitrile, extracts were centrifuged at 3,500 revolutions per minute for 10 minutes, and supernatants were used for liquid chromatography–mass spectrometry/mass spectrometry. The calibration curve with VCN concentrations from 2.5ng/ml 250ng/ml was highly linear (r = 0.999). Assay accuracy and precision were evaluated at 5ng/ml, 10ng/ml, and 200ng/ml (n = 3). Accuracy was 106% or less, and precision was 10% relative standard deviation (SD) or less.
Statistical Plan
The sample size (planned initially at 4 participants per dosing group) for the VCN-α4β2* nAChR occupancy study was based on logistical considerations. For the crossover study, we calculated that 33 participants would provide at least 80% power to detect within-patient treatment differences of 0.122m/s in gait speed and −0.131m2/s5 for JERK, assuming within-participant correlation of ≥0.64 and ≥0.72, respectively, using a paired t test and a 2-sided type I error of 0.025 (Bonferroni adjustment for coprimary endpoints). This approach is conservative, given that our analysis method uses mixed effects models. Estimates for treatment differences were based on Bohnen et al for normal pace gait speed and Mancini et al for JERK.8,28
We conducted exploratory analyses to examine the distributions of outcomes under each treatment, as well as individual and mean profiles over time. Graphical approaches such as boxplots and scatterplots with linear or nonlinear (eg, locally estimated scatterplot smoothing) methods were used, allowing identification of outliers, linearity, and correlation of measurements within participant and across time. Log transformations were applied when outcome data did not appear normally distributed.
Descriptive statistics for efficacy and safety outcomes were provided for each dosing cohort in the VCN-α4β2* nAChR occupancy study. For the crossover study, linear mixed models containing treatment sequence, treatment period, treatment group, and dependent-variable baseline value, with participant within treatment sequence as a random effect, were used for analysis of continuous outcomes. To compare differences between VCN and placebo, a test for carryover based on the sequence effect was conducted using patient with sequence as the error term. Results are presented as least squares mean and standard error. The coprimary endpoints were tested at the 2-sided 0.025 significance level. All other tests were based on a 2-sided significance level of 0.05, with no adjustments for additional multiple comparisons. Hence, p values should be interpreted in the context of hypothesis generation in this target engagement study. To estimate the magnitude of treatment effects, 95% confidence intervals are reported.
Primary and secondary efficacy endpoints were analyzed in all randomized participants (intention-to-treat [ITT] population). Secondary continuous endpoints were analyzed similarly to the primary endpoints. Categorical analyses were based on Gart test.35
Safety endpoints were analyzed in all randomized participants who received at least 1 dose of study medication. We included adverse events (AEs) that occurred in the washout period with the treatment given in period 1.
Results
Participants
Characteristics of the 15 PD participants enrolled for the initial VCN-α4β2* nAChR occupancy study are described in Table 1. Ten participants completed this phase of the study; 2 participants received 0.25mg VCN per day, 3 participants 0.25mg b.i.d. VCN per day, 3 participants 0.5mg b.i.d., and 2 participants 1.0mg b.i.d. Of the 5 PD participants not completing the imaging substudy, 1 PD participant was unable to tolerate PET imaging, tracer synthesis failed in 2 PD participants, and 2 PD participants discontinued VCN before PET imaging could be attempted. To confirm that α4β2* nAChR–VCN interactions were not grossly different in PD compared to normal brain, an additional 10 control participants were studied with ascending doses of VCN and [18F]flubatine PET in a protocol identical to that used for PD. All 10 control participants completed the imaging study protocol. Data from 1 control participant were excluded because of suspected covert tobacco abuse. Four participants received 0.25mg per day, 3 participants received 0.25mg b.i.d., and 3 participants 0.5mg b.i.d. Characteristics of participants for the VCN-α4β2* nAChR occupancy studies are described in Table 1.
TABLE 1.
Varenicline–α4β2* Nicotinic Acetylcholine Receptor Occupancy Study Participant Baseline Characteristics
| Characteristic | PD Participants, n = 15 | Control Participants, n = 10 |
|---|---|---|
| Age, yr | ||
| Mean (SD) | 67.3 (5.20) | 66.4 (8.95) |
| min, max | 52, 73 | 50, 76 |
| Male, n (%) | 14 (93%) | 8 (80%) |
| White, n (%) | 14 (93%) | 10 (100%) |
| Age at diagnosis, yr | ||
| Mean (SD) | 61.4 (5.69) | Not applicable |
| min, max | 51, 70 | |
| MDS-UPDRS III | ||
| Mean (SD) | 31.9 (12.83) | 3.5 (1.84) |
| min, max | 9, 57 | 1, 7 |
| GDS | ||
| Mean (SD) | 3.2 (3.75) | 1.6 (2.01) |
| min, max | 0, 12 | 0, 6 |
| MoCA | ||
| Mean (SD) | 25.5 (1.73) | 26.8 (2.30) |
| min, max | 23, 29 | 23, 30 |
GDS = Geriatric Depression Scale; max = maximum; MDS-UPDRS III = Movement Disorder Society Unified Parkinson’s Disease Rating Scale, part III; min = minimum; MoCA = Montreal Cognitive Assessment; PD = Parkinson disease; SD = standard deviation.
Characteristics of the crossover study participants are shown in Table 2.
TABLE 2.
Crossover Study Participant Baseline Characteristics
| Characteristic | Placebo Then Varenicline, n = 16 | Varenicline Then Placebo, n = 18 |
|---|---|---|
| Age, yr | ||
| Mean (SD) | 64.2 (5.3) | 68.1 (5.7) |
| min, max | 52, 76 | 56, 78 |
| Male, n (%) | 13 (81%) | 15 (83%) |
| White, n (%) | 16 (100%) | 17 (94%) |
| Age at diagnosis, yr | ||
| Mean (SD) | 57.7 (7.22) | 61.3 (6.66) |
| min, max | 43, 70 | 50, 74 |
| MDS-UPDRS III | ||
| Mean (SD) | 30.7 (12.4) | 33.2 (13.92) |
| min, max | 13, 62.5 | 15, 58 |
| GDS | ||
| Mean (SD) | 4.3 (4.19) | 2.4 (2.06) |
| min, max | 0, 15 | 0, 6 |
| MoCA | ||
| Mean (SD) | 26.8 (1.97) | 27.2 (2.37) |
| min, max | 24, 30 | 23, 30 |
MDS-UPDRS III: motor subscale score; range = 0–137, with higher scores indicating worse symptoms. GDS: total score; range = 0–30, with higher scores indicating worse depression. MoCA: total score; range = 0–30, with lower scores indicating worse severity.
GDS = Geriatric Depression Scale; max = maximum; MDS-UPDRS III = Movement Disorder Society Unified Parkinson’s Disease Rating Scale, part III; min = minimum; MoCA = Montreal Cognitive Assessment; SD = standard deviation.
VCN-α4β2* nAChR Occupancy Study
VCN displacement of thalamic [18F]flubatine binding to α4β2* nAChRs is reported, the thalamus being the region with the highest [18F]flubatine binding (Fig 2). Analysis of other regions gave very similar results (data not shown). The lowest daily dose of VCN, 0.25mg per day, produced significant receptor occupancy. There was little evidence of a dose–response relationship, with all VCN doses producing 60 to 70% occupancy of α4β2* nAChRs. Results in control participants were very similar (data not shown). As 0.5mg p.o. b.i.d. produced approximately the same α4β2* nAChR occupancy as 1.0mg p.o. b.i.d., 0.5mg p.o. b.i.d. was chosen as the dose for the crossover study.
FIGURE 2:
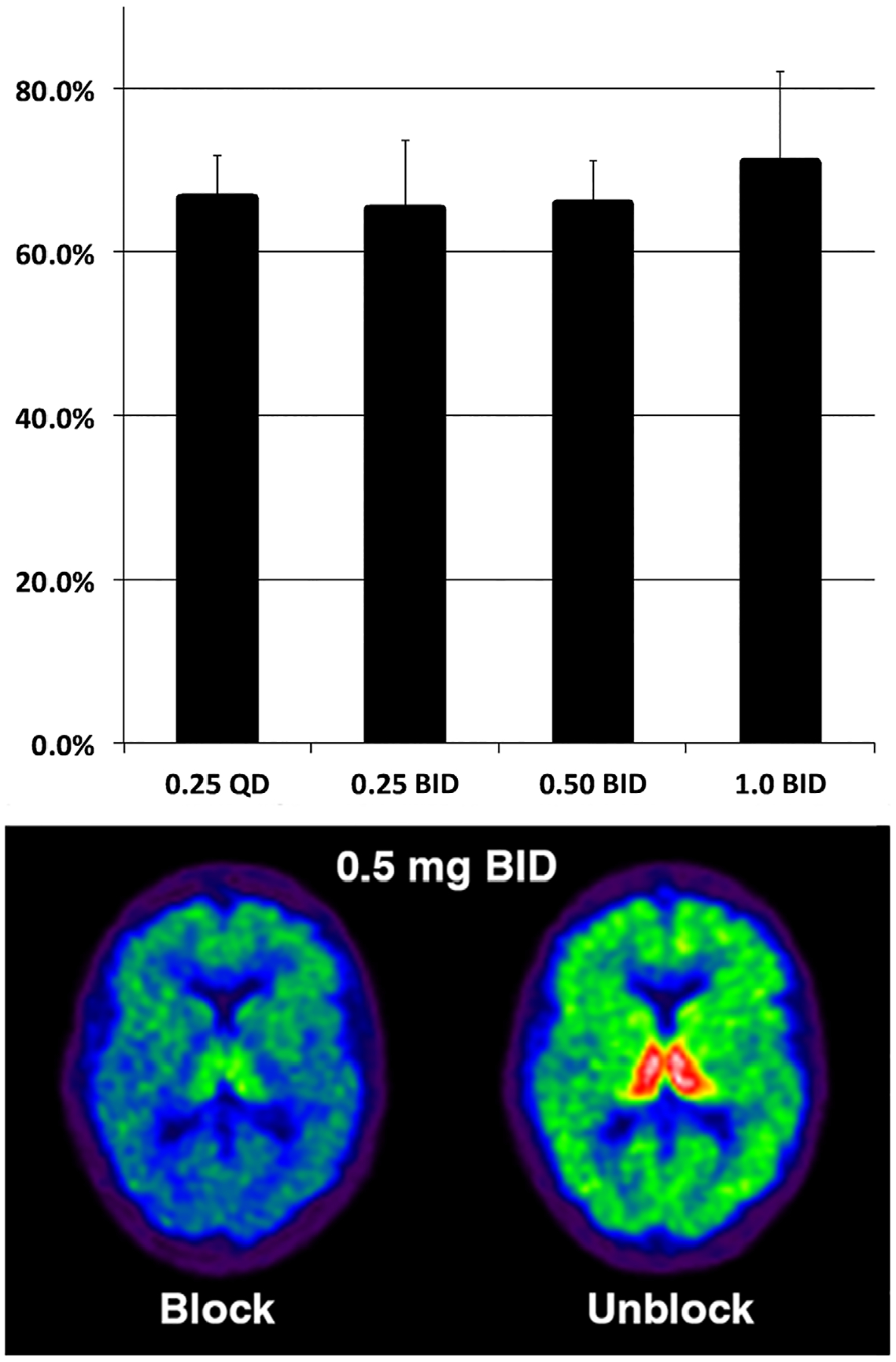
Varenicline occupancy of α4β2* nicotinic acetylcholine receptors. Top panel illustrates dose–response relationship between daily oral dose and estimated percent receptor occupancy (mean and standard deviation). X-axis units are milligrams. Bottom panel consists of parametric images of a single participant on and off 0.5mg orally twice daily. BID = twice daily; QD = once daily.
Crossover Study
ITT and Treatment-Compliant Participant Analyses
The primary analyses of the crossover study used the ITT population. Secondary analyses were conducted using identical statistical methods in participants who were compliant with study treatment. In this secondary analysis, we excluded participants without evidence of significant increases in VCN plasma levels between placebo and VCN treatment periods to define the treatment-compliant participants. Safety analyses are based on all participants.
Safety
We enrolled 34 PD participants. There was 1 dropout for reasons unrelated to the study (withdrawn 4 days into period 2 while on VCN). Among the 34 participants, there were 56 AEs (serious and nonserious) in 22 (65%) participants (Table 3). There were more AEs in the VCN periods than placebo periods; 17 participants on VCN experienced 35 AEs, whereas 8 participants on placebo experienced 18 AEs. These were largely expected AEs, such as nausea and insomnia. There were 2 serious AEs, 1 in each treatment period, neither related to VCN treatment. Two participants required dose reductions to 0.5mg p.o. per day and 1 participant to 0.25mg p.o. per day−0.25mg p.o. b.i.d. for study completion. As the VCN-α4β2* nAChR occupancy experiment demonstrated substantial nAChR occupancy at the lowest VCN dose, these participants are included in all analyses.
TABLE 3.
Crossover Study AEs
| Placebo, N = 34, n | Varenicline, N = 34, n | |
|---|---|---|
| Serious AEs | 1 | 1 |
| Participants with ≥1 serious AE | 1 | 1 |
| Nonserious AEsa | 18 | 35 |
| Participants with ≥1 nonserious AE | 8 | 17 |
| Nonserious AE severity | ||
| Mild | 15 | 29 |
| Moderate | 3 | 6 |
| Nonserious AE relatedness | ||
| Related | 4 | 16 |
| Unrelated | 14 | 19 |
Eight nonserious AEs occurred during washout, 6 in the placebo then varenicline arm and 2 in the varenicline then placebo arm; AEs during washout are categorized to the treatment in period 1 in this table; 1 subject randomized to varenicline then placebo had an unknown date of onset and thus is not included in this table (but their nonserious AE is included in summaries in the text).
AE = adverse event.
Motor Function Measures: ITT Population
Of the primary outcome measures, there was no statistically significant difference in JERK performance between VCN and placebo treatment periods (Table 4). For normal pace gait speed, VCN treatment was associated with statistically significant gait slowing, a result opposite to the hypothesized effect. Analysis of secondary/exploratory measures returned disparate results. MDS-UPDRS III scores modestly worsened during the VCN treatment periods. The PIGD subscore was not significantly different between VCN and placebo periods. Other postural stability measures, mean sway velocity and RMS, were not significantly different between placebo and VCN treatment periods. There was no significant effect on no dual task double support time. VCN did not significantly change normal pace dual task gait speed but significantly reduced the difference in normal pace gait speed between no dual task and dual task conditions. VCN treatment had no effect on gait speed under either fast pace condition. For normal pace gait speed, VCN treatment produced a modest, but significant, reduction in DTC. There was no effect on the difference between fast pace gait speed under dual task and no dual task conditions or on fast pace gait speed DTC. VCN treatment had significant and predictable effects on normal pace cadence, stride length, and stride time. Consistent with lack of VCN effect on gait speed under the fast pace condition, there was no effect on fast pace cadence, stride length, stride time, or double support time. iTUG measures showed no significant VCN effects (data not shown).
TABLE 4.
Crossover Study Motor Outcomes: Intent-to-Treat Population (N = 34)
| Outcome | Varenicline LS, Mean (SE) | Placebo LS, Mean (SE) | Treatment Difference (95% CI) | p |
|---|---|---|---|---|
| Primary outcomesa | ||||
| Gait speed–normal pace–no dual task, cm/s | 121.27 (1.36) | 124.89 (1.36) | −3.62 (−5.92, −1.32) | 0.003b |
| JERK, m2/s5 | 0.97 (0.20) | 1.04 (0.20) | −0.07 (−0.44, 0.31) | 0.73 |
| Secondary outcomes | ||||
| MDS-UPDRS III | ||||
| Total | 31.90 (1.15) | 28.77 (1.13) | 3.13 (0.45, 5.80) | 0.02b |
| PIGD subscore | 4.41 (0.32) | 4.89 (0.32) | −0.48 (−1.32, 0.36) | 0.25 |
| Gait | ||||
| Gait speed–normal pace–dual task | 114.99 (1.48) | 116.26 (1.48) | −1.27 (−4.03, 1.49) | 0.35 |
| Gait speed–fast pace–no dual task | 148.92 (1.73) | 151.26 (1.73) | −2.34 (−5.71, 1.04) | 0.17 |
| Gait speed–fast pace–dual task | 135.61 (2.08) | 137.76 (2.08) | −2.14 (−5.06, 0.78) | 0.14 |
| Gait speed–normal pace–dual task minus gait speed–normal pace–no dual task | −6.07 (1.11) | −9.10 (1.11) | 3.03 (0.53, 5.54) | 0.02b |
| Normal pace dual task cost | −5.34 (0.91) | −7.59 (0.91) | 2.25 (0.28, 4.22) | 0.03b |
| Gait speed–fast pace–dual task minus gait speed–fast pace–no dual task | −13.40 (1.37) | −13.81 (1.36) | 0.41 (−2.28, 3.11) | 0.76 |
| Fast pace dual task cost | −9.28 (0.95) | −9.26 (0.95) | 0.03 (−1.76, 1.71) | 0.98 |
| Cadence–normal pace–no dual task | 109.50 (0.58) | 111.73 (0.58) | −2.23 (−3.63, −0.83) | 0.003b |
| Cadence–fast pace–no dual task | 120.70 (0.85) | 121.97 (0.85) | −1.27 (−2.83, 0.28) | 0.10 |
| Mean stride length–normal pace–no dual task | 132.58 (1.16) | 134.56 (1.16) | −1.98 (−3.69, −0.26) | 0.03b |
| Mean stride length–fast pace–no dual task | 147.96 (1.18) | 148.75 (1.18) | −0.79 (−2.99, 1.41) | 0.47 |
| %CV stride length–normal pace–no dual task | 3.87 (0.23) | 3.67 (0.23) | 0.20 (−0.27, 0.66) | 0.39 |
| %CV stride length–fast pace–no dual task | 3.55 (0.22) | 3.41 (0.22) | 0.14 (−0.41, 0.68) | 0.61 |
| Mean stride time–normal pace–no dual task | 1.10 (0.01) | 1.08 (0.01) | 0.02 (0.008, 0.04) | 0.004b |
| Mean stride time–fast pace–no dual task | 1.00 (0.01) | 0.99 (0.01) | 0.01 (−0.003, 0.02) | 0.12 |
| %CV stride time–normal pace–no dual task | 2.55 (0.58) | 3.36 (0.58) | −0.81 (−2.42, 0.80) | 0.31 |
| %CV stride time–fast pace–no dual task | 2.54 (0.15) | 2.63 (0.15) | −0.09 (−0.50, 0.32) | 0.67 |
| Double support–normal pace–no dual task | 0.37 (0.01) | 0.37 (0.01) | 0.006 (−0.005, 0.018) | 0.25 |
| Double support–fast pace–no dual task | 0.31 (0.01) | 0.31 (0.01) | 0.002 (−0.009, 0.013) | 0.70 |
| Mean sway velocity | 0.31 (0.03) | 0.26 (0.03) | 0.05 (−0.05, 0.15) | 0.30 |
| Sway RMS | 0.15 (0.01) | 0.14 (0.01) | 0.01 (−0.02, 0.04) | 0.43 |
Estimates (least squares or adjusted means and standard errors) and p values were obtained from a linear mixed model containing treatment sequence, treatment period, treatment group, and dependent-variable baseline value, with participant within treatment sequence as a random effect. Postural measures (JERK, mean sway velocity, RMS) analysis was based on a model with baseline eyes open–foam pad covariate and baseline eyes closed–foam pad covariate.
Nominal alpha = 0.025 owing to Bonferroni correction for 2 coprimary endpoints.
Statistically significant.
%CV = percent coefficient of variation; CI = confidence interval; JERK = time-based derivative of lower trunk accelerations; LS = least squares; MDS-UPDRS III = Movement Disorder Society Unified Parkinson’s Disease Rating Scale, part III; PIGD = postural instability and gait disorder; RMS = root mean square; SE = standard error.
Cognitive Measures: ITT Population
The SAT, a measure of attentional function that reflects CNS cholinergic functions, showed positive effects of VCN treatment (Table 5). The SAT/dSAT analysis is based on 21 (out of 34) participants who completed SAT/dSAT testing at all crossover study sessions. Seven participants had complete SAT/dSAT testing at some, but not all, sessions. Two participants declined testing at some sessions because of musculoskeletal pain, 1 declined at 1 session because of a migraine headache. Data files were corrupted for some sessions for 4 participants, and these participants were excluded from the SAT/dSAT analysis. Six subjects attempted to but were unable to perform the SAT/dSAT at any session. These 6 subjects were older (mean age = 72.8 vs 64.9 years, p = 0.001, t test), had worse baseline MDS-UPDRS III scores (mean = 50.2 vs 28.1, p = 0.001, t test), were possibly more cognitively impaired (mean MoCA = 23.8 vs 26.5, p = 0.055, t test), and had slower baseline gait speed (mean normal pace gait speed = 106.1cm/s vs 127.6cm/s, p = 0.020, Satterthwaite t test). There was no significant effect of VCN on dSAT performance, which was poor in both VCN treatment and placebo periods. Neither the MoCA, nor any conventional cognitive domain–specific measures showed significant effects of VCN treatment (see Table 5).
TABLE 5.
Crossover Study Cognitive and Behavioral Outcomes: Intent-to-Treat Population (N = 34) for All Measures except SAT/dSAT (n = 21)
| Varenicline LS, Mean (SE) | Placebo LS, Mean (SE) | Treatment Difference (95% CI) | p | |
|---|---|---|---|---|
| Cognitive measures reflecting cholinergic signaling | ||||
| SATa | 0.73 (0.06) | 0.66 (0.06) | 0.076 (0.0073, 0.14) | 0.03b |
| dSATa | 0.49 (0.06) | 0.43 (0.06) | 0.058 (−0.03, 0.15) | 0.18 |
| Conventional cognitive measures | ||||
| MoCA | 26.83 (0.29) | 27.23 (0.29) | −0.40 (−1.22, 0.43) | 0.34 |
| WAIS-III digit symbol | 62.38 (1.29) | 62.34 (1.28) | 0.04 (−1.92, 2.00) | 0.97 |
| CVLT STM | 12.03 (0.32) | 12.36 (0.32) | −0.33 (−1.14, 0.48) | 0.41 |
| CVLT LTM | 12.44 (0.30) | 12.80 (0.30) | −0.36 (−0.77, 0.04) | 0.07 |
| CVLT recognition | 15.56 (0.13) | 15.47 (0.13) | 0.09 (−0.11, 0.30) | 0.36 |
| D-KEFS Stroop III, interference | 112.52 (3.42) | 115.31 (3.37) | −2.79 (−12.06, 6.47) | 0.54 |
| JOLO | 25.84 (0.48) | 24.48 (0.48) | 0.36 (−0.79, 1.52) | 0.53 |
| D-KEFS sorting total | 84.55 (1.57) | 85.63 (1.55) | −1.08 (−4.66, 2.49) | 0.54 |
| D-KEFS verbal fluency–letters total | 43.53 (1.05) | 41.69 (1.04) | 1.83 (−0.61, 4.27) | 0.14 |
| D-KEFS verbal fluency–animal | 18.19 (0.72) | 18.07 (0.71) | 0.12 (−1.33, 1.56) | 0.87 |
| Trail Making Test 4 | 103.19 (8.12) | 103.38 (8.03) | −0.19 (−16.37, 15.98) | 0.98 |
| Behavioral | ||||
| GDS | 3.56 (0.28) | 3.31 (0.27) | 0.25 (−0.33, 0.84) | 0.38 |
Estimates (least squares or adjusted means and standard errors) and p values were obtained from a linear mixed model containing treatment sequence, treatment period, treatment group, and dependent-variable baseline value, with participant within treatment sequence as a random effect.
Estimates (least squares or adjusted means and standard errors) and p values were obtained from a linear mixed model containing treatment sequence, treatment period, and treatment group, with participant within treatment sequence as a random effect.
Statistically significant.
CI = confidence interval; CVLT = California Verbal Learning Test; D-KEFS = Delis–Kaplan Executive Function System; dSAT = SAT with a distractor; GDS = Geriatric Depression Scale; JOLO = Judgment of Line Orientation; LS = least squares; LTM = long-term memory; MoCA = Montreal cognitive assessment; SAT = Sustained Attention Test; SE = standard error; STM = short-term memory; WAIS-III = Wechsler Adult Intelligence Scale, 3rd edition.
Behavioral Measures: ITT Population
VCN treatment had no effects on GDS (see Table 5) or C-SSRS scores (data not shown).
Analysis of Treatment-Compliant Population
We excluded the participant who dropped out prior to study completion. Four participants exhibited VCN levels below the level of quantification at the end of both treatment periods, suggesting noncompliance. All analyses of motor, cognitive, and behavioral measures were repeated for the 28 compliant participants (Table 6). Results were essentially identical to those of the ITT population. For the primary endpoints, VCN was associated with significantly slower normal pace gait speed and no significant effect on JERK. VCN significantly improved SAT performance. There was a similar reduction in the difference between dual task normal pace gait performance and no dual task normal pace gait performance in the compliant population (p = 0.06) and on normal pace DTC (p = 0.07); however, these were not statistically significant in this smaller sample. The magnitude of the mean VCN effect on the difference between dual task normal pace gait speed and no dual task normal pace gait speed, and normal pace DTC in the compliant population were very similar to that seen in the ITT population (see Tables 4 and 6).
TABLE 6.
Crossover Study Outcomes in Compliant Participants (n = 28)
| Varenicline LS, Mean (SE) | Placebo LS, Mean (SE) | Treatment Difference (95% CI) | p | |
|---|---|---|---|---|
| Gait speed–normal pace–no dual task, cm/s | 119.17 (1.54) | 122.06 (1.54) | −2.90 (−5.48, −0.31) | 0.03a |
| MDS-UPDRS III total | 33.54 (1.29) | 30.72 (1.27) | 2.82 (−0.18, 5.81) | 0.06 |
| Gait speed–normal pace–dual task minus gait speed–normal pace–no dual task | −6.56 (1.28) | −9.24 (1.27) | 2.68 (−0.14, 5.49) | 0.06 |
| Normal pace dual task cost | −5.78 (1.04) | −7.85 (1.04) | 2.07 (−0.18, 4.32) | 0.07 |
| Cadence–normal pace–no dual task | 109.69 (0.66) | 111.87 (0.66) | −2.17 (−3.87, −0.48) | 0.01a |
| Mean stride length–normal pace–no dual task | 130.02 (1.34) | 131.51 (1.33) | −1.49 (−3.39, 0.41) | 0.12 |
| Mean stride time–normal pace–no dual task | 1.10 (0.01) | 1.07 (0.01) | 0.02 (0.005, 0.04) | 0.02a |
| SAT | 0.71 (0.07) | 0.61 (0.07) | 0.09 (0.002, 0.18) | 0.05a |
Items shown are those with statistically significant differences in ITT analysis for comparison between ITT and compliant groups. All other items without statistically significant differences are between varenicline and placebo treatment periods in compliant population. Estimates (least squares or adjusted means and standard errors) and p values were obtained from a linear mixed model containing treatment sequence, treatment period, treatment group, and dependent-variable baseline value, with participant within treatment sequence as a random effect.
Statistically significant.
CI = confidence interval; ITT = intention-to-treat; LS = least squares; MDS-UPDRS III = Movement Disorder Society Unified Parkinson’s Disease Rating Scale, part III; SAT = sustained attention test; SE = standard error.
VCN Levels
At the end of the placebo period, VCN levels were below the level of quantification for all participants, and mean VCN levels were 13.94ng/ml (SD = 7.91) at the end of the VCN treatment periods. VCN concentrations at the end of the treatment period were consistent with previously reported pharmacokinetic data.22
Discussion
We assessed potential VCN target engagement of α4β2* nAChRs by assessing α4β2* nAChR occupancy and behavioral effects. In our first experiment, we employed [18F]flubatine PET to establish that these VCN dose schedules produce significant α4β2* nAChR occupancy without evidence of a dose–response relationship. These results are consistent with limited data suggesting that low p.o. VCN doses are effective for smoking cessation.36 We established that there is no gross difference in VCN-α4β2* nAChR interactions in PD and control CNS. Our normal control results are similar to those obtained by Lotfipour et al, who used [18F]2-fluoro-3-[2(S)-2-azetidinylmethoxy]pyridine PET to demonstrate high α4β2* nAChR occupancy in normal participants after a single, 0.5mg, p.o. VCN dose.37 Our second experiment, using a daily dose based on receptor occupancy, was a placebo-controlled, double-masked crossover study. VCN had no effect on our primary measure of postural stability or other measures of postural stability studied. VCN was associated with slower normal pace gait speed and significantly narrowed the difference between normal pace gait speed under baseline and distracting dual task conditions, with a significant effect on normal pace gait speed DTC. VCN had a significant positive effect on SAT performance, an attention measure directly linked to BFCC cholinergic functions, but no significant effects on conventional cognitive measures. We detected a significant difference in SAT performance despite a relatively low N, and the difference in SAT performance between VCN and placebo treatment periods is approximately the same as the difference in SAT performance between hypocholinergic and normocholinergic PD participants.33 Results were almost identical in analyses of ITT and treatment-compliant participants. VCN narrowing of differences in dual task normal pace gait speed and no dual task normal pace gait speed, and normal pace DTC were not statistically significant in the compliant population, although of similar magnitude, likely reflecting the reduced sample size of the compliant population.
Our results complement and partly contradict those of Hall et al, who randomized 36 PD participants to VCN, 1mg p.o. b.i.d., or placebo for an 8-week trial period.38 Participants were slightly older than our participants but comparable in PD severity and cognitive status. Their primary outcome measure was Berg Balance Scale (BBS) performance. Cognitive effects of VCN were assessed with the Mini-Mental State Examination and the Frontal Assessment Battery (FAB). There was no VCN effect with any of these measures. There are important methodological differences between this study and our work. Cholinergic systems are intact in many moderately advanced PD participants.7,9 It is likely that Hall et al enrolled some participants with normal cholinergic systems, unlike our enrollment of participants with cortical cholinergic deficits. This difference may be important in the context of a partial agonist. In the presence of normal levels of endogenous agonists, partial agonists exhibit antagonist properties, potentially impairing cholinergic signaling. Likely most important is the difference in outcome measures. The BBS is a summary ordinal measure that does not quantify gait and balance measures and does not contain any distractors. The 6-item FAB contains 2 items (4 and 5) with attentional components, but there was no measure comparable to the SAT, a specific measure of attention reflecting cholinergic functions.
Our results are complementary to those of Mancini et al.39,40 They performed a crossover study of a moderate dose, 5mg/day, of the cholinesterase inhibitor donepezil in 19 PD subjects. The demographic and clinical features of their cohort were approximately similar to our crossover study group. Mancini et al did not screen their participants for cholinergic deficits, and this dose of donepezil does not uniformly inhibit brain acetylcholinesterase.41 Using a combination of gait analysis and functional near-infrared spectroscopy, they found evidence of donepezil modulation of frontal cortical activity and attention, and enhancement of some gait parameters and dual task performance.
In terms of the selected primary endpoints, we found no effect on the measure of postural stability, JERK. We documented a significant effect on normal pace gait speed, although opposite to our hypothesis that nAChR stimulation would increase normal pace gait speed. It is plausible, however, that slower normal pace gait speed associated with VCN treatment might reflect greater attentiveness. In a rat model of variations in BFCC function, Kucinski et al found that animals with better attentional capacity secondary to more robust BFCC function were more cautious during performance of an attentionally demanding gait task under single task conditions and less likely to fall under distracting conditions.42 The difference between normal pace gait speed at baseline and with a dual task distractor showed a significant effect of VCN treatment. VCN treatment produced a significant improvement in DTC, a conventional measure of cognitive–motor integration.
Whereas there were no discernable effects of VCN on conventional measures of cognitive function, SAT performance, which reflects CNS cholinergic dysfunction in humans, including PD participants, showed a significant positive VCN effect. This positive effect of VCN parallels findings in animals that exhibit cholinergic dysfunction. Attentional and movement impairments, including a propensity for falls, are a stable trait.42 α4β2* nAChR stimulation improved the attentional performance of these rats.43
Our results are consistent also with those of Mocking et al, who showed that subacute p.o. VCN administration (0.5mg/day for 3 days, then 1.0mg/day for 4 days) in healthy participants improved working and declarative memory.44 Coupled with our [18F]flubatine PET data, we suggest that VCN treatment slowing of normal pace gait speed, narrowing of the difference between under baseline and distracting conditions, reduced DTC, and SAT results, constitute evidence of target engagement. In our analysis of exploratory/secondary endpoints, we did not correct for multiple comparisons, but it is notable that the only outcome measures with statistically significant results are plausibly related to attentional function.
We did not find any VCN effects under fast pace gait conditions. This may be because fast pace gait involves conscious focus on gait performance, strengthening attentional functions. Similarly, we did not find any VCN effect on dSAT performance, possibly due to floor effects. This conclusion is consistent with prior work indicating that the dSAT is very challenging for hypocholinergic PD participants.33 We did not find any effects on other postural control measures, mean sway velocity and RMS, studied, or on double support time during gait. None of these postural control measures is directly linked to attentional functions or cholinergic deficits, and these may not be appropriate outcome measures to assess target engagement of attention. We noted a rise in MDS-UPDRS III scores with VCN treatment. The modest magnitude of this effect is below the threshold of a minimally clinically important worsening in MDS-UPDRS III scores.45
Our results highlight some of the difficulties involved with assessing interventions for DRT-refractory gait and balance disorders. Similar to the results of Hall et al, VCN did not have effects on conventional endpoints.38 One of our primary endpoint measures, no dual task normal pace gait speed, revealed a significant VCN effect. In our secondary analyses, we found positive effects of VCN treatment on normal pace gait performance when comparing dual task and no dual task conditions. Positive effects were found with the SAT, a measure that more closely reflects the cholinergic–attentional deficits that are likely major contributors to DRT-refractory gait and balance disorders. Targeting α4β2* nAChRs may be a viable approach to mitigating this morbid PD feature. We suggest also that pursuing receptor subtype pharmacology, either for nAChRs or muscarinic cholinergic receptors, is more likely to be useful than nonspecific approaches such as use of acetylcholinesterase inhibitors.46 Even in our hypocholinergic PD participants, there are regions with relatively preserved cholinergic innervation.9 As VCN is a partial agonist, it might impede normal cholinergic neurotransmission in these regions. Evaluation of full α4β2* nAChR agonists may be worthwhile.
Although we focus on the role of α4β2* nAChRs in the BFCC system, α4β2* nAChRs are widely distributed in the CNS (see Fig 2), including high expression in the striatum, where they are located on striatal afferent terminals and likely mediate some of the effects of striatal cholinergic interneurons.44 Activation of α4β2* nAChRs on nigrostriatal dopaminergic terminals appears to enhance dopamine release. VCN effects could be mediated in part by α4β2* nAChR stimulation in the striatum and other regions, although α4β2* nAChR agonists do not have detectable motor effects in nonhuman primate models of PD.47
Future intervention studies for DRT-refractory gait–balance disorders will likely require laboratory-based measures that both are proxy measures of fall risk and permit efficient evaluation of target engagement. Future studies may also benefit from objective measures of fall risk derived from use of wearable sensors during daily life. Our experience suggests that outcome measures tied closely to underlying pathophysiologic mechanisms will be more robust biomarkers of target engagement.
Acknowledgments
This study was supported by NS091856, the Michael J. Fox Foundation, the Parkinson’s Foundation, and NIHNINDS R21 NS114749.
We thank the study participants, and the staff of the Functional Neuroimaging, Cognition, and Mobility Laboratory and the PET Center.
Footnotes
Potential Conflicts of Interest
Nothing to report.
References
- 1.van der Marck MA, Klok MP, Okun MS, et al. Consensus-based clinical practice recommendations for the examination and management of falls in patients with Parkinson’s disease. Parkinsonism Relat Disord 2014;20:360–369. [DOI] [PubMed] [Google Scholar]
- 2.Harris-Hayes M, Willis AW, Klein SE, et al. Relative mortality in U.S. Medicare beneficiaries with Parkinson disease and hip and pelvic fractures. J Bone Joint Surg Am 2014;96:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lord S, Galna B, Yarnall AJ, et al. Natural history of falls in an incident cohort of Parkinson’s disease: early evolution, risk and protective features. J Neurol 2017;264:2268–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrag A, Hommel ALAJ, Lorenzl S, et al. The late stage of Parkinson’s—results of a large multinational study on motor and non-motor complications. Parkinsonism Relat Disord 2020;75:91–96. [DOI] [PubMed] [Google Scholar]
- 5.Bohnen NI, Müller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology 2009;73:1670–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res 2011;221:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohnen NI, Müller ML, Kotagal V, et al. Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. J Cereb Blood Flow Metab 2012;32:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohnen NI, Frey KA, Studenski S, et al. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology 2013;81: 1611–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohnen NI, Kanel P, Zhou Z, et al. Cholinergic system changes of falls and freezing of gait in Parkinson’s disease. Ann Neurol 2019;85: 538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson’s disease. Mov Disord 2011;26: 2496–2503. [DOI] [PubMed] [Google Scholar]
- 11.Rochester L, Yarnall AJ, Baker MR, et al. Cholinergic dysfunction contributes to gait disturbance in early Parkinson’s disease. Brain 2012;135:2779–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarter M, Albin RL, Kucinski A, Lustig C. Where attention falls: increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function. Exp Neurol 2014;257:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris R, Martini DN, Madhyastha T, et al. Overview of the cholinergic contribution to gait, balance and falls in Parkinson’s disease. Parkinsonism Relat Disord 2019;63:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballinger EC, Ananth M, Talmage DA, Role LW. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 2016;91:1199–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gut NK, Winn P. The pedunculopontine tegmental nucleus—a functional hypothesis from the comparative literature. Mov Disord 2016; 31:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kucinski A, Paolone G, Bradshaw M, et al. Modeling fall propensity in Parkinson’s disease: deficits in the attentional control of complex movements in rats with cortical-cholinergic and striatal-dopaminergic deafferentation. J Neurosci 2013;33:16522–16539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strouwen C, Molenaar EA, Münks L, et al. Dual tasking in Parkinson’s disease: should we train hazardous behavior? Expert Rev Neurother 2015;15:1031–1039. [DOI] [PubMed] [Google Scholar]
- 18.Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol 2000;61:75–111. [DOI] [PubMed] [Google Scholar]
- 19.Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 2007;52:985–994. [DOI] [PubMed] [Google Scholar]
- 20.Jordan CJ, Xi ZX. Discovery and development of varenicline for smoking cessation. Expert Opin Drug Discov 2018;13:671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burstein AH, Fullerton T, Clark DJ, Faessel HM. Pharmacokinetics, safety, and tolerability after single and multiple oral doses of varenicline in elderly smokers. J Clin Pharmacol 2006;46:1234–1240. [DOI] [PubMed] [Google Scholar]
- 22.Faessel HM, Obach RS, Rollema H, et al. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clin Pharmacokinet 2010;49:799–816. [DOI] [PubMed] [Google Scholar]
- 23.Obach RS, Reed-Hagen AE, Krueger SS, et al. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos 2006;34: 121–130. [DOI] [PubMed] [Google Scholar]
- 24.van der Zee S, Vállez García D, Elsinga PH, et al. [18F] Fluoroethoxybenzovesamicol in Parkinson’s disease patients: quantification of a novel cholinergic positron emission tomography tracer. Mov Disord 2019;34:924–926. [DOI] [PubMed] [Google Scholar]
- 25.Sabri O, Becker GA, Meyer PM, et al. First-in-human PET quantification study of cerebral α4β2* nicotinic acetylcholine receptors using the novel specific radioligand (−)-[(18)F] flubatine. Neuroimage 2015; 118:199–208. [DOI] [PubMed] [Google Scholar]
- 26.Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res 2011;13:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hockley BG, Stewart MN, Sherman P, et al. (−)-[(18)F]Flubatine: evaluation in rhesus monkeys and a report of the first fully automated radiosynthesis validated for clinical use. J Labelled Comp Radiopharm 2013;56:595–599. [DOI] [PubMed] [Google Scholar]
- 28.Mancini M, Horak FB, Zampieri C, et al. Trunk accelerometry reveals postural instability in untreated Parkinson’s disease. Parkinsonism Relat Disord 2011;17:557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mancini M, Carlson-Kuhta P, Zampieri C, et al. Postural sway as a marker of progression in Parkinson’s disease: a pilot longitudinal study. Gait Posture 2012;36:471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly VE, Eusterbrock AJ, Shumway-Cook A. A review of dual-task walking deficits in people with Parkinson’s disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis 2012;2012:918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lustig C, Kozak R, Sarter M, et al. CNTRICS final animal model task selection: control of attention. Neurosci Biobehav Rev 2013;37: 2099–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berry AS, Demeter E, Sabhapathy S, et al. Disposed to distraction: genetic variation in the cholinergic system influences distractibility but not time-on-task effects. J Cogn Neurosci 2014;26:1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim K, Müller MLTM, Bohnen NI, et al. Thalamic cholinergic innervation makes a specific bottom-up contribution to signal detection: evidence from Parkinson’s disease patients with defined cholinergic losses. Neuroimage 2017;149:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frey PW, Colliver JA. Sensitivity and responsivity measures for discrimination learning. Learn Motiv 1973;4:327–342. [Google Scholar]
- 35.Nagelkerke NJ, Hart AA, Oosting J. The two-period binary response cross-over trial. Biom J 1986;28:863–869. [Google Scholar]
- 36.Huang Y, Li W, Yang L, et al. Long term efficacy and safety of varenicline for smoking cessation: a systematic review and meta-analysis of randomized controlled trials. J Public Health 2012;20:355–365. [Google Scholar]
- 37.Lotfipour S, Mandelkern M, Alvarez-Estrada M, Brody AL. A single administration of low-dose varenicline saturates α4β2* nicotinic acetylcholine receptors in the human brain. Neuropsychopharmacology 2012;37:1738–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall D, Kapur S, Vaughn C, et al. Varenicline for the treatment of postural and gait dysfunction in Parkinson’s disease (PD). Neurol Clin Pract (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stuart S, Morris R, Giritharan A, et al. Prefrontal cortex activity and gait in Parkinson’s disease with cholinergic and dopaminergic therapy. Mov Disord 2020;35:2019–2027. [DOI] [PubMed] [Google Scholar]
- 40.Vitorio R, Stuart S, Giritharan A, et al. Changes in prefrontal cortex activity and turning in response to dopaminergic and cholinergic therapy in Parkinson’s disease: a randomized cross-over trial. Parkinsonism Relat Disord 2021;86:10–14. [DOI] [PubMed] [Google Scholar]
- 41.Bohnen NI, Kaufer DI, Hendrickson R, et al. Degree of inhibition of cortical acetylcholinesterase and cognitive effects by donepezil in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2005;76: 315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kucinski A, Lustig C, Sarter M. Addiction vulnerability trait impacts complex movement control: evidence from sign-trackers. Behav Brain Res 2018;350:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paolone G, Angelakos CC, Meyer PJ, et al. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci 2013;33:8321–8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mocking RJ, Patrick Pflanz C, Pringle A, et al. Effects of short-term varenicline administration on emotional and cognitive processing in healthy, non-smoking adults: a randomized, double-blind, study. Neuropsychopharmacology 2013;38: 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horvath K, Aschermann Z, Acs P, et al. Minimal clinically important difference on the motor examination part of the MDS-UPDRS. Parkinsonism Relat Disord 2015;21:1421–1425. [DOI] [PubMed] [Google Scholar]
- 46.Mancini M, Chung K, Zajack A, et al. Effects of augmenting cholinergic neurotransmission on balance in Parkinson’s disease. Parkinsonism Relat Disord 2019;69:40–47. [DOI] [PubMed] [Google Scholar]
- 47.Quik M, Boyd JT, Bordia T, Perez X. Potential therapeutic application for nicotinic receptor drugs in movement disorders. Nicotine Tob Res 2019;21:357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]


