Abstract
Lens-specific beaded filament (BF) proteins CP49 and filensin interact with the C-terminus of the water channel protein Aquaporin 0 (AQP0). Previously we have reported that a C-terminally end-deleted AQP0-expressing transgenic mouse model AQP0ΔC/ΔC developed abnormal optical aberrations in the lens. This investigation was undertaken to find out whether the total loss of the BF structural proteins alter the optical properties of the lens and cause optical aberrations similar to those in AQP0ΔC/ΔC lenses; also, to map the changes in the optical quality as a function of age in the single or double BF protein knockouts as well as to assess whether there is any significant change in the water channel function of AQP0 in these knockouts. A double knockout mouse (2xKO) model for CP49 and filensin was developed by crossing CP49-KO and filensin-KO mice. Wild type, CP49-KO, filensin-KO, and 2xKO lenses at different ages, and AQP0ΔC/ΔC lenses at postnatal day-17 were imaged through the optical axis and compared for optical quality and focusing property. All three knockout models showed loss of transparency, and development of abnormal optical distortion aberration similar to that in AQP0ΔC/ΔC. Copper grid focusing by the lenses at 6, 9 and 12 months of age showed an increase in aberrations as age advanced. With progression in age, the grid images produced by the lenses of all KO models showed a transition from a positive barrel distortion aberration to a pincushion distortion aberration with the formation of three distinct aberration zones similar to those produced by AQP0ΔC/ΔC lenses. Water permeability of fiber cell membrane vesicles prepared from CP49-KO, filensin-KO and 2xKO models, measured using the osmotic shrinking method, remained similar to that of the wild type without any statistically significant alteration (P > 0.05). Western blotting and quantification revealed the expression of comparable quantities of AQP0 in all three BF protein KOs. Our study reveals that loss of single or both beaded filament proteins significantly affect lens refractive index gradient, transparency and focusing ability in an age-dependent manner and the interaction of BF proteins with AQP0 is critical for the proper functioning of the lens. The presence of BF proteins is necessary to prevent abnormal optical aberrations and maintain homeostasis in the aging lens.
Keywords: CP49, Filensin, Aquaporin 0, Abnormal distortion aberration, Lens, Cataract, Beaded filament
1. Introduction
The ocular lens assembles two separate intermediate filament (IF) systems: the canonical 8–11 nm IFs composed of vimentin, and the beaded filaments (BFs). The BFs belong to the type VI orphan filament family (Omary et al., 2004; Perng and Quinlan, 2005; Perng et al., 2007; Song et al., 2009; FitzGerald, 2009; FitzGerald et al., 2016). They structurally differ from the thin filaments, canonical IFs, and microtubules, and form a filamentous network in the elongated fiber cells at the outer cortical region (FitzGerald, 2009; Alizadeh et al., 2003). Two proteins, CP49 (phakinin or BFSP2) and filensin (BFSP1) co-assemble to form the lens-specific BFs (Ireland and Maisel, 1984, 1989; FitzGerald and Gottlieb, 1989; Perng and Quinlan, 2005; Yoon et al., 2008).
BFs first appear in the differentiating fiber cells and reach a maximum in the cells that have just completed the elongation process. In mice, both BF proteins are also found in the anterior epithelium at five weeks of age but are not assembled into BFs (FitzGerald et al., 2016). Studies revealed that both CP49 and filensin are required for BF assembly and loss of either results in a minor loss of lens transparency and significantly reduces the partner protein (CP49 or filensin) levels in the fiber cells (Alizadeh et al., 2002, 2003; Sandilands et al., 2003). During fiber cell maturation, both BF proteins undergo post-translational processing and localize in the cytoplasm (Sandilands et al., 1995a,b; Blankenship et al., 2001). BF is not required for the formation of the differentiated fiber cell phenotype but is required to maintain the differentiated state and the long-range order which characterizes the optical lens (Yoon et al., 2008). Studies on the effect of the loss of a BF protein in the lenses of single knockout mouse models have shown that in addition to maintaining the fiber cell and lens structural phenotypes, the BF proteins are essential for lens transparency (Alizadeh et al., 2002, 2003; Sandilands et al., 2003), shape, and mechanical properties (Fudge et al., 2011). Compared to the wild type (WT), lens transparency decreased in the single knockouts (Alizadeh et al., 2002; Sandilands et al., 2003). BF proteins also interact with other lens proteins such as AQP0 (Lindsey Rose et al., 2006) and modulate the gap junction function (Kumari et al., 2017) and water channel function of AQP0 (Tapodi et al., 2019). Tropomodulin 1 protein, complex with CP49 and filensin and link with the spectrin-actin network, which is critical for regulating lens fiber cell geometry, transparency, and mechanical stiffness (Fudge et al., 2011; Gokhin et al., 2012). Filensin and CP49 intermediate filaments are involved in the formation of low temperature-induced, reversible, cold cataract formation in the transparent lenses of young C57BL/6J mouse (Li et al., 2020).
AQP0 is the most profusely expressed membrane water channel protein in the lens (Bassnett et al., 2009; Schey et al., 2017). It is a multifunctional protein (Kumari and Varadaraj, 2009, 2014, 2019). Recently, we have shown that AQP0 functions as peroxiporin and aid in the passive transport of hydrogen peroxide across the membrane to reduce oxidative stress in the lens (Varadaraj and Kumari, 2020). Complete loss of AQP0 causes lens cataracts even at the embryonic stage (Shiels et al., 2001). Mutations in AQP0 also result in dominant lens cataracts (Shiels and Bassnett, 1996; Varadaraj et al., 2008; Li et al., 2021). In AQP0 heterozygous mice, loss of BF proteins prompted an increase in lens spherical aberration and accelerated cataractogenesis (Kumari and Varadaraj, 2014; Kumari et al., 2017; Wang et al., 2020, 2021). We observed a unique abnormal optical distortion aberration in the lenses of a mouse model that expressed only C-terminally end-cleaved AQP0 (Varadaraj and Kumari, 2019). It has been reported that the C-terminus of AQP0 interacts with BF proteins (Lindsey Rose et al., 2006; Wang and Schey, 2017); C-terminus is also involved in the calcium-dependent regulation of Pf by binding to Calmodulin (Lindsey Rose et al., 2008). There is a lack of data on the effect of the complete loss of both BF proteins on lens transparency, and the formation of age-related abnormal optical aberrations. In this investigation, we bridge the research gap by exploring the effect of the loss of both BF proteins CP49 and filensin on lens optical quality, aberrations and focusing, and compare the optical properties with those of single KOs and WT, as a function of age; we compared the optical aberrations of BF protein/s KO lenses with those of AQP0ΔC/ΔC lenses. We also tested whether the loss of BF proteins singly or completely would affect the expression and water permeability (Pf) function of the interacting protein AQP0.
2. Materials and methods
2.1. Animals
Wild type (WT; C57BL/6J: Jackson Laboratory), AQP0ΔC/ΔC (C-terminal end 17 amino acids (247–263) deleted AQP0 knock-in (amino acids 1–246) mouse model (Varadaraj and Kumari, 2019), CP49 knockout (CP49-KO; Bfsp2tm1Pgf; http://www.informatics.jax.org/allele/key/42966 (Alizadeh et al., 2002), filensin knockout (filensin-KO; 3641549” title = “http://www.informatics.jax.org/allele/MGI:3641549”>Bfsp1tm1Pgf; 3641549” title = “http://www.informatics.jax.org/allele/MGI:3641549”>http://www.informatics.jax.org/allele/MGI:3641549 Alizadeh et al., 2003), and double knockout of CP49 and filensin (2xKO) mouse models were used in this investigation. All the models used were in C57BL/6J genetic background which does not carry the natural CP49 gene mutation. For animal procedures, the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, the National Institutes of Health’s (NIH; Bethesda, MD, USA) “Guide for the Care and Use of Laboratory Animals” and protocols approved by Stony Brook University Animal Care and Use Committee were followed.
2.2. Generation of BF double knockout (2xKO) mouse model
CP49-KO and filensin-KO mice were crossed to generate BF double knockout (2xKO) mice. The 2xKO mice were genotyped using the genomic DNA extracted from the tail tips. Genotyping was performed using the PCR primers and optimum PCR conditions described by Alizadeh et al. (2002, 2003).
2.3. Evaluation of lens transparency and aberration
Lens transparency was assessed as described by Kumari et al. (2017). In brief, lenses of WT, CP49-KO, filensin-KO, 2xKO and postnatal day 17 (P17) of AQP0ΔC/ΔC mice that express only C-terminally end-cleaved AQP0 were dissected out. These lenses, kept in prewarmed (37 °C) mammalian physiological saline, were imaged under the same conditions and lighting, using a darkfield binocular microscope attached to a digital camera. Each lens was imaged through the optical axis, anterior side facing the camera (Fig. 1). From the darkfield images, lens transparency was quantified using ImageJ (NIH), SigmaScan Pro 5.0 and SigmaPlot 10 software programs. Pixel brightness intensity data were translated into percentage of transparency and presented as bar graphs. Darkfield optical grid focusing was performed to qualitatively evaluate aberrations in the lens. Each lens was placed over a copper electron microscope specimen grid, the anterior side of the lens facing the grid and imaged (Fig. 1). Light scatter and aberrations caused by the alterations in the refractive index gradient were evaluated.
Fig. 1.
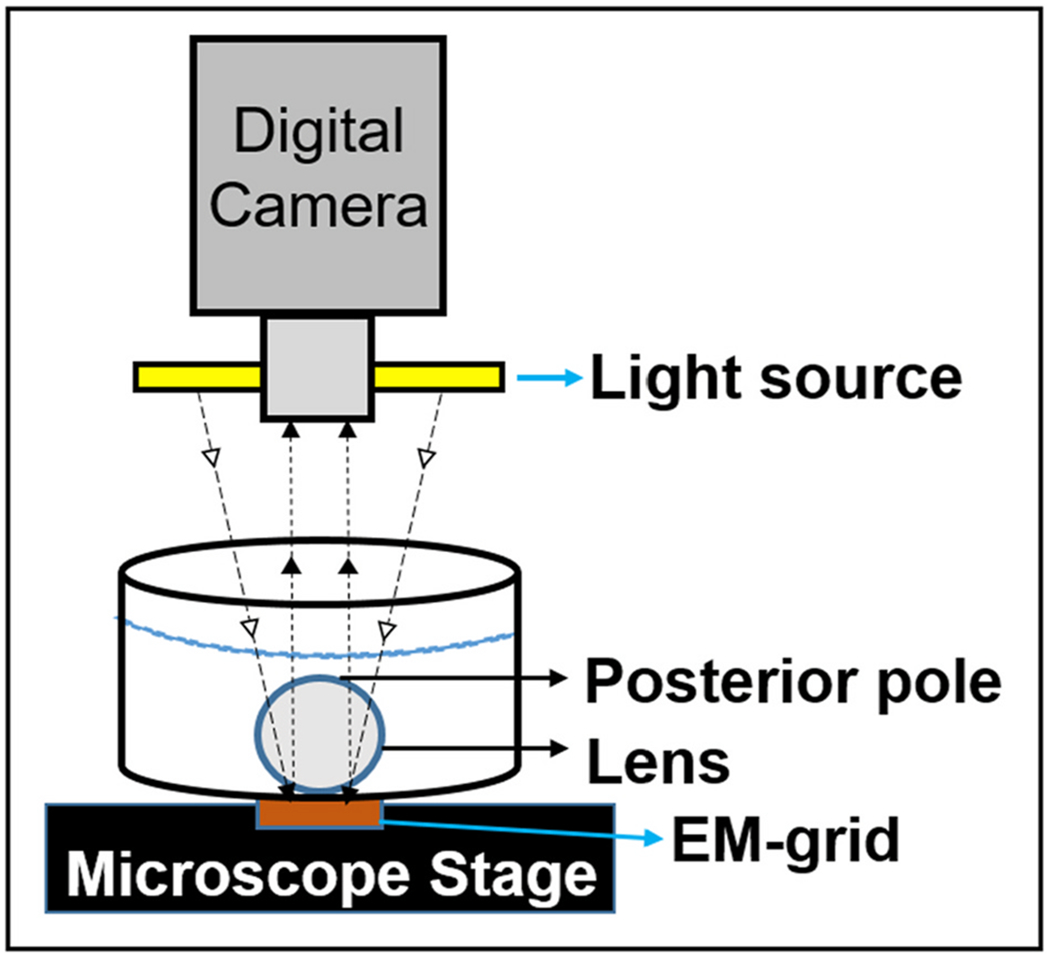
Schematic Representation of Darkfield Optical Grid Imaging. Each lens was placed over a copper electron microscope specimen grid, the anterior side of the lens facing the grid and the grid image was captured using a digital camera.
2.4. Western Blotting
Membrane proteins from WT, CP49-KO, filensin-KO and 2xKO lens fiber cells were extracted; Western blotting was done using C-terminal specific anti-AQP0, anti-CP49 and anti-filensin, as appropriate, as described by Kumari et al. (2017); immunodetection was carried out using an alkaline phosphatase kit from Vector Laboratories (Burlingame, CA, USA). The binding intensity of the immunoreactive bands was quantified using ImageJ software (NIH). The protein quantification data represent mean ± SD of four Western blot analyses using 2-month-old mouse lenses from different litters.
2.5. Mouse lens fiber cell membrane Pf Assay
Pf was measured using the rate of shrinking of lens fiber cell membrane vesicles (FCMVs), as done previously (Varadaraj et al., 1999, 2005). In brief, lens capsules were removed from WT, CP49-KO, filensin-KO and 2xKO mice, outer cortical fiber cell bundles were dissected out and FCMVs were prepared in isosmotic (300 mOsm) lens physiological saline. FCMVs were incubated in hypertonic saline (450 mOsm); using digital video microscopy, the rate of shrinkage was imaged. Pf was quantified as described by Varadaraj et al. (1999). Ten vesicles and four mice were used per study.
2.6. Statistical Analysis
The student’s t-test was done using SigmaPlot 10 software. P values ≤ 0.05 were considered significant.
3. Results and discussion
In mouse, the CP49 and filensin genes are localized in chromosomes 9 and 2, respectively. Each knockout was produced by inserting the Neo gene in the introns of the CP49 or filensin gene. The lenses of CP49 or filensin knockout mice were very similar when examined for transparency, light scattering, opacity development, morphology and ultrastructures suggesting the insertion of the Neo gene in mouse chromosome 9 or 2. The expression of the Neo gene did not cause any deleterious effect on the knockout lenses (Alizadeh et al., 2002, 2003). The CP49 (Alizadeh et al., 2002) or filensin (Alizadeh et al., 2003) knockout mice were crossed with WT C57BL/6J. Mice heterozygous for CP49 or filensin were interbred to produce 2xKO mice, which were genotyped to verify the absence of intact CP49 and filensin gene (Fig. 2A). Using the primers for CP49, and filensin + Neo gene (Alizadeh et al., 2002, 2003), two separate PCR reactions were carried out. In contrast to the WT genomic DNA, the primers for CP49 did not amplify the 450-base pair (bp) amplicon using the genomic DNA from 2xKO mice (lanes 1 and 2; samples from mouse 1 and mouse 2), indicating the disruption of CP49. After confirming the loss of CP49, genomic DNAs of mouse 1 and mouse 2 were tested for the absence of filensin. PCR using a mixture of Neo-gene primers and filensin primers amplified a 500 bp segment for the presence of the introduced Neo-gene (lanes marked as 1 and 2) indicating the presence of the disrupted allele (Alizadeh et al., 2002). Contrary to the WT, in lanes marked 1 and 2, there was no amplification of a 250 bp fragment, which represents the presence of intact filensin. These results showed the absence of both CP49 and filensin in the 2xKO knockout mouse. Individual knockout of CP49 or filensin has also been previously confirmed for the disruption of their respective loci (Alizadeh et al., 2002, 2003). Western blotting was performed to show the presence of CP49 and filensin in the WT and their absence in the double knockout (2xKO; Fig. 2B).
Fig. 2.
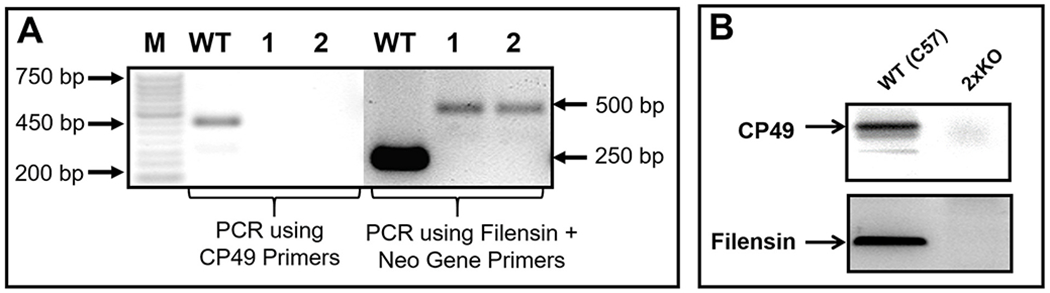
A. Genotyping. A double knockout mouse model, for lens-specific beaded filament proteins CP49 and filensin, was developed using CP49 (CP49-KO) and filensin (filensin-KO) knockout mice(Alizadeh et al., 2002, 2003), which do not have CP49 natural mutation. A Neomycin gene cassette was introduced during the process of single knockout mouse development ; genomic PCR was done to confirm the knockout of both proteins using the primer sets previously described by (Alizadeh et al., 2002, 2003). PCR of WT genomic DNA amplified a 450 bp amplicon denoting the presence of CP49. Lanes 1 and 2 ( samples from mouse 1 and mouse 2, respectively) representing samples for double knockout did not amplify the 450 bp fragment, confirming the disruption of the CP49 gene locus. With a mixture of filensin primers and Neo gene primers, WT genomic DNA amplified a 250 bp amplicon for the presence of filensin; the two double knockout samples (lanes marked as 1 and 2) amplified a 500 bp fragment (representative of the Neo gene cassette) confirming the disruption of the filensin gene locus. B. Western Blotting. WT mouse lens membrane proteins immunoreacted to CP49 and filensin antibodies indicating the expression of the respective proteins. 2xKO mouse lens membrane proteins showed no immunoreactivity to either antibody confirming the knockout status of CP49 and filensin gene loci.
Lens transparency at 2, 6, 9, and 12 months of age in WT, CP49-KO, filensin-KO, and 2xKO mice was qualitatively assessed. Darkfield images of lenses were captured using the same conditions (Fig. 3A–D, Left columns). Lens pixel brightness intensities were quantified, converted into arbitrary units and presented as bar graphs (Fig. 4). Transparency of all the tested lenses including the WT showed a decreasing trend from 2 months of age to 12-months (Fig. 3 Left columns and 4A). However, CP49-KO, filensin-KO, and 2xKO mice lenses showed an accelerated loss of lens transparency compared to WT lenses (Fig. 4B). Loss of lens transparency in the WT from 2 months to 12-months was statistically significant (P < 0.01; Fig. 4A); however, at all the tested ages, loss of transparency in the KO models was statistically significant (P < 0.01) compared to the WT lenses.
Fig. 3.
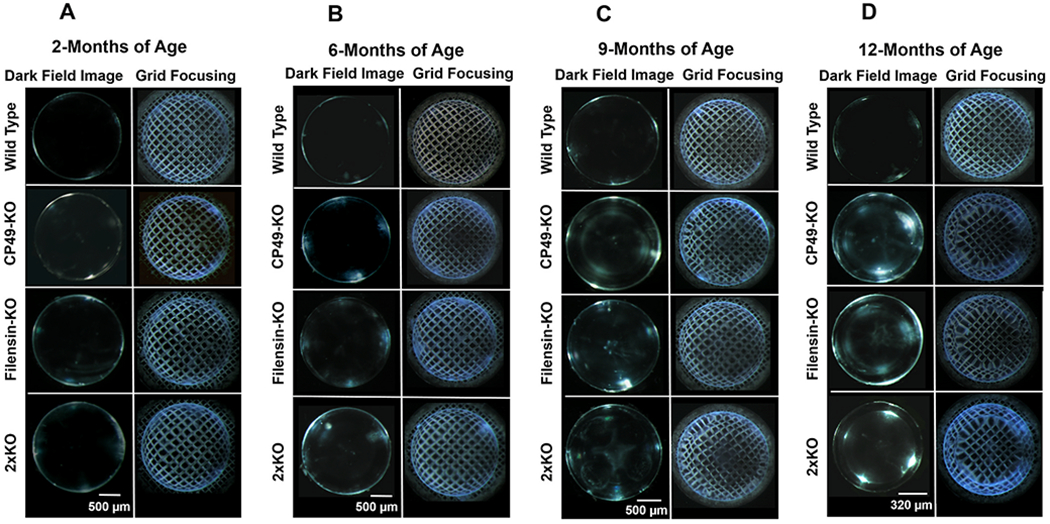
Lens transparency and optical aberration. (A-D) Progressive increase in lens opacity (left columns) and abnormal distortion aberration (right columns) in the lenses of CP49-KO, filensin-KO, and 2xKO mice compared with age-matched WT lenses. Lenses from adult mice at: (A) 2-months of age; (B) 6-months of age; (C) 9-months of age; (D) 12-months of age.
Fig. 4.
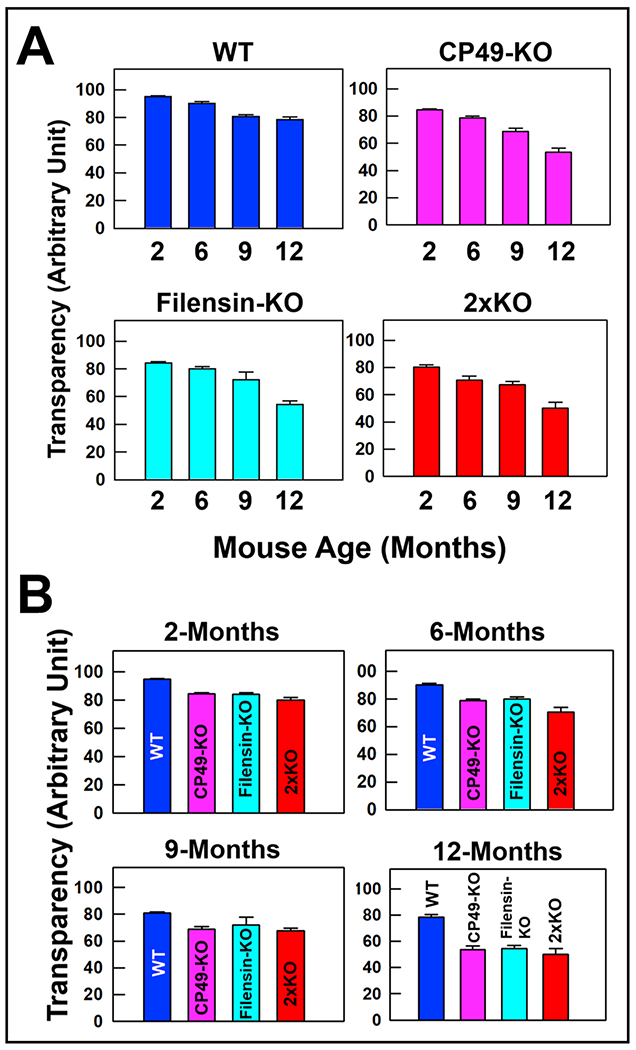
Quantification of lens transparency. Bar graph showing progressive loss of lens transparency in CP49-KO, filensin-KO and 2xKO mice compared with age-matched WT lenses. Lens transparency was calculated for (A) WT, CP49-KO, filensin-KO, and 2xKO mice at (B) 2-, 6-, 9- and 12-months of age by subtracting the pixel brightness intensity from 100. Each bar represents mean ± SD.
For analyzing the optical quality, we followed the method (Fig. 1) of imaging an electron microscopy copper grid (EM-grid) through the lenses. In general, WT lenses while magnifying the grid pattern produce a deformation called positive barrel distortion aberration in which the straight lines of the grid bend outward from the image center. This is similar to what happens while using a wide-angle (camera) lens. The opposite effect is called pincushion distortion aberration in which the straight lines bend inward. In an AQP0 mutant mouse model, we recently observed the distortion aberrations such as pincushion distortion aberration, and a mixture of the positive barrel and pincushion distortion aberrations (Varadaraj and Kumari, 2019). We are following the usage of these terms when describing the optical quality of the lenses in the current study. Lenses of WT, CP49-KO, filensin-KO, and 2xKO mice were subjected to grid focusing. WT lenses showed a positive barrel distortion aberration at the tested ages (Fig. 3A–D Right columns). At 2 months of age, CP49-KO, filensin-KO, and 2xKO lenses focused the grid lines with a positive barrel distortion aberration, very similar to those of the WT lenses (Fig. 3A Right column). At 6 months of age, all of the knockout lenses began to show an irregularity in the distortion of grid lines compared to the WT lenses (Fig. 3B Right column). By 9 months, CP49-KO, filensin-KO, and 2xKO mouse lenses started transitioning to a pincushion distortion aberration in the outer cortical region and started to form two zones (Fig. 3C Right column), which are more visible in the darkfield images (Fig. 3C Left column). The two zones in filensin-KO were not as distinct as in the CP49-KO and 2xKO lenses. By 12-months of age, the two single knockouts and the double knockout lenses completely transitioned into pincushion distortion aberration at the inner cortical and nuclear regions and showed the formation of a third zone, demarcating the first two zones (Fig. 5). The three zones are also visible in the darkfield images (Fig. 3D, KO lenses shown in the Left column). The zone formation appears to be age-dependent.
Fig. 5.
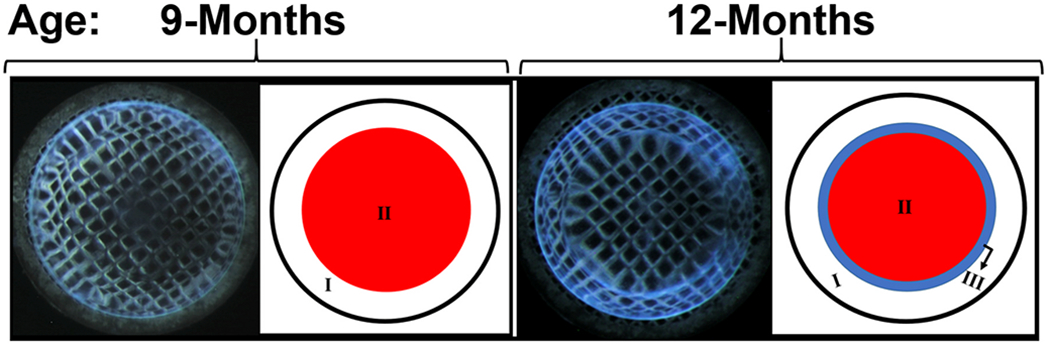
Optical aberration zones. Development of distortion aberration zones in the lenses of 2xKO mice at 9-months and 12-months of age. Schematic representation of the zones is provided parallel to each lens with zones marked as I, II, or III as appropriate.
Lenses of all three knockout mouse models showed an increase in abnormal distortion aberration pattern in an age-dependent manner from 6-months to 12-months of age (Fig. 3B–D) while focusing the grid lines. The pincushion distortion aberration (Figs. 3 and 5) progressively increased with age (compare Fig. 3C and D). The age-dependent progressive lens abnormal distortion aberration did not significantly affect the transparency but caused significant light scattering indicating refractive index gradient alteration in the lenses. In all three mouse models, abnormal distortion aberration significantly affected the focusing ability of the lenses (Fig. 3B, C, and D). Since BF proteins interact with AQP0, which is also critical for lens transparency and refractive index gradient formation (Kumari and Varadaraj, 2014), we tested to see whether there is any change in the expression levels of AQP0 in the single and double BF protein KO mice, by Western blotting and quantified the immunoreactive bands (Fig. 6A and B). There was no statistically significant difference (P > 0.05) in the expression of AQP0 in the KO lenses compared to that of the WT lenses (Fig. 6B) affirming that the negative effects observed on the lenses of the BF protein knockouts were due to the absence of one or both of the BF proteins. Next, we asked, how does the membrane Pf of AQP0 in the BF KOs compare with that of the WT? Pfof the fiber cell membrane vesicles of BF protein KOs showed no statistically significant difference from that of the WT (Fig. 7). However, through Xenopus oocyte expression studies Tapodi et al. (2019) found regulation of AQP0 Pf by caspase fragments of filensin C-terminus under different Ca2+ concentrations.
Fig. 6.
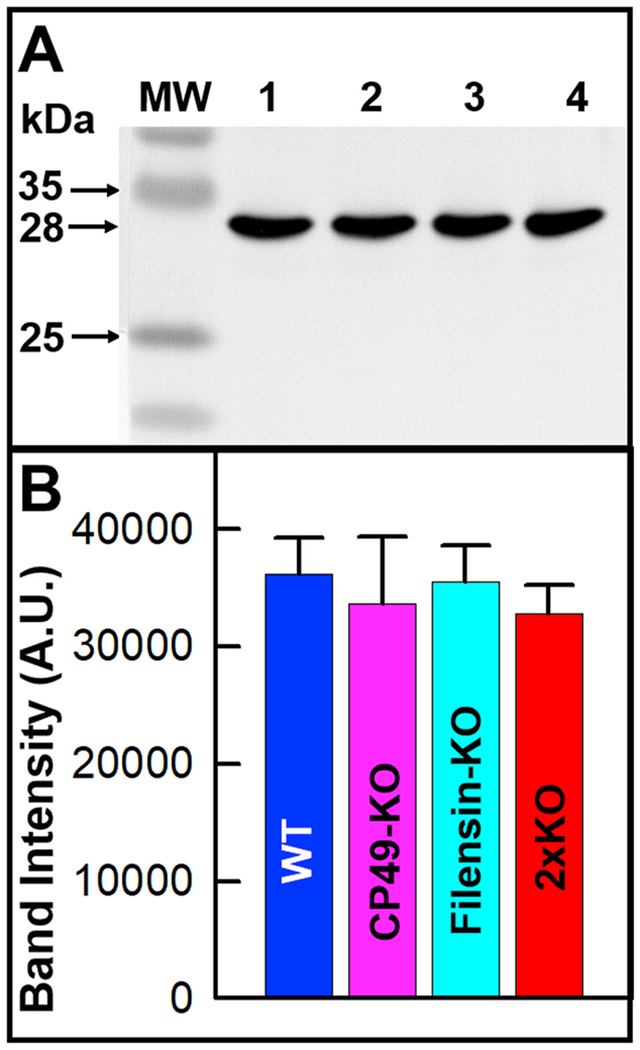
AQP0 expression in WT and BF KO lenses. A. Western blotting. Mouse lens membrane proteins immunoreacted to AQP0 antibody in WT and all KOs. B. Quantification of anti-AQP0 immunoreactive band of WT and all KOs. There was no statistically significant alteration (P > 0.05) in the AQP0 expression level in the KO lenses compared to that of WT lenses. Each bar represents mean ± SD. A.U. -Arbitrary Unit.
Fig. 7.
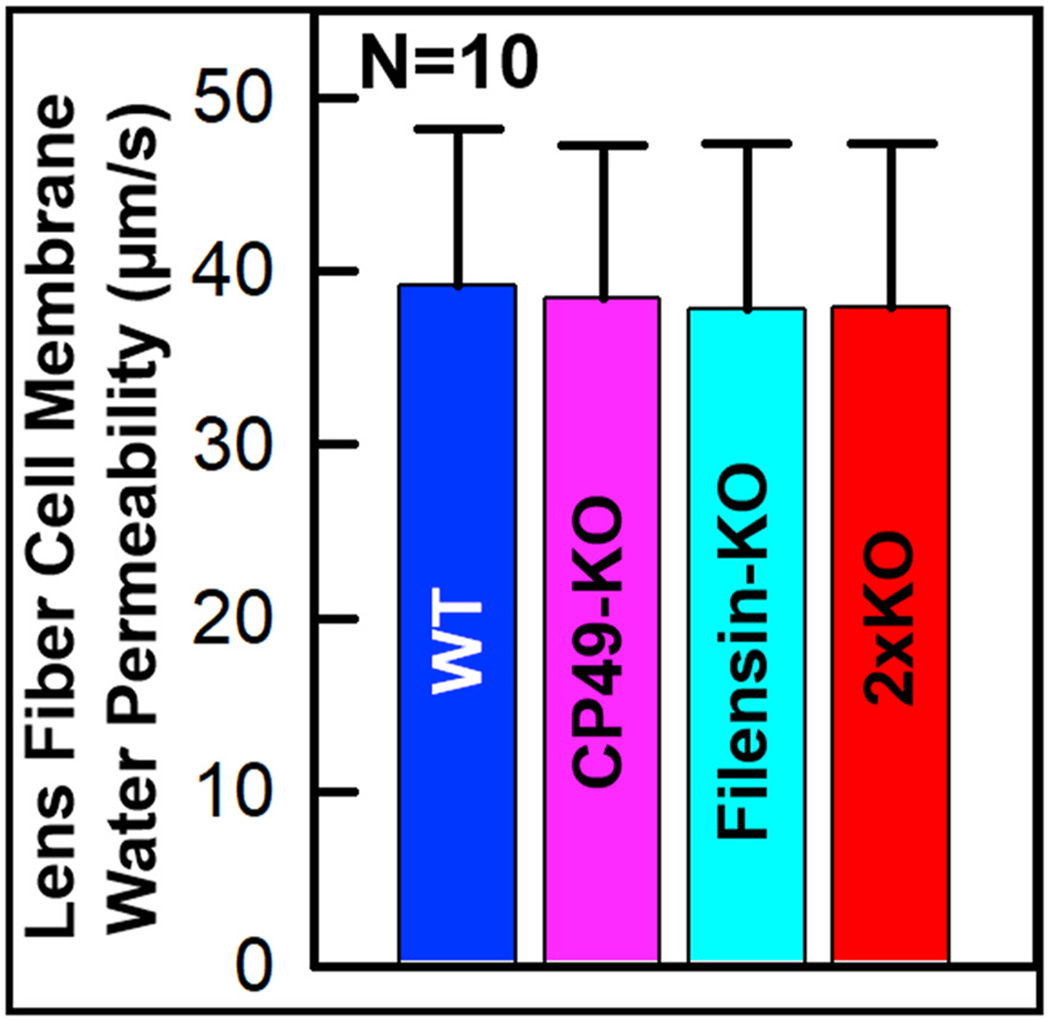
Lens fiber cell membrane water permeability. Membrane vesicles from WT, CP49-KO, filensin-KO and 2xKO lens fiber cells were tested. Each bar represents mean ± SD. SE values: WT: 2.8394; CP49-KO: 2.8016; filensin-KO: 3.0067; 2xKO: 2.9719. Fiber cell membrane vesicles exhibited no statistically significant alteration (P > 0.05) in Pf compared to those of WT. Ten vesicles and four mice were used per study.
Minogue et al. (2017) reported the formation of two zones of distortion aberrations, in the lenses of a connexin 46 (Cx46) mutant mouse model (Cx46fs380): (1) Positive barrel distortion, and (2) Pincushion distortion. Our previous study of a knockin mutant mouse model (AQP0ΔC/ΔC; Varadaraj and Kumari, 2019) expressing only C-terminally end-cleaved AQP0, in the absence of normal intact AQP0 in the lens, found three zones of the abnormal distortion aberration in the lenses; there was no alteration in membrane Pf (Kumari and Varadaraj, 2019). AQP0ΔC/ΔC lenses developed the unique optical aberration as early as P5; after P15, cataracts began to develop. Fig. 8a shows the transparency of a WT lens at P17; Fig. 8b demonstrates its focusing ability. A comparison to the age-matched AQP0ΔC/ΔC lens shows the abnormal optical distortion aberration with the development of three zones at P17 (Fig. 8c, d). Fig. 8e is a cartoon depicting the three zones. BF proteins CP49 and filensin interact with the C-terminal end of AQP0 (Lindsey Rose et al., 2006; Blankenship et al., 2001; Wang and Schey, 2017; Kumari et al., 2017). In a previous study, we have shown colocalization of CP49 and filensin with AQP0 (Kumari et al., 2017). Interaction of CP49 and filensin with other membrane proteins and the possibility of having an independent function(s) other than forming BF in the lens has been speculated (Masaki et al., 1997; Alizadeh et. al., 2003; Sandilands et. al., 2003; Wang and Schey, 2017). In the present study, the absence of BF proteins singly or together resulted in the formation of three zones of abnormal distortion aberrations in the lens and caused poor focusing ability. Pf of AQP0 remained unaffected in all three KO models (Fig. 7) similar to that in AQP0ΔC/ΔC lenses (Kumari and Varadaraj, 2019). Our data suggest that while the interaction of BF proteins with AQP0 does not influence the water channel function of the latter under the current experimental condition, BF proteins are necessary for lens transparency and optical quality. Ca2+ concentration is a regulating factor in AQP0 Pf and depends on the presence of the C-terminus of AQP0 and the interaction of BF with AQP0 (Tapodi et al., 2019). Even though the AQP0 C-terminal end cleavage we performed (247–263 amino acids) did not affect the putative calmodulin-binding site (amino acids 223–242) or the BF protein-interacting site (D246, Wang and Schey, 2017), we intend to further investigate AQP0 Pf under different Ca2+ concentrations to find out whether the similarity in the abnormal optical distortion aberration, which starts as early as postnatal day 5 in AQP0ΔC/ΔC (Varadaraj and Kumari, 2019) and around the age of 6-months in the BF KO models, could be due to an alteration in Ca2+-dependent AQP0 Pf regulation of structural interactions between BF and AQP0 or due to some other mechanism. Since pH also influences AQP0 Pf, we intend to conduct experiments under varied pH as additional future studies. Overall, this investigation documents that abnormal optical distortion aberrations occur in the BF KO lenses as a function of age and there is a similarity in the aberration pattern between the lenses of BF KOs and that in the lenses of AQP0ΔC/ΔC mice.
Fig. 8.
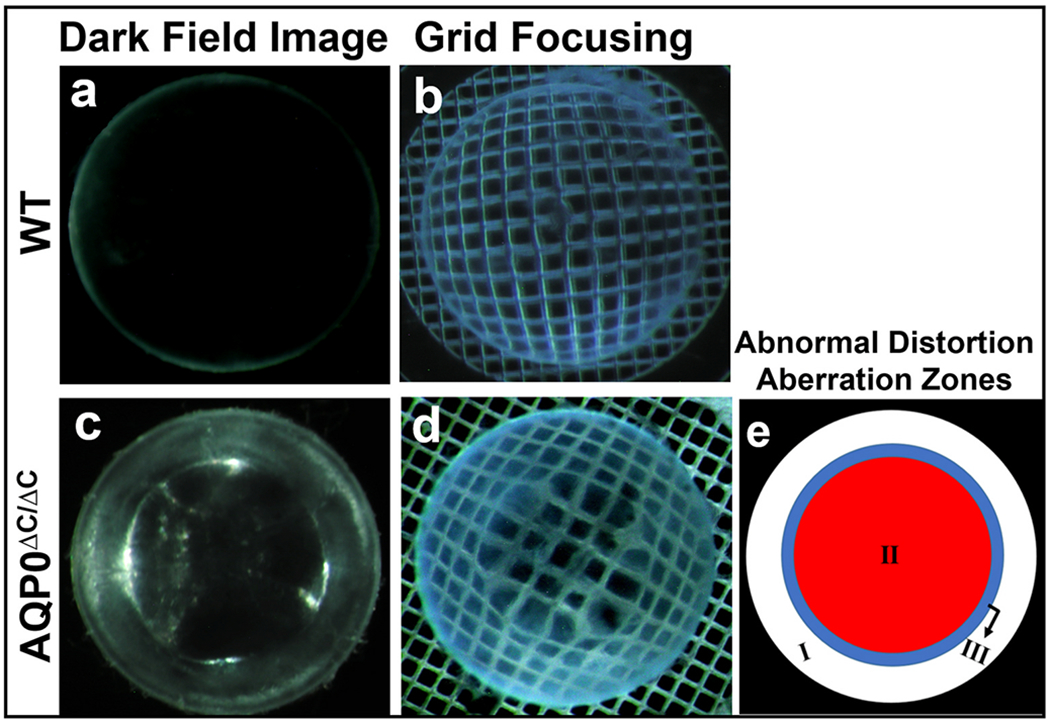
Qualitative characterization of transparency and focusing ability. (a, b) WT lenses at P17; (c, d) Age-matched AQP0ΔC/ΔC lenses that express only C-terminally end-cleaved AQP0. (e) Schematic representation of the three zones of abnormal optical distortion aberration in AQP0ΔC/ΔC lenses.
In conclusion, our results show that BF proteins and their interaction with AQP0 are essential for maintaining lens transparency, normal refractive index gradient, optical quality and homeostasis for normal focusing without the development of abnormal optical distortion aberrations. The CP49-KO, filensin-KO, 2xKO and AQP0ΔC/ΔC are suitable mouse models for studying age-related cataracts to identify therapeutic strategies for treating or preventing age-related optical aberrations and lens cataract in humans.
Acknowledgments
This work was supported by NIH-NEI grants R01 EY026155 (to K. Varadaraj) and R01 EY027430 (to P.G. FitzGerald).
Footnotes
Declaration of competing interest
None.
References
- Alizadeh A, Clark JI, Seeberger T, Hess J, Blankenship T, Spicer A, FitzGerald PG, 2002. Targeted genomic deletion of the lens-specific intermediate filament protein CP49. Invest. Ophthalmol. Vis. Sci 43, 3722–3727. [PubMed] [Google Scholar]
- Alizadeh A, Clark J, Seeberger T, Hess J, Blankenship T, FitzGerald PG, 2003. Targeted deletion of the lens fiber cell-specific intermediate filament protein Filensin. Invest. Ophthalmol. Vis. Sci 44, 5252–5258. [DOI] [PubMed] [Google Scholar]
- Blankenship TN, Hess JF, FitzGerald PG, 2001. Development- and differentiation-dependent reorganization of intermediate filaments in fiber cells. Invest. Ophthalmol. Vis. Sci 42, 735–742. [PubMed] [Google Scholar]
- FitzGerald PG, 2009. Lens intermediate filaments. Exp. Eye Res 88, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald PG, Gottlieb W, 1989. The Mr 115 kd fiber cell-specific protein is a component of the lens cytoskeleton. Curr. Eye Res 8, 801–811. [DOI] [PubMed] [Google Scholar]
- FitzGerald P, Sun N, Shibata B, Hess JF, 2016. Expression of the type VI intermediate filament proteins CP49 and filensin in the mouse lens epithelium. Mol. Vis 22, 970–989. [PMC free article] [PubMed] [Google Scholar]
- Fudge DS, McCuaig JV, Van Stralen S, Hess JF, Wang H, Mathias RT, FitzGerald PG, 2011. Intermediate filaments regulate tissue size and stiffness in the murine lens. Invest. Ophthalmol. Vis. Sci 52, 3860–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhin DS, Nowak RB, Kim NE, Arnett EE, Chen AC, Sah RL, Clark JI, Fowler VM, 2012. Tmod1 and CP49 synergize to control the fiber cell geometry, transparency, and mechanical stiffness of the mouse lens. PLoS One 7, e48734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland M, Maisel H, 1984. A cytoskeletal protein unique to lens fiber cell differentiation. Exp. Eye Res 38, 637–645. [DOI] [PubMed] [Google Scholar]
- Ireland M, Maisel H, 1989. A family of lens fiber cell specific proteins. Lens Eye Toxic. Res 6, 623–638. [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K, 2009. Intact AQP0 performs cell-to-cell adhesion. Biochem. Biophys. Res. Commun 390, 1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K, 2014. Aquaporin 0 plays a pivotal role in refractive index gradient development in mammalian eye lens to prevent spherical aberration. Biochem. Biophys. Res. Commun 45, 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Gao J, Mathias RT, Sun X, Eswaramoorthy A, Browne N, Zhang N, Varadaraj K, 2017. Aquaporin 0 modulates lens gap junctions in the presence of lens-specific beaded filament proteins. Invest. Ophthalmol. Vis. Sci 58, 6006–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K, 2019. A predominant form of C-terminally end-cleaved AQP0 functions as an open water channel and an adhesion protein in AQP0ΔC/ΔC mouse lens. Biochem. Biophys. Res. Commun 511, 626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Gu S, Quan Y, Varadaraj K, Jiang JX, 2021. Development of a potent embryonic chick lens model for studying congenital cataracts in vivo. Commun. Biol 4, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu X, Xia C, FitzGerald PG, Li R, Wang J, Gong X, 2020. CP49 and filensin intermediate filaments are essential for formation of cold cataract. Mol. Vis 26, 603–612. [PMC free article] [PubMed] [Google Scholar]
- Lindsey Rose KM, Gourdie RG, Prescott AR, Quinlan RA, Crouch RK, Schey KL, 2006. The C terminus of lens aquaporin 0 interacts with the cytoskeletal proteins filensin and CP49. Invest. Ophthalmol. Vis. Sci 47, 1562–1570. [DOI] [PubMed] [Google Scholar]
- Lindsey Rose KM, Wang Z, Magrath GN, Hazard ES, Hildebrandt JD, Schey KL, 2008. Aquaporin 0-calmodulin interaction and the effect of aquaporin 0 phosphorylation. Biochemistry 47, 339–347. [DOI] [PubMed] [Google Scholar]
- Masaki S, Quinlan RA, 1997. Gene structure and sequence comparisons of the eye lens specific protein, filensin, from rat and mouse: implications for protein classification and assembly. Gene 201, 11–20. [DOI] [PubMed] [Google Scholar]
- Minogue PJ, Gao J, Zoltoski RK, Novak LA, Mathias RT, Beyer EC, Berthoud VM, 2017. Physiological and optical alterations precede the appearance of cataracts in Cx46fs380 mice. Invest. Ophthalmol. Vis. Sci 58, 4366–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary MB, Coulombe PA, McLean WH, 2004. Intermediate filament proteins and their associated diseases. N. Engl. J. Med 351, 2087–2100. [DOI] [PubMed] [Google Scholar]
- Perng MD, Quinlan RA, 2005. Seeing is believing! the optical properties of the eye lens are dependent upon a functional intermediate filament cytoskeleton. Exp. Cell Res 305, 1–9. [DOI] [PubMed] [Google Scholar]
- Perng MD, Zhang Q, Quinlan RA, 2007. Insights into the beaded filament of the eye lens. Exp. Cell Res 313, 2180–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandilands A, Prescott AR, Carter JM, Hutcheson AM, Quinlan RA, Richards J, FitzGerald PG, 1995a. Vimentin and CP49/filensin form distinct networks in the lens which are independently modulated during lens fibre cell differentiation. J. Cell Sci 108, 1397–1406. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Prescott AR, Hutcheson AM, Quinlan RA, Casselman JT, FitzGerald PG, 1995b. Filensin is proteolytically processed during lens fiber cell differentiation by multiple independent pathways. Eur. J. Cell Biol 67, 238–253. [PubMed] [Google Scholar]
- Sandilands A, Prescott AR, Wegener A, Zoltoski RK, Hutcheson AM, Masaki S, Kuszak JR, Quinlan RA, 2003. Knockout of the intermediate filament protein CP49 destabilises the lens fibre cell cytoskeleton and decreases lens optical quality, but does not induce cataract. Exp. Eye Res 76, 385–391. [DOI] [PubMed] [Google Scholar]
- Schey KL, Petrova RS, Gletten RB, Donaldson PJ, 2017. The role of aquaporins in ocular lens homeostasis. Int. J. Mol. Sci 18, 2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bassnett S, 1996. Mutations in the founder of the MIP gene family underlie cataract development in the mouse. Nat. Genet 12, 212–215. [DOI] [PubMed] [Google Scholar]
- Song S, Landsbury A, Dahm R, Liu Y, Zhang Q, Quinlan RA, 2009. Functions of the intermediate filament cytoskeleton in the eye lens. J. Clin. Invest 119, 1837–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapodi A, Clemens DM, Uwineza A, Goldberg MW, Thinon E, Heal WP, Tate EW, Nemeth-Cahalan K, Vorontsova I, Jarrin M, Hall JE, Quinlan R, 2019. BFSP1 C-terminal domains released by post-translational processing events can alter significantly the calcium regulation of AQP0 water permeability. Exp. Eye Res 185, 107585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadaraj K, Kumari SS, 2020. Lens aquaporins function as peroxiporins to facilitate membrane transport of hydrogen peroxide. Biochem. Biophys. Res. Commun 524, 1025–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadaraj K, Kushmerick C, Baldo GJ, Bassnett S, Shiels A, Mathias RT, 1999. The role of MIP in lens fiber cell membrane transport. J. Membr. Biol 170,191–203. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kumari S, Shiels A, Mathias RT, 2005. Regulation of aquaporin water permeability in the lens. Invest. Ophthalmol. Vis. Sci 46, 1393–1402. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kumari SS, Patil R, Wax MB, Mathias RT, 2008. Functional characterization of a human aquaporin 0 mutation that leads to a congenital dominant lens cataract. Exp. Eye Res 87, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadaraj K, Kumari SS, 2019. Deletion of seventeen Amino acids at the C-terminal end of Aquaporin 0 causes distortion aberration and cataract in the lenses of AQP0ΔC/ΔC mice. Invest. Ophthalmol. Vis. Sci 60, 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Schey KL, 2017. Identification of a direct Aquaporin-0 binding site in the lens-specific cytoskeletal protein filensin. Exp. Eye Res 159, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Vorontsova I, Hoshino M, Uesugi K, Yagi N, Hall JE, Schilling TF, Pierscionek BK, 2020. Optical development in the zebrafish eye lens. Faseb. J 34, 5552–5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Vorontsova I, Hoshino M, Uesugi K, Yagi N, Hall JE, Schilling TF, Pierscionek BK, 2021. Aquaporins have regional functions in development of refractive index in the zebrafish eye lens. Invest. Ophthalmol. Vis. Sci 62, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KH, Blankenship T, Shibata B, Fitzgerald PG, 2008. Resisting the effects of aging: a function for the fiber cell beaded filament. Invest. Ophthalmol. Vis. Sci 49, 1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]


