ABSTRACT
Objective:
Chloroquine or hydroxychloroquine has demonstrated no effect on the treatment of hospitalized COVID-19 patients. This study aimed to answer questions related to the use of hydroxychloroquine for pre-exposure or post-exposure prophylaxis of SARS-CoV-2 infection and in the treatment of patients with mild COVID-19 in terms of hospitalization, adverse events, and mortality.
Methods:
This was a systematic review and meta-analysis of phase 3 randomized clinical trials, selected from various databases, which compared patients who received hydroxychloroquine for SARS-CoV-2 prophylaxis or treatment of mild COVID-19 cases with controls.
Results:
A total number of 1,376 studies were retrieved. Of those, 9 met the eligibility criteria and were included in the study. No statistically significant differences were found between the hydroxychloroquine and control groups in terms of pre- or post-exposure prophylaxis of SARS-CoV-2 infection. The use of hydroxychloroquine increased the risk of adverse events by 12% (95% CI, 6-18%; p < 0.001), and the number needed to harm was 9. In addition, no significant differences were found between the hydroxychloroquine and control groups regarding hospitalization (risk difference [RD] = −0.02; 95% CI, −0.04 to 0.00; p = 0.14) or mortality (RD = 0.00; 95% CI, −0.01 to 0.02; p = 0.98) in the treatment of mild COVID-19.
Conclusions:
The use of hydroxychloroquine for prophylaxis of SARS-CoV-2 infection or treatment of patients with mild COVID-19 is not recommended.
Keywords: Hydroxychloroquine, COVID-19, SARS-CoV-2
RESUMO
Objetivo:
A cloroquina ou hidroxicloroquina não apresentou nenhum efeito no tratamento de pacientes hospitalizados com COVID-19. O objetivo deste estudo foi responder a questões a respeito do uso de hidroxicloroquina na profilaxia da infecção por SARS-CoV-2 pré ou pós-exposição e no tratamento de pacientes com COVID-19 leve no tocante à hospitalização, eventos adversos e mortalidade.
Métodos:
Trata-se de uma revisão sistemática e meta-análise de ensaios clínicos controlados aleatórios de fase 3 que foram selecionados por meio de buscas em diversos bancos de dados e que compararam controles e pacientes que receberam hidroxicloroquina para profilaxia de SARS-CoV-2 ou tratamento de COVID-19 leve.
Resultados:
Foram identificados 1.376 estudos. Destes, 9 preencheram os critérios de elegibilidade e foram incluídos no estudo. Não foram encontradas diferenças significativas entre os grupos hidroxicloroquina e controle quanto à profilaxia da infecção por SARS-CoV-2 pré ou pós-exposição. O uso de hidroxicloroquina aumentou o risco de eventos adversos em 12% (IC95%: 6-18%; p < 0,001), e o número necessário para prejudicar foi 9. Não foram encontradas diferenças significativas entre os grupos hidroxicloroquina e controle quanto à hospitalização [diferença de risco (DR) = −0,02; IC95%: −0,04 a 0,00; p = 0,14] e mortalidade (DR = 0,00; IC95%: −0,01 a 0,02; p = 0,98) no tratamento de COVID-19 leve.
Conclusões:
Não é recomendado o uso de hidroxicloroquina nem na profilaxia da infecção por SARS-CoV-2 nem no tratamento de pacientes com COVID-19 leve.
Descritores: Hidroxicloroquina, Infecções por coronavirus, Betacoronavirus
INTRODUCTION
COVID-19 is caused by SARS-CoV-2, which emerged in China in December of 2019, and has been declared a pandemic by the World Health Organization. The economy of each country is represented by the impairment in the rate of infected cases and mortality in the population, along with access to vaccines against SARS-CoV-2, and the national policies implemented to reduce airborne transmission are represented by the load on the health care system. 1 In this context, empiric pharmacological treatment strategies to prevent or control the progression of COVID-19 have been debated in different scenarios and discussed in the scientific literature. 2 , 3 )
COVID-19 is a novel disease that required implementing rapid treatment proposals to reduce transmission, protecting exposed subjects, and decreasing mortality. The use of chloroquine or hydroxychloroquine has been suggested for reducing viral load and controlling disease severity. 4 However, after over a year of living with the COVID-19 pandemic, we have accumulated scientific evidence stating that the use of hydroxychloroquine is futile for treating hospitalized COVID-19 patients. Indeed, the actual treatment guidelines are supported by the premise of the best medical evidence, and there is none to support the use of hydroxychloroquine to reduce the need for mechanical ventilation or all-cause mortality rate. 5 Conversely, there are places where the routine use of hydroxychloroquine is still being recommended as an optimal intervention to prevent infection in subjects with a high risk of contamination (pre-exposure prophylaxis or post-exposure prophylaxis) or to control severity progression of COVID-19 after an infection. Moreover, there are no systematic reviews assessing the use of hydroxychloroquine in patients with mild COVID-19. Therefore, there is a lack of knowledge to determine whether chloroquine or hydroxychloroquine can prevent SARS-CoV-2 infection or control COVID-19 severity in non-hospitalized patients. The objective of the present study was to collect and evaluate evidence from the literature regarding these topics and to provide treatment recommendations. To that end, we addressed the following clinical questions: “Does hydroxychloroquine prevent illness in individuals who have not been diagnosed with COVID-19 but have had contact with an infected individual?” and “Does hydroxychloroquine reduce the chances of hospitalization, the development of adverse events, or the risk of mortality in patients with mild COVID-19?”
METHODS
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations. 6
Eligibility criteria
The protocol of this study was based on the Patients of interest, Intervention to be studied, Comparison of intervention, and Outcome of interest (PICO) methodology. Regarding the prophylactic use of hydroxychloroquine, the PICO framework was as follows: Patients: pre-exposure (not diagnosed with COVID-19) or post-exposure (positive RT-PCR for SARS-CoV-2) patients; Intervention: use of hydroxychloroquine; Comparison: standard treatment or placebo; and Outcome: individuals with positive RT-PCR tests, hospitalization (ward or ICU admission), mortality, and adverse events. We also investigated beneficial or harmful outcomes due to the use of hydroxychloroquine in adults at risk for SARS-CoV-2 infection. Health care workers at hospital-based units were considered at risk for being infected. Regarding patients with mild COVID-19, the PICO framework was as follows: Patients: patients with a confirmed positive RT-PCR test who had not been hospitalized prior to randomization; Intervention: use of hydroxychloroquine; and Comparison: standard treatment or placebo; and Outcome: hospitalization (ward or ICU admission), mortality, and adverse events.
The eligibility criteria for the inclusion of studies were phase 3 randomized controlled trials (RCTs) and phase 3 RCTs systematically reviewing the PICO questions. We imposed no restrictions regarding date of publication, language, or full-text availability.
Information sources and search strategy
Two of the authors developed the search strategy, which was revised and approved by the team, selected information sources, and systematically searched the following databases: MEDLINE, EMBASE, Central Cochrane, and ClinicalTrials.gov. Specific search strategies were used for each database: 1: (“COVID” OR “COV” OR “coronavirus” OR “SARS”); 2: (“chloroquine” OR “chlorochin” OR “hydroxychloroquine” OR “oxychloroquine” OR “hydroxychlorochin”) 3: 1 AND 2; and 4: 3 AND (Random*).
Study selection
Two independent researchers selected and extracted the data from the included studies. First, the articles were selected based on the title and abstract. Second, full texts were evaluated in order to include or exclude the studies; disagreements were resolved by consensus.
Data collection and investigated outcomes
Data regarding authorship, year of publication, patient description, interventions (hydroxychloroquine and control), outcomes, and follow-up period were extracted from the studies.
Regarding prophylaxis with hydroxychloroquine, the results (outcomes) collected were positive RT-PCR (longer follow-up), hospitalization, adverse events, severe adverse events, and mortality. Regarding treatment of mild COVID-19 cases with hydroxychloroquine, the outcomes were hospitalization, adverse events, severe adverse events, and mortality. Control groups varied among the studies.
Risk of bias and quality of evidence
The risk of bias was assessed using the Cochrane risk-of-bias (RoB 2) 7 tool as were other fundamental elements, being expressed as very serious, serious, or non-serious. The quality of the evidence was extrapolated from the risk of bias and was described by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) terminology as very low, low, or high, and, for meta-analyses, it was described by the GRADEpro Guideline Development Tool (GDT; McMaster University, Hamilton, ON, Canada), as very low, low, moderate, or high.
Synthesis of results and analysis
Categorical outcomes were expressed by group (hydroxychloroquine and control), number of events, and calculated risk (in %) for each group (by dividing the number of events by the total number of patients in each group). If the risk difference between the groups was significant, a 95% CI was expressed on the basis of the number needed to treat or the number needed to harm (NNH). We used fixed-effect meta-analysis to evaluate the effect of hydroxychloroquine vs. control on the outcomes when those data were available in at least two RCTs considered to have homogeneous study characteristics. Effects of meta-analyses were reported as risk differences (RD) and corresponding 95% CIs; a 95% CI including the number 0 in its range meant that there was no difference in the outcome effect between the hydroxychloroquine and control arms. The use of RD shows the absolute effect size in the meta-analysis when compared with relative risk (RR) or odds ratio, and this technique can be used when the binary outcome is zero in both study arms. Heterogeneity of effects among studies was quantified with the I2 statistic (an I2 > 50% means high heterogeneity). For the meta-analysis, we used the Review Manager software, version 5.4 (RevMan 5; Cochrane Collaboration, Oxford, United Kingdom).
RESULTS
A total of 1,376 studies were retrieved from the selected databases (Figure 1). After eliminating duplicates and including studies that met the eligibility criteria, 58 studies were selected for the assessment of their full texts (MEDLINE: 51; EMBASE: 4; and ClinicalTrials.gov: 3). Of those, 49 studies were excluded. Therefore, 9 RCTs 8 - 16 were selected, whose characteristics (Table 1), results, risk of bias, quality of evidence, and synthesis of evidence are described below (Tables 2-5).
Figure 1. Flow chart of the selection process in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations. RCT: randomized clinical trial.
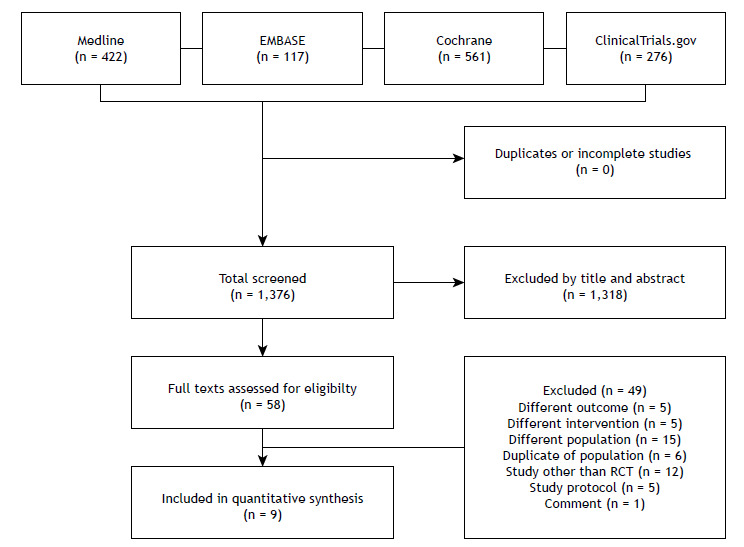
Table 1. Description of the studies included. (Continue...).
| Study/country | Participants (N) | Type/identifier | Context | Eligibility criterion | Group | Outcome | Follow-up period |
|---|---|---|---|---|---|---|---|
| Abella et al.
8
United States of America |
132 | Parallel RCT NCT04329923 | Post-exposure prophylaxis | Health care workers at COVID-19 units and no previous SARS-CoV-2 infection within the last 2 weeks | Placebo vs. Hydroxychloroquine, 600 mg/day for 8 weeks |
- Positive test for SARS-CoV-2 during 8 weeks - Adverse events |
8 weeks |
| Mitjà et al.
10
Spain |
2,485 | Cluster RCT NCT04304053 | Post-exposure prophylaxis | Health care workers, household contacts, and nursing home workers or residents with no previous SARS-CoV-2 infection within the last 2 weeks | Usual care vs. Hydroxychloroquine, 800 mg on day 1, followed by 600 mg/day for 6 days |
- Symptoms and positive test for SARS-CoV-2 - Hospitalization - Adverse events - Death |
4 weeks |
| Boulware et al.
12
Unites States of America and Canada |
821 | Parallel RCT NCT04308668 |
Post-exposure prophylaxis | Household or occupational exposure to individuals with confirmed COVID-19 (distance ≤ 6 ft for >10 min with an infected subject or no use of face mask or eye shield) | Placebo vs. Hydroxychloroquine, 800 mg on day 1 and 600 mg within 6-8 h after the first dose, followed by 600 mg/day for 4 days |
- Positive test for SARS-CoV-2 - Hospitalization - Adverse events - Deaths |
2 weeks |
| Rajasingham et al.
11
United States of America and Canada |
1,483 | Parallel RCT NCT04328467 |
Pre-exposure prophylaxis | Health care workers with high risk for SARS-CoV-2 exposure (ICU, ER, COVID-19 units) | Placebo (folic acid) vs. Hydroxychloroquine, 400 mg on day 1 and 400 mg 6-8 h later, followed by 400 mg once a week for 12 weeks vs. Hydroxychloroquine, 400 mg on day 1 and 400 mg 6-8 h later, followed by 400 mg twice a week for 12 weeks |
- COVID-19 free (no symptoms or negative RT-PCR result) - Hospitalization - Adverse events - Death |
12 weeks |
| Barnabas et al.
9
United States of America |
689 | Parallel RCT NCT04328961 |
Post-exposure prophylaxis | Contact with an index case diagnosed SARS-CoV-2 infection within 96 h | Placebo vs. Hydroxychloroquine, 400 mg for 3 days, followed by 200 mg/day for 11 days |
- Positive test for SARS-CoV-2 - Adverse events |
14 days |
| Omrani et al.
14
Qatar |
456 | Triple parallel RCT NCT04349592 |
Outpatients with mild COVID-19 | Mild disease or no symptoms, outpatients | Placebo vs. Hydroxychloroquine, 600 mg/day for 1 week vs. Hydroxychloroquine, 600 mg/day for 1 week + azithromycin |
- Viral load - Hospitalization - Severe adverse events - Death |
14 days |
| Reis et al.
13
Brazil |
685 | Triple parallel RCT NCT04403100 |
Mild COVID-19 | Outpatients reporting less than 8 days since onset of flu-like symptoms or chest CT consistent with COVID-19 | Placebo vs. Hydroxychloroquine, 800 mg as a loading dose, followed by 400 mg daily for 9 days vs. Lopinavir-ritonavir loading dose of 800 mg and 200 mg, respectively, every 12 h, followed by 400 mg and 100 mg, respectively, every 12 h for the following 9 days |
- Adverse events - Severe adverse events - Hospitalization - Deaths |
90 days |
| Mitjà et al.
15
Spain |
293 | Parallel RCT NCT04304053 |
Mild symptoms of COVID-19 | Outpatients; symptoms for less than 5 days prior to enrollment | Usual care vs. Hydroxychloroquine, 800 mg on day 1, followed by 400 mg/day for 6 days |
- Viral load - WHO progression scale - Hospitalization - Severe adverse events - Deaths |
28 days |
| Skipper et al.
16
United States of America and Canada |
491 | Parallel RCT NCT04308668 |
Mild COVID-19 | Outpatients, positive SARS-CoV-2 test and symptoms for ≤ 4 days or compatible symptoms after high-risk exposure to a contact with PCR-confirmed SARS-CoV-2 within the last 14 days | Placebo vs. Hydroxychloroquine, 800 mg once and 600 mg in 6-8 h, followed by 600 mg daily for another 4 more days |
- Hospitalization - Adverse events - Deaths |
14 days |
RCT: randomized controlled trial.
Table 2. Risk of bias of the individual studies included on the use of hydroxychloroquine for prophylaxis of SARS-CoV-2 infection.
| Study | Year | Randomization | Blinding/Allocation concealment | Double blinding | Blinding of outcome assessors | Loss | Prognostic characteristic | Appropriate outcome | Intention-to-treat analysis | Sample size calculation | Early interruption |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abella et al. 8 | 2021 | Low | Low | Low | Uncertain | Low | High | Low | High | Low | Low |
| Barnabas et al. 9 | 2021 | Low | Low | Low | Uncertain | High | Uncertain | Low | High | Low | Low |
| Mitjà et al. 10 | 2021 | Low | Low | High | Uncertain | Low | Low | Low | High | Low | Low |
| Rajasingham et al. 11 | 2020 | Low | Low | Low | Uncertain | Low | Low | Low | High | Low | Low |
| Boulware et al. 12 | 2020 | Low | Low | Low | Uncertain | Uncertain | High | Low | Uncertain | High | Low |
Table 3. Risk of bias of the individual studies included on the treatment of mild COVID-19 patients with hydroxychloroquine.
| Study | Year | Randomization | Blinding/Allocation concealment | Double blinding | Blinding of outcome assessors | Loss | Prognostic characteristic | Appropriate outcome | Intention-to-treat analysis | Sample size calculation | Early interruption |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reis et al. 13 | 2021 | Low | Low | Low | Uncertain | Low | Low | Low | Low | Low | Low |
| Omrani et al. 14 | 2020 | Low | Low | Low | Uncertain | Low | Low | Low | Low | Low | Low |
| Mitjà et al. 15 | 2020 | Low | Low | High | Uncertain | Low | Low | Low | Low | Low | Low |
| Skipper et al. 16 | 2020 | Low | Low | Low | Uncertain | Low | Low | Low | Low | Low | Low |
We assumed that the risk of bias in the studies selected to support the conclusions on the treatment was not serious. The quality of evidence in the analysis of prophylaxis varied according to the analyzed outcome: diagnosis of COVID-19 (moderate), hospitalization (moderate), adverse events (very low), serious adverse events (very low), and mortality (moderate). The quality of evidence in the analysis of mild COVID-19 treatment varied according to the analyzed outcome: hospitalization (high), adverse events (very low), serious adverse events (high), and mortality (high).
Hydroxychloroquine for pre- or post-exposure prophylaxis of SARS-CoV-2 infection
The follow-up period ranged from 2 to 8 weeks in the studies selected. No statistically significant difference was found regarding the incidence of positive COVID-19 results (RT-PCR) between the hydroxychloroquine and control groups for pre- or post-exposure prophylaxis of SARS-CoV-2 infection during the follow-up period (RD = 0.01; 95% CI, −0.01 to 0.02; p = 0.13; Figure 2A). The RR was 1.19 (95% CI, 0.95-1.50). The quality of evidence was moderate (Table 4).
Figure 2. Comparison between hydroxychloroquine and control groups for prophylaxis of SARS-CoV-2 infection regarding the incidence of positive RT-PCR results (in A); hospitalization (in B); adverse events (in C); serious adverse events (in D); and deaths (in E). HCQ: hydroxychloroquine; M-H: Mantel-Haenszel (method); and df: degrees of freedom.
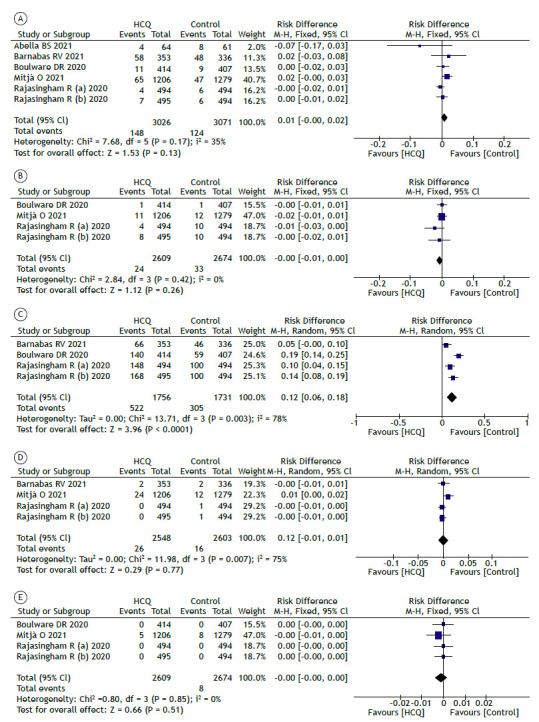
Table 4. Table of evidence of the use of hydroxychloroquine for prophylaxis of SARS-CoV-2 infection. Question: Should hydroxychloroquine, when compared with controls, be used for pre-exposure or post-exposure prophylaxis of SARS-CoV-2 infection?
| Certainty assessment | Patient (n) | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies (n) | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | HCQ | CONTROL | Relative (95% CI) | Absolute (95% CI) | ||
| POSITIVE RT-PCR | ||||||||||||
| 6 | randomized trials | seriousa,b,c,d,e | not serious | not serious | not serious | none | 148/3026 (4.9%) | 124/3071 (4.0%) | RR 1.19 (0.95 to 1.50) | 8 more per 1,000 (from 2 fewer to 20 more) | MODERATE | |
| HOSPITALIZATION | ||||||||||||
| 4 | randomized trials | seriousa,b,c,d,e | not serious | not serious | not serious | none | 24/2609 (0.9%) | 33/2674 (1.2%) | RR 0.74 (0.44 to 1.25) | 3 fewer per 1,000 (from 7 fewer to 3 more) | MODERATE | |
| ADVERSE EFFECTS | ||||||||||||
| 4 | randomized trials | seriousa,b,c,d,e | very serious f | not serious | not serious | publication bias strongly suspected g | 522/1756 (29.7%) | 305/1731 (17.6%) | RR 1.69 (1.36 to 2.09) | 122 more per 1,000 (from 63 more to 192 more) | VERY LOW | |
| SERIOUS ADVERSE EFFECTS | ||||||||||||
| 4 | randomized trials | seriousa,b,c,d,e | very serious f | not serious | not serious | publication bias strongly suspected g | 26/2548 (1.0%) | 16/2603 (0.6%) | RR 1.70 (0.91 to 3.17) | 4 more per 1,000 (from 1 fewer to 13 more) | VERY LOW | |
| DEATHS | ||||||||||||
| 4 | randomized trials | seriousa,b,c,d,e | not serious | not serious | not serious | none | 5/2609 (0.2%) | 8/2674 (0.3%) | RR 0.66 (0.22 to 2.02) | 1 fewer per 1,000 (from 2 fewer to 3 more) | MODERATE | |
HCQ: hydroxychloroquine; and RR: Risk ratio.
Explanations
a. ABSENCE OF INTENTION-TO-TREAT ANALYSIS
b. UNBALANCED PROGNOSTIC CHARACTERISTICS BETWEEN THE GROUPS
c. ABSENCE OF SAMPLE CALCULATION
d. ABSENCE OF DOUBLE BLINDING
e. LOSSES OVER 20%
f. HETEROGENEITY GREATER THAN 75%
g. OUTLIER
There was no significant difference between the hydroxychloroquine and control groups regarding the incidence of hospitalization during the follow-up period (RD = −0.00 [95% CI, −0.01 to −0.00]; p = 0.26; Figure 2B; and RR = 0.74 [95% CI, 0.44-1.25]). The quality of evidence was moderate (Table 4). The use of prophylactic hydroxychloroquine increased the risk of adverse events by 12% (95% CI, 6-8%; p < 0.001; NNH = 9) when compared with the control group (RR = 1.69 [95% CI, 1.36-2.09]; Figure 2C). However, the quality of evidence was very low (Table 4).
In terms of the incidence of serious adverse events, no statistically significant difference was found between the hydroxychloroquine and control groups (RD = 0.00 [95% CI, −0.01 to 0.01]; p = 0.77; Figure 2D; and RR = 1.70 [95% CI, 0.91-3.17]). The quality of evidence was very low (Table 4). Likewise, no statistically significant difference was found regarding the incidence of mortality between the groups (RD: −0.00 [95% CI, −0.00 to 0.00]; p = 0.51; Figure 2E; and RR = 0.66 [95% CI, 0.22-2.02]). The quality of evidence was moderate (Table 4).
Hydroxychloroquine for treating mild COVID-19
When we compared the hydroxychloroquine and control groups that included patients with mild COVID-19, no statistical differences (Figure 3) were found regarding hospitalizations (RD = −0.02 [95% CI, −0.04 to 0.00]; p = 0.14; Figure 3A; and RR = 0.68 [95% CI, 0.41-1.14]), with high quality of evidence (Table 5); adverse events (RD = 0.11 [95% CI: −0.09 to 0.31]; p = 0.27; Figure 3B; and RR = 1.47 [95% CI, 0.79-2.72]), with very low quality of evidence (Table 5); serious adverse events (RD = −0.00 [95% CI, −0.04 to 0.04]; p = 0.95); Figure 3C; and RR = 0.97 [95% CI, 0.44-2.16]); and mortality (RD = 0.00 [95% CI, −0.01 to 0.01]; p = 0.98; Figure 3D; and RR = 1.07 [95% CI, 0.15-7.86]), both with high quality of evidence (Table 5).
Figure 3. Comparison between hydroxychloroquine and control groups for the treatment of mild COVID-19 regarding the incidence of hospitalizations (in A); adverse events (in B); serious adverse events (in C);.and deaths (in D). HCQ: hydroxychloroquine; M-H: Mantel-Haenszel (method); and df: degrees of freedom.
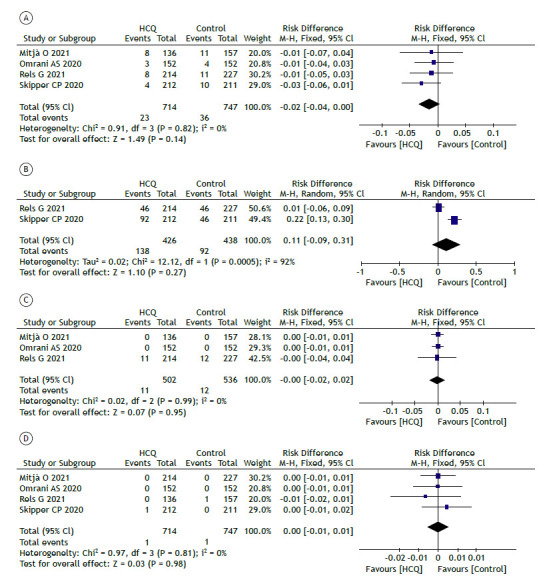
Table 5. Table of evidence of the use of hydroxychloroquine for the treatment of mild COVID-19. Question: Should hydroxychloroquine, compared with controls, be used for the treatment of mild COVID-19?
| Certainty assessment | Patient (n)s | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies (n) | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | HCQ | CONTROL | Relative (95% CI) | Absolute (95% CI) | ||
| HOSPITALIZATION | ||||||||||||
| 4 | randomized trials | not serious | not serious | not serious | not serious | none | 23/714 (3.2%) | 36/747 (4.8%) | RR 0.68 (0.41 to 1.14) | 15 fewer per 1,000 (from 28 fewer to 7 more) | HIGH | |
| ADVERSE EFFECTS | ||||||||||||
| 2 | randomized trials | not serious | very serious a | not serious | serious b | publication bias strongly suspected c | 138/426 (32.4%) | 92/438 (21.0%) | RR 1.47 (0.79 to 2.72) | 99 more per 1,000 (from 44 fewer to 361 more) | VERY LOW | |
| SERIOUS ADVERSE EFFECTS | ||||||||||||
| 3 | randomized trials | not serious | not serious | not serious | not serious | none | 11/502 (2.2%) | 12/536 (2.2%) | RR 0.97 (0.44 to 2.16) | 1 fewer per 1,000 (from 13 fewer to 26 more) | HIGH | |
| DEATHS | ||||||||||||
| 4 | randomized trials | not serious | not serious | not serious | not serious | none | 1/714 (0.1%) | 1/747 (0.1%) | RR 1.07 (0.15 to 7.86) | 0 fewer per 1,000 (from 1 fewer to 9 more) | HIGH | |
HCQ: hydroxychloroquine; and RR: Risk ratio.
Explanations
a. HETEROGENEITY GREATER THAN 75%
b. WIDE CI
c. OUTLIER
DISCUSSION
The main results of this systematic review showed that the use of hydroxychloroquine for pre- or post-exposure prophylaxis of SARS-CoV-2 had no effect on the incidence rate of confirmed SARS-CoV-2 positivity and that its use increased the risk of adverse events by 12%. In addition, the use of hydroxychloroquine in mild COVID-19 patients caused no significant differences in the rates of hospitalization, adverse events, and mortality.
The choice of relevant clinical outcomes is fundamental in defining the effectiveness of a medical treatment, and this is also true for COVID-19. treatment. For potential COVID-19 patients, prophylaxis is essential to prevent disease, and the treatment of patients with mild COVID-19 is necessary to prevent hospitalization (ward or ICU admission) and disease progression.
Our results are similar to those of a previous systematic review comprising two RCTs that studied the use of hydroxychloroquine for pre- or post-exposure prophylaxis against SARS-CoV-2 infectio. 17 - 19 However, this is the first review that studied the use of hydroxychloroquine only in patients with mild COVID-19 to assess disease progression. Our systematic review included one more RCT than did a study by Lewis et al. 19 to evaluate the efficacy of pre-exposure or post-exposure prophylaxis with hydroxychloroquine. By adding that RCT to the analysis, we obtained results that were similar to those reported by Lewis et al., 19 but we identified a decrease in the 95% CI related to risk. In other words, we reduced the uncertainty of pre- or post-exposure prophylaxis with hydroxychloroquine, and we reinforce the recommendation of not using hydroxychloroquine for that. Likewise, Hernandez et al. 18 described cohort studies and RCTs on the use of hydroxychloroquine as an intervention.
When we analyzed the results regarding the use of hydroxychloroquine in patients with mild COVID-19, most of the RoB 2 table items presented with a low risk of bias, and, concomitantly, the quality of evidence in most of the outcomes was high, which reinforces our final recommendation of not using hydroxychloroquine for the treatment of mild COVID-19 patients.
Phase 3 RCTs have several fundamental characteristics that guarantee the lowest degree of uncertainty when two forms of treatment or prophylaxis are compared: a. homogeneous samples in both groups are compared (patients with similar characteristics); b. allocation of patients to groups has no influence or interference by using random methods (unpredictability guarantees the same chance for any individual to be allocated to any of the groups); c. the population is represented (sample size estimation and power analysis that guarantees applicability and reproduction of results in practice); d. interventions are blinded (avoiding interference in the application of interventions); e. there is loss of control (avoiding manipulation in patient selection); f. procedures and interventions are standardized (avoiding variations in processes, doses, co-interventions, etc.); and g. statistical analyses are performed directly using the number of events and averages, with no need for corrections. These characteristics are absent in comparative observational studies (cohort studies).
Several barriers can hamper the performance of RCTs, including three major barriers: 1. lack of patients (rare diseases); 2. technologies that are difficult to implement (incomparable, expensive, or complex); and 3. a long time for outcomes to occur (requiring a long follow-up period). However, this is not the case with COVID-19.
The available evidence can change over time. However, there is a considerable degree of certainty that can be conferred by individual RCTs or meta-analyses using such studies, which greatly reduces the likelihood that new studies will emerge and modify the conclusions. Therefore, the use of hydroxychloroquine for prophylaxis of SARS-CoV-2 infection or for treatment of mild COVID-19 patients is unjustifiable and is currently contraindicated in order to avoid uncertainties and difficulties in making decisions.
The number of patients included in the present systematic review and meta-analysis is adequate, and the results are reproducible and can be applied in the management and care of patients.
This systematic review has limitations that need to be elucidated. First, we were unable to examine funnel plots to detect publication bias, given the small number of RCTs. However, we used a comprehensive search strategy. Second, we did not register or publish our protocol before, given the urgency to demonstrate the best evidence to be implemented in the local clinical practice. Nevertheless, all outcomes for this systematic review were defined a priori.
FINAL CONSIDERATIONS
Regarding the use of hydroxychloroquine for prophylaxis of SARS-CoV-2 infection, there were no significant differences in the incidence of infected cases (positive RT-PCR), hospitalization, serious adverse events, and mortality between the groups during the follow-up period. In addition, the use of pre- or post-exposure prophylaxis with hydroxychloroquine increased the risk of adverse events by 12% (95% CI, 6-8%; NNH = 9) when compared with controls during the follow-up period. The quality of evidence varied from very low to moderate. Likewise, no significant differences in the number of hospitalizations, serious adverse events, and deaths were found between the hydroxychloroquine and control groups in patients with mild COVID-19, and the quality of evidence was high. The same result was found regarding the incidence of adverse events, but the quality of evidence was very low. Therefore, the use of hydroxychloroquine in the prophylaxis of SARS-CoV-2 infection or treatment of patients with mild COVID-19 is not recommended.
Footnotes
Financial support: None.
Study carried out at the Faculdade de Medicina de Botucatu, Universidade Estadual Paulista, Botucatu (SP) Brasil.
REFERENCES
- 1.World Health Organization [homepage on the Internet] WHO Coronavirus (COVID-19) Dashboard. Geneva: World Health Organization; c2021. https://covid19.who.int/table [Google Scholar]
- 2.Smit M, Marinosci A, Agoritsas T, Calmy A. Prophylaxis for COVID-19 a systematic review. Clin Microbiol Infect. 2021;27(4):532–537. doi: 10.1016/j.cmi.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welte T, Ambrose LJ, Sibbring GC, Sheikh S, Müllerová H, Sabir I. Current evidence for COVID-19 therapies a systematic literature review. Eur Respir Rev. 2021;30(159):200384–200384. doi: 10.1183/16000617.0384-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16–16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Update Alert 3 Hydroxychloroquine or Chloroquine for the Treatment or Prophylaxis of COVID-19. Ann Intern Med. 2020;173(11):W156–W157. doi: 10.7326/L20-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. he PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700–b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I. RoB 2 a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898–l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 8.Abella BS, Jolkovsky EL, Biney BT, Uspal JE, Hyman MC, Frank I. Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers A Randomized Clinical Trial. JAMA Intern Med. 2021;181(2):195–202. doi: 10.1001/jamainternmed.2020.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnabas RV, Brown ER, Bershteyn A, Stankiewicz Karita HC, Johnston C, Thorpe LE. Hydroxychloroquine as Postexposure Prophylaxis to Prevent Severe Acute Respiratory Syndrome Coronavirus 2 Infection A Randomized Trial [published correction appears in Ann Intern. Med. 2021;174(3):435–435. doi: 10.7326/M20-6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitjà O, Corbacho-Monné M, Ubals M, Alemany A, Suñer C, Tebé C. A Cluster-Randomized Trial of Hydroxychloroquine for Prevention of Covid-19. N Engl J Med. 2021;384(5):417–427. doi: 10.1056/NEJMoa2021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajasingham R, Bangdiwala AS, Nicol MR, Skipper CP, Pastick KA, Axelrod ML. Hydroxychloroquine as Pre-exposure Prophylaxis for Coronavirus Disease 2019 (COVID-19) in Healthcare Workers A Randomized Trial. Clin Infect Dis. 2021;72(11):e835–e843. doi: 10.1093/cid/ciaa1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med. 2020;383(6):517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reis G, Moreira Silva EADS, Medeiros Silva DC, Thabane L, Singh G, Park JJH. Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19 The TOGETHER Randomized Clinical Trial. JAMA Netw Open. 2021;4(4):e216468. doi: 10.1001/jamanetworkopen.2021.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omrani AS, Pathan SA, Thomas SA, Harris TRE, Coyle PV, Thomas CE. Randomized double-blinded placebo-controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non-severe Covid-19. EClinicalMedicine. 2020;29:100645–100645. doi: 10.1016/j.eclinm.2020.100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitjà O, Corbacho-Monné M, Ubals M, Tebe C, Peñafiel J, Tobias A, et al. Hydroxychloroquine for Early Treatment of Adults with Mild Covid-19: A Randomized-Controlled Trial. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM. Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 A Randomized Trial [published correction appears in Ann Intern. Med. 2021;174(3):435–435. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database Syst Rev. 2021;2(2):CD013587–CD013587. doi: 10.1002/14651858.CD013587.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Hydroxychloroquine or Chloroquine for Treatment or Prophylaxis of COVID-19 A Living Systematic Review. Ann Intern Med. 2020;173(4):287–296. doi: 10.7326/M20-2496. [DOI] [PubMed] [Google Scholar]
- 19.Lewis K, Chaudhuri D, Alshamsi F, Carayannopoulos L, Dearness K, Chagla Z. The efficacy and safety of hydroxychloroquine for COVID-19 prophylaxis A systematic review and meta-analysis of randomized trials. PLoS One. 2021;16(1):e0244778. doi: 10.1371/journal.pone.0244778. [DOI] [PMC free article] [PubMed] [Google Scholar]


