Abstract
This chapter provides a general introduction to the dynorphins (DYNs) / kappa opioid receptor (KOR) system, including DYN peptides, neuroanatomy of the DYNs / KOR system, cellular signaling, and in vivo behavioral effects of KOR activation and inhibition. It is intended to serve as a primer for the book and to provide a basic background for the chapters in the book.
1. Historical perspectives
Opium, the dried latex or gum obtained from lancing the outer surface of the seed pods of the opium poppy Papaver somniferum, has been used for centuries for medicinal and recreational practices to relieve pain, cough and diarrhea, and to cause euphoria (Presley and Lindsley 2018). The euphoria produced by opium and iatrogenic-induced positive mood effects have been the basis for its abuse. It was in the 20th century when there were major advances made in understanding how the active constituents of opium, such as morphine, act to produce their beneficial and harmful effects.
Opioid receptors were first hypothesized in 1954 by Beckett and Casy (1954) and stereospecific saturable binding of levorphanol was proposed to be the opiate receptor (Goldstein et al. 1971). In 1973, the existence of specific receptors for opioid drugs was demonstrated in brain preparations by radioligand binding assays (Pert et al. 1973; Simon et al. 1973; Terenius 1973). Multiplicity of opioid receptors was reported, with different classes of opioid drugs having distinct pharmacological activities (Lord et al. 1977; Martin et al. 1976). Following the identification of opioid receptors, an intense search for the endogenous ligands ensued and cumulated in the discovery of enkephalins, β-endorphin and dynorphins (DYNs) as ligands for the delta, mu and kappa opioid receptors (DOR, MOR and KOR) (Chavkin et al. 1982; Goldstein et al. 1979; Hughes et al. 1975; Loh et al. 1976). Subsequently, the precursors of these peptides, proenkephalin, proopiomelanocortin and prodynorphin (pDYN), were cloned and their amino acid sequences deduced [see (Höllt 1986) for a review]. These precursors are synthesized as large proteins in cell bodies by the protein synthesis machinery, transported via axonal transport to nerve terminals and cleaved by peptidases to produce the final active peptides during the transport process. In the 1980s, great efforts by chemists and pharmacologists resulted in synthesis and characterization of selective MOR, KOR and DOR agonists and antagonists, which facilitated in vitro and in vivo opioid pharmacological studies. The first prototypical selective KOR agonist U50,488H and antagonist nor-binaltorphimine (norBNI) were reported by von Voigtlander et al. (1983) and Portoghese et al. (1987), respectively. Roth et al. (2002) demonstrated that salvinorin A, a compound isolated from the mint family plant Salvia divinorum, was a selective KOR agonist and also the first nonnitrogenous KOR agonist.
In 1992, the laboratories of Evans and Kieffer independently cloned the DOR (Evans et al. 1992; Kieffer et al. 1992). Subsequently, the KOR was cloned from several species, along with the MOR and a highly homologous receptor named opioid receptor-like (ORL) receptor [which subsequently renamed as nociceptin/orphanin FQ receptor (NOP receptor )] were cloned [see (Knapp et al. 1995) for a review]. Following cloning of the receptors, many studies demonstrated the structural bases of ligand binding selectivity using chimeric receptor and site-directed mutagenesis approaches. In addition, genetic deletion of the KOR or pDYN in mice revealed the in vivo physiological and pharmacological roles of the DYNs/KOR system and in sustaining drug and alcohol abuse (Chavkin 2013; Simonin et al. 1998). In the meantime, deletion of the MOR, DOR and NOP receptor was used to demonstrate their functional roles [see (Gaveriaux-Ruff and Kieffer 2002) for review]. In 2012, X-ray crystal structures of an inactive state of the human KOR complexed with the selective antagonist JDTic was revealed (Wu et al. 2012), and in 2018, the structure of an active state of the KOR was reported (Che et al. 2018). During this period, structures of active and inactive states of the MOR, DOR and NOP receptor were also published [see (Marino et al. 2018) for a review].
2. Dynorphin peptides
Endogenous opioid peptides are processed from three precursors, proopiomelanocortin, proenkephalin, and pDYN. Endogenous activation of the KOR primarily occurs via the opioid peptides derived from pDYN (Chavkin et al. 1982). Dynorphins such as DYN A (1–7, 1–8, 1–13, and 1–17), DYN B, big DYN (DYN A + DYN B), α-neoendorphin, and β-neoendorphin are derived from the processing of pDYN (Figure 1) by various non-selective proteases, including cathepsin L, prohormone convertases 1, 2 and 3 and carboxypeptidase E [see (Chavkin 2013; Fricker et al. 2020; Schwarzer 2009) for reviews]. Dynorphins and their endogenous target, the KOR, constitute the DYNs/KOR system (Chavkin et al. 1982). In addition, several peptides derived from proenkephalin, such as metorphamide and BAM-18, have high affinity for the KOR (Hurlbut et al. 1986). Although most peptide products elicit opioid-like effects, whether the different peptides have different physiological functions remains unclear. In addition, there is differential processing of pDYN throughout the brain as suggested by early work showing that the ratios of DYNs and other pDYN derivative levels varied across different regions of the mesocorticolimbic and nigrostriatal dopamine (DA) systems (Zamir et al. 1984). Further, pDYN and DYNs are colocalized mostly on presynaptic sites of hippocampal and striatal neurons, but more evenly distributed throughout the soma of amygdala and cortex neurons. Of the different DYN peptides, DYN A(1-17) is considered the primary KOR ligand having higher potency at the KOR than other DYN peptides [see (Chavkin 2013; Fricker et al. 2020; Schwarzer 2009) for reviews]. Notably, DYNs have affinity for other opioid and non-opioid receptors in addition to the KOR. For example, DYNs bind to the MOR and DOR in brain tissue, with equal or lower affinities than to the KOR [see (Chavkin 2013; Fricker et al. 2020; Schwarzer 2009) for reviews]. Des-Tyr1 DYNs have direct effects on N-methyl-D-aspartate (NMDA) receptors in the spinal cord and brain (Caudle and Dubner 1998; Shukla and Lemaire 1994). Dynorphins also show activity at bradykinin receptors, which have been implicated in pain (Lai et al. 2006). Thus, DYN peptides, typically considered a monolithic entity in the field, may be more diverse in their processing and actions than has been appreciated.
Figure 1. Summary of post-translational PDYN products, changes in chronic pain and effects on nociception, and expression patterns.
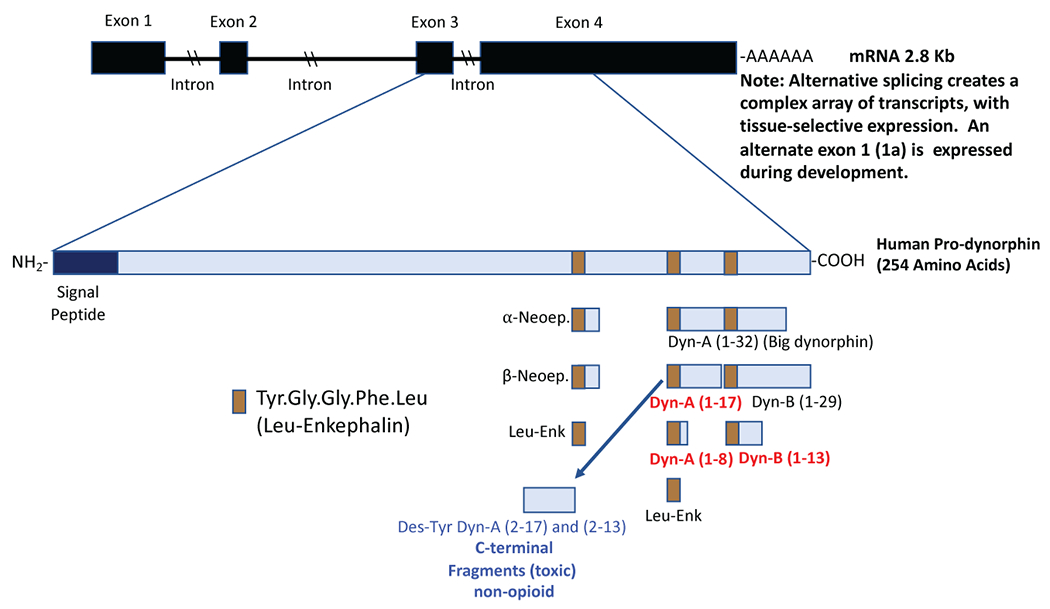
A. Dynorphin peptides are up-regulated in chronic neuropathic and inflammatory pain models (marked in red). Spinal (intrathecal) administration of DYN peptides that produce acute antinociception include Dyn A (1-8), Dyn A (1-17), Dyn B (1-13). Spinal administration of DYN peptides, Dyn A (1-17), Dyn B (1-13), Dyn A (2-17), Dyn A (2-13), produces pain-like (allodynia and hyperalgesia) responses via activation of NMDA or bradykinin receptors. [Reviewed in (Podvin et al. 2016)]. Dyn A (2-17 or 2-13) have no activity at KORs.
In contrast to classical neurotransmitter systems whereby an action potential results in pre-synaptic transmitter release from the synaptic active zone, neuropeptide signaling parameters may vary widely in terms of spatio-temporal release, target activation, and receptor fidelity (Gomes et al. 2020). Following sustained neuronal activity, DYNs are released from large dense core vesicles (Cho and Basbaum 1989; Drake et al. 1994) and decreases cellular activation following binding to KOR. Dynorphins can be released from pre-synaptic neurons onto post-synaptic neurons or act in an auto-inhibitory manner by activating presynaptic KORs in cells that release DYNs, or from be released from dendrites to produce retrograde inhibition of KOR-sensitive presynaptic inputs [see (Chavkin 2013; Schwarzer 2009) for reviews].
Dynorphin peptides are widely expressed throughout the brain and spinal cord, with only the cerebellum and dorsal thalamus having no DYNs (Fallon and Leslie 1986; Mansour et al. 1994; Mansour et al. 1988) (Figure 2). For example, expression of pDYN is high in the substantia nigra pars reticulata, various hypothalamic nuclei, nucleus of solitary tract, hippocampus, globus pallidus, spinal trigeminal nucleus and substantia gelatinosa of spinal cord. Medium to low pDYN levels are found in the amygdala, nucleus accumbens, olfactory tubercle, caudate putamen, bed nucleus stria terminalis, preoptic area, periaqueductal grey, parabrachial nucleus, raphe nucleus, cortex, periventricular nucleus of thalamus, substantia nigra pars compacta, ventral tegmental area and superior and inferior colliculus. Although it is important to consider that DYN peptides are released from the terminals of these neurons and rarely from the soma of the neurons. Thus, low pDYN levels do not mean that DYN projection neurons may not release peptide in these locations.
Figure 2.
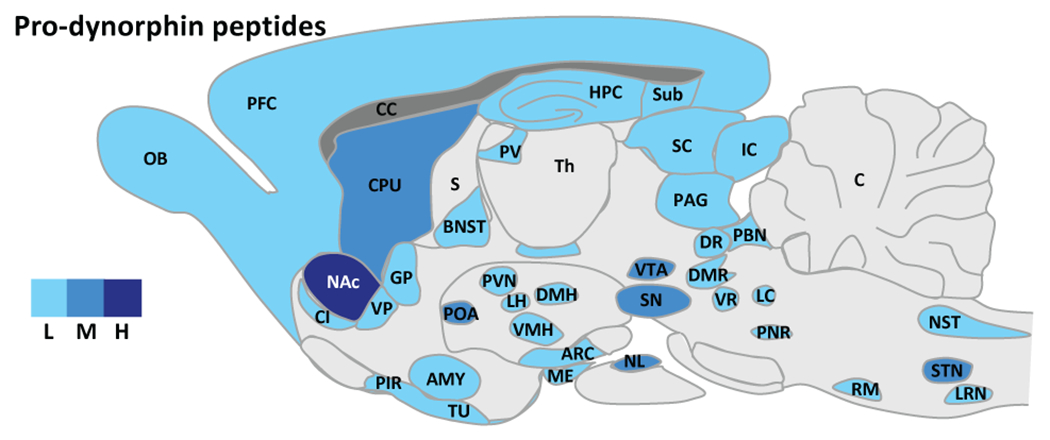
Dynorphin peptide expression in the rodent brain. L: low, M: moderate, H: high expression. Abbreviations. ARC: arcuate nucleus, hypothalamus, AMY: amygdala, BLA: basolateral nucleus, amygdala, BNST: bed nucleus of the stria terminalis, C: cerebellum, CC: corpus collosum, CeA: central nucleus, amygdala, CI: claustrum, CL: centrolateral thalamus, CM: centromedial thalamus, COA: cortical nucleus, amygdala, CPU: caudate putamen, DMH: dorsomedial hypothalamus, DMR: dorsal and medial raphe, DR: dorsal raphe, DTN: dorsal tegmental nucleus, EN: endopiriform cortex, GP: globus pallidus, HPC: hippocampus, IC: inferior colliculus, IP: interpeduncular nucleus, LC: locus coeruleus, LD: laterodorsal thalamus, LH: lateral hypothalamus, LRN: lateral reticular nucleus, ME: median eminence, MeA: median nucleus, amygdala, NAc: nucleus accumbens, NST: nucleus tractus solitarius, NL: neuronal lobe, pituitary, NRGC: nucleus reticularis gigantocellularis, OB: olfactory bulb, OCX: occipital cortex, PAG: periaqueductal gray, PBN: parabrachial nucleus, PCX: parietal cortex, PFC: prefrontal cortex, PIR: piriform cortex, PNR: pontine reticular, POA: preoptic area, PV: paraventricular thalamus, PVN: paraventricular hypothalamus, RE: reuniens thalamus, RM: raphe magnus, S: septum, SC: superior colliculus, SN: substantia nigra, STN: spinal trigeminal nucleus , Sub: subiculum, TCX: temporal cortex, Th: thalamus, TU: olfactory tubercule, VMH: ventromedial hypothalamus, VP: ventral pallidum, VR: ventral raphe, VTA: ventral tegmental area, Zi: zona incerta. [Adapted from Le Merrer et al. (2009)].
Insight into the role of pDyn-derived peptides in behaviors has been gained utilizing mutant mice lacking pDyn-derived peptides (Schunk et al. 2008; Sharifi et al. 2001). Mice with genetic deletion of pDyn have been demonstrated to (1) show impaired extinction learning of contextual fear (Bilkei-Gorzo et al. 2012); (2) have diminished impairment in cognition associated with aging and stress (Carey et al. 2009; Ménard et al. 2013; Nguyen et al. 2005); (3) exhibit increased alcohol and drug self-administration and decreased stress-induced reinstatement of drug preference (Femenía and Manzanares 2012; Galeote et al. 2009; Redila and Chavkin 2008); (4) display anxiolytic phenotypes relative to controls (Ménard et al. 2013; Wittmann et al. 2009) or show increased anxiety-like behavior relative to controls (Femenía et al. 2011); (5) have decreased pain in models of chronic pain [see (Tseng and Hoon 2020) for a review]. Therefore, endogenous dynorphins likely play a pivotal role in regulating cognition, fear-related behaviors, reward, anxiety, and nociception.
Cloning of the KOR
The KOR has been cloned from several species including humans (Mansson et al. 1994; Simonin et al. 1995; Zhu et al. 1995). The KOR genes were mapped at position q11-12 in human chromosome 8 (Simonin et al. 1995) and mouse chromosome 1A2-3 (Nishi et al. 1994). Like all other G protein-coupled receptors (GPCRs), the KORs have seven transmembrane domains with a N terminal outside the cell and an intracellular C terminal domain. The sequences of mouse, rat, guinea pig and human KORs have high homology. The amino acid sequence of the human KOR is 94% identical to those of the rat and mouse and 91% identical to that of the guinea pig (Simonin et al. 1995). The N- and C-terminal domains have more sequence variations than the transmembrane domains among the KORs of different species. The amino acid sequences of the human KOR, MOR, DOR and NOP receptor share ~ 60% overall identity and ~80% identity among the transmembrane domains (Zhu et al. 1995). The N- and C-terminal domains have the highest sequence divergence among the four opioid receptors, hence antibodies are typically generated against the N- and C-terminal domain peptides.
Neuroanatomy of the KOR
The distribution of the KOR has been investigated with several different methods, including biochemical techniques, i.e. receptor autoradiography and immunohistochemistry (IHC), and recently with mutant mouse lines expressing KOR-Cre or KOR-tdTomato. The KOR IHC, discussed below, has been plagued by lack of highly specific antibodies. The KOR mRNA distribution has been examined using in situ hybridization.
Receptor autoradiography was performed on sections of fresh frozen tissues with radioactive labeled KOR-selective ligand such as [3H]U69,593 (Mansour et al. 1994; Unterwald et al. 1991; Wang et al. 2011) or [3H]CI-977 (Slowe et al. 1999), and the sections were exposed to 3H-senstive films or screens. The most important advantage of receptor autoradiography is its high specificity because of the use of highly selective ligands and anatomical definition. However, it takes a relatively long exposure time to obtain the results due to the low energy of β-particles emitted from 3H-labeled ligands. In addition, autoradiography has low resolutions, making it difficult to visualize small brain regions.
Immunohistochemistry of the KOR has been carried out with antibodies against synthetic peptide fragments of partial sequences of N- and C-terminal domains the KOR [for example, (Appleyard et al. 1997; Arvidsson et al. 1995; Drake et al. 1996; Mansour et al. 1996)]. Because these studies yielded significant different results among themselves and also from those of receptor autoradiography, these studies are not described here. Issues related to KOR antibodies are discussed elsewhere in this book.
Recently, reporter protein approaches were employed to map the distribution of the KOR in the mouse brain. The Ross lab generated a KOR-Cre mouse line, in which the exon 2 coding region of the oprk1 gene was replaced with that of Cre recombinase (Cai et al. 2016). They then bred KOR-Cre mice with mice expressing the Cre-dependent allele Rosalsl tdTomato (also known as Ai14), which resulted in tdTomato (tdT) being expressed in KOR-containing cells. The KOR distribution in the KOR-Cre x Ai14 mouse brain was similar to that of receptor autoradiography in most regions, however, there were significant differences, such as the presence of the KOR in the cerebellum, high expression in the striatum and low expression in the claustrum. These differences are due to constitutive and cumulative expression of Cre from all the developmental stages. This can be circumvented by viral injection of Cre-dependent reporter gene into brain regions of interests.
Liu-Chen’s lab produced a knockin mouse line that expresses a fusion protein of KOR conjugated in frame with tdTomato 5’ to the stop codon (KOR-tdT) (Chen et al. 2020). The KOR-tdT has similar distribution as KOR in receptor autoradiography of adult mouse brains. As the expression of KOR-tdT is under the control of KOR promotor like the wildtype KOR, KOR-tdT mice do not have developmental issues as KOR-Cre mice. One unique feature is that these mice display prominent KOR-tdT-containing neuronal fibers not seen with receptor autoradiography, for example, projections from the striatum to substantia innominata and substantia nigra reticulata. The KOR-Cre x Ai14 and KOR-tdT mouse lines provide resolutions at the cellular level and are valuable tools for KOR neuroanatomy studies. Liu-Chen’s lab, employing the CLARITY technique (Chung and Deisseroth 2013) to clear mouse brains followed by 3-D imaging, produced the first 3-D images of the KOR in the brain, which is also the first for any GPCRs (Chen et al. 2020)(Video 1).
Results from receptor autoradiography and reporter protein approaches show that the KOR has a widespread distribution in the brain. The claustrum, a brain region implicated in consciousness, has the highest level, followed by the endopiriform nucleus (separate regions in rodents that are a single continuous structure in primates); however, the functions of KOR in these areas are not clear. The KOR is present in the areas involved in mood, reward, motivation and addiction, including the ventral tegmental area, nucleus accumbens, prefrontal cortex, anterior cingulate cortex, amygdala nuclei, bed nucleus of stria terminalis and raphe nucleus, with the nucleus accumbens shell exhibiting particularly intense signal. Moderate levels of KOR are observed in pain pathways, including the periaqueductal gray, parabrachial nucleus, some nuclei in thalamus, primary and secondary somatosensory cortices. The KOR is found in several nuclei in the hypothalamus, indicating roles of the KOR in neuroendocrine regulation. The KOR is also found in paraventricular nucleus and nucleus reunion of thalamus, but its functions in these brain regions is currently unknown (Figure 3, for KOR distribution, signaling and ligands see https://www.guidetoimmunopharmacology.org/GRAC/ObjectDisplayForward?objectId=318). Discrepancies in KOR binding versus KOR mRNA expression may in part be due to trafficking of KOR to terminals of long-range neuronal projections. The KOR mRNA is localized in cell bodies, whereas KOR binding in any given region is comprised of KOR in local cells and KORs localized on afferent inputs to that region.
Figure 3.
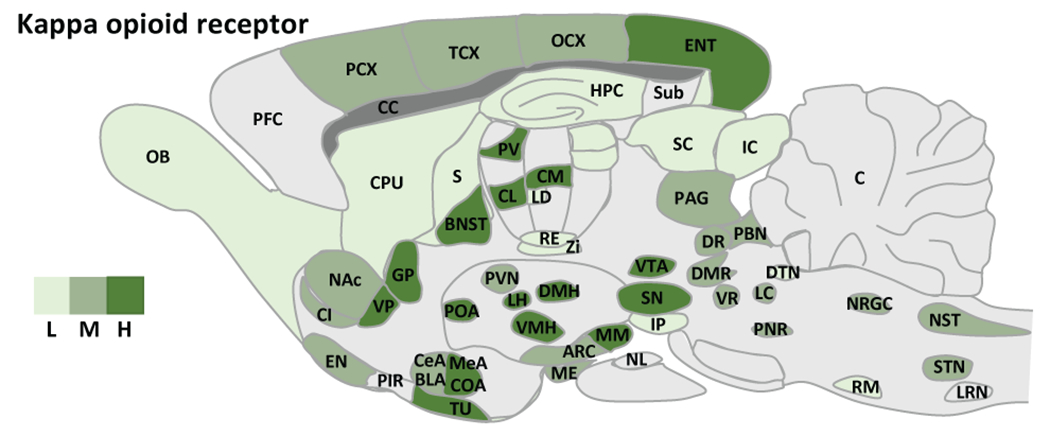
Expression of the KOR in rat brain. L: low, M: moderate, H: high expression. Abbreviations. ARC: arcuate nucleus, hypothalamus, AMY: amygdala, BLA: basolateral nucleus, amygdala, BNST: bed nucleus of the stria terminalis, C: cerebellum, CC: corpus collosum, CeA: central nucleus, amygdala, CI: claustrum, CL: centrolateral thalamus, CM: centromedial thalamus, COA: cortical nucleus, amygdala, CPU: caudate putamen, DMH: dorsomedial hypothalamus, DMR: dorsal and medial raphe, DR: dorsal raphe, DTN: dorsal tegmental nucleus, EN: endopiriform cortex, GP: globus pallidus, HPC: hippocampus, IC: inferior colliculus, IP: interpeduncular nucleus, LC: locus coeruleus, LD: laterodorsal thalamus, LH: lateral hypothalamus, LRN: lateral reticular nucleus, ME: median eminence, MeA: median nucleus, amygdala, NAc: nucleus accumbens, NST: nucleus tractus solitarius, NL: neuronal lobe, pituitary, NRGC: nucleus reticularis gigantocellularis, OB: olfactory bulb, OCX: occipital cortex, PAG: periaqueductal gray, PBN: parabrachial nucleus, PCX: parietal cortex, PFC: prefrontal cortex, PIR: piriform cortex, PNR: pontine reticular, POA: preoptic area, PV: paraventricular thalamus, PVN: paraventricular hypothalamus, RE: reuniens thalamus, RM: raphe magnus, S: septum, SC: superior colliculus, SN: substantia nigra, STN: spinal trigeminal nucleus , Sub: subiculum, TCX: temporal cortex, Th: thalamus, TU: olfactory tubercule, VMH: ventromedial hypothalamus, VP: ventral pallidum, VR: ventral raphe, VTA: ventral tegmental area, Zi: zona incerta. [Adapted from Le Merrer et al. (2009)].
The KOR is also found in the dorsal horn of the spinal cord, and is involved in pain and itch regulation. Additionally, the KOR is distributed throughout the body, including in the lungs, heart, spleen, kidneys, liver and small intestines (Peckys and Landwehrmeyer 1999; Peng et al. 2012), though its precise function in each of these regions is yet to be fully described.
There are species differences in the KOR distribution in the brain, with the receptor distribution in the human brain being more similar to that in the guinea pig than in rat or mouse. For example, in the guinea pig and human, but not the rat or mouse, the KOR is abundant in the cerebellum and deep layers (layers V and VI) of the cortex, and is found in striosomes of the striatum (having patchy distribution) (Mansour et al. 1988; Quirion and Pilapil 1991; Quirion et al. 1987). Transcript for the KOR also shows some divergence between humans and rodents where unlike rodents, there was low level of transcript in the human substantia nigra and hippocampus (Simonin et al. 1995).
There are also differences in the KOR level in the brain among species. Kitchen et al. (1990) reported that Bmax value of [3H]U69,593 binding to the KOR in adult rat brain was 7.3 fmol/mg protein. The KOR levels in the human frontal cortex and forebrains of animals are in the order of pigeon (8x) > guinea pig (~3x) ~ human (~2.5x) > mouse (~1.5x) > rat brain (1x) (Mansour et al. 1988; Quirion and Pilapil 1991; Quirion et al. 1987). Moreover, the KOR level in the adult rat brain is about 1/20 of that of the MOR, which is second to the NOP receptor that has the highest expression in the brain of all opioid receptors. However, it was reported that the KOR mRNA was the most abundantly expressed over MOR and DOR in the human during development (Wang et al. 2006).
KOR signaling at the cellular level
In the brain, DYNs are released upon membrane depolarization and activates the KOR to modulate presynaptic and postsynaptic neural activity (Chavkin et al. 1983; Wagner et al. 1991). Opioid receptors, including the KOR, belong to the rhodopsin sub-family of GPCRs. At the cellular level, activation of the KOR stimulates Gi/o proteins and promotes receptor phosphorylation. Phosphorylated KOR, in turn, recruits β-arrestins, which lead to β-arrestin-mediated signaling (Bruchas et al. 2006; Law 2011; McLennan et al. 2008). It has also been reported that the KOR signals via Gz and G16 proteins (Lee et al. 1998; Tso and Wong 2000).
Activation of KOR results in dissociation of guanosine 5’-diphosphate (GDP) from Gαi and association of guanosine 5’-triphosphate (GTP) with Gαi/o as well as dissociation of the Gβγ from Gα subunits. Activated Gαi/o proteins inhibit adenylate cyclase resulting in decreases in cyclic AMP production and a host of effects in downstream targets, and inhibit Ca2+-channel activity and attenuate presynaptic neurotransmitter release [see (Law 2011) for a review]. Gβγ proteins activate G protein-gated inwardly rectifying potassium channels (GIRKs) and other potassium channels, which causes a reduction in neuronal or axonal excitability [see (Law 2011) for a review]. Gβγ proteins also enhance ERK1/2 phosphorylation (early phase) via L-type Ca2+ channels, phospholipase C (PLC), intracellular Ca2+ release, protein kinase C (PKC) (Bohn et al. 2000; Law 2011). β-Arrestins-mediated signaling includes activation of ERK1/2 (late phase) and p38 MAPK (Al-Hasani and Bruchas 2011; Bruchas and Chavkin 2010). The KOR also activates c-Jun N-terminal kinase (JNK) and protein kinase B (Akt) pathways. Hence, activation of the KOR results in signal transduction via a variety of intracellular pathways with diverse effects on cells (Bruchas and Chavkin 2010; Pradhan et al. 2012). Biased agonism for the KOR has been demonstrated in vitro, in which agonists preferentially activate G protein- or β-arrestin-mediated pathways [for a review, (Bohn and Aubé 2017)]. Several KOR biased agonists have been reported, which may provide beneficial pharmacological effects with reduced unwanted side effects [see (Bohn and Aubé 2017; Faouzi et al. 2020; Mores et al. 2019) for reviews]. The predominance of one signaling cascade over another may vary by brain region, demonstrating the potential utility of biased KOR ligands that may target one downstream cascade over another (Crowley and Kash 2015). Activation of JNK signaling was reported to inactivate the KOR, which is one of the mechanisms for prolonged durations of action (up to weeks) of KOR antagonists, such as norBNI and JDTic (Bruchas et al. 2007).
Agonist-promoted KOR phosphorylation and regulation
Binding of an agonist, such as U50,488H to the KOR, caused KOR phosphorylation at S356, T357, T362 and S369 in the C-terminal domain (Chen et al. 2016) in cultured cells. The KOR phosphorylation at all the residues was mediated by Gi/o alpha proteins and G protein-coupled receptor kinases (GRK2, GRK3, GRK5, GRK6), and PKC (Chiu et al. 2017). GRKs-mediated, but not PKC-mediated, KOR phosphorylation followed by β-arrestin recruitment desensitized U50,488H-induced ERK1/2 response and [35S]GTPgS binding and was involved in agonist-induced KOR internalization (Liu-Chen 2004). PKC activation by phorbol ester induced agonist-independent KOR phosphorylation. Compared with U50,488H, PKC activation induced much higher S356/T357 phosphorylation, much lower T363 phosphorylation, and similar levels of S369 phosphorylation. PKC activation caused a lower level of agonist-independent KOPR internalization, compared with U50,488H.
U50,488H promoted KOR internalization in a process that depends on GRKs, β-arrestin, and dynamin proteins (Li et al. 1999). KOR internalization precedes downregulation which, in addition, involves Rab5- and Rab7-dependent process and requires ubiquitination of the KOR (Li et al. 2000). The KOR appears to be internalized into early endosomes then trafficked via late endosomes to lysosomes and proteosomes for degradation (Liu-Chen 2004).
X-ray Crystal Structures of the KOR
The understanding of the DYNs / KOR system function and signaling continues to be enhanced by the publication of the high-resolution crystal structures of the receptor. Wu et al. (2012) reported an X-ray crystal structure of an inactive state of the human KOR in complex with the selective KOR antagonist JDTic. Che et al. (2018) provided a crystal structure of an active state of the human KOR in complex with the epoxymorphinan opioid agonist MP1104 and an active-state-stabilizing nanobody. The active structure provides significant information for the structural basis of biased agonism (Che et al. 2018) and allosteric modulation (Che et al. 2020). Comparisons between inactive- and active-state KOR structures reveal substantial conformational changes in the binding pocket and intracellular and extracellular regions. The characterization of the crystal structures of inactive and active states of the KOR has provided insight for ligand-receptor interactions allowing new concepts for novel drug design. This structural characterization together with identification of the signaling events that elicit antinociceptive versus dysphoric and psychotomimetic effects, has provided extensive advancement in novel chemical entities that hold promise as new pain treatments with minimal aversive effects including depressive or addictive properties.
In vivo pharmacology of the DYNs/KOR system
Activation of the KOR in vivo produces many physiological effects and behavioural responses, including analgesia, antipruritic effects, water diuresis, dysphoria / aversion, sedation, motor incoordination and hypothermia (Mucha and Herz 1985; Pfeiffer et al. 1986; Simonin et al. 1998; Togashi et al. 2002; von Voigtlander et al. 1983). KOR agonists are effective analgesics without causing respiratory depression and abuse liabilities associated with MOR-selective or preferring analgesics (Paton et al. 2020). In light of the recent opioid epidemic, KOR agonists may be re-examined as analgesics, either alone or as part of pharmacological regimen. Generation of KOR-deficient animals has provided significant knowledge on the in vivo physiological role of the DYNs / KOR system (Ansonoff et al. 2006; Simonin et al. 1998). Dynorphins are released in the central nervous system during pain states and activate KORs to produce analgesia, with KOR-knockout mice exhibiting enhanced pain sensitivity (Simonin et al. 1998).
The KOR is also involved in the pathophysiology of pruritus, with KOR agonists as promising antipruritic agents [for a review see Cowan et al. (2015)]. However, to date, nalfurafine is the only KOR agonist approved for clinical use (marketed in Japan). One of the limitations that has hampered the development of KOR agonists as safer analgesics and effective anti-pruritic agents is the negative effects that emerge following KOR agonist administration. KOR agonists produce dysphoria, depressive-like symptoms and psychotomimetic effects in humans (Pfeiffer et al. 1986; Wadenberg 2003; Walsh et al. 2001), and elicit place aversion and depressive-like behaviors (Mucha and Herz 1985; Shippenberg and Herz 1986) as well as stimulate drug-seeking (Bruchas et al. 2010; Chavkin and Koob 2016; Knoll and Carlezon 2010) in rodents. Activation of the DYNs/KOR system also elicits signs of anxiety and fear in animals and humans (Chartoff and Mavrikaki 2015; Chavkin and Koob 2016; Darcq and Kieffer 2018).
Chavkin and colleagues have proposed that antinociception produced by KOR agonists is mediated by G protein pathways, whereas aversion is mediated by β-arrestin2-dependent p38 MAP kinase phosphorylation [reviewed in (Bruchas and Chavkin 2010)]. However, White et al. (2015) showed that U69,593, salvinorin A and RB-64 (a salvinorin A analog) produced similar levels of aversion in the conditioned place preference (CPA) test in wildtype and β-arrestin2−/− mice, indicating that either β-arrestin2 is not involved in CPA or other pathways besides β-arrestin2 are involved. In addition, βarrestin2 deletion impaired KOR-mediated motor incoordination, but did not affect antinociception or hypolocomotion (White et al. 2015). Morgenweck et al. (2015) demonstrated that β-arrestin2 deletion did not affect the anti-scratch effects of U50,488H or the G protein-biased KOR agonist isoquinone 2.1, indicating that the anti-scratch effect of KOR agonists is not mediated by β-arrestin2.
Several groups have been actively searching for G protein-biased KOR agonists [see (Bohn and Aubé 2017; Faouzi et al. 2020; Mores et al. 2019) for reviews] to avoid the dysphoric and psychotomimetic effects. Whether G protein-biased KOR agonists will fulfill the promise of retaining beneficial analgesic and antipruritic effects with reduced side effects in humans remains to be determined. Another approach is to develop peripherally acting KOR agonists to avoid the central nervous system-mediated side effects. An example is CR845/difelikefalin, which is under clinical trials for treatment of pruritus associated with chronic kidney or liver disease or atopic dermatitis and treatment of pain in post-operative settings (Fishbane et al. 2020; Therapeutics 2020) (https://www.caratherapeutics.com/pipeline-technology/our-pipeline/).
Kappa opioid receptor antagonists have been demonstrated to have antidepressant- and anxiolytic-like activities in rodents. In addition, KOR antagonists were found to reduce stress-induced instatement of drug seeking for several drugs of abuse in animals. Thus, KOR antagonists may be useful for treatment of depression, anxiety and drug addiction [see (Al-Hasani and Bruchas 2011; Bruchas et al. 2010; Carroll and Carlezon 2013; Van’t Veer and Carlezon 2013) for reviews]. Buprenorphine, having MOR partial agonist and KOR antagonist activities, in combination with the MOR antagonist samidorphan was investigated in clinical trials for treatment of depression, but the combination did not meet the primary endpoint goal (Zajecka et al. 2019). JDTic underwent clinical trials for cocaine use disorder, but failed in phase I due to cardiac side effects (Buda et al. 2015). Aticaprant (formerly JNJ-67953964, CERC-501 and LY2456302) is currently in clinical trials for management of anhedonia (an important symptom of major depressive disorder) (Krystal et al. 2020; Pizzagalli et al. 2020).
Conclusion
The KOR is found throughout the peripheral and central nervous systems and participate in a range of physiological functions. The potential therapeutic use of KOR ligands as analgesics, anti-pruritic, mood and substance use disorders are of continued interest and being pursued by various pharmaceutical companies. The following chapters will provide more thorough insights of KOR functions in various physiological and pathological states, together with recent developments in chemistry and pharmacology of novel KOR ligands and their therapeutic potentials.
Supplementary Material
Video 1 A video clip of 3-D images of KtdT/KtdT mouse brains showing KtdT distribution. Adult KtdT/KtdT mice were perfused and brains were cleared via CLARITY. Brains were imaged without IHC from dorsal and ventral sides and images were digitally re-constructed into 3-D images. Experiments were performed on three brains with similar results. [Reproduced from Chen et al. (2020)].
Acknowledgements:
The writing of this manuscript was supported by the NIH Grants R01DA041781, 1UG3TR003148-01 and 2P50 DA005010, and the Department of Defense Grant W81XWH-15-1-0435 (CMC), the National Institute of Mental Health Intramural Research Program and a Brain and Behavior Research Foundation NARSAD Young Investigator Award (HAT), the Austrian Science Fund (FWF: I4697) (MS), the NIH grants R01DA041359, R21DA045274 and P30DA013429 (LYLC).
Footnotes
Conflict of interests: The authors declare no conflict of interests.
References
- Al-Hasani R, Bruchas MR (2011) Molecular mechanisms of opioid receptor-dependent signaling and behavior Anesthesiology 115:1363–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansonoff MA, Zhang J, Czyzyk T, Rothman RB, Stewart J, Xu H, Zjwiony J, Siebert DJ, Yang F, Roth BL, Pintar JE (2006) Antinociceptive and hypothermic effects of Salvinorin A are abolished in a novel strain of kappa-opioid receptor-1 knockout mice J Pharmacol Exp Ther 318:641–648 [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Patterson TA, Jin WZ, Chavkin C (1997) Agonist-induced phosphorylation of the kappa-opioid receptor J Neurochem 69:2405–2412 [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee JH, Nakano AH, Lin X, Loh HH, Law P-Y, Wessendorf MW, Elde R (1995) The kappa-opioid receptor is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids Proc Natl Acad Sci U S A 92:5062–5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett AH, Casy AF (1954) Stereochemistry of certain analgesics Nature 173:1231–1232 doi: 10.1038/1731231a0 [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Erk S, Schürmann B, Mauer D, Michel K, Boecker H, Scheef L, Walter H, Zimmer A (2012) Dynorphins regulate fear memory: from mice to men J Neurosci 32:9335–9343 doi: 10.1523/jneurosci.1034-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Aubé J (2017) Seeking (and Finding) Biased Ligands of the Kappa Opioid Receptor ACS Med Chem Lett 8:694–700 doi: 10.1021/acsmedchemlett.7b00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Belcheva MM, Coscia CJ (2000) Mitogenic signaling via endogenous kappa-opioid receptors in C6 glioma cells: Evidence for the involvement of protein kinase C and the mitogen-activated protein kinase signaling cascade J Neurochem 74:564–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C (2010) Kinase cascades and ligand-directed signaling at the kappa opioid receptor Psychopharmacology (Berl) 210:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C (2010) The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors Brain Res 1314:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Macey TA, Lowe JD, Chavkin C (2006) Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem 281:18081–18089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C (2007) Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase J Biol Chem 282:29803–29811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buda JJ, Carroll FI, Kosten TR, Swearingen D, Walters BB (2015) A Double-Blind, Placebo-Controlled Trial to Evaluate the Safety, Tolerability, and Pharmacokinetics of Single, Escalating Oral Doses of JDTic Neuropsychopharmacology 40:2059–2065 doi: 10.1038/npp.2015.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Huang H, Kuzirian MS, Snyder LM, Matsushita M, Lee MC, Ferguson C, Homanics GE, Barth AL, Ross SE (2016) Generation of a KOR-Cre knockin mouse strain to study cells involved in kappa opioid signaling Genesis 54:29–37 doi: 10.1002/dvg.22910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Lyons AM, Shay CF, Dunton O, McLaughlin JP (2009) Endogenous kappa opioid activation mediates stress-induced deficits in learning and memory J Neurosci 29:4293–4300 doi: 10.1523/JNEUROSCI.6146-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Carlezon WA Jr. (2013) Development of kappa opioid receptor antagonists J Med Chem 56:2178–2195 doi: 10.1021/jm301783x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle RM, Dubner R (1998) Ifenprodil blocks the excitatory effects of the opioid peptide dynorphin 1–17 on NMDA receptor-mediated currents in the CA3 region of the guinea pig hippocampus Neuropeptides 32:87–95 doi: 10.1016/s0143-4179(98)90022-1 [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Mavrikaki M (2015) Sex Differences in Kappa Opioid Receptor Function and Their Potential Impact on Addiction Front Neurosci 9:466 doi: 10.3389/fnins.2015.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C (2013) Dynorphin--still an extraordinarily potent opioid peptide Mol Pharmacol 83:729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Bakhit C, Weber E, Bloom FE (1983) Relative contents and concomitant release of prodynorphin/neoendorphin-derived peptides in rat hippocampus Proc Natl Acad Sci U S A 80:7669–7673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A (1982) Dynorphin is a specific endogenous ligand of the kappa opioid receptor Science 215:413–415 [DOI] [PubMed] [Google Scholar]
- Chavkin C, Koob GF (2016) Dynorphin, Dysphoria, and Dependence: the Stress of Addiction Neuropsychopharmacology 41:373–374 doi: 10.1038/npp.2015.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che T, English J, Krumm BE, Kim K, Pardon E, Olsen RHJ, Wang S, Zhang S, Diberto JF, Sciaky N, Carroll FI, Steyaert J, Wacker D, Roth BL (2020) Nanobody-enabled monitoring of kappa opioid receptor states Nat Commun 11:1145 doi: 10.1038/s41467-020-14889-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che T, Majumdar S, Zaidi SA, Ondachi P, McCorvy JD, Wang S, Mosier PD, Uprety R, Vardy E, Krumm BE, Han GW, Lee MY, Pardon E, Steyaert J, Huang XP, Strachan RT, Tribo AR, Pasternak GW, Carroll FI, Stevens RC, Cherezov V, Katritch V, Wacker D, Roth BL (2018) Structure of the Nanobody-Stabilized Active State of the Kappa Opioid Receptor Cell 172:55–67 e15 doi: 10.1016/j.cell.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chiu YT, Wu W, Huang P, Mann A, Schulz S, Liu-Chen LY (2016) Determination of sites of U50,488H-promoted phosphorylation of the mouse kappa opioid receptor (KOPR): Disconnect between KOPR phosphorylation and internalization Biochem J 473 497–508 doi: 10.1042/BJ20141471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Willhouse AH, Huang P, Ko N, Wang Y, Xu B, Huang LHM, Kieffer B, Barbe MF, Liu-Chen LY (2020) Characterization of a Knock-In Mouse Line Expressing a Fusion Protein of kappa Opioid Receptor Conjugated with tdTomato: 3-Dimensional Brain Imaging via CLARITY eNeuro 7 doi: 10.1523/ENEURO.0028-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YT, Chen C, Yu D, Schulz S, Liu-Chen LY (2017) Agonist-dependent and -independent kappa opioid receptor phosphorylation: Distinct phosphorylation patterns and different cellular outcomes Mol Pharmacol 92:588–600 doi: 10.1124/mol.117.108555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Basbaum AI (1989) Ultrastructural analysis of dynorphin B-immunoreactive cells and terminals in the superficial dorsal horn of the deafferented spinal cord of the rat Journal of Comparative Neurology 281:193–205 [DOI] [PubMed] [Google Scholar]
- Chung K, Deisseroth K (2013) CLARITY for mapping the nervous system Nat Methods 10:508–513 doi: 10.1038/nmeth.2481 [DOI] [PubMed] [Google Scholar]
- Cowan A, Kehner GB, Inan S (2015) Targeting itch with ligands selective for kappa opioid receptors Handb Exp Pharmacol 226:291–314 doi: 10.1007/978-3-662-44605-8_16 [DOI] [PubMed] [Google Scholar]
- Crowley NA, Kash TL (2015) Kappa opioid receptor signaling in the brain: Circuitry and implications for treatment Prog Neuropsychopharmacol Biol Psychiatry 62:51–60 doi: 10.1016/j.pnpbp.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Kieffer BL (2018) Opioid receptors: drivers to addiction? Nat Rev Neurosci 19:499–514 doi: 10.1038/s41583-018-0028-x [DOI] [PubMed] [Google Scholar]
- Drake CT, Patterson TA, Simmons ML, Chavkin C, Milner TA (1996) Kappa opioid receptor-like immunoreactivity in guinea pig brain: ultrastructural localization in presynaptic terminals in hippocampal formation J Comp Neurol 370:377–395 [DOI] [PubMed] [Google Scholar]
- Drake CT, Terman GW, Simmons ML, Milner TA, Kunkel DD, Schwartzkroin PA, Chavkin C (1994) Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters J Neurosci 14:3736–3750 doi: 10.1523/jneurosci.14-06-03736.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, Keith DE, Morrison H, Magendzo K, Edwards RH (1992) Cloning of a delta opioid receptor by functional expression Science 258:1952–1955 [DOI] [PubMed] [Google Scholar]
- Fallon JH, Leslie FM (1986) Distribution of dynorphin and enkephalin peptides in the rat brain J Comp Neurol 249:293–336 doi: 10.1002/cne.902490302 [DOI] [PubMed] [Google Scholar]
- Faouzi A, Varga BR, Majumdar S (2020) Biased Opioid Ligands Molecules 25 doi: 10.3390/molecules25184257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femenía T, Manzanares J (2012) Increased ethanol intake in prodynorphin knockout mice is associated to changes in opioid receptor function and dopamine transmission Addict Biol 17:322–337 doi: 10.1111/j.1369-1600.2011.00378.x [DOI] [PubMed] [Google Scholar]
- Femenía T, Pérez-Rial S, Urigüen L, Manzanares J (2011) Prodynorphin gene deletion increased anxiety-like behaviours, impaired the anxiolytic effect of bromazepam and altered GABAA receptor subunits gene expression in the amygdala J Psychopharmacol 25:87–96 doi: 10.1177/0269881110367724 [DOI] [PubMed] [Google Scholar]
- Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F, Investigators K-T (2020) A Phase 3 Trial of Difelikefalin in Hemodialysis Patients with Pruritus N Engl J Med 382:222–232 doi: 10.1056/NEJMoa1912770 [DOI] [PubMed] [Google Scholar]
- Fricker LD, Margolis EB, Gomes I, Devi LA (2020) Five Decades of Research on Opioid Peptides: Current Knowledge and Unanswered Questions Mol Pharmacol 98:96–108 doi: 10.1124/mol.120.119388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeote L, Berrendero F, Bura SA, Zimmer A, Maldonado R (2009) Prodynorphin gene disruption increases the sensitivity to nicotine self-administration in mice Int J Neuropsychopharmacol 12:615–625 doi: 10.1017/s1461145708009450 [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL (2002) Opioid receptor genes inactivated in mice: the highlights Neuropeptides 36:62–71 [DOI] [PubMed] [Google Scholar]
- Goldstein A, Lowney LI, Pal BK (1971) Stereospecific and nonspecific interactions of the morphine congener levorphanol in subcellular fractions of mouse brain Proc Natl Acad Sci U S A 68:1742–1747 doi: 10.1073/pnas.68.8.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L (1979) Dynorphin-(1–13), an extraordinarily potent opioid peptide Proc Natl Acad Sci U S A 76:6666–6670 doi: 10.1073/pnas.76.12.6666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Sierra S, Lueptow L, Gupta A, Gouty S, Margolis EB, Cox BM, Devi LA (2020) Biased signaling by endogenous opioid peptides Proc Natl Acad Sci U S A 117:11820–11828 doi: 10.1073/pnas.2000712117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höllt V (1986) Opioid peptide processing and receptor selectivity Annu Rev Pharmacol Toxicol 26:59–77 doi: 10.1146/annurev.pa.26.040186.000423 [DOI] [PubMed] [Google Scholar]
- Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR (1975) Identification of two related pentapeptides from the brain with potent opiate agonist activity Nature 258:577–580 [DOI] [PubMed] [Google Scholar]
- Hurlbut DE, Evans CJ, Barchas JD, Leslie FM (1986) The pharmacological profile of BAM 18 NIDA Res Monogr 75:81–84 [PubMed] [Google Scholar]
- Kieffer B, Befort K, Gaveriaux-Ruff C, Hirth CG (1992) The d-opioid receptor: Isolation of a cDNA by expression cloning and pharmacological characterization [published erratum appears in Proc.Natl.Acad. Sci.U.S.A. 1994 Feb 1;91(3):1193] Proc Natl Acad Sci U S A 89:12048–12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen I, Kelly M, Viveros MP (1990) Ontogenesis of kappa-opioid receptors in rat brain using [3H]U-69593 as a binding ligand Eur J Pharmacol 175:93–96 [DOI] [PubMed] [Google Scholar]
- Knapp RJ, Malatynska E, Collins N, Fang L, Wang JY, Hruby VJ, Roeske WR, Yamamura HI (1995) Molecular biology and pharmacology of cloned opioid receptors [review] FASEB J 9:516–525 [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA, Jr. (2010) Dynorphin, stress, and depression Brain Res 1314:56–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Pizzagalli DA, Smoski M, Mathew SJ, Nurnberger J Jr, Lisanby SH, Iosifescu D, Murrough JW, Yang H, Weiner RD, Calabrese JR, Sanacora G, Hermes G, Keefe RSE, Song A, Goodman W, Szabo ST, Whitton AE, Gao K, Potter WZ (2020) A randomized proof-of-mechanism trial applying the ‘fast-fail’ approach to evaluating kappa-opioid antagonism as a treatment for anhedonia Nat Med 26:760–768 doi: 10.1038/s41591-020-0806-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Luo MC, Chen Q, Ma S, Gardell LR, Ossipov MH, Porreca F (2006) Dynorphin A activates bradykinin receptors to maintain neuropathic pain Nat Neurosci 9:1534–1540 [DOI] [PubMed] [Google Scholar]
- Law P-Y (2011) Opioid receptor signal transduction mechanisms. In: Pasternak GW (ed) The Opiate Receptors. 2 edn. The Humana Press, New York, NY, pp 195–238 [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL (2009) Reward processing by the opioid system in the brain Physiol Rev 89:1379–1412 doi: 10.1152/physrev.00005.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Joshi S, Chan JS, Wong YH (1998) Differential coupling of mu-, delta-, and kappa-opioid receptors to G alpha16-mediated stimulation of phospholipase C J Neurochem 70:2203–2211 [PubMed] [Google Scholar]
- Li J-G, Benovic JL, Liu-Chen L-Y (2000) Mechanisms of agonist-induced down-regulation of the human kappa-opioid receptor: internalization is required for down-regulation Mol Pharmacol 58:795–801 [PubMed] [Google Scholar]
- Li J-G, Luo LY, Krupnick JG, Benovic JL, Liu-Chen L-Y (1999) U50,488H-induced internalization of the human kappa opioid receptor involves a beta-arrestin- and dynamin-dependent mechanism. Kappa receptor internalization is not required for mitogen-activated protein kinase activation J Biol Chem 274:12087–12094 [DOI] [PubMed] [Google Scholar]
- Liu-Chen L-Y (2004) Agonist-induced regulation and trafficking of kappa opioid receptors Life Sci 75:511–536 [DOI] [PubMed] [Google Scholar]
- Loh HH, Tseng LF, Wei E, Li CH (1976) beta-endorphin is a potent analgesic agent Proc Natl Acad Sci U S A 73:2895–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JAH, Waterfield AA, Hughes J, Kosterlitz H (1977) Endogenous opioid peptides: multiple agonists and receptors Nature (London) 267:495–499 [DOI] [PubMed] [Google Scholar]
- Mansour A, Burke S, Pavlic RJ, Akil H, Watson SJ (1996) Immunohistochemical localization of the cloned kappa 1 receptor in the rat CNS and pituitary Neuroscience 71:671–690 [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Meng F, Akil H, Watson SJ (1994) Kappa 1 receptor mRNA distribution in the rat CNS: comparison to kappa receptor binding and prodynorphin mRNA Mol Cell Neurosci 5:124–144 [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ (1988) Anatomy of CNS opioid receptors Trends Neurosci 11:308–314 [DOI] [PubMed] [Google Scholar]
- Mansson E, Bare L, Yang D (1994) Isolation of a human k opioid receptor cDNA from placenta Biochem Biophys Res Comm 202:1431–1437 [DOI] [PubMed] [Google Scholar]
- Marino KA, Shang Y, Filizola M (2018) Insights into the function of opioid receptors from molecular dynamics simulations of available crystal structures Br J Pharmacol 175:2834–2845 doi: 10.1111/bph.13774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gibert PE (1976) The effects of morphine and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog J Pharmacol Exp Ther 197:517–532 [PubMed] [Google Scholar]
- McLennan GP, Kiss A, Miyatake M, Belcheva MM, Chambers KT, Pozek JJ, Mohabbat Y, Moyer RA, Bohn LM, Coscia CJ (2008) Kappa opioids promote the proliferation of astrocytes via Gbetagamma and beta-arrestin 2-dependent MAPK-mediated pathways J Neurochem 107:1753–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard C, Tse YC, Cavanagh C, Chabot JG, Herzog H, Schwarzer C, Wong TP, Quirion R (2013) Knockdown of prodynorphin gene prevents cognitive decline, reduces anxiety, and rescues loss of group 1 metabotropic glutamate receptor function in aging J Neurosci 33:12792–12804 doi: 10.1523/jneurosci.0290-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mores KL, Cummins BR, Cassell RJ, van Rijn RM (2019) A Review of the Therapeutic Potential of Recently Developed G Protein-Biased Kappa Agonists Front Pharmacol 10:407 doi: 10.3389/fphar.2019.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenweck J, Frankowski KJ, Prisinzano TE, Aube J, Bohn LM (2015) Investigation of the role of betaarrestin2 in kappa opioid receptor modulation in a mouse model of pruritus Neuropharmacology 99:600–609 doi:S0028–3908(15)30072–1 [pii]; 10.1016/j.neuropharm.2015.08.027 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Herz A (1985) Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning Psychopharmacology (Berl) 86:274–280 [DOI] [PubMed] [Google Scholar]
- Nguyen XV, Masse J, Kumar A, Vijitruth R, Kulik C, Liu M, Choi DY, Foster TC, Usynin I, Bakalkin G, Bing G (2005) Prodynorphin knockout mice demonstrate diminished age-associated impairment in spatial water maze performance Behav Brain Res 161:254–262 doi: 10.1016/j.bbr.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Nishi M, Takeshima H, Mori M, Nakagawara K, Takeuchi T (1994) Structure and chromosomal mapping of genes for the mouse kappa-opioid receptor and an opioid receptor homologue (MOR-C) Biochem Biophys Res Commun 205:1353–1357 [DOI] [PubMed] [Google Scholar]
- Paton KF, Atigari DV, Kaska S, Prisinzano T, Kivell BM (2020) Strategies for Developing κ Opioid Receptor Agonists for the Treatment of Pain with Fewer Side Effects J Pharmacol Exp Ther 375:332–348 doi: 10.1124/jpet.120.000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB (1999) Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study Neuroscience 88:1093–1135 doi: 10.1016/s0306-4522(98)00251-6 [DOI] [PubMed] [Google Scholar]
- Peng J, Sarkar S, Chang SL (2012) Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR Drug Alcohol Depend 124:223–228 doi: 10.1016/j.drugalcdep.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert CB, Pasternak G, Snyder SH (1973) Opiate agonists and antagonists discriminated by receptor binding in brain Science 182:1359–1361 [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM (1986) Psychotomimesis mediated by kappa opiate receptors Science 233:774–776 doi: 10.1126/science.3016896 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Smoski M, Ang YS, Whitton AE, Sanacora G, Mathew SJ, Nurnberger J Jr, Lisanby SH, Iosifescu DV, Murrough JW, Yang H, Weiner RD, Calabrese JR, Goodman W, Potter WZ, Krystal AD (2020) Selective kappa-opioid antagonism ameliorates anhedonic behavior: evidence from the Fast-fail Trial in Mood and Anxiety Spectrum Disorders (FAST-MAS) Neuropsychopharmacology 45:1656–1663 doi: 10.1038/s41386-020-0738-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese PS, Lipkowski AW, Takemori AE (1987) Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists Life Sci 40:1287–1292 [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, Kieffer BL, Evans CJ (2012) Ligand-directed signalling within the opioid receptor family Br J Pharmacol 167:960–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley CC, Lindsley CW (2018) DARK Classics in Chemical Neuroscience: Opium, a Historical Perspective ACS Chem Neurosci 9:2503–2518 doi: 10.1021/acschemneuro.8b00459 [DOI] [PubMed] [Google Scholar]
- Quirion R, Pilapil C (1991) Distribution of multiple opioid receptors in the human brain. In: Mendelsohn FAO, Paxinos G (eds) Receptors in the human nervous system. Academic Press, Inc., San Diego, CA, pp 103–121 [Google Scholar]
- Quirion R, Pilapil C, Magnan J (1987) Localization of kappa opioid receptor binding sites in human forebrain using [3H]U69,593: comparison with [3H]bremazocine Cell Mol Neurobiol 7:303–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Chavkin C (2008) Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system Psychopharmacology (Berl) 200:59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB (2002) Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist Proc Natl Acad Sci U S A 99:11934–11939 doi: 10.1073/pnas.182234399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunk E, Rosskothen I, Wittmann W, Gaburro S, Singewald N, Herzog H, Schwarzer C (2008) Behavioural characterization of prodynorphin knockout mice BMC Pharmacol 8 (Suppl. 1):A5 doi:doi: 10.1186/1471-2210-8-S1-A5 [DOI] [Google Scholar]
- Schwarzer C (2009) 30 years of dynorphins--new insights on their functions in neuropsychiatric diseases Pharmacol Ther 123:353–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi N, Diehl N, Yaswen L, Brennan MB, Hochgeschwender U (2001) Generation of dynorphin knockout mice Brain Res Mol Brain Res 86:70–75 doi: 10.1016/s0169-328x(00)00264-3 [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A (1986) Differential effects of mu and kappa opioid systems on motivational processes NIDA Res Monogr 75:563–566 [PubMed] [Google Scholar]
- Shukla VK, Lemaire S (1994) Non-opioid effects of dynorphins: possible role of the NMDA receptor Trends Pharmacol Sci 15:420–424 doi: 10.1016/0165-6147(94)90091-4 [DOI] [PubMed] [Google Scholar]
- Simon EJ, Hiller JM, Edelman I (1973) Stereospecific binding of the potent narcotic analgesic (3H) Etorphine to rat-brain homogenate Proceedings of the National Academy of Sciences of the United States of America 70:1947–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Gaveriaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattei MG, Charron G, Bloch B, Kieffer B (1995) kappa-Opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system Proc Natl Acad Sci U S A 92:7006–7010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le M, Roques BP, Maldonado R, Kieffer BL (1998) Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal EMBO Journal 17:886–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowe SJ, Simonin F, Kieffer B, Kitchen I (1999) Quantitative autoradiography of mu-,delta- and kappa1 opioid receptors in kappa-opioid receptor knockout mice Brain Res 818:335–345 [DOI] [PubMed] [Google Scholar]
- Terenius L (1973) Stereospecific interaction between narcotic analgesics and a synaptic plasm a membrane fraction of rat cerebral cortex Acta Pharmacol Toxicol (Copenh) 32:317–320 doi: 10.1111/j.1600-0773.1973.tb01477.x [DOI] [PubMed] [Google Scholar]
- Therapeutics C (2020) https://www.caratherapeutics.com. Accessed December 8, 2020
- Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J, Tanaka T, Nagase H (2002) Antipruritic activity of the kappa-opioid receptor agonist, TRK-820 Eur J Pharmacol 435:259–264 [DOI] [PubMed] [Google Scholar]
- Tseng PY, Hoon MA (2020) Molecular Genetics of Kappa Opioids in Pain and Itch Sensations Handb Exp Pharmacol doi: 10.1007/164_2020_397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso PH, Wong YH (2000) G(z) can mediate the acute actions of mu- and kappa-opioids but is not involved in opioid-induced adenylyl cyclase supersensitization J Pharmacol Exp Ther 295:168–176 [PubMed] [Google Scholar]
- Unterwald EM, Knapp C, Zukin RS (1991) Neuroanatomical localization of kappa 1 and kappa 2 opioid receptors in rat and guinea pig brain Brain Res 562:57–65 [DOI] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WA Jr. (2013) Role of kappa-opioid receptors in stress and anxiety-related behavior Psychopharmacology (Berl) 229:435–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Voigtlander PF, Lahti RA, Ludens JH (1983) U-50,488: a selective and structurally novel non-Mu (kappa) opioid agonist J Pharmacol Exp Ther 224:7–12 [PubMed] [Google Scholar]
- Wadenberg ML (2003) A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist CNS Drug Rev 9:187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JJ, Evans CJ, Chavkin C (1991) Focal stimulation of the mossy fibers releases endogenous dynorphins that bind kappa 1-opioid receptors in guinea pig hippocampus J Neurochem 57:333–343 doi: 10.1111/j.1471-4159.1991.tb02132.x [DOI] [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Abreu ME, Bigelow GE (2001) Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans Psychopharmacology (Berl) 157:151–162 [DOI] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Anderson V, Minkoff H, Hurd YL (2006) Discrete opioid gene expression impairment in the human fetal brain associated with maternal marijuana use Pharmacogenomics J 6:255–264 doi: 10.1038/sj.tpj.6500375 [DOI] [PubMed] [Google Scholar]
- Wang YJ, Rasakham K, Huang P, Chudnovskaya D, Cowan A, Liu-Chen LY (2011) Sex difference in ê-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5’-O-(3-[35S]thiotriphosphate) binding in the guinea pig J Pharmacol Exp Ther 339:438–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KL, Robinson JE, Zhu H, DiBerto JF, Polepally PR, Zjawiony JK, Nichols DE, Malanga CJ, Roth BL (2015) The G protein-biased kappa-opioid receptor agonist RB-64 Is analgesic with a unique spectrum of activities in vivo J Pharmacol Exp Ther 352:98–109 doi:jpet.114.216820 [pii]; 10.1124/jpet.114.216820 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C (2009) Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone Neuropsychopharmacology 34:775–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC (2012) Structure of the human kappa-opioid receptor in complex with JDTic Nature 485:327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajecka JM, Stanford AD, Memisoglu A, Martin WF, Pathak S (2019) Buprenorphine/samidorphan combination for the adjunctive treatment of major depressive disorder: results of a phase III clinical trial (FORWARD-3) Neuropsychiatr Dis Treat 15:795–808 doi: 10.2147/ndt.S199245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir N, Weber E, Palkovits M, Brownstein M (1984) Differential processing of prodynorphin and proenkephalin in specific regions of the rat brain Proc Natl Acad Sci U S A 81:6886–6889 doi: 10.1073/pnas.81.21.6886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chen C, Xue JC, Kunapuli S, DeRiel JK, Liu-Chen L-Y (1995) Cloning of a human kappa opioid receptor from the brain Life Sci 56:L201–L207 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1 A video clip of 3-D images of KtdT/KtdT mouse brains showing KtdT distribution. Adult KtdT/KtdT mice were perfused and brains were cleared via CLARITY. Brains were imaged without IHC from dorsal and ventral sides and images were digitally re-constructed into 3-D images. Experiments were performed on three brains with similar results. [Reproduced from Chen et al. (2020)].


