Abstract
Thrombin is a multifunctional serine proteinase that induces a variety of responses from neural cells by cleavage of proteinase-activated receptors (PARs) including PAR1 and PAR4. Thrombin/PAR signaling has been implicated in the neuroinflammatory response that occurs in the brain following stroke and other CNS pathologies. The neuroinflammatory response involves astrocytes and results in induction of pro-inflammatory chemokines including interleukin-8 (IL-8 or CXCL8) and interferon-gamma-induced protein-10 (IP-10 or CXCL10) in these cells. Astroctyes are known to express PARs, however the effect of thrombin on astrocytic chemokine secretion is unknown. Here we characterize the ability of thrombin to induce proliferation/metabolic activity and chemokine secretion in primary human fetal astrocytes. Thrombin induced dose-dependent astrocyte proliferation as well as release of both IL-8 and IP-10, but not interleukin-6 or the chemokine regulated and normal T cell expressed and secreted (RANTES). The chemokine responses were mimicked by PAR1, but not PAR4, activating peptides. Our data indicate that astrocytic chemokine release is part of the neuroinflammatory response triggered by the exposure of the CNS to thrombin.
Keywords: Thrombin, astrocyte, proteinase-activated receptor (PAR), chemokine, interleukin-8 (IL-8 or CXCL8), interferon-gamma-induced protein-10 (IP-10 or CXCL10)
Introduction
Thrombin is a serine proteinase best known for its role in blood coagulation [1]. In addition to its hemostatic effects, thrombin can induce numerous effects on neural cells through activation of proteinase-activated receptors (PARs) [2]. PARs are a family (PAR1-PAR4) of G-protein coupled receptors whose activation can trigger multiple intracellular signaling pathways [1]. Picomolar concentrations of thrombin cause process retraction in neurons and astrocytes while higher concentrations induce astrocyte proliferation [2]. Thrombin/PAR signaling can modulate the viability of both astrocytes and neurons [2] and has been directly implicated in the pathophysiology of a number of CNS diseases including stroke [3] and traumatic brain injury [4].
Astrocytes are the most numerous cell-type in the brain and play a critical role in CNS function [5]. Astrocytes are central actors in both neuroinflammation and the response to cerebral ischemia [6]. Astrocytes, along with microglia, are the primary sources of chemokine production following CNS injury [7]. Chemokines are chemotactic cytokines that direct the movement of circulating leukocytes to sites of inflammation or injury [8]. Chemokines regulate cell-cell interactions in both the immune and nervous systems and have broad function in both development and disease [9]. In the brain, chemokines initiate inflammatory responses through their chemoattractive properties but they can also exert direct effects on neural cells [7].
Rodent astrocytes express all four PAR subtypes [10] but only PAR1 and PAR4 are capable of independent thrombin-induced signaling in these cells [1]. Several studies on rat astrocytes [11, 12] have demonstrated that thrombin can induce expression and release of the chemokine growth-regulated oncogene/cytokine-induced neutrophil chemoattractant-1 (GRO/CINC-1); a chemokine with structural and functional similarities to human IL-8 [13]. Immunohistochemical studies on human autopsy tissue demonstrated that PAR1 expression is most robust in astrocytes where it co-localized with GFAP in all brain regions [14]. In the human glioblastoma cell line U178MG, thrombin induced dose-dependent phosphoinositide hydrolysis and Ca++ mobilization in a PAR1-dependent manner [14]. Several studies using the human astrocytoma cell line 1321N1 have also demonstrated thrombin-induced responses including Ca++-dependent morphological changes [15] and nucleoside release [16]. However, the ability of thrombin to induce responses in non-transformed primary human astrocytes remains unknown. We therefore investigated the ability of thrombin to induce proliferation/metabolic activity and chemokine release in cultured human fetal astrocytes.
Methods
Materials
Pharmaceutical-grade recombinant human α-thrombin (Recothrom™ was obtained from ZymoGenetics, Inc., Seattle, WA. Thrombin-specific proteolytic activity inhibitor, D-Phenylalanyl-L-prolyl-L-arginine chloromethyl ketone dihydrochloride (PPACK), was obtained from Calbiochem, La Jolla, CA. PAR agonist and scrambled peptides (mouse sequence, >98% purity) were purchased from Genscript Corporation, Scotch Plains, NJ. PAR peptide amino acid sequences were as follows: PAR1 selective activating peptide, TFLLR; PAR1 scrambled peptide, FSLLR; PAR4 selective activating peptide, GYPGQV.
Cell culture
Human fetal tissue was obtained from Birth Defects Research Laboratories at the University of Washington (UW) in accordance with federal and UW institutional guidelines (E4 exempt). Cultures of primary human fetal astrocytes were prepared according to previously described protocol [17]. For experiments, astrocytes were washed twice with phosphate buffered saline (PBS) without calcium and magnesium (Gibco/Life Technologies, Grand Island, NY) and detached in 0.05% Tris-Ethylenediaminetetraacetic acid (EDTA) containing 10 μg/ml DNase. Cells were gently triturated and plated at 3 × 104 cells per well in a 96 well plate. Cells were washed 8–12 hours later with PBS containing calcium and magnesium (Gibco) and then incubated for 5 – 7 days in fresh Dulbecco’s modified Eagle’s Medium (DMEM) + 5% horse serum + G5 supplement (Gibco). For all experiments, cells were washed three times in DMEM and serum starved in DMEM for 24 hours prior to stimulation. Cells were stimulated with indicated concentrations of thrombin or PAR activating peptides for 24 hours. All experiments with PAR peptides were carried out in the presence of polymyxin B (10 μg/ml). For proteolysis inhibition experiments, 5 μl of thrombin (1,130 U/ml) was pre-incubated with 1.3 μl PPACK (5 μg/ml) for 30 min and the reaction mix was diluted to the indicated thrombin concentrations in DMEM before being added to cells.
Proliferation/metabolic activity and chemokine release assays
Quantification of proliferation/metabolic activity was determined with the WST-1 reagent (Boehringer Mannheim, Indianapolis, IN) as described [19]. Quantification of IL-8, IP-10, IL-6 and RANTES was assessed using the Luminex® multibead array system (Upstate/Millipore, Billerica, MA) and the Luminex® IS100™ analyzer according to manufacturer’s instructions.
Statistics
Statistical evaluation was carried out using PRISM software (GraphPad, San Diego, CA). Comparisons were made using one-way ANOVA with Bonferroni’s post-test. p<0.05 was considered to be significant. Data are given as mean ± S.E.M.
Results
Thrombin induces proliferation/metabolic activity in human fetal astrocytes
Human fetal astrocytes were treated with increasing concentrations of thrombin for 24 hours. Results from WST-1 assay showed that thrombin induced a dose-dependent increase in proliferation/metabolic activity starting at 0.01 U/ml (Fig.1). This effect reached 2.15-fold baseline control levels at a dose of 1 U/ml (~7.7 nM) (Fig.1). The thrombin-induced increase was reduced 84% (down to 1.19-fold baseline) by pre-incubation with PPACK (Fig.1).
Figure 1: Thrombin-induced proliferation/metabolic activity.
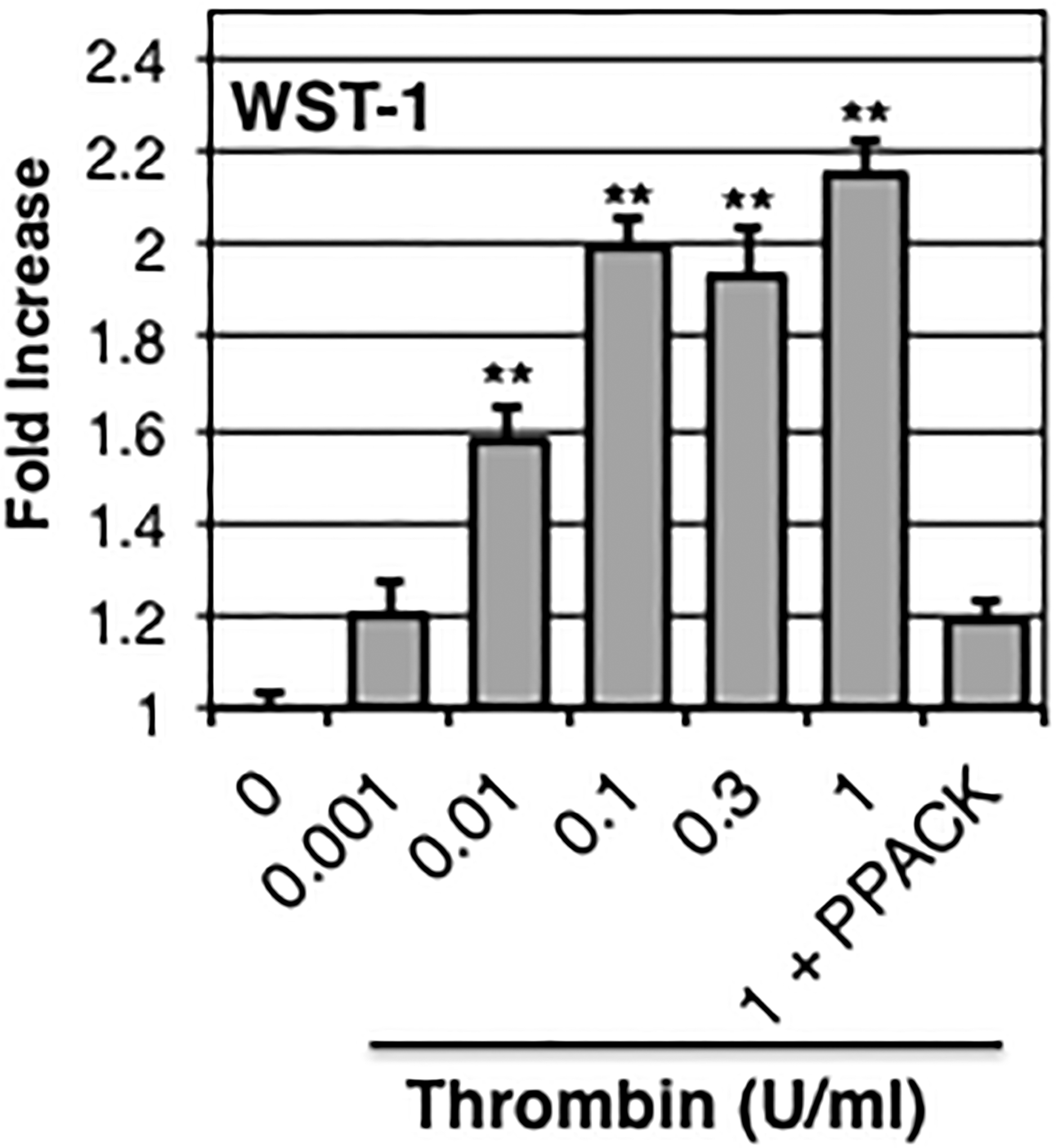
Human fetal astrocytes were stimulated with increasing concentrations of thrombin ± PPACK (10 μg/ml) as indicated. Proliferation/metabolic activity was assessed 24 h later using the WST-1 assay. Data are normalized to unstimulated control and expressed as mean ± S.E.M. n≥9, exp≥3, ** indicates p< 0.01.
Thrombin induces release of chemokines IL-8 and IP-10 in human fetal astrocytes
In the human fetal astrocytes, treatment with thrombin also induced a dose-dependent increase in release of IL-8 and IP-10 starting at 0.1 U/ml (Fig.2A) and 0.01 U/ml (Fig.2B), respectively. The concentrations of IL-8 and IP-10 reached peak levels of 955±157 and 707±133 pg/ml following treatment with 1.0 or 0.3 U/ml thrombin, respectively. The thrombin-induced effects on both chemokines were completely inhibited by PPACK (Fig.2A&B). Neither IL-6 nor RANTES were consistently detected above background levels following thrombin stimulation (data not shown).
Figure 2: Thrombin-induced chemokine release.
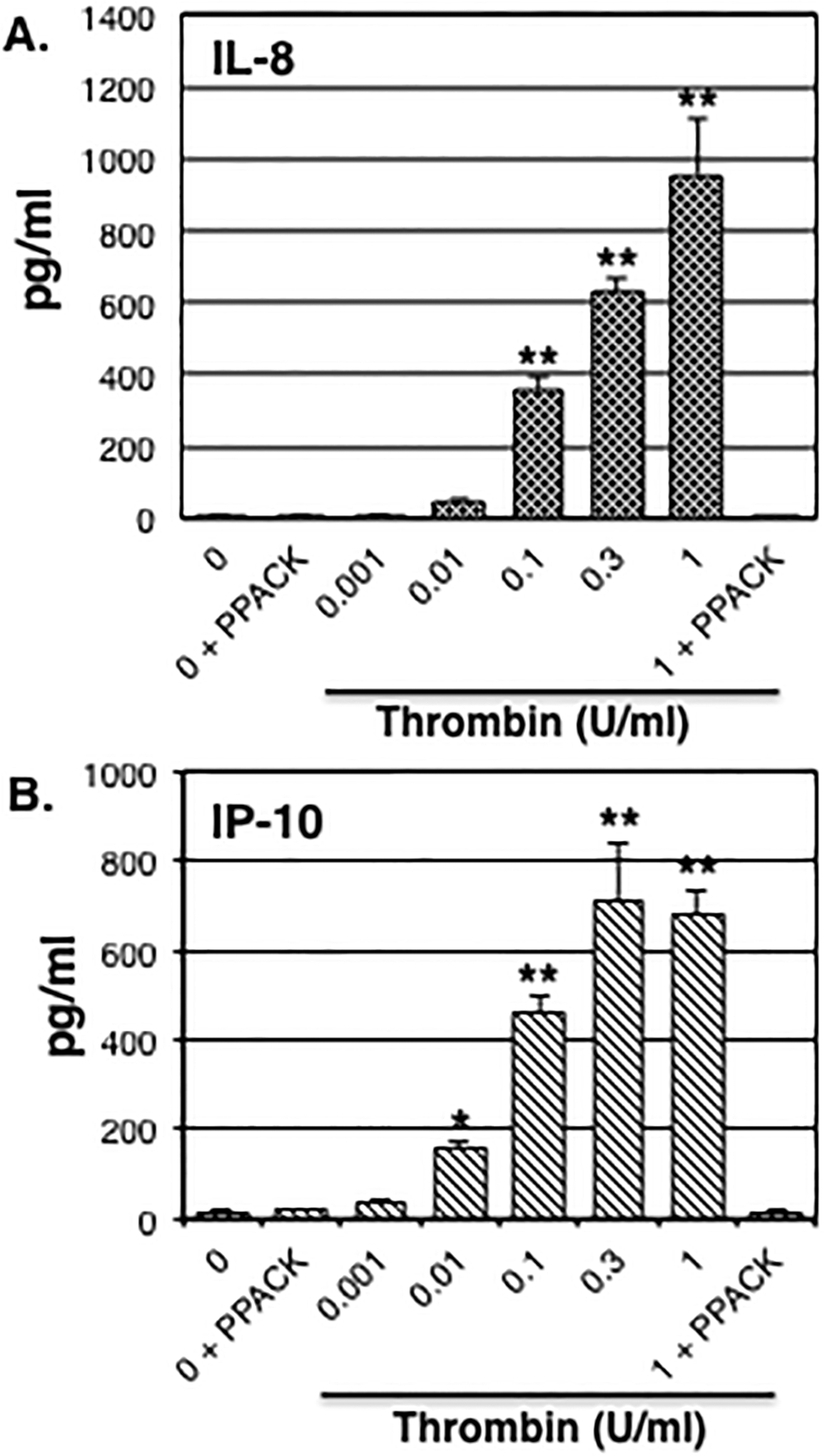
Human fetal astrocytes were stimulated with increasing concentrations of thrombin ± PPACK (10 μg/ml) as indicated. IL-8 (A) and IP-10 (B) release was assessed 24 h later in conditioned medium as described in Methods. Data are expressed as mean ± S.E.M. n≥6, exp≥2, * indicates p< 0.05, ** indicates p< 0.01.
PAR1, but not PAR4, activating peptide induces IL-8 and IP-10 release
PAR1 selective agonist peptide TFLLR induced a dose-dependent release of both IL-8 (Fig.3A) and IP-10 (Fig.3B) from human fetal astrocytes. Following treatment with 100 μM TFLLR, the concentrations of IL-8 and IP-10 reached peak levels of 86±16 and 118±33 pg/ml, respectively (Fig.3A&B). Neither 100 μM PAR1 scrambled peptide FSLLR nor 100 μM PAR4 activating peptide GYPGQV induced chemokine release (Fig.3A&B). PAR1 selective agonist peptide TFLLR (100 μM) did not induce release of either IL-6 or RANTES (data not shown).
Figure 3: Effect of PAR activating peptides on chemokine release.
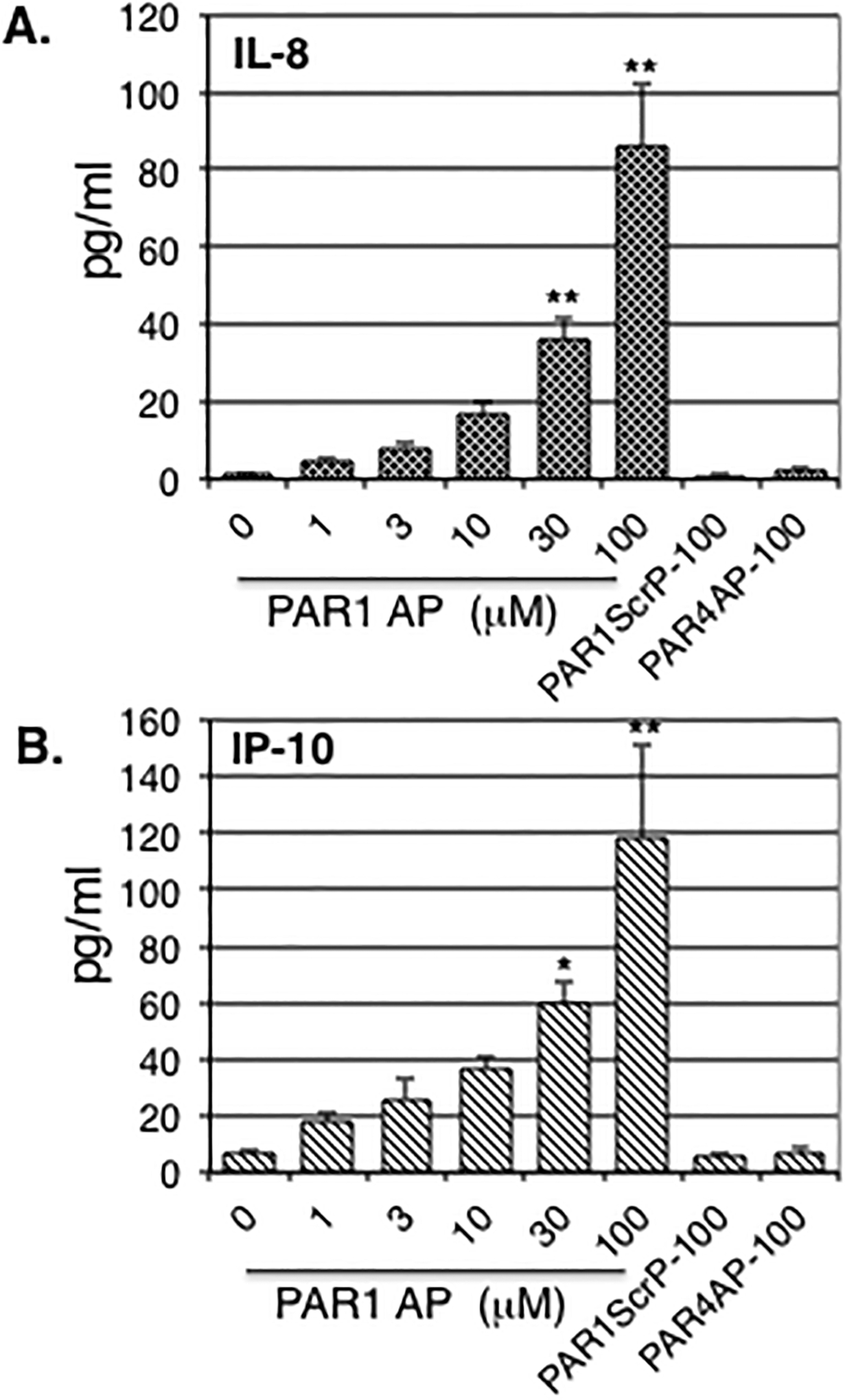
Human fetal astrocytes were stimulated with indicated concentrations of PAR1 agonist peptide (PAR1 AP), PAR1 scrambled peptide (PAR1ScrP) or PAR4 agonist peptide (PAR4 AP). IL-8 (A) and IP-10 (B) release was assessed 24 h later in conditioned medium as described in Methods. Data are expressed as mean ± S.E.M. percent response relative to thrombin plus DMSO (vehicle) control. n≥6, exp≥2, * indicates p< 0.05, ** indicates p< 0.01.
Discussion
The central findings in this report are that thrombin induces a dose-dependent increase in proliferation/metabolic activity as well as release of chemokines IL-8 (CXCL8) and IP-10 (CXCL10) from non-transformed cultured human fetal astrocytes. Our studies using PAR sub-type selective agonist peptides suggest that the above effects are mediated by PAR1. These findings are consistent with, and expand upon, previous work on rat astrocytes demonstrating both the proliferative [2] and chemokine-inducing [11, 12] effects of thrombin/PAR signaling. In particular, these results add to emerging evidence that astrocytes are a primary and functionally important cellular target for thrombin-induced PAR activation in the human brain [14–16].
In this study, the PAR1 selective peptide agonist induced a dose-dependent chemokine release profile similar to thrombin whereas equimolar concentrations of PAR4 activating peptide failed to induce a response (Fig.3). While these results clearly implicate PAR1 in the regulation of astrocytic chemokine release, we cannot completely exclude a contribution from PAR4 as PAR activating peptides have variable potencies [1] and very high doses are necessary to reproduce some thrombin-like responses [2]. Nonetheless, the PPACK inhibition (Fig.2) and PAR activating peptide (Fig.3) results confirm the chemokine release response as a bona fide thrombin/PAR-mediated effect. Similarly, we cannot entirely exclude the possibility that a small number of microglia could exist in our human fetal astrocyte cultures and contribute to these chemokine release responses. However, this possibility is unlikely given that prior studies on thrombin-induced chemokine release in microglia have failed to detect either IL-8 or IP-10 [20, 21]. Finally, our use of pharmaceutical grade thrombin [19] in these studies along with the results seen with our PPACK and polymyxin B controls effectively exclude the possibility that LPS contamination [18] might be contributing to the thrombin- and/or PAR1 activating peptide-induced effects seen.
The thrombin-induced release of IL-8 from human fetal astrocytes is interesting in light of previous findings demonstrating thrombin-induced release of GRO/CINC-1 from rat astrocytes [11, 12]. GRO/CINC-1 (homologous to GRO-α or CXCL1 in humans) shares structural and functional similarities to human IL-8 [13]. Both GRO/CINC-1 and IL-8 belong to the glutamic acid-leucine-arginine (ELR)+ sub-class of CXC chemokines. ELR+ chemokines tend to be regulated in tandem by inflammatory or immune stimuli such as IL-1, tumor necrosis factor-α (TNF-α) and LPS [8]. The latter three stimuli can all induce IL-8 expression specifically in human fetal astrocytes [22]. The IL-8 promoter contains binding sites for pro-inflammatory transcription factors such as NFκB. The latter has recently been implicated in thrombin-induced expression of IL-8 in human lung epithelial cells [23].
The ability of thrombin to induce both GRO/CINC-1 in rat astrocytes and IL-8 in human fetal astrocytes suggest a common functional consequence of thrombin exposure to these two distinct but related chemokines. GRO/CINC-1 and IL-8 share a common receptor (CXCR2) and actively participate in inflammatory reactions by inducing leukocyte (particularly neutrophil) recruitment and the generation of reactive oxygen species [8]. Thrombin is generated immediately at sites of vascular injury in stroke [2] whereas neutrophil recruitment into the ischemic hemisphere typically follows one to two days later [24]. Thrombin-induced release of IL-8 from astrocytes in the ischemic penumbra could be a possible mechanism for post-stroke neutrophil recruitment to the CNS. In addition, CXCR2 is present on neurons and astrocytes and its activation by GRO/CINC-1 protects both cell types against C2-ceramide-induced apoptotic cell death [11, 12]. Thus, thrombin-induced release of GRO/CINC-1 from rat astrocytes has been suggested as a key step in a novel CXCR2-mediated neuroprotective pathway [11]. Although it is uncertain whether or not IL-8 has the same capability as GRO/CINC-1 to alter neural cell viability, thrombin-induced astrocytic release of IL-8 could potentially function in a neuroprotective manner analogous to GRO/CINC-1.
This is the first report of thrombin-induced expression of IP-10 in any species or cell type. IP-10, which activates the pro-inflammatory chemokine receptor CXCR3, has been implicated in the pathophysiology of Multiple Sclerosis as well as other neurological disorders [25]. From a functional standpoint, IP-10 is critical in the recruitment of effector T-lymphocytes, natural killer cells, macrophages and dendritic cells [25]. IP-10 also induces inhibition of both angiogenesis and fibrosis and helps promote recovery of neural tissue following injury [24]. The direct effects of IP-10 on neural cell viability and function are largely unknown. Thrombin-induced IP-10 release from astrocytes may play an important and complementary role to IL-8 in directing the brain’s neuroinflammatory response to various CNS injuries. In addition, thrombin-induced IP-10 release from astrocytes could facilitate both glial-glial and glial-neuronal cell interactions by activating CXCR3 expressed on nearby microglia and neurons, respectively [25].
Conclusion
Thrombin induces a dose-dependent increase in proliferation/metabolic activity as well as release of chemokines IL-8 (CXCL8) and IP-10 (CXCL10) from human fetal astrocytes. These effects are mediated by PAR1. The selective thrombin-induced chemokine release profile observed (IL-8 and IP-10, but not IL-6 or RANTES) could help determine the character and kinetics of immune cell infiltration into the CNS under pathological conditions.
Funding Sources:
National Institute of Health
Footnotes
Conflicts of Interest: None declared
Please direct requests for reprints to corresponding author
References
- 1.Coughlin SR, Protease-activated receptors in hemostasis, thrombosis and vascular biology. Journal of thrombosis and haemostasis : JTH, 2005. 3(8): p. 1800–14. [DOI] [PubMed] [Google Scholar]
- 2.Grand RJ, Turnell AS, and Grabham PW, Cellular consequences of thrombin-receptor activation. The Biochemical journal, 1996. 313 (Pt 2): p. 353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, et al. , The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America, 2003. 100(22): p. 13019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicole O, Goldshmidt A, Hamill CE, Sorensen SD, Sastre A, Lyuboslavsky P, et al. , Activation of protease-activated receptor-1 triggers astrogliosis after brain injury. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2005. 25(17): p. 4319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuroglia. 2nd ed, ed. Kettenmann H and Ransom BR. Vol. one. 2005, New York, NY: Oxford University Press. [Google Scholar]
- 6.Nedergaard M and Dirnagl U, Role of glial cells in cerebral ischemia. Glia, 2005. 50(4): p. 281–6. [DOI] [PubMed] [Google Scholar]
- 7.Trendelenburg G and Dirnagl U, Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning. Glia, 2005. 50(4): p. 307–20. [DOI] [PubMed] [Google Scholar]
- 8.Charo IF and Ransohoff RM, The many roles of chemokines and chemokine receptors in inflammation. The New England journal of medicine, 2006. 354(6): p. 610–21. [DOI] [PubMed] [Google Scholar]
- 9.Ransohoff RM, Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity, 2009. 31(5): p. 711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Ubl JJ, and Reiser G, Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia, 2002. 37(1): p. 53–63. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Luo W, and Reiser G, Activation of protease-activated receptors in astrocytes evokes a novel neuroprotective pathway through release of chemokines of the growth-regulated oncogene/cytokine-induced neutrophil chemoattractant family. The European journal of neuroscience, 2007. 26(11): p. 3159–68. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Luo W, Stricker R, and Reiser G, Protease-activated receptor-1 protects rat astrocytes from apoptotic cell death via JNK-mediated release of the chemokine GRO/CINC-1. Journal of neurochemistry, 2006. 98(4): p. 1046–60. [DOI] [PubMed] [Google Scholar]
- 13.Ramos CD, Heluy-Neto NE, Ribeiro RA, Ferreira SH, and Cunha FQ, Neutrophil migration induced by IL-8-activated mast cells is mediated by CINC-1. Cytokine, 2003. 21(5): p. 214–23. [DOI] [PubMed] [Google Scholar]
- 14.Junge CE, Lee CJ, Hubbard KB, Zhang Z, Olson JJ, Hepler JR, et al. , Protease-activated receptor-1 in human brain: localization and functional expression in astrocytes. Experimental neurology, 2004. 188(1): p. 94–103. [DOI] [PubMed] [Google Scholar]
- 15.Nakao K, Shirakawa H, Sugishita A, Matsutani I, Niidome T, Nakagawa T, et al. , Ca2+ mobilization mediated by transient receptor potential canonical 3 is associated with thrombin-induced morphological changes in 1321N1 human astrocytoma cells. Journal of neuroscience research, 2008. 86(12): p. 2722–32. [DOI] [PubMed] [Google Scholar]
- 16.Kreda SM, Seminario-Vidal L, Heusden C, and Lazarowski ER, Thrombin-promoted release of UDP-glucose from human astrocytoma cells. British journal of pharmacology, 2008. 153(7): p. 1528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yagle K, Lu H, Guizzetti M, Moller T, and Costa LG, Activation of mitogen-activated protein kinase by muscarinic receptors in astroglial cells: role in DNA synthesis and effect of ethanol. Glia, 2001. 35(2): p. 111–20. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein JR, Swarts S, Bishop C, Hanisch UK, and Moller T, Lipopolysaccharide is a frequent and significant contaminant in microglia-activating factors. Glia, 2008. 56(1): p. 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein JR, Hong S, Kulman JD, Bishop C, Kuniyoshi J, Andersen H, et al. , Unraveling thrombin’s true microglia-activating potential: markedly disparate profiles of pharmaceutical-grade and commercial-grade thrombin preparations. J Neurochem, 2005. 95(4): p. 1177–87. [DOI] [PubMed] [Google Scholar]
- 20.Hanisch UK, van Rossum D, Xie Y, Gast K, Misselwitz R, Auriola S, et al. , The microglia-activating potential of thrombin: the protease is not involved in the induction of proinflammatory cytokines and chemokines. The Journal of biological chemistry, 2004. 279(50): p. 51880–7. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe Y, Miura I, Ohgami Y, and Fujiwara M, Extracellular presence of IL-8 in the astrocyte-rich cultured cerebellar granule cells under acidosis. Life sciences, 1998. 63(12): p. 1037–46. [DOI] [PubMed] [Google Scholar]
- 22.Hua LL and Lee SC, Distinct patterns of stimulus-inducible chemokine mRNA accumulation in human fetal astrocytes and microglia. Glia, 2000. 30(1): p. 74–81. [DOI] [PubMed] [Google Scholar]
- 23.Lin CH, Cheng HW, Ma HP, Wu CH, Hong CY, and Chen BC, Thrombin induces NF-kappaB activation and IL-8/CXCL8 expression in lung epithelial cells by a Rac1-dependent PI3K/Akt pathway. The Journal of biological chemistry, 2011. 286(12): p. 10483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frangogiannis NG, Chemokines in ischemia and reperfusion. Thrombosis and haemostasis, 2007. 97(5): p. 738–47. [PubMed] [Google Scholar]
- 25.Groom JR and Luster AD, CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunology and cell biology, 2011. 89(2): p. 207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


